Antidiabetic and Anticancer Potentials of Mangifera indica L. from Different Geographical Origins
Abstract
1. Introduction
2. Results
2.1. Determination of the Cytotoxic Activities
2.2. The effect of Extracts on Glucose Uptake Using HepG2
2.3. The Effect of Extracts on Glutathione Peroxidase Activity (GPx) Using HepG2
2.4. The Effect of Extracts on the α-Amylase Activity
2.5. Statistical Analysis
2.5.1. Descriptive Analysis
2.5.2. Correlation Analysis
Pearson’s Correlation
Component Analysis for Variance
Variables Correlation with ANOVA
3. Discussion
4. Materials and Methods
4.1. Collection and Preparation of Samples
4.2. Cells and Microorganisms
4.3. Chemicals and Reagents
4.4. Characterization and Standardization of the Extracts
4.5. Cell Culture
4.6. Determination of Cytotoxicity and Selectivity
4.7. Glucose Uptake Assay
4.8. Determination of Glutathione Peroxidase Activity
4.9. α-Amylase Inhibition Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, K.; Patel, M.; Patel, R.; Parmar, P. Mangifera Indica (Mango). Pharmacogn. Rev. 2010, 4, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; He, Q. Mango bioactive compounds and related nutraceutical properties—A review. Food Rev. Int. 2009, 25, 346–370. [Google Scholar] [CrossRef]
- Guevara, M.; Tamayo, D.; González, S.; Núñez, A. Vimang as natural antioxidant supplementation in patients with malignant tumours. Minerva Med. 2001, 92, 95–97. [Google Scholar]
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R. A review on ethnopharmacological applications, pharmacological activities, and bioactive compounds of Mangifera indica (Mango). Evid. Based Complement. Alternat. Med. 2017, 2017, 6949835. [Google Scholar] [CrossRef] [PubMed]
- Umamahesh, K.; Ramesh, B.; Kumar, B.V.; Reddy, O.V.S. In vitro anti-oxidant, anti-microbial and anti-inflammatory activities of five Indian cultivars of mango (Mangifera indica L.) fruit peel extracts. J. Herbmed Pharmacol. 2019, 8, 238–247. [Google Scholar] [CrossRef]
- Bai, X.; Lai, T.; Zhou, T.; Li, Y.; Li, X.; Zhang, H. In Vitro Antioxidant Activities of Phenols and Oleanolic Acid from Mango Peel and Their Cytotoxic Effect on A549 Cell Line. Molecules 2018, 23, 1395. [Google Scholar] [CrossRef]
- Abbasi, A.M.; Liu, F.; Guo, X.; Fu, X.; Li, T.; Liu, R.H. Phytochemical composition, cellular antioxidant capacity and antiproliferative activity in mango (Mangifera indica L.) pulp and peel. Int. J. Food Sci. Technol. 2017, 52, 817–826. [Google Scholar] [CrossRef]
- Abdullah, A.-S.H.; Mohammed, A.S.; Rasedee, A.; Mirghani, M.E.S. Oxidative stress-mediated apoptosis induced by ethanolic mango seed extract in cultured estrogen receptor positive breast cancer MCF-7 cells. Int. J. Mol. Sci. 2015, 16, 3528–3536. [Google Scholar] [CrossRef]
- Gondi, M.; Basha, S.A.; Bhaskar, J.J.; Salimath, P.V.; Prasada Rao, U.J.S. Anti-diabetic effect of dietary mango (Mangifera indica L.) peel in streptozotocin-induced diabetic rats. J. Sci. Food Agric. 2015, 95, 991–999. [Google Scholar] [CrossRef]
- Sekar, V.; Chakraborty, S.; Mani, S.; Sali, V.K.; Vasanthi, H.R. Mangiferin from Mangifera indica fruits reduces post-prandial glucose level by inhibiting α-glucosidase and α-amylase activity. S. Afr. J. Bot. 2019, 120, 129–134. [Google Scholar] [CrossRef]
