Pentosan Polysulfate Affords Pleotropic Protection to Multiple Cells and Tissues
Abstract
1. Introduction
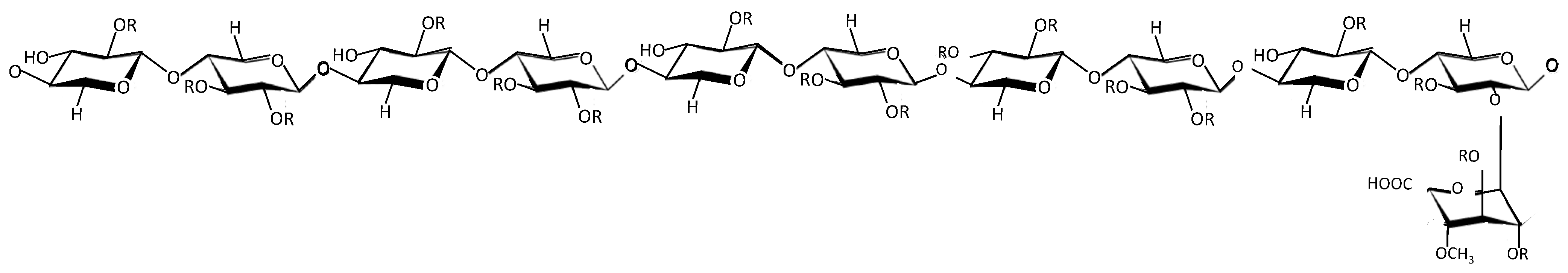
2. Overview of PPS
3. PPS Competes with and Mimics HS
3.1. PPS as a Therapy
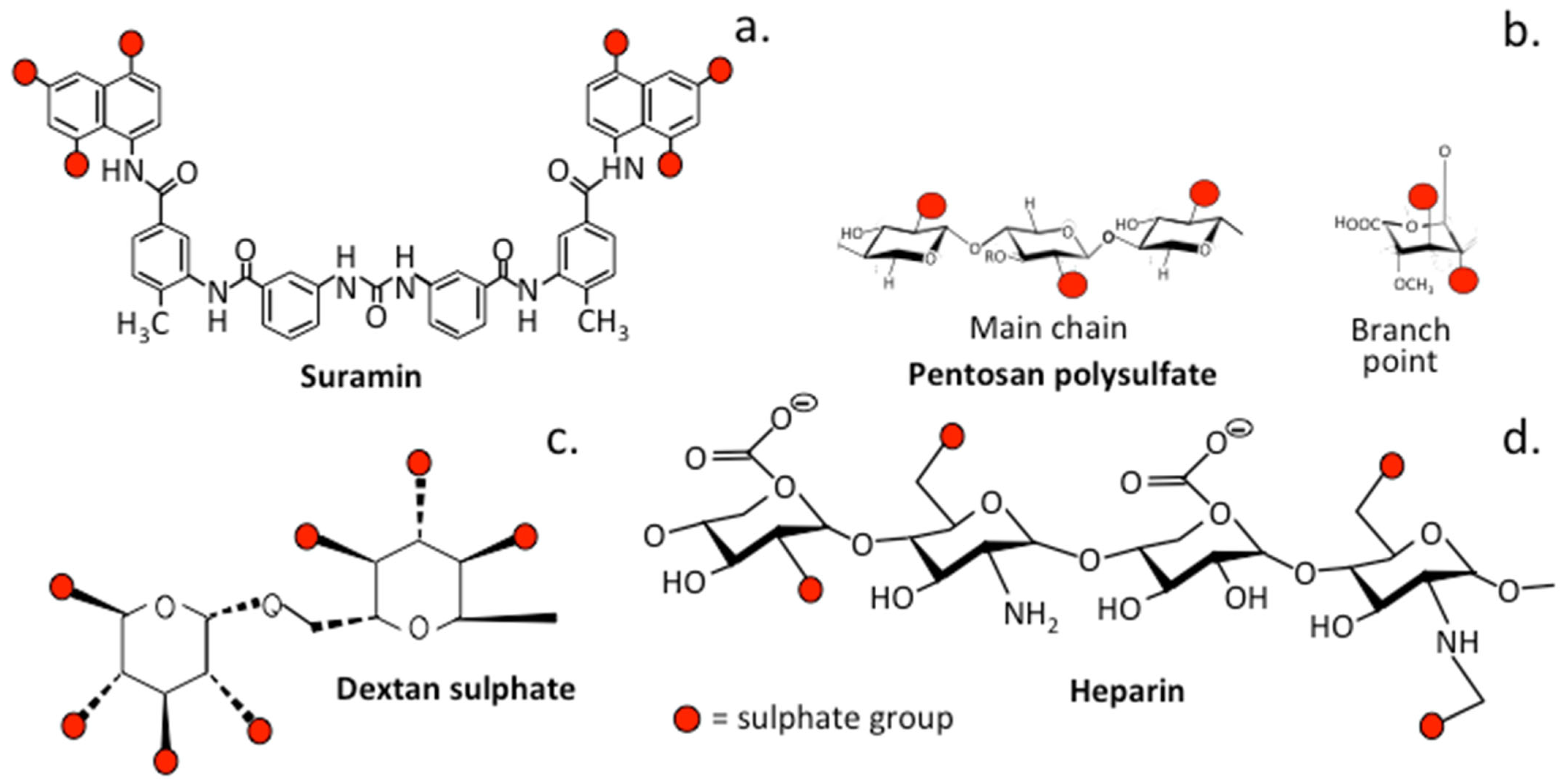
3.2. The Role of Sulfation in PPS and Related Sulfated Molecules
3.2.1. Suramin
3.2.2. Dextran Sulfate
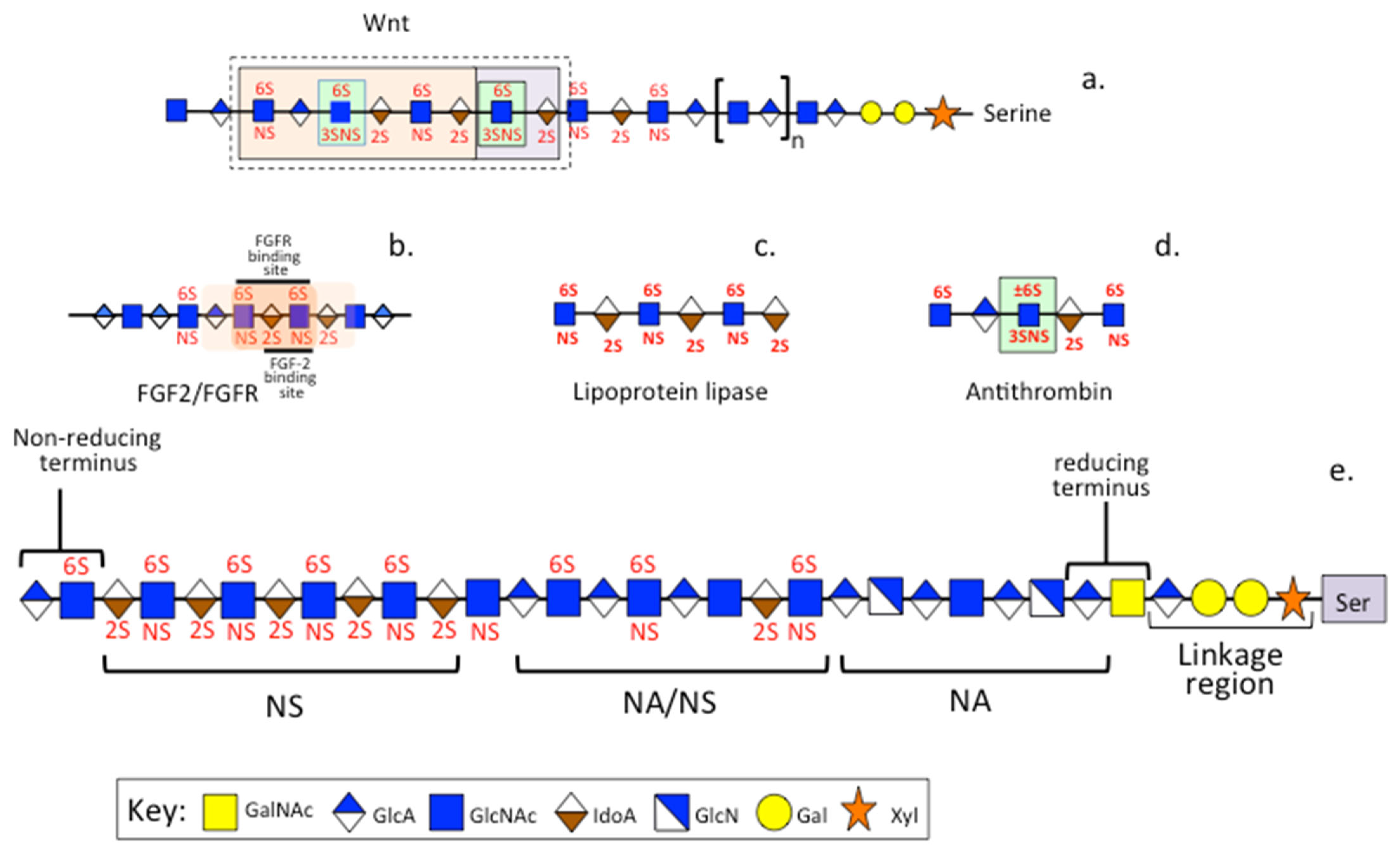
3.3. PPS, Heparin and HS
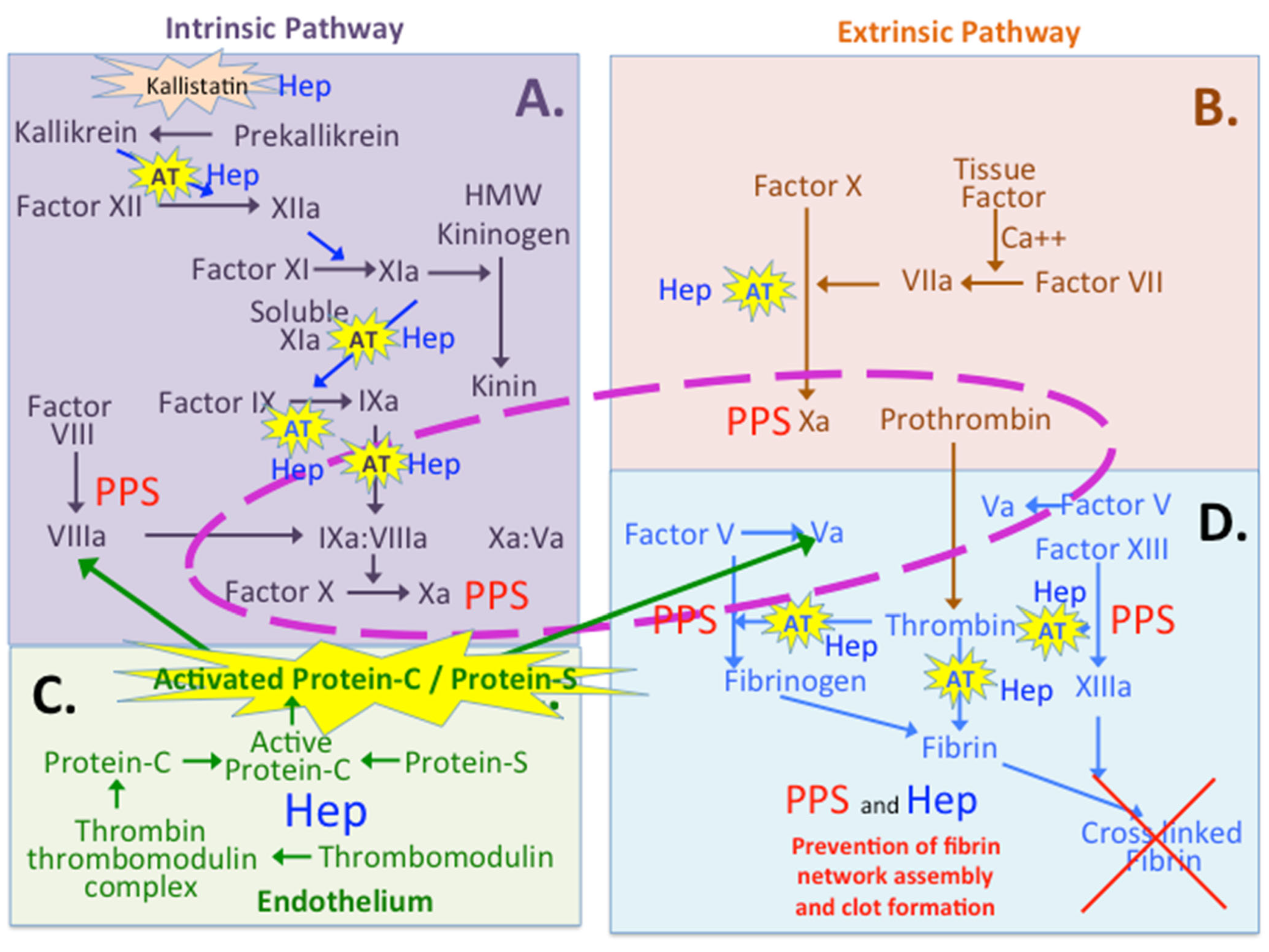
4. PPS and Coagulation
5. PPS and the Gut
5.1. A Potential Health Promoting Role for PPS Processed by Gut Bacteria to a Pre-Biotic Xylo-Oligosaccharide
5.2. Gut Content Transit Time and the Incidence of Bowel Cancer
6. The Chondroprotective Properties of PPS
7. PPS and Stem Cells Used in the Repair of the Degenerate IVD
8. A Comparison of the Anticoagulant Properties of Heparin and PPS
8.1. Multiple Roles for Heparin and PPS in the Coagulation Cascades
8.2. Protein C Inhibitor and Thrombomodulin
8.3. Kallistatin
9. Heparin Inhibits Metastatic Events That Promote Cancer Development
10. Heparin and PPS in Bone Health
11. Regulation of Hepcidin and Iron Metabolism by PPS in Chronic Diseases
12. PPS as an Anti-Tumor Agent in a Model of Gastric Cancer
13. Beneficial Combination PPS-MSC Therapy in a Model of Interstitial Cystitis
14. Anti-Viral Properties of PPS
14.1. Animal Studies Demonstrating PPS as an Antiviral
14.2. Clinical Trials of PPS as an Antiviral
15. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Anderson, V.; Perry, C.M. Pentosan polysulfate: A review of its use in the relief of bladder pain or discomfort in interstitial cystitis. Drugs 2006, 66, 821–835. [Google Scholar] [CrossRef] [PubMed]
- Nickel, J.; Barkin, J.; Forrest, J.; Mosbaugh, P.G.; Hernandez-Graulau, J.; Kaufman, D.; Lloyd, K.; Evans, R.J.; Parsons, C.L.; Atkinson, L.E.; et al. Randomized, double-blind, dose-ranging study of pentosan polysulfate sodium for interstitial cystitis. Urology 2005, 65, 654–658. [Google Scholar] [CrossRef]
- Nickel, J.; Herschorn, S.; Whitmore, K.E.; Forrest, J.B.; Hu, P.; Friedman, A.J.; Baseman, A.S. Pentosan polysulfate sodium for treatment of interstitial cystitis/bladder pain syndrome: Insights from a randomized, double-blind, placebo controlled study. J. Urol. 2015, 193, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Teichman, J. The role of pentosan polysulfate in treatment approaches for interstitial cystitis. Rev. Urol. 2002, 4 (Suppl. S1), S21–S27. [Google Scholar] [PubMed]
- Deshpande, P.; Luthra, P.; Pandey, A.K.; Paghdar, D.J.; Govardhana, P.S.G.V. Process for the Preparation of Pentosan Polysulfate or Salts Thereof. U.S. Patent 20100105889, 29 April 2010. Available online: https://www.freepatentsonline.com/y2010/0105889.html (accessed on 11 March 2021).
