One-Pot Synthesis of 1-Thia-4-azaspiro[4.4/5]alkan-3-ones via Schiff Base: Design, Synthesis, and Apoptotic Antiproliferative Properties of Dual EGFR/BRAFV600E Inhibitors
Abstract
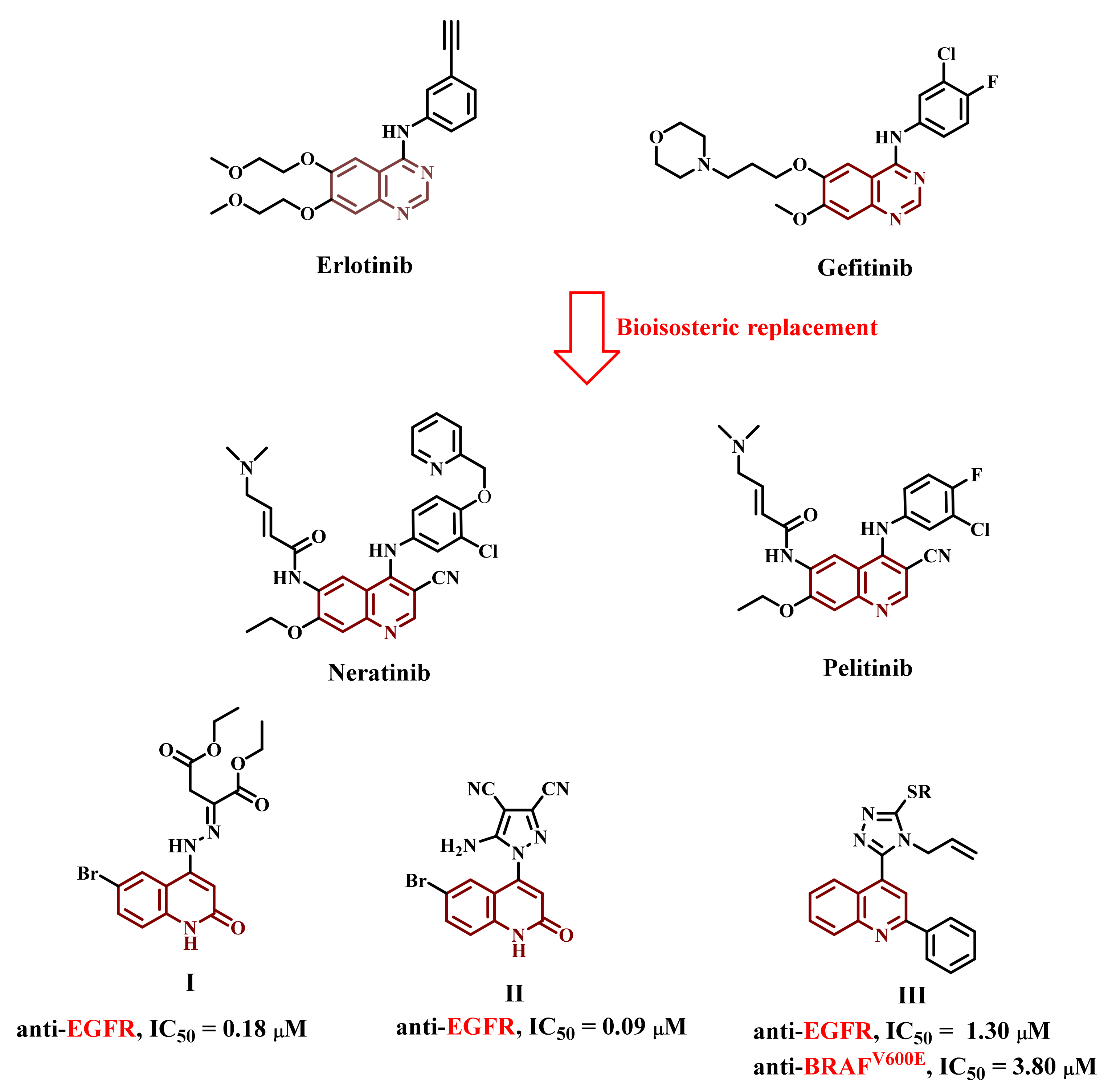
:1. Introduction
2. Results and Discussion
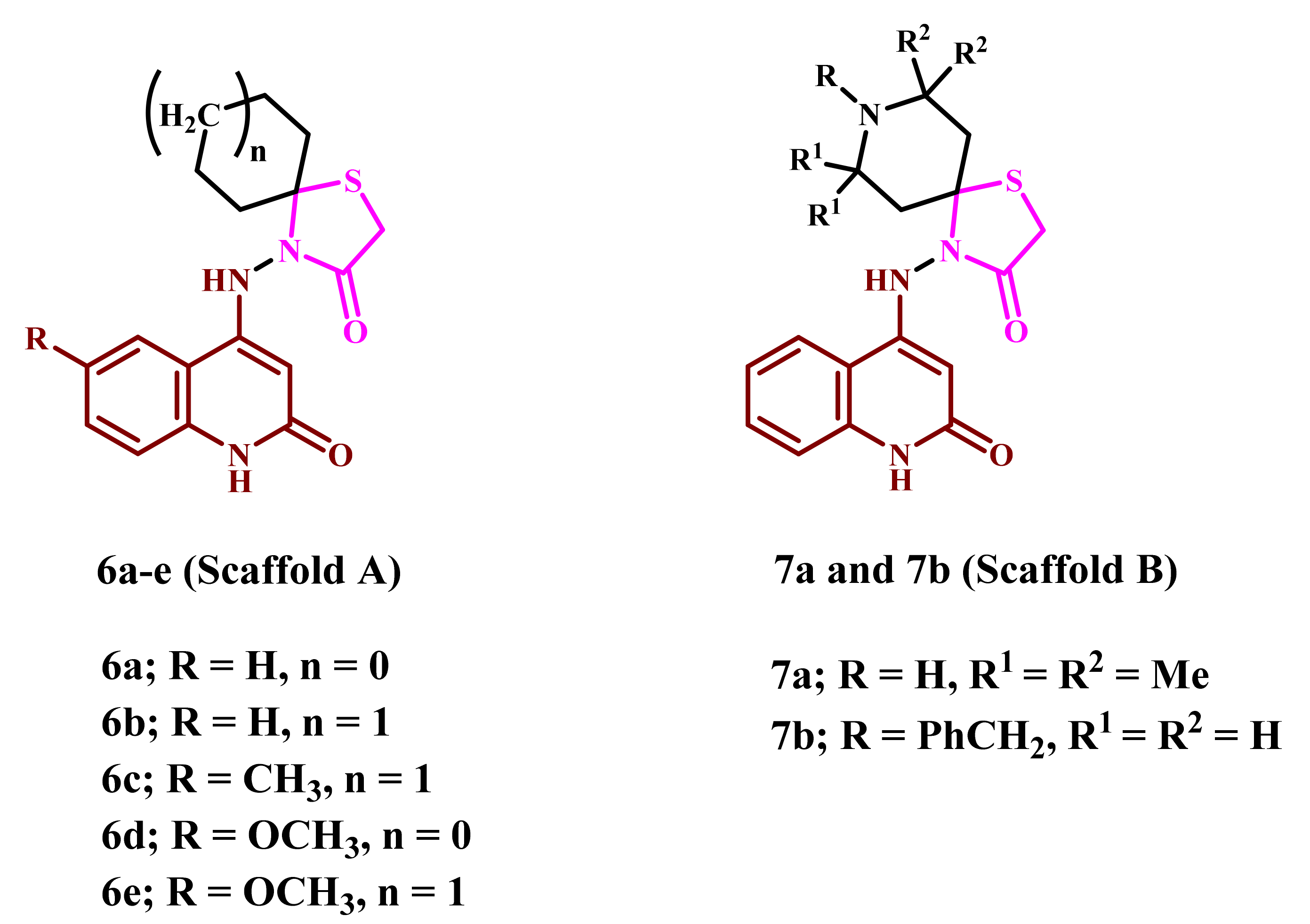
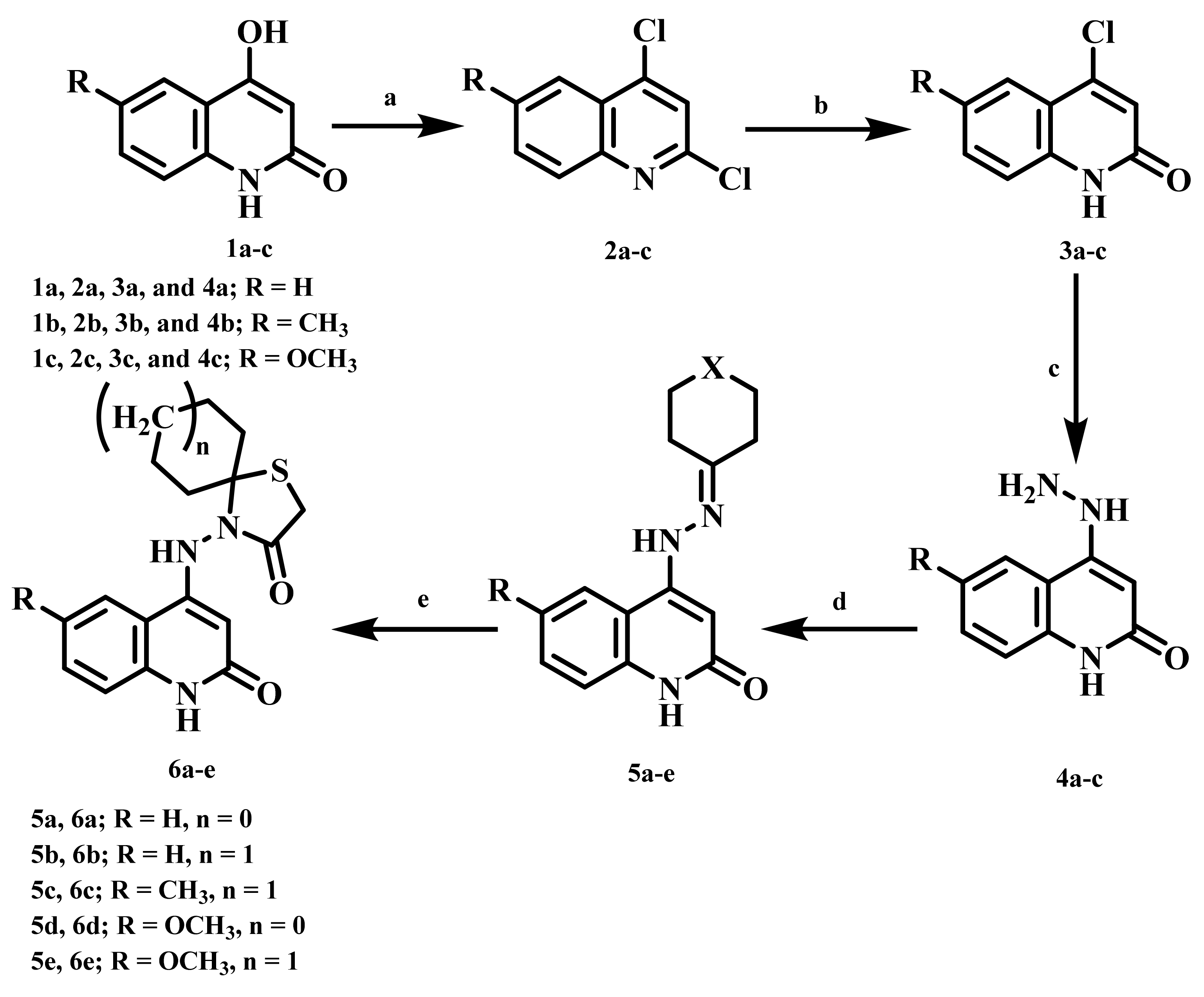
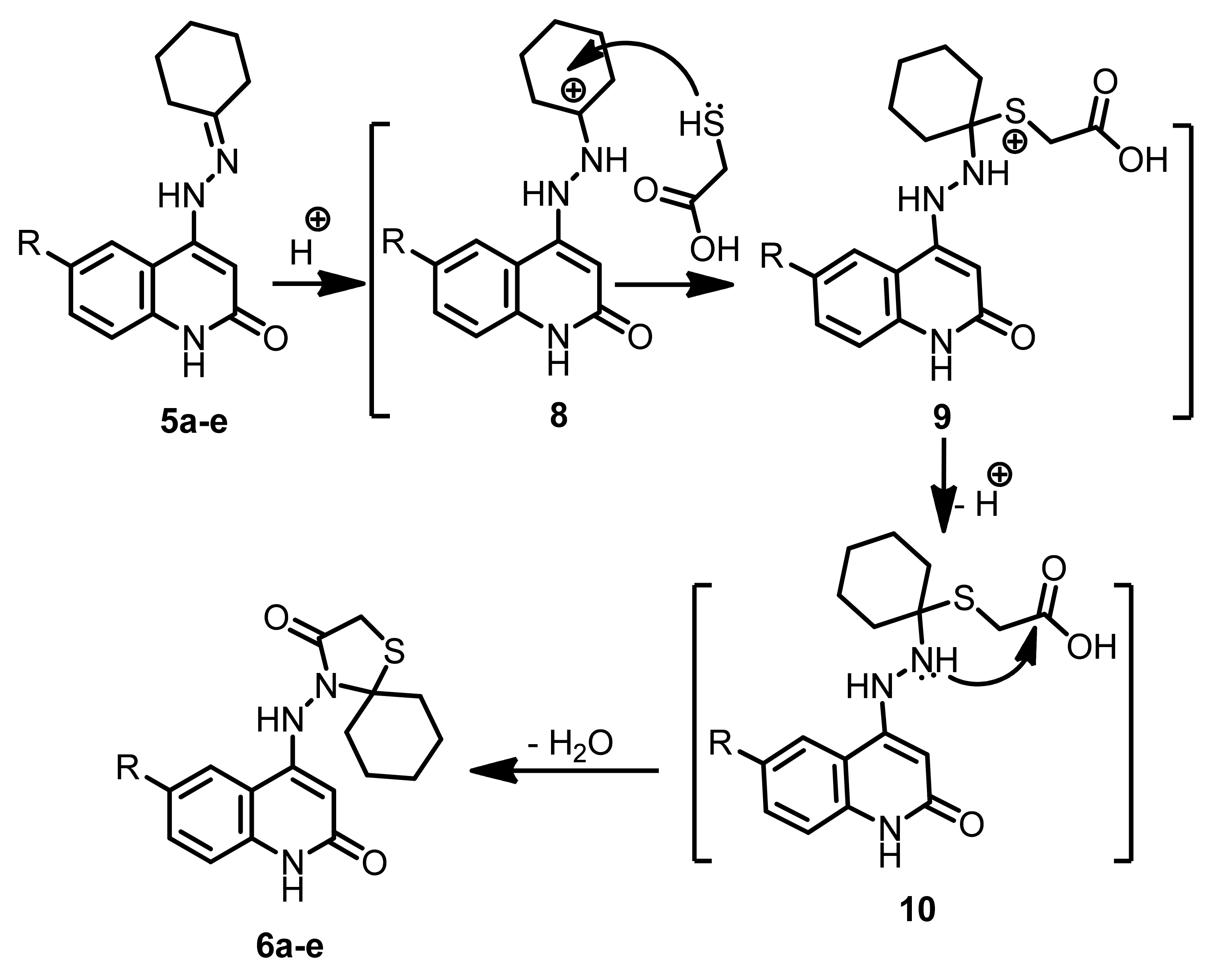
2.1. Chemistry
2.2. Biology
2.2.1. Cell Viability Assay
2.2.2. Antiproliferative Assay
2.2.3. EGFR Inhibitory Assay
2.2.4. BRAFV600E Inhibitory Assay
2.2.5. Apoptotic Markers Activation Assay
Caspase 3 Activation Assay
Caspase-8, Bax and Bcl-2 Levels Assay
3. Conclusions
4. Experimental
4.1. Chemistry
4.1.1. 4-((2-Oxo-1,2-dihydroquinolin-4-yl)amino)-1-thia-4-azaspiro[4.4]nonan-3-one (6a)
4.1.2. 4-((2-Oxo-1,2-dihydroquinolin-4-yl)amino)-1-thia-4-azaspiro[4.5]decan-3-one (6b)
4.1.3. 4-((6-Methyl-2-oxo-1,2-dihydroquinolin-4-yl)amino)-1-thia-4-azaspiro[4.5]decan-3-one (6c)
4.1.4. 4-((6-Methoxy-2-oxo-1,2-dihydroquinolin-4-yl)amino)-1-thia-4-azaspiro[4.4]nonan-3-one (6d)
4.1.5. 4-((6-Methoxy-2-oxo-1,2-dihydroquinolin-4-yl)amino)-1-thia-4-azaspiro[4.5]decan-3-one (6e)
4.1.6. 7,7,9,9-Tetramethyl-4-((2-oxo-1,2-dihydroquinolin-4-yl)amino)-1-thia-4,8-diazaspiro-[4.5]decan-3-one (7a)
4.1.7. 8-Benzyl-4-((2-oxo-1,2-dihydroquinolin-4-yl)amino)-1-thia-4,8-diazaspiro[4.5]decan-3-one (7b)
4.2. Biology
4.2.1. Cell Viability Assay
4.2.2. Antiproliferative Assay
4.2.3. EGFR Inhibitory Assay
4.2.4. BRAFV600E Inhibitory Assay
4.2.5. Apoptotic Markers Assay
Caspase 3 Activation Assay
Caspase-8, Bax and Bcl-2 Levels Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stanković, T.; Dinić, J.; Podolski-Renić, A.; Musso, L.; Burić, S.S.; Dallavalle, S.; Pešić, M. Dual Inhibitors as a New Challenge for Cancer Multidrug Resistance Treatment. Curr. Med. Chem. 2019, 26, 6074–6106. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, N.M.; Pingili, D.; Kadasi, S.; Mettu, A.; Prasad, S.V.U.M. Dual or multi-targeting inhibitors: The next generation anti-cancer agents. Eur. J. Med. Chem. 2018, 143, 1277–1300. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.; Andersson, D.I. Evolutionary consequences of drug resistance: Shared principles across diverse targets and organisms. Nat. Rev. Genet. 2015, 16, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Staunton, J.E.; Jin, X.; Lee, M.S.; Zimmermann, G.R.; Borisy, A.A. Synergistic drug combinations tend to improve therapeutically relevant selectivity. Nat. Biotechnol. 2009, 27, 659–666. [Google Scholar] [CrossRef]
