Ethanolic Extract from Limonia acidissima L. Fruit Attenuates Serum Uric Acid Level via URAT1 in Potassium Oxonate-Induced Hyperuricemic Rats
Abstract
1. Introduction
2. Results
2.1. Phytochemical Profile
2.2. Antioxidant Profile
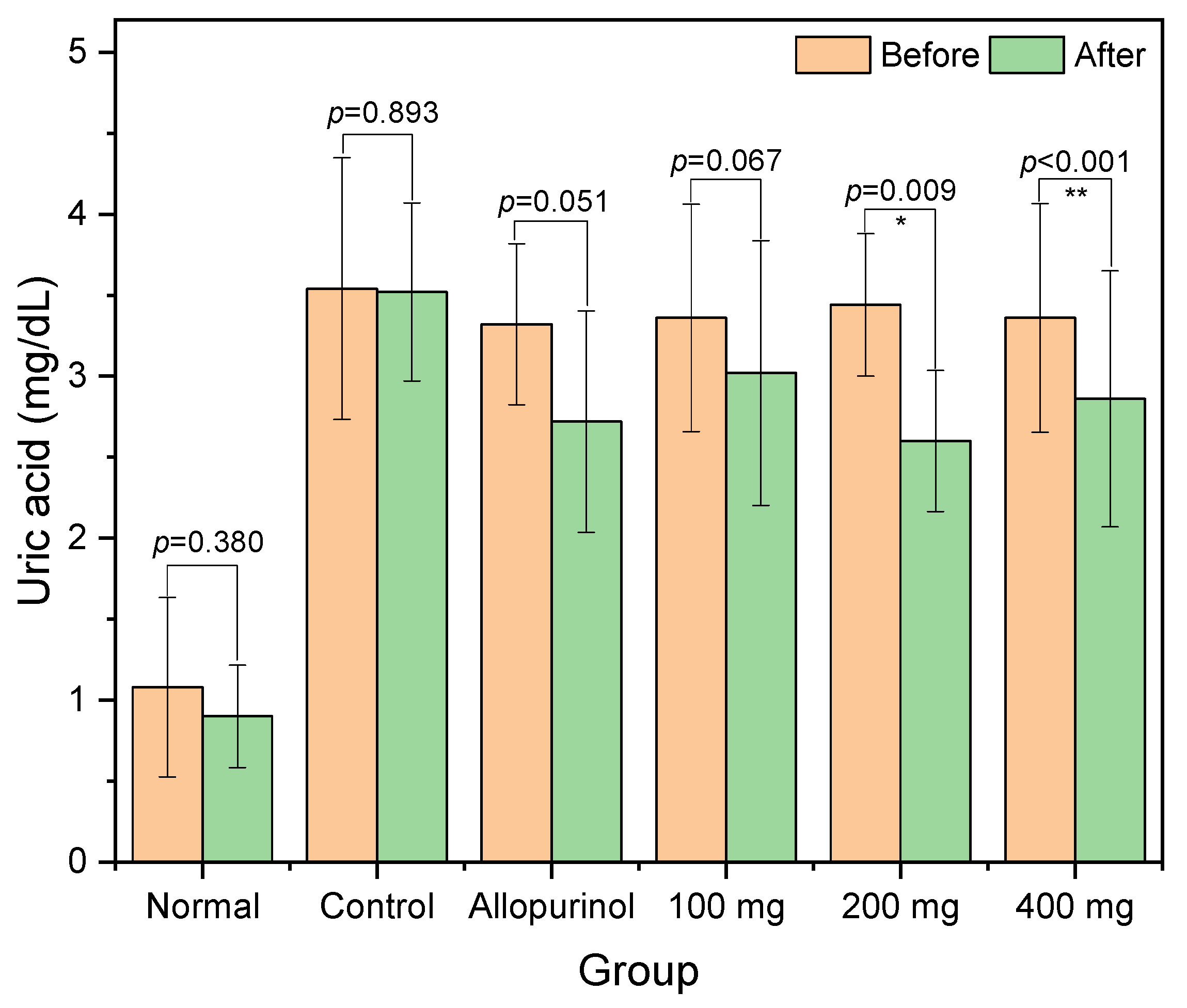
2.3. Serum Uric Acid
2.4. Kidney Parameters
2.5. Liver Parameters
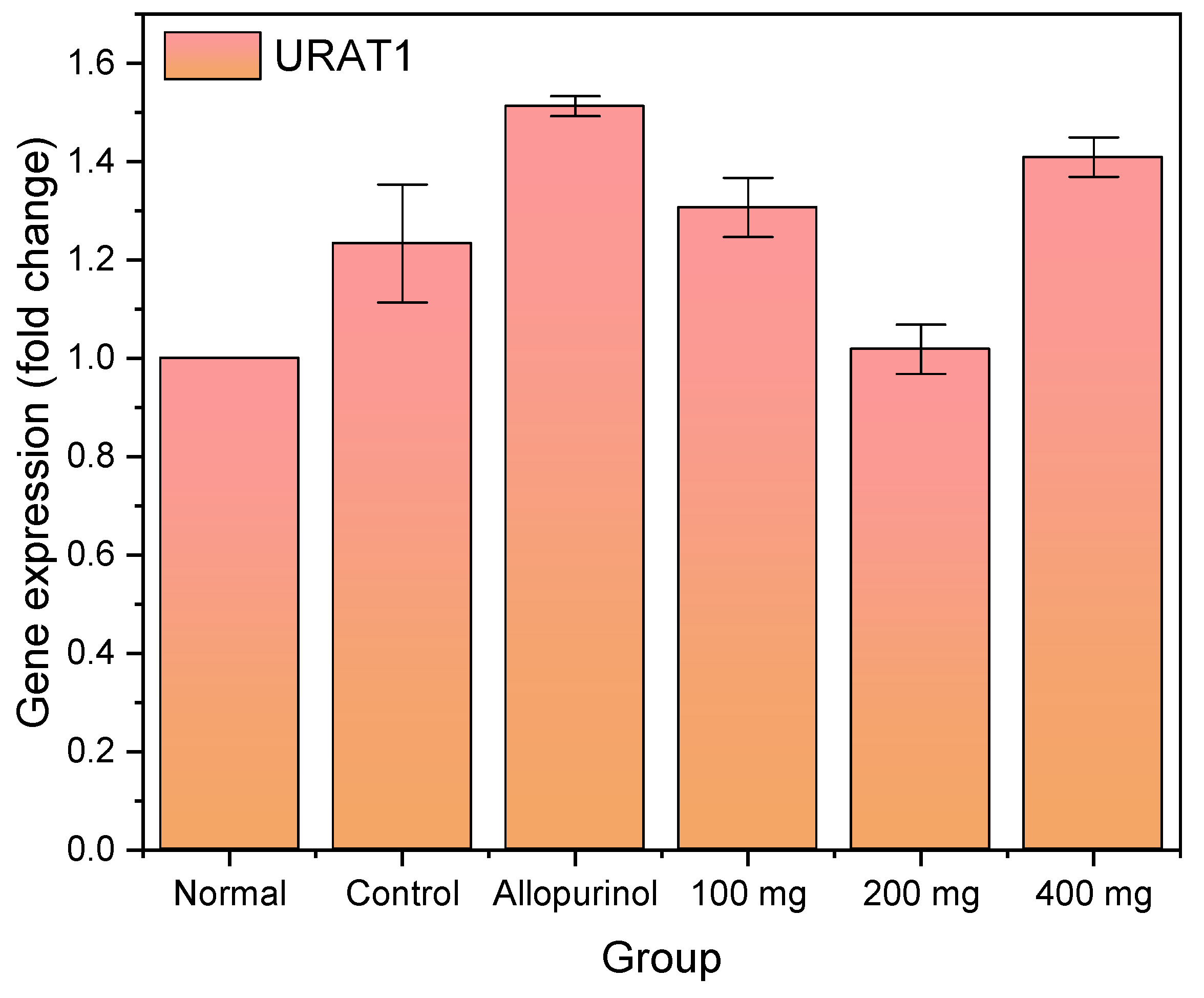
2.6. Expression Profile of URAT1
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Extraction
| Column oven temperature | 45 °C |
| Injection temperature | 280 °C |
| Pressure | 51.5 kPa |
| Total flow | 14 mL/min |
| Column flow | 1 mL/min |
| Linear velocity | 36.2 cm/s |
4.3. Animal Treatment
4.4. Determination of Serum Markers
4.5. Determination of URAT1 Gene Expression
4.6. DPPH Scavenging Assay
4.7. Determination of Total Phenolic and Flavonoid Contents
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Han, S.; Liu, F.; Chen, S.; Chen, X.; Chen, H. Global prevalence of hyperuricemia in adolescents from 2000 to 2019: A meta-analysis. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Ma, W.-G.; Wang, J.; Bu, X.-W.; Zhang, H.-H.; Zhang, J.-P.; Zhang, X.-X.; He, Y.-X.; Wang, D.-L.; Zhang, Z.-J.; Meng, F.-X. Effects of polygonum cuspidatum on AMPK-FOXO3α signaling pathway in rat model of uric acid-induced renal damage. Chin. J. Integr. Med. 2019, 25, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Gaubert, M.; Bardin, T.; Cohen-Solal, A.; Diévart, F.; Fauvel, J.-P.; Guieu, R.; Sadrin, S.; Maixent, J.M.; Galinier, M.; Paganelli, F. Hyperuricemia and hypertension, coronary artery disease, kidney disease: From concept to practice. Int. J. Mol. Sci. 2020, 21, 4066. [Google Scholar] [CrossRef]
- Sun, H.-L.; Wu, Y.-W.; Bian, H.-G.; Yang, H.; Wang, H.; Meng, X.-M.; Jin, J. Function of Uric Acid Transporters and Their Inhibitors in Hyperuricaemia. Front. Pharmacol. 2021, 12, 667753. [Google Scholar] [CrossRef] [PubMed]
