Recent Progress of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Ocular Drug Delivery Platforms
Abstract
1. Introduction
2. Eye Anatomy, Barriers and Routes in Ocular Drug Delivery
2.1. Anterior Segment of the Eye
2.1.1. Tear Film
2.1.2. Cornea
2.1.3. Conjunctiva
2.1.4. Iris
2.1.5. Ciliary Body
2.1.6. Lens
2.2. Posterior Segment of the Eye
2.2.1. Sclera
2.2.2. Choroid
2.2.3. Retina
2.2.4. Vitreous Body
2.3. Alternative Routes of Ocular Delivery
3. Feasibility of Lipid Nanoparticles in Ophthalmology
3.1. Lipid Nanoparticles—Structural Features and Recent Progress in Ocular Therapeutics
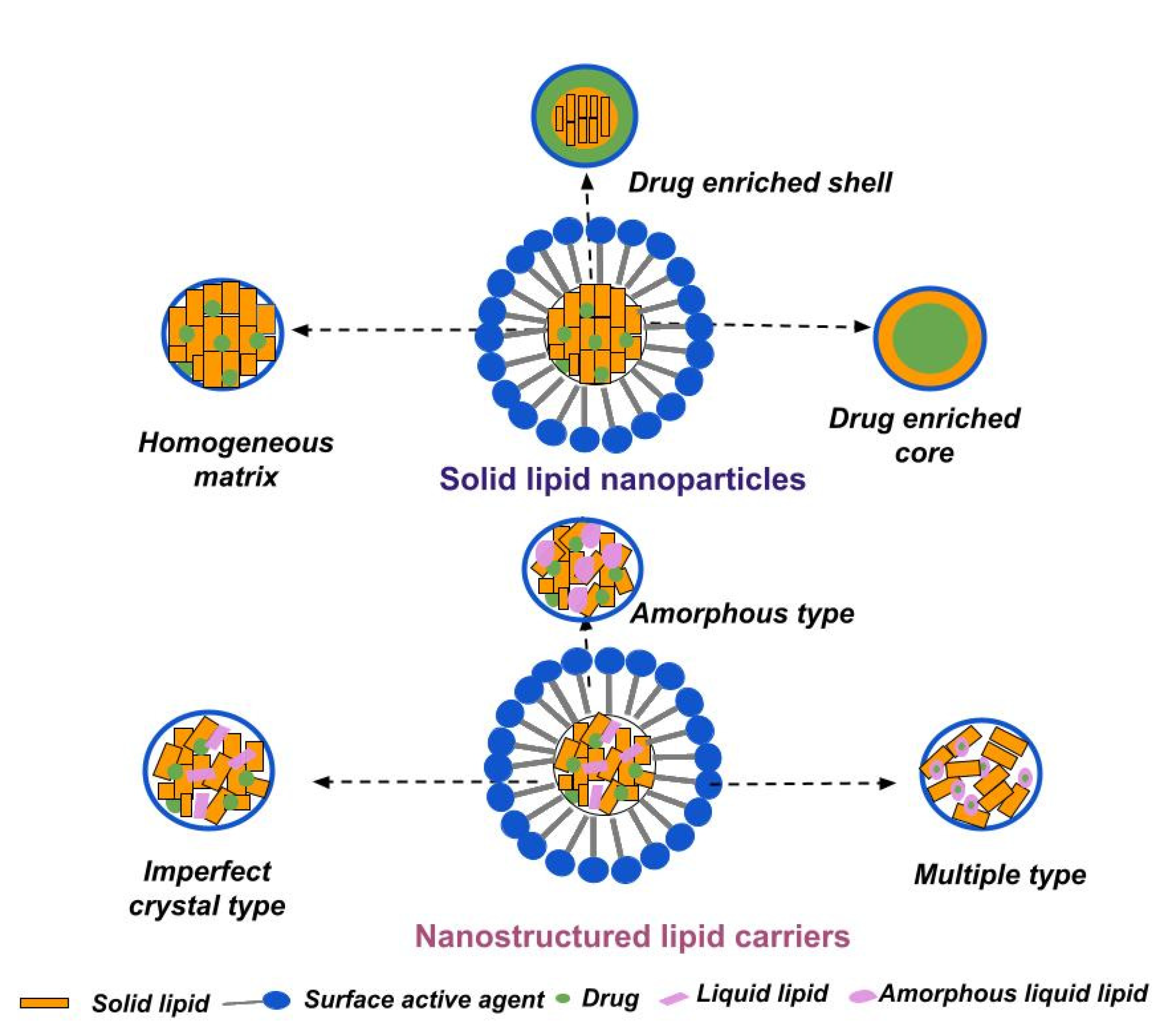
3.1.1. Solid Lipid Nanoparticles
3.1.2. Nanostructured Lipid Carriers
3.2. Sterilization Feasibility of SLNs and NLCs
3.3. Clinical Application of SLNs and NLCs in Ocular Therapeutics
4. Conclusions and Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. World Report on Vision; World Health Organization: Geneva, Switzerland, 2019; ISBN 9789241516570. [Google Scholar]
- Usgaonkar, U.; Shet Parkar, S.R.; Shetty, A. Impact of the use of digital devices on eyes during the lockdown period of COVID-19 pandemic. Ind. J. Ophthalmol. 2021, 69, 1901–1906. [Google Scholar] [CrossRef]
- Shalini, S.; Ipsita, P.; Abhay, P.; Pandey, A.K. Life style disorders in ophthalmology and their management. Environ. Conserv. J. 2019, 20, 67–72. [Google Scholar]
- García-Marqués, J.V.; Talens-Estarelles, C.; García-Lázaro, S.; Wolffsohn, J.S.; Cerviño, A. Systemic, environmental and lifestyle risk factors for dry eye disease in a mediterranean caucasian population. Cont. Lens Anterior Eye. 2022, 45, 101539. [Google Scholar] [CrossRef] [PubMed]
- Bourne, R.R.A.; Steinmetz, J.D.; Flaxman, S.; Briant, P.S.; Taylor, H.R.; Resnikoff, S.; Casson, R.J.; Abdoli, A.; Gharbieh, E.A.; Afshin, A.; et al. Trends in prevalence of blindness and distance and near vision impairment over 30 years: An analysis for the Global Burden of Disease Study. Lancet Glob. Health. 2021, 9, e130–e143. [Google Scholar] [CrossRef]
- Gote, V.; Sikder, S.; Sicotte, J.; Pal, D. Ocular Drug Delivery: Present Innovations and Future Challenges. J. Pharmacol. Exp. Ther. 2019, 370, 602–624. [Google Scholar] [CrossRef]
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Nayak, K.; Misra, M. A review on recent drug delivery systems for posterior segment of eye. Biomed. Pharmacother. 2018, 107, 1564–1582. [Google Scholar] [CrossRef]
- Bachu, R.D.; Chowdhury, P.; Al-Saedi, Z.H.F.; Karla, P.K.; Boddu, S.H.S. Ocular Drug Delivery Barriers-Role of Nanocarriers in the Treatment of Anterior Segment Ocular Diseases. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef]
- Wilson, C.G. Ophthalmic Formulation. In Specialized Pharmaceutical Formulation: The Science and Technology of Dosage Forms, 1st ed.; Tovey, G.D., Ed.; Royal Society of Chemistry: London, UK, 2022; pp. 1–44. [Google Scholar]
- Kuno, N.; Fujii, S. Recent Advances in Ocular Drug Delivery Systems. Polymers 2011, 3, 193–221. [Google Scholar] [CrossRef]
- Jünemann, A.G.M.; Chorągiewicz, T.; Ozimek, M.; Grieb, P.; Rejdak, R. Drug bioavailability from topically applied ocular drops. Does drop size matter? Ophthalmol. J. 2016, 1, 29–35. [Google Scholar] [CrossRef]
- Taghe, S.; Mirzaeei, S. Preparation and characterization of novel, mucoadhesive ofloxacin nanoparticles for ocular drug delivery. Braz. J. Pharm. Sci. 2019, 55, e17105. [Google Scholar] [CrossRef]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular drug delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef]
- Baranowski, P.; Karolewicz, B.; Gajda, M.; Pluta, J. Ophthalmic drug dosage forms: Characterisation and research methods. Sci. World J. 2014, 2014, 861904. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Li, X.; Kebebe, D.; Zhang, B.; Ren, J.; Lu, J.; Li, J.; Du, S.; Liu, Z. Research progress of in-situ gelling ophthalmic drug delivery system. Asian J. Pharm. Sci. 2019, 14, 1–15. [Google Scholar] [CrossRef]
- Bastawrous, A.; Burgess, P.I.; Mahdi, A.M.; Kyari, F.; Burton, M.J.; Kuper, H. Posterior segment eye disease in sub-Saharan Africa: Review of recent population-based studies. Trop. Med. Int. Health. 2014, 19, 600–609. [Google Scholar] [CrossRef]
- Tóth, G.; Szabó, D.; Sándor, G.L.; Nagy, Z.Z.; Limburg, H.; Németh, J. Hátsószegmens-betegségek okozta látásromlás és vakság Magyarországon az 50 évnél idosebb korú lakosság körében [Visual impairment and blindness caused by posterior segment diseases in Hungary in people aged 50 years and older]. Orv. Hetil. 2022, 163, 624–630. [Google Scholar] [CrossRef]
- Varela-Fernández, R.; Díaz-Tomé, V.; Luaces-Rodríguez, A.; Conde-Penedo, A.; García-Otero, X.; Luzardo-Álvarez, A.; Fernández-Ferreiro, A.; Otero-Espinar, F.J. Drug Delivery to the Posterior Segment of the Eye: Biopharmaceutic and Pharmacokinetic Considerations. Pharmaceutics 2020, 12, 269. [Google Scholar] [CrossRef] [PubMed]
- Otero-Espinar, F.J.; Fernández-Ferreiro, A.; González-Barcia, M.; Blanco-Méndez, J.; Luzardo, A. Stimuli sensitive ocular drug delivery systems. In Drug Targeting and Stimuli Sensitive Drug Delivery Systems, 1st ed.; Grumezescu, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 211–270. [Google Scholar]
- Sánchez-López, E.; Espina, M.; Doktorovova, S.; Souto, E.B.; García, M.L. Lipid nanoparticles (SLN, NLC): Overcoming the anatomical and physiological barriers of the eye—Part I—Barriers and determining factors in ocular delivery. Eur. J. Pharm. Biopharm. 2017, 110, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, G.A.; Ferreira-Nunes, R.; Dalmolin, L.F.; Dos Santos Ré, A.C.; Anjos, J.L.V.; Mendanha, S.A.; Aires, C.P.; Lopez, R.F.V.; Cunha-Filho, M.; Gelfuso, G.M.; et al. Besifloxacin liposomes with positively charged additives for an improved topical ocular delivery. Sci. Rep. 2020, 10, 19285. [Google Scholar] [CrossRef]
- Chen, X.; Wu, J.; Lin, X.; Wu, X.; Yu, X.; Wang, B.; Xu, W. Tacrolimus Loaded Cationic Liposomes for Dry Eye Treatment. Front. Pharmacol. 2022, 13, 838168. [Google Scholar] [CrossRef] [PubMed]
- Fathalla, D.; Fouad, E.A.; Soliman, G.M. Latanoprost niosomes as a sustained release ocular delivery system for the management of glaucoma. Drug Dev. Ind. Pharm. 2020, 46, 806–813. [Google Scholar] [CrossRef] [PubMed]
- El-Nabarawi, M.A.; Abd El Rehem, R.T.; Teaima, M.; Abary, M.; El-Mofty, H.M.; Khafagy, M.M.; Lotfy, N.M.; Salah, M. Natamycin niosomes as a promising ocular nanosized delivery system with ketorolac tromethamine for dual effects for treatment of candida rabbit keratitis; in vitro/in vivo and histopathological studies. Drug Dev. Ind. Pharm. 2019, 45, 922–936. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Pascual, M.; Gómez-Aguado, I.; Rodríguez-Castejón, J.; Rodríguez-Gascón, A.; Muntoni, E.; Battaglia, L.; del Pozo-Rodríguez, A.; Solinís Aspiazu, M.Á. Topical Administration of SLN-Based Gene Therapy for the Treatment of Corneal Inflammation by De Novo IL-10 Production. Pharmaceutics 2020, 12, 584. [Google Scholar] [CrossRef]
- Wang, L.; Liu, W.; Huang, X. An approach to revolutionize cataract treatment by enhancing drug probing through intraocular cell line. Libyan J. Med. 2018, 13, 1500347. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.; Hu, Y.; Zhang, H.; Liu, J.; Zhao, Y. Polydopamine nanoparticles attenuate retina ganglion cell degeneration and restore visual function after optic nerve injury. J. Nanobiotechnology 2021, 19, 436. [Google Scholar] [CrossRef]
- Francia, S.; Shmal, D.; Di Marco, S.; Chiaravalli, G.; Maya-Vetencourt, J.F.; Mantero, G.; Michetti, C.; Cupini, S.; Manfredi, G.; DiFrancesco, M.L.; et al. Light-induced charge generation in polymeric nanoparticles restores vision in advanced-stage retinitis pigmentosa rats. Nat. Commun. 2022, 13, 3677. [Google Scholar] [CrossRef]
- Rincón, M.; Espinoza, L.C.; Silva-Abreu, M.; Sosa, L.; Pesantez-Narvaez, J.; Abrego, G.; Calpena, A.C.; Mallandrich, M. Quality by Design of Pranoprofen Loaded Nanostructured Lipid Carriers and Their Ex Vivo Evaluation in Different Mucosae and Ocular Tissues. Pharmaceuticals 2022, 15, 1185. [Google Scholar] [CrossRef] [PubMed]
- Polat, H.K.; Kurt, N.; Aytekin, E.; Akdağ Çaylı, Y.; Bozdağ Pehlivan, S.; Çalış, S. Design of Besifloxacin HCl-Loaded Nanostructured Lipid Carriers: In Vitro and Ex Vivo Evaluation. J. Ocul. Pharmacol. Ther. 2022, 38, 412–423. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, M.; Shi, Y.; Fang, L.; Cao, F. Rational design of mixed nanomicelle eye drops with structural integrity investigation. Acta Biomater. 2022, 141, 164–177. [Google Scholar] [CrossRef]
