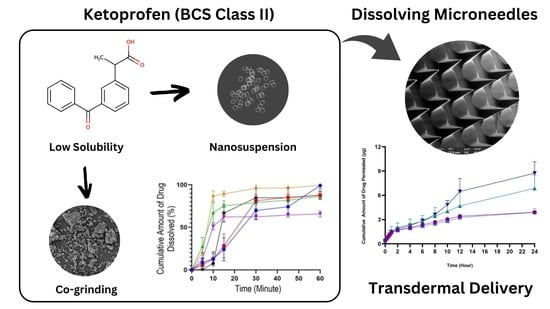
Combination of Dissolving Microneedles with Nanosuspension and Co-Grinding for Transdermal Delivery of Ketoprofen
Abstract
1. Introduction
2. Results and Discussion
2.1. UV-Vis Spectrophotometric Condition
2.2. High-Performance Liquid Chromatography (HPLC) Validation Method
2.3. Characterization of Nanosuspension Ketoprofen
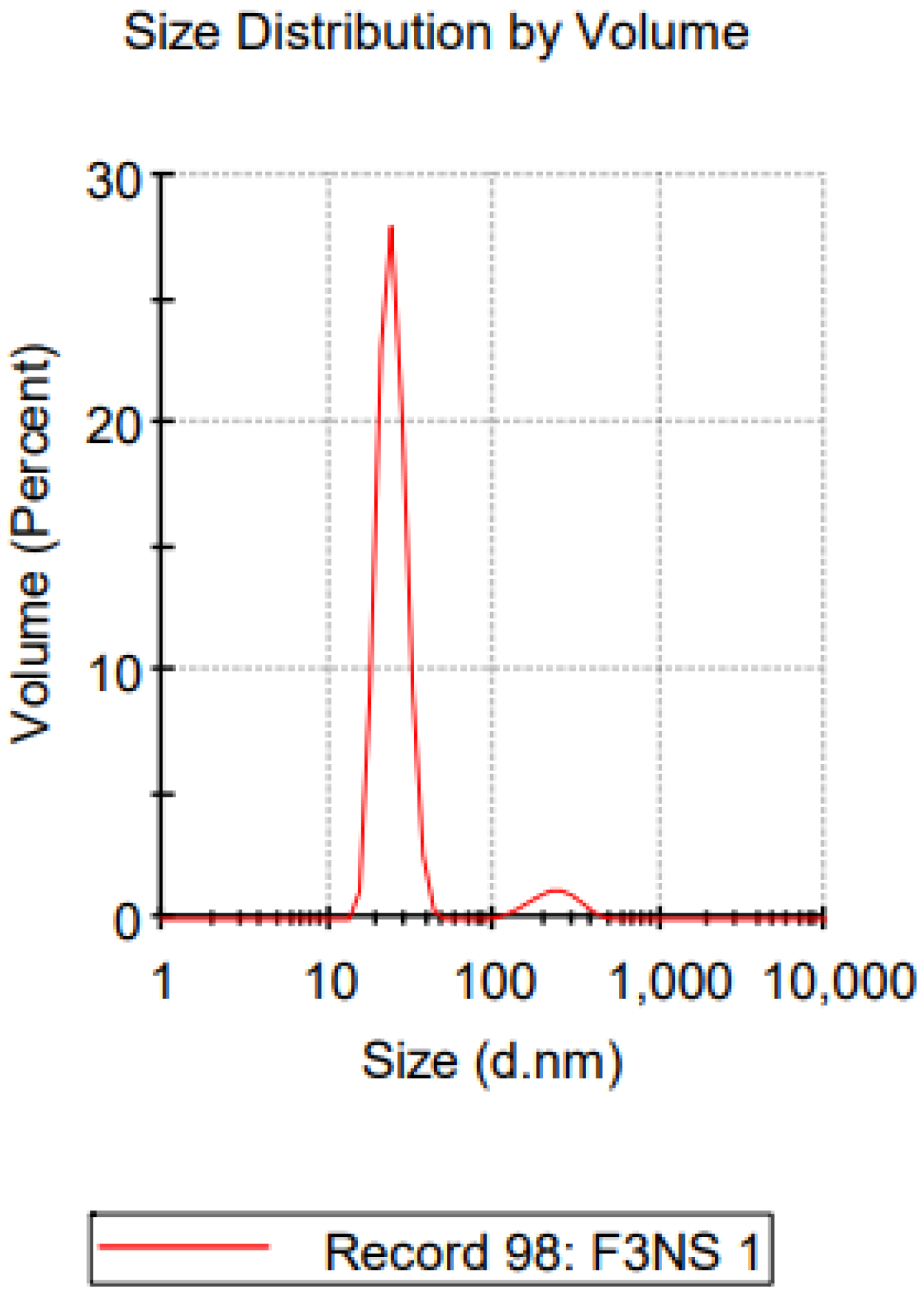
2.3.1. Particle Size and Zeta Potential Analysis
