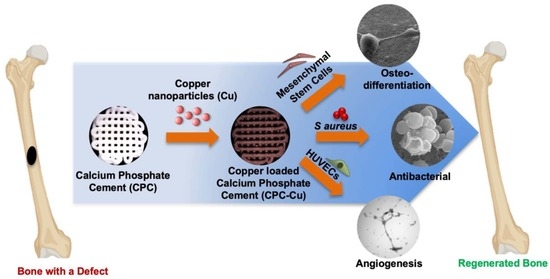
Multifunctional Three-Dimensional Printed Copper Loaded Calcium Phosphate Scaffolds for Bone Regeneration
Abstract
1. Introduction
2. Results
2.1. Injectability
2.2. 3D Printing
2.3. Scaffold Characterization
2.3.1. Optical Microscopy
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. Contact Angle Measurement
2.3.4. Fourier Transform Infrared Spectroscopy
2.3.5. Powder X-ray Diffraction (XRD)
2.3.6. Copper Ion Release Study
2.4. In Vitro Studies
2.4.1. Cell Attachment
2.4.2. Cell Proliferation
2.4.3. Alkaline Phosphatase Activity
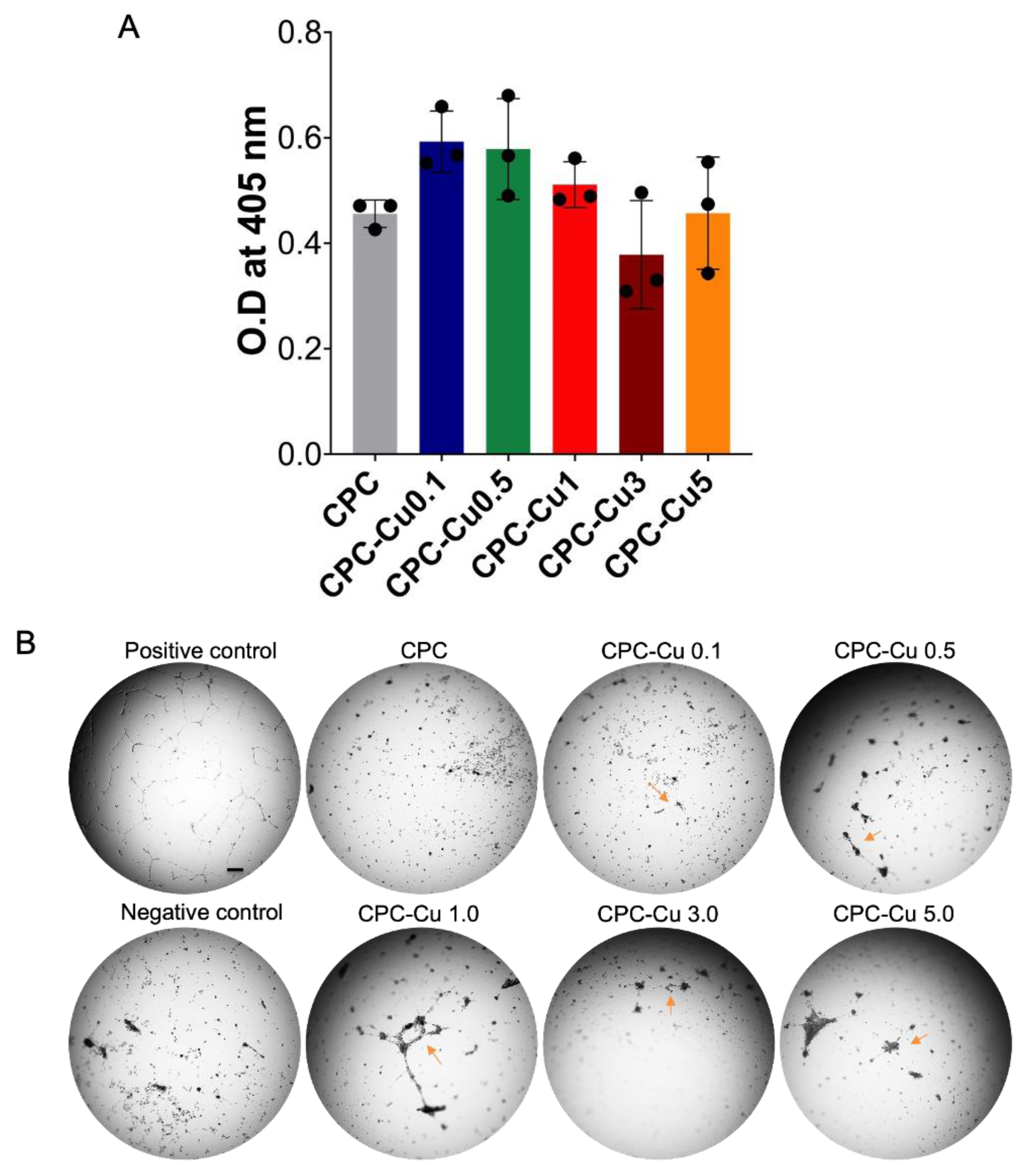
2.4.4. Angiogenic Activity
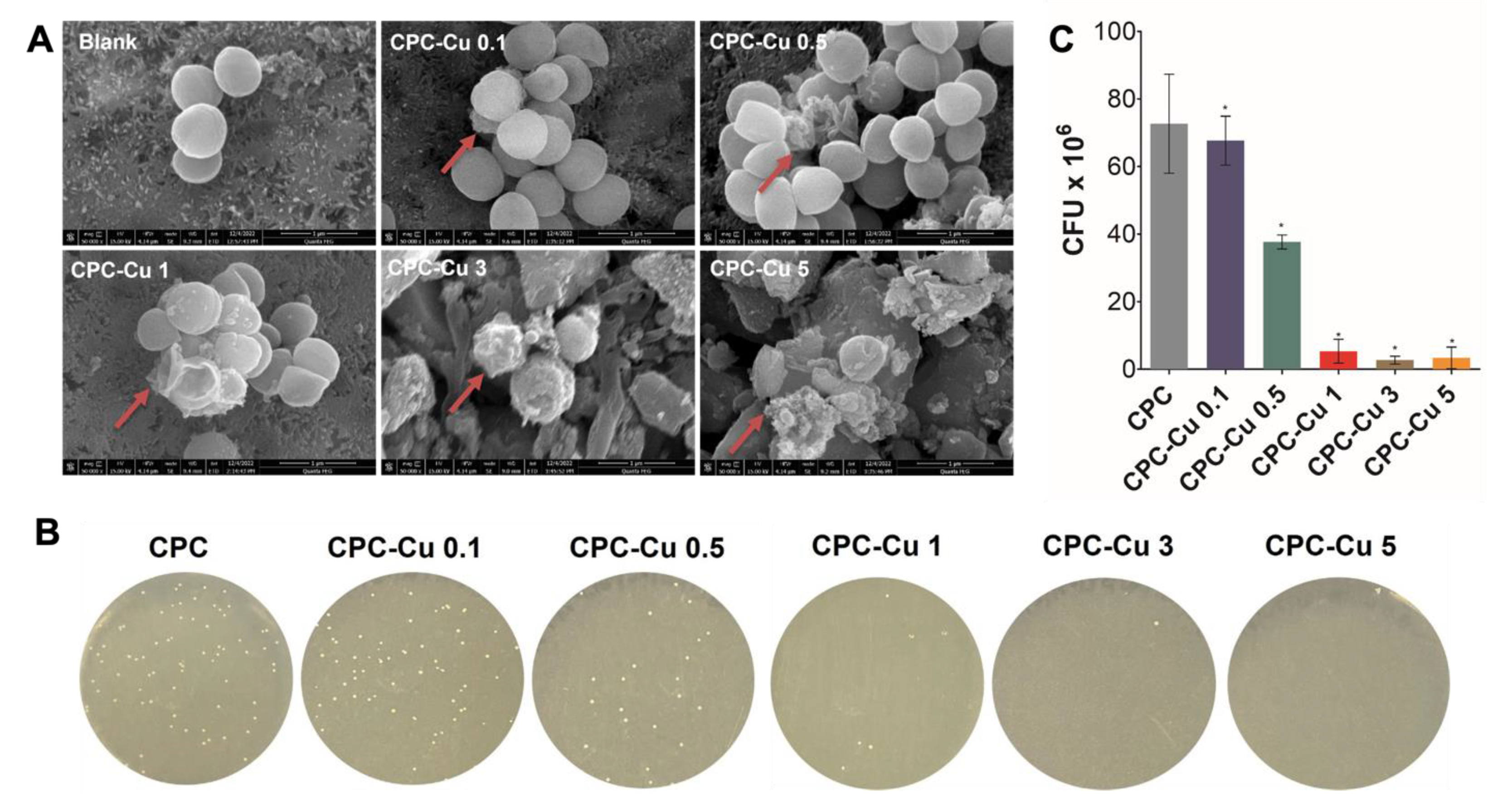
2.4.5. Antibacterial Study
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Copper Loaded CPC
4.3. Printing of Copper Loaded CPC
4.4. Scaffold Characterization
4.4.1. Injectability
4.4.2. Microscopy Imaging
4.4.3. Contact Angle Measurement
4.4.4. Fourier Transform Infrared Spectroscopy (FTIR)
4.4.5. Powder X-ray Diffraction (XRD)
4.4.6. Copper Ion Release Study
4.5. In Vitro Studies
4.5.1. Cell Attachment
4.5.2. Cell Proliferation
4.5.3. Alkaline Phosphatase Activity
4.5.4. Angiogenic Activity
4.6. Antibacterial Study
4.7. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lode, A.; Meissner, K.; Luo, Y.; Sonntag, F.; Glorius, S.; Nies, B.; Vater, C.; Despang, F.; Hanke, T.; Gelinsky, M. Fabrication of Porous Scaffolds by Three-Dimensional Plotting of a Pasty Calcium Phosphate Bone Cement under Mild Conditions: 3D Plotting of a Pasty Calcium Phosphate Bone Cement. J. Tissue Eng. Regen. Med. 2014, 8, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.; Makaram, N.; Simpson, A.; Keating, J. Fracture Nonunion in Long Bones: A Literature Review of Risk Factors and Surgical Management. Injury 2021, 52, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Zheng, F.; Zhao, W.; Zhang, Y.; Yuan, J.; Zhang, B.; Li, L. Prevalence and Influencing Factors of Nonunion in Patients with Tibial Fracture: Systematic Review and Meta-Analysis. J. Orthop. Surg. 2020, 15, 377. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.W.; Muschler, G.F. Bone Graft Materials: An Overview of the Basic Science. Clin. Orthop. Relat. Res. 2000, 371, 10. [Google Scholar] [CrossRef]