- Ghosh, P. The pathobiology of osteoarthritis and the rationale for the use of pentosan polysulfate for its treatment. Semin. Arthritis Rheum. 1999, 28, 211–267. [Google Scholar] [CrossRef]
- Ennemoser, M.; Rieger, J.; Muttenthaler, E.; Gerlza, T.; Zatloukal, K.; Kungl, A.J. Enoxaparin and pentosan polysulfate bind to the SARS-CoV-2 spike protein and human ACE2 receptor, inhibiting Vero cell infection. Biomedicines 2022, 10, 49. [Google Scholar] [CrossRef]
- Subha, K.; Varalakshmi, P. Effect of sodium pentosan polysulphate on tissue lipids in control and glycollate treated rats. Pharm. Res. 1993, 27, 289–297. [Google Scholar] [CrossRef]
- Parsons, C.; Pollen, J.J.; Anwar, H.; Stauffer, C.; Schmidt, J.D. Antibacterial activity of bladder surface mucin duplicated in the rabbit bladder by exogenous glycosaminoglycan (sodium pentosanpolysulfate). Infect. Immun. 1980, 27, 876–881. [Google Scholar] [CrossRef]
- Schamhart, D.; de Boer, E.C.; Kurth, K.H. Interaction between bacteria and the lumenal bladder surface: Modulation by pentosan polysulfate, an experimental and theoretical approach with clinical implication. World J. Urol. 1994, 12, 27–37. [Google Scholar] [CrossRef]
- Klegeris, A.; Singh, E.A.; McGeer, P.L. Effects of C-reactive protein and pentosan polysulphate on human complement activation. Immunology 2002, 106, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Tran-Lundmark, K.; Tran, P.K.; Paulsson-Berne, G.; Fridén, V.; Soininen, R.; Tryggvason, K.; Wight, T.N.; Kinsella, M.G.; Borén, J.; Hedin, U. Heparan sulfate in perlecan promotes mouse atherosclerosis: Roles in lipid permeability, lipid retention, and smooth muscle cell proliferation. Circ. Res. 2008, 103, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.; Whitelock, J.M.; Williams, H.; Kim, H.N.; Medbury, H.J.; Lord, M.S. Macrophages bind LDL using heparan sulfate and the perlecan protein core. J. Biol. Chem. 2021, 296, 100520. [Google Scholar] [CrossRef]
- Wilsie, L.; Chanchani, S.; Navaratna, D.; Orlando, R.A. Cell surface heparan sulfate proteoglycans contribute to intracellular lipid accumulation in adipocytes. Lipids Health Dis. 2005, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Lupia, E.; Zheng, F.; Grosjean, F.; Tack, I.; Doublier, S.; Elliot, S.J.; Vlassara, H.; Striker, G.E. Pentosan polysulfate inhibits atherosclerosis in Watanabe heritable hyperlipidemic rabbits: Differential modulation of metalloproteinase-2 and -9. Lab. Investig. 2012, 92, 236–245. [Google Scholar] [CrossRef] [PubMed]
- MacArthur, J.; Bishop, J.R.; Stanford, K.I.; Wang, L.; Bensadoun, A.; Witztum, J.L.; Esko, J.D. Liver heparan sulfate proteoglycans mediate clearance of triglyceride-rich lipoproteins independently of LDL receptor family members. J. Clin. Investig. 2007, 117, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Virk, S.; Federova, T.; Oo, W.M.; Hunter, D.J. The effect of pentosan polysulfate sodium for improving dyslipidaemia and knee pain in people with knee osteoarthritis: A pilot study. Osteoarthr. Cartil. Open. 2023, 10030343. [Google Scholar] [CrossRef]
- Ghosh, P.; Cheras, P.A. Vascular mechanisms in osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2001, 15, 693–709. [Google Scholar] [CrossRef]
- Whitelock, J.; Iozzo, R.V. Heparan sulfate: A complex polymer charged with biological activity. Chem. Rev. 2005, 105, 2745–2764. [Google Scholar] [CrossRef]
- Smith, M.M.; Melrose, J. Xylan Prebiotics and the Gut Microbiome Promote Health and Wellbeing: Potential Novel Roles for Pentosan Polysulfate. Pharmaceuticals 2022, 15, 1151. [Google Scholar] [CrossRef]
- Zhang, Q.; Ran, X.; He, Y.; Ai, Q.; Shi, Y. Acetate downregulates the activation of NLRP3 inflammasomes and attenuates lung injury in neonatal mice with bronchopulmonary dysplasia. Front. Pediatr. 2021, 8, 985. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Junior, A.; Borgonovi, T.F.; De Salis, L.V.V.; Leite, A.Z.; Dantas, A.S.; De Salis, G.V.V.; Cruz, G.N.F.; De Oliveira, L.F.V.; Gomes, E.; Penna, A.L.B.; et al. Detection of Intestinal Dysbiosis in Post-COVID-19 Patients One to Eight Months after Acute Disease Resolution. Int. J. Environ. Res. Public Health 2022, 19, 10189. [Google Scholar] [CrossRef]
- Giannos, P.; Prokopidis, K. Gut dysbiosis and long COVID-19: Feeling gutted. J. Med. Virol. 2022, 94, 2917–2918. [Google Scholar] [CrossRef]
- Bertini, S.; Alekseeva, A.; Elli, S.; Pagani, I.; Zanzoni, S.; Eisele, G.; Krishnan, R.; Maag, K.P.; Reiter, C.; Lenhart, D.; et al. Pentosan Polysulfate Inhibits Attachment and Infection by SARS-CoV-2 In Vitro: Insights into Structural Requirements for Binding. Thromb. Haemost. 2022, 122, 984–997. [Google Scholar] [CrossRef]
- Bendas, G. Pentosan Polysulfate-A “Better Heparin” as Potential Medication for the Treatment of SARS-CoV-2 Infections? Thromb. Haemost. 2022, 122, 870. [Google Scholar] [CrossRef]
- Arepally, G. Heparin-induced thrombocytopenia. Blood 2017, 129, 2864–2872. [Google Scholar] [CrossRef] [PubMed]
- Arepally, G.; Padmanabhan, A. Heparin-Induced Thrombocytopenia: A Focus on Thrombosis. Arter. Thromb. Vasc. Biol. 2021, 41, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Baroletti, S.; Goldhaber, S.Z. Heparin-induced thrombocytopenia. Circulation 2006, 114, e355–e356. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.; Farrugia, B.L.; Biose, I.J.; Bix, G.J.; Melrose, J.; Perlecan, A. Multi-Functional, Cell-Instructive, Matrix-Stabilizing Proteoglycan With Roles in Tissue Development Has Relevance to Connective Tissue Repair and Regeneration. Front. Cell Dev. Biol. 2022, 10, 856261. [Google Scholar] [CrossRef] [PubMed]
- Al-Zahrani, A.; Gajewski, J.B. Long-term efficacy and tolerability of pentosan polysulphate sodium in the treatment of bladder pain syndrome. Can Urol. Assoc. J. 2011, 5, 113–118. [Google Scholar] [CrossRef][Green Version]
- Nickel, J.; Forrest, J.B.; Tomera, K.; Hernandez-Graulau, J.; Moon, T.D.; Schaeffer, A.J.; Krieger, J.N.; Zeitlin, S.I.; Evans, R.J.; Lama, D.J.; et al. Pentosan polysulfate sodium therapy for men with chronic pelvic pain syndrome: A multicenter, randomized, placebo controlled study. J. Urol. 2005, 173, 1252–1255. [Google Scholar] [CrossRef]
- Senthil, D.; Malini, M.M.; Varalakshmi, P. Sodium pentosan polysulphate--a novel inhibitor of urinary risk factors and enzymes in experimental urolithiatic rats. Ren. Fail. 1998, 20, 573–580. [Google Scholar] [CrossRef][Green Version]
- Wallius, B.; Tidholm, A.E. Use of pentosan polysulphate in cats with idiopathic, non-obstructive lower urinary tract disease: A double-blind, randomised, placebo-controlled trial. J. Feline Med. Surg. 2009, 11, 409–412. [Google Scholar] [CrossRef]
- Andrews, J.; Ghosh, P.; Lentini, A.; Ternai, B. The interaction of pentosan polysulphate (SP54) with human neutrophil elastase and connective tissue matrix components. Chem. Biol. Interact. 1983, 47, 157–173. [Google Scholar] [CrossRef]
- Takizawa, M.; Ohuchi, E.; Yamanaka, H.; Nakamura, H.; Ikeda, E.; Ghosh, P.; Okada, Y. Production of tissue inhibitor of metalloproteinases 3 is selectively enhanced by calcium pentosan polysulfate in human rheumatoid synovial fibroblasts. Arthritis Rheum. 2000, 43, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, M.; Yatabe, T.; Okada, A.; Chijiiwa, M.; Mochizuk, I.S.; Ghosh, P.; Okada, Y. Calcium pentosan polysulfate directly inhibits enzymatic activity of ADAMTS4 (aggrecanase-1) in osteoarthritic chondrocytes. FEBS Lett. 2008, 582, 2945–2949. [Google Scholar] [CrossRef]
- Troeberg, L.; Fushimi, K.; Khokha, R.; Emonard, H.; Ghosh, P.; Nagase, H. Calcium pentosan polysulfate is a multifaceted exosite inhibitor of aggrecanases. FASEB J. 2008, 22, 3515–3524. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, K.; Shirabe, S.; Miyata, N.; Murata, M.; Yamauchi, A.; Kataoka YNiwa, M. Sodium pentosan polysulfate resulted in cartilage improvement in knee osteoarthritis--an open clinical trial. BMC Clin. Pharm. 2010, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Oehme, D.; Ghosh, P.; Goldschlager, T.; Itescu, S.; Shimon, S.; Wu, J.; McDonald, C.; Troupis, J.M.; Rosenfeld, J.V.; Jenkin, G. Reconstitution of degenerated ovine lumbar discs by STRO-3-positive allogeneic mesenchymal precursor cells combined with pentosan polysulfate. J. Neurosurg. Spine 2016, 24, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Ghatak, S.; Maytin, E.V.; Mack, J.A.; Hascall, V.C.; Atanelishvili, I.; Moreno Rodriguez, R.; Markwald, R.R.; Misra, S. Roles of Proteoglycans and Glycosaminoglycans in Wound Healing and Fibrosis. Int. J. Cell Biol. 2015, 2015, 834893. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Edelman, J.; March, L.; Smith, M. Effects of pentosan polysulfate in osteoarthritis of the knee: A randomized, double-blind, placebo-controlled pilot study. Curr. Res. Clin. Exp. 2005, 66, 552–571. [Google Scholar] [CrossRef] [PubMed]
- Koenig, T.; Dart, A.J.; McIlwraith, C.W.; Horadagoda, N.; Bell, R.J.; Perkins, N.; Dart, C.; Krockenberger, M.; Jeffcott, L.B.; Little, C.B. Treatment of experimentally induced osteoarthritis in horses using an intravenous combination of sodium pentosan polysulfate, N-acetyl glucosamine, and sodium hyaluronan. Vet. Surg. 2014, 43, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Olczyk, P.; Mencner, Ł.; Komosinska-Vassev, K. Diverse Roles of Heparan Sulfate and Heparin in Wound Repair. Biomed. Res. Int. 2015, 2015, 549417. [Google Scholar] [CrossRef]
- Rogachefsky, R.; Dean, D.D.; Howell, D.S.; Altman, R.D. Treatment of canine osteoarthritis with sodium pentosan polysulfate and insulin-like growth factor-1. Ann. N. Y. Acad. Sci. 1994, 732, 392–394. [Google Scholar] [CrossRef] [PubMed]
- Sampson, M.; Kabbani, M.; Krishnan, R.; Nganga, M.; Theodoulou, A.; Krishnan, J. Improved clinical outcome measures of knee pain and function with concurrent resolution of subchondral Bone Marrow Edema Lesion and joint effusion in an osteoarthritic patient following Pentosan Polysulphate Sodium treatment: A case report. BMC Musculoskelet. Disord. 2017, 18, 396. [Google Scholar] [CrossRef] [PubMed]
- Suranji Wijekoon, H.; Kim, S.; Bwalya, E.C.; Fang, J.; Aoshima, K.; Hosoya, K.; Okumura, M. Anti-arthritic effect of pentosan polysulfate in rats with collagen-induced arthritis. Res. Vet. Sci. 2019, 122, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Frith, J.; Cameron, A.R.; Menzies, D.J.; Ghosh, P.; Whitehead, D.L.; Gronthos, S.; Zannettino, A.C.; Cooper-White, J.J. An injectable hydrogel incorporating mesenchymal precursor cells and pentosan polysulphate for intervertebral disc regeneration. Biomaterials 2013, 34, 9430–9440. [Google Scholar] [CrossRef] [PubMed]