2.3.2. Determination of Ketoprofen Content in Nanosuspension
2.4. Characterization of Co-grinded Ketoprofen
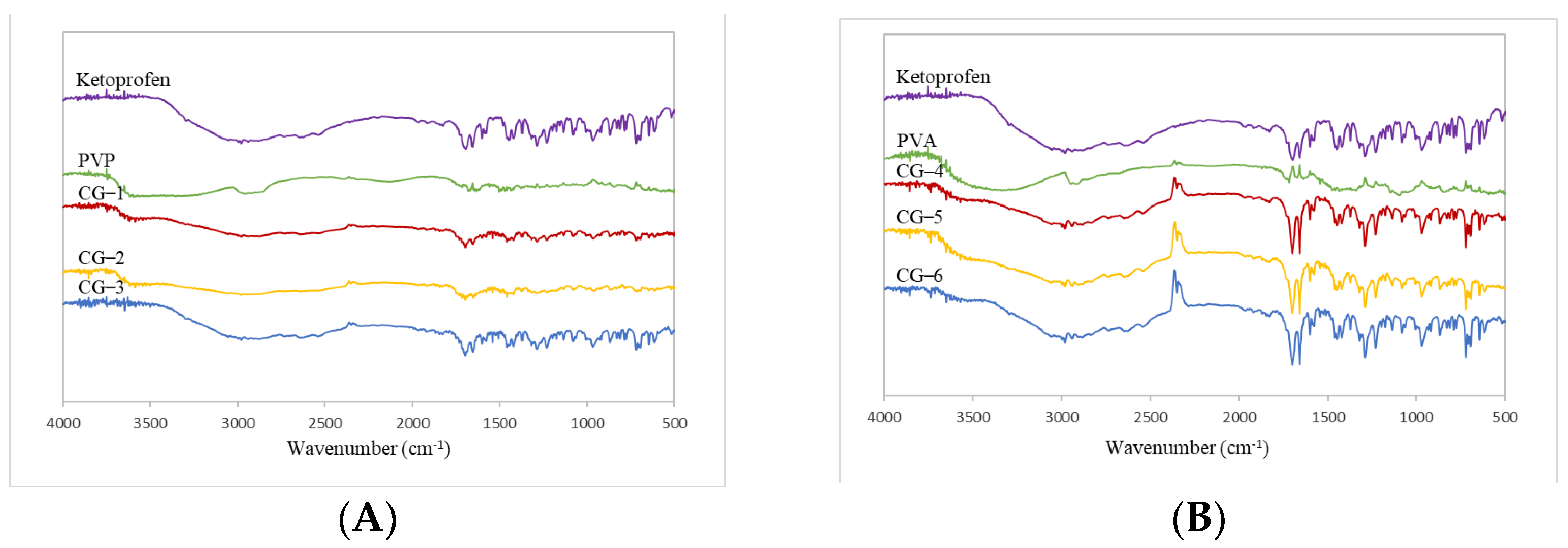
2.4.1. FT-IR Spectrophotometer
2.4.2. XRD
2.4.3. Differential Scanning Calorimetry (DSC) Analysis of Co-grinded Ketoprofen
2.4.4. Scanning Electron Microscopy (SEM)
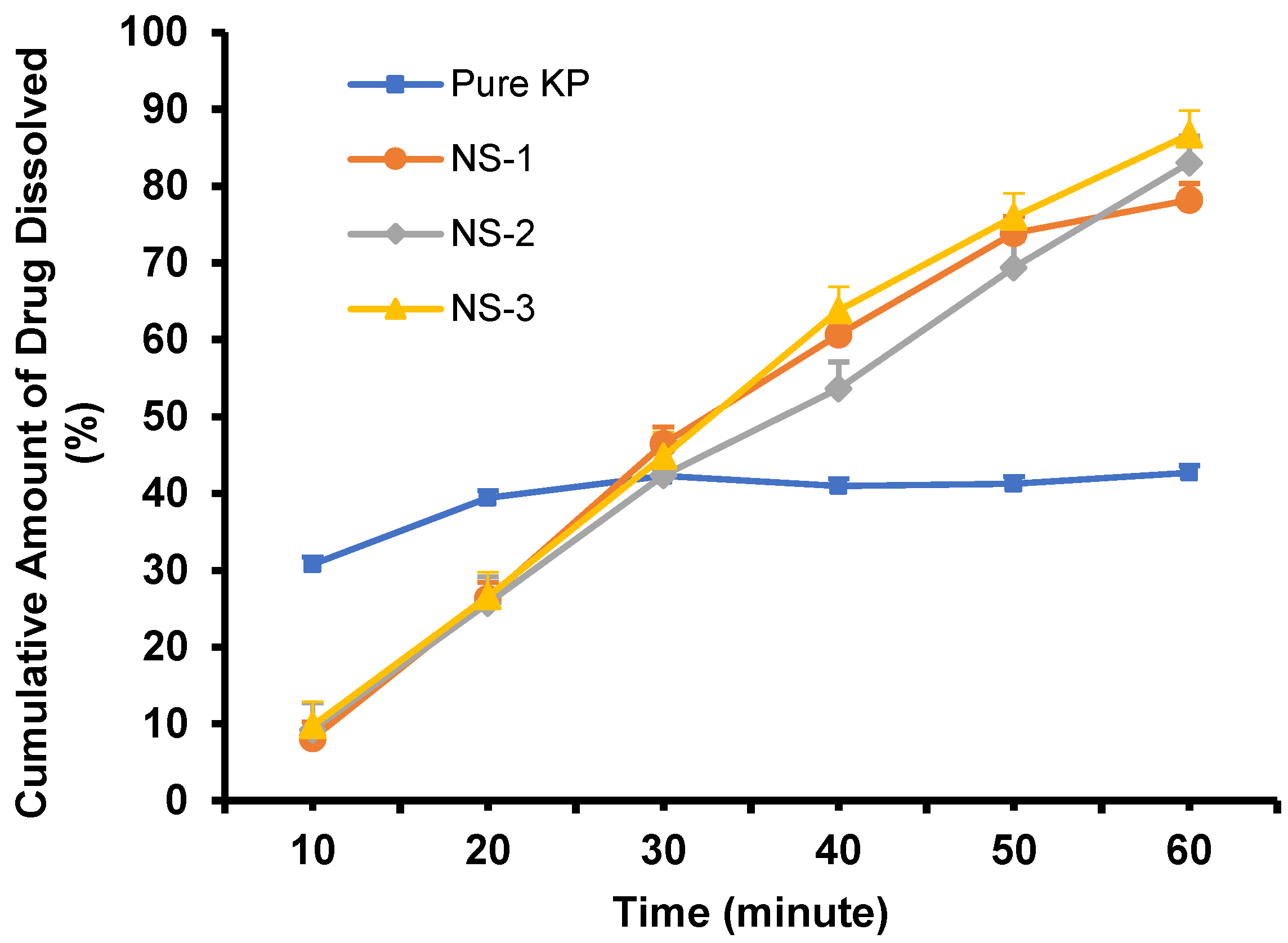
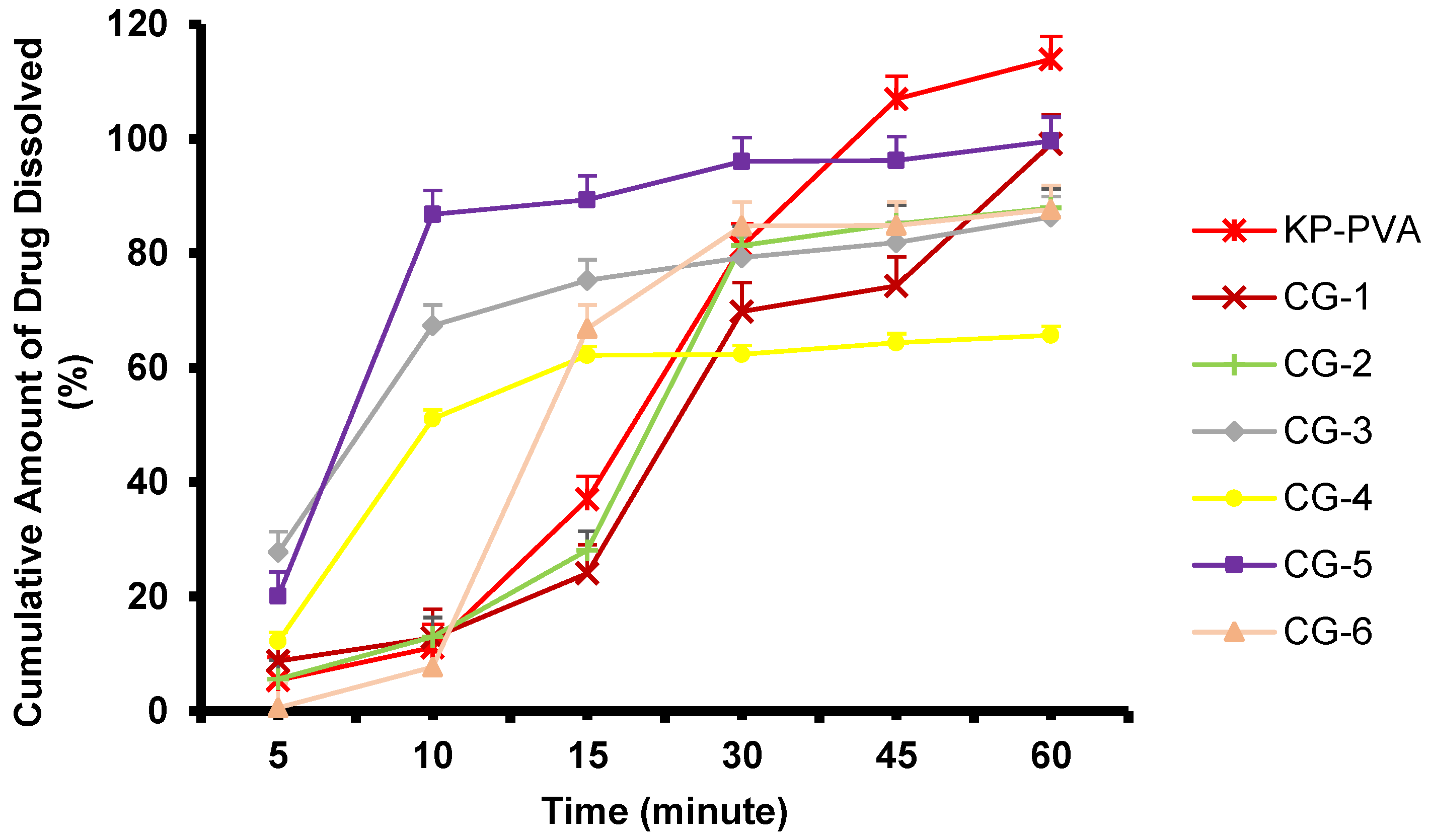
2.5. Dissolution Study of Nanosuspension and Co-grinded Ketoprofen