- Wu, Y.; Woodbine, L.; Carr, A.M.; Pillai, A.R.; Nokhodchi, A.; Maniruzzaman, M. 3D Printed Calcium Phosphate Cement (CPC) Scaffolds for Anti-Cancer Drug Delivery. Pharmaceutics 2020, 12, 1077. [Google Scholar] [CrossRef]
- Tronco, M.C.; Cassel, J.B.; dos Santos, L.A. α-TCP-Based Calcium Phosphate Cements: A Critical Review. Acta Biomater. 2022, 151, 70–87. [Google Scholar] [CrossRef]
- Carey, L.E.; Xu, H.H.K.; Simon, C.G.; Takagi, S.; Chow, L.C. Premixed Rapid-Setting Calcium Phosphate Composites for Bone Repair. Biomaterials 2005, 26, 5002–5014. [Google Scholar] [CrossRef]
- Vorndran, E.; Geffers, M.; Ewald, A.; Lemm, M.; Nies, B.; Gbureck, U. Ready-to-Use Injectable Calcium Phosphate Bone Cement Paste as Drug Carrier. Acta Biomater. 2013, 9, 9558–9567. [Google Scholar] [CrossRef]
- Ahlfeld, T.; Doberenz, F.; Kilian, D.; Vater, C.; Korn, P.; Lauer, G.; Lode, A.; Gelinsky, M. Bioprinting of Mineralized Constructs Utilizing Multichannel Plotting of a Self-Setting Calcium Phosphate Cement and a Cell-Laden Bioink. Biofabrication 2018, 10, 045002. [Google Scholar] [CrossRef]
- Cui, X.; Huang, C.; Chen, Z.; Zhang, M.; Liu, C.; Su, K.; Wang, J.; Li, L.; Wang, R.; Li, B.; et al. Hyaluronic Acid Facilitates Bone Repair Effects of Calcium Phosphate Cement by Accelerating Osteogenic Expression. Bioact. Mater. 2021, 6, 3801–3811. [Google Scholar] [CrossRef]
- Hettich, G.; Schierjott, R.A.; Epple, M.; Gbureck, U.; Heinemann, S.; Mozaffari-Jovein, H.; Grupp, T.M. Calcium Phosphate Bone Graft Substitutes with High Mechanical Load Capacity and High Degree of Interconnecting Porosity. Materials 2019, 12, 3471. [Google Scholar] [CrossRef]
- Barralet, J.; Gbureck, U.; Habibovic, P.; Vorndran, E.; Gerard, C.; Doillon, C.J. Angiogenesis in Calcium Phosphate Scaffolds by Inorganic Copper Ion Release. Tissue Eng. Part A 2009, 15, 1601–1609. [Google Scholar] [CrossRef]
- Kurobane, T.; Shiwaku, Y.; Anada, T.; Hamai, R.; Tsuchiya, K.; Baba, K.; Iikubo, M.; Takahashi, T.; Suzuki, O. Angiogenesis Involvement by Octacalcium Phosphate-Gelatin Composite-Driven Bone Regeneration in Rat Calvaria Critical-Sized Defect. Acta Biomater. 2019, 88, 514–526. [Google Scholar] [CrossRef]