- Gliozzi, M.; Malara, N.; Muscoli, S.; Mollace, V. The treatment of hyperuricemia. Int. J. Cardiol. 2016, 213, 23–27. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Cincione, R.I.; Tocci, G.; Borghi, C. Clinical Effects of Xanthine Oxidase Inhibitors in Hyperuricemic Patients. Med. Princ. Pract. 2021, 30, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Stamp, L.K.; Chapman, P.T. Allopurinol hypersensitivity: Pathogenesis and prevention. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101501. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, L.; Ren, L.; Xie, Y. Advances in structures required of polyphenols for xanthine oxidase inhibition. Food Front. 2020, 1, 152–167. [Google Scholar] [CrossRef]
- Haidari, F.; Keshavarz, S.A.; Rashidi, M.R.; Shahi, M.M. Orange juice and hesperetin supplementation to hyperuricemic rats alter oxidative stress markers and xanthine oxidoreductase activity. J. Clin. Biochem. Nutr. 2009, 45, 285–291. [Google Scholar] [CrossRef]
- Ooi, K.L.; Zakaria, R.; Tan, M.L.; Sulaiman, S.F. The influence of chemical composition of potent inhibitors in the hydrolyzed extracts of anti-hyperuricemic plants to their xanthine oxidase activities. J. Ethnopharmacol. 2021, 278, 114294. [Google Scholar] [CrossRef]
- Rahmi, E.P.; Kumolosasi, E.; Jalil, J.; Husain, K.; Buang, F.; Abd. Razak, A.F.; Jamal, J.A. Anti-hyperuricemic and anti-inflammatory effects of Marantodes pumilum as potential treatment for gout. Front. Pharmacol. 2020, 11, 289. [Google Scholar] [CrossRef]
- Sánchez-Lozada, L.G.; Soto, V.; Tapia, E.; Avila-Casado, C.; Sautin, Y.Y.; Nakagawa, T.; Franco, M.; Rodríguez-Iturbe, B.; Johnson, R.J. Role of oxidative stress in the renal abnormalities induced by experimental hyperuricemia. Am. J. Physiol.-Ren. Physiol. 2008, 295, F1134–F1141. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Xu, H.; Sun, Q.; Yu, X.; Chen, W.; Wei, H.; Jiang, J.; Xu, Y.; Lu, W. The Role of Oxidative Stress in Hyperuricemia and Xanthine Oxidoreductase (XOR) Inhibitors. Oxidative Med. Cell. Longev. 2021, 2021, 1470380. [Google Scholar] [CrossRef] [PubMed]
- Lamani, S.; Anu-Appaiah, K.A.; Murthy, H.N.; Dewir, Y.H.; Rihan, H.Z. Fatty Acid Profile, Tocopherol Content of Seed Oil, and Nutritional Analysis of Seed Cake of Wood Apple (Limonia acidissima L.), an Underutilized Fruit-Yielding Tree Species. Horticulturae 2021, 7, 275. [Google Scholar] [CrossRef]