- Shah, K.N.; Bhatt, R.; Rotow, J.; Rohrberg, J.; Olivas, V.; Wang, V.E.; Hemmati, G.; Martins, M.M.; Maynard, A.; Kuhn, J.; et al. Aurora kinase A drives the evolution of resistance to third-generation EGFR inhibitors in lung cancer. Nat. Med. 2019, 25, 111–118. [Google Scholar] [CrossRef]
- Notarangelo, T.; Sisinni, L.; Condelli, V.; Landriscina, M. Dual EGFR and BRAF blockade overcomes resistance to vemurafenib in BRAF mutated thyroid carcinoma cells. Cancer Cell Int. 2017, 17, 86–94. [Google Scholar] [CrossRef] [Green Version]
- Mondaca, S.; Lacouture, M.; Hersch, J.; Yaeger, R. Balancing RAF, MEK, and EGFR inhibitor doses to achieve clinical responses and modulate toxicity in BRAF V600E colorectal cancer. JCO Precis. Oncol. 2018, 10, 1–5. [Google Scholar] [CrossRef]
- Zhang, Q.; Diao, Y.; Wang, F.; Fu, Y.; Tang, F.; You, Q.; Zhou, H. Design and discovery of 4-anilinoquinazoline ureas as multikinase inhibitors targeting BRAF, VEGFR-2 and EGFR. MedChemComm 2013, 4, 979–986. [Google Scholar] [CrossRef]
- Okaniwa, M.; Hirose, M.; Imada, T.; Ohashi, T.; Hayashi, Y.; Miyazaki, T.; Arita, T.; Yabuki, M.; Kakoi, K.; Kato, J.; et al. Design and synthesis of novel DFG-out RAF/vascular endothelial growth factor receptor 2 (VEGFR2) inhibitors. 1. Exploration of [5,6]-fused bicyclic scaffolds. J. Med. Chem. 2012, 55, 3452–3478. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Properties of FDA-approved small molecule protein kinase inhibitors. Pharmacol. Res. 2019, 144, 19–50. [Google Scholar] [CrossRef]
- Prajapati, S.M.; Patel, K.D.; Vekariya, R.H.; Panchal, S.N.; Patel, H.D. Recent advances in the synthesis of quinolines: A review. RSC Adv. 2014, 4, 24463–24476. [Google Scholar] [CrossRef]
- Navneetha, O.; Deepthi, K.; Rao, A.M.; Jyostna, T.S. A review on chemotherapeutic activities of quinolone. Int. J. Pharm. Chem. Biol. Sci. 2017, 7, 364–372. [Google Scholar]
- Barlési, F.; Tchouhadjian, C.; Doddoli, C.; Villani, P.; Greillier, L.; Kleisbauer, J.P.; Thomas, P.; Astoul, P. Gefitinib (ZD1839, Iressa®) in non-small-cell lung cancer: A review of clinical trials from a daily practice perspective. Fund. Clin. Pharmacol. 2005, 19, 385–393. [Google Scholar] [CrossRef]
- Iyer, R.; Bharthuar, A. A review of erlotinib–an oral, selective epidermal growth factor receptor tyrosine kinase inhibitor. Exp. Opin. Pharmacother. 2010, 11, 311–320. [Google Scholar] [CrossRef]
- Bao, B.; Mitrea, C.; Wijesinghe, P.; Marchetti, L.; Girsch, E.; Farr, R.L.; Boerner, J.L.; Mohammad, R.; Dyson, G.; Terlecky, S.R.; et al. Treating triple negative breast cancer cells with erlotinib plus a select antioxidant overcomes drug resistance by targeting cancer cell heterogeneity. Sci. Rep. 2017, 7, 44125. [Google Scholar] [CrossRef] [Green Version]