- Raja, N.; Han, S.H.; Cho, M.; Choi, Y.-J.; Jin, Y.-Z.; Park, H.; Lee, J.H.; Yun, H. Effect of Porosity and Phase Composition in 3D Printed Calcium Phosphate Scaffolds on Bone Tissue Regeneration in vivo. Mater. Des. 2022, 219, 110819. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Aghda, N.H.; Pillai, A.R.; Thakkar, R.; Nokhodchi, A.; Maniruzzaman, M. Emerging 3D Printing Technologies for Drug Delivery Devices: Current Status and Future Perspective. Adv. Drug Deliv. Rev. 2021, 174, 294–316. [Google Scholar] [CrossRef] [PubMed]
- Landers, R.; Mülhaupt, R. Desktop Manufacturing of Complex Objects, Prototypes and Biomedical Scaffolds by Means of Computer-Assisted Design Combined with Computer-Guided 3D Plotting of Polymers and Reactive Oligomers. Macromol. Mater. Eng. 2000, 282, 17–21. [Google Scholar] [CrossRef]
- Malhotra, A.; Habibovic, P. Calcium Phosphates and Angiogenesis: Implications and Advances for Bone Regeneration. Trends Biotechnol. 2016, 34, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.; Renaudin, G.; Forestier, C.; Nedelec, J.-M.; Descamps, S. Biological Properties of Copper-Doped Biomaterials for Orthopedic Applications: A Review of Antibacterial, Angiogenic and Osteogenic Aspects. Acta Biomater. 2020, 117, 21–39. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, H.; He, F.; Wu, T.; Zhou, L.; Ye, J. Concentration-Dependent Osteogenic and Angiogenic Biological Performances of Calcium Phosphate Cement Modified with Copper Ions. Mater. Sci. Eng. C 2019, 99, 1199–1212. [Google Scholar] [CrossRef]
- Tao, B.; Lin, C.; Deng, Y.; Yuan, Z.; Shen, X.; Chen, M.; He, Y.; Peng, Z.; Hu, Y.; Cai, K. Copper-Nanoparticle-Embedded Hydrogel for Killing Bacteria and Promoting Wound Healing with Photothermal Therapy. J. Mater. Chem. B 2019, 7, 2534–2548. [Google Scholar] [CrossRef]
- Shimabukuro, M. Antibacterial Property and Biocompatibility of Silver, Copper, and Zinc in Titanium Dioxide Layers Incorporated by One-Step Micro-Arc Oxidation: A Review. Antibiotics 2020, 9, 716. [Google Scholar] [CrossRef]
- Mitra, D.; Kang, E.-T.; Neoh, K.G. Antimicrobial Copper-Based Materials and Coatings: Potential Multifaceted Biomedical Applications. ACS Appl. Mater. Interfaces 2020, 12, 21159–21182. [Google Scholar] [CrossRef]
