Plasma and Cerebrospinal Fluid Concentrations of Micafungin Administered at High Doses in Critically Ill Infants with Systemic Candidiasis: A Pooled Analysis of Two Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Design of the Study
2.2. Outcomes
2.3. Ethical Approval
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Yanni, S.B.; Smith, P.B.; Benjamin, D.K., Jr.; Augustijns, P.F.; Thakker, D.R.; Annaert, P.P. Higher clearance of micafungin in neonates compared with adults: Role of age-dependent micafungin serum binding. Biopharm. Drug Dispos. 2011, 32, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Hope, W.W.; Mickiene, D.; Petraitis, V.; Petraitiene, R.; Kelaher, A.M.; Hughes, J.E.; Cotton, M.P.; Bacher, J.; Keirns, J.J.; Buell, D.; et al. The Pharmacokinetics and Pharmacodynamics of Micafungin in Experimental Hematogenous Candida meningoencephalitis: Implications for Echinocandin Therapy in Neonates. J. Infect. Dis. 2008, 197, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Hope, W.W.; Castagnola, E.; Groll, A.H.; Roilides, E.; Akova, M.; Arendrup, M.C.; Arikan-Akdagli, S.; Bassetti, M.; Bille, J.; Cornely, O.A.; et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Prevention and management of invasive infections in neonates and children caused by Candida spp. Clin. Microbiol. Infect. 2012, 18, 38–52. [Google Scholar] [CrossRef]
- O’Hara, K.; Wright, I.M.; Schneider, J.J.; Jones, A.L.; Martin, J.H. Pharmacokinetics in neonatal prescribing: Evidence base, paradigms and the future. Br. J. Clin. Pharmacol. 2015, 80, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Rodieux, F.; Wilbaux, M.; van den Anker, J.N.; Pfister, M. Effect of Kidney Function on Drug Kinetics and Dosing in Neonates, Infants, and Children. Clin. Pharmacokinet. 2015, 54, 1183–1204. [Google Scholar] [CrossRef]
- van den Anker, J.; Reed, M.D.; Allegaert, K.; Kearns, G.L. Developmental Changes in Pharmacokinetics and Pharmacodynamics. J. Clin. Pharmacol. 2018, 58 (Suppl. 10), S10–S25. [Google Scholar] [CrossRef]
- Ruggiero, A.; Ariano, A.; Triarico, S.; Capozza, M.A.; Ferrara, P.; Attinà, G. Neonatal pharmacology and clinical implications. Drugs Context 2019, 8, 212608. [Google Scholar] [CrossRef]
- Hope, W.W.; Kaibara, A.; Roy, M.; Arrieta, A.; Azie, N.; Kovanda, L.L.; Benjamin, D.K., Jr. Population pharmacokinetics of micafungin and its metabolites M1 and M5 in children and adolescents. Antimicrob. Agents Chemother. 2015, 59, 905–913. [Google Scholar] [CrossRef]
- Queiroz-Telles, F.; Berezin, E.; Leverger, G.; Freire, A.; van der Vyver, A.; Chotpitayasunondh, T.; Konja, J.; Diekmann-Berndt, H.; Koblinger, S.; Groll, A.H.; et al. Micafungin versus liposomal amphotericin b for pediatric patients with invasive candidiasis: Substudy of a randomized Double-blind trial. Pediatr. Infect. Dis. J. 2008, 27, 820–826. [Google Scholar] [CrossRef]
- Auriti, C.; Falcone, M.; Ronchetti, M.P.; Goffredo, B.M.; Cairoli, S.; Crisafulli, R.; Piersigilli, F.; Corsetti, T.; Dotta, A.; Pai, M.P. High-Dose Micafungin for Preterm Neonates and Infants with Invasive and Central Nervous System Candidiasis. Antimicrob. Agents. Chemother. 2016, 60, 7333–7339. [Google Scholar] [CrossRef]
- Auriti, C.; Goffredo, B.M.; Ronchetti, M.P.; Piersigilli, F.; Cairoli, S.; Bersani, I.; Dotta, A.; Bagolan, P.; Pai, M.P. High-Dose Micafungin in Neonates and Young Infants with Invasive Candidiasis: Results of a Phase 2 Study. Antimicrob. Agents Chemother. 2021, 65, e02494-20. [Google Scholar] [CrossRef] [PubMed]
- Auriti, C.; Goffredo, B.M.; Ronchetti, M.P.; Piersigilli, F.; Cairoli, S.; Bersani, I.; Dotta, A.; Pai, M.P. Validation of heel stick microsampling to optimize micafungin doses in neonates and young infants. Antimicrob. Agents Chemother. 2018, 62, e01199-18. [Google Scholar] [CrossRef]
- De Rose, D.U.; Cairoli, S.; Dionisi, M.; Santisi, A.; Massenzi, L.; Goffredo, B.M.; Dionisi-Vici, C.; Dotta, A.; Auriti, C. Therapeutic Drug Monitoring Is a Feasible Tool to Personalize Drug Administration in Neonates Using New Techniques: An Overview on the Pharmacokinetics and Pharmacodynamics in Neonatal Age. Int. J. Mol. Sci. 2020, 21, 5898. [Google Scholar] [CrossRef] [PubMed]
- Martens-Lobenhoffer, J.; Rupprecht, V.; Bode-Böger, S.M. Determination of micafungin and anidulafungin in human plasma: UV- or mass spectrometric quantification? J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2011, 879, 2051–2056. [Google Scholar] [CrossRef] [PubMed]
- Astellas Pharma US Inc. Mycamine: Highlights of Prescribing Information. 2020. Available online: https://www.astellas.us/docs/mycamine.pdf (accessed on 6 March 2023).