- Frith, J.; Menzies, D.J.; Cameron, A.R.; Ghosh, P.; Whitehead, D.L.; Gronthos, S.; Zannettino, A.C.; Cooper-White, J.J. Effects of bound versus soluble pentosan polysulphate in PEG/HA-based hydrogels tailored for intervertebral disc regeneration. Biomaterials 2014, 35, 1150–1162. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, N.; Schirmer, L.; Atallah, P.; Wandel, E.; Ferrer, R.A.; Werner, C.; Simon, J.C.; Franz, S.; Freudenberg, U. Glycosaminoglycan-based hydrogels capture inflammatory chemokines and rescue defective wound healing in mice. Sci. Transl. Med. 2017, 9, eaai9044. [Google Scholar] [CrossRef]
- Kilgore, K.; Naylor, K.B.; Tanhehco, E.J.; Park, J.L.; Booth, E.A.; Washington, R.A.; Lucchesi, B.R. The semisynthetic polysaccharide pentosan polysulfate prevents complement-mediated myocardial injury in the rabbit perfused heart. J. Pharm. Exp. 1998, 285, 987–994. [Google Scholar]
- Fischer, A.; Barrowcliffe, T.W.; Thomas, D.P. A comparison of pentosan polysulphate (SP54) and heparin. I: Mechanism of action on blood coagulation. Thromb. Haemost. 1982, 47, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Giedrojć, J.; Radziwon, P.; Klimiuk, M.; Bielawiec, M.; Breddin, H.K.; Kłoczko, J. Experimental studies on the anticoagulant and antithrombotic effects of sodium and calcium pentosan polysulphate. J. Physiol. Pharm. 1999, 50, 111–119. [Google Scholar]
- Goad, K.; Horne, M.K.; Gralnick, H.R. Pentosan-induced thrombocytopenia: Support for an immune complex mechanism. Br. J. Haematol. 1994, 88, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Orme, C.; Harris, R.C. A comparison of the lipolytic and anticoagulative properties of heparin and pentosan polysulphate in the thoroughbred horse. Acta Physiol. Scand. 1997, 59, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Vinazzer, H. Effect of pentosan polysulfate on fibrinolysis: Basic tests and clinical application. Semin. Thromb. Hemost. 1991, 17, 375–378. [Google Scholar] [CrossRef]
- Tardy-Poncet, B.; Tardy, B.; Grelac, F.; Reynaud, J.; Mismetti, P.; Bertrand, J.C.; Guyotat, D. Pentosan polysulfate-induced thrombocytopenia and thrombosis. Am. J. Hematol. 1994, 45, 252–257. [Google Scholar] [CrossRef]
- Francis, D.; Hutadilok, N.; Kongtawelert, P.; Ghosh, P. Pentosan polysulphate and glycosaminoglycan polysulphate stimulate the synthesis of hyaluronan in vivo. Rheumatol. Int. 1993, 13, 61–64. [Google Scholar] [CrossRef]
- Verbruggen, G.; Veys, E.M. Intra-articular injection pentosan polysulphate results in increased hyaluronan molecular weight in joint fluid. Clin. Exp. Rheumatol. 1992, 10, 249–254. [Google Scholar]
- Stapledon, C.; Tsangari, H.; Solomon, L.B.; Campbell, D.G.; Hurtado, P.; Krishnan, R.; Atkins, G.J. Human osteocyte expression of Nerve Growth Factor: The effect of Pentosan Polysulphate Sodium (PPS) and implications for pain associated with knee osteoarthritis. PLoS ONE 2019, 14, e0222602. [Google Scholar] [CrossRef] [PubMed]
- Oehme, D.; Ghosh, P.; Shimmon, S.; Wu, J.; McDonald, C.; Troupis, J.M.; Goldschlager, T.; Rosenfeld, J.V.; Jenkin, G. Mesenchymal progenitor cells combined with pentosan polysulfate mediating disc regeneration at the time of microdiscectomy: A preliminary study in an ovine model. J. Neurosurg. Spine 2014, 20, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Hayes, A.J.; Melrose, J. Pentosan Polysulfate, a Semisynthetic Heparinoid Disease-Modifying Osteoarthritic Drug with Roles in Intervertebral Disc Repair Biology Emulating the Stem Cell Instructive and Tissue Reparative Properties of Heparan Sulfate. Stem. Cells Dev. 2022, 31, 406–430. [Google Scholar] [CrossRef] [PubMed]
- Elliot, S.; Zorn, B.H.; McLeod, D.G.; Moul, J.W.; Nyberg, L.; Striker, L.J.; Striker, G.E. Pentosan polysulfate decreases prostate smooth muscle proliferation and extracellular matrix turnover. Prostate Cancer Prostatic Dis. 2003, 6, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Rusnati, M.; Urbinati, C.; Caputo, A.; Possati, L.; Lortat-Jacob, H.; Giacca, M.; Ribatti, D.; Presta, M. Pentosan polysulfate as an inhibitor of extracellular HIV-1 Tat. J. Biol. Chem. 2001, 276, 22420–22425. [Google Scholar] [CrossRef]
- Veszelka, S.; Pásztói, M.; Farkas, A.E.; Krizbai, I.; Ngo, T.K.; Niwa, M.; Abrahám, C.S.; Deli, M.A. Pentosan polysulfate protects brain endothelial cells against bacterial lipopolysaccharide-induced damages. Neurochem. Int. 2007, 50, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Guan, T.-J.; Zheng, S.; Grosjean, F.; Liu, W.; Xiong, H.; Gordon, R.; Vlassara, H.; E Striker, G.; Zheng, F. Inhibition of inflammation by pentosan polysulfate impedes the development and progression of severe diabetic nephropathy in aging C57B6 mice. Lab. Investig. 2011, 91, 1459–1471. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Chen, A.L.; Gao, Q.; Xu, B.; Guo, X.; Guan, T. Pentosan polysulfate ameliorates fibrosis and inflammation markers in SV40 MES13 cells by suppressing activation of PI3K/AKT pathway via miR-446a-3p. BMC Nephrol. 2022, 23, 105. [Google Scholar] [CrossRef]
- Daly, C.; Ghosh, P.; Zannettino, A.C.W.; Badal, T.; Shimmon, R.; Jenkin, G.; Oehme, D.; Jain, K.; Sher, I.; Vais, A.; et al. Mesenchymal progenitor cells primed with pentosan polysulfate promote lumbar intervertebral disc regeneration in an ovine model of microdiscectomy. Spine J. 2018, 18, 491–506. [Google Scholar] [CrossRef]
- Chen, P.; Yuan, Y.; Zhang, T.; Xu, B.; Gao, Q.; Guan, T. Pentosan polysulfate ameliorates apoptosis and inflammation by suppressing activation of the p38 MAPK pathway in high glucose treated HK 2 cells. Int. J. Mol. Med. 2018, 41, 908–914. [Google Scholar] [CrossRef]
- Smith, M.; Ghosh, P.; Numata, Y.; Bansal, M.K. The effects of orally administered calcium pentosan polysulfate on inflammation and cartilage degradation produced in rabbit joints by intraarticular injection of a hyaluronate-polylysine complex. Arthritis Rheum. 1994, 37, 125–136. [Google Scholar] [CrossRef]
- Baba, M.; Nakajima, M.; Schols, D.; Pauwels, R.; Balzarini, J.; De Clercq, E. Pentosan polysulfate, a sulfated oligosaccharide, is a potent and selective anti-HIV agent in vitro. Antivir. Res. 1988, 9, 335–343. [Google Scholar] [CrossRef] [PubMed]
- García-Villalón, D.; Gil-Fernández, C. Antiviral activity of sulfated polysaccharides against African swine fever virus. Antivir. Res. 1991, 15, 139–148. [Google Scholar] [CrossRef]
- Herrero, L.; Foo, S.S.; Sheng, K.C.; Chen, W.; Forwood, M.R.; Bucala, R.; Mahalingam, S. Pentosan Polysulfate: A Novel Glycosaminoglycan-Like Molecule for Effective Treatment of Alphavirus-Induced Cartilage Destruction and Inflammatory Disease. J. Virol. 2015, 89, 8063–8076. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.; Duiker, M.; Rudd, P.A.; Skerrett, D.; Pollard, J.G.D.; Siddel, C.; Rifat, R.; Ng, J.H.K.; Georgius, P.; Hererro, L.J.; et al. Pentosan polysulfate sodium for Ross River virus-induced arthralgia: A phase 2a, randomized, double-blind, placebo-controlled study. BMC Musculoskelet. Disord. 2021, 22, 271. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Yasunaga, J.I.; Ohshima, K.; Matsumoto, T.; Matsuoka, M. Pentosan Polysulfate Demonstrates Anti-human T-Cell Leukemia Virus Type 1 Activities In Vitro and In Vivo. J. Virol. 2019, 93, e00413–e00419. [Google Scholar] [CrossRef]
- Pluda, J.; Shay, L.E.; Foli, A.; Tannenbaum, S.; Cohen, P.J.; Goldspiel, B.R.; Adamo, D.; Cooper, M.R.; Broder, S.; Yarchoan, R. Administration of pentosan polysulfate to patients with human immunodeficiency virus-associated Kaposi’s sarcoma. J. Natl. Cancer Inst. 1993, 85, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Sékaly, R.P.; Chiasson, J.L. Pentosan polysulfate, a potent anti HIV and anti tumor agent, inhibits protein serine/threonine and tyrosine kinases. Mol. Cell Biochem. 1993, 120, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Wu, J.; Shimmon, S.; Zannettino, A.C.; Gronthos, S.; Itescu, S. Pentosan polysulfate promotes proliferation and chondrogenic differentiation of adult human bone marrow-derived mesenchymal precursor cells. Arthritis Res. 2010, 12, R28. [Google Scholar] [CrossRef]
- Troeberg, L.; Mulloy, B.; Ghosh, P.; Lee, M.H.; Murphy, G.; Nagase, H. Pentosan polysulfate increases affinity between ADAMTS-5 and TIMP-3 through formation of an electrostatically driven trimolecular complex. Biochem. J. 2012, 443, 307–315. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yamamoto, K.; Wilkinson, D.; Bou-Gharios, G. Targeting Dysregulation of Metalloproteinase Activity in Osteoarthritis. Calcif. Tiss. Int. 2021, 109, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Innes, J.; Barr, A.R.; Sharif, M. Efficacy of oral calcium pentosan polysulphate for the treatment of osteoarthritis of the canine stifle joint secondary to cranial cruciate ligament deficiency. Vet. Rec. 2000, 146, 433–437. [Google Scholar] [CrossRef]
- Read, R.; Cullis-Hill, D.; Jones, M.P. Systemic use of pentosan polysulphate in the treatment of osteoarthritis. J. Small Anim. Pract. 1996, 37, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Bwalya, E.; Kim, S.; Fang, J.; Wijekoon, H.M.S.; Hosoya, K.; Okumura, M. Pentosan polysulfate inhibits IL-1β-induced iNOS, c-Jun and HIF-1α upregulation in canine articular chondrocytes. PLoS ONE 2017, 12, e0177144. [Google Scholar] [CrossRef] [PubMed]
- Clark, E.; Nava, B.; Caputi, M. Tat is a multifunctional viral protein that modulates cellular gene expression and functions. Oncotarget 2017, 8, 27569–27581. [Google Scholar] [CrossRef] [PubMed]
- Vistnes, M.; Aronsen, J.M.; Lunde, I.G.; Sjaastad, I.; Carlson, C.R.; Christensen, G. Pentosan polysulfate decreases myocardial expression of the extracellular matrix enzyme ADAMTS4 and improves cardiac function in vivo in rats subjected to pressure overload by aortic banding. PLoS ONE 2014, 9, e89621. [Google Scholar] [CrossRef]
- Kirker-Head, C.; Feldmann, H. Chapter 23. Pharmacotherapy of joint and tendon disease. In Equine Sports Medicine and Surgery, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 473–502. [Google Scholar] [CrossRef]
- Wedrén, H. Effects of sodium pentosanpolysulphate on symptoms related to chronic non-bacterial prostatitis. A double-blind randomized study. Scand. J. Urol. Nephrol. 1987, 21, 81–88. [Google Scholar] [CrossRef]
- Baytas, S.; Linhardt, R.J. Advances in the preparation and synthesis of heparin and related products. Drug Discov. Today 2020, 25, 2095–2109. [Google Scholar] [CrossRef]
- Colombo, E.; Mauri, L.; Marinozzi, M.; Rudd, T.R.; Yates, E.A.; Ballabio, D.; Guerrini, M. NMR spectroscopy and chemometric models to detect a specific non-porcine ruminant contaminant in pharmaceutical heparin. J. Pharm. Biomed. Anal. 2022, 214, 114724. [Google Scholar] [CrossRef] [PubMed]