- Xu, X.; Sun, L.; Zhou, L.; Cheng, Y.; Cao, F. Functional chitosan oligosaccharide nanomicelles for topical ocular drug delivery of dexamethasone. Carbohydr. Polym. 2020, 227, 115356. [Google Scholar] [CrossRef]
- Nayak, K.; Misra, M. PEGylated microemulsion for dexamethasone delivery to posterior segment of eye. J. Biomater. Sci. Polym. Ed. 2020, 31, 1071–1090. [Google Scholar] [CrossRef] [PubMed]
- Bachu, R.D.; Stepanski, M.; Alzhrani, R.M.; Jung, R.; Boddu, S.H.S. Development and Evaluation of a Novel Microemulsion of Dexamethasone and Tobramycin for Topical Ocular Administration. J. Ocul. Pharmacol. Ther. 2018, 34, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Osuna, I.; Vicario-de-la-Torre, M.; Andrés-Guerrero, V.; Sánchez-Nieves, J.; Guzmán-Navarro, M.; de la Mata, F.J.; Gómez, R.; de Las Heras, B.; Argüeso, P.; Ponchel, G.; et al. Novel Water-Soluble Mucoadhesive Carbosilane Dendrimers for Ocular Administration. Mol. Pharm. 2016, 13, 2966–2976. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res. Pharm. Sci. 2018, 13, 288–303. [Google Scholar]
- Costa, C.P.; Barreiro, S.; Moreira, J.N.; Silva, R.; Almeida, H.; Sousa Lobo, J.M.; Silva, A.C. In Vitro Studies on Nasal Formulations of Nanostructured Lipid Carriers (NLC) and Solid Lipid Nanoparticles (SLN). Pharmaceuticals 2021, 14, 711. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Thuy, V.N.; Van, T.V.; Dao, A.H.; Lee, B.-J. Nanostructured lipid carriers and their potential applications for versatile drug delivery via oral administration. OpenNano 2022, 8, 100064. [Google Scholar] [CrossRef]
- Czajkowska-Kośnik, A.; Szekalska, M.; Winnicka, K. Nanostructured lipid carriers: A potential use for skin drug delivery systems. Pharmacol. Rep. 2019, 71, 156–166. [Google Scholar] [CrossRef]
- Musielak, E.; Feliczak-Guzik, A.; Nowak, I. Optimization of the Conditions of Solid Lipid Nanoparticles (SLN) Synthesis. Molecules 2022, 27, 2202. [Google Scholar] [CrossRef] [PubMed]
- Poonia, N.; Kharb, R.; Lather, V.; Pandita, D. Nanostructured lipid carriers: Versatile oral delivery vehicle. Future Sci. OA 2016, 2, FSO135. [Google Scholar] [CrossRef]
- Abo El-Enin, H.A.; Elkomy, M.H.; Naguib, I.A.; Ahmed, M.F.; Alsaidan, O.A.; Alsalahat, I.; Ghoneim, M.M.; Eid, H.M. Lipid Nanocarriers Overlaid with Chitosan for Brain Delivery of Berberine via the Nasal Route. Pharmaceuticals 2022, 15, 281. [Google Scholar] [CrossRef]
- Mirchandani, Y.; Patravale, V.B.; Brijesh, S. Solid lipid nanoparticles for hydrophilic drugs. J. Control Release. 2021, 335, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.R.; Queiroz, J.F.; Weksler, B.; Romero, I.A.; Couraud, P.O.; Reis, S. Solid lipid nanoparticles as a vehicle for brain-targeted drug delivery: Two new strategies of functionalization with apolipoprotein E. Nanotechnology 2015, 26, 495103. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J. Eye. In Vitamin C in Human Health and Disease, 1st ed.; Lee, W.J., Ed.; Springer: Dordrecht, The Netherlands, 2019; pp. 177–182. [Google Scholar]
- Watson, P.G.; Young, R.D. Scleral structure, organisation and disease. A review. Exp. Eye Res. 2004, 78, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [CrossRef]
- Labelle, P. The Eye. In Pathologic Basis of Veterinary Disease, 6th ed.; Zachary, J.F., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1265–1318.e1. [Google Scholar]
- Moiseev, R.V.; Morrison, P.W.J.; Steele, F.; Khutoryanskiy, V.V. Penetration Enhancers in Ocular Drug Delivery. Pharmaceutics 2019, 11, 321. [Google Scholar] [CrossRef]
- Salazar, J.J.; Ramírez, A.I.; De Hoz, R.; Salobrar-Garcia, E.; Rojas, P.; Fernández-Albarral, J.A.; López-Cuenca, I.; Rojas, B.; Triviño, A.; Ramírez, J.M. Anatomy of the Human Optic Nerve: Structure and Function; Ferreri, F.M., Ed.; IntechOpen: London, UK, 2018; pp. 1–46. [Google Scholar]
- Lin, S.; Ge, C.; Wang, D.; Xie, Q.; Wu, B.; Wang, J.; Nan, K.; Zheng, Q.; Chen, W. Overcoming the Anatomical and Physiological Barriers in Topical Eye Surface Medication Using a Peptide-Decorated Polymeric Micelle. ACS Appl. Mater. Interfaces 2019, 11, 39603–39612. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zuo, Y.Y. Nanomedicine for Ocular Drug Delivery. In Nanomedicine, 1st ed.; Gu, N., Ed.; Springer: Singapore, 2022; pp. 755–786. [Google Scholar]
- McDermott, A.M. Antimicrobial Compounds in Tears. Exp. Eye Res. 2013, 117, 53–61. [Google Scholar] [CrossRef]
- Kopacz, D.; Niezgoda, Ł.; Fudalej, E.; Nowak, A.; Maciejewicz, P. Tear film—Physiology and Disturbances in Various Diseases and Disorders. In Ocular Surface Diseases—Some Current Date on Tear Film Problem and Keratoconic Diagnosis, 1st ed.; Kopacz, D., Ed.; IntechOpen: London, UK, 2021; pp. 1–17. [Google Scholar]
- Willcox, M.D.P.; Argüeso, P.; Georgiev, G.A.; Holopainen, J.M.; Laurie, G.W.; Millar, T.J.; Papas, E.B.; Rolland, J.P.; Schmidt, T.A.; Stahl, U.; et al. TFOS DEWS II Tear Film Report. Ocul. Surf. 2017, 15, 366–403. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lockwood, A. Topical ocular drug delivery systems: Innovations for an unmet need. Exp. Eye Res. 2022, 218, 109006. [Google Scholar] [CrossRef]
- Jumelle, C.; Gholizadeh, S.; Annabi, N.; Dana, R. Advances and limitations of drug delivery systems formulated as eye drops. J. Control Release. 2020, 321, 1–22. [Google Scholar] [CrossRef]
- Shafaie, S.; Hutter, V.; Cook, M.T.; Brown, M.B.; Chau, D.Y.S. In Vitro Cell Models for Ophthalmic Drug Development Applications. Biores. Open Access. 2016, 5, 94–108. [Google Scholar] [CrossRef]
- Peris-Martínez, C.; García-Domene, M.C.; Penadés, M.; Luque, M.J.; Fernández-López, E.; Artigas, J.M. Spectral Transmission of the Human Corneal Layers. J. Clin. Med. 2021, 10, 4490. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Jiang, S.; Musayeva, A.; Pfeiffer, N.; Gericke, A. Corneal Epithelial Stem Cells-Physiology, Pathophysiology and Therapeutic Options. Cells 2021, 10, 2302. [Google Scholar] [CrossRef] [PubMed]
- Shastri, D.H.; Silva, A.C.; Almeida, H. Ocular Delivery of Therapeutic Proteins: A Review. Pharmaceutics 2023, 15, 205. [Google Scholar] [CrossRef]
- Wang, R.; Gao, Y.; Liu, A.; Zhai, G. A review of nanocarrier-mediated drug delivery systems for posterior segment eye disease: Challenges analysis and recent advances. J. Drug Target 2021, 29, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Lockhart, C.M.; Kelly, E.J.; Rettie, A.E. Ocular cytochrome P450s and transporters: Roles in disease and endobiotic and xenobiotic disposition. Drug Metab. Rev. 2014, 46, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Lagali, N. Corneal Stromal Regeneration: Current Status and Future Therapeutic Potential. Curr. Eye Res. 2020, 45, 278–290. [Google Scholar] [CrossRef]
- Morrison, P.W.; Khutoryanskiy, V.V. Advances in ophthalmic drug delivery. Ther. Deliv. 2014, 5, 1297–1315. [Google Scholar] [CrossRef]
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A.K. A comprehensive insight on ocular pharmacokinetics. Drug Deliv. Transl. Res. 2016, 6, 735–754. [Google Scholar] [CrossRef]
- Pak, J.; Chen, Z.J.; Sun, K.; Przekwas, A.; Walenga, R.; Fan, J. Computational Modeling of Drug Transport Across the In Vitro Cornea. Comput. Biol. Med. 2018, 92, 139–146. [Google Scholar] [CrossRef]
- Löscher, M.; Seiz, C.; Hurst, J.; Schnichels, S. Topical Drug Delivery to the Posterior Segment of the Eye. Pharmaceutics 2022, 14, 134. [Google Scholar] [CrossRef]
- Harikumar, S.I.; Sonia, A. Nanotechnological approaches in Ophthalmic delivery systems. Int. J. Drug Dev. Res. 2011, 3, 9–19. [Google Scholar]
- Kwatra, D.; Mitra, A.K. Drug delivery in ocular diseases: Barriers and strategies. World J. Pharmacol. 2013, 2, 78–83. [Google Scholar] [CrossRef]
- Akhter, M.H.; Ahmad, I.; Alshahrani, M.Y.; Al-Harbi, A.I.; Khalilullah, H.; Afzal, O.; Altamimi, A.S.A.; Najib Ullah, S.N.M.; Ojha, A.; Karim, S. Drug Delivery Challenges and Current Progress in Nanocarrier-Based Ocular Therapeutic System. Gels 2022, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, I.F.; Barbosa, E.J.; Peters, M.C.C.; Henostroza, M.A.B.; Yukuyama, M.N.; Dos Santos Neto, E.; Löbenberg, R.; Bou-Chacra, N. Cutting-edge advances in therapy for the posterior segment of the eye: Solid lipid nanoparticles and nanostructured lipid carriers. Int. J. Pharm. 2020, 589, 119831. [Google Scholar] [CrossRef]
