Encapsulation: A Strategy to Deliver Therapeutics and Bioactive Compounds?
Abstract
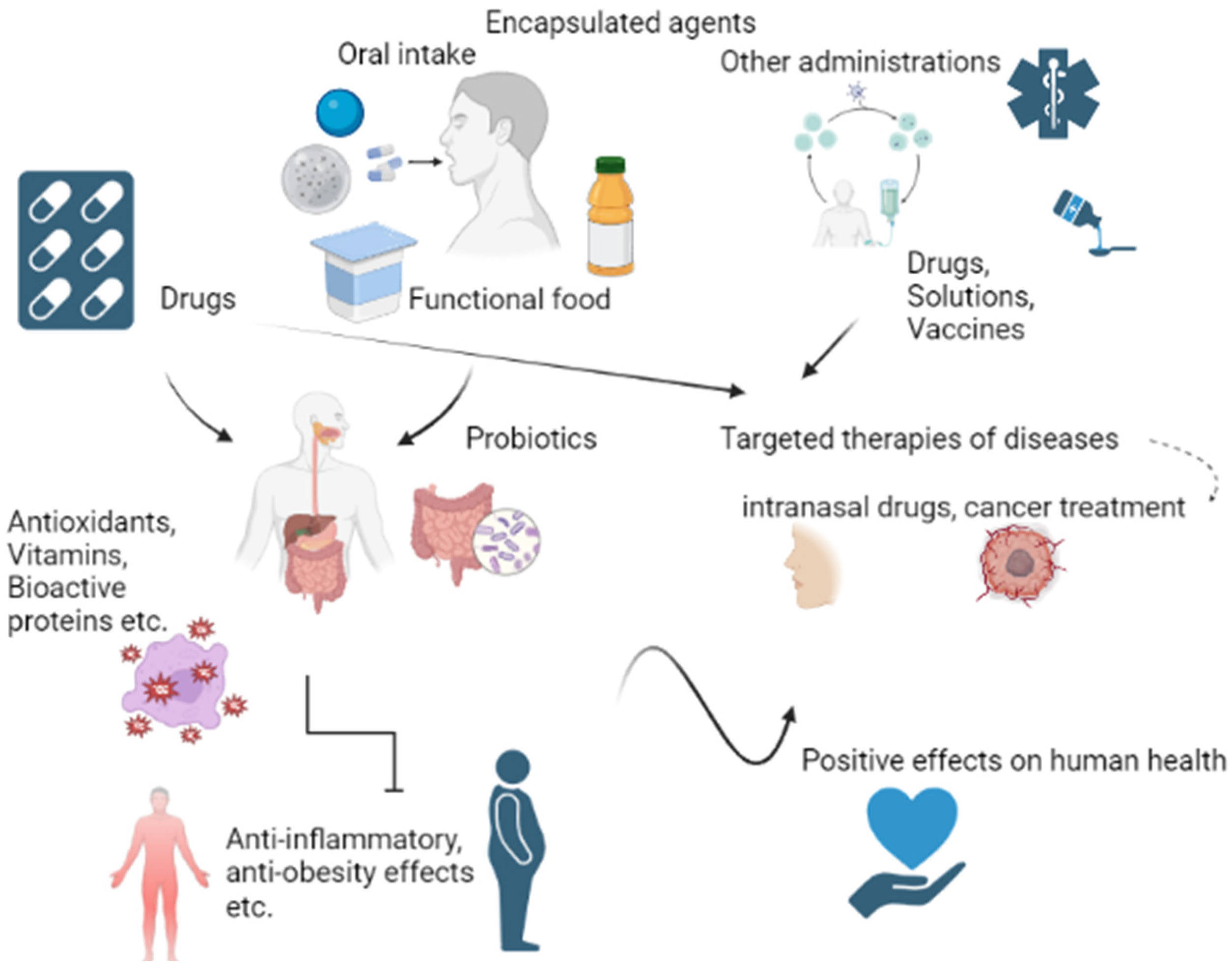
1. Introduction
2. Encapsulated Agents
2.1. Medicine
2.1.1. Drugs
2.1.2. Cells
2.2. Food Supplements and Functional Foods
2.2.1. Vitamins
2.2.2. Probiotics
2.2.3. Extracts
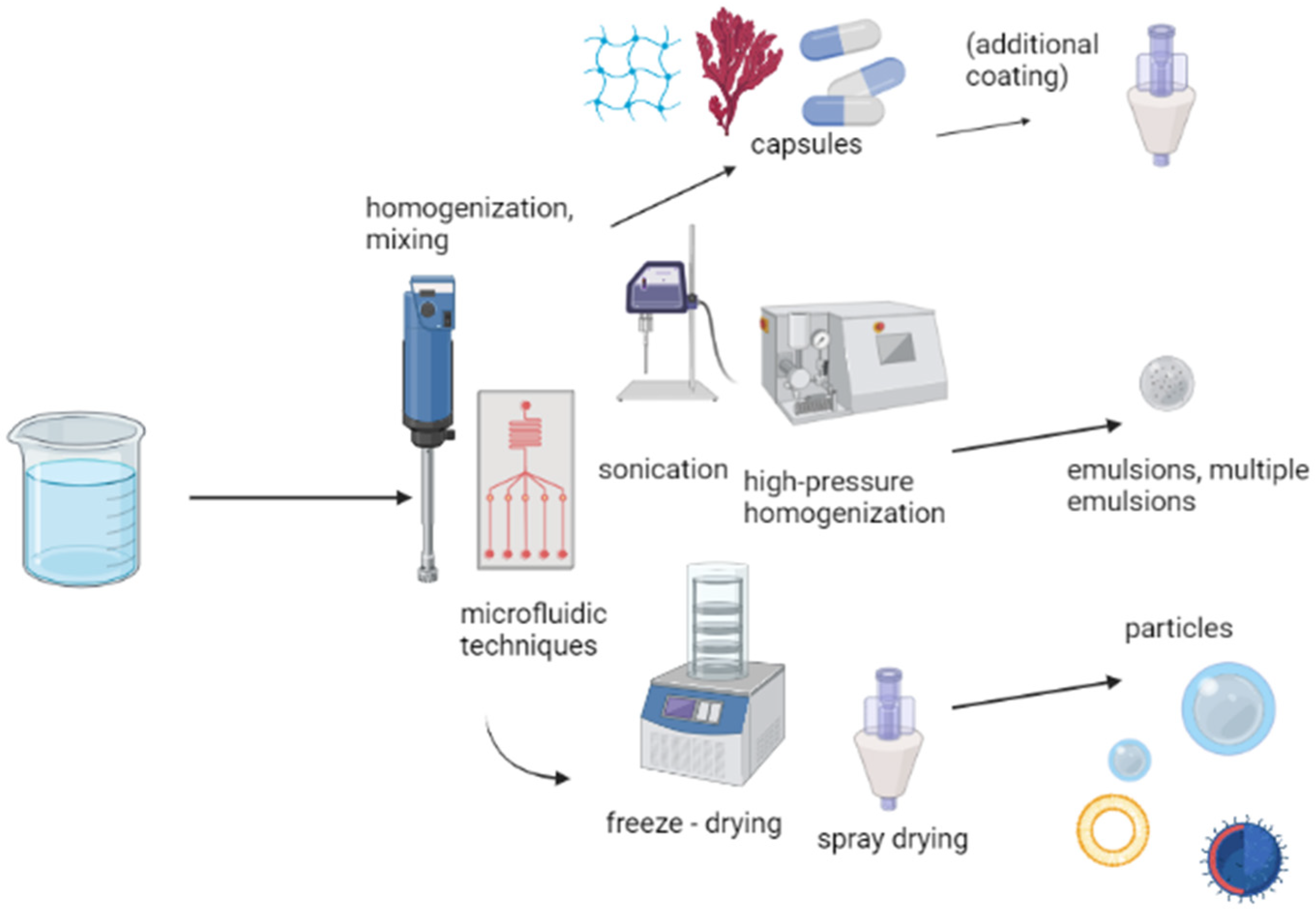
3. Encapsulation Methods and Techniques
3.1. Capsules
3.2. Emulsions
3.2.1. Simple Emulsions
3.2.2. Multiple Emulsions
3.3. Particles
3.3.1. Janus Particles
| Encapsulated Agent | Micro/Nano | Intended Use | Reference |
|---|---|---|---|
| Resveratrol | Micro/Nano (less than 1 µm) | Prevention of ageing, cancer, inflammation, neurodegenerative, and cardiac diseases | [119] |
| Green tea extract | Micro | Prevention of ageing | [120] |
| Quercetin | Micro/Nano (less than 1 µm) | Therapeutic agent and a food component | [121] |
| Alpha-tocopherol (active form of vitamin E) | Micro/Nano (less than 1 µm) | Foods, improvement of light, heat and oxygen stability | [122] |
3.3.2. Liposomes