- Lemmnitzer, K.; Köhling, S.; Freyse, J.; Rademann, J.; Schiller, J. Characterization of defined sulfated heparin-like oligosaccharides by electrospray ionization ion trap mass spectrometry. J. Mass Spectrom. 2021, 56, e4692. [Google Scholar] [CrossRef]
- Shriver, Z.; Sasisekharan, R. Capillary Electrophoretic Analysis of Isolated Sulfated Polysaccharides to Characterize Pharmaceutical Products. Methods Mol. Biol. 2022, 2303, 329–339. [Google Scholar] [PubMed]
- Zhang, Z.; Li, B.; Suwan, J.; Zhang, F.; Wang, Z.; Liu, H.; Mulloy, B.; Linhardt, R.J. Analysis of pharmaceutical heparins and potential contaminants using (1)H-NMR and PAGE. J. Pharm. Sci. 2009, 98, 4017–4026. [Google Scholar] [CrossRef]
- Schwartsmann, G.; Sprinz, E.; Kalakun, L.; Yamagushi, N.; Sander, E.; Grivicich, I.; Koya, R.; Mans, D.R. Phase II study of pentosan polysulfate (PPS) in patients with AIDS-related Kaposi’s sarcoma. Tumori 1996, 82, 360–363. [Google Scholar] [CrossRef]
- Zugmaier, G.; Lippman, M.E.; Wellstein, A. Inhibition by pentosan polysulfate (PPS) of heparin-binding growth factors released from tumor cells and blockage by PPS of tumor growth in animals. J. Natl. Cancer Inst. 1992, 84, 1716–1724. [Google Scholar] [CrossRef] [PubMed]
- Rha, S.; Noh, S.H.; Kwak, H.J.; Wellstein, A.; Kim, J.H.; Roh, J.K.; Min, J.S.; Kim, B.S.; Chung, H.C. Comparison of biological phenotypes according to midkine expression in gastric cancer cells and their autocrine activities could be modulated by pentosan polysulfate. Cancer Lett. 1997, 118, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Jones, D. Measuring midkine: The utility of midkine as a biomarker in cancer and other diseases. Br. J. Pharm. 2014, 171, 2925–2939. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Jo, J.Y.; Kim, S.H.; Choi, M.; Han, C.; Choi, B.K.; Kim, S.S. Midkine Is a Potential Therapeutic Target of Tumorigenesis, Angiogenesis, and Metastasis in Non-Small Cell Lung Cancer. Cancers 2020, 12, 2402. [Google Scholar] [CrossRef]
- Parker, B.; Swain, S.M.; Zugmaier, G.; DeLap, R.L.; Lippman, M.E.; Wellstein, A. Detectable inhibition of heparin-binding growth factor activity in sera from patients treated with pentosan polysulfate. J. Natl. Cancer Inst. 1993, 85, 1068–1073. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, Q.; Li, R.; Chen, S.; Tan, J.; Li, L.; Dong, X.; Huang, C.; Wen, T.; Liu, J. Targeting MDK Abrogates IFN-γ-Elicited Metastasis in Cancers of Various Origins. Front. Oncol. 2022, 12, 885656. [Google Scholar] [CrossRef] [PubMed]
- Budsberg, S.; Bergh, M.S.; Reynolds, L.R.; Streppa, H.K. Evaluation of pentosan polysulfate sodium in the postoperative recovery from cranial cruciate injury in dogs: A randomized, placebo-controlled clinical trial. Vet. Surg. 2007, 36, 234–244. [Google Scholar] [CrossRef]
- Kramer, C.; Tsang, A.S.; Koenig, T.; Jeffcott, L.B.; Dart, C.M.; Dart, A.J. Survey of the therapeutic approach and efficacy of pentosan polysulfate for the prevention and treatment of equine osteoarthritis in veterinary practice in Australia. Aust. Vet. J. 2014, 92, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.; Sugahara, K.; Farrugia, B.; Whitelock, J.M.; Caterson, B.; Melrose, J. Biodiversity of CS-proteoglycan sulphation motifs: Chemical messenger recognition modules with roles in information transfer, control of cellular behaviour and tissue morphogenesis. Biochem. J. 2018, 475, 587–620. [Google Scholar] [CrossRef]
- Melrose, J. Glycosaminoglycans in Wound Healing. Bone Tissue Regen. Insights 2016, 7, 29–50. [Google Scholar] [CrossRef]
- Mikami, T.; Kitagawa, H. Chondroitin sulfate glycosaminoglycans function as extra/pericellular ligands for cell surface receptors. J. Biochem. 2023, mvac110. [Google Scholar] [CrossRef]
- Walimbe, T.; Panitch, A. Proteoglycans in Biomedicine: Resurgence of an Underexploited Class of ECM Molecules. Front. Pharm. 2020, 10, 1661. [Google Scholar] [CrossRef]
- Wiedemar, N.; Hauser, D.A.; Mäser, P. 100 Years of Suramin. Antimicrob. Agents Chemother. 2020, 64, e01168-19. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Y.; Chu, H.; Guan, Y.; Bi, J.; Wang, B. Multiple functions of the RNA-binding protein HuR in cancer progression, treatment responses and prognosis. Int. J. Mol. Sci. 2013, 14, 10015–10041. [Google Scholar] [CrossRef]
- Wu, M.; Tong, C.W.S.; Yan, W.; To, K.K.W.; Cho, W.C.S. The RNA Binding Protein HuR: A Promising Drug Target for Anticancer Therapy. Curr. Cancer Drug Targets 2019, 19, 382–399. [Google Scholar] [CrossRef]
- Kakuguchi, W.; Nomura, T.; Kitamura, T.; Otsuguro, S.; Matsushita, K.; Sakaitani, M.; Maenaka, K.; Tei, K. Suramin, screened from an approved drug library, inhibits HuR functions and attenuates malignant phenotype of oral cancer cells. Cancer Med. 2018, 7, 6269–6280. [Google Scholar] [CrossRef]
- Ahles, T.; Herndon, J.E.; Small, E.J.; Vogelzang, N.J.; Kornblith, A.B.; Ratain, M.J.; Stadler, W.; Palchak, D.; Marshall, M.E.; Wilding, G.; et al. Quality of life impact of three different doses of suramin in patients with metastatic hormone-refractory prostate carcinoma: Results of Intergroup O159/Cancer and Leukemia Group B 9480. Cancer 2004, 101, 2202–2208. [Google Scholar] [CrossRef]
- Parveen, N.; Lin, Y.L.; Chou, R.H.; Sun, C.M.; Yu, C. Synthesis of Novel Suramin Analogs With Anti-Proliferative Activity via FGF1 and FGFRD2 Blockade. Front. Chem. 2022, 9, 764200. [Google Scholar] [CrossRef] [PubMed]
- Spirig, R.; Gajanayake, T.; Korsgren, O.; Nilsson, B.; Rieben, R. Low molecular weight dextran sulfate as complement inhibitor and cytoprotectant in solid organ and islet transplantation. Mol. Immunol. 2008, 45, 4084–4094. [Google Scholar] [CrossRef] [PubMed]
- Laumonier, T.; Walpen, A.J.; Maurus, C.F.; Mohacsi, P.J.; Matozan, K.M.; Korchagina, E.Y.; Bovin, N.V.; Vanhove, B.; Seebach, J.D.; Rieben, R. Dextran sulfate acts as an endothelial cell protectant and inhibits human complement and natural killer cell-mediated cytotoxicity against porcine cells. Transplantation 2003, 76, 838–843. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Spirig, R.; van Kooten, C.; Obregon, C.; Nicod, L.; Daha, M.; Rieben, R. The complement inhibitor low molecular weight dextran sulfate prevents TLR4-induced phenotypic and functional maturation of human dendritic cells. J. Immunol. 2008, 181, 878–890. [Google Scholar] [CrossRef]
- Stein, C.; LaRocca, R.V.; Thomas, R.; McAtee, N.; Myers, C.E. Suramin: An anticancer drug with a unique mechanism of action. J. Clin. Oncol. 1989, 7, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, W.; Xu, Y.Y.; Guo, J.; Jiao, L.; Wang, H.; Li, M.; Yang, Q. Dextran Sulfate Inhibits Cell Proliferation and Induces Apoptosis by Regulating EZH2 in Gastric Carcinoma. Curr. Cancer Drug Targets 2021, 21, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Sakakura, C.; Kin, S.; Nakase, Y.; Fukuda, K.; Shimomura, K.; Ito, T.; Fujiyama, J.; Yamasaki, J.; Tsujimoto, H.; et al. Dextran sulfate suppresses cell adhesion and cell cycle progression of melanoma cells. Anticancer Res. 2005, 25, 895–902. [Google Scholar]
- Xu, Y.; Jin, X.; Huang, Y.; Wang, J.; Wang, X.; Wang, H. Dextran sulfate inhibition on human gastric cancer cells invasion, migration and epithelial mesenchymal transformation. Oncol. Lett. 2018, 16, 5041–5049. [Google Scholar] [CrossRef]
- Wellstein, A.; Zugmaier, G.; Califano JA 3rd Kern, F.; Paik, S.; Lippman, M.E. Tumor growth dependent on Kaposi’s sarcoma-derived fibroblast growth factor inhibited by pentosan polysulfate. J. Natl. Cancer Inst. 1991, 83, 716–720. [Google Scholar] [CrossRef]
- McLeskey, S.; Zhang, L.; Trock, B.J.; Kharbanda, S.; Liu, Y.; Gottardis, M.M.; Lippman, M.E.; Kern, F.G. Effects of AGM-1470 and pentosan polysulphate on tumorigenicity and metastasis of FGF-transfected MCF-7 cells. Br. J. Cancer 1996, 73, 1053–1062. [Google Scholar] [CrossRef]
- Grigoryan, B.; Kasyan, G.; Pivazyan, L.; Pushkar, D. Pentosan polysulfate in patients with bladder pain syndrome/interstitial cystitis with Hunner’s lesions or glomerulations: Systematic review and meta-analysis. Adv. Urol. 2022, 14, 17562872221102809. [Google Scholar] [CrossRef]
- Mulholland, S.; Hanno, P.; Parsons, C.L.; Sant, G.R.; Staskin, D.R. Pentosan polysulfate sodium for therapy of interstitial cystitis. A double-blind placebo-controlled clinical study. Urology 1990, 35, 552–558. [Google Scholar] [CrossRef]
- Herrera-Heredia, S.; Hsu, H.P.; Kao, C.Y.; Tsai, Y.H.; Yamaguchi, Y.; Roers, A.; Hsu, C.L.; Dzhagalov, I.L. Heparin is required for the formation of granules in connective tissue mast cells. Front. Immunol. 2022, 13, 1000405. [Google Scholar] [CrossRef] [PubMed]
- Fareed, J.; Leong, W.; Hoppensteadt, D.A.; Jeske, W.P.; Walenga, J.; Bick, R.L. Development of generic low molecular weight heparins: A perspective. Hematol. Oncol. Clin. N. Am. 2005, 19, 53–68. [Google Scholar] [CrossRef]
- Hirsh, J.; Ofosu, F.; Buchanan, M. Rationale behind the development of low molecular weight heparin derivatives. Semin. Thromb. Hemost. 1985, 11, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Hoppensteadt, D.; Walenga, J.M.; Fareed, J.; Bick, R.L. Heparin, low-molecular-weight heparins, and heparin pentasaccharide: Basic and clinical differentiation. Hematol. Oncol. Clin. N. Am. 2003, 17, 313–341. [Google Scholar] [CrossRef]
- Zhang, W.; Wei, X.; Yang, S.; Du, C.; Hu, B. Unfractionated heparin or low-molecular-weight heparin for venous thromboembolism prophylaxis after hepatic resection: A meta-analysis. Medicine 2022, 101, e31948. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Xia, B.; Yuan, Y.; Wang, Y.; Wang, Y. Low-molecular-weight heparin in addition to low-dose aspirin for preventing preeclampsia and its complications: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2022, 9, 1073148. [Google Scholar] [CrossRef] [PubMed]
- Reverter, J. Fondaparinux sodium. Drugs Today 2002, 38, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Walenga, J.; Fareed, J.; Jeske, W.P.; Bıck, R.L.; Samama, M.M. Development of a Synthetic Heparin Pentasaccharide: Fondaparinux. Turk. J. Haematol. 2002, 19, 137–150. [Google Scholar]
- Bauersachs, R. Fondaparinux Sodium: Recent Advances in the Management of Thrombosis. J. Cardiovasc Pharm. 2023, 28, 10742484221145010. [Google Scholar] [CrossRef]
- Chen, L.; Khan, N.; Lindenbauer, A.; Nguyen, T.H. When Will Fondaparinux Induce Thrombocytopenia? Bioconjug Chem. 2022, 33, 1574–1583. [Google Scholar] [CrossRef]