- Pescina, S.; Lucca, L.G.; Govoni, P.; Padula, C.; Favero, E.D.; Cantù, L.; Santi, P.; Nicoli, S. Ex Vivo Conjunctival Retention and Transconjunctival Transport of Poorly Soluble Drugs Using Polymeric Micelles. Pharmaceutics 2019, 11, 476. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, K.; Lee, V.H.; Kim, K.J. Roles of the conjunctiva in ocular drug delivery: A review of conjunctival transport mechanisms and their regulation. Eur. J. Pharm. Biopharm. 2005, 60, 227–240. [Google Scholar] [CrossRef]
- Szabadi, E. Functional Organization of the Sympathetic Pathways Controlling the Pupil: Light-Inhibited and Light-Stimulated Pathways. Front. Neurol. 2018, 9, 1069. [Google Scholar] [CrossRef]
- Jakubiak, P.; Cantrill, C.; Urtti, A.; Alvarez-Sánchez, R. Establishment of an In vitro-In vivo Correlation for Melanin Binding and the Extension of the Ocular Half-Life of Small-Molecule Drugs. Mol. Pharm. 2019, 16, 4890–4901. [Google Scholar] [CrossRef]
- Tangri, P.; Khurana, S. Basics of ocular drug delivery systems. Int. J. Res. Pharm. Biomed. Sci. 2011, 2, 1541–1552. [Google Scholar]
- Rimpelä, A.K.; Reinisalo, M.; Hellinen, L.; Grazhdankin, E.; Kidron, H.; Urtti, A.; Del Amo, E.M. Implications of melanin binding in ocular drug delivery. Adv. Drug Deliv. Rev. 2018, 126, 23–43. [Google Scholar] [CrossRef]
- Rimpelä, A.K.; Hagström, M.; Kidron, H.; Urtti, A. Melanin targeting for intracellular drug delivery: Quantification of bound and free drug in retinal pigment epithelial cells. J. Control Release 2018, 283, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Achouri, D.; Alhanout, K.; Piccerelle, P.; Andrieu, V. Recent advances in ocular drug delivery. Drug Dev. Ind. Pharm. 2013, 39, 1599–1617. [Google Scholar] [CrossRef] [PubMed]
- Dubald, M.; Bourgeois, S.; Andrieu, V.; Fessi, H. Ophthalmic Drug Delivery Systems for Antibiotherapy—A Review. Pharmaceutics 2018, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Seyfoddin, A.; Shaw, J.; Al-Kassas, R. Solid lipid nanoparticles for ocular drug delivery. Drug Deliv. 2010, 7, 467–489. [Google Scholar] [CrossRef]
- Ali, J.; Fazil, M.; Qumbar, M.; Khan, N.; Ali, A. Colloidal drug delivery system: Amplify the ocular delivery. Drug Deliv. 2014, 23, 700–716. [Google Scholar]
- Hejtmancik, J.F.; Shiels, A. Overview of the Lens. Prog. Mol. Biol. Transl. Sci. 2015, 134, 119–127. [Google Scholar]
- Ruan, X.; Liu, Z.; Luo, L.; Liu, Y. The structure of the lens and its associations with the visual quality. BMJ Open Ophthalmol. 2020, 5, e000459. [Google Scholar] [CrossRef]
- Coudrillier, B.; Pijanka, J.; Jefferys, J.; Sorensen, T.; Quigley, H.A.; Boote, C.; Nguyen, T.D. Collagen structure and mechanical properties of the human sclera: Analysis for the effects of age. J. Biomech. Eng. 2015, 137, 410061–4100614. [Google Scholar] [CrossRef]
- Alshaikh, R.A.; Waeber, C.; Ryan, K.B. Polymer based sustained drug delivery to the ocular posterior segment: Barriers and future opportunities for the treatment of neovascular pathologies. Adv. Drug Deliv. Rev. 2022, 187, 114342. [Google Scholar] [CrossRef]
- Yadav, D.; Varma, L.T.; Yadav, K. Drug Delivery to Posterior Segment of the Eye: Conventional Delivery Strategies, Their Barriers, and Restrictions. In Drug Delivery for the Retina and Posterior Segment Disease, 1st ed.; Patel, J.K., Sutariya, V., Kanwar, J.R., Pathak, Y.V., Eds.; Springer: Cham, Switzerland, 2018; pp. 51–67. [Google Scholar]
- Djigo, A.D.; Bérubé, J.; Landreville, S.; Proulx, S. Characterization of a tissue-engineered choroid. Acta Biomater. 2019, 84, 305–316. [Google Scholar] [CrossRef]
- Hurley, J.B. Retina Metabolism and Metabolism in the Pigmented Epithelium: A Busy Intersection. Annu. Rev. Vis. Sci. 2021, 7, 665–692. [Google Scholar] [CrossRef] [PubMed]
- Del Amo, E.M.; Rimpelä, A.K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic aspects of retinal drug delivery. Prog. Retin. Eye Res. 2017, 57, 134–185. [Google Scholar] [CrossRef]
- Naylor, A.; Hopkins, A.; Hudson, N.; Campbell, M. Tight Junctions of the Outer Blood Retina Barrier. Int. J. Mol. Sci. 2019, 21, 211. [Google Scholar] [CrossRef]
- Willermain, F.; Libert, S.; Motulsky, E.; Salik, D.; Caspers, L.; Perret, J.; Delporte, C. Origins and consequences of hyperosmolar stress in retinal pigmented epithelial cells. Front. Physiol. 2014, 5, 199. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Coránguez, M.; Ramos, C.; Antonetti, D.A. The inner blood-retinal barrier: Cellular basis and development. Vision Res. 2017, 139, 123–137. [Google Scholar] [CrossRef]
- Asencio-Duran, M.; Dabad-Moreno, J.; Vicandi-Plaza, B.; Muñoz, M.; Capote-Díez, M.; Santisteban, G. The Vitreous Body and Its Role in the Diagnosis of Eye Pathologies. Med. Res. Arch. 2021, 9, 9. [Google Scholar] [CrossRef]
- Mishra, D.; Gade, S.; Glover, K.; Sheshala, R.; Singh, T.R.R. Vitreous Humor: Composition, Characteristics and Implication on Intravitreal Drug Delivery. Curr. Eye Res. 2022, 48, 208–218. [Google Scholar] [CrossRef]
- Stevens, S. Administering a subconjunctival injection. Community Eye Health J. 2009, 22, 15. [Google Scholar]
- Rafiei, F.; Tabesh, H.; Farzad, F. Sustained subconjunctival drug delivery systems: Current trends and future perspectives. Int. Ophthalmol. 2020, 40, 2385–2401. [Google Scholar] [CrossRef]
- Nebbioso, M.; Livani, M.L.; Santamaria, V.; Librando, A.; Sepe, M. Intracameral lidocaine as supplement to classic topical anesthesia for relieving ocular pain in cataract surgery. Int. J. Ophthalmol. 2018, 11, 1932–1935. [Google Scholar]
- Alghamdi, E.A.S.; Al Qahtani, A.Y.; Sinjab, M.M.; Alyahya, K.M. Intracameral Injections. In Extemporaneous Ophthalmic Preparations, 1st ed.; Alghamdi, E.A.S., Al Qahtani, A.Y., Sinjab, M.M., Alyahya, K.M., Eds.; Springer: Cham, Switzerland, 2020; pp. 67–68. [Google Scholar]
- Shah, T.J.; Conway, M.D.; Peyman, G.A. Intracameral dexamethasone injection in the treatment of cataract surgery induced inflammation: Design, development, and place in therapy. Clin. Ophthalmol. 2018, 12, 2223–2235. [Google Scholar] [CrossRef]
- Adrianto, M.F.; Annuryanti, F.; Wilson, C.G.; Sheshala, R.; Thakur, R.R.S. In vitro dissolution testing models of ocular implants for posterior segment drug delivery. Drug Deliv. Transl. Res. 2022, 12, 1355–1375. [Google Scholar] [CrossRef] [PubMed]
- Marashi, A.; Zazo, A. Suprachoroidal injection of triamcinolone acetonide using a custom-made needle to treat diabetic macular edema post pars plana vitrectomy: A case series. J. Int. Med. Res. 2022, 50, 1–10. [Google Scholar] [CrossRef]
- Chiang, B.; Jung, J.H.; Prausnitz, M.R. The suprachoroidal space as a route of administration to the posterior segment of the eye. Adv. Drug Deliv. Rev. 2018, 126, 58–66. [Google Scholar] [CrossRef]
- Martin, D.F. Evolution of Intravitreal Therapy for Retinal Diseases-From CMV to CNV: The LXXIV Edward Jackson Memorial Lecture. Am. J. Ophthalmol. 2018, 191, xli–lviii. [Google Scholar] [CrossRef] [PubMed]
- Gorantla, S.; Rapalli, V.K.; Waghule, T.; Singh, P.P.; Dubey, S.K.; Saha, R.N.; Singhvi, G. Nanocarriers for ocular drug delivery: Current status and translational opportunity. RSC Adv. 2020, 10, 27835–27855. [Google Scholar] [CrossRef]
- Peng, C.; Kuang, L.; Zhao, J.; Ross, A.E.; Wang, Z.; Ciolino, J.B. Bibliometric and visualized analysis of ocular drug delivery from 2001 to 2020. J. Control Release. 2022, 345, 625–645. [Google Scholar] [CrossRef]
- Das, B.; Nayak, A.K.; Mallick, S. Lipid-based nanocarriers for ocular drug delivery: An updated review. J. Drug Deliv. Sci. Technol. 2022, 76, 103780. [Google Scholar] [CrossRef]
- Shahraeini, S.S.; Akbari, J.; Saeedi, M.; Morteza-Semnani, K.; Abootorabi, S.; Dehghanpoor, M.; Rostamkalaei, S.S.; Nokhodchi, A. Atorvastatin Solid Lipid Nanoparticles as a Promising Approach for Dermal Delivery and an Anti-inflammatory Agent. AAPS PharmSciTech. 2020, 21, 263. [Google Scholar] [CrossRef] [PubMed]
- Essaghraoui, A.; Belfkira, A.; Hamdaoui, B.; Nunes, C.; Lima, S.A.C.; Reis, S. Improved Dermal Delivery of Cyclosporine A Loaded in Solid Lipid Nanoparticles. Nanomaterials 2019, 9, 1204. [Google Scholar] [CrossRef]
- Kakkar, S.; Singh, M.; Mohan Karuppayil, S.; Raut, J.S.; Giansanti, F.; Papucci, L.; Schiavone, N.; Nag, T.C.; Gao, N.; Yu, F.X.; et al. Lipo-PEG nano-ocular formulation successfully encapsulates hydrophilic fluconazole and traverses corneal and non-corneal path to reach posterior eye segment. J. Drug Target 2021, 29, 631–650. [Google Scholar] [CrossRef]