- Priya Darsini, D.T.; Maheshu, V.; Vishnupriya, M.; Nishaa, S.; Sasikumar, J.M. Antioxidant potential and amino acid analysis of underutilized tropical fruit Limonia acidissima L. Free Radic. Antioxid. 2013, 3, S62–S69. [Google Scholar] [CrossRef]
- Ilango, K.; Chitra, V. Wound healing and anti-oxidant activities of the fruit pulp of Limonia acidissima Linn (Rutaceae) in rats. Trop. J. Pharm. Res. 2010, 9, 3. [Google Scholar] [CrossRef]
- Pradhan, D.; Tripathy, G.; Patanaik, S. Anticancer activity of Limonia acidissima Linn (Rutaceae) fruit extracts on human breast cancer cell lines. Trop. J. Pharm. Res. 2012, 11, 413–419. [Google Scholar] [CrossRef]
- Neelamadhab, P.; Patro, V.J.; Jena, B.K.; Panda, P. Evaluation of phytochemical and anti-microbial activity of ethanolic extract of Limonia acidissima L. leaves. Int. J. Herb. Med. 2013, 1, 22–27. [Google Scholar]
- Veryanti, P.R.; Kusuma, I.M. Uji efektivitas ekstrak buah kawista (Limonia acidissima) terhadap penurunan kadar asam urat darah pada mencit jantan. Media Farm. 2020, 17, 105–144. [Google Scholar] [CrossRef]
- Singh, K.G.; Ramya, G.S.; Purohit, D. In-vitro and ex-vivo studies on synergistic effects of Limonia acidissima and apple cider vinegar on anti-urolithiatic activity. Int. J. Pharm. Sci. Res. 2020, 11, 3347–3354. [Google Scholar]
- Quang, D.N.; Hashimoto, T.; Tanaka, M.; Takaoka, S.; Asakawa, Y. Tyromycic acids B− E, new lanostane triterpenoids from the mushroom Tyromyces fissilis. J. Nat. Prod. 2004, 67, 148–151. [Google Scholar] [CrossRef]
- Luo, L.-S.; Wang, Y.; Dai, L.-J.; He, F.-X.; Zhang, J.-L.; Zhou, Q. Triterpenoid acids from medicinal mushroom Inonotus obliquus (Chaga) alleviate hyperuricemia and inflammation in hyperuricemic mice: Possible inhibitory effects on xanthine oxidase activity. J. Food Biochem. 2022, 46, e13932. [Google Scholar] [CrossRef] [PubMed]
- de Sá Müller, C.M.; Coelho, G.B.; Araújo, M.C.d.P.M. Lychnophora pinaster ethanolic extract and its chemical constituents ameliorate hyperuricemia and related inflammation. J. Ethnopharmacol. 2019, 242, 112040. [Google Scholar] [CrossRef]