- Tanguay, M.; Séguin, C. Recurrent thrombosis rescued by fondaparinux in high-risk patients: A case series. Res. Pract. Thromb. Haemost. 2022, 6, e12773. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Tan, L.; Pan, N.; Zhang, L. The clinical use of Fondaparinux: A synthetic heparin pentasaccharide. Prog. Mol. Biol. Transl. Sci. 2019, 163, 41–53. [Google Scholar]
- Zhou, Z.; Zhang, L.; Wu, X.; Luo, L.; Wu, J.; Xu, D.; Wu, M. Chemical synthesis and pharmacological properties of heparin pentasaccharide analogues. Eur. J. Med. Chem. 2022, 234, 114256. [Google Scholar] [CrossRef]
- Quinsey, N.; Greedy, A.L.; Bottomley, S.P.; Whisstock, J.C.; Pike, R.N. Antithrombin: In control of coagulation. Int. J. Biochem. Cell Biol. 2004, 36, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Quinsey, N.; Whisstock, J.C.; Le Bonniec, B.; Louvain, V.; Bottomley, S.P.; Pike, R.N. Molecular determinants of the mechanism underlying acceleration of the interaction between antithrombin and factor Xa by heparin pentasaccharide. J. Biol. Chem. 2022, 277, 15971–15978. [Google Scholar] [CrossRef]
- Imakiire, S.; Takedatsu, H.; Mitsuyama, K.; Sakisaka, H.; Tsuruta, K.; Morita, M.; Kuno, N.; Abe, K.; Funakoshi, S.; Ishibashi, H.; et al. Role of Serum Proteinase 3 Antineutrophil Cytoplasmic Antibodies in the Diagnosis, Evaluation of Disease Severity, and Clinical Course of Ulcerative Colitis. Gut Liver 2022, 16, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Thuy-Boun, P.; Wang, A.Y.; Crissien-Martinez, A.; Xu, J.H.; Chatterjee, S.; Stupp, G.S.; Su, A.I.; Coyle, W.J.; Wolan, D.W. Quantitative Metaproteomics and Activity-based Protein Profiling of Patient Fecal Microbiome Identifies Host and Microbial Serine-type Endopeptidase Activity Associated With Ulcerative Colitis. Mol. Cell Proteom. 2022, 21, 100197. [Google Scholar] [CrossRef] [PubMed]
- Buttacavoli, M.; Di Cara, G.; Roz, E.; Pucci-Minafra, I.; Feo, S.; Cancemi, P. Integrated Multi-Omics Investigations of Metalloproteinases in Colon Cancer: Focus on MMP2 and MMP9. Int. J. Mol. Sci. 2021, 22, 12389. [Google Scholar] [CrossRef]
- Damodharan, U.; Ganesan, R.; Radhakrishnan, U.C. Expression of MMP2 and MMP9 (gelatinases A and B) in human colon cancer cells. Appl. Biochem. Biotechnol. 2011, 165, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhu, J.; Luo, H.H.; Yu, S.W.; Wang, L. Pro-protein convertase subtilisin/kexin type 9 promotes intestinal tumor development by activating Janus kinase 2/signal transducer and activator of transcription 3/SOCS3 signaling in ApcMin/+ mice. Int. J. Immunopathol. Pharm. 2021, 35, 20587384211038345. [Google Scholar] [CrossRef]
- Cooley, J.; Takayama, T.K.; Shapiro, S.D.; Schechter, N.M.; Remold-O’Donnell, E. The serpin MNEI inhibits elastase-like and chymotrypsin-like serine proteases through efficient reactions at two active sites. Biochemistry 2001, 40, 15762–15770. [Google Scholar] [CrossRef]
- Kelly-Robinson, G.; Reihill, J.A.; Lundy, F.T.; McGarvey, L.P.; Lockhart, J.C.; Litherland, G.J.; Thornbury, K.D.; Martin, S.L. The Serpin Superfamily and Their Role in the Regulation and Dysfunction of Serine Protease Activity in COPD and Other Chronic Lung Diseases. Int. J. Mol. Sci. 2021, 22, 6351. [Google Scholar] [CrossRef]
- Wang, S.; Pang, L.; Liu, Z.; Meng, X. SERPINE1 associated with remodeling of the tumor microenvironment in colon cancer progression: A novel therapeutic target. BMC Cancer 2021, 21, 767. [Google Scholar] [CrossRef]
- Mkaouar, H.; Akermi, N.; Mariaule, V.; Boudebbouze, S.; Gaci, N.; Szukala, F.; Pons, N.; Marquez, J.; Gargouri, A.; Maguin, E.; et al. Siropins, novel serine protease inhibitors from gut microbiota acting on human proteases involved in inflammatory bowel diseases. Microb. Cell Fact. 2016, 15, 201. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Kim, M.; Harada, A.; Idowu, M.O.; Sundaresan, G.; Zweit, J.; Oh, Y. Alpha-1 Antitrypsin Inhibits Tumorigenesis and Progression of Colitis-Associated Colon Cancer through Suppression of Inflammatory Neutrophil-Activated Serine Proteases and IGFBP-3 Proteolysis. Int. J. Mol. Sci. 2022, 23, 13737. [Google Scholar] [CrossRef]
- Mkaouar, H.; Mariaule, V.; Rhimi, S.; Hernandez, J.; Kriaa, A.; Jablaoui, A.; Akermi, N.; Maguin, E.; Lesner, A.; Korkmaz, B.; et al. Gut Serpinome: Emerging Evidence in IBD. Int. J. Mol. Sci. 2021, 22, 6088. [Google Scholar] [CrossRef]
- Ivanov, D.; Emonet, C.; Foata, F.; Affolter, M.; Delley, M.; Fisseha, M.; Blum-Sperisen, S.; Kochhar, S.; Arigoni, F. A serpin from the gut bacterium Bifidobacterium longum inhibits eukaryotic elastase-like serine proteases. J. Biol. Chem. 2006, 281, 17246–17252. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, B.; Amorim, C.; Silvério, S.C.; Rodrigues, L.R. Novel and emerging prebiotics: Advances and opportunities. Adv. Food Nutr. Res. 2021, 95, 41–95. [Google Scholar]
- Kondepudi, K.; Ambalam, P.; Nilsson, I.; Wadström, T.; Ljungh, A. Prebiotic-non-digestible oligosaccharides preference of probiotic bifidobacteria and antimicrobial activity against Clostridium difficile. Anaerobe 2012, 18, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Rycroft, C.; Jones, M.R.; Gibson, G.R.; Rastall, R.A. A comparative in vitro evaluation of the fermentation properties of prebiotic oligosaccharides. J. Appl. Microbiol. 2001, 91, 878–887. [Google Scholar] [CrossRef]
- Asadpoor, M.; Ithakisiou, G.N.; Henricks, P.A.J.; Pieters, R.; Folkerts, G.; Braber, S. Non-Digestible Oligosaccharides and Short Chain Fatty Acids as Therapeutic Targets against Enterotoxin-Producing Bacteria and Their Toxins. Toxins 2021, 13, 175. [Google Scholar] [CrossRef]
- Divyashri, G.; Sadanandan, B.; Chidambara Murthy, K.N.; Shetty, K.; Mamta, K. Neuroprotective Potential of Non-Digestible Oligosaccharides: An Overview of Experimental Evidence. Front. Pharm. 2021, 12, 712531. [Google Scholar] [CrossRef]
- Klurfeld, D. Dietary fiber-mediated mechanisms in carcinogenesis. Cancer Res. 1992, 52 (Suppl. S7), 2055s–2059s. [Google Scholar]
- Pool-Zobel, B.; van Loo, J.; Rowland, I.; Roberfroid, M.B. Experimental evidences on the potential of prebiotic fructans to reduce the risk of colon cancer. Br. J. Nutr. 2002, 87 (Suppl. S2), S273–S281. [Google Scholar] [CrossRef] [PubMed]
- Calman, K. Why are small bowel tumours rare? An experimental model. Gut 1974, 15, 552–554. [Google Scholar] [CrossRef][Green Version]
- Ghosh, P.; Hutadilok, N. Interactions of pentosan polysulfate with cartilage matrix proteins and synovial fibroblasts derived from patients with osteoarthritis. Osteoarthr. Cartil. 1996, 4, 43–53. [Google Scholar] [CrossRef][Green Version]
- Wu, J.; Shimmon, S.; Paton, S.; Daly, C.; Goldschlager, T.; Gronthos, S.; Zannettino, A.C.W.; Ghosh, P. Pentosan polysulfate binds to STRO-1+ mesenchymal progenitor cells, is internalized, and modifies gene expression: A novel approach of pre-programing stem cells for therapeutic application requiring their chondrogenesis. Stem. Cell Res. 2017, 8, 278. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Merchán, E. Molecular Mechanisms of Cartilage Repair and Their Possible Clinical Uses: A Review of Recent Developments. Int. J. Mol. Sci. 2022, 23, 14272. [Google Scholar] [CrossRef] [PubMed]
- Bwalya, E.; Kim, S.; Fang, J.; Wijekoon, H.M.S.; Hosoya, K.; Okumura, M. Effects of pentosan polysulfate and polysulfated glycosaminoglycan on chondrogenesis of canine bone marrow-derived mesenchymal stem cells in alginate and micromass culture. J. Vet. Med. Sci. 2017, 79, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Goldschlager, T.; Ghosh, P.; Zannettino, A.; Gronthos, S.; Rosenfeld, J.V.; Itescu, S.; Jenkin, G. Cervical motion preservation using mesenchymal progenitor cells and pentosan polysulfate, a novel chondrogenic agent: Preliminary study in an ovine model. Neurosurg. Focus 2010, 28, E4. [Google Scholar] [CrossRef] [PubMed]
- Ofosu, F.; Blajchman, M.A.; Modi, G.J.; Smith, L.M.; Buchanan, M.R.; Hirsh, J. The importance of thrombin inhibition for the expression of the anticoagulant activities of heparin, dermatan sulphate, low molecular weight heparin and pentosan polysulphate. Br. J. Haematol. 1985, 60, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.; Kakkar, V.V. The antiheparin effect of a heparinoid, pentosan polysulphate. Investigation of a mechanism. Biochem. J. 1984, 218, 657–665. [Google Scholar] [CrossRef]
- Fischer, A.; Dautzenberg, M.D.; Aurousseau, M.H.; Béguin, S.; Goudemand, J.; Hemker, H.C. Comparison between the effect of pentosan polysulphate heparin and antithrombin III injections in antithrombin III deficient patients. Thromb. Res. 1985, 37, 295–307. [Google Scholar] [CrossRef]
- Scully, M.; Kakkar, V.V. Effect of a pentosan polysulphate upon thrombin and factor Xa inactivation by antithrombin III. Biochem. J. 1984, 222, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Neese, L.; Pratt, C.W.; Church, F.C. Modulation of protein C inhibitor activity. Blood Coagul. Fibrinolysis 1994, 5, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Pratt, C.W.; Whinna, H.C.; Church, F.C. A comparison of three heparin-binding serine proteinase inhibitors. J. Biol. Chem. 1992, 267, 8795–8801. [Google Scholar] [CrossRef]
- Pratt, C.; Church, F.C. General features of the heparin-binding serpins antithrombin, heparin cofactor II and protein C inhibitor. Blood Coagul. Fibrinolysis 1993, 4, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Wagenvoord, R.; Hendrix, H.; Soria, C.; Hemker, H.C. Localization of the inhibitory site(s) of pentosan polysulphate in blood coagulation. Thromb. Haemost. 1988, 60, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Kohli, S.; Shahzad, K.; Jouppila, A.; Holthöfer, H.; Isermann, B.; Lassila, R. Thrombosis and Inflammation-A Dynamic Interplay and the Role of Glycosaminoglycans and Activated Protein C. Front. Cardiovasc. Med. 2022, 9, 866751. [Google Scholar] [CrossRef]
- Bhakuni, T.; Ali, M.F.; Ahmad, I.; Bano, S.; Ansari, S.; Jairajpuri, M.A. Role of heparin and non heparin binding serpins in coagulation and angiogenesis: A complex interplay. Arch. Biochem. Biophys. 2016, 604, 128–142. [Google Scholar] [CrossRef]
- Gray, E.; Hogwood, J.; Mulloy, B. The anticoagulant and antithrombotic mechanisms of heparin. Handb. Exp. Pharm. 2012, 207, 43–61. [Google Scholar]
- Schwarz, N.; Müller, J.; Yadegari, H.; McRae, H.L.; Reda, S.; Hamedani, N.S.; Oldenburg, J.; Pötzsch, B.; Rühl, H. Ex Vivo Modeling of the PC (Protein C) Pathway Using Endothelial Cells and Plasma: A Personalized Approach. Arter. Thromb. Vasc. Biol. 2023, 43, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Esmon, C. The protein C pathway. Chest 2003, 124 (Suppl. S3), 26S–32S. [Google Scholar] [CrossRef]