- Rajshekar, M.S.; Rajshekar, J. Assesment of Antidiabetic Activity of Mangifera Indica Seed Kernel Extracts in Streptozotocin Induced Diabetic Rats. J. Nat. Remedies 2013, 14, 33–40. [Google Scholar] [CrossRef]
- Mirza, B.; Croley, C.R.; Ahmad, M.; Pumarol, J.; Das, N.; Sethi, G.; Bishayee, A. Mango (Mangifera indica L.): A magnificent plant with cancer preventive and anticancer therapeutic potential. Crit. Rev. Food Sci. Nutr. 2021, 61, 2125–2151. [Google Scholar] [CrossRef]
- Du, S.; Liu, H.; Lei, T.; Xie, X.; Wang, H.; He, X.; Tong, R.; Wang, Y. Mangiferin: An effective therapeutic agent against several disorders (Review). Mol. Med. Rep. 2018, 18, 4775–4786. [Google Scholar] [CrossRef] [PubMed]
- Taing, M.-W.; Pierson, J.-T.; Shaw, P.N.; Dietzgen, R.G.; Roberts-Thomson, S.J.; Gidley, M.J.; Monteith, G.R. Mango fruit extracts differentially affect proliferation and intracellular calcium signalling in MCF-7 human breast cancer cells. J. Chem. 2015, 2015, 613268. [Google Scholar] [CrossRef]
- Ahmad, R.; Aldholmi, M.; Mostafa, A.; Alqathama, A.; Aldarwish, A.; Abuhassan, A.; Alateeq, L.; Bubshait, S.; Aljaber, M.; Aldossary, S. A novel green extraction and analysis technique for the comprehensive characterization of mangiferin in different parts of the fresh mango fruit (Mangifera indica). LWT 2022, 159, 113176. [Google Scholar] [CrossRef]
- Corrales-Bernal, A.; Urango, L.A.; Rojano, B.; Maldonado, M.E. In vitro and in vivo effects of mango pulp (Mangifera indica cv. Azucar) in colon carcinogenesis. Arch. Latinoam. Nutr. 2014, 64, 16–23. [Google Scholar]
- Noratto, G.D.; Bertoldi, M.C.; Krenek, K.; Talcott, S.T.; Stringheta, P.C.; Mertens-Talcott, S.U. Anticarcinogenic effects of polyphenolics from mango (Mangifera indica) varieties. J. Agric. Food Chem. 2010, 58, 4104–4112. [Google Scholar] [CrossRef]
- Prasad, S.; Kalra, N.; Shukla, Y. Induction of apoptosis by lupeol and mango extract in mouse prostate and LNCaP cells. Nutr. Cancer 2007, 60, 120–130. [Google Scholar] [CrossRef]
- Abdullah, A.-S.H.; Mohammed, A.S.; Rasedee, A.; Mirghani, M.E.S.; Al-Qubaisi, M.S. Induction of apoptosis and oxidative stress in estrogen receptor-negative breast cancer, MDA-MB231 cells, by ethanolic mango seed extract. BMC Complement. Altern. Med. 2015, 15, 1–7. [Google Scholar] [CrossRef]
- Nguyen, H.X.; Do, T.N.V.; Le, T.H.; Nguyen, M.T.T.; Nguyen, N.T.; Esumi, H.; Awale, S. Chemical constituents of Mangifera indica and their antiausterity activity against the PANC-1 Human pancreatic cancer cell line. J. Nat. Prod. 2016, 79, 2053–2059. [Google Scholar] [CrossRef]
- Navarro, M.; Arnaez, E.; Moreira, I.; Quesada, S.; Azofeifa, G.; Wilhelm, K.; Vargas, F.; Chen, P. Polyphenolic Characterization, Antioxidant, and Cytotoxic Activities of Mangifera indica Cultivars from Costa Rica. Foods 2019, 8, 384. [Google Scholar] [CrossRef]
- Kim, H.; Moon, J.Y.; Kim, H.; Lee, D.-S.; Cho, M.; Choi, H.-K.; Kim, Y.S.; Mosaddik, A.; Cho, S.K. Antioxidant and antiproliferative activities of mango (Mangifera indica L.) flesh and peel. Food Chem. 2010, 121, 429–436. [Google Scholar] [CrossRef]
