Recent Advances in Anti-Tuberculosis Drug Discovery Based on Hydrazide–Hydrazone and Thiadiazole Derivatives Targeting InhA
Abstract
1. Introduction
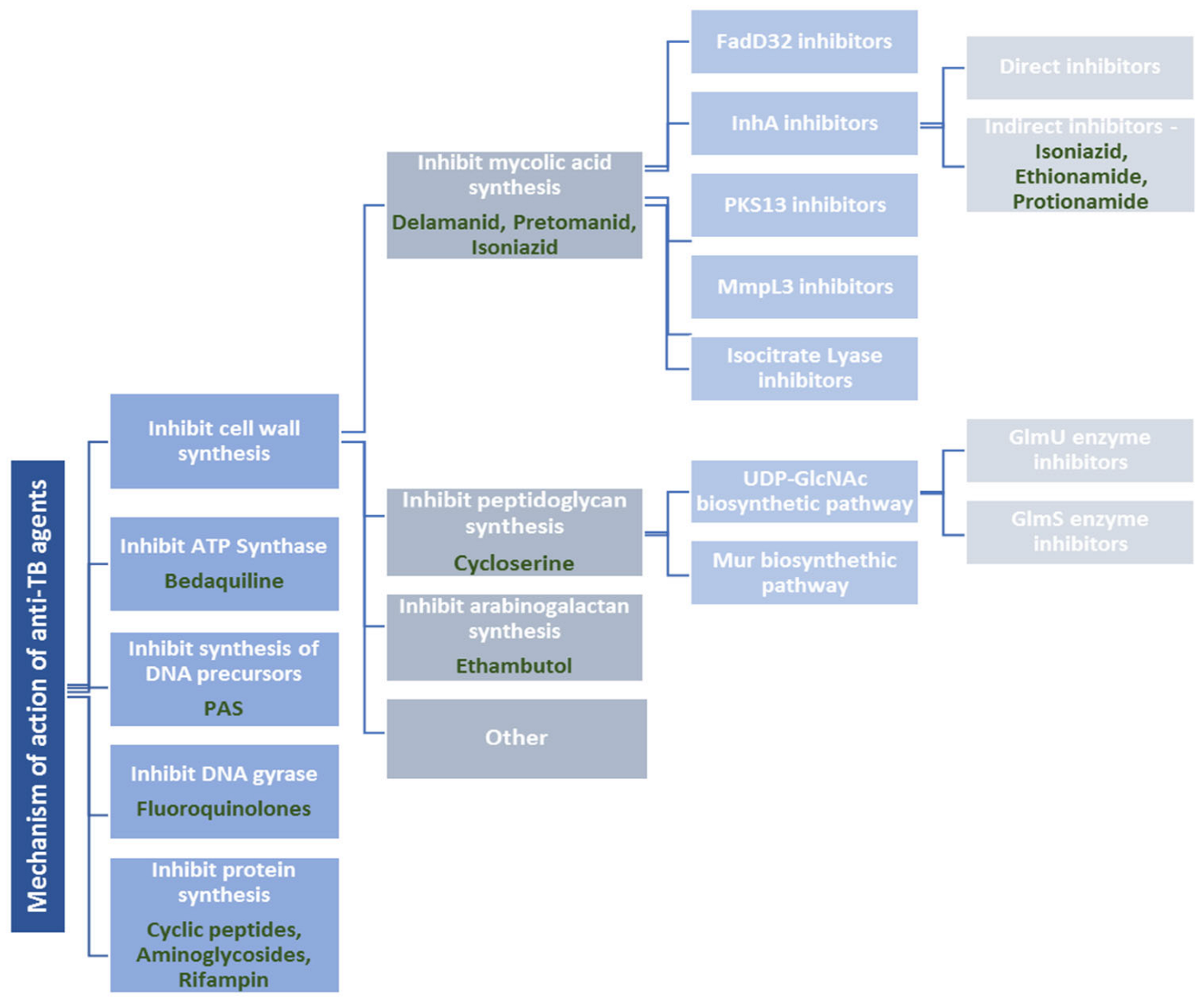
2. Drug-Resistant Tuberculosis and Mechanism of Action of Currently Available Anti-Tuberculosis Agents
2.1. Cell Wall Synthesis Inhibitors
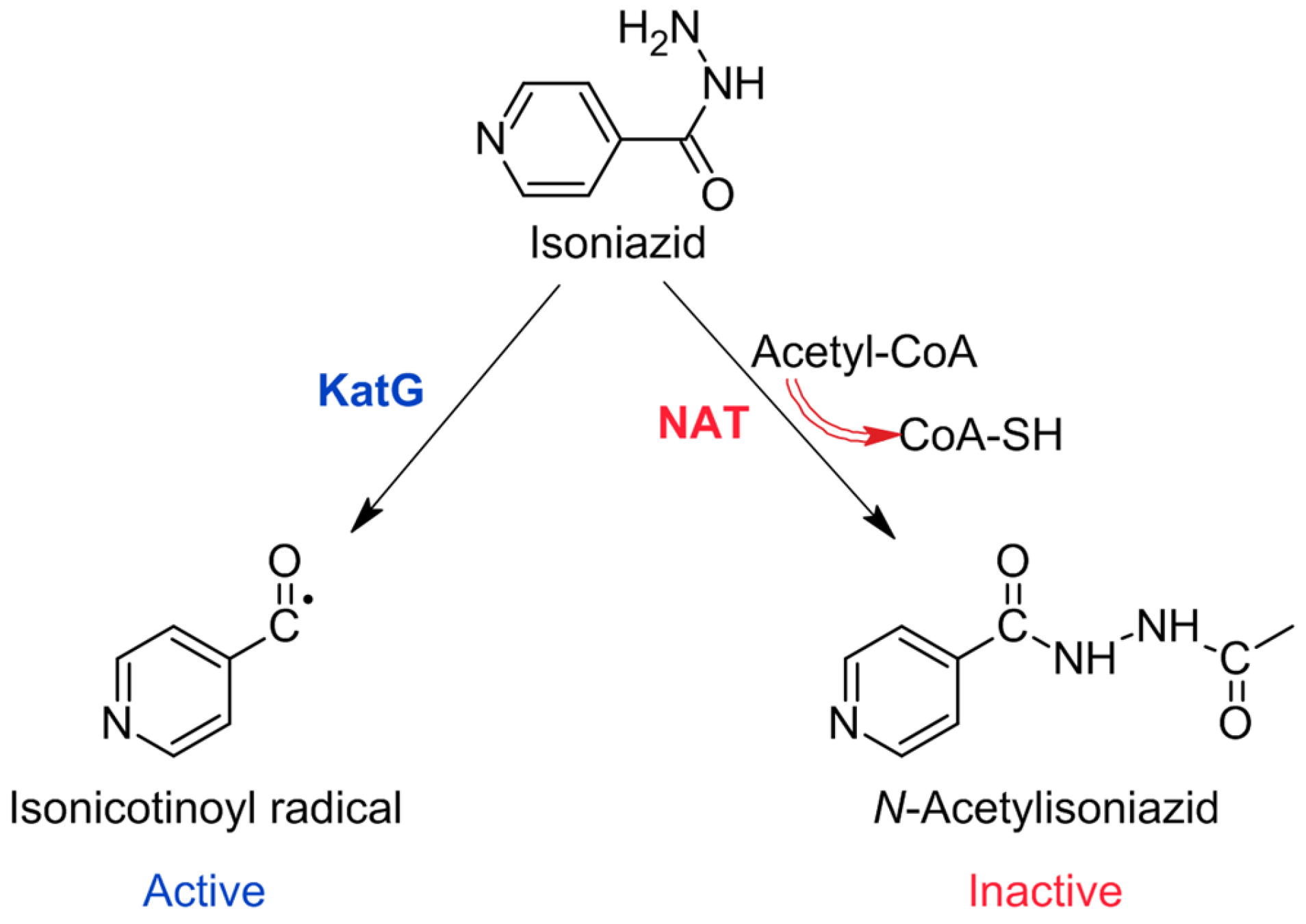
2.1.1. Drugs Affecting Mycolic Acid (MA) Synthesis
2.1.2. Drugs Affecting Peptidoglycan Synthesis
2.1.3. Drugs Affecting Arabinogalactan Synthesis
2.2. ATP-Synthase Inhibitors
2.3. Inhibitors of Synthesis of DNA Precursors and DNA Gyrase
2.4. Protein Synthesis Inhibitors
2.5. Drugs with Another Mechanism of Action
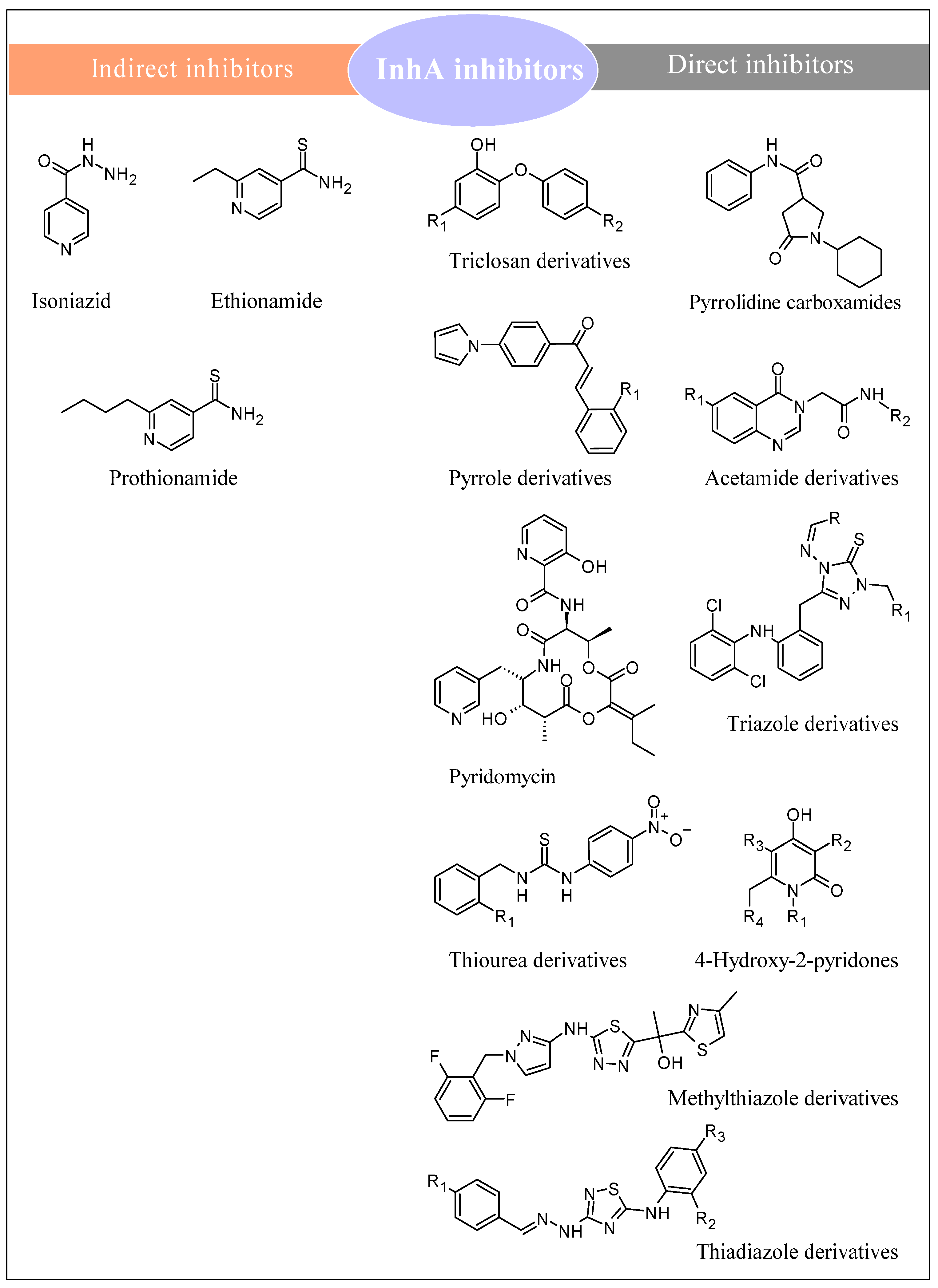
3. Anti-Tubercular Structures Targeting InhA
3.1. InhA and Its Role in M. tuberculosis
3.2. Hydrazide-Hydrazones
3.3. Thiadiazoles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. World Tuberculosis Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- WHO. WHO Consolidated Guidelines on Tuberculosis Module 4: Treatment Drug-Susceptible Tuberculosis Treatment; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Migliori, G.B.; Tiberi, S. WHO drug-resistant TB guidelines 2022: What is new? Int. J. Tuberc. Lung Dis. 2022, 26, 590–591. [Google Scholar] [CrossRef] [PubMed]
- Mi, J.; Gong, W.; Wu, X. Advances in Key Drug Target Identification and New Drug Development for Tuberculosis. BioMed Res. Int. 2022, 2022, 5099312. [Google Scholar] [CrossRef] [PubMed]
- Doğan, Ş.D.; Gündüz, M.G.; Doğan, H.; Krishna, V.S.; Lherbet, C.; Sriram, D. Design and synthesis of thiourea-based derivatives as Mycobacterium tuberculosis growth and enoyl acyl carrier protein reductase (InhA) inhibitors. Eur. J. Med. Chem. 2020, 199, 112402. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.S.; Bhole, R.P.; Khedekar, P.B.; Chikhale, R.V. Mycobacterium enoyl acyl carrier protein reductase (InhA): A key target for antitubercular drug discovery. Bioorg. Chem. 2021, 115, 105242. [Google Scholar] [CrossRef] [PubMed]
- Rožman, K.; Sosič, I.; Fernandez, R.; Young, R.J.; Mendoza, A.; Gobec, S.; Encinas, L. A new ‘golden age’ for the antitubercular target InhA. Drug Discov. Today 2017, 22, 492–502. [Google Scholar] [CrossRef]
- Shirude, P.S.; Madhavapeddi, P.; Naik, M.; Murugan, K.; Shinde, V.; Nandishaiah, R.; Bhat, J.; Kumar, A.; Hameed, S.; Holdgate, G.; et al. Methyl-thiazoles: A novel mode of inhibition with the potential to develop novel inhibitors targeting InhA in Mycobacterium tuberculosis. J. Med. Chem. 2013, 56, 8533–8542. [Google Scholar] [CrossRef]
- Sharma, P.C.; Sharma, D.; Sharma, A.; Saini, N.; Goyal, R.; Ola, M.; Chawla, R.; Thakur, V.K. Hydrazone comprising compounds as promising anti-infective agents: Chemistry and structure-property relationship. Mater. Today Chem. 2020, 18, 100349. [Google Scholar] [CrossRef]
- Peixoto, A.d.S.; Araújo, R.M.; Schindler, H.C.; Pimentel, L.M.L.M. Treatment of Sensitive Tuberculosis—Mechanisms of Action and Resistance. Biomed. J. Sci. Tech. Res. 2019, 18, 13715–13718. [Google Scholar] [CrossRef]