2.6. Evaluation of a Dissolving Microneedle (DMN) Containing Nanosuspension and Co-grinded Ketoprofen
2.6.1. Physical Evaluation of Dissolving Microneedle
2.6.2. Mechanical Properties
2.6.3. Loss of Mass
2.6.4. Insertion Ability Study
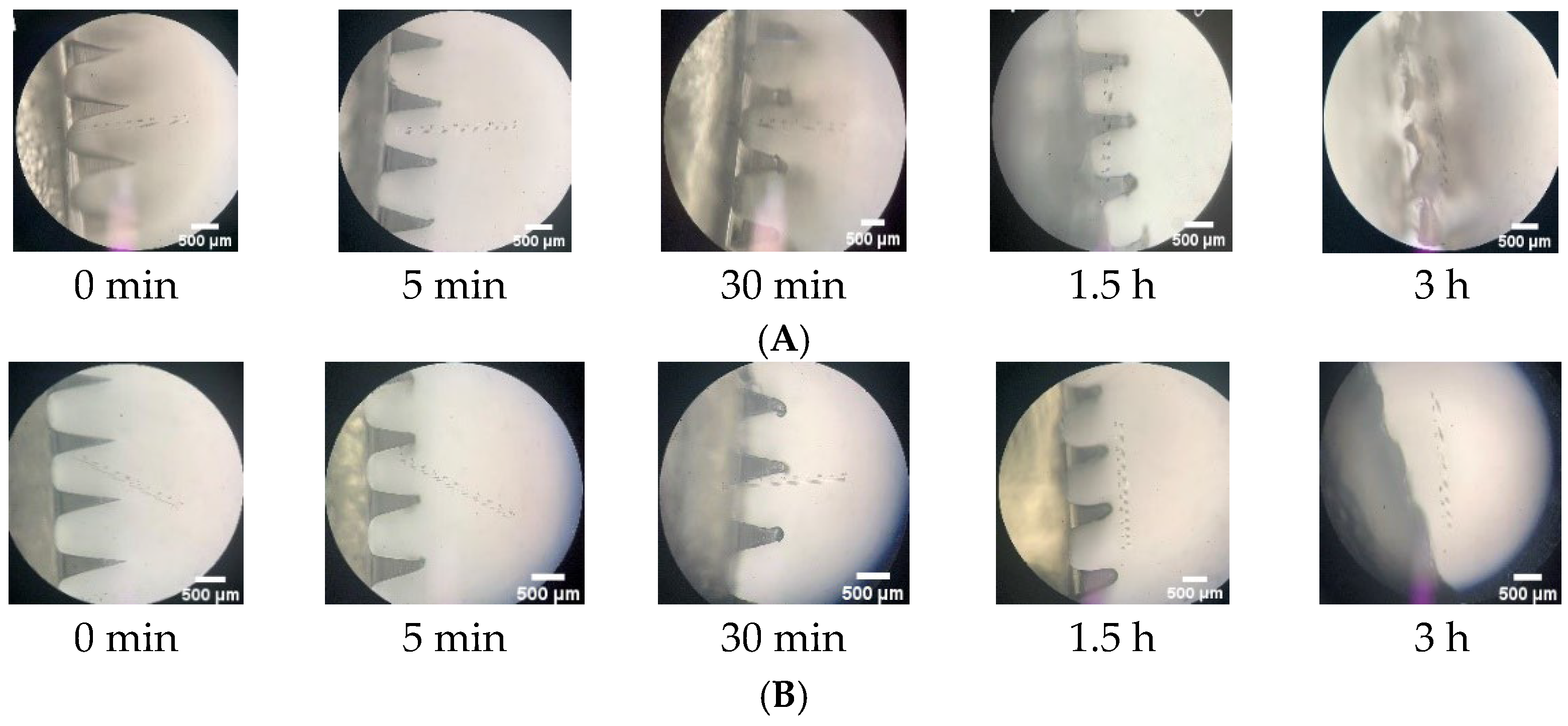
2.6.5. In-Skin Dissolution Study
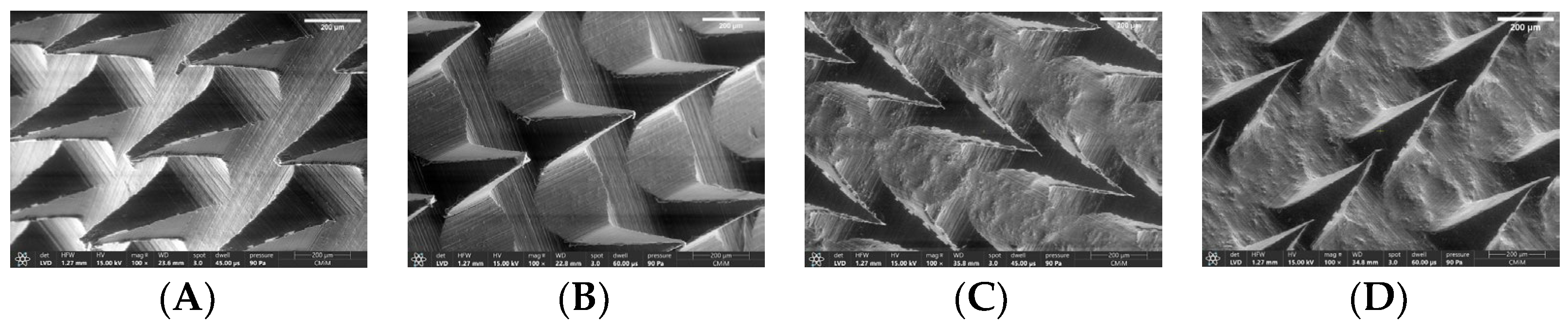
2.6.6. Morphological Observation of Selected Formulation
2.6.7. Determination of Ketoprofen Content
2.6.8. Differential Scanning Calorimetry (DSC) Analysis of DMN