- Egunsola, O.; Adefurin, A.; Fakis, A.; Jacqz-Aigrain, E.; Choonara, I.; Sammons, H. Safety of fluconazole in paediatrics: A systematic review. Eur. J. Clin. Pharmacol. 2013, 69, 1211–1221. [Google Scholar] [CrossRef]
- European Medicines Agency. Mycamine (R). Summary of Product Characteristics. 2022. Available online: https://www.ema.europa.eu/en/documents/product-information/mycamine-epar-product-information_en.pdf (accessed on 6 March 2023).
- Scott, B.L.; Hornik, C.D.; Zimmerman, K. Pharmacokinetic, efficacy, and safety considerations for the use of antifungal drugs in the neonatal population. Expert Opin. Drug Metab. Toxicol. 2020, 16, 605–616. [Google Scholar] [CrossRef]
- Taormina, G.; Gopinath, R.; Moore, J.; Yasinskaya, Y.; Colangelo, P.; Reynolds, K.; Nambiar, S. A Regulatory Review Approach for Evaluation of Micafungin for Treatment of Neonatal Candidiasis. Clin. Infect. Dis. 2021, 73, 2335–2340. [Google Scholar] [CrossRef]
- Robati Anaraki, M.; Nouri-Vaskeh, M.; Abdoli Oskoei, S. Fluconazole prophylaxis against invasive candidiasis in very low and extremely low birth weight preterm neonates: A systematic review and meta-analysis. Clin. Exp. Pediatr. 2021, 64, 172–179. [Google Scholar] [CrossRef]
- Pansieri, C.; Pandolfini, C.; Jacqz-Aigrain, E.; van den Anker, J.; Bonati, M. Fluconazole prophylaxis in neonates. Arch. Dis. Child. 2015, 100, 75–76. [Google Scholar] [CrossRef]
- Che, D.; Zhou, H.; Li, T.; Wu, B. Duration and intensity of fluconazole for prophylaxis in preterm neonates: A meta-analysis of randomized controlled trials. BMC Infect. Dis. 2016, 16, 312. [Google Scholar] [CrossRef]
- Fly, J.H.; Kapoor, S.; Bobo, K.; Stultz, J.S. Updates in the Pharmacologic Prophylaxis and Treatment of Invasive Candidiasis in the Pediatric and Neonatal Intensive Care Units. Curr. Treat. Options Infect. Dis. 2022, 14, 15–34. [Google Scholar] [CrossRef]