- Kamsri, P.; Hanwarinroj, C.; Phusi, N.; Pornprom, T.; Chayajarus, K.; Punkvang, A.; Suttipanta, N.; Srimanote, P.; Suttisintong, K.; Songsiriritthigul, C.; et al. Discovery of New and Potent InhA Inhibitors as Anti-tuberculosis Agents: Structure Based Virtual Screening Validated by Biological Assays and X-ray Crystallography. J. Chem. Inf. Model. 2020, 60, 226–234. [Google Scholar] [CrossRef]
- Doğan, H.; Doğan, Ş.D.; Gündüz, M.G.; Krishna, V.S.; Lherbet, C.; Sriram, D.; Şahin, O.; Sarıpınar, E. Discovery of hydrazone containing thiadiazoles as Mycobacterium tuberculosis growth and enoyl acyl carrier protein reductase (InhA) inhibitors. Eur. J. Med. Chem. 2020, 188, 112035. [Google Scholar] [CrossRef]
- Fahmi, M.R.G.; Kurniawan, Y.S. Heterocyclic hydrazone derivatives as potential antitubercular agent against Mycobacterium tuberculosis. J. Exp. Clin. Microbiol. 2019, 2, 16. [Google Scholar]
- Saifullah, B.; Hussein, M.Z.B.; Al Ali, S.H.H. Controlled-release approaches towards the chemotherapy of tuberculosis. Int. J. Nanomed. 2012, 7, 5451–5463. [Google Scholar] [CrossRef] [PubMed]
- Šink, R.; Sosič, I.; Živec, M.; Fernandez-Menendez, R.; Turk, S.; Pajk, S.; Alvarez-Gomez, D.; Lopez-Roman, E.M.; Gonzales-Cortez, C.; Rullas-Triconado, J.; et al. Design, synthesis, and evaluation of new thiadiazole-based direct inhibitors of enoyl acyl carrier protein reductase (InhA) for the treatment of tuberculosis. J. Med. Chem. 2015, 58, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Kuang, W.; Zhang, H.; Wang, X.; Yang, P. Overcoming Mycobacterium tuberculosis through small molecule inhibitors to break down cell wall synthesis. Acta Pharm. Sinica. B 2022, 12, 3201–3214. [Google Scholar] [CrossRef] [PubMed]
- NIAID. Tսbercսlosis Drugs aոԁ Mechanisms of Action. Available online: https://www.niaid.nih.gov/diseases-conditions/tbdrugs (accessed on 6 February 2023).
- Perrin, C.; Athersuch, K.; Elder, G.; Martin, M.; Alsalhani, A. Recently developed drugs for the treatment of drug-resistant tuberculosis: A research and development case study. BMJ Glob. Health 2022, 7, e007490. [Google Scholar] [CrossRef] [PubMed]
- Dartois, V.A.; Rubin, E.J. Anti-tuberculosis treatment strategies and drug development: Challenges and priorities. Nat. Rev. Microbiol. 2022, 20, 685–701. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6234, Cycloserine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cycloserine (accessed on 6 February 2023).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 14052, Ethambutol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ethambutol (accessed on 6 February 2023).
- Fox, G.J.; Menzies, D. A Review of the Evidence for Using Bedaquiline (TMC207) to Treat Multi-Drug Resistant Tuberculosis. Infect. Dis. 2013, 2, 123–144. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 4649, 4-Aminosalicylic acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/4-Aminosalicylic-acid (accessed on 6 February 2023).