- Gómez-Aguado, I.; Rodríguez-Castejón, J.; Beraza-Millor, M.; Vicente-Pascual, M.; Rodríguez-Gascón, A.; Garelli, S.; Battaglia, L.; del Pozo-Rodríguez, A.; Solinís, M.Á. mRNA-Based Nanomedicinal Products to Address Corneal Inflammation by Interleukin-10 Supplementation. Pharmaceutics 2021, 13, 1472. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Hanafy, M.S.; Xu, H.; Leal, J.; Zhai, Y.; Ghosh, D.; Williams, R.O., III; Smyth, H.D.C.; Cui, Z. Aerosolizable siRNA-encapsulated solid lipid nanoparticles prepared by thin-film freeze-drying for potential pulmonary delivery. Int. J. Pharm. 2021, 596, 120215. [Google Scholar] [CrossRef] [PubMed]
- Elbrink, K.; Van Hees, S.; Chamanza, R.; Roelant, D.; Loomans, T.; Holm, R.; Kiekens, F. Application of solid lipid nanoparticles as a long-term drug delivery platform for intramuscular and subcutaneous administration: In vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2021, 163, 158–170. [Google Scholar] [CrossRef]
- Khanna, K.; Sharma, N.; Rawat, S.; Khan, N.; Karwasra, R.; Hasan, N.; Kumar, A.; Jain, G.K.; Nishad, D.K.; Khanna, S.; et al. Intranasal solid lipid nanoparticles for management of pain: A full factorial design approach, characterization & Gamma Scintigraphy. Chem. Phys. Lipids 2021, 236, 105060. [Google Scholar] [CrossRef]
- Parvez, S.; Yadagiri, G.; Gedda, M.R.; Singh, A.; Singh, O.P.; Verma, A.; Sundar, S.; Mudavath, S.L. Modified solid lipid nanoparticles encapsulated with Amphotericin B and Paromomycin: An effective oral combination against experimental murine visceral leishmaniasis. Sci. Rep. 2020, 10, 12243. [Google Scholar] [CrossRef]
- Angelova, A.; Angelov, B. Dual and multi-drug delivery nanoparticles towards neuronal survival and synaptic repair. Neural Regen Res. 2017, 12, 886–889. [Google Scholar] [CrossRef]
- Sahoo, R.K.; Biswas, N.; Guha, A.; Sahoo, N.; Kuotsu, K. Nonionic surfactant vesicles in ocular delivery: Innovative approaches and perspectives. Biomed Res. Int. 2014, 2014, 263604. [Google Scholar] [CrossRef]
- Youssef, A.A.A.; Dudhipala, N.; Majumdar, S. Dual Drug Loaded Lipid Nanocarrier Formulations for Topical Ocular Applications. Int. J. Nanomed. 2022, 17, 2283–2299. [Google Scholar] [CrossRef] [PubMed]
- Alvi, M.M.; Chatterjee, P. A prospective analysis of co-processed non-ionic surfactants in enhancing permeability of a model hydrophilic drug. AAPS PharmSciTech. 2014, 15, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B.; Shah, J.; Gupta, S.; Boddu, S.H.S.; Sreeharsha, N.; Joseph, A.; Shinu, P.; Morsy, M.A. Lipid Nanoparticles as a Promising Drug Delivery Carrier for Topical Ocular Therapy—An Overview on Recent Advances. Pharmaceutics 2022, 14, 533. [Google Scholar] [CrossRef] [PubMed]
- Malvajerd, S.S.; Azadi, A.; Izadi, Z.; Kurd, M.; Dara, T.; Dibaei, M.; Zadeh, M.S.; Javar, H.A.; Hamidi, M. Brain delivery of curcumin using solid lipid nanoparticles and nanostructured lipid carriers: Preparation, optimization, and pharmacokinetic evaluation. ACS Chem. Neurosci. 2019, 10, 728–739. [Google Scholar] [CrossRef]
- Nagai, N.; Ogata, F.; Otake, H.; Nakazawa, Y.; Kawasaki, N. Energy-dependent endocytosis is responsible for drug transcorneal penetration following the instillation of ophthalmic formulations containing indomethacin nanoparticles. Int. J. Nanomedicine 2019, 14, 1213–1227. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, F.M.; Bianchera, A.; Gasco, P.; Nicoli, S.; Pescina, S. Lipid-Based Nanocarriers for Ophthalmic Administration: Towards Experimental Design Implementation. Pharmaceutics 2021, 13, 447. [Google Scholar] [CrossRef]
- Amrite, A.C.; Edelhauser, H.F.; Singh, S.R.; Kompella, U.B. Effect of circulation on the disposition and ocular tissue distribution of 20 nm nanoparticles after periocular administration. Mol. Vis. 2008, 14, 150–160. [Google Scholar]
- Niamprem, P.; Srinivas, S.P.; Tiyaboonchai, W. Penetration of Nile red-loaded nanostructured lipid carriers (NLCs) across the porcine cornea. Colloids Surf. B Biointerfaces. 2019, 176, 371–378. [Google Scholar] [CrossRef]
- Scioli Montoto, S.; Muraca, G.; Ruiz, M.E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front. Mol. Biosci. 2020, 7, 587997. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Dhar, A.; Patel, C.; Khimani, M.; Neogi, S.; Sharma, P.; Kumar, N.S.; Vekariya, R.L. A brief review on solid lipid nanoparticles: Part and parcel of contemporary drug delivery systems. RSC Adv. 2020, 10, 26777–26791. [Google Scholar] [CrossRef] [PubMed]
- Attama, A.A.; Momoh, M.A.; Builders, P.F. Lipid Nanoparticulate Drug Delivery Systems: A Revolution in Dosage Form Design and Development. In Recent Advances in Novel Drug Carrier Systems; Sezer, A.D., Ed.; IntechOpen: London, UK, 2012; pp. 107–140. [Google Scholar]
- Gordillo-Galeano, A.; Mora-Huertas, C.E. Solid lipid nanoparticles and nanostructured lipid carriers: A review emphasizing on particle structure and drug release. Eur. J. Pharm. Biopharm. 2018, 133, 285–308. [Google Scholar] [CrossRef]
- Boonme, P.; Souto, E.B.; Wuttisantikul, N.; Jongjit, T.; Pichayakorn, W. Influence of lipids on the properties of solid lipid nanoparticles from microemulsion technique. Eur. J. Lipid Sci. Technol. 2013, 115, 820–824. [Google Scholar] [CrossRef]
- Paliwal, R.; Rai, S.; Vaidya, B.; Khatri, K.; Goyal, A.K.; Mishra, N.; Mehta, A.; Vyas, S.P. Effect of lipid core material on characteristics of solid lipid nanoparticles designed for oral lymphatic delivery. Nanomedicine 2009, 5, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Cavendish, M.; Nalone, L.; Barbosa, T.; Barbosa, R.; Costa, S.; Nunes, R.; da Silva, C.F.; Chaud, M.V.; Souto, E.B.; Hollanda, L.; et al. Study of pre-formulation and development of solid lipid nanoparticles containing perillyl alcohol. J. Therm. Anal. Calorim. 2019, 141, 767–774. [Google Scholar] [CrossRef]
- Hernández-Esquivel, R.-A.; Navarro-Tovar, G.; Zárate-Hernández, E.; Aguirre-Bañuelos, P. Solid Lipid Nanoparticles (SLN). In Nanocomposite Materials for Biomedical and Energy Storage Applications, 1st ed.; Sharma, A., Ed.; IntechOpen: London, UK, 2022; pp. 1–27. [Google Scholar]
- Balamurugan, K.; Chintamani, P. Lipid nano particulate drug delivery: An overview of the emerging trend. Pharma Innov. J. 2018, 7, 779–789. [Google Scholar]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54, S131–S155. [Google Scholar] [CrossRef]
- Sumera; Anwar, A.; Ovais, M.; Khan, A.; Raza, A. Docetaxel-loaded solid lipid nanoparticles: A novel drug delivery system. IET Nanobiotechnol. 2017, 11, 621–629. [Google Scholar] [CrossRef]
- Aguirre-Ramírez, M.; Silva-Jiménez, H.; Banat, I.M.; Díaz De Rienzo, M.A. Surfactants: Physicochemical interactions with biological macromolecules. Biotechnol. Lett. 2021, 43, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-T.-L.; Duong, V.-A. Solid Lipid Nanoparticles. Encyclopedia 2022, 2, 952–973. [Google Scholar] [CrossRef]
- Silva, A.; Martins-Gomes, C.; Coutinho, T.; Fangueiro, J.; Sanchez-Lopez, E.; Pashirova, T.; Andreani, T.; Souto, E.B. Soft Cationic Nanoparticles for Drug Delivery: Production and Cytotoxicity of Solid Lipid Nanoparticles (SLNs). Appl. Sci. 2019, 9, 4438. [Google Scholar] [CrossRef]
- Amis, T.M.; Renukuntla, J.; Bolla, P.K.; Clark, B.A. Selection of Cryoprotectant in Lyophilization of Progesterone-Loaded Stearic Acid Solid Lipid Nanoparticles. Pharmaceutics 2020, 12, 892. [Google Scholar] [CrossRef]
- Dhiman, N.; Awasthi, R.; Sharma, B.; Kharkwal, H.; Kulkarni, G.T. Lipid Nanoparticles as Carriers for Bioactive Delivery. Front. Chem. 2021, 9, 580118. [Google Scholar] [CrossRef]
- Siram, K.; Karuppaiah, A.; Gautam, M.; Sankar, V. Fabrication of Hyaluronic Acid Surface Modified Solid Lipid Nanoparticles Loaded with Imatinib Mesylate for Targeting Human Breast Cancer MCF-7 Cells. J. Clust. Sci. 2022; in press. [Google Scholar]