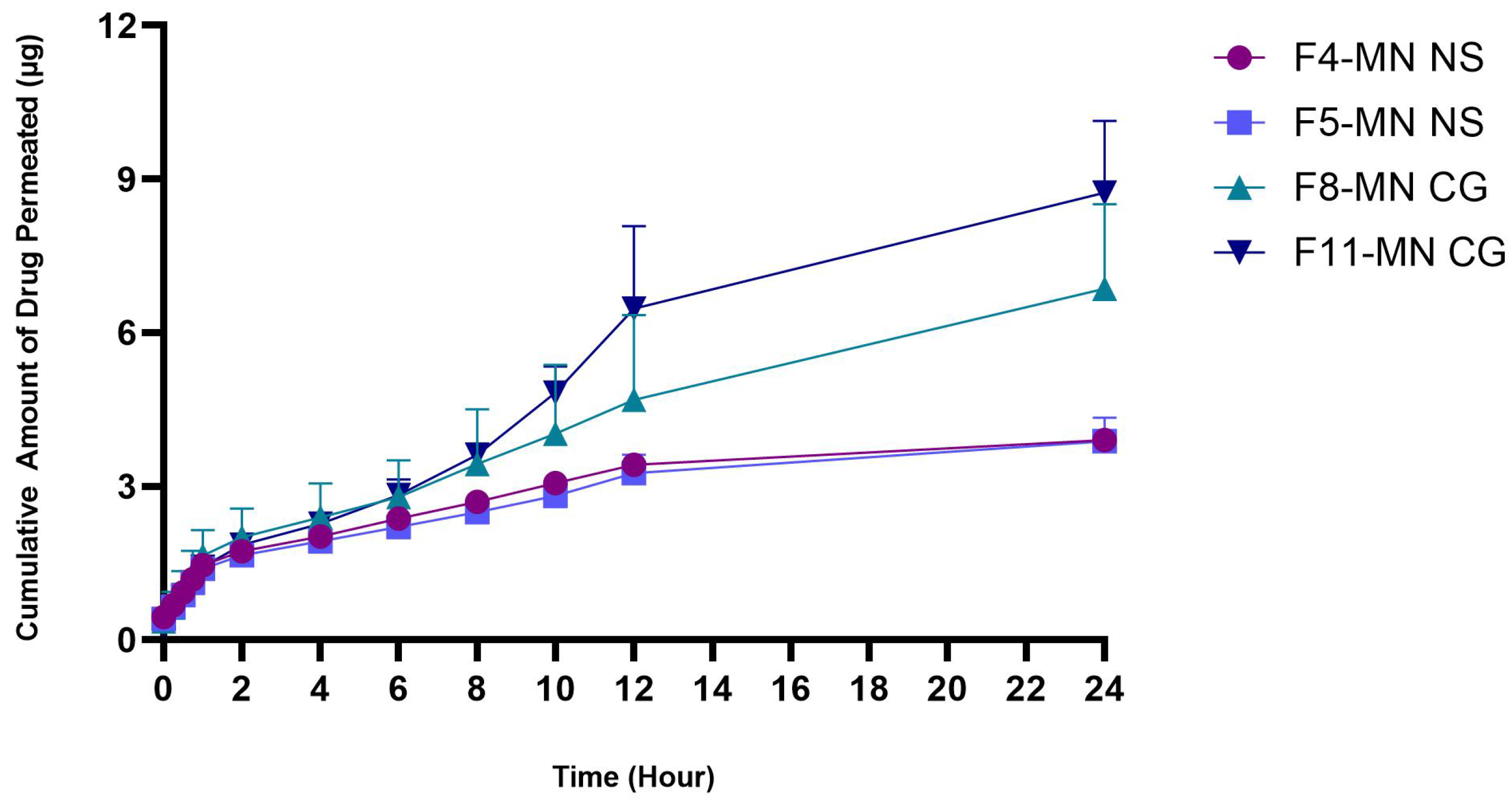
2.6.9. In Vitro Permeation Studies
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Analytical Method Condition
UV-Vis Spectrophotometric Condition
Validation of UV-Vis Spectrophotometer
Chromatographic Condition
Validation of High-Performance Liquid Chromatography (HPLC) Method
- Specificity
- b.
- Linearity
- c.
- Limit of Detection (LOD)
- d.
- Limit of Quantification (LOQ)
- e.
- Accuracy
- f.