- Rasheed, A. A review on the role of InhA inhibitors in Tuberculosis (TB). Med. Med. Sci. Res. 2021, 1, 1–9. [Google Scholar]
- Joshi, S.D.; Dixit, S.R.; Basha, J.; Kulkarni, V.H.; Aminabhavi, T.M.; Nadagouda, M.N.; Lherbet, C. Pharmacophore mapping, molecular docking, chemical synthesis of some novel pyrrolyl benzamide derivatives and evaluation of their inhibitory activity against enoyl-ACP reductase (InhA) and Mycobacterium tuberculosis. Bioorg. Chem. 2018, 81, 440–453. [Google Scholar] [CrossRef]
- Marrakchi, H.; Lanéelle, G.; Quémard, A.K. InhA, a target of the antituberculous drug isoniazid, is involved in a mycobacterial fatty acid elongation system, FAS-II. Microbiology 2000, 146, 289–296. [Google Scholar] [CrossRef]
- Aslan, E.K.; Krishna, V.S.; Armaković, S.J.; Armaković, S.; Şahin, O.; Tønjum, T.; Gündüz, M.G. Linking azoles to isoniazid via hydrazone bridge: Synthesis, crystal structure determination, antitubercular evaluation and computational studies. J. Mol. Liq. 2022, 354, 118873. [Google Scholar] [CrossRef]
- Angelova, V.T.; Valcheva, V.; Vassilev, N.G.; Buyukliev, R.; Momekov, G.; Dimitrov, I.; Saso, L.; Djukic, M.; Shivachev, B. Antimycobacterial activity of novel hydrazide-hydrazone derivatives with 2H-chromene and coumarin scaffold. Bioorg. Med. Chem. Lett. 2017, 27, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Angelova, V.T.; Valcheva, V.; Pencheva, T.; Voynikov, Y.; Vassilev, N.; Mihaylova, R.; Momekov, G.; Shivachev, B. Synthesis, antimycobacterial activity and docking study of 2-aroyl-[1]benzopyrano[4,3-c]pyrazol-4(1H)-one derivatives and related hydrazide-hydrazones. Bioorganic Med. Chem. Lett. 2017, 27, 2996–3002. [Google Scholar] [CrossRef] [PubMed]
- Angelova, V.T.; Pencheva, T.; Vassilev, N.; Simeonova, R.; Momekov, G.; Valcheva, V. New indole and indazole derivatives as potential antimycobacterial agents. Med. Chem. Res. 2019, 28, 485–497. [Google Scholar] [CrossRef]
- Angelova, V.T.; Pencheva, T.; Vassilev, N.; K-Yovkova, E.; Mihaylova, R.; Petrov, B.; Valcheva, V. Development of New Antimycobacterial Sulfonyl Hydrazones and 4-Methyl-1,2,3-Thiadiazole-Based Hydrazone Derivatives. Antibiotics 2022, 11, 562. [Google Scholar] [CrossRef]
- Angelova, V.T.; Simeonova, R. Effects of a new 1,2,3-thiadiazole containing hydrazone antimycobacterial agent on serum and liver biochemical parameters in female mice. Drug Chem. Toxicol. 2022, 45, 113–119. [Google Scholar] [CrossRef]
- Valcheva, V.; Simeonova, R.; Mileva, M.; Philipov, S.; Petrova, R.; Dimitrov, S.; Georgieva, A.; Tsvetanova, E.; Teneva, Y.; Angelova, V.T. In Vivo Toxicity, Redox-Modulating Capacity and Intestinal Permeability of Novel Aroylhydrazone Derivatives as Anti-Tuberculosis Agents. Pharmaceutics 2022, 15, 79. [Google Scholar] [CrossRef]
- Naveen Kumar, H.S.; Parumasivam, T.; Ibrahim, P.; Asmawi, M.Z.; Sadikun, A. Synthesis of hydrophobic N-acylated isonicotinic acid hydrazide derivatives as potential enoyl–acyl carrier protein reductase (InhA) inhibitors. Med. Chem. Res. 2014, 23, 1267–1277. [Google Scholar] [CrossRef]
- Pahlavani, E.; Kargar, H.; Rad, N.S. A Study on Antitubercular and Antimicrobial Activity of Isoniazid Derivative. Zahedan J. Res. Med. Sci. 2015, 17, e1010. [Google Scholar] [CrossRef]