- Stamos, J.; Sliwkowski, M.X.; Eigenbrot, C. Structure of the epidermal growth factor receptor kinase domain alone and in complex with a 4-anilinoquinazoline inhibitor. J. Biol. Chem. 2002, 277, 46265–46272. [Google Scholar] [CrossRef] [Green Version]
- Wissner, A.; Berger, D.M.; Boschelli, D.H.; Floyd, M.B.; Greenberger, L.M.; Gruber, B.C.; Johnson, B.D.; Mamuya, N.; Nilakantan, R.; Reich, M.F.; et al. 4-Anilino-6,7-dialkoxyquinoline-3-carbonitrile inhibitors of epidermal growth factor receptor kinase and their bioisosteric relationship to the 4-anilino-6,7-dialkoxyquinazoline inhibitors. J. Med. Chem. 2000, 43, 3244–3256. [Google Scholar] [CrossRef]
- Wissner, A.; Mansour, T.S. The development of HKI-272 and related compounds for the treatment of cancer. Arch. Pharm. 2008, 341, 465–477. [Google Scholar] [CrossRef]
- Kiesel, B.F.; Parise, R.A.; Wong, A.; Keyvanjah, K.; Jacobs, S.; Beumer, J.H. LC–MS/MS assay for the quantitation of the tyrosine kinase inhibitor neratinib in human plasma. J. Pharm. Biomed. Anal. 2017, 134, 130–136. [Google Scholar] [CrossRef] [Green Version]
- Pisaneschi, F.; Nguyen, Q.-D.; Shamsaei, E.; Glaser, M.; Robins, E.; Kaliszczak, M.; Smith, G.; Spivey, A.C.; Aboagye, E.O. Development of a new epidermal growth factor receptor positron emission tomography imaging agent based on the 3-cyanoquinoline core: Synthesis and biological evaluation. Bioorg. Med. Chem. 2010, 18, 6634–6645. [Google Scholar] [CrossRef]
- Lü, S.; Zheng, W.; Ji, L.; Luo, Q.; Hao, X.; Li, X.; Wang, F. Synthesis, characterization, screening and docking analysis of 4-anilinoquinazoline derivatives as tyrosine kinase inhibitors. Eur. J. Med. Chem. 2013, 61, 84–94. [Google Scholar] [CrossRef]
- Luethi, D.; Durmus, S.; Schinkel, A.H.; Schellens, J.H.M.; Beijnen, J.H.; Sparidans, R.W. Liquid chromatography–tandem mass spectrometry assay for the EGFR inhibitor pelitinib in plasma. J. Chromatogr. B 2013, 934, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Pawar, V.G.; Sos, M.L.; Rode, H.B.; Rabiller, M.; Heynck, S.; van Otterlo, W.A.L.; Thomas, R.K.; Rauh, D. Synthesis and biological evaluation of 4-anilinoquinolines as potent inhibitors of epidermal growth factor receptor. J. Med. Chem. 2010, 53, 2892–2901. [Google Scholar] [CrossRef] [PubMed]
- Elbastawesy, M.A.I.; Aly, A.A.; Ramadan, M.; Elshaier, Y.A.M.M.; Youssif, B.G.M.; Brown, A.B.; Abuo-Rahma, G.E.A. Novel Pyrazoloquinolin-2-ones: Design, Synthesis, Docking Studies, and Biological Evaluation as Antiproliferative EGFR- TK Inhibitors. Bioorg. Chem. 2019, 90, 103045–103060. [Google Scholar] [CrossRef] [PubMed]