- Kumar, B.D.; Krishnakumar, K.; Jaganathan, S.K.; Mandal, M. Effect of mangiferin and mahanimbine on glucose utilization in 3T3-L1 cells. Pharmacogn. Mag. 2013, 9, 72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Kong, X.; Yuan, H.; Guan, H.; Li, Y.; Niu, Y. Mangiferin Improved Palmitate-Induced-Insulin Resistance by Promoting Free Fatty Acid Metabolism in HepG2 and C2C12 Cells via PPAR. J. Diabetes Res. 2019, 2019, 2052675. [Google Scholar] [CrossRef]
- Wang, H.L.; Li, C.Y.; Zhang, B.; Liu, Y.D.; Lu, B.M.; Shi, Z.; An, N.; Zhao, L.K.; Zhang, J.J.; Bao, J.K.; et al. Mangiferin facilitates islet regeneration and β-cell proliferation through upregulation of cell cycle and β-cell regeneration regulators. Int. J. Mol. Sci. 2014, 15, 9016–9035. [Google Scholar] [CrossRef] [PubMed]
- Mannervik, B. Glutathione peroxidase. In Methods Enzymol.; Elsevier: Amsterdam, The Netherlands, 1985; Volume 113, pp. 490–495. [Google Scholar]
- Maiorino, F.M.; Brigelius-Flohé, R.; Aumann, K.; Roveri, A.; Schomburg, D.; Flohé, L. Diversity of glutathione peroxidases. Methods Enzym. 1995, 252, 38–53. [Google Scholar] [CrossRef]
- Gupta, B.L.; Baquer, N.Z. Hexokinase, glucose-6-phosphate dehydrogenase and antioxidant enzymes in diabetic reticulocytes: Effects of insulin and vanadate. IUBMB Life 1998, 46, 1145–1152. [Google Scholar] [CrossRef]
- Brigelius-Flohe, R.; Kipp, A. Glutathione peroxidases in different stages of carcinogenesis. Biochim. Biophys. Acta (BBA) Gen. Subj. 2009, 1790, 1555–1568. [Google Scholar] [CrossRef]
- Sellamuthu, P.S.; Arulselvan, P.; Kamalraj, S.; Fakurazi, S.; Kandasamy, M. Protective nature of mangiferin on oxidative stress and antioxidant status in tissues of streptozotocin-induced diabetic rats. ISRN Pharmacol. 2013, 2013, 750109. [Google Scholar] [CrossRef]
- Al Omairi, N.E.; Radwan, O.K.; Alzahrani, Y.A.; Kassab, R.B. Neuroprotective efficiency of Mangifera indica leaves extract on cadmium-induced cortical damage in rats. Metab. Brain Dis. 2018, 33, 1121–1130. [Google Scholar] [CrossRef]
- Mccue, P.; KWON, Y.I.; Shetty, K. Anti-amylase, anti-glucosidase and anti-angiotensin i-converting enzyme potential of selected foods. J. Food Biochem. 2005, 29, 278–294. [Google Scholar] [CrossRef]
- Ogunwande, I.A.; Matsui, T.; Fujise, T.; Matsumoto, K. α-Glucosidase inhibitory profile of Nigerian medicinal plants in immobilized assay system. Food Sci. Technol. Res. 2007, 13, 169–172. [Google Scholar] [CrossRef]
- Gondi, M.; Prasada Rao, U.J.S. Ethanol extract of mango (Mangifera indica L.) peel inhibits α-amylase and α-glucosidase activities, and ameliorates diabetes related biochemical parameters in streptozotocin (STZ)-induced diabetic rats. J. Food Sci. Technol. 2015, 52, 7883–7893. [Google Scholar] [CrossRef]
- Dineshkumar, B.; Mitra, A.; Manjunatha, M. Studies on the anti-diabetic and hypolipidemic potentials of mangiferin (xanthone glucoside) in streptozotocin-induced type 1 and type 2 diabetic model rats. Int. J. Adv. Pharm. Sci. 2010, 1, 75–85. [Google Scholar] [CrossRef]
- Preciado-Saldaña, A.M.; Domínguez-Avila, J.A.; Ayala-Zavala, J.F.; Astiazaran-Garcia, H.F.; Montiel-Herrera, M.; Villegas-Ochoa, M.A.; González-Aguilar, G.A.; Wall-Medrano, A. Mango “Ataulfo” Peel Extract Improves Metabolic Dysregulation in Prediabetic Wistar Rats. Life 2022, 12, 532. [Google Scholar] [CrossRef] [PubMed]