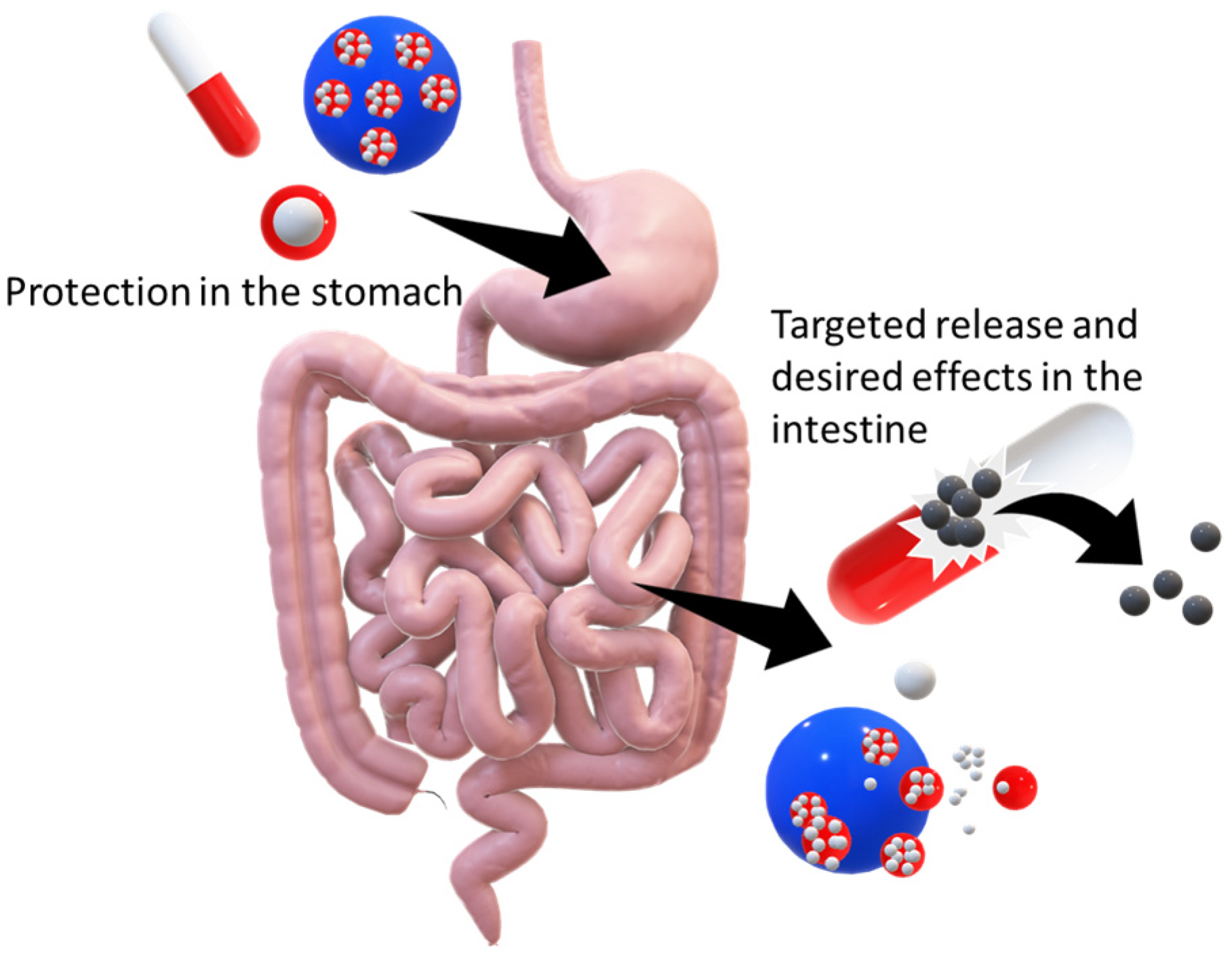
4. Behaviour and Distributions of Dispersions in the Human Body
4.1. Gastrointestinal Tract
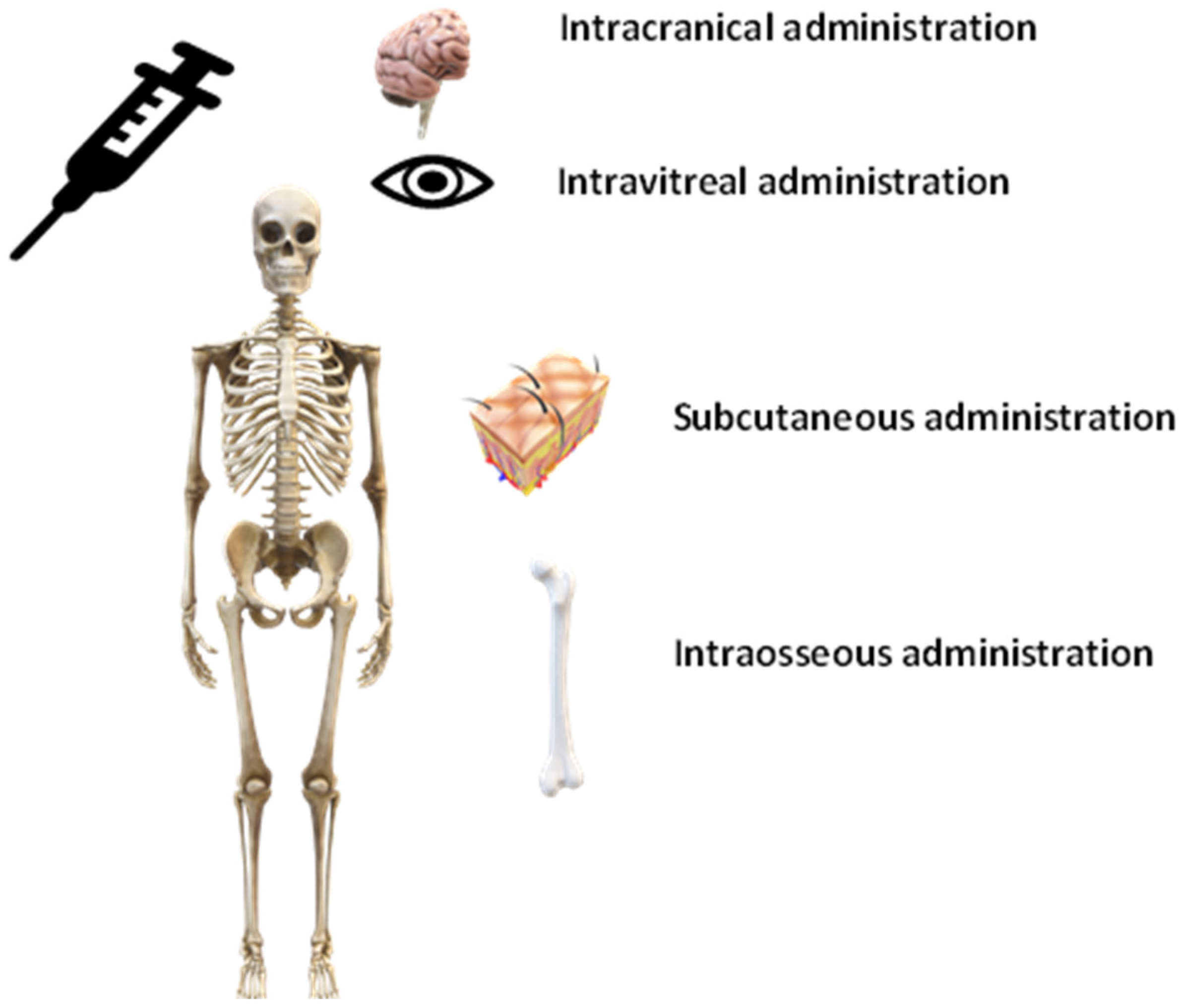
4.2. Other Methods of Administration
4.3. Immunogenic Properties
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rooke, J. Advancing Health Equity With Lifestyle Medicine. Am. J. Lifestyle Med. 2018, 12, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Sanford, A.M. Population Health and Aging. J. Nutr. Health Aging 2019, 23, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Martirosyan, D.; von Brugger, J.; Bialow, S. Functional Food Science: Differences and Similarities with Food Science. Funct. Foods Health Dis. 2021, 11, 408–430. [Google Scholar] [CrossRef]
- Allison, D.B.; Fontaine, K.R.; Manson, J.A.E.; Stevens, J.; VanItallie, T.B. Annual Deaths Attributable to Obesity in the United States. JAMA 1999, 282, 1530–1538. [Google Scholar] [CrossRef]
- WHO. Obesity and Overweight; Fact Sheet No 311 January 2015; WHO: Geneva, Switzerland, 2015.