- Mohassab, A.M.; Hassan, H.A.; Abdelhamid, D.; Gouda, A.M.; Youssif, B.G.M.; Tateishi, H.; Fujita, M.; Otsuka, M.; Abdel-Aziz, M. Design and Synthesis of Novel quinoline/chalcone/1,2,4-triazole hybrids as potent antiproliferative agent targeting EGFR and BRAFV600E kinases. Bioorg. Chem. 2021, 106, 104510. [Google Scholar] [CrossRef]
- Zheng, Y.; Tice, C.M.; Singh, S.B. The use of spirocyclic scaffolds in drug discovery. Bioorg. Med. Chem. Lett. 2014, 24, 3673–3682. [Google Scholar] [CrossRef] [Green Version]
- Batista, V.F.; Pinto, D.C.G.A.; Silva, A.M.S. Recent in vivo advances of spirocyclic scaffolds for drug discovery. Expert Opin. Drug Discov. 2022, 17, 603–618. [Google Scholar] [CrossRef]
- Hiesinger, K.; Dar’in, D.; Proschak, E.; Krasavin, M. Spirocyclic Scaffolds in Medicinal Chemistry. J. Med. Chem. 2021, 64, 150–183. [Google Scholar] [CrossRef]
- Ayati, A.; Emami, S.; Asadipour, A.; Shafiee, A.; Foroumadi, A. Recent applications of 1,3-thiazole core structure in the identification of new lead compounds and drug discovery. Eur. J. Med. Chem. 2015, 97, 699–718. [Google Scholar] [CrossRef]
- Sharma, P.C.; Jain, A.; Yar, M.S.; Pahwa, R.; Singh, J.; Chanalia, P. Novel fluoroquinolone derivatives bearing N-thiomide linkage with 6-substituted-2-aminobenzothiazoles: Synthesis and antibacterial evaluation. Arab. J. Chem. 2017, 10, S568–S575. [Google Scholar] [CrossRef] [Green Version]
- Petrou, A.; Fesatidou, M.; Geronikaki, A. Thiazole Ring—A Biologically Active Scaffold. Molecules 2021, 26, 3166. [Google Scholar] [CrossRef]
- Othman, I.M.M.; Alamshany, Z.M.; Tashkandi, N.Y.; Gad-Elkareem, M.A.M.; Abd El-Karim, S.S.; Nossier, E.S. Synthesis and biological evaluation of new derivatives of thieno-thiazole and dihydrothiazolo-thiazole scaffolds integrated with a pyrazoline nucleus as anticancer and multi-targeting kinase inhibitor. RSC Adv. 2022, 12, 561–577. [Google Scholar] [CrossRef]
- Nafie, M.S.; Kishk, S.M.; Mahgoub, S.; Amer, A.M. Quinoline-based thiazolidinone derivatives as potent cytotoxic and apoptosis-inducing agents through EGFR inhibition. Chem. Biol. Drug Des. 2022, 99, 547–560. [Google Scholar] [CrossRef]
- Xiong, L.; He, H.; Fan, M.; Hu, L.; Wang, F.; Song, X.; Shi, S.; Qi, B. Discovery of novel conjugates of quinoline and thiazolidinone urea as potential anti-colorectal cancer agent. J. Enzym. Inhib. Med. Chem. 2022, 37, 2334–2347. [Google Scholar] [CrossRef]