- Song, S.-H.; Park, D.-H.; Bae, M.-S.; Choi, C.-Y.; Shim, J.-H.; Yoon, G.; Cho, Y.-C.; Oh, D.-S.; Yoon, I.-S.; Cho, S.-S. Ethanol Extract of Cudrania tricuspidata Leaf Ameliorates Hyperuricemia in Mice via Inhibition of Hepatic and Serum Xanthine Oxidase Activity. Evid.-Based Complement. Altern. Med. 2018, 2018, 8037925. [Google Scholar] [CrossRef]
- Mahour, K.; Mishra, A.; Kumar, A.; Vihan, V. Preliminary Pharmacognostical and phytochemical investigation on Feronia elephantum corr. Fruit. J. Pharm. Res. 2008, 1, 45–48. [Google Scholar]
- Toyoda, Y.; Takada, T.; Saito, H.; Hirata, H.; Ota-Kontani, A.; Tsuchiya, Y.; Suzuki, H. Identification of Inhibitory Activities of Dietary Flavonoids against URAT1, a Renal Urate Re-Absorber: In Vitro Screening and Fractional Approach Focused on Rooibos Leaves. Nutrients 2022, 14, 575. [Google Scholar] [CrossRef]
- Akhigbe, R.E.; Hamed, M.A.; Odetayo, A.F.; Akhigbe, T.M.; Ajayi, A.F.; Ajibogun, F.A.H. Omega-3 fatty acid rescues ischaemia/perfusion-induced testicular and sperm damage via modulation of lactate transport and xanthine oxidase/uric acid signaling. Biomed. Pharmacother. 2021, 142, 111975. [Google Scholar] [CrossRef]
- Zhang, B.; Zeng, M.; Wang, Y.; Li, M.; Wu, Y.; Xu, R.; Zhang, Q.; Jia, J.; Huang, Y.; Zheng, X. Oleic acid alleviates LPS-induced acute kidney injury by restraining inflammation and oxidative stress via the Ras/MAPKs/PPAR-γ signaling pathway. Phytomedicine 2022, 94, 153818. [Google Scholar] [CrossRef]
- Deb, S.; Sakharkar, P. A Population Based Study of Liver Function amongst Adults with Hyperuricemia and Gout in the United States. Diseases 2021, 9, 61. [Google Scholar] [CrossRef]
- Rodríguez, F.; Aller, R.; ML, G.G.; Ampuero, J.; Gómez-Camarero, J.; Martín-Mateos, R.; Burgos-Santamaría, D.; Rosales, J.M.; Aspichueta, P.; Buque, X. Higher levels of serum uric acid influences hepatic damage in patients with non-alcoholic fatty liver disease (NAFLD). Rev. Esp. Enferm. Dig. Organo Of. Soc. Esp. Patol. Dig. 2019, 111, 264–269. [Google Scholar]
- Ali, S.A.; Sharief, N.H.; Mohamed, Y.S. Hepatoprotective activity of some medicinal plants in Sudan. Evid.-Based Complement. Altern. Med. 2019, 2019, 2196315. [Google Scholar] [CrossRef] [PubMed]