- Manzoni, P.; Wu, C.; Tweddle, L.; Roilides, E. Micafungin in premature and non-premature infants: A systematic review of 9 clinical trials. Pediatr. Infect. Dis. J. 2014, 33, e291–e298. [Google Scholar] [CrossRef] [PubMed]
- Walraven, C.J.; Lee, S.A. Antifungal lock therapy. Antimicrob. Agents Chemother. 2013, 57, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Katragkou, A.; Roilides, E.; Walsh, T.J. Role of Echinocandins in Fungal Biofilm-Related Disease: Vascular Catheter-Related Infections, Immunomodulation, and Mucosal Surfaces. Clin. Infect. Dis. 2015, 61, S622–S629. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Giacobbe, D.R.; Vena, A.; Esposito, S. An overview of micafungin as a treatment option for invasive candidiasis in pediatric patients younger than 4 months old. Expert. Opin. Pharmacother. 2022, 23, 1987–1994. [Google Scholar] [CrossRef]
- Hsieh, E.; Smith, P.B.; Jacqz-Aigrain, E.; Kaguelidou, F.; Cohen-Wolkowiez, M.; Manzoni, P.; Benjamin, D.K., Jr. Neonatal fungal infections: When to treat? Early Hum. Dev. 2012, 88 (Suppl. 2), S6–S10. [Google Scholar] [CrossRef]
- Benjamin, D.K., Jr.; Kaufman, D.A.; Hope, W.W.; Smith, P.B.; Arrieta, A.; Manzoni, P.; Kovanda, L.L.; Labemacher, C.; Isaacson, B.; Jednachowski, D.; et al. A Phase 3 Study of Micafungin Versus Amphotericin B Deoxycholate in Infants with Invasive Candidiasis. Pediatr. Infect. Dis. J. 2018, 37, 992–998. [Google Scholar] [CrossRef]
- Benjamin, D.K., Jr.; Stoll, B.J.; Fanaroff, A.A.; McDonald, S.A.; Oh, W.; Higgins, R.D.; Duara, S.; Poole, K.; Laptook, A.; Goldberg, R. Neonatal Candidiasis Among Extremely Low Birth Weight Infants: Risk Factors, Mortality Rates, and Neurodevelopmental Outcomes at 18 to 22 Months. Pediatrics 2006, 117, 84–92. [Google Scholar] [CrossRef]