- Yadav, J.; Chaudhary, R.P. A review on advances in synthetic methodology and biological profile of spirothiazolidin-4-ones. J. Heterocycl. Chem. 2022, 59, 1839–1878. [Google Scholar] [CrossRef]
- Lozynskyi, A.; Zimenkovsky, B.; Lesyk, R. Synthesis and Anticancer Activity of New Thiopyrano[2,3-d]thiazoles Based on Cinnamic Acid Amides. Sci. Pharm. 2014, 82, 723–733. [Google Scholar] [CrossRef] [Green Version]
- Hu-Lieskovan, S.; Mok, S.; Homet Moreno, B.; Tsoi, J.; Robert, L.; Goedert, L.; Pinheiro, E.; Koya, R.; Graeber, T.; Comin-Anduix, B.; et al. Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAFV600E melanoma. Sci. Transl. Med. 2015, 7, 279ra41. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Maksoud, M.S.; Kim, M.-R.; El-Gamal, M.I.; Gamal El-Din, M.M.; Tae, J.; Choi, H.S.; Lee, K.-T.; Yoo, K.H.; Oh, C.-H. Design, synthesis, in vitro antiproliferative evaluation, and kinase inhibitory effects of a new series of imidazo[2,1-b]thiazole derivatives. Eur. J. Med. Chem. 2015, 95, 453–463. [Google Scholar] [CrossRef]
- Aly, A.A.; Alshammari, M.B.; Ahmad, A.; Gomaa, H.A.M.; Youssif, B.G.M.; Bräse, S.; Ibrahim, M.A.A.; Mohamed, A.H. Design, synthesis, docking, and mechanistic studies of new thiazolyl/thiazolidinylpyrimidine-2,4-dione antiproliferative agents. Arab. J. Chem. 2023, 16, 104612. [Google Scholar] [CrossRef]
- Al-Wahaibi, L.H.; Gouda, A.M.; Abou-Ghadir, O.F.; Salem, O.I.A.; Ali, A.T.; Farghaly, H.S.; Abdelrahman, M.H.; Trembleau, L.; Abdu-Allah, H.H.M.; Youssif, B.G.M. Design and synthesis of novel 2,3-dihydropyrazino[1,2-a]indole-1,4-dione derivatives as antiproliferative EGFR and BRAFV600E dual inhibitors. Bioorg. Chem. 2020, 104, 104260. [Google Scholar] [CrossRef]
- Gomaa, H.A.M.; Shaker, M.E.; Alzarea, S.I.; Hendawy, O.M.; Mohamed, F.A.M.; Gouda, A.M.; Ali, A.T.; Morcoss, M.M.; Abdelrahman, M.H.; Trembleau, L.; et al. Optimization and SAR investigation of novel 2,3-dihydropyrazino[1,2-a]indole-1,4-dione derivatives as EGFR and BRAFV600E dual inhibitors with potent antiproliferative and antioxidant activities. Bioorg. Chem. 2022, 120, 105616. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, M.B.; Aly, A.A.; Youssif, B.G.M.; Bräse, S.; Ahmad, A.; Brown, A.B.; Ibrahim, M.A.A.; Mohamed, A.H. Design and synthesis of new thiazolidinone/uracil derivatives as antiproliferative agents targeting EGFR and/or BRAFV600E. Front. Chem. 2022, 10, 1076383. [Google Scholar] [CrossRef] [PubMed]