- Okamoto, T.; Tanigami, H.; Suzuki, K.; Shimaoka, M. Thrombomodulin: A bifunctional modulator of inflammation and coagulation in sepsis. Crit. Care Res. Pract. 2012, 2012, 614545. [Google Scholar] [CrossRef]
- Yang, L.; Manithody, C.; Walston, T.D.; Cooper, S.T.; Rezaie, A.R. Thrombomodulin enhances the reactivity of thrombin with protein C inhibitor by providing both a binding site for the serpin and allosterically modulating the activity of thrombin. J. Biol. Chem. 2003, 278, 37465–37470. [Google Scholar] [CrossRef]
- Li, W.; Huntington, J.A. The heparin binding site of protein C inhibitor is protease-dependent. J. Biol. Chem. 2008, 283, 36039–36045. [Google Scholar] [CrossRef] [PubMed]
- Freyssinet, J.; Wiesel, M.L.; Grunebaum, L.; Pereillo, J.M.; Gauchy, J.; Schuhler, S.; Freund, G.; Cazenave, J.P. Activation of human protein C by blood coagulation factor Xa in the presence of anionic phospholipids. Enhancement by sulphated polysaccharides. Biochem. J. 1989, 261, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.; Chao, L. Biochemistry, regulation and potential function of kallistatin. Biol. Chem. Hoppe Seyler 1995, 376, 705–713. [Google Scholar] [PubMed]
- Chao, J.; Bledsoe, G.; Chao, L. Protective Role of Kallistatin in Vascular and Organ Injury. Hypertension 2016, 68, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.; Li, P.; Chao, L. Kallistatin suppresses cancer development by multi-factorial actions. Crit. Rev. Oncol. Hematol. 2017, 113, 71–78. [Google Scholar] [CrossRef]
- Chao, J.; Guo, Y.; Chao, L. Protective Role of Endogenous Kallistatin in Vascular Injury and Senescence by Inhibiting Oxidative Stress and Inflammation. Oxid. Med. Cell Longev. 2018, 2018, 4138560. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Qian, L.L.; Zhu, Y.; Jiang, Y.; Guo, J.Q.; Wu, Y.; Yang, Z.W.; Yao, Y.Y.; Ma, G.S. Kallistatin/Serpina3c inhibits cardiac fibrosis after myocardial infarction by regulating glycolysis via Nr4a1 activation. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1861, 166441. [Google Scholar] [CrossRef]
- Wu, H.; Li, R.; Zhang, Z.; Jiang, H.; Ma, H.; Yuan, C.; Sun, C.; Li, Y.; Kong, B. Kallistatin inhibits tumour progression and platinum resistance in high-grade serous ovarian cancer. J. Ovarian Res. 2019, 12, 125. [Google Scholar] [CrossRef]
- Yiu, W.; Li, Y.; Lok, S.W.Y.; Chan, K.W.; Chan, L.Y.Y.; Leung, J.C.K.; Lai, K.N.; Tsu, J.H.L.; Chao, J.; Huang, X.R.; et al. Protective role of kallistatin in renal fibrosis via modulation of Wnt/β-catenin signaling. Clin. Sci. 2021, 135, 429–446. [Google Scholar] [CrossRef]
- Yin, H.; Gao, L.; Shen, B.; Chao, L.; Chao, J. Kallistatin inhibits vascular inflammation by antagonizing tumor necrosis factor-alpha-induced nuclear factor kappaB activation. Hypertension 2010, 56, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Engelberg, H. Actions of heparin that may affect the malignant process. Cancer 1999, 85, 257–272. [Google Scholar] [CrossRef]
- Pintus, G.; Tadolini, B.; Maioli, M.; Posadino, A.M.; Bennardini, F.; Bettuzzi, S.; Ventura, C. Heparin inhibits phorbol ester-induced ornithine decarboxylase gene expression in endothelial cells. FEBS Lett. 1998, 423, 98–104. [Google Scholar] [CrossRef]
- Wright, T.; Pukac, L.A.; Castellot, J.J.; Karnovsky, M.J.; Levine, R.A.; Kim-Park, H.-Y.; Campisi, J. Heparin suppresses the induction of c-fos and c-myc mRNA in murine fibroblasts by selective inhibition of a protein kinase C-dependent pathway. Proc. Natl. Acad. Sci. USA 1989, 86, 3199–3203. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Kindy, M.S.; Sonenstein, G.E. Expression of the c-myb proto-oncogene in bovine vascular smooth muscle cells. J. Biol. Chem. 1992, 267, 4625–4630. [Google Scholar] [CrossRef] [PubMed]
- Busch, S.; Martin, G.A.; Barnhart, R.L.; Mano, M.; Cardin, A.D.; Jackson, R.L. Trans-repressor activity of nuclear glycosaminoglycans on Fos and Jun/AP-1 oncoprotein-mediated transcription. J. Cell. Biol. 1992, 116, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Castellot, J.J.; Pukac, L.A.; Caleb, B.L.; Wright, T.C., Jr.; Karnovsky, M.J. Heparin selectively inhibits a protein kinase C-dependent mechanism of cell cycle progression in calf aortic smooth muscle cells. J. Cell. Biol. 1989, 109 Pt 1, 3147–3155. [Google Scholar] [CrossRef]
- Dratwa, M.; Wysoczańska, B.; Łacina, P.; Kubik, T.; Bogunia-Kubik, K. TERT-Regulation and Roles in Cancer Formation. Front. Immunol. 2020, 11, 589929. [Google Scholar] [CrossRef] [PubMed]
- Higgins, W.; Fox, D.M.; Kowalski, P.S.; Nielsen, J.E.; Worrall, D.M. Heparin enhances serpin inhibition of the cysteine protease cathepsin L. J. Biol. Chem. 2010, 285, 3722–3729. [Google Scholar] [CrossRef]
- Bauman, S.; Church, F.C. Enhancement of heparin cofactor II anticoagulant activity. J. Biol. Chem. 1999, 274, 34556–34565. [Google Scholar] [CrossRef] [PubMed]
- Kurahashi, K.; Inoue, S.; Yoshida, S.; Ikeda, Y.; Morimoto, K.; Uemoto, R.; Ishikawa, K.; Kondo, T.; Yuasa, T.; Endo, I.; et al. The Role of Heparin Cofactor Ⅱ in the Regulation of Insulin Sensitivity and Maintenance of Glucose Homeostasis in Humans and Mice. J. Atheroscler. Thromb. 2017, 24, 1215–1230. [Google Scholar] [CrossRef]
- Cool, S.; Nurcombe, V. The osteoblast-heparan sulfate axis: Control of the bone cell lineage. Int. J. Biochem. Cell Biol. 2005, 37, 1739–1745. [Google Scholar] [CrossRef]
- Eikelboom, J.; Hankey, G.J. Low molecular weight heparins and heparinoids. Med. J. Aust. 2002, 177, 379–383. [Google Scholar] [CrossRef]
- Jochmann, K.; Bachvarova, V.; Vortkamp, A. Heparan sulfate as a regulator of endochondral ossification and osteochondroma development. Matrix Biol. 2014, 34, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Kram, V.; Zcharia, E.; Yacoby-Zeevi, O.; Metzger, S.; Chajek-Shaul, T.; Gabet, Y.; Müller, R.; Vlodavsky, I.; Bab, I. Heparanase is expressed in osteoblastic cells and stimulates bone formation and bone mass. J. Cell. Physiol. 2006, 207, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, S.; Inubushi, T.; Irie, F.; Takigami, I.; Matsumoto, K.; Shimizu, K.; Akiyama, H.; Yamaguchi, Y. Osteoblastic heparan sulfate regulates osteoprotegerin function and bone mass. JCI Insight 2018, 3, e89624. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, S.; Feng, D.; Liu, Y.; Wang, Q.; Gao, T.; Liu, Z.; Zhang, Y.; Chen, J.; Qiu, L. Biological role of heparan sulfate in osteogenesis: A review. Carbohydr. Polym. 2021, 272, 118490. [Google Scholar] [CrossRef]
- Todosenko, N.; Yurova, K.; Khaziakhmatova, O.; Malashchenko, V.; Khlusov, I.; Litvinova, L. Heparin and Heparin-Based Drug Delivery Systems: Pleiotropic Molecular Effects at Multiple Drug Resistance of Osteosarcoma and Immune Cells. Pharmaceutics 2022, 14, 2181. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Luo, Y.; Xu, D.; Ke, X.; Ci, T. Low molecular weight heparin modified bone targeting liposomes for orthotopic osteosarcoma and breast cancer bone metastatic tumors. Int. J. Biol. Macromol. 2020, 164, 2583–2597. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Cao, D.; Liu, Y.; Wang, H.; Ke, X.; Ci, T. Low molecular weight heparin-based reduction-sensitive nanoparticles for antitumor and anti-metastasis of orthotopic breast cancer. Biomater. Sci. 2018, 6, 2172–2188. [Google Scholar] [CrossRef]
- Sun, H.; Cao, D.; Wu, H.; Liu, H.; Ke, X.; Ci, T. Development of low molecular weight heparin based nanoparticles for metastatic breast cancer therapy. Int. J. Biol. Macromol. 2018, 112, 343–355. [Google Scholar] [CrossRef]
- Ibrahim, S.; Osman, R.; Awad, G.A.; Mortada, N.D.; Geneidy, A.S. Low molecular weight heparins for current and future uses: Approaches for micro- and nano-particulate delivery. Drug Deliv. 2016, 23, 2661–2667. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; McGee, A.; Strauss, E.; Youm, T.; Jazrawi, L. Subchondral Calcium Phosphate is Ineffective for Bone Marrow Edema Lesions in Adults With Advanced Osteoarthritis. Clin. Orthop. Relat. Res. 2015, 473, 2334–2342. [Google Scholar] [CrossRef] [PubMed]
- Wijekoon, S.; Sunaga, T.; Wang, Y.; Mwale, C.; Kim, S.; Okumura, M. Pentosan polysulfate regulates hepcidin 1-facilitated forman and function of osteoclast derived from canine bone marrow. PLoS ONE 2022, 17, e0265596. [Google Scholar] [CrossRef] [PubMed]
- Asperti, M.; Denardo, A.; Gryzik, M.; Castagna, A.; Girelli, D.; Naggi, A.; Arosio, P.; Poli, M. Pentosan polysulfate to control hepcidin expression in vitro and in vivo. Biochem. Pharm. 2020, 175, 113867. [Google Scholar] [CrossRef] [PubMed]
- Schuchman, E.; Ge, Y.; Lai, A.; Borisov, Y.; Faillace, M.; Eliyahu, E.; He, X.; Iatridis, J.; Vlassara, H.; Striker, G.; et al. Pentosan polysulfate: A novel therapy for the mucopolysaccharidoses. PLoS ONE 2013, 8, e54459. [Google Scholar] [CrossRef] [PubMed]
- Jayaranee, S.; Sthaneshwar, P.; Sokkalingam, S. Serum prohepcidin concentrations in rheumatoid arthritis. Pathology 2009, 41, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Koca, S.; Isik, A.; Ustundag, B.; Metin, K.; Aksoy, K. Serum pro-hepcidin levels in rheumatoid arthritis and systemic lupus erythematosus. Inflammation 2008, 31, 146–153. [Google Scholar] [CrossRef]
- Masson, C. Rheumatoid anemia. Jt. Bone Spine 2011, 78, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, A.; Zhang, H.; Liu, B.; Geng, Y.; Xu, Y.; Zuo, G.; Jia, P. Iron overload induced osteocytes apoptosis and led to bone loss in Hepcidin-/- mice through increasing sclerostin and RANKL/OPG. Bone 2022, 164, 116511. [Google Scholar] [CrossRef]
- Sato, H.; Takai, C.; Kazama, J.J.; Wakamatsu, A.; Hasegawa, E.; Kobayashi, D.; Kondo, N.; Nakatsue, T.; Abe, A.; Ito, S.; et al. Serum hepcidin level, iron metabolism and osteoporosis in patients with rheumatoid arthritis. Sci. Rep. 2020, 10, 9882. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, W.; Song, S.; Li, J.C.; Gan, K.; Shen, C.; Holzbeierlein, J.; Li, B. The iron-modulating hormone hepcidin is upregulated and associated with poor survival outcomes in renal clear cell carcinoma. Front. Pharm. 2022, 13, 1080055. [Google Scholar] [CrossRef] [PubMed]
- Mathew, M.; Sivaprakasam, S.; Phy, J.L.; Bhutia, Y.D.; Ganapathy, V. Polycystic ovary syndrome and iron overload: Biochemical link and underlying mechanisms with potential novel therapeutic avenues. Biosci. Rep. 2022, 43, BSR20212234. [Google Scholar] [CrossRef]
- Luo, G.; Xiang, L.; Xiao, L. Iron Restriction Alleviates Atherosclerosis in ApoE KO Mice: An iTRAQ Proteomic Analysis. Int. J. Mol. Sci. 2022, 23, 15915. [Google Scholar] [CrossRef]