- Schüller, S.S.; Bauer, C.; Unterasinger, L.; Berger, A. Safety and Efficacy of Micafungin in Extremely Low Birth Weight Infants. Pediatr. Infect. Dis. J. 2018, 37, e169–e172. [Google Scholar] [CrossRef]
- Mesini, A.; Saffioti, C.; Mariani, M.; Florio, A.; Medici, C.; Moscatelli, A.; Castagnola, E. First case of Candida auris colonization in a preterm, extremely low-birth-weight newborn after vaginal delivery. J. Fungi 2021, 7, 649. [Google Scholar] [CrossRef]
- Ramya, G.M.; Balakrishnan, U.; Chandrasekaran, A.; Abiramalatha, T.; Amboiram, P.; Sekar, U.; UshaDevi, R. Candida auris, an emerging pathogen—Challenge in the survival of microprimies. Indian J. Med. Microbiol. 2021, 39, 367–369. [Google Scholar] [CrossRef] [PubMed]
| Mean ± Standard Deviation | Minimum Value | Maximum Value | |
|---|---|---|---|
| Birth weight (grams) | 1610 ± 940 | 400 | 3800 |
| Gestational age (weeks) | 31.2 ± 5.3 | 23.0 | 40.0 |
| Weight at micafungin infusion (grams) | 2620 ± 1380 | 600 | 6800 |
| Age at micafungin infusion (months) | 2.5 ± 2.1 | 0.30 | 8.10 |
| 0 to ≤28 Days (n = 16) | >28 Days ≤120 Days (n = 23) | >120 Days (n = 13) | |
|---|---|---|---|
| Clearance (L/h), mean ± SD | 0.072 ± 0.066 | 0.087 ± 0.049 | 0.109 ± 0.071 |
| Clearance/weight (L/h/kg), mean ± SD | 0.036 ± 0.016 | 0.036 ± 0.010 | 0.028 ± 0.011 |
| AUC (mg·h/L), mean ± SD | 259.0 ± 92.7 | 236.8 ± 63.9 | 346.4 ± 145.0 |
| Half-life (h), mean ± SD | 13.5 ± 7.6 | 13.4 ± 4.3 | 14.4 ± 4.2 |
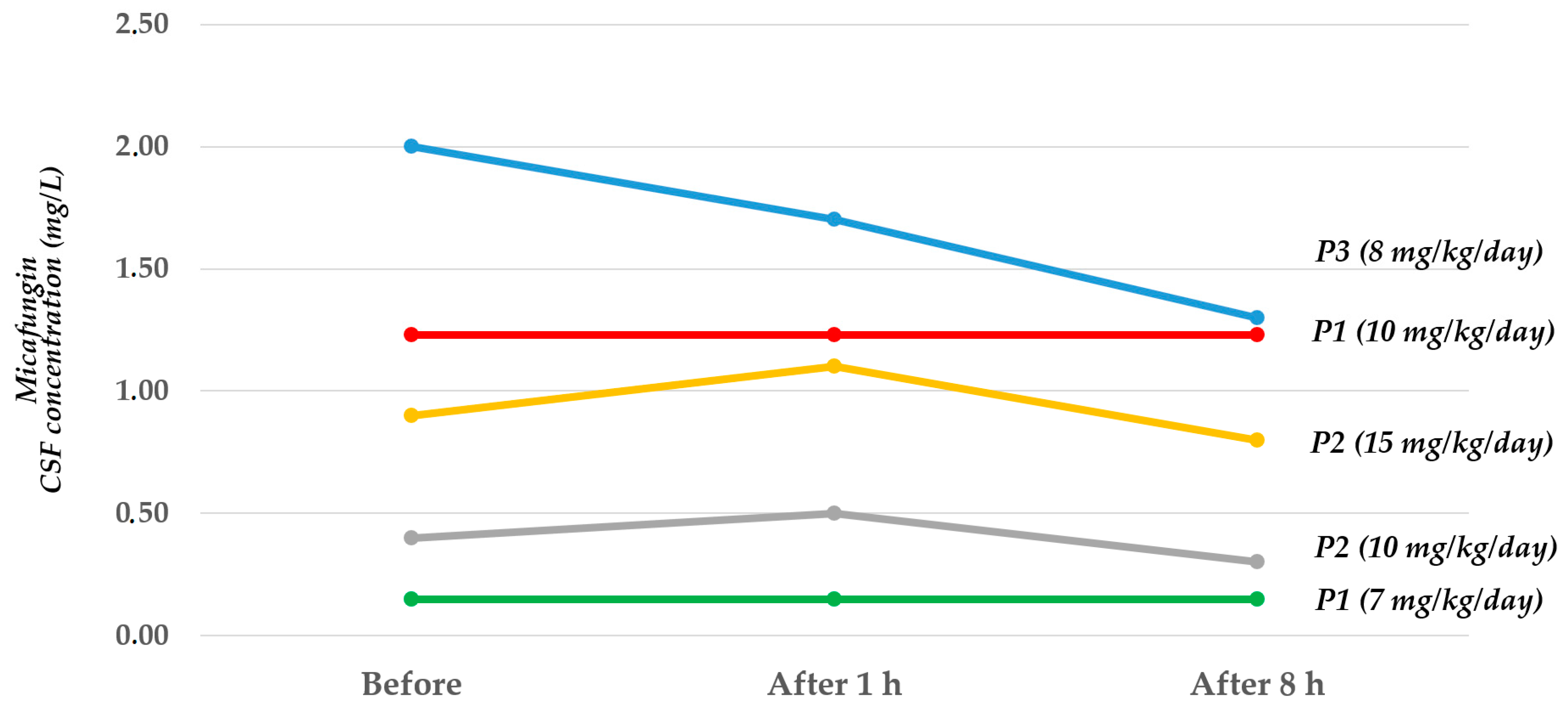
| CSF Values before Infusion (mg/L) | CSF Values after 1 h (mg/L) | CSF Values after 8 h (mg/L) | |
|---|---|---|---|
| Patient 1 (dose 7 mg/kg/day) | 0.15 | 0.15 | 0.15 |
| Patient 1 (dose 10 mg/kg/day) | 1.23 | 1.23 | 1.23 |
| Patient 2 (dose 10 mg/kg/day) | 0.40 | 0.50 | 0.30 |
| Patient 2 (dose 15 mg/kg/day) | 0.90 | 1.10 | 0.80 |
| Patient 3 (dose 8 mg/kg/day) | 2.00 | 1.70 | 1.30 |