- Kuo, Y.-C.; Chao, I.-W. Conjugation of melanotransferrin antibody on solid lipid nanoparticles for mediating brain cancer malignancy. Biotechnol. Prog. 2015, 32, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Onugwu, A.L.; Attama, A.A.; Nnamani, P.O.; Onugwu, S.O.; Onuigbo, E.B.; Khutoryanskiy, V.V. Development and optimization of solid lipid nanoparticles coated with chitosan and poly(2-ethyl-2-oxazoline) for ocular drug delivery of ciprofloxacin. J. Drug Deliv. Sci.Technol. 2022, 74, 103527. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Eid, H.M.; Elkomy, M.H.; El Menshawe, S.F.; Salem, H.F. Development, Optimization, and In Vitro/In Vivo Characterization of Enhanced Lipid Nanoparticles for Ocular Delivery of Ofloxacin: The Influence of Pegylation and Chitosan Coating. AAPS PharmSciTech. 2019, 20, 183. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.; Dong, C.; Zhang, L. Sustained latanoprost release from PEGylated solid lipid nanoparticle-laden soft contact lens to treat glaucoma. Pharm. Dev. Technol. 2022, 27, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Hu, K. Preparation and Characterization of Tacrolimus-Loaded SLNs in situ Gel for Ocular Drug Delivery for the Treatment of Immune Conjunctivitis. Drug Des. Devel. Ther. 2021, 15, 141–150. [Google Scholar] [CrossRef]
- El-Emam, G.A.; Girgis, G.N.; Hamed, M.F.; El-Azeem Soliman, O.A.; Abd El Gawad, A.E.G.H. Formulation and Pathohistological Study of Mizolastine–Solid Lipid Nanoparticles–Loaded Ocular Hydrogels. Int. J. Nanomed. 2021, 16, 7775–7799. [Google Scholar] [CrossRef]
- Carbone, C.; Fuochi, V.; Zielińska, A.; Musumeci, T.; Souto, E.B.; Bonaccorso, A.; Puglia, C.; Petronio, G.P.; Furneri, P.M. Dual-drugs delivery in solid lipid nanoparticles for the treatment of Candida albicans mycosis. Colloids Surf. B Biointerfaces 2020, 186, 110705. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, Z.; Yang, J.; Lu, P.; Zhou, T.; Li, J.; Zhang, J. Assessment to the Antifungal Effects in vitro and the Ocular Pharmacokinetics of Solid-Lipid Nanoparticle in Rabbits. Int. J. Nanomed. 2021, 16, 7847–7857. [Google Scholar] [CrossRef]
- Khames, A.; Khaleel, M.A.; El-Badawy, M.F.; El-Nezhawy, A.O.H. Natamycin solid lipid nanoparticles—Sustained ocular delivery system of higher corneal penetration against deep fungal keratitis: Preparation and optimization. Int. J. Nanomedicine 2019, 14, 2515–2531. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Guzman-Aranguez, A.; Hussain, A.; Srinivas, C.S.; Kaur, I.P. Solid lipid nanoparticles for ocular delivery of isoniazid: Evaluation, proof of concept and in vivo safety & kinetics. Nanomedicine 2019, 14, 465–491. [Google Scholar] [PubMed]
- Nair, A.B.; Shah, J.; Al-Dhubiab, B.E.; Jacob, S.; Patel, S.S.; Venugopala, K.N.; Morsy, M.A.; Gupta, S.; Attimarad, M.; Sreeharsha, N.; et al. Clarithromycin Solid Lipid Nanoparticles for Topical Ocular Therapy: Optimization, Evaluation and In Vivo Studies. Pharmaceutics 2021, 13, 523. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorso, A.; Pepe, V.; Zappulla, C.; Cimino, C.; Pricoco, A.; Puglisi, G.; Giuliano, F.; Pignatello, R.; Carbone, C. Sorafenib Repurposing for Ophthalmic Delivery by Lipid Nanoparticles: A Preliminary Study. Pharmaceutics 2021, 13, 1956. [Google Scholar] [CrossRef]
- Yadav, M.; Schiavone, N.; Guzman-Aranguez, A.; Giansanti, F.; Papucci, L.; Perez de Lara, M.J.; Singh, M.; Kaur, I.P. Atorvastatin-loaded solid lipid nanoparticles as eye drops: Proposed treatment option for age-related macular degeneration (AMD). Drug Deliv. Transl. Res. 2020, 10, 919–944. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Li, Y.; Wang, K.; Zhu, X.; Tharkar, P.; Shu, W.; Zhang, T.; Zeng, S.; Zhu, L.; Murray, M.; et al. Compritol solid lipid nanoparticle formulations enhance the protective effect of betulinic acid derivatives in human Müller cells against oxidative injury. Exp. Eye Res. 2022, 215, 108906. [Google Scholar] [CrossRef]
- Ahmad, I.; Pandit, J.; Sultana, Y.; Mishra, A.K.; Hazari, P.P.; Aqil, M. Optimization by design of etoposide loaded solid lipid nanoparticles for ocular delivery: Characterization, pharmacokinetic and deposition study. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 959–970. [Google Scholar] [CrossRef]
- Freitas, L.G.A.; Isaac, D.L.C.; Lima, E.M.; Souza, L.G.; Abud, M.A.; Reis, R.G.D.; Tannure, W.T.; Ávila, M.P. Retinal changes in rabbit after intravitreal injection of sunitinib encapsulated into solid lipid nanoparticles and polymeric nanocapsules. Arq. Bras. Oftalmol. 2018, 81, 408–413. [Google Scholar] [CrossRef]
- Wang, F.Z.; Zhang, M.W.; Zhang, D.S.; Huang, Y.; Chen, L.; Jiang, S.M.; Shi, K.; Li, R. Preparation, optimization, and characterization of chitosan-coated solid lipid nanoparticles for ocular drug delivery. J. Biomed. Res. 2018, 32, 411–423. [Google Scholar]
- Taskar, P.S.; Patil, A.; Lakhani, P.; Ashour, E.; Gul, W.; ElSohly, M.A.; Murphy, B.; Majumdar, S. Δ9-Tetrahydrocannabinol Derivative-Loaded Nanoformulation Lowers Intraocular Pressure in Normotensive Rabbits. Transl. Vis. Sci. Technol. 2019, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, F.; Liu, R.; Chen, J.; Zhang, Q.; Lao, R.; Wang, Z.; Jin, X.; Liu, C. Novel cationic lipid nanoparticles as an ophthalmic delivery system for multicomponent drugs: Development, characterization, in vitro permeation, in vivo pharmacokinetic, and molecular dynamics studies. Int. J. Nanomed. 2017, 12, 8115–8127. [Google Scholar] [CrossRef]
- Gomes Souza, L.; Antonio Sousa-Junior, A.; Alves Santana Cintra, B.; Vieira Dos Anjos, J.L.; Leite Nascimento, T.; Palmerston Mendes, L.; de Souza Vieira, M.; do Nascimento Ducas, R.; Campos Valadares, M.; Antônio Mendanha, S.; et al. Pre-clinical safety of topically administered sunitinib-loaded lipid and polymeric nanocarriers targeting corneal neovascularization. Int. J. Pharm. 2023, 635, 122682. [Google Scholar] [CrossRef]
- Jaiswal, P.; Gidwani, B.; Vyas, A. Nanostructured lipid carriers and their current application in targeted drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 27–40. [Google Scholar] [CrossRef]
- Dhiman, S.; Mishra, N.; Sharma, S. Development of PEGylated solid lipid nanoparticles of pentoxifylline for their beneficial pharmacological potential in pathological cardiac hypertrophy. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1901–1908. [Google Scholar] [CrossRef]
- Elmowafy, M.; Al-Sanea, M.M. Nanostructured lipid carriers (NLCs) as drug delivery platform: Advances in formulation and delivery strategies. Saudi Pharm. J. 2021, 29, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Shahzadi, I.; Fürst, A.; Knoll, P.; Bernkop-Schnürch, A. Nanostructured Lipid Carriers (NLCs) for Oral Peptide Drug Delivery: About the Impact of Surface Decoration. Pharmaceutics 2021, 13, 1312. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, I.; Yasir, M.; Verma, M.; Singh, A.P. Nanostructured Lipid Carriers: A Groundbreaking Approach for Transdermal Drug Delivery. Adv. Pharm. Bull. 2020, 10, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Kiss, E.L.; Berkó, S.; Gácsi, A.; Kovács, A.; Katona, G.; Soós, J.; Csányi, E.; Gróf, I.; Harazin, A.; Deli, M.A.; et al. Design and Optimization of Nanostructured Lipid Carrier Containing Dexamethasone for Ophthalmic Use. Pharmaceutics 2019, 11, 679. [Google Scholar] [CrossRef]
- El-Salamouni, N.S.; Farid, R.M.; El-Kamel, A.H.; El-Gamal, S.S. Effect of sterilization on the physical stability of brimonidine-loaded solid lipid nanoparticles and nanostructured lipid carriers. Int. J. Pharm. 2015, 496, 976–983. [Google Scholar] [CrossRef]
- Apostolou, M.; Assi, S.; Fatokun, A.A.; Khan, I. The Effects of Solid and Liquid Lipids on the Physicochemical Properties of Nanostructured Lipid Carriers. J. Pharm. Sci. 2021, 110, 2859–2872. [Google Scholar] [CrossRef]
- Malik, D.S.; Kaur, G. Nanostructured gel for topical delivery of azelaic acid: Designing, characterization, and in-vitro evaluation. J. Drug Deliv. Sci. Technol. 2018, 47, 123–136. [Google Scholar] [CrossRef]
- Bang, K.-H.; Na, Y.-G.; Huh, H.W.; Hwang, S.-J.; Kim, M.-S.; Kim, M.; Lee, H.-K.; Cho, C.-W. The Delivery Strategy of Paclitaxel Nanostructured Lipid Carrier Coated with Platelet Membrane. Cancers 2019, 11, 807. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Wang, Q.; Liu, Y. Lung cancer combination therapy: Doxorubicin and β-elemene co-loaded, pH-sensitive nanostructured lipid carriers. Drug Des. Devel. Ther. 2019, 13, 1087–1098. [Google Scholar] [CrossRef]
- Javed, S.; Mangla, B.; Almoshari, Y.; Sultan, M.; Ahsan, W. Nanostructured lipid carrier system: A compendium of their formulation development approaches, optimization strategies by quality by design, and recent applications in drug delivery. Nanotechnol. Rev. 2022, 11, 1744–1777. [Google Scholar] [CrossRef]