- Deyneko, D.V.; Fadeeva, I.V.; Borovikova, E.Y.; Dzhevakov, P.B.; Slukin, P.V.; Zheng, Y.; Xia, D.; Lazoryak, B.I.; Rau, J.V. Antimicrobial Properties of Co-Doped Tricalcium Phosphates Ca3−2x(M′M″)x(PO4)2 (M = Zn2+, Cu2+, Mn2+ and Sr2+). Ceram. Int. 2022, 48, 29770–29781. [Google Scholar] [CrossRef]
- Lin, Z.; Cao, Y.; Zou, J.; Zhu, F.; Gao, Y.; Zheng, X.; Wang, H.; Zhang, T.; Wu, T. Improved Osteogenesis and Angiogenesis of a Novel Copper Ions Doped Calcium Phosphate Cement. Mater. Sci. Eng. C 2020, 114, 111032. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Kim, Y.K.; Mofrad, M.R.K. Chapter 5—Mechanobiological Approaches for the Control of Cell Motility. In Microfluidic Cell Culture Systems; Bettinger, C., Borenstein, J.T., Tao, S.L., Eds.; Micro and Nano Technologies; William Andrew Publishing: Oxford, UK, 2013; pp. 105–136. ISBN 978-1-4377-3459-1. [Google Scholar]
- Lee, H.J. 15—Improving Superhydrophobic Textile Materials. In Functional Textiles for Improved Performance, Protection and Health; Pan, N., Sun, G., Eds.; Woodhead Publishing Series in Textiles; Woodhead Publishing: Cambridge, UK, 2011; pp. 339–359. ISBN 978-1-84569-723-5. [Google Scholar]
- Jaidev, L.R.; Chatterjee, K. Surface Functionalization of 3D Printed Polymer Scaffolds to Augment Stem Cell Response. Mater. Des. 2019, 161, 44–54. [Google Scholar] [CrossRef]
- Bohner, M.; Van Landuyt, P.; Merkle, H.P.; Lemaitre, J. Composition Effects on the PH of a Hydraulic Calcium Phosphate Cement. J. Mater. Sci. Mater. Med. 1997, 8, 675–681. [Google Scholar] [CrossRef]
- Betancourt-Galindo, R.; Reyes-Rodriguez, P.Y.; Puente-Urbina, B.A.; Avila-Orta, C.A.; Rodríguez-Fernández, O.S.; Cadenas-Pliego, G.; Lira-Saldivar, R.H.; García-Cerda, L.A. Synthesis of Copper Nanoparticles by Thermal Decomposition and Their Antimicrobial Properties. J. Nanomater. 2014, 2014, 980545. [Google Scholar] [CrossRef]
- Maheo, A.R. Biosynthesis and Characterization of Eupatorium Adenophorum and Chitosan Mediated Copper Oxide Nanoparticles and Their Antibacterial Activity. Results Surf. Interfaces 2022, 6, 100048. [Google Scholar] [CrossRef]
- Hydroxyapatite, Powder X-Ray Diffraction, Crystal Structure Modelling. Am. J. Mater. Sci. 2013.