- Buckle, D.R.; Cantello, B.C.C.; Smith, H.; Spicer, B.A. 4-Hydroxy-3-nitro-2-quinolones and related compounds as inhibitors of allergic reactions. J. Med. Chem. 1975, 18, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Bhudevi, B.; Ramana, P.V.; Mudiraj, A.; Reddy, A.R. Synthesis of 4-hydroxy-3-formylideneamino-lH/methyl/phenylquinolin2-ones. Indian J. Chem. B 2009, 48, 255–260. [Google Scholar]
- Yang, Z.; Sun, P. Compare of three ways of synthesis of simple Schiff bas. Molbank 2006, 2006, M514. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, M.A.; Mohammed, A.F.; Salem, O.I.A.; Gomaa, H.A.M.; Youssif, B.G.M. New 1,3,4-oxadiazoles linked 1,2,3-triazole moiety as antiproliferative agents targeting EGFR-TK. Arch. Der Pharm. 2022, 355, e2200009. [Google Scholar] [CrossRef]
- Ramadan, M.; Abd El-Aziz, M.; Elshaier, Y.A.M.M.; Youssif, B.G.M.; Brown, A.B.; Fathy, H.M.; Aly, A.A. Design and synthesis of new pyranoquinolinone heteroannulated to triazolopyrimidine of potential apoptotic antiproliferative activity. Bioorg. Chem. 2020, 105, 104392. [Google Scholar] [CrossRef]
- Al-Sanea, M.M.; Gotina, L.; Mohamed, M.F.A.; Parambi, D.G.T.; Gomaa, H.A.M.; Mathew, B.; Youssif, B.G.M.; Alharbi, K.S.; Elsayed, Z.M.; Abdelgawad, M.A.; et al. Design, Synthesis and Biological Evaluation of New HDAC1 and HDAC2 Inhibitors Endowed with Ligustrazine as a Novel Cap Moiety. Drug Des. Dev. Ther. 2020, 14, 497–508. [Google Scholar] [CrossRef] [Green Version]
- Hisham, M.; Hassan, H.A.; Gomaa, H.A.M.; Youssif, B.G.M.; Hayallah, A.M.; Abdel-Aziz, M. Structure-based design, synthesis and antiproliferative action of new quinazoline-4-one/chalcone hybrids as EGFR inhibitors. J. Mol. Struct. 2022, 1254, 132422. [Google Scholar] [CrossRef]
- Mohamed, F.A.M.; Gomaa, H.A.M.; Hendawy, O.M.; Ali, A.T.; Farghaly, H.S.; Gouda, A.M.; Abdelazeem, A.H.; Abdelrahman, M.H.; Trembleau, L.; Youssif, B.G.M. Design, synthesis, and biological evaluation of novel EGFR inhibitors containing 5-chloro-3-hydroxymethyl-indole-2-carboxamide scaffold with apoptotic antiproliferative activity. Bioorg. Chem. 2021, 112, 104960. [Google Scholar] [CrossRef]
- Abou-Zied, H.A.; Beshr, E.A.M.; Gomaa, H.A.M.; Mostafa, Y.A.; Youssif, B.G.M.; Hayallah, A.M.; Abdel-Aziz, M. Discovery of new cyanopyridine/chalcone hybrids as dual inhibitors of EGFR/BRAFV600E with promising antiproliferative properties. Arch. Der Pharm. 2022, 355, e2200464. [Google Scholar] [CrossRef]
- Hisham, M.; Youssif, B.G.M.; Osman, E.E.A.; Hayallah, A.M.; Abdel-Aziz, M. Synthesis and biological evaluation of novel xanthine derivatives as potential apoptotic antitumor agents. Eur. J. Med. Chem. 2019, 176, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.J. Caspases: Executioners of apoptosis. Pathobiol. Hum. Dis. 2014, 145, 145–152. [Google Scholar]