- Cihan-Üstündağ, G.; Şatana, D.; Özhan, G.; Çapan, G. Indole-based hydrazide-hydrazones and 4-thiazolidinones: Synthesis and evaluation as antitubercular and anticancer agents. J. Enzym. Inhib. Med. Chem. 2016, 31, 369–380. [Google Scholar] [CrossRef]
- Velezheva, V.; Brennan, P.; Ivanov, P.; Kornienko, A.; Lyubimov, S.; Kazarian, K.; Nikonenko, B.; Majorov, K.; Apt, A. Synthesis and antituberculosis activity of indole-pyridine derived hydrazides, hydrazide-hydrazones, and thiosemicarbazones. Bioorg. Med. Chem. Lett. 2016, 26, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Ghiano, D.G.; Recio-Balsells, A.; Bortolotti, A.; Defelipe, L.A.; Turjanski, A.; Morbidoni, H.R.; Labadie, G.R. New one-pot synthesis of anti-tuberculosis compounds inspired on isoniazid. Eur. J. Med. Chem. 2020, 208, 112699. [Google Scholar] [CrossRef] [PubMed]
- Santoso, M.; Fahmi, M.R.; Kurniawan, Y.S.; Ersam, T.; Fatmawati, S.; Martak, F.; Fadlan, A.T.i.S. Isoniazid-Isatin Hydrazone Derivatives: Synthesis, Antitubercular Activity and Molecular Docking Studies. Trends Sci. 2021, 18, 39. [Google Scholar] [CrossRef]
- Koçak Aslan, E.; Han, M.İ.; Krishna, V.S.; Tamhaev, R.; Dengiz, C.; Doğan, Ş.D.; Lherbet, C.; Mourey, L.; Tønjum, T.; Gündüz, M.G. Isoniazid Linked to Sulfonate Esters via Hydrazone Functionality: Design, Synthesis, and Evaluation of Antitubercular Activity. Pharmaceuticals 2022, 15, 1301. [Google Scholar] [CrossRef]
- dos Santos Fernandes, G.F.; De Souza, P.C.; Moreno-Viguri, E.; Santivañez-Veliz, M.; Paucar, R.; Pérez-Silanes, S.; Chegaev, K.; Guglielmo, S.; Lazzarato, L.; Fruttero, R. Design, synthesis, and characterization of N-oxide-containing heterocycles with in vivo sterilizing antitubercular activity. J. Med. Chem. 2017, 60, 8647–8660. [Google Scholar] [CrossRef]
- Naveen Kumar, H.S.; Parumasivam, T.; Jumaat, F.; Ibrahim, P.; Asmawi, M.Z.; Sadikun, A. Synthesis and evaluation of isonicotinoyl hydrazone derivatives as antimycobacterial and anticancer agents. Med. Chem. Res. 2014, 23, 269–279. [Google Scholar] [CrossRef]
- More, U.A.; Joshi, S.D.; Aminabhavi, T.M.; Gadad, A.K.; Nadagouda, M.N.; Kulkarni, V.H. Design, synthesis, molecular docking and 3D-QSAR studies of potent inhibitors of enoyl-acyl carrier protein reductase as potential antimycobacterial agents. Eur. J. Med. Chem. 2014, 71, 199–218. [Google Scholar] [CrossRef]
- Ghiya, S.; Joshi, Y.C. Synthesis and antimicrobial evaluation of hydrazones derived from 4-methylbenzenesulfonohydrazide in aqueous medium. Med. Chem. Res. 2016, 25, 970–976. [Google Scholar] [CrossRef]
- Joshi, S.D.; Kumar, D.; Dixit, S.R.; Tigadi, N.; More, U.A.; Lherbet, C.; Aminabhavi, T.M.; Yang, K.S. Synthesis, characterization and antitubercular activities of novel pyrrolyl hydrazones and their Cu-complexes. Eur. J. Med. Chem. 2016, 121, 21–39. [Google Scholar] [CrossRef]
- Concha, C.; Quintana, C.; Klahn, A.H.; Artigas, V.; Fuentealba, M.; Biot, C.; Halloum, I.; Kremer, L.; López, R.; Romanos, J.; et al. Organometallic tosyl hydrazones: Synthesis, characterization, crystal structures and in vitro evaluation for anti-Mycobacterium tuberculosis and antiproliferative activities. Polyhedron 2017, 131, 40–45. [Google Scholar] [CrossRef]