- Alexopoulou, M.; Mystiridou, E.; Mouzakis, D.; Zaoutsos, S.; Fatouros, D.G.; Bouropoulos, N. Preparation, Characterization and in Vitro Assessment of Ibuprofen Loaded Calcium Phosphate/Gypsum Bone Cements. Cryst. Res. Technol. 2016, 51, 41–48. [Google Scholar] [CrossRef]
- Wang, L.; Qiu, Y.; Lv, H.; Si, Y.; Liu, L.; Zhang, Q.; Cao, J.; Yu, J.; Li, X.; Ding, B. 3D Superelastic Scaffolds Constructed from Flexible Inorganic Nanofibers with Self-Fitting Capability and Tailorable Gradient for Bone Regeneration. Adv. Funct. Mater. 2019, 29, 1901407. [Google Scholar] [CrossRef]
- Guo, C.; Niu, D.; Liu, J.; Bao, X.; Xu, G. Application of Biodegradable PLGA-PEG-PLGA/CPC Composite Bone Cement in the Treatment of Osteoporosis. Coatings 2021, 11, 827. [Google Scholar] [CrossRef]
- Moreau, J.L.; Xu, H.H.K. Mesenchymal Stem Cell Proliferation and Differentiation on an Injectable Calcium Phosphate—Chitosan Composite Scaffold. Biomaterials 2009, 30, 2675–2682. [Google Scholar] [CrossRef]
- Lew, D.P.; Waldvogel, F.A. Osteomyelitis. Lancet 2004, 364, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Patzakis, M.J.; Zalavras, C.G. Chronic Posttraumatic Osteomyelitis and Infected Nonunion of the Tibia: Current Management Concepts. JAAOS—J. Am. Acad. Orthop. Surg. 2005, 13, 417. [Google Scholar] [CrossRef] [PubMed]
- Carroll Woodard, J.; Riser, W.H. Morphology of Fracture Nonunion and Osteomyelitis. Vet. Clin. N. Am. Small Anim. Pract. 1991, 21, 813–844. [Google Scholar] [CrossRef] [PubMed]