- Kita, K.; Dittrich, C. Drug Delivery Vehicles with Improved Encapsulation Efficiency: Taking Advantage of Specific Drug-Carrier Interactions. Expert Opin. Drug Deliv. 2011, 8, 329–342. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Hausman-Cohen, S.; Pizano, J.; Schmidt, M.A.; Minich, D.M.; Joffe, Y.; Brandhorst, S.; Evans, S.J.; Brady, D.M. Personalized Nutrition: Translating the Science of NutriGenomics Into Practice: Proceedings From the 2018 American College of Nutrition Meeting. J. Am. Coll. Nutr. 2019, 38, 287–301. [Google Scholar] [CrossRef]
- Martirosyan, D.; Kanya, H.; Nadalet, C. Can Functional Foods Reduce the Risk of Disease? Advancement of Functional Food Definition and Steps to Create Functional Food Products. Funct. Foods Health Dis. 2021, 11, 213–221. [Google Scholar] [CrossRef]
- Otunola, G.A.; Martiryosan, D. Choosing Suitable Food Vehicles for Functional Food Products. Funct. Foods Health Dis. 2021, 11, 44–55. [Google Scholar] [CrossRef]
- Reque, P.M.; Brandelli, A. Encapsulation of Probiotics and Nutraceuticals: Applications in Functional Food Industry. Trends Food Sci. Technol. 2021, 114, 1–10. [Google Scholar] [CrossRef]
- Chen, L.; Yokoyama, W.; Alves, P.; Tan, Y.; Pan, J.; Zhong, F. Effect of Encapsulation on β-Carotene Absorption and Metabolism in Mice. Food Hydrocoll. 2021, 121, 107009. [Google Scholar] [CrossRef]
- Madene, A.; Jacquot, M.; Scher, J.; Desobry, S. Flavour Encapsulation and Controlled Release—A Review. Int. J. Food Sci. Technol. 2006, 41, 1–21. [Google Scholar] [CrossRef]
- Speranza, B.; Petruzzi, L.; Bevilacqua, A.; Gallo, M.; Campaniello, D.; Sinigaglia, M.; Corbo, M.R. Encapsulation of Active Compounds in Fruit and Vegetable Juice Processing: Current State and Perspectives. J. Food Sci. 2017, 82, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Abd El Kader, A.E.; Abu Hashish, H.M. Encapsulation Techniques of Food Bioproduct. Egypt. J. Chem. 2020, 63, 1881–1909. [Google Scholar] [CrossRef]
- Bhujbal, S.V.; de Vos, P.; Niclou, S.P. Drug and Cell Encapsulation: Alternative Delivery Options for the Treatment of Malignant Brain Tumors. Adv. Drug Deliv. Rev. 2014, 67–68, 142–153. [Google Scholar] [CrossRef]
- Li, W.; Lei, X.; Feng, H.; Li, B.; Kong, J.; Xing, M. Layer-by-Layer Cell Encapsulation for Drug Delivery: The History, Technique Basis, and Applications. Pharmaceutics 2022, 14, 297. [Google Scholar] [CrossRef]
- Risch, S.J. Encapsulation: Overview of Uses and Techniques; ACS Publications: Washington, DC, USA, 1995. [Google Scholar]
- Subirana, M.; Solá, I.; Garcia, J.M.; Gich, I.; Urrútia, G. A Nursing Qualitative Systematic Review Required MEDLINE and CINAHL for Study Identification. J. Clin. Epidemiol. 2005, 58, 20–25. [Google Scholar] [CrossRef]
- Gasparyan, A.Y.; Ayvazyan, L.; Blackmore, H.; Kitas, G.D. Writing a Narrative Biomedical Review: Considerations for Authors, Peer Reviewers, and Editors. Rheumatol. Int. 2011, 31, 1409–1417. [Google Scholar] [CrossRef]
- Tan, M.X.L.; Danquah, M.K. Drug and Protein Encapsulation by Emulsification: Technology Enhancement Using Foam Formulations. Chem. Eng. Technol. 2012, 35, 618–626. [Google Scholar] [CrossRef]
- Martínez Rivas, C.J.; Tarhini, M.; Badri, W.; Miladi, K.; Greige-Gerges, H.; Nazari, Q.A.; Galindo Rodríguez, S.A.; Román, R.Á.; Fessi, H.; Elaissari, A. Nanoprecipitation Process: From Encapsulation to Drug Delivery. Int. J. Pharm. 2017, 532, 66–81. [Google Scholar] [CrossRef]
- Yadav, N.; Francis, A.P.; Priya, V.V.; Patil, S.; Mustaq, S.; Khan, S.S.; Alzahrani, K.J.; Banjer, H.J.; Mohan, S.K.; Mony, U.; et al. Polysaccharide-Drug Conjugates: A Tool for Enhanced Cancer Therapy. Polymers 2022, 14, 950. [Google Scholar] [CrossRef]
- Ge, D.; Zou, L.; Li, C.; Liu, S.; Li, S.; Sun, S.; Ding, W. Simulation of the Osmosis-Based Drug Encapsulation in Erythrocytes. Eur. Biophys. J. 2018, 47, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Alhajamee, M.; Marai, K.; al Abbas, S.M.N.; Homayouni Tabrizi, M. Co-Encapsulation of Curcumin and Tamoxifen in Lipid-Chitosan Hybrid Nanoparticles for Cancer Therapy. Mater. Technol. 2022, 37, 1183–1194. [Google Scholar] [CrossRef]
- Kim, M.R.; Feng, T.; Zhang, Q.; Chan, H.Y.E.; Chau, Y. Co-Encapsulation and Co-Delivery of Peptide Drugs via Polymeric Nanoparticles. Polymers 2019, 11, 288. [Google Scholar] [CrossRef]
- Gómez-Gaete, C.; Lorena Bustos, G.; Ricardo Godoy, R.; Katia Saez, C.; Pedro Novoa, G.; Marcos Fernández, E.; Tsapis, N.; Fattal, E. Successful Factorial Design for the Optimization of Methylprednisolone Encapsulation in Biodegradable Nanoparticles. Drug Dev. Ind. Pharm. 2013, 39, 310–320. [Google Scholar] [CrossRef]
- Hunt, N.C.; Grover, L.M. Cell Encapsulation Using Biopolymer Gels for Regenerative Medicine. Biotechnol. Lett. 2010, 32, 733–742. [Google Scholar] [CrossRef]
- Ghidoni, I.; Chlapanidas, T.; Bucco, M.; Crovato, F.; Marazzi, M.; Vigo, D.; Torre, M.L.; Faustini, M. Alginate Cell Encapsulation: New Advances in Reproduction and Cartilage Regenerative Medicine. Cytotechnology 2008, 58, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Gazda, L.S.; Vinerean, H.V.; Laramore, M.A.; Diehl, C.H.; Hall, R.D.; Rubin, A.L.; Smith, B.H. Encapsulation of Porcine Islets Permits Extended Culture Time and Insulin Independence in Spontaneously Diabetic BB Rats. Cell Transplant. 2007, 16, 609–620. [Google Scholar] [CrossRef]
- Lahooti, S.; Sefton, M.V. Effect of an Immobilization Matrix and Capsule Membrane Permeability on the Viability of Encapsulated HEK Cells. Biomaterials 2000, 21, 987–995. [Google Scholar] [CrossRef]
- Hortelano, G.; Al-Hendy, A.; Ofosu, F.A.; Chang, P.L. Delivery of Human Factor IX in Mice by Encapsulated Recombinant Myoblasts: A Novel Approach towards Allogeneic Gene Therapy of Hemophilia B. Blood 1996, 87, 5095–5103. [Google Scholar] [CrossRef]
- Liu, H.W.; Ofosu, F.A.; Chang, P.L. Expression of Human Factor IX by Microencapsulated Recombinant Fibroblasts. Hum. Gene Ther. 1993, 4, 291–301. [Google Scholar] [CrossRef]
- Cheema, U.; Nazhat, S.N.; Alp, B.; Foroughi, F.; Anandagoda, N.; Mudera, V.; Brown, R.A. Fabricating Tissues: Analysis of Farming versus Engineering Strategies. Biotechnol. Bioprocess Eng. 2007, 12, 9–14. [Google Scholar] [CrossRef]
- Hunt, N.C.; Shelton, R.M.; Grover, L.M. An Alginate Hydrogel Matrix for the Localised Delivery of a Fibroblast/Keratinocyte Co-Culture. Biotechnol. J. 2009, 4, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Bitar, M.; Salih, V.; Brown, R.A.; Nazhat, S.N. Effect of Multiple Unconfined Compression on Cellular Dense Collagen Scaffolds for Bone Tissue Engineering. J. Mater. Sci. Mater. Med. 2007, 18, 237–244. [Google Scholar] [CrossRef]
- Chawda, P.J.; Shi, J.; Xue, S.; Young Quek, S. Co-Encapsulation of Bioactives for Food Applications. Food Qual. Saf. 2017, 1, 302–309. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Estevinho, B.N.; Rocha, F. The Progress and Application of Vitamin E Encapsulation—A Review. Food Hydrocoll. 2021, 121, 106998. [Google Scholar] [CrossRef]
- Gonnet, M.; Lethuaut, L.; Boury, F. New Trends in Encapsulation of Liposoluble Vitamins. J. Control. Release 2010, 146, 276–290. [Google Scholar] [CrossRef]