- Wijekoon, S.; Tsogbadrakh, M.; Sunaga, T.; Wang, Y.; Mwale, C.; Kim, S.; Alimaa, D.; Okumura, M. Pentosan polysulfate regulates hepcidin expression in native Mongolian horses. J. Vet. Med. Sci. 2022, 84, 1437–1441. [Google Scholar] [CrossRef] [PubMed]
- Rha, S.; Noh, S.H.; Kim, T.S.; Yoo, N.C.; Roh, J.K.; Min, J.S.; Kim, B.S. Modulation of biological phenotypes for tumor growth and metastasis by target-specific biological inhibitors in gastric cancer. Int. J. Mol. Med. 1999, 4, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Chun, S.Y.; Lee, E.H.; Chung, J.W.; Lee, J.N.; Ha, Y.S.; Choi, J.Y.; Song, P.H.; Kwon, T.G.; Han, M.H.; et al. Efficacy of combination therapy with pentosan polysulfate sodium and adipose tissue-derived stem cells for the management of interstitial cystitis in a rat model. Stem. Cell Res. 2020, 45, 101801. [Google Scholar] [CrossRef]
- Lee, E.; Pavy, M.; Young, N.; Freeman, C.; Lobigs, M. Antiviral effect of the heparan sulfate mimetic, PI-88, against dengue and encephalitic flaviviruses. Antivir. Res. 2006, 69, 31–38. [Google Scholar] [CrossRef]
- Andrei, G.; De Clercq, E. Inhibitory effect of selected antiviral compounds on arenavirus replication in vitro. Antivir. Res. 1990, 14, 287–299. [Google Scholar] [CrossRef]
- Baba, M.; Snoeck, R.; Pauwels, R.; De Clercq, E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob. Agents Chemother. 1988, 32, 1742–1745. [Google Scholar] [CrossRef]
- Andrei, G.; Snoeck, R.; Schols, D.; Goubau, P.; Desmyter, J.; De Clercq, E. Comparative activity of selected antiviral compounds against clinical isolates of human cytomegalovirus. Eur. J. Clin. Microbiol. Infect. Dis. 1991, 10, 1026–1033. [Google Scholar] [CrossRef]
- Nyberg, K.; Ekblad, M.; Bergström, T.; Freeman, C.; Parish, C.; Ferro, V.; Trybala, E. The low molecular weight heparan sulfate-mimetic, PI-88, inhibits cell-to-cell spread of herpes simplex virus. Antivir. Res. 2004, 63, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Schmidtke, M.; Karger, A.; Meerbach, A.; Egerer, R.; Stelzner, A.; Makarov, V. Binding of a N,N′-bisheteryl derivative of dispirotripiperazine to heparan sulfate residues on the cell surface specifically prevents infection of viruses from different families. Virology 2003, 311, 134–143. [Google Scholar] [CrossRef]
- Esté, J.; De Vreese, K.; Witvrouw, M.; Schmit, J.-C.; Vandamme, A.-M.; Anné, J.; Desmyter, J.; Henson, G.W.; Bridger, G.; De Clercq, E. Antiviral activity of the bicyclam derivative JM3100 against drug-resistant strains of human immunodeficiency virus type 1. Antivir. Res. 1996, 29, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Schols, D.; Pauwels, R.; Desmyter, J.; De Clercq, E. Dextran sulfate and other polyanionic anti-HIV compounds specifically interact with the viral gp120 glycoprotein expressed by T-cells persistently infected with HIV-1. Virology 1990, 175, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Schols, D.; Pauwels, R.; Baba, M.; Desmyter, J.; De Clercq, E. Syncytium formation and destruction of bystander CD4+ cells cocultured with T cells persistently infected with human immunodeficiency virus as demonstrated by flow cytometry. J. Gen. Virol. 1989, 70, 2397–2408. [Google Scholar] [CrossRef]
- Nakashima, H.; Balzarini, J.; Pauwels, R.; Schols, D.; Desmyter, J.; De Clercq, E. Anti-HIV-1 activity of antiviral compounds, as quantitated by a focal immunoassay in CD4+ HeLa cells and a plaque assay in MT-4 cells. J. Virol. Methods 1990, 29, 197–208. [Google Scholar] [CrossRef]
- Lanza, P.; Washington, L.C.; Zanetti, M. A method to analyze the interaction between gp120 of human immunodeficiency virus and CD4. Viral. Immunol. 1992, 5, 305–310. [Google Scholar] [CrossRef]
- Esté, J.A.; Schols, D.; De Vreese, K.; Van Laethem, K.; Vandamme, A.-M.; Desmyter, J.; De Clercq, E. Development of resistance of human immunodeficiency virus type 1 to dextran sulfate associated with the emergence of specific mutations in the envelope gp120 glycoprotein. Mol. Pharm. 1997, 52, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Parish, C.; Low, L.; Warren, H.S.; Cunningham, A.L. A polyanion binding site on the CD4 molecule. Proximity to the HIV-gp120 binding region. J. Immunol. 1990, 145, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Schols, D.; Baba, M.; Pauwels, R.; Desmyter, J.; De Clercq, E. Specific interaction of aurintricarboxylic acid with the human immunodeficiency virus/CD4 cell receptor. Proc. Natl. Acad. Sci. USA 1989, 86, 3322–3326. [Google Scholar] [CrossRef] [PubMed]
- Thiele, B.; Braig, H.R.; Ehm, I.; Kunze, R.; Ruf, B. Influence of sulfated carbohydrates on the accessibility of CD4 and other CD molecules on the cell surface and implications for human immunodeficiency virus infection. Eur. J. Immunol. 1989, 19, 1161–1164. [Google Scholar] [CrossRef]
- Witvrouw, M.; De Clercq, E. Sulfated polysaccharides extracted from sea algae as potential antiviral drugs. Gen. Pharmacol. Vasc. Syst. 1997, 29, 497–511. [Google Scholar] [CrossRef]
- Lynch, G.; Low, L.; Li, S.; Sloane, A.; Adams, S.; Parish, C.; Kemp, B.; Cunningham, A.L. Sulfated polyanions prevent HIV infection of lymphocytes by disruption of the CD4-gp120 interaction, but do not inhibit monocyte infection. J. Leukoc. Biol. 1994, 56, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Mondor, I.; Ugolini, S.; Sattentau, Q.J. Human immunodeficiency virus type 1 attachment to HeLa CD4 cells is CD4 independent and gp120 dependent and requires cell surface heparans. J. Virol. 1998, 72, 3623–3634. [Google Scholar] [CrossRef] [PubMed]
- Cos, P.; Maes, L.; Vlietinck, A.; Pieters, L. Plant-Derived Leading Compounds for Chemotherapy of Human Immunodefiency Virus (HIV) Infection—An Update (1998–2007). Planta. Med. 2008, 74, 1323–1337. [Google Scholar] [CrossRef]
- Mann, D.; Frankel, A.D. Endocytosis and targeting of exogenous HIV-1 Tat protein. EMBO J. 1991, 10, 1733–1739. [Google Scholar] [CrossRef]
- Moelling, K.; Schulze, T.; Diringer, H. Inhibition of human immunodeficiency virus type 1 RNase H by sulfated polyanions. J. Virol. 1989, 63, 5489–5491. [Google Scholar] [CrossRef] [PubMed]
- Neyts, J.; Snoeck, R.; Schols, D.; Balzarini, J.; Esko, J.D.; Van Schepdael, A.; De Clercq, E. Sulfated polymers inhibit the interaction of human cytomegalovirus with cell surface heparan sulfate. Virology 1992, 189, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Dogra, P.; Martin, E.B.; Williams, A.; Richardson, R.L.; Foster, J.S.; Hackenback, N.; Kennel, S.J.; Sparer, T.E.; Wall, J.S. Novel heparan sulfate-binding peptides for blocking herpesvirus entry. PLoS ONE 2015, 10, e0126239. [Google Scholar] [CrossRef] [PubMed]
- Kaltenbach, D.D.; Jaishankar, D.; Hao, M.; Beer, J.C.; Volin, M.V.; Desai, U.R.; Tiwari, V. Sulfotransferase and heparanase: Remodeling engines in promoting virus infection and disease development. Front. Pharm. 2018, 9, 1315. [Google Scholar] [CrossRef]
- Tiwari, V.; Liu, J.; Valyi-Nagy, T.; Shukla, D. Anti-heparan sulfate peptides that block Herpes simplex virus infection in vivo. J. Biol. Chem. 2011, 286, 25406–25415. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.; Tarbutton, M.; Shukla, D. Diversity of heparan sulfate and HSV entry: Basic understanding and treatment strategies. Molecules 2015, 20, 2707–2727. [Google Scholar] [CrossRef]
- Baldwin, J.; Maus, E.; Zanotti, B.; Volin, M.V.; Tandon, R.; Shukla, D.; Tiwari, V. A role for 3-O-sulfated heparan sulfate in promoting human cytomegalovirus infection in human iris cells. J. Virol. 2015, 89, 5185–5192. [Google Scholar] [CrossRef] [PubMed]
- Compton, T.; Nowlin, D.M.; Cooper, N.R. Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate. Virology 1993, 193, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Elste, J.; Kaltenbach, D.; Patel, V.R.; Nguyen, M.T.; Sharthiya, H.; Tandon, R.; Mehta, S.K.; Volin, M.V.; Fornaro, M.; Tiwari, V.; et al. Inhibition of human cytomegalovirus entry into host cells through a pleiotropic small molecule. Int. J. Mol. Sci. 2020, 21, 1676. [Google Scholar] [CrossRef]
- Iqbal, M.; Flick-Smith, H.; McCauley, J.W. Interactions of bovine viral diarrhoea virus glycoprotein E(rns) with cell surface glycosaminoglycans. J. Gen. Virol. 2000, 81 Pt 2, 451–459. [Google Scholar]
- Zautner, A.; Körner, U.; Henke, A.; Badorff, C.; Schmidtke, M. Heparan sulfates and coxsackievirus-adenovirus receptor: Each one mediates coxsackievirus B3 PD infection. J. Virol. 2003, 77, 10071–10077. [Google Scholar] [CrossRef] [PubMed]
- Crance, J.; Gratier, D.; Guimet, J.; Jouan, A. Inhibition of sandfly fever Sicilian virus (Phlebovirus) replication in vitro by antiviral compounds. Res. Virol. 1997, 148, 353–365. [Google Scholar] [CrossRef]
- Pourianfar, H.; Kirk, K.; Grollo, L. Initial evidence on differences among Enterovirus 71, Coxsackievirus A16 and Coxsackievirus B4 in binding to cell surface heparan sulphate. Virus Dis. 2014, 25, 277–284. [Google Scholar] [CrossRef]
- Pourianfar, H.; Poh, C.L.; Fecondo, J.; Grollo, L. In vitro evaluation of the antiviral activity of heparan sulfate mimetic compounds against Enterovirus 71. Virus Res. 2012, 169, 22–29. [Google Scholar] [CrossRef]
- Zhang, Y.; Schols, D.; De Clercq, E. Selective activity of various antiviral compounds against HHV-7 infection. Antivir. Res. 1999, 43, 23–35. [Google Scholar] [CrossRef]
- Hidari, K.; Abe, T.; Suzuki, T. Carbohydrate-related inhibitors of Dengue virus entry. Viruses 2013, 5, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Yim, J.H.; Kim, S.-Y.; Kim, H.S.; Lee, W.G.; Kim, S.J.; Kang, P.-S.; Lee, C.-K. In vitro inhibition of influenza A virus infection by marine microalga-derived sulfated polysaccharide p-KG03. Antivir. Res. 2012, 93, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Lüscher-Mattli, M.; Glück, R.; Kempf, C.; Zanoni-Grassi, M. A comparative study of the effect of dextran sulfate on the fusion and the in vitro replication of influenza A and B, Semliki Forest, vesicular stomatitis, rabies, Sendai, and mumps virus. Arch. Virol. 1993, 130, 317–326. [Google Scholar] [CrossRef]
- Pujol, C.; Carlucci, M.J.; Matulewicz, M.C.; Damonte, E.B. Natural sulfated polysaccharides for the prevention and control of viral infections. Top. Heterocycl. Chem. 2007, 11, 259–281. [Google Scholar]
- Kumar, S. COVID-19: A drug repurposing and biomarker identification by using comprehensive gene-disease associations through protein-protein interaction network analysis. Wwwpreprintsorg 2020. preprint. [Google Scholar]
- Basha, S. Corona virus drugs–a brief overview of past, present and future. J. Peer Sci. 2020, 2, e1000013. [Google Scholar]