- Gérard, C.; Bordeleau, L.-J.; Barralet, J.; Doillon, C.J. The Stimulation of Angiogenesis and Collagen Deposition by Copper. Biomaterials 2010, 31, 824–831. [Google Scholar] [CrossRef]
- Richter, R.F.; Ahlfeld, T.; Gelinsky, M.; Lode, A. Composites Consisting of Calcium Phosphate Cements and Mesoporous Bioactive Glasses as a 3D Plottable Drug Delivery System. Acta Biomater. 2022, 156, 146–157. [Google Scholar] [CrossRef]
- Hellerbrand, K.; Siedler, M.; Schutz, A.; Pompe, C.; Friess, W. In Situ Hardening Paste, Its Manufacturing and Use. U.S. Patent US20090048145A1, 19 February 2009. [Google Scholar]
- Huhtamäki, T.; Tian, X.; Korhonen, J.T.; Ras, R.H.A. Surface-Wetting Characterization Using Contact-Angle Measurements. Nat. Protoc. 2018, 13, 1521–1538. [Google Scholar] [CrossRef]
- Zanchetta, P.; Guezennec, J. Surface Thermodynamics of Osteoblasts: Relation between Hydrophobicity and Bone Active Biomaterials. Colloids Surf. B Biointerfaces 2001, 22, 301–307. [Google Scholar] [CrossRef]
- Strnad, G.; Chirila, N.; Petrovan, C.; Russu, O. Contact Angle Measurement on Medical Implant Titanium Based Biomaterials. Procedia Technol. 2016, 22, 946–953. [Google Scholar] [CrossRef]