- Oliveira, P.F.M.; Guidetti, B.; Chamayou, A.; André-Barrès, C.; Madacki, J.; Korduláková, J.; Mori, G.; Orena, B.S.; Chiarelli, L.R.; Pasca, M.R.; et al. Mechanochemical Synthesis and Biological Evaluation of Novel Isoniazid Derivatives with Potent Antitubercular Activity. Molecules 2017, 22, 1457. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.W.; Saudi, M.N.; Abdel-Ghany, Y.S.; Ismail, A.; Elzahhar, P.A.; Sriram, D.; Nassra, R.; Abdel-Aziz, M.M.; El-Hawash, S.A. Novel pyrazine based anti-tubercular agents: Design, synthesis, biological evaluation and in silico studies. Bioorg. Chem. 2020, 96, 103610. [Google Scholar] [CrossRef] [PubMed]
- Bonnett, S.A.; Dennison, D.; Files, M.; Bajpai, A.; Parish, T. A class of hydrazones are active against non-replicating Mycobacterium tuberculosis. PLoS ONE 2018, 13, e0198059. [Google Scholar] [CrossRef]
- Bonnett, S.A.; Ollinger, J.; Chandrasekera, S.; Florio, S.; O’Malley, T.; Files, M.; Jee, J.A.; Ahn, J.; Casey, A.; Ovechkina, Y.; et al. A Target-Based Whole Cell Screen Approach to Identify Potential Inhibitors of Mycobacterium tuberculosis Signal Peptidase. ACS Infect. Dis. 2016, 2, 893–902. [Google Scholar] [CrossRef]
- Nogueira, T.; Cruz, L.; Lourenço, M.; Souza, M. Design, Synthesis and Anti-tuberculosis Activity of Hydrazones and N-acylhydrazones Containing Vitamin B6 and Different Heteroaromatic Nucleus. Lett. Drug Des. Discov. 2018, 15, 792–798. [Google Scholar] [CrossRef]
- Sampiron, E.G.; Costacurta, G.F.; Baldin, V.P.; Almeida, A.L.; Ieque, A.L.; Santos, N.C.; Alves-Olher, V.G.; Vandresen, F.; Gimenes, A.C.; Siqueira, V.L.; et al. Hydrazone, benzohydrazones and isoniazid-acylhydrazones as potential antituberculosis agents. Future Microbiol. 2019, 14, 981–994. [Google Scholar] [CrossRef]
- Rohane, S.H.; Chauhan, A.J.; Fuloria, N.K.; Fuloria, S. Synthesis and in vitro antimycobacterial potential of novel hydrazones of eugenol. Arab. J. Chem. 2020, 13, 4495–4504. [Google Scholar] [CrossRef]
- G, S.T.; Subramanian, S.; Eswaran, S. Design, Synthesis and Study of Antibacterial and Antitubercular Activity of Quinoline Hydrazone Hybrids. Heterocycl. Commun. 2020, 26, 137–147. [Google Scholar] [CrossRef]
- Padmini, T.; Bhikshapathi, D.; Suresh, K.; Kulkarni, R.; Kamal, B.R. Novel Aminopyrazole Tagged Hydrazones as Anti-Tubercular Agents: Synthesis and Molecular Docking Studies. Med. Chem. 2021, 17, 344–351. [Google Scholar] [CrossRef]
- de Faria, C.F.; Moreira, T.; Lopes, P.; Costa, H.; Krewall, J.R.; Barton, C.M.; Santos, S.; Goodwin, D.; Machado, D.; Viveiros, M.; et al. Designing new antitubercular isoniazid derivatives with improved reactivity and membrane trafficking abilities. Biomed. Pharm. 2021, 144, 112362. [Google Scholar] [CrossRef]
- Karunanidhi, S.; Chandrasekaran, B.; Karpoormath, R.; Patel, H.M.; Kayamba, F.; Merugu, S.R.; Kumar, V.; Dhawan, S.; Kushwaha, B.; Mahlalela, M.C. Novel thiomorpholine tethered isatin hydrazones as potential inhibitors of resistant Mycobacterium tuberculosis. Bioorg. Chem. 2021, 115, 105133. [Google Scholar] [CrossRef] [PubMed]
- Pflégr, V.; Horváth, L.; Stolaříková, J.; Pál, A.; Korduláková, J.; Bősze, S.; Vinšová, J.; Krátký, M. Design and synthesis of 2-(2-isonicotinoylhydrazineylidene)propanamides as InhA inhibitors with high antitubercular activity. Eur. J. Med. Chem. 2021, 223, 113668. [Google Scholar] [CrossRef]