- Precision
3.2.2. Formulation of Ketoprofen Nanosuspension
3.2.3. Characterization of Nanosuspension Ketoprofen
Particle Size and Zeta Potential Analysis
Determination of Ketoprofen Content in Nanosuspension
Determination of Selected Nanosuspension Ketoprofen Formula
3.2.4. Formulation of Co-grinded Ketoprofen
3.2.5. Characterization of Co-grinded Ketoprofen
FT-IR Spectrometer
XRD (X-ray Diffraction)
Differential Scanning Calorimetry (DSC) Analysis of Co-grinded Ketoprofen
SEM (Scanning Electron Microscopy)
3.2.6. Dissolution Study
3.2.7. Formulation and Evaluation of Dissolving Microneedle (DMN) Containing Ketoprofen Nanosuspension or Co-Grinding
Physical Evaluation of Microneedle Array
Mechanical Properties
Loss of Mass While Drying
Insertion Ability Study
In-Skin Dissolution Study
Determination of Ketoprofen Content in Dissolving Microneedles
3.2.8. Differential Scanning Calorimetry (DSC) Analysis of DMN
3.2.9. In Vitro Permeation Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, V.; Abbas, A.; Aster, J. Robbins and Cotran Pathologic Basis of Disease, 9th ed.; Elsevier: New York, NY, USA, 2017. [Google Scholar]
- Sasono, B.; Amanda, N.A.; Dewi, D.N.S.S. Faktor Dominan pada Penderita Osteoarthritis di RSUD dr. Mohamad Soewandhie, Surabaya, Indonesia. J. Med. Udayana. 2020, 9, 3–8. [Google Scholar]
- Kemenkes, R.I. Hasil Riset Kesehatan Dasar Tahun 2018. Kementrian Kesehat RI 2018, 53, 1689–1699. [Google Scholar]
- Sweetman, S.C. Martindale The Complete Drug Reference 36th Edition. J. Chem. Inf. Model. 2009, 53, 1689–1699. [Google Scholar]
- Vučen, S.R.; Vuleta, G.; Crean, A.M.; Moore, A.C.; Ignjatovic, N.; Uskokovic, D. Improved percutaneous delivery of ketoprofen using combined application of nanocarriers and silicon microneedles. J. Pharm. Pharmacol. 2013, 65, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Vonkeman, H.E.; van de Laar, M.A.F.J. Nonsteroidal Anti-Inflammatory Drugs: Adverse Effects and Their Prevention. Semin. Arthritis Rheum. 2010, 39, 294–312. [Google Scholar] [CrossRef] [PubMed]
- Ramadon, D.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Enhancement strategies for transdermal drug delivery systems: Current trends and applications. Drug Deliv. Transl. Res. 2021, 12, 758–791. [Google Scholar] [CrossRef]
- Manasco, J.L.; Tang, C.; Burns, N.A.; Saquing, C.D.; Khan, S.A. Rapidly dissolving poly(vinyl alcohol)/cyclodextrin electrospun nanofibrous membranes. RSC Adv. 2014, 4, 13274–13279. [Google Scholar] [CrossRef]
- Fukushima, K.; Ise, A.; Morita, H.; Hasegawa, R.; Ito, Y.; Sugioka, N.; Takada, K. Two-Layered Dissolving Microneedles for Percutaneous Delivery of Peptide/Protein Drugs in Rats. Pharm. Res. 2010, 28, 7–21. [Google Scholar] [CrossRef]
- Dragicevic, N.; Maibach, H.I. Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Nanocarriers; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–384. [Google Scholar]
- Mahato, R. Microneedles in Drug Delivery. Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2017; pp. 331–353. [Google Scholar]
- Larrañeta, E.; Lutton, R.E.M.; Woolfson, A.D.; Donnelly, R.F. Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Mater. Sci. Eng. R Rep. 2016, 104, 1–32. [Google Scholar] [CrossRef]
- Duarah, S.; Sharma, M.; Wen, J. Recent advances in microneedle-based drug delivery: Special emphasis on its use in paediatric population. Eur. J. Pharm. Biopharm. 2019, 136, 48–69. [Google Scholar] [CrossRef]
- Lahiji, S.F.; Dangol, M.; Jung, H. A patchless dissolving microneedle delivery system enabling rapid and efficient transdermal drug delivery. Sci. Rep. 2015, 5, srep07914. [Google Scholar] [CrossRef]
- Dangol, M.; Yang, H.; Li, C.G.; Lahiji, S.F.; Kim, S.; Ma, Y.; Jung, H. Innovative polymeric system (IPS) for solvent-free lipophilic drug transdermal delivery via dissolving microneedles. J. Control. Release 2016, 223, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.; Mohammed, Y.; Pastore, M.; Namjoshi, S.; Yousef, S.; Alinaghi, A.; Haridass, I.; Abd, E.; Leite-Silva, V.; Benson, H.; et al. Topical and cutaneous delivery using nanosystems. J. Control. Release 2017, 247, 86–105. [Google Scholar] [CrossRef]
- Hilaliyati, N.; Ben, E.S.; Zaini, E. Enhanced Dissolution Rate of Ketoprofen by Co-grinding Technique with Hydroxypropyl Methylcellulose E6 polymer. J. Sains Farm Klin. 2017, 3, 193–201. [Google Scholar] [CrossRef]
- Garg, A.; Singh, S.; Rao, V.U.; Bindu, K.; Balasubramaniam, J. Solid State Interaction of Raloxifene HCl with Different Hydrophilic Carriers During Co-grinding and its Effect on Dissolution Rate. Drug Dev. Ind. Pharm. 2009, 35, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Shantier, S.W.G.-K. Development and Validation of UV-Visible Spectrophotometric Method for Estimation of Ketoprofen in Capsule and Tablet Dosage. Indo. Am. J. Pharm. Res. 2019, 9, 1866–1869. [Google Scholar]
- ICH. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), Validation of Analytical Procedures: Text and Methodology Q2 (R2). Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-q2r2-validation-analytical-procedures-step-2b_en.pdf (accessed on 24 February 2022).
- Ferraz-Carvalho, R.; Mendonça, E.A.M.; Silva, J.P.A.; Cavalcanti, I.M.F.; Galdino, S.L.; Pitta, I.R.; Lima, M.D.C.A.; Santos-Magalhães, N.S.; Lira-Nogueira, M.C.B. Validation of a UV-spectrophotometric analytical method for determination of LPSF/AC04 from inclusion complex and liposomes. Braz. J. Pharm. Sci. 2015, 51, 183–191. [Google Scholar] [CrossRef]
- Chourasia, M.K.; Kang, L.; Chan, S.Y. Nanosized ethosomes bearing ketoprofen for improved transdermal delivery. Results Pharma Sci. 2011, 1, 60–67. [Google Scholar] [CrossRef]
- European Medicine Agency. Guideline On Bioanalytical Method Validation. 2011. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf (accessed on 26 February 2022).