- Abdelbaset, M.S.; Abdel-Aziz, M.; Abuo-Rahma, G.E.A.; Abdelrahman, M.H.; Ramadan, M.; Youssif, B.G.M. Novel quinoline derivatives carrying nitrones/oximes nitric oxide donors: Design, synthesis, antiproliferative and caspase-3 activation activities. Arch. Der Pharm. 2018, 352, 1800270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abou-Zied, H.A.; Youssif, B.G.M.; Mohamed, M.F.A.; Hayallah, A.M.; Abdel-Aziz, M. EGFR inhibitors and apoptotic inducers: Design, synthesis, anticancer activity and docking studies of novel xanthine derivatives carrying chalcone moiety as hybrid molecules. Bioorg. Chem. 2019, 89, 102997. [Google Scholar] [CrossRef]

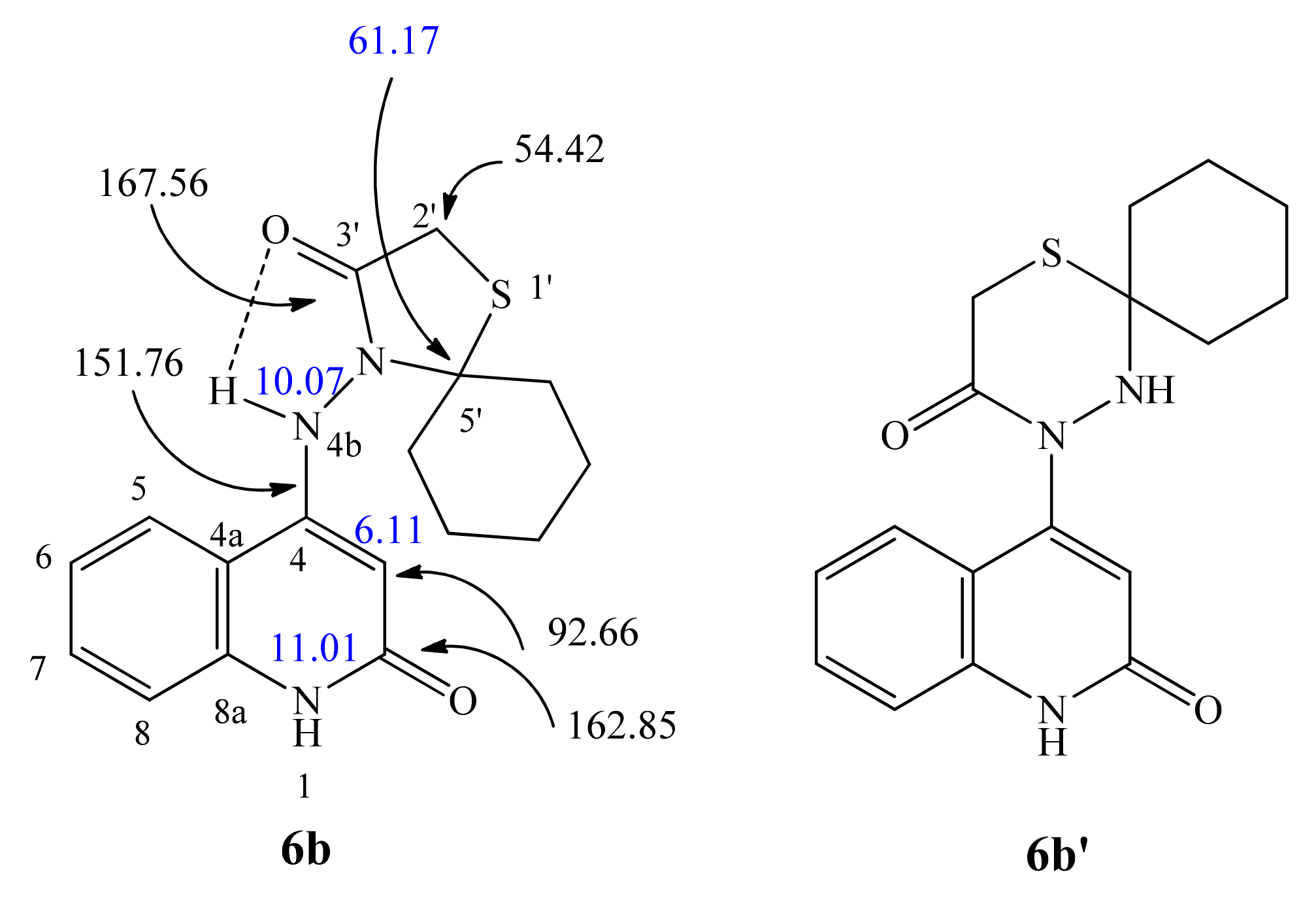
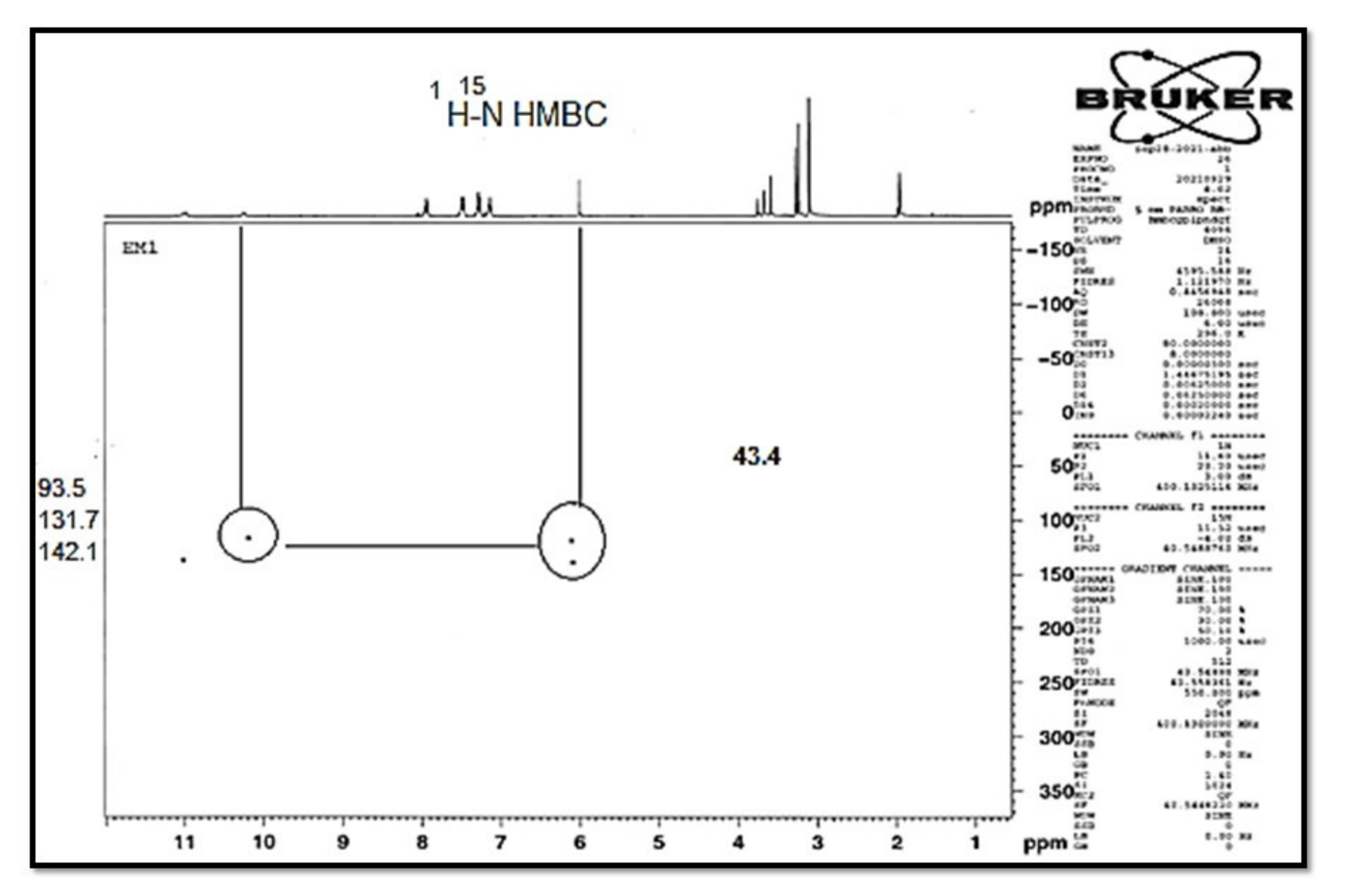


| 1H NMR | 1H-1H COSY | Assignment | |
|---|---|---|---|
| 11.01 (bs; 1H) | 6.08 | NH-1 | |
| 10.09 (s; 1H) | NH-4b | ||
| 7.98 (d, J = 8.1; 1H) | 7.46, 7.12 | H-5 | |
| 7.46 (dd, J = 7.7, 7.5; 1H) | 7.98, 7.28, 7.12 | H-7 | |
| 7.35 (m; 4H) | 7.28 | H-o, m | |
| 7.28 (m; 3H) | 7.46, 7.35, 7.12 | H-8, p | |
| 7.12 (dd, J = 7.7, 7.4; 1H) | 7.98, 7.46, 7.28 | H-6 | |
| 6.08 (s; 1H) | 11.01 | H-3 | |
| 3.71 (s; 2H) | H-2′ | ||
| 3.56 (s; 2H) | H-8″ | ||
| 2.67 (t, J = 5.6; 2H) | 2.55, 2.44 | H-6′/6″ | |
| 2.55 (m; 4H) | 2.67, 2.44 | H-7′,7″ | |
| 2.44 (t, J = 5.4; 2H) | 2.67, 2.55 | H-6″/6′ | |
| 13C NMR | HSQC | HMBC | Assignment |
| 167.33 | 10.09, 3.71 | C-3′ | |
| 162.90 | 10.09, 6.08 | C-2 | |
| 149.51 | 10.09, 7.98, 7.28, 6.08 | C-4 | |
| 139.23 | 7.98, 7.46 | C-8a | |
| 138.31 | 7.35, 7.28, 3.56 | C-i | |
| 130.13 | 7.46 | 7.98, 7.46, 7.28 | C-7 |
| 128.73 | 7.35 | 7.35, 7.35, 3.56 | C-o |
| 128.16 | 7.35 | 7.35, 7.35 | C-m |
| 126.93 | 7.28 | 7.28 | C-p |
| 122.44 | 7.98 | 7.98, 7.46, 6.08 | C-5 |
| 120.31 | 7.12 | 7.28, 7.12 | C-6 |
| 115.45 | 7.28 | 7.46, 7.12 | C-8 |
| 112.26 | 10.09, 7.98, 7.28, 7.12, 6.08 | C-4a | |
| 92.33 | 6.08 | 10.09, 6.08 | C-3 |
| 61.36 | 3.56, 2.55 | C-8″ | |
| 54.11 | 3.71 | 2.67, 244 | C-2′ |
| 53.23, 52.07 | 2.55 | 3.56, 2.67, 2.55, 2.55, 2.44 | C-7′,7″ |
| 47.33 | 3.56 | 2.67, 2.44 | C-5′ |
| 34.46 | 2.44 | 2.67, 2.55 | C-6′/6″ |
| 27.26 | 2.67 | 2.67, 2.55, 2.55 | C-6″/6′ |
| 15N NMR: | HSQC | HMBC | Assignment |
| 141.9 | 11.00 | 7.28, 6.08 | N-1 |
| 132.0 | 10.09 | 10.09, 6.08 | N-4b |
| 49.7 | 2.67, 2.44 | N-8′ | |
| Compd. | Cell Viability % | Antiproliferative Activity IC50 ± SEM (µM) | ||||
|---|---|---|---|---|---|---|
| A-549 | MCF-7 | Panc-1 | HT-29 | Average (GI50) | ||
| 6a | 90 | 40 ± 4 | 44 ± 4 | 46 ± 4 | 48 ± 4 | 45 |