- Wojtczak, E.; Gadzinowski, M.; Makowski, T.; Maresz, K.; Kubisa, P.; Bednarek, M.; Pluta, M. Encapsulation of Hydrophobic Vitamins by Polylactide Stereocomplexation and Their Release Study. Polym. Int. 2018, 67, 1523–1534. [Google Scholar] [CrossRef]
- Mujica-Álvarez, J.; Gil-Castell, O.; Barra, P.A.; Ribes-Greus, A.; Bustos, R.; Faccini, M.; Matiacevich, S. Encapsulation of Vitamins A and e as Spray-Dried Additives for the Feed Industry. Molecules 2020, 25, 1357. [Google Scholar] [CrossRef]
- Ivana, M.; SKOVáPetra, M.; Dana, B.; Klára, P.; Jitka, B.; Jana, H.; SOVáPavla, B. Preparation and Stability of Organic Core-Shell Particles with Encapsulated Complex Natural Plant Sources of Phenolics, Caffeine and Vitamins. In Proceedings of the NANOCON 2014—Conference Proceedings, 6th International Conference, Brno, Czech Republic, 5–7 November 2014. [Google Scholar]
- Zhang, W.; Zhang, L.; Zhu, D.; Wu, Y.; Qin, Y.; Ou, W.; Song, L.; Zhang, Q. Influence of Composition on the Encapsulation Properties of P/O/W Multiple Emulsions for Vitamin C. J. Dispers. Sci. Technol. 2019, 40, 1637–1644. [Google Scholar] [CrossRef]
- Yan, B.; Davachi, S.M.; Ravanfar, R.; Dadmohammadi, Y.; Deisenroth, T.W.; van Pho, T.; Odorisio, P.A.; Darji, R.H.; Abbaspourrad, A. Improvement of Vitamin C Stability in Vitamin Gummies by Encapsulation in Casein Gel. Food Hydrocoll. 2021, 113, 106414. [Google Scholar] [CrossRef]
- Binda, S.; Hill, C.; Johansen, E.; Obis, D.; Pot, B.; Sanders, M.E.; Tremblay, A.; Ouwehand, A.C. Criteria to Qualify Microorganisms as “Probiotic” in Foods and Dietary Supplements. Front. Microbiol. 2020, 11, 1662. [Google Scholar] [CrossRef] [PubMed]
- Šipailienė, A.; Petraitytė, S. Encapsulation of Probiotics: Proper Selection of the Probiotic Strain and the Influence of Encapsulation Technology and Materials on the Viability of Encapsulated Microorganisms. Probiot. Antimicrob. Proteins 2018, 10, 1–10. [Google Scholar] [CrossRef]
- Martín, M.J.; Lara-Villoslada, F.; Ruiz, M.A.; Morales, M.E. Microencapsulation of Bacteria: A Review of Different Technologies and Their Impact on the Probiotic Effects. Innov. Food Sci. Emerg. Technol. 2015, 27, 15–25. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Bahrami, A.; Rehman, A.; Rezaei, A.; Babazadeh, A.; Singh, H.; Jafari, S.M. Co-Encapsulation of Probiotics with Prebiotics and Their Application in Functional/Synbiotic Dairy Products. Crit. Rev. Food Sci. Nutr. 2022, 62, 2470–2494. [Google Scholar] [CrossRef]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, Prebiotics and Synbiotics—A Review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef]
- Fiocco, D.; Longo, A.; Arena, M.P.; Russo, P.; Spano, G.; Capozzi, V. How Probiotics Face Food Stress: They Get by with a Little Help. Crit. Rev. Food Sci. Nutr. 2020, 60, 1552–1580. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.M.C.; Gibson, G.R.; Rowland, I. Health Benefits of Probiotics: Are Mixtures More Effective than Single Strains? Eur. J. Nutr. 2011, 50, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, R.; Shah, N.P. Immune System Stimulation by Probiotic Microorganisms. Crit. Rev. Food Sci. Nutr. 2014, 54, 938–956. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.J.; Cedran, M.F.; Bicas, J.L.; Sato, H.H. Encapsulated Probiotic Cells: Relevant Techniques, Natural Sources as Encapsulating Materials and Food Applications—A Narrative Review. Food Res. Int. 2020, 137, 109682. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The Pros, Cons, and Many Unknowns of Probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Didari, T.; Solki, S.; Mozaffari, S.; Nikfar, S.; Abdollahi, M. A Systematic Review of the Safety of Probiotics. Expert Opin. Drug Saf. 2014, 13, 227–239. [Google Scholar] [CrossRef]
- Armendáriz-Barragán, B.; Zafar, N.; Badri, W.; Galindo-Rodríguez, S.A.; Kabbaj, D.; Fessi, H.; Elaissari, A. Plant Extracts: From Encapsulation to Application. Expert Opin. Drug Deliv. 2016, 13, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Bryła, A.; Lewandowicz, G.; Juzwa, W. Encapsulation of Elderberry Extract into Phospholipid Nanoparticles. J. Food Eng. 2015, 167, 189–195. [Google Scholar] [CrossRef]
- Castromonte, M.; Wacyk, J.; Valenzuela, C. Encapsulation of Antioxidant Extracts from Agroindustrial By-Products: A Review. Rev. Chil. Nutr. 2020, 47, 836–847. [Google Scholar] [CrossRef]
- Vinceković, M.; Viskić, M.; Jurić, S.; Giacometti, J.; Bursać Kovačević, D.; Putnik, P.; Donsì, F.; Barba, F.J.; Režek Jambrak, A. Innovative Technologies for Encapsulation of Mediterranean Plants Extracts. Trends Food Sci. Technol. 2017, 69, 1–12. [Google Scholar] [CrossRef]
- Su, T.; Huang, C.; Yang, C.; Jiang, T.; Su, J.; Chen, M.; Fatima, S.; Gong, R.; Hu, X.; Bian, Z.; et al. Apigenin Inhibits STAT3/CD36 Signaling Axis and Reduces Visceral Obesity. Pharmacol. Res. 2020, 152, 104586. [Google Scholar] [CrossRef]
- Kandasamy, S.; Naveen, R. A Review on the Encapsulation of Bioactive Components Using Spray-drying and Freeze-drying Techniques. J. Food Process Eng. 2022, 45, e14059. [Google Scholar] [CrossRef]
- Kaushal, A.M.; Gupta, P.; Bansal, A.K. Amorphous Drug Delivery Systems: Molecular Aspects, Design, and Performance. Crit. Rev. Ther. Drug Carr. Syst. 2004, 21, 62. [Google Scholar] [CrossRef]
- Jing, Z.; Ma, Y.; Zhu, J. Application of a Novel Electrostatic Dry Powder Coating Technology on Capsules for Enteric Release. J. Drug Deliv. Sci. Technol. 2022, 68, 103058. [Google Scholar] [CrossRef]
- Oliveira, S.M.; Gruppi, A.; Vieira, M.V.; Matos, G.S.; Vicente, A.A.; Teixeira, J.A.C.; Fuciños, P.; Spigno, G.; Pastrana, L.M. How Additive Manufacturing Can Boost the Bioactivity of Baked Functional Foods. J. Food Eng. 2021, 294, 110394. [Google Scholar] [CrossRef]
- Di, A.; Zhang, S.; Liu, X.; Tong, Z.; Sun, S.; Tang, Z.; Chen, X.D.; Wu, W.D. Microfluidic Spray Dried and Spray Freeze Dried Uniform Microparticles Potentially for Intranasal Drug Delivery and Controlled Release. Powder Technol. 2021, 379, 144–153. [Google Scholar] [CrossRef]
- Ke, W.R.; Kwok, P.C.L.; Khanal, D.; Chang, R.Y.K.; Chan, H.K. Co-Spray Dried Hydrophobic Drug Formulations with Crystalline Lactose for Inhalation Aerosol Delivery. Int. J. Pharm. 2021, 602, 120608. [Google Scholar] [CrossRef] [PubMed]
- Slavutsky, A.M.; Chávez, M.C.; Favaro-trindade, C.S.; Bertuzzi, M.A. Encapsulation of Lactobacillus Acidophilus in a Pilot-Plant Spray-Dryer. Effect of Process Parameters on Cell Viability. J. Food Process Eng. 2017, 40, e12394. [Google Scholar] [CrossRef]
- Ceja-Medina, L.I.; Ortiz-Basurto, R.I.; Medina-Torres, L.; Calderas, F.; Bernad-Bernad, M.J.; González-Laredo, R.F.; Ragazzo-Sánchez, J.A.; Calderón-Santoyo, M.; González-ávila, M.; Andrade-González, I.; et al. Microencapsulation of Lactobacillus plantarum by Spray Drying with Mixtures of Aloe Vera Mucilage and Agave Fructans as Wall Materials. J. Food Process Eng. 2020, 43, e13436. [Google Scholar] [CrossRef]