- FDA. Analytical Method Validation. In New Drug Development: Regulatory Paradigms for Clinical Pharmacology and Biopharmaceutics; CRC Press: Boca Raton, FL, USA, 2016; pp. 138–159. [Google Scholar]
- Vora, L.; Vavia, P.R.; Larrañeta, E.; Bell, S.E.; Donnelly, R.F. Novel nanosuspension-based dissolving microneedle arrays for transdermal delivery of a hydrophobic drug. J. Interdiscip. Nanomed. 2018, 3, 89–101. [Google Scholar] [CrossRef]
- Chonkar, A.D.; Rao, J.V.; Managuli, R.S.; Mutalik, S.; Dengale, S.; Jain, P.; Udupa, N. Development of fast dissolving oral films containing lercanidipine HCl nanoparticles in semicrystalline polymeric matrix for enhanced dissolution and ex vivo permeation. Eur. J. Pharm. Biopharm. 2016, 103, 179–191. [Google Scholar] [CrossRef]
- Bartos, C.; Jójárt-Laczkovich, O.; Katona, G.; Budai-Szűcs, M.; Ambrus, R.; Bocsik, A.; Gróf, I.; Deli, M.A.; Szabó-Révész, P. Optimization of a combined wet milling process in order to produce poly(vinyl alcohol) stabilized nanosuspension. Drug Des. Dev. Ther. 2018, 12, 1567–1580. [Google Scholar] [CrossRef] [PubMed]
- Wada, K. FTIR Talk Letter. Shimadzu. 2006. Available online: https://www.shimadzu.eu/ftir-talk-letter-archive (accessed on 2 May 2022).
- Wicaksono, Y.; Setyawan, D.; Siswandono, S. Multicomponent Crystallization of for Improving the Solubility. Chem. J. Mold. 2018, 13, 74–81. [Google Scholar] [CrossRef]
- Chan, S.-Y.; Chung, Y.-Y.; Cheah, X.-Z.; Tan, E.Y.-L.; Quah, J. The characterization and dissolution performances of spray dried solid dispersion of ketoprofen in hydrophilic carriers. Asian J. Pharm. Sci. 2015, 10, 372–385. [Google Scholar] [CrossRef]
- Halim, A.; Hamdeni, S.; Zaini, E. Peningkatan Laju Disolusi Trimetoprim dengan Teknik Co-Grinding Menggunakan Polimer Polivinilpirolidon K-30 (Enhanced Dissolution Rate of Trimethoprim by Co-grinding Technique with Polyvinylpyrrolidone K-30 Polymer). J. Ilmu. Kefarmasian Indones. 2013, 11, 1–6. [Google Scholar]
- Thomas, D.; Zhuravlev, E.; Wurm, A.; Schick, C.; Cebe, P. Fundamental thermal properties of polyvinyl alcohol by fast scanning calorimetry. Polymer 2018, 137, 145–155. [Google Scholar] [CrossRef]
- Chauhan, A. Powder XRD Technique and its Applications in Science and Technology. J. Anal. Bioanal. Tech. 2014, 5, 212. [Google Scholar] [CrossRef]
- Browne, E.; Worku, Z.A.; Healy, A.M. Physicochemical Properties of Poly-vinyl Polymers and Their Influence on Ketoprofen Amorphous Solid Dispersion Performance: A Polymer Selection Case Study. Pharmaceutics 2020, 12, 433. [Google Scholar] [CrossRef]
- Ardiansyah, S.; Nasrul, E.; Rivai, H.; Ben, E.S.; Zaini, E. Physicochemical Physicochemical characterization of amorphous solid dispersion of ketoprofen–polyvinylpyrrolidone K-30. Int. J. Pharm. Pharm. Sci. 2015, 7, 5–8. [Google Scholar]
- Nada, A.; Bandarkar, F.; Al-basarah, Y. Formulation of Ibuprofen Nanoparticles and Nanosuspensions with Enhanced Dissolution Rate using Ultra-Homogenization Technique. Asian J. Pharm. 2017, 2017, 4–10. [Google Scholar]
- Liu, T.; Wang, B.; Dong, W.; Gong, J. Solution-Mediated Phase Transformation of a Hydrate to its Anhydrous Form of Donepezil Hydrochloride. Chem. Eng. Technol. 2013, 36, 1327–1334. [Google Scholar] [CrossRef]
- Permana, A.D.; Tekko, I.A.; McCrudden, M.T.C.; Anjani, Q.K.; Ramadon, D.; McCarthy, H.O.; Donnelly, R.F. Solid lipid nanoparticle-based dissolving microneedles: A promising intradermal lymph targeting drug delivery system with potential for enhanced treatment of lymphatic filariasis. J. Control. Release 2019, 316, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Hutton, A.R.J.; Quinn, H.L.; McCague, P.J.; Jarrahian, C.; Rein-Weston, A.; Coffey, P.S.; Gerth-Guyette, E.; Zehrung, D.; Larrañeta, E.; Donnelly, R.F. Transdermal delivery of vitamin K using dissolving microneedles for the prevention of vitamin K deficiency bleeding. Int. J. Pharm. 2018, 541, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Teodorescu, M.; Bercea, M. Poly(vinylpyrrolidone)—A Versatile Polymer for Biomedical and Beyond Medical Applications. Polym. Plast. Technol. Eng. 2015, 54, 923–943. [Google Scholar] [CrossRef]
- Wang, Q.L.; Ren, J.W.; Chen, B.Z.; Jin, X.; Zhang, C.Y.; Guo, X.D. Effect of humidity on mechanical properties of dissolving microneedles for transdermal drug delivery. J. Ind. Eng. Chem. 2018, 59, 251–258. [Google Scholar] [CrossRef]
- Choudhary, S.; Sengwa, R. ZnO nanoparticles dispersed PVA–PVP blend matrix based high performance flexible nanodielectrics for multifunctional microelectronic devices. Curr. Appl. Phys. 2018, 18, 1041–1058. [Google Scholar] [CrossRef]
- Larrañeta, E.; Moore, J.; Vicente-Pérez, E.M.; González-Vázquez, P.; Lutton, R.; Woolfson, A.D.; Donnelly, R.F. A proposed model membrane and test method for microneedle insertion studies. Int. J. Pharm. 2014, 472, 65–73. [Google Scholar] [CrossRef]