| Plasma Values before Infusion (mg/L) | Plasma Values after 1 h (mg/L) | Plasma Values after 8 h (mg/L) | |
|---|---|---|---|
| Patient 1 (dose 7 mg/kg/day) | 2.90 | 15.60 | 8.90 |
| Patient 1 (dose 10 mg/kg/day) | 3.20 | 21.70 | 6.58 |
| Patient 2 (dose 10 mg/kg/day) | 6.70 | 17.40 | 8.90 |
| Patient 2 (dose 15 mg/kg/day) | 5.80 | 21.90 | 12.20 |
| Patient 3 (dose 8 mg/kg/day) | 9.12 | 27.00 | 16.84 |
| CSF/Plasma Ratio before Infusion | CSF/Plasma Ratio after 1 h | CSF/Plasma Ratio after 8 h | |
|---|---|---|---|
| Patient 1 (dose 7 mg/kg/day) | 0.05 | 0.01 | 0.02 |
| Patient 1 (dose 10 mg/kg/day) | 0.38 | 0.06 | 0.19 |
| Patient 2 (dose 10 mg/kg/day) | 0.06 | 0.03 | 0.03 |
| Patient 2 (dose 15 mg/kg/day) | 0.16 | 0.05 | 0.07 |
| Patient 3 (dose 8 mg/kg/day) | 0.22 | 0.06 | 0.08 |
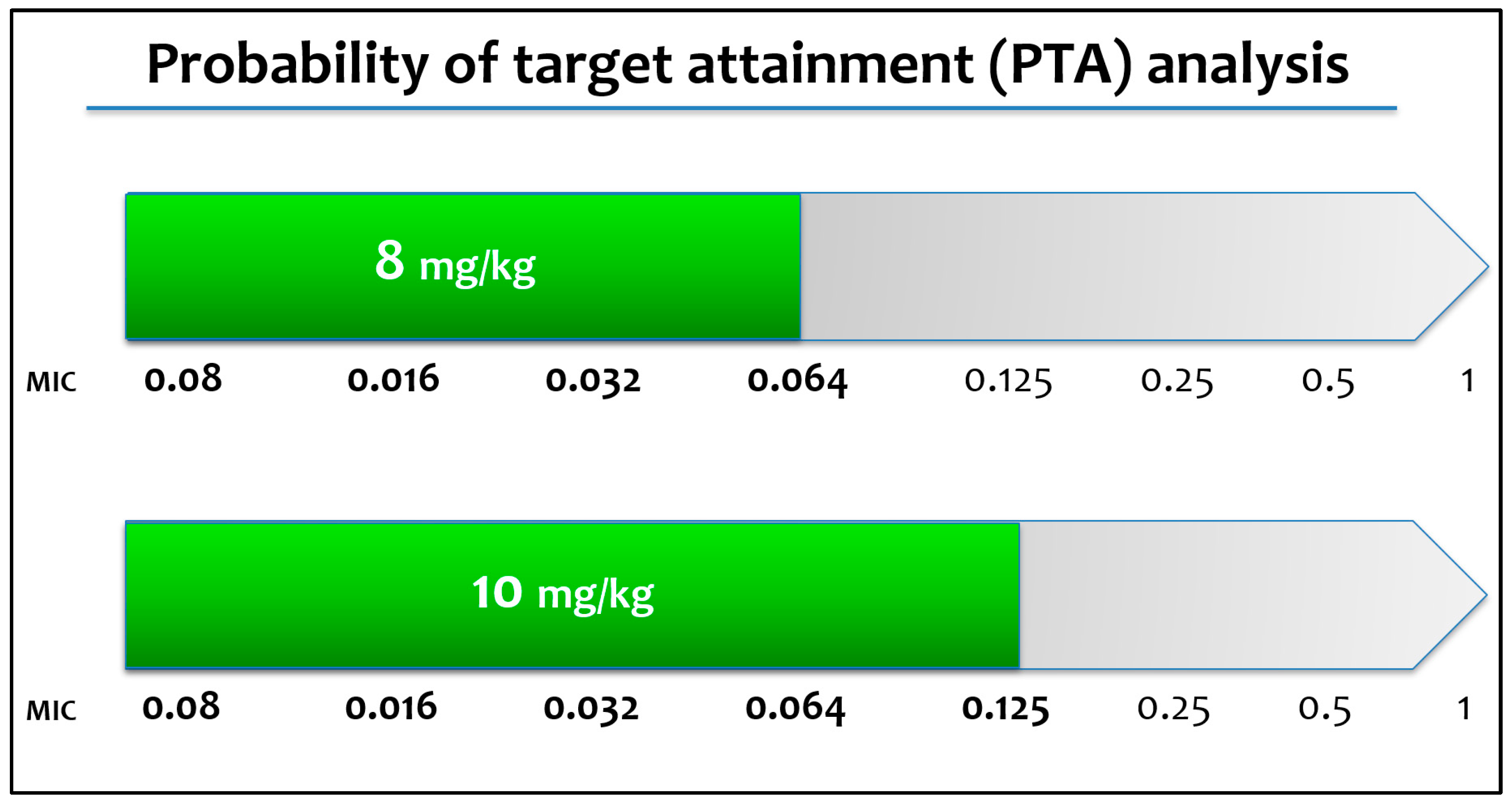
| Target | AUC/MIC ≥ 3000 | ||||
|---|---|---|---|---|---|
| Dose (mg/kg) | 2 | 4 | 8 | 10 | |
| MIC (mg/L) | 0.008 | 88.8% | 97.3% | 99.7% | 99.9% |
| 0.016 | 56.3% | 88.8% | 97.3% | 98.6% | |
| 0.032 | 11.2% | 56.3% | 88.8% | 92.9% | |
| 0.064 | 0.3 % | 11.2% | 56.3% | 70.7% | |
| 0.125 | 0.0% | 0.3% | 11.2% | 23.5% | |
| 0.25 | 0.0% | 0.0% | 0.3% | 1.3% | |
| 0.5 | 0.0% | 0.0% | 0.0% | 0.0% | |
| 1 | 0.0% | 0.0% | 0.0% | 0.0% | |
| Target | AUC/MIC ≥ 2000 | ||||
| Dose (mg/kg) | 2 | 4 | 8 | 10 | |
| MIC (mg/L) | 0.008 | 94.9% | 99.1% | 99.9% | 100.0% |
| 0.016 | 79.7% | 94.9% | 99.1% | 99.5% | |
| 0.032 | 34.2% | 79.7% | 94.9% | 96.9% | |
| 0.064 | 3.4% | 34.2% | 79.7% | 87.0% | |
| 0.125 | 0.0% | 3.8% | 35.9% | 53.3% | |
| 0.25 | 0.0% | 0.0% | 3.8% | 9.9% | |
| 0.5 | 0.0% | 0.0% | 0.0% | 0.2% | |
| 1 | 0.0% | 0.0% | 0.0% | 0.0% | |
| Target | AUC/MIC ≥ 1000 | ||||
| Dose (mg/kg) | 2 | 4 | 8 | 10 | |
| MIC (mg/L) | 0.008 | 99.1% | 99.9% | 100.0% | 100.0% |
| 0.016 | 94.9% | 99.1% | 99.9% | 100.0% | |
| 0.032 | 79.7% | 94.9% | 99.1% | 99.5% | |