- Desale, V.J.; Mali, S.N.; Thorat, B.R.; Yamgar, R.S.; Dharanguttikar, S.V.; Dharanguttikar, V.R.; Chtita, S.; Oliveira, M.; Cruz, J.N. Synthesis, Characterization, ‘ADMET-SAR’ Prediction, DPPH Assay, and Anti-Mycobacterium Study of 4-[(substituted benzyl) amino]benzohydrazides and its Hydrazones as the Acyl-CoA Carboxylase, AccD5 Inhibitors. Curr. Comput. Aided Drug Des. 2022. Advance online publication. [Google Scholar] [CrossRef]
- Gobis, K.; Szczesio, M.; Olczak, A.; Korona-Głowniak, I.; Augustynowicz-Kopeć, E.; Mazernt-Politowicz, I.; Ziembicka, D.; Główka, M.L. Differences in the Structure and Antimicrobial Activity of Hydrazones Derived from Methyl 4-Phenylpicolinimidate. Materials 2022, 15, 3085. [Google Scholar] [CrossRef] [PubMed]
- Briffotaux, J.; Xu, Y.; Huang, W.; Hui, Z.; Wang, X.; Gicquel, B.; Liu, S. A Hydrazine-Hydrazone Adamantine Compound Shows Antimycobacterial Activity and Is a Probable Inhibitor of MmpL3. Molecules 2022, 27, 7130. [Google Scholar] [CrossRef]
- Akki, M.; Reddy, D.S.; Katagi, K.S.; Kumar, A.; Devarajegowda, H.C.; Kumari, M.S.; Babagond, V.; Joshi, S.D. Coumarin Hydrazone Oxime Scaffolds as Potent Anti-tubercular Agents: Synthesis, X-ray crystal and Molecular Docking Studies. ChemistrySelect 2022, 7, e202203260. [Google Scholar] [CrossRef]
- Abdelhamid, E.; Mahfouz, N.; Omar, F.; Ibrahim, Y.; Abouwarda, A. Synthesis, Antitubercular Activity and in Silico Studies of Arylidene Hydrazinylimidazolinones. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Senthilkumar, S.; Seralathan, J.; Muthukumaran, G. Synthesis, structure analysis, biological activity and molecular docking studies of some hydrazones derived from 4-aminobenzohydrazide. J. Mol. Struct. 2021, 1226, 129354. [Google Scholar] [CrossRef]
- Lone, M.; Mubarak, M.; Nabi, S.; Amin, S.; Nabi, S.; Kantroo, H.; Samim, M.; Shafi, S.; Ahmad, S.; Ahmad, Z.; et al. Isonicotinoyl-butanoic acid hydrazone derivatives as anti-tubercular agents: In-silico studies, synthesis, spectral characterization and biological evaluation. Med. Chem. Res. 2023, 1–19. [Google Scholar] [CrossRef]
- Hemanth, K.; Lakshmanan, K.; Rajagopal, K.; Byran, P.A.G. A review on biological activities: 1,3,4-thiadiazole and its derivatives. Rasāyan J. Chem. 2022, 15, 1573–1587. [Google Scholar] [CrossRef]
- Irfan, A.; Ullah, S.; Anum, A.; Jabeen, N.; Zahoor, A.F.; Kanwal, H.; Kotwica-Mojzych, K.; Mojzych, M. Synthetic transformations and medicinal significance of 1, 2, 3-thiadiazoles derivatives: An update. Appl. Sci. 2021, 11, 5742. [Google Scholar] [CrossRef]
- Pages, L.B.; Pichel, J.C.; Menendez, R.F.; Velando, E.P.F.; Del Valle, S.G.; Diaz, M.L.L.; Losana, A.M.; Wolfendale, M.J. Glaxo Group: (Pyrazol-3-yl)-1,3,4-thiadiazol-2-amine and (Pyrazol-3-yl)-1,3,4-thiazol-2-amine Compounds. International Patent Application No. WO2010118852A1, 21 October 2010. [Google Scholar]
- Pichel, J.C.; Menendez, R.F.; Velando EP, F.; Del Valle, S.G. Glaxo Group: 3-Amino-pyrazole Derivatives Useful Against Tuberculosis. International Patent Application No. WO2012049161A1, 19 April 2012. [Google Scholar]
- Joshi, S.D.; More, U.A.; Koli, D.; Kulkarni, M.S.; Nadagouda, M.N.; Aminabhavi, T.M. Synthesis, evaluation and in silico molecular modeling of pyrroyl-1,3,4-thiadiazole inhibitors of InhA. Bioorg. Chem. 2015, 59, 151–167. [Google Scholar] [CrossRef]
- Karabanovich, G.; Zemanová, J.; Smutný, T.; Székely, R.; Šarkan, M.; Centárová, I.; Vocat, A.; Pávková, I.; Čonka, P.; Němeček, J.; et al. Development of 3,5-Dinitrobenzylsulfanyl-1,3,4-oxadiazoles and Thiadiazoles as Selective Antitubercular Agents Active against Replicating and Nonreplicating Mycobacterium tuberculosis. J. Med. Chem. 2016, 59, 2362–2380. [Google Scholar] [CrossRef] [PubMed]