- Putri, H.E.; Utami, R.N.; Wahyudin, E.; Oktaviani, W.W.; Mudjahid, M.; Permana, A.D. Dissolving Microneedle Formulation of Ceftriaxone: Effect of Polymer Concentrations on Characterisation and Ex Vivo Permeation Study. J. Pharm. Innov. 2021, 17, 1176–1188. [Google Scholar] [CrossRef]
- Ramadon, D.; Permana, A.D.; Courtenay, A.J.; McCrudden, M.T.C.; Tekko, I.A.; McAlister, E.; Anjani, Q.K.; Utomo, E.; McCarthy, H.O.; Donnelly, R.F. Development, Evaluation, and Pharmacokinetic Assessment of Polymeric Microarray Patches for Transdermal Delivery of Vancomycin Hydrochloride. Mol. Pharm. 2020, 17, 3353–3368. [Google Scholar] [CrossRef]
- González-Vázquez, P.; Larrañeta, E.; McCrudden, M.T.C.; Jarrahian, C.; Rein-Weston, A.; Quintanar-Solares, M.; Zehrung, D.; McCarthy, H.; Courtenay, A.J.; Donnelly, R.F. Transdermal delivery of gentamicin using dissolving microneedle arrays for potential treatment of neonatal sepsis. J. Control. Release 2017, 265, 30–40. [Google Scholar] [CrossRef]
- Nornoo, A.O.; Wulz, J.; Yoon, H.; Nan, Y.; Lese, M. Impact of the chemical and physical stability of ketoprofen compounded in various pharmaceutical bases on its topical and transdermal delivery. Pharm. Dev. Technol. 2014, 21, 204–213. [Google Scholar] [CrossRef]
- Anjani, Q.K.; Sabri, A.H.; Castellanos, N.M.; Utomo, E.; Martinez, A.C.; Robles, D.J.; Wardoyo, L.A.H.; Donnelly, R.F. Soluplus-based dissolving microarray patches loaded with colchicine: Towards a minimally invasive treatment and management of gout. Biomater. Sci. 2022, 10, 5838–5855. [Google Scholar] [CrossRef] [PubMed]
- Zsikó, S.; Csányi, E.; Kovács, A.; Budai-Szűcs, M.; Gácsi, A.; Berkó, S. Methods to Evaluate Skin Penetration In Vitro. Sci. Pharm. 2019, 87, 19. [Google Scholar] [CrossRef]
- Ramadon, D.; Anwar, E.; Harahap, Y. In vitro Penetration and Bioavailability of Novel Transdermal Quercetin-loaded Ethosomal Gel. Indian J. Pharm. Sci. 2017, 79, 948–956. [Google Scholar] [CrossRef]
- McGrath, M.G.; Vucen, S.; Vrdoljak, A.; Kelly, A.; O’Mahony, C.; Crean, A.M.; Moore, A. Production of dissolvable microneedles using an atomised spray process: Effect of microneedle composition on skin penetration. Eur. J. Pharm. Biopharm. 2014, 86, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Garland, M.J.; Migalska, K.; Tuan-Mahmood, T.-M.; Singh, T.R.R.; Majithija, R.; Caffarel-Salvador, E.; McCrudden, C.M.; McCarthy, H.O.; Woolfson, A.D.; Donnelly, R.F. Influence of skin model on in vitro performance of drug-loaded soluble microneedle arrays. Int. J. Pharm. 2012, 434, 80–89. [Google Scholar] [CrossRef]
- Kaleemullah, M.; Jiyauddin, K.; Thiban, E.; Rasha, S.; Al-Dhalli, S.; Budiasih, S.; Gamal, O.; Fadli, A.; Eddy, Y. Development and evaluation of Ketoprofen sustained release matrix tablet using Hibiscus rosa-sinensis leaves mucilage. Saudi Pharm. J. 2016, 25, 770–779. [Google Scholar] [CrossRef]
- He, M.; Yang, G.; Zhang, S.; Zhao, X.; Gao, Y. Dissolving Microneedles Loaded with Etonogestrel Microcrystal Particles for Intradermal Sustained Delivery. J. Pharm. Sci. 2018, 107, 1037–1045. [Google Scholar] [CrossRef]
- Du, G.; Sun, X. Current Advances in Sustained Release Microneedles. Pharm. Front. 2020, 2, e11–e22. [Google Scholar] [CrossRef]
- Tekko, I.; Permana, A.D.; Vora, L.; Hatahet, T.; McCarthy, H.; Donnelly, R.F. Localised and sustained intradermal delivery of methotrexate using nanocrystal-loaded microneedle arrays: Potential for enhanced treatment of psoriasis. Eur. J. Pharm. Sci. 2020, 152, 105469. [Google Scholar] [CrossRef]
- Kim, B.; Cho, H.-E.; Moon, S.H.; Ahn, H.-J.; Bae, S.; Cho, H.-D.; An, S. Transdermal delivery systems in cosmetics. Biomed. Dermatol. 2020, 4, 10. [Google Scholar] [CrossRef]
- Ameri, M.; Lewis, H.; Lehman, P. Effect of Skin Model on In Vitro Performance of an Adhesive Dermally Applied Microarray Coated with Zolmitriptan. J. Pharm. 2018, 2018, 7459124. [Google Scholar] [CrossRef] [PubMed]
- Iskandarsyah, I.; Mutakim, A. Preparasi dan Karakterisasi Nanosuspensi dengan Polivinilpirolidon (PVP) Sebagai Pembawa Nanopartikel Senyawa Asam Mefenamat. Pharm. Sci. Res. 2010, 7, 6. [Google Scholar]
- Zafar, F.; Shoaib, M.H.; Naz, A.; Yousuf, R.I.; Ali, H. Determination of Ketoprofen in Human Plasma by RP-HPLC. Am. J. Anal. Chem. 2013, 04, 252–257. [Google Scholar] [CrossRef]
- Guo, B.; Liu, H.; Li, Y.; Zhao, J.; Yang, D.; Wang, X.; Zhang, T. Application of phospholipid complex technique to improve the dissolution and pharmacokinetic of probucol by solvent-evaporation and co-grinding methods. Int. J. Pharm. 2014, 474, 50–56. [Google Scholar] [CrossRef]
- Watson, D. Pharmaceutical Analysis: A Textbook for Pharmacy Students and Pharmaceutical Chemists, 5th ed.; Elsevier: London, UK, 2020. [Google Scholar]
- Yadav, P.S.; Kumar, V.; Singh, U.P.; Bhat, H.R.; Mazumder, B. Physicochemical characterization and in vitro dissolution studies of solid dispersions of ketoprofen with PVP K30 and d-mannitol. Saudi Pharm. J. 2011, 21, 77–84. [Google Scholar] [CrossRef]