- Sahmani, S.; Shahali, M.; Ghadiri Nejad, M.; Khandan, A.; Aghdam, M.M.; Saber-Samandari, S. Effect of Copper Oxide Nanoparticles on Electrical Conductivity and Cell Viability of Calcium Phosphate Scaffolds with Improved Mechanical Strength for Bone Tissue Engineering. Eur. Phys. J. Plus 2019, 134, 7. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, S.; Zhou, J.; Shen, Y.; Huang, W.; Zhang, C.; Rahaman, M.N.; Wang, D. Evaluation of Borate Bioactive Glass Scaffolds as a Controlled Delivery System for Copper Ions in Stimulating Osteogenesis and Angiogenesis in Bone Healing. J. Mater. Chem. B 2014, 2, 8547–8557. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, N.J.; Kidney, B.A. Alkaline Phosphatase: Beyond the Liver. Vet. Clin. Pathol. 2007, 36, 223–233. [Google Scholar] [CrossRef]
- Deyneko, D.; Zheng, Y.; Barbaro, K.; Lebedev, V.N.; Aksenov, S.M.; Borovikova, E.Y.; Gafurov, M.R.; Fadeeva, I.V.; Antoniac, I.; Lazoryak, B.; et al. Dependence of Antimicrobial Properties on Site-Selective Arrangement and Concentration of Bioactive Cu2+ Ions in Tricalcium Phosphate. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Sears, N.; Dhavalikar, P.; Whitely, M.; Cosgriff-Hernandez, E. Fabrication of Biomimetic Bone Grafts with Multi-Material 3D Printing. Biofabrication 2017, 9, 025020. [Google Scholar] [CrossRef]
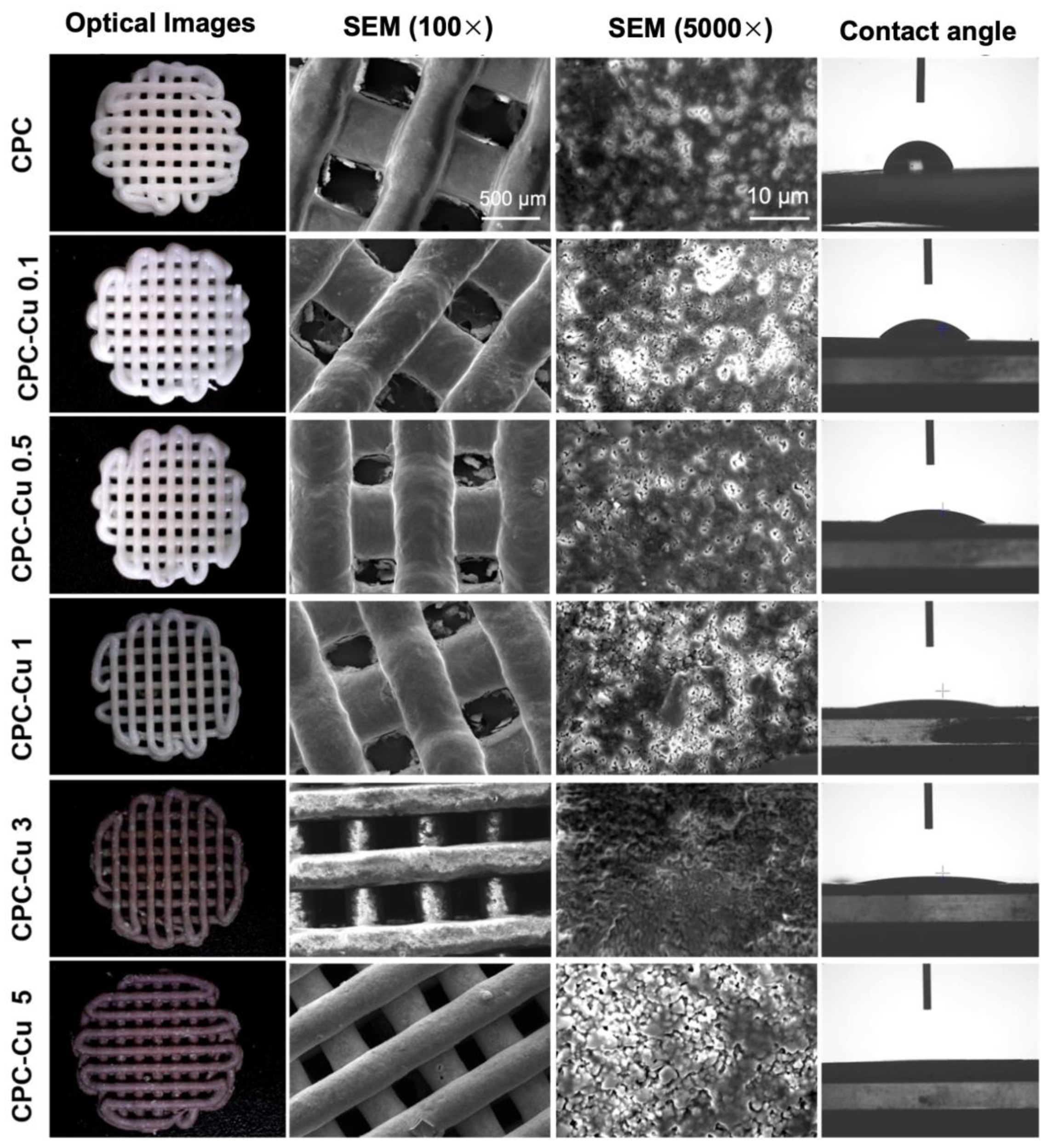

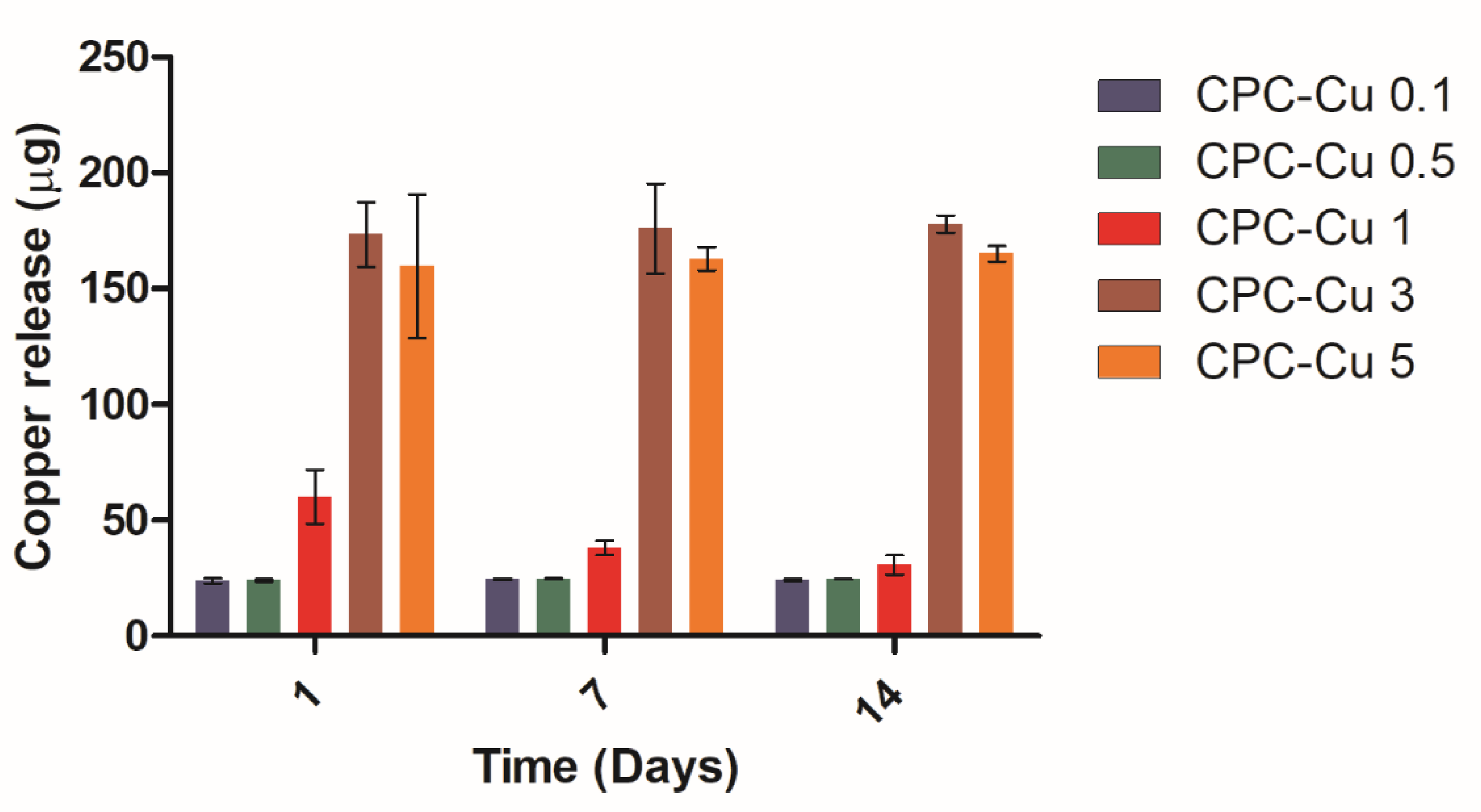
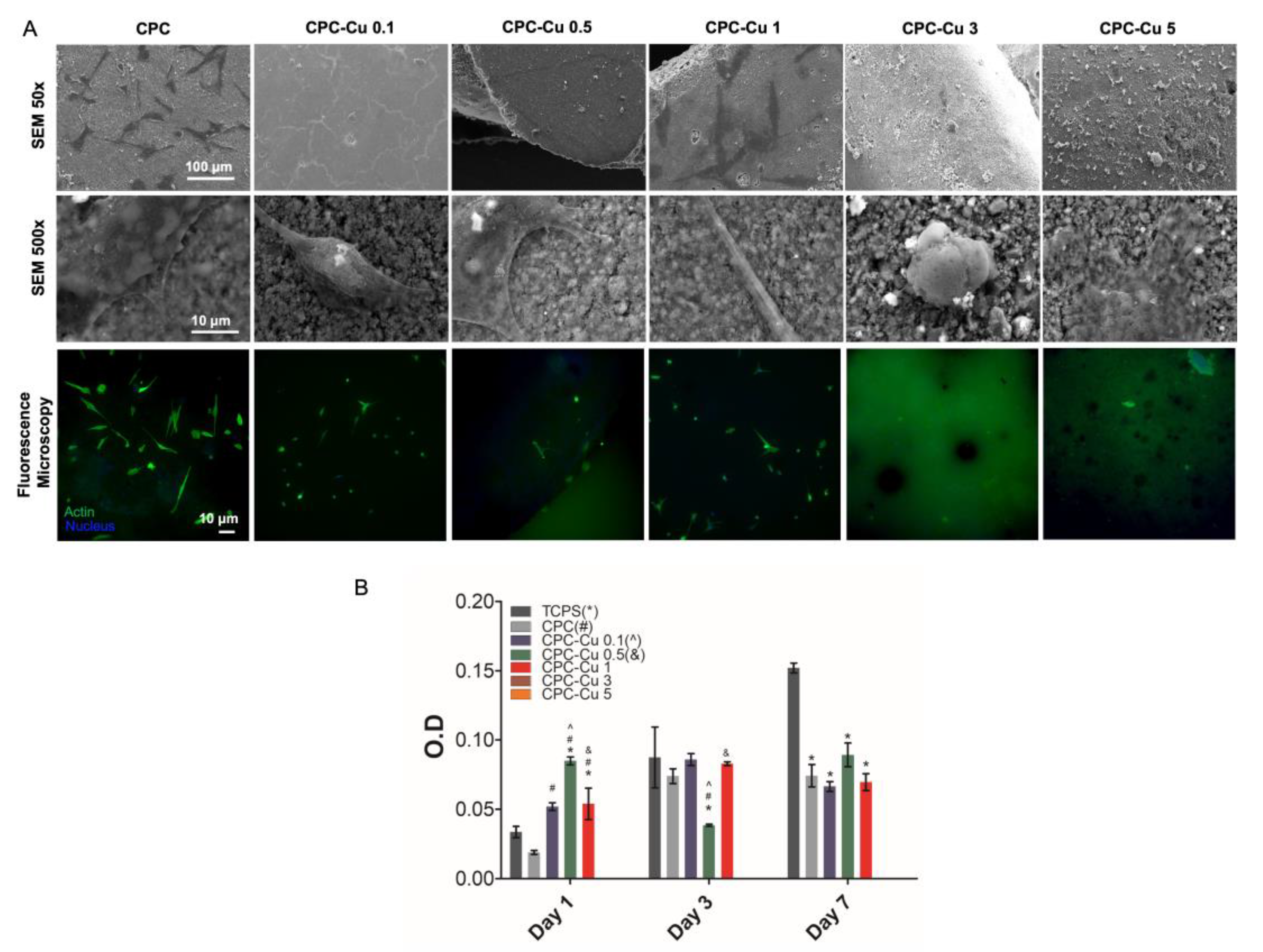
| Sample | CPC (wt%) | Cu-NP (wt%) | Strut Diameter (µm) | Pore Diameter (µm) | Extrusion Peak Force (g) | Contact Angle (°) |
|---|---|---|---|---|---|---|
| CPC | 100 | 0 | 434 ± 25 | 434 ± 17 | 3090 ± 41 | 75 ± 9 |