| 6b | 89 | 33 ± 3 | 35 ± 3 | 36 ± 3 | 36 ± 3 | 35 |
| 6c | 91 | 72 ± 7 | 74 ± 7 | 73 ± 7 | 72 ± 7 | 73 |
| 6d | 91 | 48 ± 4 | 52 ± 5 | 54 ± 5 | 54 ± 5 | 52 |
| 6e | 92 | 36 ± 3 | 40 ± 3 | 42 ± 4 | 42 ± 4 | 40 |
| 7a | 89 | 80 ± 8 | 82 ± 8 | 81 ± 8 | 81 ± 8 | 81 |
| 7b | 91 | 30 ± 3 | 34 ± 3 | 32 ± 3 | 32 ± 3 | 32 |
| Erlotinib | ND | 30 ± 3 | 40 ± 3 | 30 ± 3 | 30 ± 3 | 33 |
| Compd. | EGFR Inhibition IC50 ± SEM (nM) | BRAFV600E Inhibition IC50 ± SEM (nM) |
|---|---|---|
| 6a | 97 ± 07 | 137 ± 12 |
| 6b | 84 ± 06 | 108 ± 09 |
| 6c | 135 ± 11 | 164 ± 15 |
| 6d | 104 ± 08 | 159 ± 14 |
| 6e | 92 ± 07 | 129 ± 10 |
| 7a | 149 ± 12 | 187 ± 16 |
| 7b | 78 ± 05 | 96 ± 8 |
| Erlotinib | 80 ± 05 | 60 ± 05 |
| Compound Number | Caspase-3 | |
|---|---|---|
| Conc (Pg/mL) | Fold Change | |
| 6b | 487.50 ± 4 | 7.50 |
| 7b | 544.50 ± 5 | 8.5 |
| Staurosporine | 503.00 ± 4 | 8.0 |
| Control | 65.50 | 1 |
| Compound Number | Caspase-8 | Bax | Bcl-2 | |||
|---|---|---|---|---|---|---|
| Conc (ng/mL) | Fold Change | Conc (Pg/mL) | Fold Change | Conc (ng/mL) | Fold Reduction | |
| 6b | 1.50 | 17 | 220 | 28 | 1.30 | 4 |
| 7b | 1.90 | 21 | 295 | 37 | 1.00 | 5 |
| Staurosporine | 1.80 | 20 | 280 | 35 | 1.10 | 5 |
| Control | 0.09 | 1 | 8 | 1 | 5 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Wahaibi, L.H.; El-Sheref, E.M.; Hammouda, M.M.; Youssif, B.G.M. One-Pot Synthesis of 1-Thia-4-azaspiro[4.4/5]alkan-3-ones via Schiff Base: Design, Synthesis, and Apoptotic Antiproliferative Properties of Dual EGFR/BRAFV600E Inhibitors. Pharmaceuticals 2023, 16, 467. https://doi.org/10.3390/ph16030467
Al-Wahaibi LH, El-Sheref EM, Hammouda MM, Youssif BGM. One-Pot Synthesis of 1-Thia-4-azaspiro[4.4/5]alkan-3-ones via Schiff Base: Design, Synthesis, and Apoptotic Antiproliferative Properties of Dual EGFR/BRAFV600E Inhibitors. Pharmaceuticals. 2023; 16(3):467. https://doi.org/10.3390/ph16030467
Chicago/Turabian StyleAl-Wahaibi, Lamya H., Essmat M. El-Sheref, Mohamed M. Hammouda, and Bahaa G. M. Youssif. 2023. "One-Pot Synthesis of 1-Thia-4-azaspiro[4.4/5]alkan-3-ones via Schiff Base: Design, Synthesis, and Apoptotic Antiproliferative Properties of Dual EGFR/BRAFV600E Inhibitors" Pharmaceuticals 16, no. 3: 467. https://doi.org/10.3390/ph16030467
APA StyleAl-Wahaibi, L. H., El-Sheref, E. M., Hammouda, M. M., & Youssif, B. G. M. (2023). One-Pot Synthesis of 1-Thia-4-azaspiro[4.4/5]alkan-3-ones via Schiff Base: Design, Synthesis, and Apoptotic Antiproliferative Properties of Dual EGFR/BRAFV600E Inhibitors. Pharmaceuticals, 16(3), 467. https://doi.org/10.3390/ph16030467