- Haider, M.; Abdin, S.M.; Kamal, L.; Orive, G. Nanostructured Lipid Carriers for Delivery of Chemotherapeutics: A Review. Pharmaceutics 2020, 12, 288. [Google Scholar] [CrossRef] [PubMed]
- Khosa, A.; Reddi, S.; Saha, R.N. Nanostructured lipid carriers for site-specific drug delivery. Biomed. Pharmacother. 2018, 103, 598–613. [Google Scholar] [CrossRef]
- Selvaraj, K.; Kuppusamy, G.; Krishnamurthy, J.; Mahalingam, R.; Singh, S.K.; Gulati, M. Repositioning of Itraconazole for the Management of Ocular Neovascularization Through Surface-Modified Nanostructured Lipid Carriers. Assay Drug Dev. Technol. 2019, 17, 178–190. [Google Scholar] [CrossRef]
- Sharma, D.S.; Wadhwa, S.; Gulati, M.; Kumar, B.; Chitranshi, N.; Gupta, V.K.; Alrouji, M.; Alhajlah, S.; AlOmeir, O.; Vishwas, S.; et al. Chitosan modified 5-fluorouracil nanostructured lipid carriers for treatment of diabetic retinopathy in rats: A new dimension to an anticancer drug. Int. J. Biol. Macromol. 2023, 224, 810–830. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Yi, J.; Lv, S.; Zhang, B. Ocular amphotericin B delivery by chitosan-modified nanostructured lipid carriers for fungal keratitis-targeted therapy. J. Liposome Res. 2017, 27, 228–233. [Google Scholar] [CrossRef]
- Pai, R.V.; Vavia, P.R. Chitosan oligosaccharide enhances binding of nanostructured lipid carriers to ocular mucins: Effect on ocular disposition. Int. J. Pharm. 2020, 577, 119095. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jin, X.; Yang, Y.; Zhang, L.; Liu, R.; Li, Z. Trimethyl chitosan nanoparticles for ocular baicalein delivery: Preparation, optimization, in vitro evaluation, in vivo pharmacokinetic study and molecular dynamics simulation. Int. J. Biol. Macromol. 2020, 156, 749–761. [Google Scholar] [CrossRef]
- Tan, G.; Li, J.; Song, Y.; Yu, Y.; Liu, D.; Pan, W. Phenylboronic acid-tethered chondroitin sulfate-based mucoadhesive nanostructured lipid carriers for the treatment of dry eye syndrome. Acta Biomater. 2019, 99, 350–362. [Google Scholar] [CrossRef]
- Zhu, R.; Chen, W.; Gu, D.; Wang, T.; Li, J.; Pan, H. Chondroitin sulfate and L-Cysteine conjugate modified cationic nanostructured lipid carriers: Pre-corneal retention, permeability, and related studies for dry eye treatment. Int. J. Biol. Macromol. 2023, 228, 624–637. [Google Scholar] [CrossRef]
- Abdelhakeem, E.; El-Nabarawi, M.; Shamma, R. Effective Ocular Delivery of Eplerenone Using Nanoengineered Lipid Carriers in Rabbit Model. Int. J. Nanomed. 2021, 16, 4985–5002. [Google Scholar] [CrossRef]
- Yan, T.; Ma, Z.; Liu, J.; Yin, N.; Lei, S.; Zhang, X.; Li, X.; Zhang, Y.; Kong, J. Thermoresponsive Genistein NLC-dexamethasone-moxifloxacin multi drug delivery system in lens capsule bag to prevent complications after cataract surgery. Sci. Rep. 2021, 11, 181. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, N.; Taymouri, S.; Saeidi, A.; Akbari, V. Thermosensitive hydrogel containing sertaconazole loaded nanostructured lipid carriers for potential treatment of fungal keratitis. Pharm. Dev. Technol. 2019, 24, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Feng, R.; Li, J.; Wang, Y.; Song, Y.; Tan, G.; Liu, D.; Liu, W.; Yang, X.; Pan, H.; et al. A hybrid genipin-crosslinked dual-sensitive hydrogel/nanostructured lipid carrier ocular drug delivery platform. Asian J. Pharm. Sci. 2019, 14, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xu, S.; Yu, S.; Li, J.; Tan, G.; Li, S.; Pan, W. A Hybrid Genipin-Cross-Linked Hydrogel/Nanostructured Lipid Carrier for Ocular Drug Delivery: Cellular, ex Vivo, and in Vivo Evaluation. ACS Biomater. Sci. Eng. 2020, 6, 1543–1552. [Google Scholar] [CrossRef]
- Chen, L.; Wu, R. Brinzolamide- and latanoprost-loaded nano lipid carrier prevents synergistic retinal damage in glaucoma. Acta Biochim. Pol. 2022, 69, 423–428. [Google Scholar] [CrossRef]
- Varela-Fernández, R.; García-Otero, X.; Díaz-Tomé, V.; Regueiro, U.; López-López, M.; González-Barcia, M.; Isabel Lema, M.; Javier Otero-Espinar, F. Lactoferrin-loaded nanostructured lipid carriers (NLCs) as a new formulation for optimized ocular drug delivery. Eur. J. Pharm. Biopharm. 2022, 172, 144–156. [Google Scholar] [CrossRef]
- Kumari, S.; Dandamudi, M.; Rani, S.; Behaeghel, E.; Behl, G.; Kent, D.; O’Reilly, N.J.; O’Donovan, O.; McLoughlin, P.; Fitzhenry, L. Dexamethasone-Loaded Nanostructured Lipid Carriers for the Treatment of Dry Eye Disease. Pharmaceutics 2021, 13, 905. [Google Scholar] [CrossRef] [PubMed]
- Zahir-Jouzdani, F.; Khonsari, F.; Soleimani, M.; Mahbod, M.; Arefian, E.; Heydari, M.; Shahhosseini, S.; Dinarvand, R.; Atyabi, F. Nanostructured lipid carriers containing rapamycin for prevention of corneal fibroblasts proliferation and haze propagation after burn injuries: In vitro and in vivo. J. Cell Physiol. 2019, 234, 4702–4712. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Tiwari, A.; Asdaq, S.M.B.; Nair, A.B.; Bhatt, S.; Shinu, P.; Al Mouslem, A.K.; Jacob, S.; Alamri, A.S.; Alsanie, W.F.; et al. Itraconazole loaded nano-structured lipid carrier for topical ocular delivery: Optimization and evaluation. Saudi J. Biol. Sci. 2022, 29, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.; Lakhani, P.; Taskar, P.; Wu, K.W.; Sweeney, C.; Avula, B.; Wang, Y.H.; Khan, I.A.; Majumdar, S. Formulation Development, Optimization, and In Vitro-In Vivo Characterization of Natamycin-Loaded PEGylated Nano-Lipid Carriers for Ocular Applications. J. Pharm. Sci. 2018, 107, 2160–2171. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, X.; Zhang, P.; Mo, F.; Si, P.; Kang, X.; Wang, M.; Zhang, J. Dasatinib loaded nanostructured lipid carriers for effective treatment of corneal neovascularization. Biomater. Sci. 2021, 9, 2571–2583. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Yang, J.; Xu, H.; Shi, J.; Liang, Z.; Zhang, R.; Lu, P.; Pu, G.; Zhao, N.; Zhang, J. Sorafenib-loaded nanostructured lipid carriers for topical ocular therapy of corneal neovascularization: Development, in-vitro and in vivo study. Drug Deliv. 2022, 29, 837–855. [Google Scholar] [CrossRef] [PubMed]
- Nirbhavane, P.; Sharma, G.; Singh, B.; Begum, G.; Jones, M.-C.; Rauz, S.; Vincent, R.; Denniston, A.K. Triamcinolone acetonide loaded-cationic nano-lipoidal formulation for uveitis: Evidences of improved biopharmaceutical performance and anti-inflammatory activity. Colloids Surf. B. Biointerfaces 2020, 190, 110902. [Google Scholar] [CrossRef]
- Jounaki, K.; Makhmalzadeh, B.S.; Feghhi, M.; Heidarian, A. Topical ocular delivery of vancomycin loaded cationic lipid nanocarriers as a promising and non-invasive alternative approach to intravitreal injection for enhanced bacterial endophthalmitis management. Eur. J. Pharm. Sci. 2021, 167, 105991. [Google Scholar] [CrossRef]
- Puglia, C.; Blasi, P.; Ostacolo, C.; Sommella, E.; Bucolo, C.; Platania, C.B.M.; Romano, G.L.; Geraci, F.; Drago, F.; Santonocito, D.; et al. Innovative Nanoparticles Enhance N-Palmitoylethanolamide Intraocular Delivery. Front. Pharmacol. 2018, 9, 285. [Google Scholar] [CrossRef]
- Zingale, E.; Rizzo, S.; Bonaccorso, A.; Consoli, V.; Vanella, L.; Musumeci, T.; Spadaro, A.; Pignatello, R. Optimization of Lipid Nanoparticles by Response Surface Methodology to Improve the Ocular Delivery of Diosmin: Characterization and In-Vitro Anti-Inflammatory Assessment. Pharmaceutics 2022, 14, 1961. [Google Scholar] [CrossRef]
- Santonocito, D.; Vivero-Lopez, M.; Lauro, M.R.; Torrisi, C.; Castelli, F.; Sarpietro, M.G.; Puglia, C. Design of Nanotechnological Carriers for Ocular Delivery of Mangiferin: Preformulation Study. Molecules 2022, 27, 1328. [Google Scholar] [CrossRef]
- González-Fernández, F.M.; Delledonne, A.; Nicoli, S.; Gasco, P.; Padula, C.; Santi, P.; Sissa, C.; Pescina, S. Nanostructured Lipid Carriers for Enhanced Transscleral Delivery of Dexamethasone Acetate: Development, Ex Vivo Characterization and Multiphoton Microscopy Studies. Pharmaceutics 2023, 15, 407. [Google Scholar] [CrossRef] [PubMed]
- Khairnar, S.V.; Pagare, P.; Thakre, A.; Nambiar, A.R.; Junnuthula, V.; Abraham, M.C.; Kolimi, P.; Nyavanandi, D.; Dyawanapelly, S. Review on the Scale-Up Methods for the Preparation of Solid Lipid Nanoparticles. Pharmaceutics 2022, 14, 1886. [Google Scholar] [CrossRef] [PubMed]
- Duong, V.-A.; Nguyen, T.-T.-L.; Maeng, H.-J. Preparation of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Drug Delivery and the Effects of Preparation Parameters of Solvent Injection Method. Molecules 2020, 25, 4781. [Google Scholar] [CrossRef]