- Mali, J.K.; Sutar, Y.B.; Pahelkar, A.R.; Verma, P.M.; Telvekar, V.N. Novel fatty acid-thiadiazole derivatives as potential antimycobacterial agents. Chem. Biol. Drug Des. 2020, 95, 174–181. [Google Scholar] [CrossRef]
- Patel, H.; Jadhav, H.; Ansari, I.; Pawara, R.; Surana, S. Pyridine and nitro-phenyl linked 1,3,4-thiadiazoles as MDR-TB inhibitors. Eur. J. Med. Chem. 2019, 167, 1–9. [Google Scholar] [CrossRef] [PubMed]
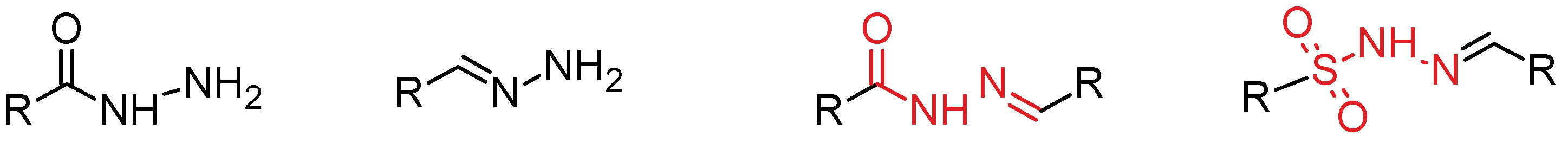
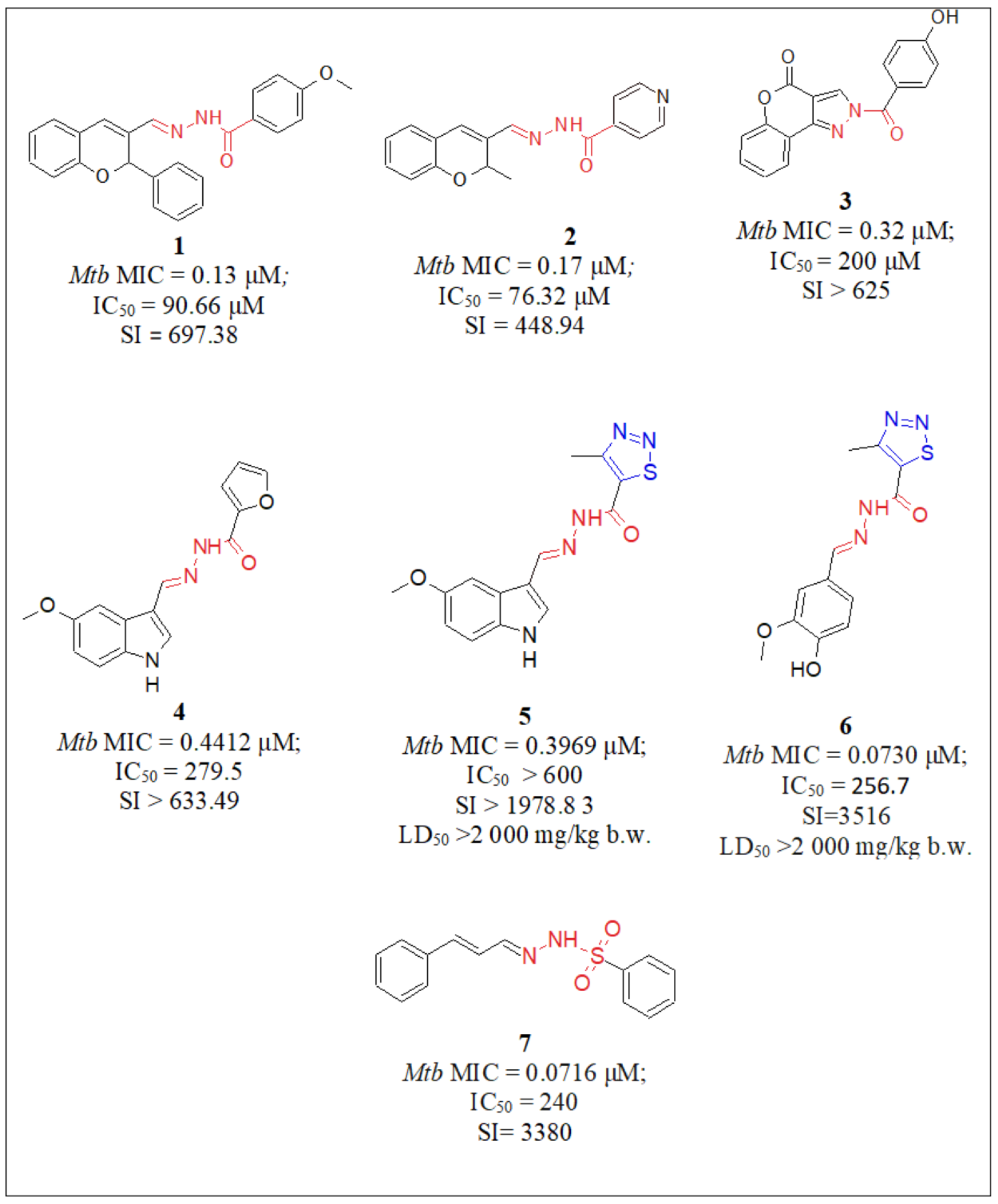
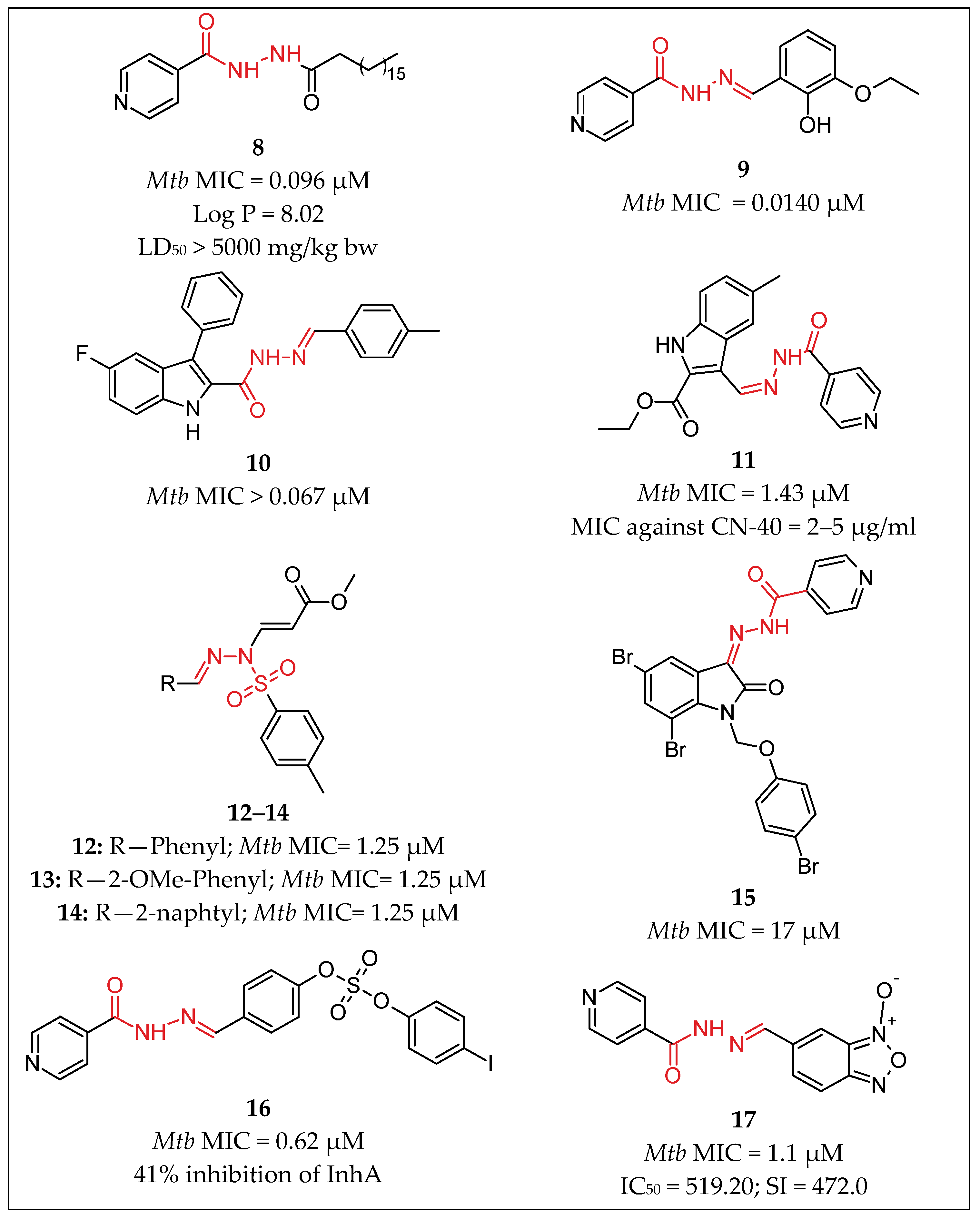
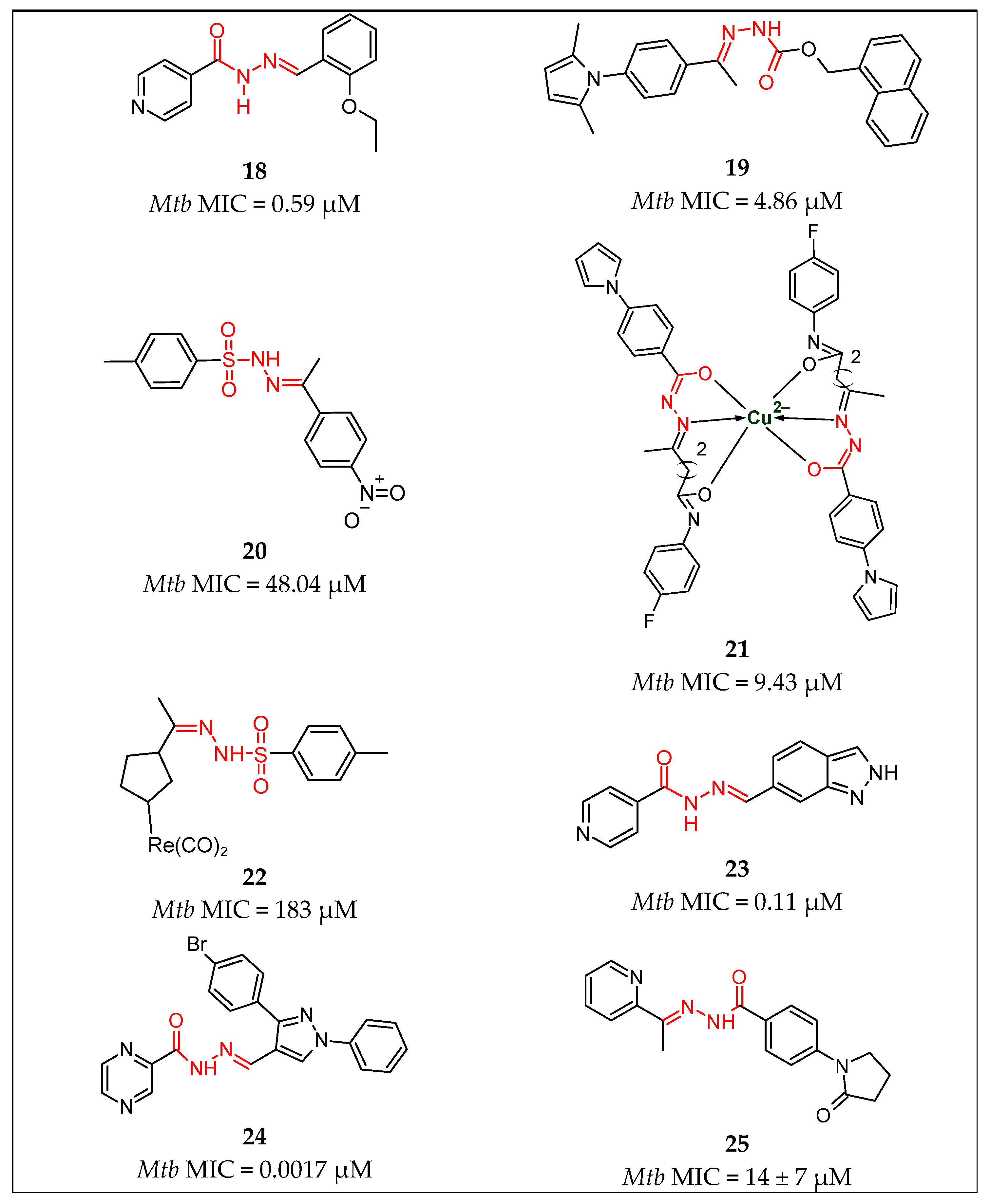
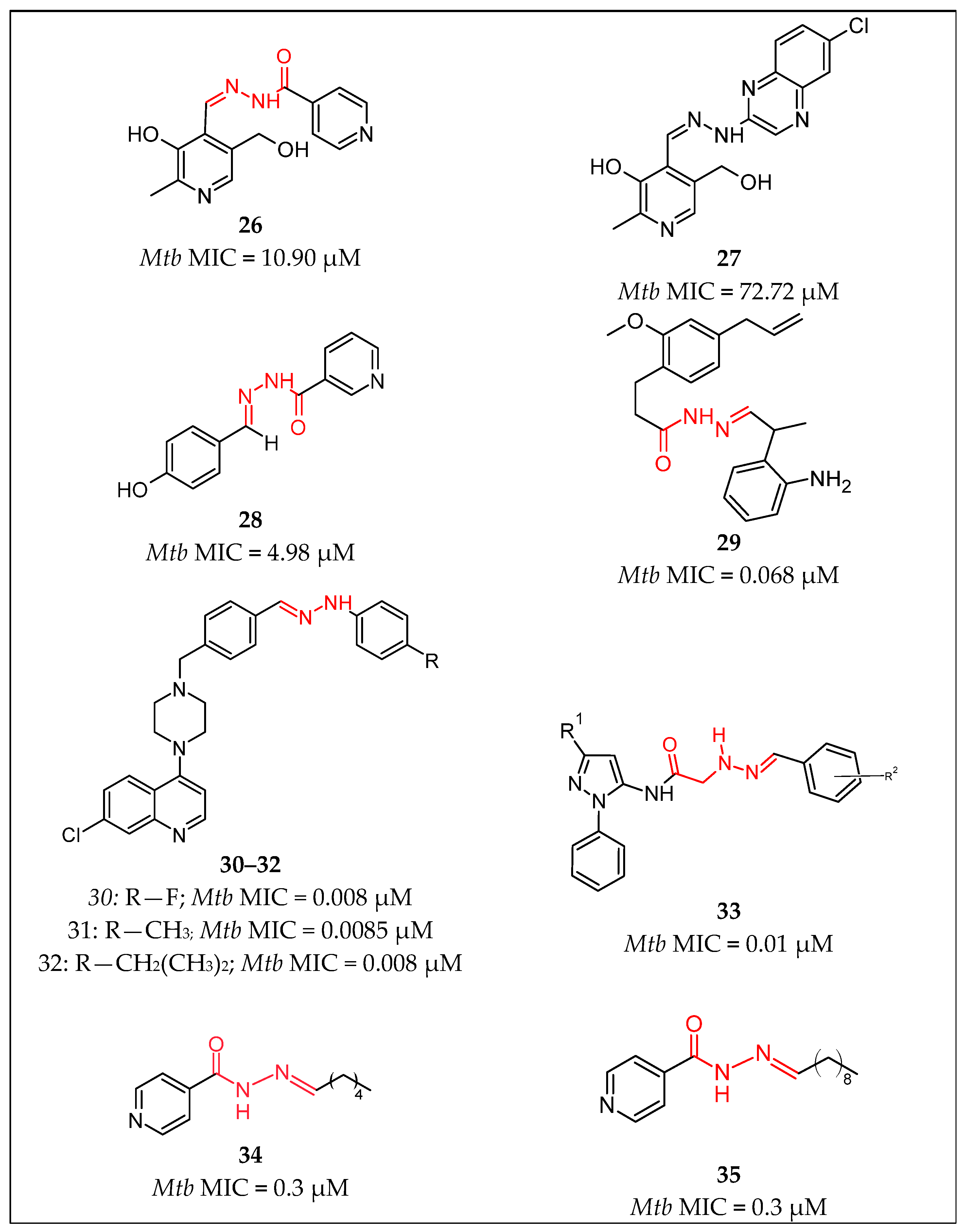
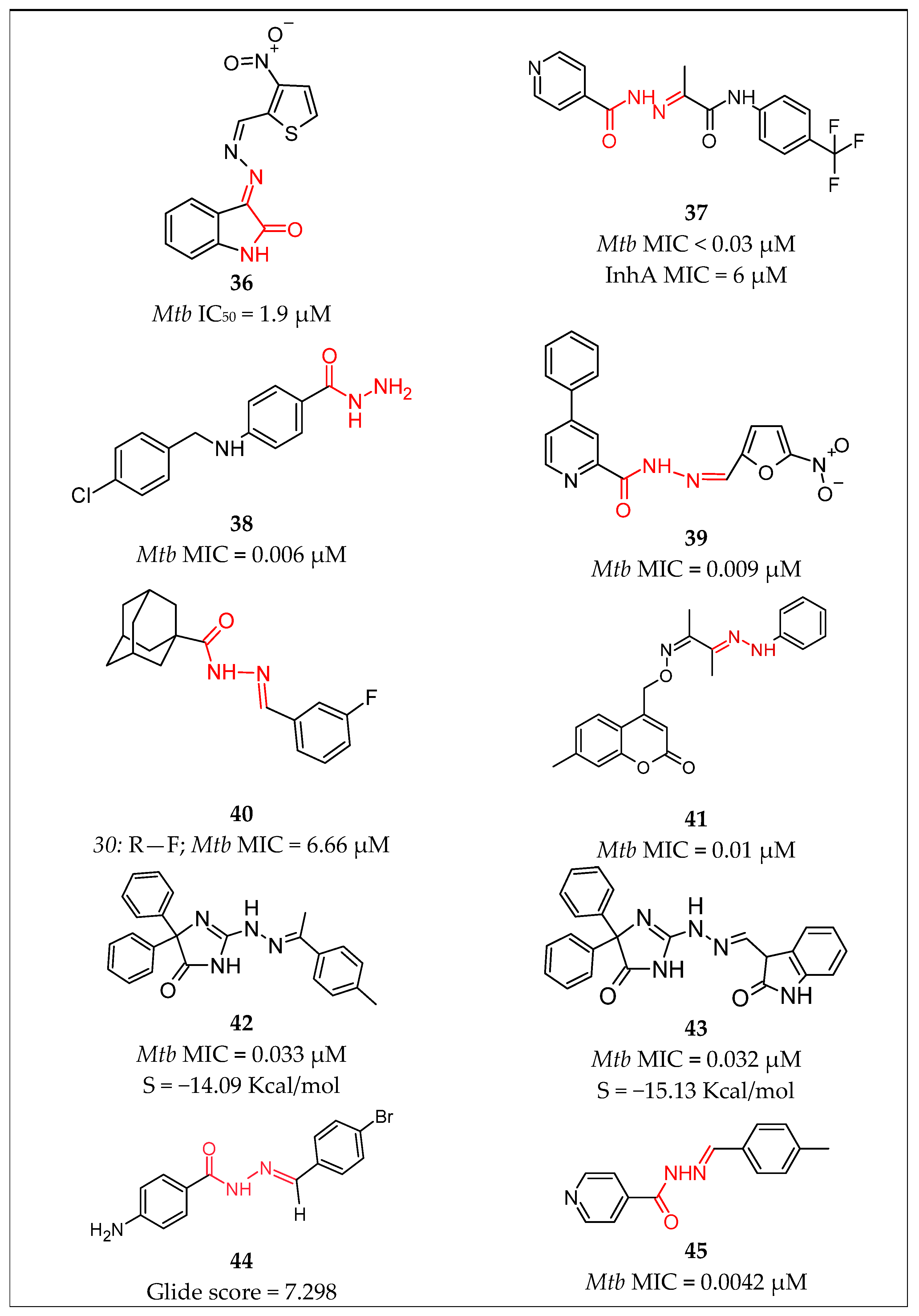
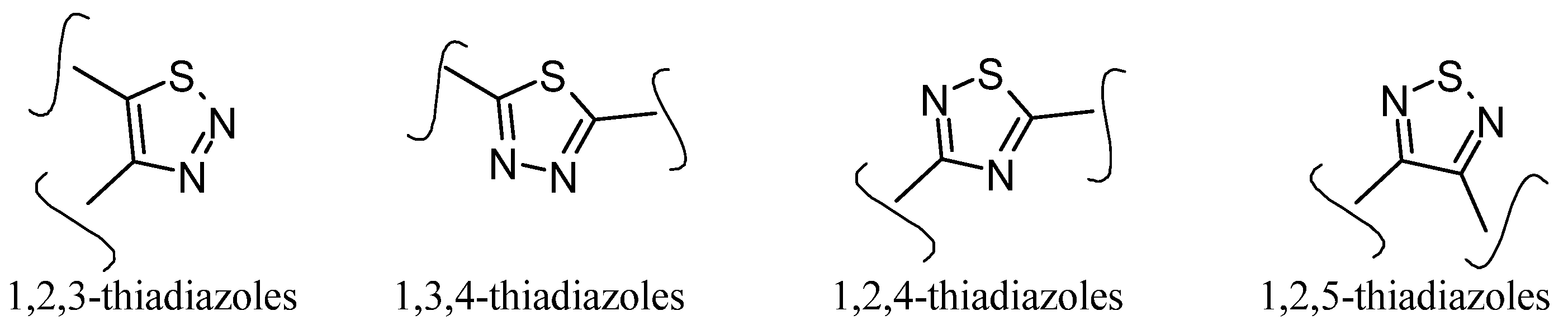
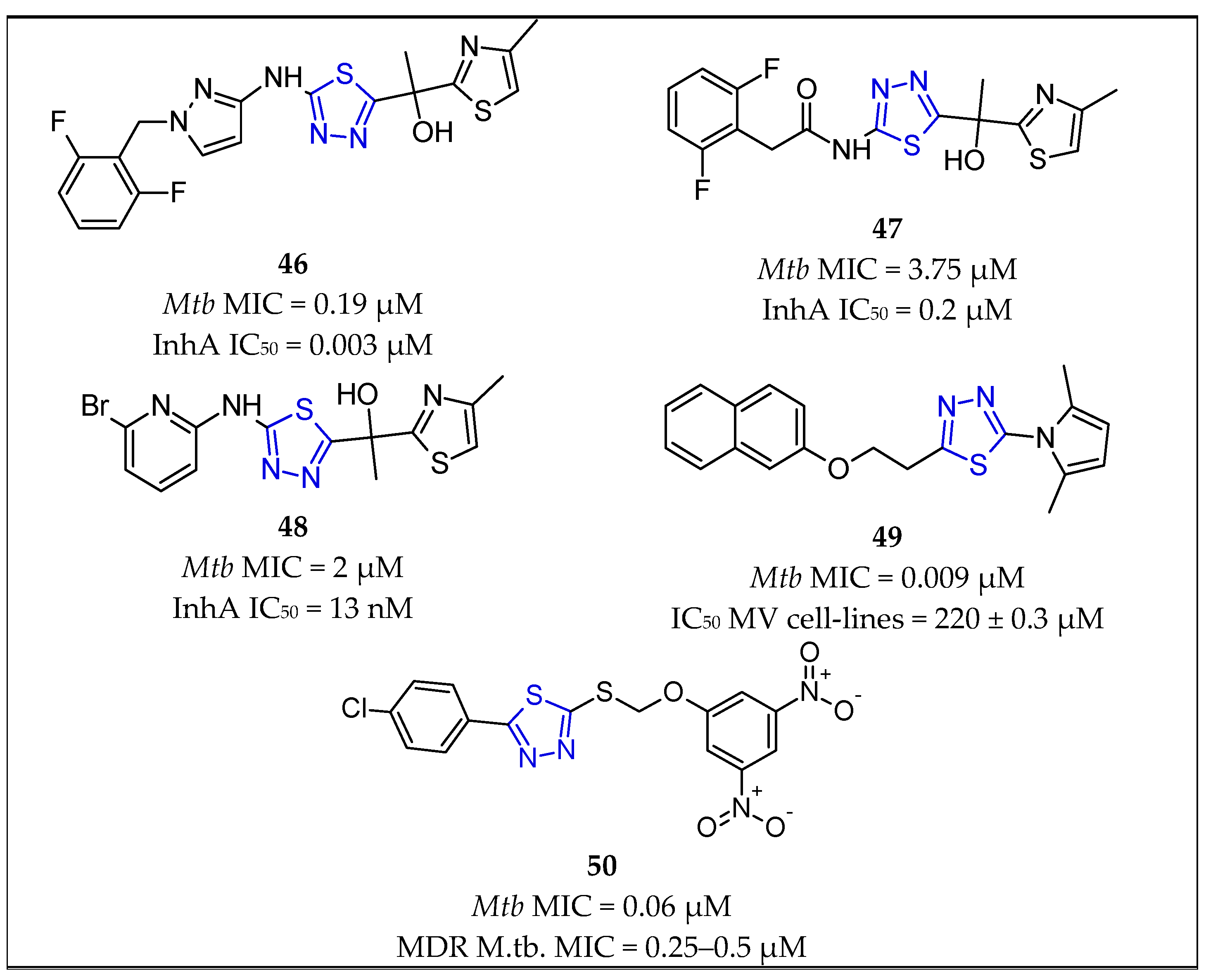
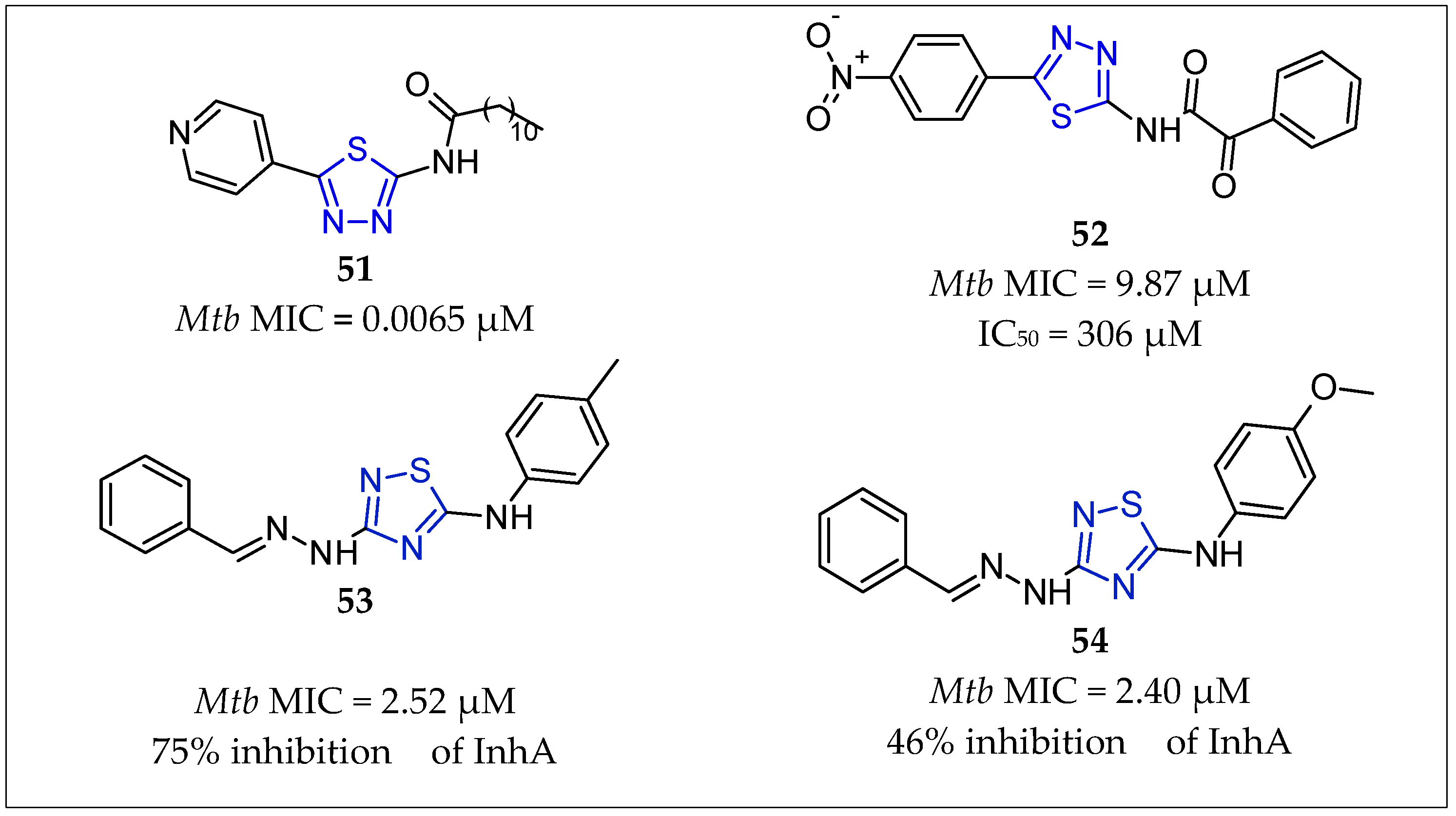
| No. | Substituents | Compd. | MIC (µM) | SI | LD50 (mg/kg b.w.) | InhA | References | |
|---|---|---|---|---|---|---|---|---|
| Hydrazide-hydrazones | 1 | Pyridine/2H-chromene | 2 | 0.17 | 449 | - | - | Angelova et al., 2017 [28] |
| 2 | Pyridine/(CH2)15-CH3 | 8 | 0.096 | - | >5000 | - | Kumar et al., 2014 [34] | |
| 3 | Pyridine/Ar | 9 | 0.014 | - | - | - | Pahlavani et al., 2015 [35] | |
| 4 | Pyridine/Indole | 11 | 1.43 | 300 | - | - | Velezheva et al., 2016 [37] | |
| 5 | Pyridine/Isatin | 15 | 0.17 | - | - | - | Santoso et al., 2021 [39] | |
| 6 | Pyridine/Ar | 16 | 0.62 | - | - | 41% inhibition | Koçak Aslan et al., 2022 [40] | |
| 7 | Pyridine/2,1,3-benzoxadiazole | 17 | 1.1 | 472 | - | - | Fernandes et al., 2017 [41] | |
| 8 | Pyridine/Ar | 18 | 0.59 | - | - | - | Kumar et al., 2014 [42] | |
| 9 | Pyridine/2H-indazol | 23 | 0.11 | - | - | 19% Inhibition | Oliveira et al., 2017 [47] | |
| 10 | Pyridine/Ph | 26 | 10.90 | - | - | - | Nogueira et al., 2018 [51] | |
| 11 | Pyridine/2-hydroxyPh | 28 | 4.98 | - | - | - | Sampiron et al., 2019 [52] | |
| 12 | Pyridine/(CH2)4-CH3 | 34 | 0.03 | - | - | Faria et al., 2021 [56] | ||
| 13 | Pyridine/(CH2)8-CH3 | 35 | 0.03 | - | - | - | Faria et al., 2021 [56] | |
| 14 | Pyridine/Ph-propanamide | 37 | 0.03 | - | - | MIC = 6 µM | Pflégr et al., 2021 [58] | |
| 15 | Pyridine/5-nitrofuran | 39 | 0.009 | - | - | - | Gobis et al., 2022 [60] | |
| 16 | Pyridine/Ar | 45 | 0.004 | - | - | - | Lone et al., 2023 [65] | |
| 17 | 1,2,3-Thiadiazole/Indole | 5 | 0.396 | 1979 | >2000 | - | Angelova et al., 2019 [30] | |
| 18 | 1,2,3-Thiadiazole/Ar | 6 | 0.073 | 3516 | >2000 | - | Angelova et al., 2022 [31] | |
| 19 | Ar/2H-Chromene | 1 | 0.13 | 697 | - | - | Angelova et al., 2017 [28] | |
| 20 | Ar/Coumarin | 3 | 0.32 | >625 | - | - | Angelova et al., 2017 [29] | |
| 21 | Ar/pyridin, Me | 25 | 14 | - | - | - | Bonnett et al., 2018 [49,50] | |
| 22 | Ar/NH-pyrazole | 33 | 0.01 | - | - | - | Padmini et al., 2021 [55] | |
| 23 | Ar/Ar | 44 | 7.29 | - | - | - | Senthilkumar et al., 2016 [64] | |
| 24 | R/2-(propan-2-yl)aniline | 29 | 0.068 | - | - | - | Rohane et al., 2020 [53] | |
| 25 | furan/Indole | 4 | 0.44 | >634 | - | - | Angelova et al., 2019 [30] | |
| 26 | Indole/Ar | 10 | 0.067 | - | - | - | Cihan-Üstündağ et al., 2016 [36] | |
| 27 | Naphthalen-1-yl-methyl/pyrrole | 19 | 4.86 | - | - | - | More et al., 2014 [43] | |