- Rosaini, H.; Sari, Y.E.N.; Makmur, I.; Halim, A.; Sidoretno, W.M. Karakterisasi Sifat Fisikokimia Sistem Dispersi Padat Nimodipin Dengan Poloxamer 188 Menggunakan Metode Penggilingan Bersama. JOPS J. Pharm. Sci. 2020, 4, 18–26. [Google Scholar] [CrossRef]
- Khaleel, N.Y.; Abdulrasool, A.A.; Ghareeb, M.M.; Hussain, S.A. Solubility and dissolution improvement of ketoprofen by solid dispersion in polymer and surfactant using solvent evaporation method. Int. J. Pharm. Pharm. Sci. 2011, 3, 431–435. [Google Scholar]
- Ramadon, D.; Sutrisna, L.F.P.; Harahap, Y.; Putri, K.S.S.; Ulayya, F.; Hartrianti, P.; Anjani, Q.K.; Donnelly, R.F. Enhancing Intradermal Delivery of Lidocaine by Dissolving Microneedles: Comparison between Hyaluronic Acid and Poly(Vinyl Pyrrolidone) Backbone Polymers. Pharmaceutics 2023, 15, 289. [Google Scholar] [CrossRef]
- Abdelghany, S.; Tekko, I.A.; Vora, L.; Larrañeta, E.; Permana, A.D.; Donnelly, R.F. Nanosuspension-Based Dissolving Microneedle Arrays for Intradermal Delivery of Curcumin. Pharmaceutics 2019, 11, 308. [Google Scholar] [CrossRef]







| Range (µg/mL) | Slope | Intercept | R2 | LOD (µg/mL) | LOQ (µg/mL) |
|---|---|---|---|---|---|
| 5–30 | 1.7569 | −0.0257 | 0.9995 | 0.7056 | 2.1382 |
| Formulations | PVA (%) | Dv-90 (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|---|
| NS-1 | 0.5 | 578.67 ± 30.82 | 0.467 ± 0.07 | −19.17 ± 3.70 |
| NS-2 | 1 | 256.33 ± 44.07 | 0.471 ± 0.11 | −17.63 ± 1.90 |
| NS-3 | 2 | 124.3 ± 26.15 | 0.265 ± 0.02 | −15.7 ± 3.72 |
| Formulation | Composition |
|---|---|
| CG-1 | K:P = 3:1 |
| CG-2 | K:P = 5:1 |
| CG-3 | K:P = 10:1 |
| CG-4 | K:A = 1:1 |
| CG-5 | K:A = 1:2 |
| CG-6 | K:A = 2:1 |
| Formulations | Composition | Morphology | |
|---|---|---|---|
| F1-MN NS | PVA 10% |  |  |
| F2-MN NS | PVP 30% |  |  |
| F3-MN NS | PVA 5% PVP 5% |  |  |
| F4-MN NS | PVA 5% PVP 10% |  |  |
| F5-MN NS | PVA 5% PVP 15% |  |  |
| Formulations | Composition | Morphology | |
|---|---|---|---|
| F1-MN CG | Co-grinded Keto 25% PVA 10% |  |  |
| F2-MN CG | Co-grinded Keto 25% PVA 30% |  |  |
| F3-MN CG | Co-grinded Keto 25% PVA 2.5% PVP 5% |  |  |
| F4-MN CG | Co-grinded Keto 25% PVA 2.5% PVP 10% |  |  |
| F5-MN CG | Co-grinded Keto 25% PVA 2.5% PVP 15% |  |  |
| F6-MN CG | Co-grinded Keto 25% PVA 5% PVP 5% |  |  |
| F7-MN CG | Co-grinded Keto 25% PVA 5% PVP 10% |  | 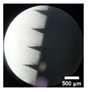 |
| F8-MN CG | Co-grinded Keto 25% PVA 5% PVP 15% |  |  |
| F9-MN CG | Co-grinded Keto 25% PVA 7.5% PVP 5% |  |  |
| F10-MN CG | Co-grinded Keto 25% PVA 7.5% PVP 10% |  |  |
| F11-MN CG | Co-grinded Keto 25% PVA 7.5% PVP 15% |  |  |
| Formulations | Polymer | Height Reduction (%) ± SD |
|---|---|---|
| F1-MN NS | PVA 10% | 13.33 ± 1.655 |
| F2-MN NS | PVA 30% | 18.989 ± 2.935 |
| F3-MN NS | PVA 5% PVP 5% | 14.57 ± 0.631 |
| F4-MN NS | PVA 5% PVP 10% | 12.06 ± 0.481 |
| F5-MN NS | PVA 5% PVP 15% | 4.239 ± 0.452 |
| F6-MN CG | PVA 5% PVP 5% | 6.77 ± 2.79 |
| F7-MN CG | PVA 5% PVP 10% | 2.38 ± 1.52 |
| F8-MN CG | PVA 5% PVP 15% | 0.58 ± 0.21 |
| F9-MN CG | PVA 7.5% PVP 5% | 20.89 ± 1.17 |
| F10-MN CG | PVA 7.5% PVP 10% | 6.20 ± 0.45 |
| F11-MN CG | PVA 7.5% PVP 15% | 1.26 ± 0.56 |
| Formulations | Polymer | Loss of Mass (%) ± SD |
|---|---|---|
| F1-MN NS | PVA 10% | 88.10 ± 0.063 |
| F3-MN NS | PVA 5% PVP 5% | 92.16 ± 2.937 |
| F4-MN NS | PVA 5% PVP 10% | 79.56 ± 0.410 |
| F5-MN NS | PVA 5% PVP 15% | 79.24 ± 0.392 |
| F6-MN CG | PVA 5% PVP 5% | 71.26 ± 0.084 |
| F7-MN CG | PVA 5% PVP 10% | 67.50 ± 0.58 |
| F8-MN CG | PVA 5% PVP 15% | 61.79 ± 1.051 |
| F9-MN CG | PVA 7.5% PVP 5% | 65.66 ± 1.17 |
| F10-MN CG | PVA 7.5% PVP 10% | 59.50 ± 0.365 |
| F11-MN CG | PVA 7.5% PVP 15% | 54.53 ± 1.144 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramadon, D.; Ulayya, F.; Qur’ani, A.S.; Iskandarsyah, I.; Harahap, Y.; Anjani, Q.K.; Aileen, V.; Hartrianti, P.; Donnelly, R.F. Combination of Dissolving Microneedles with Nanosuspension and Co-Grinding for Transdermal Delivery of Ketoprofen. Pharmaceuticals 2023, 16, 378. https://doi.org/10.3390/ph16030378
Ramadon D, Ulayya F, Qur’ani AS, Iskandarsyah I, Harahap Y, Anjani QK, Aileen V, Hartrianti P, Donnelly RF. Combination of Dissolving Microneedles with Nanosuspension and Co-Grinding for Transdermal Delivery of Ketoprofen. Pharmaceuticals. 2023; 16(3):378. https://doi.org/10.3390/ph16030378
Chicago/Turabian StyleRamadon, Delly, Fathin Ulayya, Annisa Sakinah Qur’ani, Iskandarsyah Iskandarsyah, Yahdiana Harahap, Qonita Kurnia Anjani, Vania Aileen, Pietradewi Hartrianti, and Ryan F. Donnelly. 2023. "Combination of Dissolving Microneedles with Nanosuspension and Co-Grinding for Transdermal Delivery of Ketoprofen" Pharmaceuticals 16, no. 3: 378. https://doi.org/10.3390/ph16030378
APA StyleRamadon, D., Ulayya, F., Qur’ani, A. S., Iskandarsyah, I., Harahap, Y., Anjani, Q. K., Aileen, V., Hartrianti, P., & Donnelly, R. F. (2023). Combination of Dissolving Microneedles with Nanosuspension and Co-Grinding for Transdermal Delivery of Ketoprofen. Pharmaceuticals, 16(3), 378. https://doi.org/10.3390/ph16030378