- Zielińska, A.; Soles, B.B.; Lopes, A.R.; Vaz, B.F.; Rodrigues, C.M.; Alves, T.F.R.; Klensporf-Pawlik, D.; Durazzo, A.; Lucarini, M.; Severino, P.; et al. Nanopharmaceuticals for Eye Administration: Sterilization, Depyrogenation and Clinical Applications. Biology 2020, 9, 336. [Google Scholar] [CrossRef]
- Pardeike, J.; Weber, S.; Haber, T.; Wagner, J.; Zarfl, H.P.; Plank, H.; Zimmer, A. Development of an itraconazole-loaded nanostructured lipid carrier (NLC) formulation for pulmonary application. Int. J. Pharm. 2011, 419, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Gokce, E.H.; Sandri, G.; Bonferoni, M.C.; Rossi, S.; Ferrari, F.; Güneri, T.; Caramella, C. Cyclosporine A loaded SLNs: Evaluation of cellular uptake and corneal cytotoxicity. Int. J. Pharm. 2008, 364, 76–86. [Google Scholar] [CrossRef]
- Youshia, J.; Kamel, A.O.; El Shamy, A.; Mansour, S. Gamma sterilization and in vivo evaluation of cationic nanostructured lipid carriers as potential ocular delivery systems for antiglaucoma drugs. Eur. J. Pharm. Sci. 2021, 163, 105887. [Google Scholar] [CrossRef]
- Thi, T.T.H.; Suys, E.J.A.; Lee, J.S.; Nguyen, D.H.; Park, K.D.; Truong, N.P. Lipid-Based Nanoparticles in the Clinic and Clinical Trials: From Cancer Nanomedicine to COVID-19 Vaccines. Vaccines 2021, 9, 359. [Google Scholar] [CrossRef]
- Khiev, D.; Mohamed, Z.A.; Vichare, R.; Paulson, R.; Bhatia, S.; Mohapatra, S.; Lobo, G.P.; Valapala, M.; Kerur, N.; Passaglia, C.L.; et al. Emerging Nano-Formulations and Nanomedicines Applications for Ocular Drug Delivery. Nanomaterials 2021, 11, 173. [Google Scholar] [CrossRef]
- Buttini, F.; Rozou, S.; Rossi, A.; Zoumpliou, V.; Rekkas, D.M. The application of Quality by Design framework in the pharmaceutical development of dry powder inhalers. Eur. J. Pharm. Sci. 2018, 113, 64–76. [Google Scholar] [CrossRef] [PubMed]
- Janagam, D.R.; Wu, L.; Lowe, T.L. Nanoparticles for drug delivery to the anterior segment of the eye. Adv. Drug Deliv. Rev. 2017, 122, 31–64. [Google Scholar] [CrossRef] [PubMed]

| Alternative Route | Specifics | Benefits | Limitations | References |
|---|---|---|---|---|
| Sub- conjunctival (SC) | SC route includes SC injections, administered in the lower or upper fornix, as well as instillation of SC implants; Clinical indications include corneal/scleral lesions, glaucoma, cytomegalovirus rhinitis. | Possibility to ensure high local drug concentration; Improved penetration of water-soluble drugs due to the bypassing of the corneal epithelium. | Conjunctival and choroidal blood/lymphatic flow; Temporary pain at the injection site; Local irritations. | [98,99] |
| Intracameral (IC) | Injections applied in the anterior chamber, often as a prevention of postoperative endophthalmitis after cataract surgery; Delivery of antibiotics, steroids, anesthetics. | Lower drug concentration needed; Decreased side effects vs. topical steroid application; Increased anesthesia during surgery when co-administered with topical anesthetics. | Potential complications, such as toxic anterior segment syndrome, corneal endothelial toxicity. | [100,101,102] |
| Transscleral | Drug delivery to the posterior segment of the eye; The sclera is thinnest around the equator, therefore, it is the preferred area for injection. | Obviates the corneal and conjunctival barrier; Less-invasive procedure compared to intravitreal injections. | Static barriers (sclera, choroid, retina) and dynamic barriers (choroidal blood flow) reduce drug bioavailability; Necessity of high doses. | [84,99,103] |
| Supra-choroidal (SC) | Drug injection under the choroid, targeting the following areas: choroid and retina; Microneedles have also been used for drug deposition into the SC space; Clinical indications include: posterior uveitis, macular edema. | Obviates the sclera and improves drug bioavailability within the choroid and retina; Effective for the delivery of small molecules; Lower risk of intraocular pressure spikes. | Choroidal circulation; Risk of occurrence of choroidal hemorrhage or detachment. | [99,104,105] |
| Intravitreal (IV) | Direct injection to the vitreous body targeting posterior eye segment; Drug delivery of vascular endothelial growth factor (VEGF) inhibitors, antibiotics, corticosteroids; IV injections are applied in the therapy of age-related macular degeneration, cytomegalovirus retinitis, diabetic macular edema, retinal vein occlusions. | Bypasses the BRB; Provides high local therapeutic concentration and prolonged drug levels; Reduced systemic side effects. | Repetitive instillations lead to serious ocular complications and patient non-compliance. Eye discomfort and pain were reported following IV injections. | [53,106] |
| Systemic/Oral | Drugs are administered orally or intravenously; Therapeutic applications include: scleritis, cytomegalovirus retinitis. | Acceptance by the patients. | Low bioavailability (<2%)— barrier role of BAB, BRB; Necessity of high doses, corresponding to increased risk of side effects. | [107] |
| Composition | Drug/Disease | Method of Preparation | Physicochemical Characteristics | Results | References |
|---|---|---|---|---|---|
| Tripalmitin Tween 80 Glycerol | Econazole/ Fungal keratitis | Microemulsion method | Size 19.05 ± 0.28 nm PDI 0.21 ± 0.01 ζ potential −2.20 ± 0.10 mV EE = 94.18 ± 1.86% | Slow and controlled drug release (within 96 h); Improved antifungal activity; Enhanced bioavailability—drug concentration was above MIC within 3 h after application. | [153] |
| Precirol ATO 5 Pluronic F68 Stearyl amine | Natamycin/ Fungal keratitis | Hot emulsification-ultrasonication technique | Size 42 nm PDI 0.224 ζ potential 26 mV EE ≈ 85% | Prolonged drug release (within 8 h); Improved corneal penetration; Superior antifungal activity vs. free drug; Excellent ocular tolerability. | [154] |
| Compritol 888 ATO Stearic acid Tween 80 Soy lecithin | Isoniazid/ Ocular tuberculosis | Microemulsion method | Size 149.2 ± 4.9 nm PDI 0.15 ± 0.02 ζ potential −0.35 ± 0.28 mV EE = 65.2 ± 2.2% | Prolonged drug release (48 h); Enhanced corneal permeability (1.6 fold); Improved ocular bioavailability (4.2 fold) vs. drug solution. | [155] |
| Stearic acid Tween 80 Transcutol P | Clarithromycin/ Bacterial endophthalmitis | High-speed mixing and the ultrasonication method | Size 157 ± 42.4 nm PDI 0.13 ± 0.02 ζ potential −17.2 ± 3.1 mV EE = 81.3 ± 4.6 | Sustained drug release (~80% in 8 h); Improved transcorneal permeation and bioavailability compared to drug solution. | [156] |
| Softisan 100 (Hydrogenated Coco-Glycerides) Suppocire NB (C10–C18 Triglycerides) Tween 80 Tegin O DOTAP DDAB | Sorafenib/ Uveal melanoma | Phase inversion temperature method | Size 127.85 ± 1.50 nm PDI 0.215 ± 0.014 ζ potential 20 mV EE= 75.0 ± 2.1% | Sustained drug release (less than 25% of encapsulated drug released after 72 h); Good physical stability, cytocompatibility and mucoadhesive properties of elaborated SLNs. | [157] |
| Compritol 888ATO PEG 400 Poloxamer 188 Phospholipon 90H | Atorvastatin/ Age-related macular degeneration | Hot high-pressure homogenization | Size 256.3 ± 10.5 nm PDI 0.26 ± 0.02 ζ potential −2.65 mV EE= 73.1 ± 1.52% | Improved bioavailability (8-fold in aqueous humor and 12-fold in vitreous humor) vs. free drug; Proven safety in corneal/retinal cell lines; Successful delivery to the retina, confirmed by intact fluorescein-labeled SLNs. | [158] |
| Com- pritol 888 ATO/Compritol HD5 ATO Pluronic F127 | Betulinic acid (BA) derivatives H3, H5 and H7/ Retinal diseases (diabetic retinopathy, age-related macular degeneration, choroidal neovascularization) | Microemulsion method | Size 58.5± 9.8 nm PDI 0.246 ζ potential 6.45 ± 5.58 mV EE = 75.10% | Improved drug delivery and enhanced anti-oxidative efficacy of BA derivatives; Suppressed glutamate-induced ROS production/necrosis in human Müller cells. | [159] |
| Gelucire 44/14 Compritol ATO 888 Tween 80 | Etoposide/ Posterior segment-related diseases (e.g., age-related macular degeneration, diabetic retinopathy) | Melt- emulsification and ultrasonication technique | Size 239.43 ± 2.35 nm PDI 0.261 ± 0.001 EE 80.96 ± 2.21% | Sustained etoposide concentration of etoposide in vitreous body for 7 days after IV injection Better toxicological profile vs. etoposide solution. | [160] |