| CPC-Cu 0.1 | 99.9 | 0.1 | 457 ± 38 | 433 ± 20 | 4018 ± 37 | 35 ± 9 |
| CPC-Cu 0.5 | 99.5 | 0.5 | 462 ± 15 | 466 ± 28 | 4292 ± 137 | 9 ± 2 |
| CPC-Cu 1 | 99 | 1 | 467 ± 22 | 457 ± 13 | 3675 ± 101 | 10 ± 2 |
| CPC-Cu 3 | 97 | 3 | 453 ± 28 | 453 ± 24 | 4524 ± 150 | 8 ± 2 |
| CPC-Cu 5 | 95 | 5 | 451 ± 12 | 451 ± 13 | 3615 ± 56 | 3 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pillai, A.; Chakka, J.; Heshmathi, N.; Zhang, Y.; Alkadi, F.; Maniruzzaman, M. Multifunctional Three-Dimensional Printed Copper Loaded Calcium Phosphate Scaffolds for Bone Regeneration. Pharmaceuticals 2023, 16, 352. https://doi.org/10.3390/ph16030352
Pillai A, Chakka J, Heshmathi N, Zhang Y, Alkadi F, Maniruzzaman M. Multifunctional Three-Dimensional Printed Copper Loaded Calcium Phosphate Scaffolds for Bone Regeneration. Pharmaceuticals. 2023; 16(3):352. https://doi.org/10.3390/ph16030352
Chicago/Turabian StylePillai, Amit, Jaidev Chakka, Niloofar Heshmathi, Yu Zhang, Faez Alkadi, and Mohammed Maniruzzaman. 2023. "Multifunctional Three-Dimensional Printed Copper Loaded Calcium Phosphate Scaffolds for Bone Regeneration" Pharmaceuticals 16, no. 3: 352. https://doi.org/10.3390/ph16030352
APA StylePillai, A., Chakka, J., Heshmathi, N., Zhang, Y., Alkadi, F., & Maniruzzaman, M. (2023). Multifunctional Three-Dimensional Printed Copper Loaded Calcium Phosphate Scaffolds for Bone Regeneration. Pharmaceuticals, 16(3), 352. https://doi.org/10.3390/ph16030352