| 28 | Adamantyl/Ar | 40 | 6.66 | - | - | - | Briffotaux et al., 2022 [61] | |
| 29 | 4-Chlorobenzyl)amino] Benzohydrazide | 38 | 0.006 | - | - | - | Desale et al., 2022 [59] | |
| 30 | 6-Chloroquinoxalin-2-yl/Ar | 27 | 72.72 | - | - | - | Nogueira et al., 2018 [51] | |
| Hydrazones | 31 | Ar/Ar | 30–32 | 0.008 | - | - | Sruthi et al., 2020 [54] | |
| 32 | thiophen-2-yl/1,3-dihydro-2H-indol-2-one | 36 | 1.9 | - | - | Karunanidhi et al., 2021 [57] | ||
| 33 | Ph/coumarin | 41 | 0.021 | - | - | Akki et al., 2022 [62] | ||
| 34 | Imidazol-4-one/Ar | 42 | 0.033 | - | - | Abdelhamid et al., 2022 [63] | ||
| 35 | Imidazol-4-one/2H-indol-2-one imidazol-4-one | 43 | 0.032 | - | - | Abdelhamid et al., 2022 [63] | ||
| Sulfonyl hydrazones | 36 | Ar/ethenylbenzene | 7 | 0.0716 | 3380 | Angelova et al., 2022 [31] | ||
| 37 | para-tolyl/Ph para-tolyl/2-OMePh para-tolyl/2-naphtyl | 12 13 14 | 1.25 1.25 1.25 | - | - | - | Ghiano et al., 2020 [38] Ghiano et al., 2020 [38] Ghiano et al., 2020 [38] | |
| 38 | para-tolyl/4-nitroPh | 20 | 48.04 | - | - | - | Ghiya and Joshi et al., 2016 [44] | |
| 39 | para-tolyl/R1R2 | 22 | 183 | - | - | - | Concha et al., 2017 [46] | |
| 40 | Pyrazine/1H-pyrazole | 24 | 0.0017 | 1085.7 | 846.9 | - | Hassan et al., 2020 [48] | |
| Thiadiazoles | 41 | 5-amino-1,3,4-thiadiazole | 46 | 0.19 | - | - | MIC = 0.003 μM | Ballell et al., 2010 [68] Castro et al., 2012 [69] Shirude et al., 2013 [8] |
| 42 | 5-amino-1,3,4-thiadiazole | 47 | 3.75 | - | - | MIC = 0.2 μM | Shirude et al., 2013 [8] | |
| 43 | 5-amino-1,3,4-thiadiazole | 48 | 2.00 | - | - | MIC = 13 nM | Šink et al., 2018 [15] | |
| 44 | 5-pyrrolyl-1,3,4-thiadiazole | 49 | 0.009 | - | - | - | of Joshi et al., 2018 [70] | |
| 45 | 5-sulfanyl-1,3,4-thiadiazoe | 50 | 0.06 | - | - | - | Karabanovich et al., 2016 [71] | |
| 46 | 5-amino-1,3,4-thiadiazol | 51 | 2.25 | - | - | - | Mali et al., 2020 [72] | |
| 47 | 5-amino-1,3,4-thiadiazol | 52 | 9.87 | - | - | - | Patel et al., 2019 [73] | |
| 48 | 3-benzylidenehydrazinyl-1,2,4-thiadiazol-5-amine | 53 | 2.52 | - | - | 75% inhibition | Doğan et al., 2020 [12] | |
| 49 | 3-benzylidenehydrazinyl-1,2,4-thiadiazol-5-amine | 54 | 2.40 | - | - | 46% inhibition | Doğan et al., 2020 [12] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teneva, Y.; Simeonova, R.; Valcheva, V.; Angelova, V.T. Recent Advances in Anti-Tuberculosis Drug Discovery Based on Hydrazide–Hydrazone and Thiadiazole Derivatives Targeting InhA. Pharmaceuticals 2023, 16, 484. https://doi.org/10.3390/ph16040484
Teneva Y, Simeonova R, Valcheva V, Angelova VT. Recent Advances in Anti-Tuberculosis Drug Discovery Based on Hydrazide–Hydrazone and Thiadiazole Derivatives Targeting InhA. Pharmaceuticals. 2023; 16(4):484. https://doi.org/10.3390/ph16040484
Chicago/Turabian StyleTeneva, Yoanna, Rumyana Simeonova, Violeta Valcheva, and Violina T. Angelova. 2023. "Recent Advances in Anti-Tuberculosis Drug Discovery Based on Hydrazide–Hydrazone and Thiadiazole Derivatives Targeting InhA" Pharmaceuticals 16, no. 4: 484. https://doi.org/10.3390/ph16040484
APA StyleTeneva, Y., Simeonova, R., Valcheva, V., & Angelova, V. T. (2023). Recent Advances in Anti-Tuberculosis Drug Discovery Based on Hydrazide–Hydrazone and Thiadiazole Derivatives Targeting InhA. Pharmaceuticals, 16(4), 484. https://doi.org/10.3390/ph16040484