| Stearic acid Sodium taurodeoxycholate Phosphati- dylcholine | Sutinib (Sb)/ Retinal diseases (age-related macular degeneration, diabetic retinopathy, retinal vein occlusions) | Microemulsion method | Size 140 nm PDI 0.20 | Excellent tolerability profile based on in vivo study on 20 albino rabbits; After IV injections, Sb SLNs didn’t cause any abnormalities in ocular morphology in contrast to polymeric nanocapsules. | [161] |
| Chitosan Phospholipids (Lipoid S100) Glyceryl mono- stearate Tween 80 PEG 400 | Methazolamide/ Glaucoma | Emulsion-solvent evaporation method | Size 247.7 ± 17.3 nm PDI ζ potential 33.5 ± 3.9 mV EE = 58.5 ± 4.5% | Prolonged drug release compared to drug solution; Excellent tolerability and marked reduction in IOP vs. uncoated methazolamide SLNs. | [162] |
| Compritol 888 ATO Pluronic F68 Tween 80 Glycerol | Δ9 -Tetrahydrocannabinol-valine-hemisuccinate/ Glaucoma | Ultrasonication | Size 287.80 ± 7.35 nm PDI 0.29 ± 0.01 EE = 93.57 ± 4.68% | Greater reduction in the IOP with respect to intensity and duration compared to pilocarpine/timolol maleate eye drops; High drug concentration in the iris/ciliary body and choroid/ retina. | [163] |
| Composition | Drug/Disease | Method of Preparation | Physicochemical Characteristics | Results | References |
|---|---|---|---|---|---|
| Glycerol monostearate 40–55 Soy lecithin Compritol 888 ATO Cholesterol Capryol 90 Miglyol 812 N Kolliphor P 407 Kolliphor P 188 α-Tocopherol-PEG | Lactoferrin/ Keratoconus | Double emulsion/ solvent evaporation method. | Size 119.45 ± 11.44 nm PDI 0.151 ± 0.045 ζ potential 17.50 ± 2.53 mV EE ≈ 75% | Controlled release profile; Good physical stability (up to 3 months); Muco-adhesive properties (for at least 240 min); Ocular tolerability. | [193] |
| Labrafac lipophile WL1349 Cholesterol Tween 80 | Dexamethasone (DXM)/ Dry Eye Disease | Solvent diffusion method | Size 19.51 ± 0.5 nm PDI 0.08 ζ potential 9.8 mV EE = 99.6 ± 0.5% | Cellular internalization in HCECs and corneal distribution in ex vivo porcine cornea; Significant reduction in inflammatory cytokines (MMP-9, IL-6 and TNF-α) related to DED pathogenesis vs. free DXM. | [194] |
| Precirol ATO5 Capryol PGMC Stearylamine Tween 80 Poloxamer 188 | Rapamycin/ Corneal alkaline burn injury | Emulsification solvent diffusion and evaporation method | Size 216 ± 40 nm ζ potential 14 ± 2.6 mV EE = 97.66 ± 0.57% | Improved fibroblast uptake of encapsulated cargo via NLCs (1.5 times); Superior in vivo corneal healing properties of NLCs vs. control groups. | [195] |
| Stearic acid, oleic acid Poloxamer 407 | Itraconazole/ Fungal keratitis | High-speed homogenization technique | Size 150.67 nm ζ potential −28 mV EE = 94.65% | Ocular safe formulation according to HET−CAM test; Enhanced antifungal activity of the NLCs compared to commercial eye drops. | [196] |
| PrecirolATO 5,Castor oil, Span 80, mPEG-2K-DSPE sodium salt Poloxamer 188, Tween 80, glycerin | Natamycin/ Fungal keratitis | High-pressure homogenization | Size 241.96 nm, PDI 0.406 EE = 95.35% | Improved in vitro transcorneal permeation and flux of formulated NT compared to drug suspension. | [197] |
| Glycerin monostearate Miglyol 812 N Solutol HS 15 Gelucire 44/14 Soy lecithin | Dasatinib (DAS)/ Corneal neovascularization | Melt-emulsification method | Size 78.53 ± 0.36 nm PDI 0.21 ± 0.01 ζ potential −29.6 ± 1.0 mV EE = 97.71% ± 0.89% | Enhanced solubility of DAS (1200-fold) after inclusion in NLCs; Inhibition of the development of CNV and associated corneal pathological alterations in a mouse model of CNV. | [198] |
| Monolaurin Capryol-90 Cremophor RH40 Transcutol P Glycerin | Sorafenib/ Corneal neovascularization | Microemulsion method | Size 111.87 ± 0.93 nm PDI 0.15 ± 0.01 ζ potential−0.35 ± 0.08 mV EE = 99.20 ± 0.86% | Excellent ocular tolerability (in vivo test on rabbits), non-toxic in HCEC; Approximately 6.7- and 1.3-fold higher drug concentrations in rabbit cornea and conjunctiva vs. free drug. | [199] |
| Compritol 888 ATO Apifil (PEG-8 beeswax) Miglyol 812N Labrasol, Kolliphor EL Cremophor RH60 | Dexamethasone/ Ophthalmic inflammatory diseases, severe uveitis | Ultrasonication method | Size 92.18 ± 0.49 nm PDI 0.12 ± 0.02 ζ potential −7.62 ± 0.26, EE = 88.31% | Good ocular tolerability; Ability to penetrate across the cornea; High concentration of NLCs in the stroma, according to porcine corneal penetration study. | [171] |
| Capmul MCM C10 Soya lecithin Captex 200 P Transcutol P Polysorbate 80 Stearylamine | Triamcinolone acetonide/ Uveitis | Hot microemulsion method | Size 198.95 ± 12.82 nm PDI 0.326 ± 0.04 ζ potential 35.8 ± 1.94 mV EE = 88.14 ± 3.03 % | Sustained drug release (84% within 24 h); Ex vivo corneal permeation of 51%; Biocompatible and ocular tolerable formulation (HET-CAM test). | [200] |
| Cholesterol Stearic acid Stearylamine Oleic acid Labrafil M 1944 Tween 80 | Vancomycin (VMC)/ Bacterial endophthalmitis | Cold homogenization technique | Size 96.40 ± 0.71 nm PDI 0.352 ± 0.011 ζ potential 29.7 ± 0.47 mV, EE = 74.80 ± 4.30% | Improved transcorneal penetration; Biocompatible, non-irritant formulation (in vitro RBC hemolytic assay); Enhanced (3-fold) intravitreal VMC concentration after topical application compared to drug solution. | [201] |
| Miglyol 812 Compritol 888 ATO Lutrol F68 | Palmitoylethanolamide (PEA)/ Retinal diseases (diabetic retinopathy, glaucoma) | High shear homogenization | Size 208.6 ± 10.2 nm PDI 0.18 ζ potential > 20 mV | Improved ocular bioavailability: 40% and 100% higher PEA levels in vitreous body and retina compared to free drug. | [202] |
| Glyceryl monostearate Labrafil M 2125 CS Tween 80 Transcutol HP Chitosan | 5-Fluorouracil (5-FU)/ Diabetic retinopathy | Melt emulsification-ultrasonication method | Size 163.2 ± 2.3 nm PDI 0.28 ± 1.52 ζ potential 21.4 ± 0.5 mV EE = 85.0 ± 0.2 % | Higher and sustained 5-FU release vs. free drug; Non-irritant formulations; Antiangiogenic effect confirmed by in vivo study in a diabetic retinopathy rat model. | [181] |
| Capryol 90 Softisan 100 Tween 80 | Diosmin/ Diabetic retinopathy | Melt emulsification method and ultrasonication | Size 83.58 ± 0.77 nm PDI 0.263 ± 0.067 ζ potential −18.5 ± 0.60 mV EE = 99.53± 2.50 | Very good physical stability of NLCs up to 60 days; Cytocompatibility assessed on ARPE-19 cells, Cytoprotective effects. | [203] |
| Compritol 888 ATO Miglyol 812 Lutrol F68 | Mangiferin (MNG)/ Oxidative stress related diseases, macular degeneration, diabetic retinopathy | High shear homogenization and ultrasound | Size 148.9 ± 0.1 nm PDI 0.21 ± 0.02 ζ potential −23.5 ± 0.2 mV, EE ≈ 92% | Higher antioxidant activity of MNG NLCs vs. free compound according to ORAC assay; Non-irritant formulations according to HET−CAM Assay. | [204] |
| Glyceryl monostearate Castor oil Poloxamer 188 | Brimonidine/ Glaucoma, ocular hypertension | High shear homogenization | Size 151.97 ±1.98 nm PDI 0.230 ± 0.01 ζ potential −44.2 ± 7.81 mV EE = 83.631 ± 0.495% | Improved permeability compared to analogous model SLNs; Highest reduction in the IOP in rabbits (vs. SLNs and free drug). | [172] |
| Captex 200P (propylene glycol dicaprate) Soya lecithin Capmul® MCM C10 (glyceryl monocaprate) Tween 80 Transcutol P Stearylamine Captex 200P | Brinzolamide (Brla) Latanoprost (Ltp)/ Glaucoma | Hot microemulsion method | Size165.28 ± 2.36 nm PDI 0.31 ± 0.015 ζ potential 35.33 ± 0.37 mV EE = 97.5 ± 2.16% | Adequate transcorneal permeation (Brla and Ltp levels after 24 h were ≈82% and ≈84%, respectively); Effective reduction of IOP in rats’ eyes with laser-induced glaucoma. | [192] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gugleva, V.; Andonova, V. Recent Progress of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Ocular Drug Delivery Platforms. Pharmaceuticals 2023, 16, 474. https://doi.org/10.3390/ph16030474
Gugleva V, Andonova V. Recent Progress of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Ocular Drug Delivery Platforms. Pharmaceuticals. 2023; 16(3):474. https://doi.org/10.3390/ph16030474
Chicago/Turabian StyleGugleva, Viliana, and Velichka Andonova. 2023. "Recent Progress of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Ocular Drug Delivery Platforms" Pharmaceuticals 16, no. 3: 474. https://doi.org/10.3390/ph16030474
APA StyleGugleva, V., & Andonova, V. (2023). Recent Progress of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Ocular Drug Delivery Platforms. Pharmaceuticals, 16(3), 474. https://doi.org/10.3390/ph16030474