- Anthero, A.G.d.S.; Bezerra, E.O.; Comunian, T.A.; Procópio, F.R.; Hubinger, M.D. Effect of Modified Starches and Gum Arabic on the Stability of Carotenoids in Paprika Oleoresin Microparticles. Dry. Technol. 2021, 39, 1927–1940. [Google Scholar] [CrossRef]
- Roque, M.; Geraldes, D.; da Silva, C.; Oliveira, M.; Nascimento, L. Long-Circulating and Fusogenic Liposomes Loaded with Paclitaxel and Doxorubicin: Effect of Excipient, Freezing, and Freeze-Drying on Quality Attributes. Pharmaceutics 2022, 15, 86. [Google Scholar] [CrossRef]
- Sweeney, L.G.; Wang, Z.; Loebenberg, R.; Wong, J.P.; Lange, C.F.; Finlay, W.H. Spray-Freeze-Dried Liposomal Ciprofloxacin Powder for Inhaled Aerosol Drug Delivery. Int. J. Pharm. 2005, 305, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Zuanon, L.A.C.; Malacrida, C.R.; Telis, V.R.N. Effect of Ultrasound on the Stability of Turmeric Oleoresin Microencapsulated in Gelatin-Collagen Matrices. J. Food Process Eng. 2017, 40, e12360. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, L.; Tan, X.; Wu, Z.; Zhou, N.; Dong, N.; Zhang, Y.; Yin, T.; He, H.; Gou, J.; et al. Preclinical Evaluations of Norcantharidin Liposome and Emulsion Hybrid Delivery System with Improved Encapsulation Efficiency and Enhanced Antitumor Activity. Expert Opin. Drug Deliv. 2022, 19, 451–464. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, E.; Xie, Y.; Liu, D.; Hu, Y.; Shi, Z.; Xiong, C.; Yang, Q. Hydrangea-like Nanocellulose Microspheres with High Dye Adsorption and Drug Encapsulation Prepared by Emulsion Method. Carbohydr. Polym. 2022, 296, 119947. [Google Scholar] [CrossRef]
- Debotton, N.; Garsiani, S.; Cohen, Y.; Dahan, A. Enabling Oral Delivery of Antiviral Drugs: Double Emulsion Carriers to Improve the Intestinal Absorption of Zanamivir. Int. J. Pharm. 2022, 629, 122392. [Google Scholar] [CrossRef] [PubMed]
- Seddari, S.; ben Seghier, N.E.W.; Moulai-Mostefa, N. Formulation and Characterization of W/O/W Crystallizable Double Emulsions Stabilized by OSA Starch/Xanthan Gum Mixture as Drug Delivery Systems. J. Dispers. Sci. Technol. 2022, 1–9. [Google Scholar] [CrossRef]
- Kabakci, C.; Sumnu, G.; Sahin, S.; Oztop, M.H. Encapsulation of Magnesium with Lentil Flour by Using Double Emulsion to Produce Magnesium Enriched Cakes. Food Bioprocess Technol. 2021, 14, 1773–1790. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, Y.; Liu, H.; McClements, D.J.; Cheng, C.; Zou, L.; Liu, W.; Liu, W. Probiotic Encapsulation in Water-in-Oil High Internal Phase Emulsions: Enhancement of Viability under Food and Gastrointestinal Conditions. LWT 2022, 163, 113499. [Google Scholar] [CrossRef]
- Varankovich, N.V.; Khan, N.H.; Nickerson, M.T.; Kalmokoff, M.; Korber, D.R. Evaluation of Pea Protein-Polysaccharide Matrices for Encapsulation of Acid-Sensitive Bacteria. Food Res. Int. 2015, 70, 118–124. [Google Scholar] [CrossRef]
- Sun, W.; Griffiths, M.W. Survival of Bifidobacteria in Yogurt and Simulated Gastric Juice Following Immobilization in Gellan-Xanthan Beads. Int. J. Food Microbiol. 2000, 61, 17–25. [Google Scholar] [CrossRef]
- Ashimova, A.; Yegorov, S.; Negmetzhanov, B.; Hortelano, G. Cell Encapsulation Within Alginate Microcapsules: Immunological Challenges and Outlook. Front. Bioeng. Biotechnol. 2019, 7, 380. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.S.; Veloso, C.M. Microencapsulation of Natural Dyes with Biopolymers for Application in Food: A Review. Food Hydrocoll. 2021, 112, 106374. [Google Scholar] [CrossRef]
- Castro-Rosas, J.; Ferreira-Grosso, C.R.; Gómez-Aldapa, C.A.; Rangel-Vargas, E.; Rodríguez-Marín, M.L.; Guzmán-Ortiz, F.A.; Falfan-Cortes, R.N. Recent Advances in Microencapsulation of Natural Sources of Antimicrobial Compounds Used in Food—A Review. Food Res. Int. 2017, 102, 575–587. [Google Scholar] [CrossRef]
- Da Silva, P.T.; Fries, L.L.M.; de Menezes, C.R.; Holkem, A.T.; Schwan, C.L.; Wigmann, É.F.; Bastos, J.D.O.; Silva, C.D.B.D. Microencapsulation: Concepts, Mechanisms, Methods and Some Applications in Food Technology. Ciência Rural 2014, 44, 1304–1311. [Google Scholar] [CrossRef]
- Yang, T.; Qin, W.; Zhang, Q.; Luo, J.; Lin, D.; Chen, H. Essential-Oil Capsule Preparation and Its Application in Food Preservation: A Review. Food Rev. Int. 2022, 1–35. [Google Scholar] [CrossRef]
- Akdeniz, B.; Sumnu, G.; Sahin, S. Microencapsulation of Phenolic Compounds Extracted from Onion (Allium Cepa) Skin. J. Food Process. Preserv. 2018, 42, e13648. [Google Scholar] [CrossRef]
- Betz, M.; Kulozik, U. Microencapsulation of Bioactive Bilberry Anthocyanins by Means of Whey Protein Gels. Procedia Food Sci. 2011, 1, 2047–2056. [Google Scholar] [CrossRef]
- Narin, C.; Ertugrul, U.; Tas, O.; Sahin, S.; Oztop, M.H. Encapsulation of Pea Protein in an Alginate Matrix by Cold Set Gelation Method and Use of the Capsules in Fruit Juices. J. Food Sci. 2020, 85, 3423–3431. [Google Scholar] [CrossRef]
- Vega-Sagardía, M.; Rocha, J.; Sáez, K.; Smith, C.T.; Gutierrez-Zamorano, C.; García-Cancino, A. Encapsulation, with and without Oil, of Biofilm Forming Lactobacillus fermentum UCO-979C Strain in Alginate-Xanthan Gum and Its Anti-Helicobacter Pylori Effect. J. Funct. Foods 2018, 46, 504–513. [Google Scholar] [CrossRef]
- Petraitytė, S.; Šipailienė, A. Enhancing Encapsulation Efficiency of Alginate Capsules Containing Lactic Acid Bacteria by Using Different Divalent Cross-Linkers Sources. LWT 2019, 110, 307–315. [Google Scholar] [CrossRef]
- Lamsen, M.R.L.; Wang, T.; D’Souza, D.; Dia, V.; Chen, G.; Zhong, Q. Encapsulation of Vitamin D3 in Gum Arabic to Enhance Bioavailability and Stability for Beverage Applications. J. Food Sci. 2020, 85, 2368–2379. [Google Scholar] [CrossRef]
- Dimopoulos, G.; Katsimichas, A.; Tsimogiannis, D.; Oreopoulou, V.; Taoukis, P. Cell Permeabilization Processes for Improved Encapsulation of Oregano Essential Oil in Yeast Cells. J. Food Eng. 2021, 294, 110408. [Google Scholar] [CrossRef]
- Narasimha Murthy, S.; Shivakumar, H.N. CHAPTER 1—Topical and Transdermal Drug Delivery A2. In Handbook of Non-Invasive Drug Delivery Systems; Kulkarni, V.S., Ed.; Personal Care & Cosmetic Technology; William Andrew Publishing: Norwich, NY, USA, 2010. [Google Scholar]
- Lu, W.; Kelly, A.L.; Miao, S. Emulsion-Based Encapsulation and Delivery Systems for Polyphenols. Trends Food Sci. Technol. 2016, 47, 1–9. [Google Scholar] [CrossRef]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An Advanced Mode of Drug Delivery System. 3 Biotech 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Dickinson, E. Advances in Food Emulsions and Foams: Reflections on Research in the Neo-Pickering Era. Curr. Opin. Food Sci. 2020, 33, 52–60. [Google Scholar] [CrossRef]
- Klojdová, I.; Stathopoulos, C. The Potential Application of Pickering Multiple Emulsions in Food. Foods 2022, 11, 1558. [Google Scholar] [CrossRef]
- Aserin, A. Multiple Emulsions: Technology and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Dickinson, E. Double Emulsions Stabilized by Food Biopolymers. Food Biophys. 2011, 6, 1–11. [Google Scholar] [CrossRef]
- Muschiolik, G.; Dickinson, E. Double Emulsions Relevant to Food Systems: Preparation, Stability, and Applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 532–555. [Google Scholar] [CrossRef]