- Chitra, V. Hepatoprotective and antioxidant activities of fruit pulp of Limonia acidissima Linn. Int. J. Health Res. 2009, 2, 4. [Google Scholar]
- Lee, C.-T.; Chang, L.-C.; Liu, C.-W.; Wu, P.-F. Negative correlation between serum uric acid and kidney URAT1 mRNA expression caused by resveratrol in rats. Mol. Nutr. Food Res. 2017, 61, 1601030. [Google Scholar] [CrossRef]
- Song, D.; Zhao, X.; Wang, F.; Wang, G. A brief review of urate transporter 1 (URAT1) inhibitors for the treatment of hyperuricemia and gout: Current therapeutic options and potential applications. Eur. J. Pharmacol. 2021, 907, 174291. [Google Scholar] [CrossRef]
- Jansen, T.L.; Perez-Ruiz, F.; Tausche, A.-K.; Richette, P. International position paper on the appropriate use of uricosurics with the introduction of lesinurad. Clin. Rheumatol. 2018, 37, 3159–3165. [Google Scholar] [CrossRef]
- Chen, Y.; You, R.; Wang, K.; Wang, Y. Recent Updates of Natural and Synthetic URAT1 Inhibitors and Novel Screening Methods. Evid.-Based Complement. Altern. Med. 2021, 2021, 5738900. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Wei, R.; Han, D.; Zhu, J.; Luo, W.; Ao, W.; Zhong, G. Hypouricemic Effects of Extracts from Urtica hyperborea Jacq. ex Wedd. in Hyperuricemia Mice through XOD, URAT1, and OAT1. BioMed Res. Int. 2020, 2020, 2968135. [Google Scholar] [CrossRef] [PubMed]
- Huda, M.; Pralampita, P.W.; Agustina, D.; Abrori, C.; Wahyudi, S.S. The Effect of Alopurinol on Blood Urea Nitrogen and Creatinine Serum Levels in Patients with Chronic Kidney Disease. J. Agromed. Med. Sci. 2021, 7, 8–15. [Google Scholar] [CrossRef]
- Hasballah, K.; Sarong, M.; Rusly, R.; Fitria, H.; Maida, D.R.; Iqhrammullah, M. Antiproliferative Activity of Triterpenoid and Steroid Compounds from Ethyl Acetate Extract of Calotropis gigantea Root Bark against P388 Murine Leukemia Cell Lines. Sci. Pharm. 2021, 89, 21. [Google Scholar] [CrossRef]

| Peak | Retention Time (Min) | Area (%) | Phytocompound |
|---|---|---|---|
| 1 | 4.025 | 0.56 | (3-Methyl-oxiran-2-yl)-methanol |
| 2 | 4.090 | 6.51 | 2-Pentanone, 4-hydroxy-4-methyl |