| 0.064 | 34.2% | 79.7% | 94.9% | 96.9% | |
| 0.125 | 3.8% | 35.9% | 79.7% | 87.8% | |
| 0.25 | 3.8% | 3.8% | 35.9% | 53.3% | |
| 0.5 | 0.0% | 0.0% | 3.8% | 9.9% | |
| 1 | 0.0% | 0.0% | 0.0% | 0.0% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Rose, D.U.; Bersani, I.; Ronchetti, M.P.; Piersigilli, F.; Cairoli, S.; Dotta, A.; Desai, A.; Kovanda, L.L.; Goffredo, B.M.; Auriti, C. Plasma and Cerebrospinal Fluid Concentrations of Micafungin Administered at High Doses in Critically Ill Infants with Systemic Candidiasis: A Pooled Analysis of Two Studies. Pharmaceuticals 2023, 16, 472. https://doi.org/10.3390/ph16030472
De Rose DU, Bersani I, Ronchetti MP, Piersigilli F, Cairoli S, Dotta A, Desai A, Kovanda LL, Goffredo BM, Auriti C. Plasma and Cerebrospinal Fluid Concentrations of Micafungin Administered at High Doses in Critically Ill Infants with Systemic Candidiasis: A Pooled Analysis of Two Studies. Pharmaceuticals. 2023; 16(3):472. https://doi.org/10.3390/ph16030472
Chicago/Turabian StyleDe Rose, Domenico Umberto, Iliana Bersani, Maria Paola Ronchetti, Fiammetta Piersigilli, Sara Cairoli, Andrea Dotta, Amit Desai, Laura Lynn Kovanda, Bianca Maria Goffredo, and Cinzia Auriti. 2023. "Plasma and Cerebrospinal Fluid Concentrations of Micafungin Administered at High Doses in Critically Ill Infants with Systemic Candidiasis: A Pooled Analysis of Two Studies" Pharmaceuticals 16, no. 3: 472. https://doi.org/10.3390/ph16030472
APA StyleDe Rose, D. U., Bersani, I., Ronchetti, M. P., Piersigilli, F., Cairoli, S., Dotta, A., Desai, A., Kovanda, L. L., Goffredo, B. M., & Auriti, C. (2023). Plasma and Cerebrospinal Fluid Concentrations of Micafungin Administered at High Doses in Critically Ill Infants with Systemic Candidiasis: A Pooled Analysis of Two Studies. Pharmaceuticals, 16(3), 472. https://doi.org/10.3390/ph16030472