- Sarkar, A.; Dickinson, E. Sustainable Food-Grade Pickering Emulsions Stabilized by Plant-Based Particles. Curr. Opin. Colloid Interface Sci. 2020, 49, 69–81. [Google Scholar] [CrossRef]
- Muschiolik, G. Multiple Emulsions for Food Use. Curr. Opin. Colloid Interface Sci. 2007, 12, 213–220. [Google Scholar] [CrossRef]
- Klojdová, I.; Štětina, J.; Horáčková, Š. W/O/W Multiple Emulsions as the Functional Component of Dairy Products. Chem. Eng. Technol. 2019, 42, 715–727. [Google Scholar] [CrossRef]
- Matos, M.; Gutiérrez, G.; Martínez-Rey, L.; Iglesias, O.; Pazos, C. Encapsulation of Resveratrol Using Food-Grade Concentrated Double Emulsions: Emulsion Characterization and Rheological Behaviour. J. Food Eng. 2018, 226, 73–81. [Google Scholar] [CrossRef]
- Hemar, Y.; Cheng, L.J.; Oliver, C.M.; Sanguansri, L.; Augustin, M. Encapsulation of Resveratrol Using Water-in-Oil-in-Water Double Emulsions. Food Biophys. 2010, 5, 120–127. [Google Scholar] [CrossRef]
- Akhtar, M.; Murray, B.S.; Afeisume, E.I.; Khew, S.H. Encapsulation of Flavonoid in Multiple Emulsion Using Spinning Disc Reactor Technology. Food Hydrocoll. 2014, 34, 62–67. [Google Scholar] [CrossRef]
- Mishra, B.; Panyam, J.; Sharma, A.v. Mechanisms of Drug Release Control by Osmotic Additives from Multiple w/o/w Emulsions Containing Diclofenac Sodium. Acta Pharm. Turc. 1999, 41, 58–61. [Google Scholar]
- Lindenstruth, K.; Müller, B.W. W/O/W Multiple Emulsions with Diclofenac Sodium. Eur. J. Pharm. Biopharm. 2004, 58, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Dluska, E.; Metera, A.; Markowska-Radomska, A.; Tudek, B. Effective Cryopreservation and Recovery of Living Cells Encapsulated in Multiple Emulsions. Biopreserv. Biobank. 2019, 17, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Cournarie, F.; Rosilio, V.; Chéron, M.; Vauthier, C.; Lacour, B.; Grossiord, J.L.; Seiller, M. Improved Formulation of W/O/W Multiple Emulsion for Insulin Encapsulation. Influence of the Chemical Structure of Insulin. Colloid Polym. Sci. 2004, 282, 562–568. [Google Scholar] [CrossRef]
- Verma, K.; Pandey, S.P.; Mishra, P. Development of Multiple Emulsion of Andrographolide for Taste Masking. Asian J. Pharm. 2018, 12, S1501. [Google Scholar]
- Suñer-Carbó, J.; Calpena-Campmany, A.; Halbaut-Bellowa, L.; Clares-Naveros, B.; Rodriguez-Lagunas, M.J.; Barbolini, E.; Zamarbide-Losada, J.; Boix-Montañés, A. Biopharmaceutical Development of a Bifonazole Multiple Emulsion for Enhanced Epidermal Delivery. Pharmaceutics 2019, 11, 66. [Google Scholar] [CrossRef]
- Brannon-Peppas, L.; Ghosn, B.; Roy, K.; Cornetta, K. Encapsulation of Nucleic Acids and Opportunities for Cancer Treatment. Pharm. Res. 2007, 24, 618–627. [Google Scholar] [CrossRef]
- Kalani, M.; Yunus, R. Application of Supercritical Antisolvent Method in Drug Encapsulation: A Review. Int. J. Nanomed. 2011, 6, 1429–1442. [Google Scholar] [CrossRef]
- Campuzano, S.; Esteban-Fernández De Ávila, B.; Yáñez-Sedeño, P.; Pingarrón, J.M.; Wang, J. Nano/Microvehicles for Efficient Delivery and (Bio)Sensing at the Cellular Level. Chem. Sci. 2017, 8, 6750–6763. [Google Scholar] [CrossRef]
- Hu, J.; Zhou, S.; Sun, Y.; Fang, X.; Wu, L. Fabrication, Properties and Applications of Janus Particles. Chem. Soc. Rev. 2012, 41, 4356–4378. [Google Scholar] [CrossRef]
- Lim, Y.G.J.; Poh, K.C.W.; Loo, S.C.J. Hybrid Janus Microparticles Achieving Selective Encapsulation for Theranostic Applications via a Facile Solvent Emulsion Method. Macromol. Rapid Commun. 2019, 40, 1800801. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zhao, X.; Sun, M.; Hao, H. Research Advances in the Synthesis, Application, Assembly, and Calculation of Janus Materials. Ind. Eng. Chem. Res. 2021, 60, 1071–1095. [Google Scholar] [CrossRef]
- Zhou, W.; Sun, W.; Yang, P. Preparation and Functional Application of Janus Particles. Prog. Chem. 2018, 30, 1601–1614. [Google Scholar]
- Istenič, K.; Balanč, B.D.; Djordjević, V.B.; Bele, M.; Nedović, V.A.; Bugarski, B.M.; Ulrih, N.P. Encapsulation of Resveratrol into Ca-Alginate Submicron Particles. J. Food Eng. 2015, 167, 196–203. [Google Scholar] [CrossRef]
- Elabbadi, A.; Jeckelmann, N.; Haefliger, O.P.; Ouali, L. Complexation/Encapsulation of Green Tea Polyphenols in Mixed Calcium Carbonate and Phosphate Micro-Particles. J. Microencapsul. 2011, 28, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ghatak, D.; Iyyaswami, R. Selective Encapsulation of Quercetin from Dry Onion Peel Crude Extract in Reassembled Casein Particles. Food Bioprod. Process. 2019, 115, 100–109. [Google Scholar] [CrossRef]
- Fang, Z.; Xu, X.; Cheng, H.; Li, J.; Guang, C.; Liang, L. Comparison of Whey Protein Particles and Emulsions for the Encapsulation and Protection of α-Tocopherol. J. Food Eng. 2019, 247, 56–63. [Google Scholar] [CrossRef]
- Gonzalez Gomez, A.; Hosseinidoust, Z. Liposomes for Antibiotic Encapsulation and Delivery. ACS Infect. Dis. 2020, 6, 896–908. [Google Scholar] [CrossRef]
- Eloy, J.O.; Claro de Souza, M.; Petrilli, R.; Barcellos, J.P.A.; Lee, R.J.; Marchetti, J.M. Liposomes as Carriers of Hydrophilic Small Molecule Drugs: Strategies to Enhance Encapsulation and Delivery. Colloids Surf. B Biointerfaces 2014, 123, 345–363. [Google Scholar] [CrossRef]
- Lin, W.; Goldberg, R.; Klein, J. Poly-Phosphocholination of Liposomes Leads to Highly-Extended Retention Time in Mice Joints. J. Mater. Chem. B 2022, 10, 2820–2827. [Google Scholar] [CrossRef]
- Sherry, M.; Charcosset, C.; Fessi, H.; Greige-Gerges, H. Essential Oils Encapsulated in Liposomes: A Review. J. Liposome Res. 2013, 23, 268–275. [Google Scholar] [CrossRef]
- Mohammadi, A.; Jafari, S.M.; Mahoonak, A.S.; Ghorbani, M. Liposomal/Nanoliposomal Encapsulation of Food-Relevant Enzymes and Their Application in the Food Industry. Food Bioprocess Technol. 2021, 14, 23–38. [Google Scholar] [CrossRef]
- Mohan, A.; McClements, D.J.; Udenigwe, C.C. Encapsulation of Bioactive Whey Peptides in Soy Lecithin-Derived Nanoliposomes: Influence of Peptide Molecular Weight. Food Chem. 2016, 213, 143–148. [Google Scholar] [CrossRef]
- Tavakoli, H.; Hosseini, O.; Jafari, S.M.; Katouzian, I. Evaluation of Physicochemical and Antioxidant Properties of Yogurt Enriched by Olive Leaf Phenolics within Nanoliposomes. J. Agric. Food Chem. 2018, 66, 9231–9240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Song, X.; Liang, C.; Yi, X.; Song, G.; Chao, Y.; Yang, Y.; Yang, K.; Feng, L.; Liu, Z. Catalase-Loaded Cisplatin-Prodrug-Constructed Liposomes to Overcome Tumor Hypoxia for Enhanced Chemo-Radiotherapy of Cancer. Biomaterials 2017, 138, 13–21. [Google Scholar] [CrossRef]
- Jahadi, M.; Keighobadi, K.; Azimzadeh, B.; Keivani, H.; Khosravi-Darani, K. Liposomes as Herbal Compound Carriers: An Updated Review. Curr. Nutr. Food Sci. 2021, 17, 790–797. [Google Scholar] [CrossRef]