| 3 | 4.305 | 0.80 | Ethylbenzene |
| 4 | 19.042 | 0.41 | Hexadecanoic acid, methyl ester |
| 5 | 19.454 | 3.23 | n-Hexadecanoic acid |
| 6 | 20.799 | 0.14 | 11,14-Eicosadienoic acid |
| 7 | 20.853 | 0.82 | 9-Octadecenoic acid (Z)-, methyl ester |
| 8 | 21.264 | 4.38 | Oleic acid |
| 9 | 21.481 | 1.73 | Octadecanoic acid |
| 10 | 23.871 | 11.61 | 3β-Ergost-5-en-3-ol |
| 11 | 24.120 | 0.22 | 2′-O-methyl-guanosine |
| 12 | 24.584 | 13.35 | Stigmasterol |
| 13 | 24.803 | 7.93 | Bis(2-ethylhexyl) phthalate |
| 14 | 24.906 | 7.98 | Tris(2,4-di-tert-butylphenyl) phosphate |
| 15 | 25.535 | 0.27 | N-Decanoylmorpholine |
| 16 | 26.029 | 27.62 | ɣ-Sitosterol |
| 17 | 26.995 | 4.33 | Cholest-4-en-3-ol |
| 18 | 27.401 | 0.53 | N-pentyl-arachidamide |
| 19 | 27.678 | 0.31 | Stigmasterone |
| 20 | 28.008 | 2.64 | Lanosterol |
| 21 | 28.307 | 1.17 | Lupeol |
| 22 | 29.389 | 3.46 | ɣ-Sitostenone |
| Variable | Value, Mean ± SD |
|---|---|
| TPC (mg GAE/g extract) | 143.9 ± 5.24 |
| TFC (mg QE/g extract) | 390.2 ± 3.66 |
| IC50 of DPPH Inhibition (mg/L) | 0.14 ± 0.02 |
| Parameters a | Before | After | p-Value |
|---|---|---|---|
| Blood urea nitrogen, Mean± SD (mg/dL) | |||
| Normal | 23.00 ± 9.35 | 22.80 ± 8.47 | 0.704 |
| Control | 19.80 ± 5.26 | 20.60 ± 7.16 | 0.700 |
| Allopurinol | 22.80 ± 10.38 | 19.40 ± 4.04 | 0.449 |
| 100 mg | 19.80 ± 3.77 | 21.00 ± 2.00 | 0.468 |
| 200 mg | 23.80 ± 4.15 | 21.60 ± 4.83 | 0.216 |
| 400 mg | 17.60 ± 3.29 | 22.80 ± 3.56 | 0.007 ** |
| Creatinine, Mean± SD (mg/dL) | |||
| Normal b | 0.66 ± 0.13 | 0.68 ± 0.19 | >0.999 |
| Control | 0.70 ± 0.23 | 0.62 ± 0.11 | 0.512 |
| Allopurinol b | 0.68 ± 0.19 | 0.64 ± 0.09 | 0.750 |
| 100 mg | 0.54 ± 0.30 | 0.70 ± 0.16 | 0.140 |
| 200 mg | 0.78 ± 0.26 | 0.74 ± 0.24 | 0.670 |
| 400 mg | 0.64 ± 0.21 | 0.62 ± 0.27 | 0.621 |
| Parameters a | Before | After | p-Value |
|---|---|---|---|
| Aspartate aminotransferase, Mean ± SD (IU/L) | |||
| Normal | 139.2 ± 11.43 | 140.4 ± 2.07 | 0.833 |
| Control | 208.4 ± 49.13 | 206.4 ± 55.13 | 0.742 |
| Allopurinol | 224.4 ± 47.68 | 151.2 ± 22.07 | 0.020 * |
| 100 mg | 197.5 ± 30.40 | 170.8 ± 43.06 | 0.107 |