- Fuming, Z.; He, P.; Rodrigues, A.L.; Jeske, W.; Tandon, R.; Bates, J.T.; Bierdeman, M.A.; Fareed, J.; Dordick, J.; Linhardt, R.J. Potential anti-SARS-CoV-2 activity of pentosan polysulfate and mucopolysaccharide polysulfate. Pharmaceuticals 2022, 15, 258. [Google Scholar]
- Nie, C.; Pouyan, P.; Lauster, D.; Trimpert, J.; Kerkhoff, Y.; Szekeres, G.P.; Wallert, M.; Block, S.; Sahoo, A.K.; Dernedde, J.; et al. Polysulfates Block SARS-CoV-2 Uptake through Electrostatic Interactions. Angew. Chem. Int. Ed. Engl. 2021, 60, 15870–15878. [Google Scholar] [CrossRef]
- Gessain, A.; Cassar, O. Epidemiological aspects and world distribution of HTLV-1 infection. Front. Microbiol. 2012, 3, 388. [Google Scholar] [CrossRef]
- Bangham, C.; Araujo, A.; Yamano, Y.; Taylor, G.P. HTLV-1-associated myelopathy/tropical spastic paraparesis. Nat. Rev. Dis. Prim. 2015, 1, 15012. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Satoh, K.; Fukuda, T.; Kinoshita, I.; Nishiura, Y.; Nagasato, K.; Yamauchi, A.; Kataoka, Y.; Nakamura, T.; Sasaki, H.; et al. Pentosan polysulfate treatment ameliorates motor function with increased serum soluble vascular cell adhesion molecule-1 in HTLV-1-associated neurologic disease. J. Neurovirol. 2014, 20, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Thormar, H.; Balzarini, J.; Debyser, Z.; Witvrouw, M.; Desmyter, J.; De Clercq, E. Inhibition of visna virus replication and cytopathic effect in sheep choroid plexus cell cultures by selected anti-HIV agents. Antivir. Res. 1995, 27, 49–57. [Google Scholar] [CrossRef]
- Hosoya, M.; Balzarini, J.; Shigeta, S.; De Clercq, E. Differential inhibitory effects of sulfated polysaccharides and polymers on the replication of various myxoviruses and retroviruses, depending on the composition of the target amino acid sequences of the viral envelope glycoproteins. Antimicrob. Agents Chemother. 1991, 35, 2515–2520. [Google Scholar] [CrossRef]
- Rudd, P.; Lim, E.X.Y.; Stapledon, C.J.M.; Krishnan, R.; Herrero, L.J. Pentosan polysulfate sodium prevents functional decline in chikungunya infected mice by modulating growth factor signalling and lymphocyte activation. PLoS ONE 2021, 16, e0255125. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Nayyar, S.; Galvin, T.A.; Merril, C.R.; Bigelow, L.B. Sodium pentosan polysulfate (PPS), an anti-HIV agent also exhibits synergism with AZT, lymphoproliferative activity, and virus enhancement. AIDS Res. Hum. Retrovir. 1990, 6, 679–689. [Google Scholar] [CrossRef]
- Bianculli, R.; Mase, J.D.; Schulz, M.D. Antiviral polymers: Past approaches and future possibilities. Macromolecules 2020, 53, 9158–9186. [Google Scholar] [CrossRef]
- Sun, E.; He, J.; Zhuang, X. Live cell imaging of viral entry. Curr. Opin. Virol. 2013, 3, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Sapp, M.; Bienkowska-Haba, M. Viral entry mechanisms: Human papillomavirus and a long journey from extracellular matrix to the nucleus. FEBS J. 2009, 276, 7206–7216. [Google Scholar] [CrossRef]
- Müller, W.; Schröder, H.C.; Neufurth, M.; Wang, X. An unexpected biomaterial against SARS-CoV-2: Bio-polyphosphate blocks binding of the viral spike to the cell receptor. Mater. Today 2021, 51, 504–524. [Google Scholar] [CrossRef]
- Muschin, T.; Budragchaa, D.; Kanamoto, T.; Nakashima, H.; Ichiyama, K.; Yamamoto, N.; Shuqin, H.; Yoshida, T. Chemically sulfated natural galactomannans with specific antiviral and anticoagulant activities. Int. J. Biol. Macromol. 2016, 89, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Neufurth, M.; Wang, X.; Tolba, E.; Lieberwirth, I.; Wang, S.; Schröder, H.C.; Müller, W.E.G. The inorganic polymer, polyphosphate, blocks binding of SARS-CoV-2 spike protein to ACE2 receptor at physiological concentrations. Biochem. Pharm. 2020, 182, 114215. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; He, P.; Rodrigues, A.L.; Datta, P.; Tandon, R.; Bates, J.T.; Bierdeman, M.A.; Chen, C.; Dordick, J.; Zhang, F.; et al. Anti-SARS-CoV-2 Activity of Rhamnan Sulfate from Monostroma nitidum. Mar. Drugs 2021, 19, 685. [Google Scholar] [CrossRef]
- Müller, W.; Wang, X.; Neufurth, M.; Schröder, H.C. Polyphosphate in Antiviral Protection: A Polyanionic Inorganic Polymer in the Fight Against Coronavirus SARS-CoV-2 Infection. Prog. Mol. Subcell Biol. 2022, 61, 145–189. [Google Scholar]
- Shi, D.; He, P.; Song, Y.; Cheng, S.; Linhardt, R.J.; Dordick, J.S.; Chi, L.; Zhang, F. Kinetic and Structural Aspects of Glycosaminoglycan-Monkeypox Virus Protein A29 Interactions Using Surface Plasmon Resonance. Molecules 2022, 27, 5898. [Google Scholar] [CrossRef] [PubMed]
| Property | Refs |
|---|---|
| Treatment of cystitis, painful bladder syndrome, chronic pelvic pain | [1,2,3,31] |
| Promotion of repair of the degenerate intervertebral disc | [61,67] |
| Regulation of Complement Activation | [50] |
| Modulation of vascular coagulation, fibrinolysis and thrombocytopenia | [11,50,53,55,56] |
| Stimulation of hyaluronan synthesis by synoviocytes, fibroblasts and chondrocytes | [57,58] |
| Inhibition of NGF production by osteocytes providing pain relief in OA/RA | [59] |
| Lipid removal from engorged subchondral blood vessels in OA/RA and pain alleviation | [8,15,17,45] |
| Regulation of cytokine and inflammatory mediator production in ARDS | [65,66,68,69] |
| Disrupts cell surface viral HS interactions, prevents host cell infection/viral replication | [65,66,68,70,71,72,73,74] |
| Anti-tumor agent in many cancer types | [62,75,76] |
| Promotes BM stromal MSC differentiation/proliferation/expansion in tissue repair progenitor cell lineages | [61,67,77] |
| Tissue protective protease inhibitor. Multifaceted exosite inhibitor of aggrecanases, inhibits ADAMTS4 in OA chondrocytes, improves inhibitory properties of TIMP-3. Inhibits IGFBP- 5 proteolysis in articular cartilage in OA preserving IGF-I and II levels, cartilage integrity and functional properties | [37,78,79] |
| Protects cartilage from degradation in tendon transection models of joint destabilization that induce OA | [41,42,44,80,81] |
| Cartilage protective effects of PPS arise from its stimulation of proteoglycan synthesis by chondrocytes cultured in the presence or absence of IL-1, and stimulation of HA synthesis by RA and OA synoviocytes. HA also has cell protective properties in the glycocalyx | [38,69] |
| PPS inhibits IL-1β-induced iNOS, c-Jun and HIF-1α upregulation in canine articular chondrocytes in OA models | [82] |
| Inhibitor of extracellular HIV-1 Tat (trans-activator of transcription) | [63,83] |
| Improves cardiac function and tissue protection from action of ADAMTS4 | [84] |
| Tissue protective properties in tendon PPS is a potent inhibitor of human granulocyte elastase, cathepsin B, cathepsin G, testicular and arterial hyaluronidase, N-acetylglucosaminidase | [85] |
| Protection of brain endothelial cells from damage by bacterial LPS-induced neuroinflammation | [64] |
| PPS inhibits inflammation and impedes progression of severe diabetic nephropathy | [65] |
| Amelioration of tissue fibrosis and inflammation through suppression of PI3K/AKT cell signaling | [66] |
| Decreases prostate smooth muscle cell proliferation and ECM production | [62] |
| PPS has tissue protective properties in chronic non-bacterial prostatitis | [86] |
| Virus | Inhibitor in Order of Potency | Study Type | References |
|---|---|---|---|
| African swine fever virus | λ-carrageenan, | IVL | [71] |
| PPS, | |||
| κ-carrageenan, | |||
| Fucoidin | |||
| Bovine viral diarrhea virus | PPS, | IVL | [252] |
| Fucoidin, | |||
| Suramin, | |||
| Heparin, | |||
| Dermatan sulfate | |||
| Coxsackievirus B3 | PPS, | IVL | [253] |
| Heparin | |||
| Coxsackievirus A16 | Heparin, | IVL | [255] |
| PPS | |||
| Dengue virus | Heparin, | IVL | [223] |
| PPS, | |||
| Suramin, | |||
| PI-88 | |||
| Enterovirus 71 | Heparin, | IVL | [255] |
| PPS | |||
| HIN1 influenza virus, | PPS, | IVL | [260] |
| H3N2 influenza virus | Dextran sulfate | ||
| Herpes simplex virus-1, Herpes simplex virus-2 | Dextran sulfate, | IVL | [226,227,228] |
| PI-88, | |||
| Heparin, | |||
| PPS | |||
| Herpes simplex virus, Human immunodeficiency virus-1, Vesicular stomatitis virus, Human cytomegalovirus | Dextran sulfate, | IVL | [225] |
| λ-carrageenan, | |||
| PPS, | |||
| Fucoidin, | |||
| κ-carrageenan, | |||
| Heparin, | |||
| Human cytomegalovirus | Dextran sulfate, | IVL | [226,244] |
| PPS, | |||
| Heparin | |||
| Human herpes virus 7 | PPS, | IVL | [257] |
| Dextran sulfate, | |||
| Heparin | |||
| Human immunodeficiency virus-1 | PPS, | IVL | [91] |
| Dextran sulfate, | |||
| Heparin, | |||
| Fucoidin, | |||
| λ-carrageenan | |||
| κ-carrageenan, | |||
| Human immunodeficiency virus-1 | PS, | IVL | [229] |
| Suamin, | [231] | ||
| Hepa | [233] | ||
| Dextran sulfate | [240] | ||
| Human immunodeficiency virus-1 | Dextran sulfate, | IVL | [233] |
| PPS, | [239] | ||
| Heparin, Fucoidin | [240] | ||
| Human T-cell leukemia virus type-1 | PPS | IVL | [74] |
| Japanese encephalitis virus | PPS, | IVL | [223] |
| Heparin, | |||
| PI-88, | |||
| Suramin | |||
| Junin virus, Tacaribe virus | Fucoidin, | IVL | [224] |
| λ-carrageenan, | |||
| Dextran sulfate, | |||
| PPS, | |||
| Heparin | |||
| Monkey pox virus | PPS | IVL | [281] |
| Sandfly fever Sicilian virus | Heparin, | IVL | [254] |
| Suramin, | |||
| PPS, | |||
| κ-carrageenan, | |||
| λ-carrageenan, | |||
| Dextran sulfate, | |||
| Fucoidin | |||
| SARS-CoV-2 | PPS, | IVL | [7,24,264,265] |
| Heparin | |||
| Visna virus | PPS | IVL | [269] |
| Chikungunya virus | PPS | PCL | [271] |
| Ross River virus, Chikungunya virus | PPS | PCL | [72] |
| Human T cell leukemia virus type-1 | PPS | PCL | [72] |
| AIDS-Karposi’s sarcoma Phase I CT | PPS | CT | [75] |
| AIDS-Karposi’s sarcoma Phase II CT | PPS | CT | [92] |
| Human T-cell leukemia virus type-1 | PPS | CT | [268] |
| Ross River Virus Induced Arthralgia Phase IIa | PPS | CT | [73] |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, M.M.; Melrose, J. Pentosan Polysulfate Affords Pleotropic Protection to Multiple Cells and Tissues. Pharmaceuticals 2023, 16, 437. https://doi.org/10.3390/ph16030437
Smith MM, Melrose J. Pentosan Polysulfate Affords Pleotropic Protection to Multiple Cells and Tissues. Pharmaceuticals. 2023; 16(3):437. https://doi.org/10.3390/ph16030437
Chicago/Turabian StyleSmith, Margaret M., and James Melrose. 2023. "Pentosan Polysulfate Affords Pleotropic Protection to Multiple Cells and Tissues" Pharmaceuticals 16, no. 3: 437. https://doi.org/10.3390/ph16030437
APA StyleSmith, M. M., & Melrose, J. (2023). Pentosan Polysulfate Affords Pleotropic Protection to Multiple Cells and Tissues. Pharmaceuticals, 16(3), 437. https://doi.org/10.3390/ph16030437