- Wang, X.; Song, Y.; Su, Y.; Tian, Q.; Li, B.; Quan, J.; Deng, Y. Are PEGylated Liposomes Better than Conventional Liposomes? A Special Case for Vincristine. Drug Deliv. 2016, 23, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Badri, W.; Miladi, K.; Nazari, Q.A.; Greige-Gerges, H.; Fessi, H.; Elaissari, A. Encapsulation of NSAIDs for Inflammation Management: Overview, Progress, Challenges and Prospects. Int. J. Pharm. 2016, 515, 757–773. [Google Scholar] [CrossRef]
- Chatzikleanthous, D.; O’Hagan, D.T.; Adamo, R. Lipid-Based Nanoparticles for Delivery of Vaccine Adjuvants and Antigens: Toward Multicomponent Vaccines. Mol. Pharm. 2021, 18, 2867–2888. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Ray, S.; Thakur, R.S. Solid Lipid Nanoparticles: A Modern Formulation Approach in Drug Delivery System. Indian J. Pharm. Sci. 2009, 71, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid Nanoparticles for MRNA Delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Scioli Montoto, S.; Muraca, G.; Ruiz, M.E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front. Mol. Biosci. 2020, 7, 587997. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Chen, Z.; Zhang, Y.; Lin, X.; Li, B. Novel Food-Grade Pickering Emulsions Stabilized by Tea Water-Insoluble Protein Nanoparticles from Tea Residues. Food Hydrocoll. 2019, 96, 322–330. [Google Scholar] [CrossRef]
- McQuilken, S.A. The Mouth, Stomach and Intestines. Anaesth. Intensive Care Med. 2021, 22, 330–335. [Google Scholar] [CrossRef]
- Mackie, A.; Mulet-Cabero, A.I.; Torcello-Gomez, A. Simulating Human Digestion: Developing Our Knowledge to Create Healthier and More Sustainable Foods. Food Funct. 2020, 11, 9397–9431. [Google Scholar] [CrossRef] [PubMed]
- Urbain, J.L.C.; Siegel, J.A.; Charkes, N.D.; Maurer, A.H.; Malmud, L.S.; Fisher, R.S. The Two-Component Stomach: Effects of Meal Particle Size on Fundal and Antral Emptying. Eur. J. Nucl. Med. 1989, 15, 254–259. [Google Scholar] [CrossRef]
- Cardoso, A.; Gonzaga Vaz Coelho, L.; Savassi-Rocha, P.R.; Vignolo, M.C.; Abrantes, M.M.; Miranda De Almeida, A.; Dias, E.E.; Vieira, G.; Moreira De Castro, M.; Vieira Lemos, Y. Gastric Emptying of Solids and Semi-Solids in Morbidly Obese and Non-Obese Subjects: An Assessment Using the 13C-Octanoic Acid and 13C-Acetic Acid Breath Tests. Obes. Surg. 2007, 17, 236–241. [Google Scholar] [CrossRef]
- Wu, P.; Chen, X.D. On Designing Biomimic in Vitro Human and Animal Digestion Track Models: Ideas, Current and Future Devices. Curr. Opin. Food Sci. 2020, 35, 10–19. [Google Scholar] [CrossRef]
- Li, C.; Yu, W.; Wu, P.; Chen, X.D. Current in Vitro Digestion Systems for Understanding Food Digestion in Human Upper Gastrointestinal Tract. Trends Food Sci. Technol. 2020, 96, 114–126. [Google Scholar] [CrossRef]
- Klojdová, I.; Kumherová, M.; Veselá, K.; Horáčková, Š.; Štětina, J. Functional W1/o/W2 Model Food Product with Encapsulated Colostrum and High Protein Content. Eur. Food Res. Technol. 2022, 248, 899–903. [Google Scholar] [CrossRef]
- Akbari, A.; Jabbari, N.; Sharifi, R.; Ahmadi, M.; Vahhabi, A.; Seyedzadeh, S.J.; Nawaz, M.; Szafert, S.; Mahmoodi, M.; Jabbari, E.; et al. Free and Hydrogel Encapsulated Exosome-Based Therapies in Regenerative Medicine. Life Sci. 2020, 249, 117447. [Google Scholar] [CrossRef] [PubMed]
- Rajkovic, O.; Potjewyd, G.; Pinteaux, E. Regenerative Medicine Therapies for Targeting Neuroinflammation after Stroke. Front. Neurol. 2018, 9, 734. [Google Scholar] [CrossRef]
- Gonzalez-Pujana, A.; Santos, E.; Orive, G.; Pedraz, J.L.; Hernandez, R.M. Cell Microencapsulation Technology: Current Vision of Its Therapeutic Potential through the Administration Routes. J. Drug Deliv. Sci. Technol. 2017, 42, 49–62. [Google Scholar] [CrossRef]
- Garate, A.; Ciriza, J.; Casado, J.G.; Blazquez, R.; Pedraz, J.L.; Orive, G.; Hernandez, R.M. Assessment of the Behavior of Mesenchymal Stem Cells Immobilized in Biomimetic Alginate Microcapsules. Mol. Pharm. 2015, 12, 3953–3962. [Google Scholar] [CrossRef]
- Short, A.R.; Koralla, D.; Deshmukh, A.; Wissel, B.; Stocker, B.; Calhoun, M.; Dean, D.; Winter, J.O. Hydrogels That Allow and Facilitate Bone Repair, Remodeling, and Regeneration. J. Mater. Chem. B 2015, 3, 7818–7830. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Jeon, O.; Bouhadir, K.H.; Mansour, J.M.; Alsberg, E. Photocrosslinked Alginate Hydrogels with Tunable Biodegradation Rates and Mechanical Properties. Biomaterials 2009, 30, 2724–2734. [Google Scholar] [CrossRef] [PubMed]
- Murua, A.; Orive, G.; Hernández, R.M.; Pedraz, J.L. Emerging Technologies in the Delivery of Erythropoietin for Therapeutics. Med. Res. Rev. 2011, 31, 284–309. [Google Scholar] [CrossRef]
- Selimoglu, S.M.; Elibol, M. Alginate as an Immobilization Material for MAb Production via Encapsulated Hybridoma Cells. Crit. Rev. Biotechnol. 2010, 30, 145–159. [Google Scholar] [CrossRef]
- Kwak, N.; Okamoto, N.; Wood, J.M.; Campochiaro, P.A. VEGF Is Major Stimulator in Model of Choroidal Neovascularization. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3158–3164. [Google Scholar]
- De Vos, P.; van Hoogmoed, C.G.; van Zanten, J.; Netter, S.; Strubbe, J.H.; Busscher, H.J. Long-Term Biocompatibility, Chemistry, and Function of Microencapsulated Pancreatic Islets. Biomaterials 2003, 24, 305–312. [Google Scholar] [CrossRef]
- Del Giudice, G.; Rappuoli, R.; Didierlaurent, A.M. Correlates of Adjuvanticity: A Review on Adjuvants in Licensed Vaccines. Semin. Immunol. 2018, 39, 14–21. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, K. Overview of Vaccine Adjuvants. Med. Drug Discov. 2021, 11, 100103. [Google Scholar] [CrossRef]
- Pulendran, B.; Arunachalam, P.S.; O’Hagan, D.T. Emerging Concepts in the Science of Vaccine Adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, T.R. The Mechanisms of Action of Vaccines Containing Aluminum Adjuvants: An In Vitro vs In Vivo Paradigm. Springerplus 2015, 4, 181. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D.T.; Ott, G.S.; van Nest, G.; Rappuoli, R.; del Giudice, G. The History of MF59® Adjuvant: A Phoenix That Arose from the Ashes. Expert Rev. Vaccines 2013, 12, 13–30. [Google Scholar] [CrossRef]
- Garçon, N.; Vaughn, D.W.; Didierlaurent, A.M. Development and Evaluation of AS03, an Adjuvant System Containing α-Tocopherol and Squalene in an Oil-in-Water Emulsion. Expert Rev. Vaccines 2012, 11, 349–366. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Contant, P.; Sangster, M.Y.; Topham, D.J. Squalene-Based Influenza Vaccine Adjuvants and Their Impact on the Hemagglutinin-Specific b Cell Response. Pathogens 2021, 10, 355. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.J.; Kang, S.M. Immunology and Efficacy of MF59-Adjuvanted Vaccines. Hum. Vaccines Immunother. 2018, 14, 3041–3045. [Google Scholar] [CrossRef]
- Goll, J.B.; Jain, A.; Jensen, T.L.; Assis, R.; Nakajima, R.; Jasinskas, A.; Coughlan, L.; Cherikh, S.R.; Gelber, C.E.; Khan, S.; et al. The Antibody Landscapes Following AS03 and MF59 Adjuvanted H5N1 Vaccination. NPJ Vaccines 2022, 7, 103. [Google Scholar] [CrossRef]
- Wang, N.; Chen, M.; Wang, T. Liposomes Used as a Vaccine Adjuvant-Delivery System: From Basics to Clinical Immunization. J. Control. Release 2019, 303, 130–150. [Google Scholar] [CrossRef]