| 200 mg | 201.4 ± 17.80 | 162.8 ± 23.26 | 0.068 |
| 400 mg | 216.0 ± 21.06 | 170.2 ± 26.29 | 0.010 ** |
| Alanine aminotransferase, Mean ± SD (IU/L) | |||
| Normal b | 80.0 ± 7.91 | 80.80 ± 5.85 | 0.750 |
| Control b | 136.2 ± 41.57 | 133.8 ± 36.95 | 0.875 |
| Allopurinol b | 138.6 ± 82.86 | 95.60 ± 11.48 | 0.188 |
| 100 mg | 145.2 ± 50.54 | 125.0 ± 28.20 | 0.352 |
| 200 mg | 142.0 ± 55.76 | 122.0 ± 53.44 | 0.005 ** |
| 400 mg | 144.8 ± 21.04 | 94.20 ± 17.77 | 0.003 ** |
| Molecule | Sequence | PCR Product Size (bp) | NCBI Reference Sequence |
|---|---|---|---|
| β-actin | F: 5′-CCTAAGGCCAACCGTGAAAAGATG-3 | 219 | NM_007393.3 |
| R: 5′-GTCCCGGCCAGCCAGGTCCAG-3′ | |||
| URAT1 | F: 5′-TTCATGCCCACCTTCCCCCTC TAC-3′ | 207 | NM_009203.3 |
| R: 5′-CATCCTCCAGCTGCGCACACCATA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yusnaini, R.; Nasution, R.; Saidi, N.; Arabia, T.; Idroes, R.; Ikhsan, I.; Bahtiar, R.; Iqhrammullah, M. Ethanolic Extract from Limonia acidissima L. Fruit Attenuates Serum Uric Acid Level via URAT1 in Potassium Oxonate-Induced Hyperuricemic Rats. Pharmaceuticals 2023, 16, 419. https://doi.org/10.3390/ph16030419
Yusnaini R, Nasution R, Saidi N, Arabia T, Idroes R, Ikhsan I, Bahtiar R, Iqhrammullah M. Ethanolic Extract from Limonia acidissima L. Fruit Attenuates Serum Uric Acid Level via URAT1 in Potassium Oxonate-Induced Hyperuricemic Rats. Pharmaceuticals. 2023; 16(3):419. https://doi.org/10.3390/ph16030419
Chicago/Turabian StyleYusnaini, Rika, Rosnani Nasution, Nurdin Saidi, Teti Arabia, Rinaldi Idroes, Ikhsan Ikhsan, Rahmad Bahtiar, and Muhammad Iqhrammullah. 2023. "Ethanolic Extract from Limonia acidissima L. Fruit Attenuates Serum Uric Acid Level via URAT1 in Potassium Oxonate-Induced Hyperuricemic Rats" Pharmaceuticals 16, no. 3: 419. https://doi.org/10.3390/ph16030419
APA StyleYusnaini, R., Nasution, R., Saidi, N., Arabia, T., Idroes, R., Ikhsan, I., Bahtiar, R., & Iqhrammullah, M. (2023). Ethanolic Extract from Limonia acidissima L. Fruit Attenuates Serum Uric Acid Level via URAT1 in Potassium Oxonate-Induced Hyperuricemic Rats. Pharmaceuticals, 16(3), 419. https://doi.org/10.3390/ph16030419