- Rao, M.; Peachman, K.K.; Alving, C.R. Liposome Formulations as Adjuvants for Vaccines. In Current Topics in Microbiology and Immunology; Springer: Cham, Switzerland, 2021; Volume 433. [Google Scholar]
- Chen, C.; Zhang, C.; Li, R.; Wang, Z.; Yuan, Y.; Li, H.; Fu, Z.; Zhou, M.; Zhao, L. Monophosphoryl-Lipid A (MPLA) Is an Efficacious Adjuvant for Inactivated Rabies Vaccines. Viruses 2019, 11, 1118. [Google Scholar] [CrossRef] [PubMed]
- Rajput, Z.I.; Hu, S.H.; Xiao, C.-W.; Arijo, A.G. Adjuvant Effects of Saponins on Animal Immune Responses. J. Zhejiang Univ. Sci. B 2007, 8, 153–161. [Google Scholar] [CrossRef]
- Zhu, B.; He, T.; Gao, X.; Shi, M.; Sun, H. Evaluation and Characteristics of Immunological Adjuvant Activity of Purified Fraction of Albizia Julibrissin Saponins. Immunol. Investig. 2019, 48, 283–302. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Oh, S.W.; Fujita, T. RIG-I-like Receptors and Type I Interferonopathies. J. Interferon Cytokine Res. 2017, 37, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.; Hou, B. TLR Signaling in B-Cell Development and Activation. Cell Mol. Immunol. 2013, 10, 103–106. [Google Scholar] [CrossRef]
- Ablasser, A.; Poeck, H.; Anz, D.; Berger, M.; Schlee, M.; Kim, S.; Bourquin, C.; Goutagny, N.; Jiang, Z.; Fitzgerald, K.A.; et al. Selection of Molecular Structure and Delivery of RNA Oligonucleotides to Activate TLR7 versus TLR8 and to Induce High Amounts of IL-12p70 in Primary Human Monocytes. J. Immunol. 2009, 182, 6824–6833. [Google Scholar] [CrossRef]
- Swaminathan, G.; Thoryk, E.A.; Cox, K.S.; Meschino, S.; Dubey, S.A.; Vora, K.A.; Celano, R.; Gindy, M.; Casimiro, D.R.; Bett, A.J. A Novel Lipid Nanoparticle Adjuvant Significantly Enhances B Cell and T Cell Responses to Sub-Unit Vaccine Antigens. Vaccine 2016, 34, 110–119. [Google Scholar] [CrossRef]
- Shirai, S.; Kawai, A.; Shibuya, M.; Munakata, L.; Omata, D.; Suzuki, R.; Yoshioka, Y. Lipid Nanoparticle Acts as a Potential Adjuvant for Influenza Split Vaccine without Inducing Inflammatory Responses. Vaccines 2020, 8, 433. [Google Scholar] [CrossRef]
- Awasthi, S.; Hook, L.M.; Swaminathan, G.; Cairns, T.M.; Brooks, B.; Smith, J.S.; Ditto, N.T.; Gindy, M.E.; Bett, A.J.; Espeseth, A.S.; et al. Antibody Responses to Crucial Functional Epitopes as a Novel Approach to Assess Immunogenicity of Vaccine Adjuvants. Vaccine 2019, 37, 3770–3778. [Google Scholar] [CrossRef]
- Alameh, M.G.; Tombácz, I.; Bettini, E.; Lederer, K.; Sittplangkoon, C.; Wilmore, J.R.; Gaudette, B.T.; Soliman, O.Y.; Pine, M.; Hicks, P.; et al. Lipid Nanoparticles Enhance the Efficacy of MRNA and Protein Subunit Vaccines by Inducing Robust T Follicular Helper Cell and Humoral Responses. Immunity 2021, 54, 2877–2892.e7. [Google Scholar] [CrossRef] [PubMed]
- Dolgin, E. The Tangled History of MRNA Vaccines. Nature 2021, 597, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Strizova, Z.; Smetanova, J.; Bartunkova, J.; Milota, T. Principles and Challenges in Anti-COVID-19 Vaccine Development. Int. Arch. Allergy Immunol. 2021, 182, 339–349. [Google Scholar] [CrossRef] [PubMed]
| Technique(s) Used | Encapsulated Agents | Coating Materials for Encapsulation | Application Areas | References |
|---|---|---|---|---|
| spray drying | resveratrol | polysaccharide—chitosan | intranasal drugs | [64] |
| budesonide or rifampicin | oligosaccharide—lactose | intranasal drugs | [65] | |
| Lactobacillus Acidophilus | milk proteins, polysaccharides—pectin and maltodextrin | functional food | [66] | |
| Lactobacillus plantarum | polysaccharides (extract from Aloe vera) | functional food | [67] | |
| oleoresin from paprika | polysaccharides—gum arabic, starch | food supplement | [68] | |
| (spray) freeze-drying | paclitaxel and doxorubicin | liposome | anticancer treatment | [69] |
| ciprofloxacin | liposome | intranasal drugs | [70] | |
| turmeric oleoresin | protein-gelatine | food supplement | [71] | |
| emulsion techniques | norcantharidin | liposome-emulsion hybrid delivery system | anticancer treatment | [72] |
| Doxorubicin (Adriamycin) | polysaccharides—nanocellulose | anticancer treatment | [73] | |
| Zanamivir | polysaccharides—cellulose, gum arabic | intranasal drugs | [74] | |
| chlortetracycline | polysaccharides—starch, xanthan gum | model drug preparation | [75] | |
| magnesium | plant oil, lentil flour | functional food | [76] | |
| Bifidobacterium lactis | plant oil, beeswax | functional food | [77] |
| Encapsulated Agent | Intended Use | Reference |
|---|---|---|
| Plant essential oils (rich in terpenes and terpenoids), extracted from thyme, oregano, lemongrass) | Antibacterial and antioxidant agents | [84] |
| Phenolic compounds extracted from onion | Antioxidant agent | [85] |
| Phenolic compounds extracted from bilberry | Antioxidant agent | [86] |
| Pea protein | Food (encapsulation due to taste masking) | [87] |
| Lactobacillus fermentum strain UCO-979C | Inhibition of Helicobacter pylori | [88] |
| Lactobacillus plantarum F1, Lactobacillus reuteri 182, Lactobacillus helveticus 305 | Alginate capsules with reduced mortality of the cells during gelation | [89] |
| Vitamin D3 | Food (enhanced D3 stability) | [90] |
| Essential oil encapsulated in yeast cells | Improved stability of essential oil | [91] |
| Encapsulated Agent | Intended Use | Reference |
|---|---|---|
| Polyphenols | Prevention of ageing, cancer, inflammation and neurodegenerative diseases | [93,103,104] |
| Phenolic compounds | Antioxidants | [105] |
| Diclofenac sodium | Anti-inflammatory agent | [106,107] |
| Living cells | Cell therapy for regenerative, reproductive and transfusion medicine | [108] |
| Insulin | Diabetes treatment | [109] |
| Bioactive proteins | Functional food | [102] |
| Andrographolide (diterpenoid lactone) | Formulation with hepatoprotective activity | [110] |
| Bifonazole [1-[[1,1′-biphenyl)-4-phenylmethyl]-1H-imidazole) | Topical delivery of bifonazole to maximize its efficacy | [111] |
| Encapsulated Agent | Intended Use | Reference |
|---|---|---|
| Catalase (EC 1.11.1.6) | Cancer therapy | [130] |
| Herbal phytochemicals (quercetin, vinblastine, hesperidin etc.) | Food supplements | [131] |
| Vincristine | Cancer therapy | [132] |
| Non-steroidal anti-inflammatory treatment | Drugs | [133] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klojdová, I.; Milota, T.; Smetanová, J.; Stathopoulos, C. Encapsulation: A Strategy to Deliver Therapeutics and Bioactive Compounds? Pharmaceuticals 2023, 16, 362. https://doi.org/10.3390/ph16030362
Klojdová I, Milota T, Smetanová J, Stathopoulos C. Encapsulation: A Strategy to Deliver Therapeutics and Bioactive Compounds? Pharmaceuticals. 2023; 16(3):362. https://doi.org/10.3390/ph16030362
Chicago/Turabian StyleKlojdová, Iveta, Tomáš Milota, Jitka Smetanová, and Constantinos Stathopoulos. 2023. "Encapsulation: A Strategy to Deliver Therapeutics and Bioactive Compounds?" Pharmaceuticals 16, no. 3: 362. https://doi.org/10.3390/ph16030362
APA StyleKlojdová, I., Milota, T., Smetanová, J., & Stathopoulos, C. (2023). Encapsulation: A Strategy to Deliver Therapeutics and Bioactive Compounds? Pharmaceuticals, 16(3), 362. https://doi.org/10.3390/ph16030362