- Irondi, E.A.; Oboh, G.; Akindahunsi, A.A. Antidiabetic effects of Mangifera indica Kernel Flour-supplemented diet in streptozotocin-induced type 2 diabetes in rats. Food Sci. Nutr. 2016, 4, 828–839. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Chen, S.; Chen, H.; Wang, Y.; Wang, Y.; Hochstetter, D.; Xu, P. Studies on the bioactivity of aqueous extract of pu-erh tea and its fractions: In vitro antioxidant activity and α-glycosidase inhibitory property, and their effect on postprandial hyperglycemia in diabetic mice. Food Chem. Toxicol. 2013, 53, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Alqathama, A.; Aldholmi, M.; Riaz, M.; Mukhtar, M.H.; Aljishi, F.; Althomali, E.; Alamer, M.A.; Alsulaiman, M.; Ayashy, A. Biological Screening of Glycyrrhiza glabra L. from Different Origins for Antidiabetic and Anticancer Activity. Pharmaceuticals 2022, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Al-Salem, H.S.; Arifuzzaman, M.; Alkahtani, H.M.; Abdalla, A.N.; Issa, I.S.; Alqathama, A.; Albalawi, F.S.; Rahman, A.M. A series of isatin-hydrazones with cytotoxic activity and CDK2 kinase inhibitory activity: A potential type II ATP competitive inhibitor. Molecules 2020, 25, 4400. [Google Scholar] [CrossRef] [PubMed]
- Odeyemi, S.; Dewar, J. In vitro antidiabetic activity affecting glucose uptake in hepg2 cells following their exposure to extracts of Lauridia tetragona (Lf) RH Archer. Processes 2019, 8, 33. [Google Scholar] [CrossRef]
- Quan, N.V.; Tran, H.-D.; Xuan, T.D.; Ahmad, A.; Dat, T.D.; Khanh, T.D.; Teschke, R. Momilactones A and B are α-amylase and α-glucosidase inhibitors. Molecules 2019, 24, 482. [Google Scholar] [CrossRef] [PubMed]
| Original Code | HCT116 | MCF7 | Glucose Uptake | GPx Activity | α-Amylase Inhibition | MG Amount (mg/10 g) | |
|---|---|---|---|---|---|---|---|
| India Badami (large green) epicarp | 91 ± 0.25 | 71 ± 0.03 | 101 ± 0.04 | 0.50 ± 0.31 | 7 ± 0.04 | 0.81 | |
| India Badami (large green) endocarp | 78 ± 0.11 | 73 ± 0.16 | 98 ± 0.02 | 0.90 ± 0.16 | 10 ± 0.09 | 0.44 | |
| India Badami (large green) mesocarp | 72 ± 0.38 | 65 ± 0.21 | 99 ± 0.01 | 0.61 ± 0.23 | 16 ± 0.01 | 1.07 | |
| India Badami (large green) seed | 37 ± 0.11 | 37 ± 0.01 | 96 ± 0.08 | 0.90 ± 0.19 | 55 ± 0.13 | 0.51 | |
| India Totapuri (long green) epicarp | 63 ± 0.11 | 81 ± 0.06 | 99 ± 0.06 | 0.72± 0.21 | 3 ± 0.03 | 1.10 | |
| India Totapuri (long green) endocarp | 86 ± 0.12 | 80 ± 0.01 | 99 ± 0.03 | 0.81 ± 0.12 | 3 ± 0.04 | 1.10 | |
| India Totapuri (long green) mesocarp | 74 ± 0.09 | 67 ± 0.07 | 99 ± 0.03 | 0.92 ± 0.13 | 9 ± 0.12 | 1.49 | |
| India Totapuri (long green) seed | 45 ± 0.16 | 34 ± 0.01 | 101 ± 0.06 | 0.53 ± 0.14 ** | 4 ± 0.04 | 1.39 | |
| Indian Alphonso (small round green) epicarp | 74 ± 0.24 | 73 ± 0.03 | 100 ± 0.04 | 1.09 ± 0.17 | 13 ± 0.04 | 1.25 | |
| Indian Alphonso (small round green) endocarp | 81 ± 0.14 | 82 ± 0.02 | 99 ± 0.04 | 0.72 ± 0.33 | 4 ± 0.03 | 1.81 | |
| Indian Alphonso (small round green) mesocarp | 80 ± 0.16 | 74 ± 0.03 | 114 ± 0.05 ** | 0.96 ± 0.23 | 39 ± 0.12 | 2.09 | |
| Indian Alphonso (small round green) seed | 70 ± 0.37 | 58 ± 0.30 | 100 ± 0.06 | 0.57 ± 0.32 | 30 ± 0.08 | 0.99 | |
| Egypt (reddish green) epicarp | 41 ± 0.12 | 38 ± 0.08 | 98 ± 0.05 | 0.63 ± 0.23 | 21 ± 0.16 | 0.65 | |
| Egypt (reddish green) endocarp | 83 ± 0.12 | 95 ± 0.02 | 99 ± 0.02 | 0.25 ± 0.13 *** | 9 ± 0.03 | 0.87 | |
| Egypt (reddish green) mesocarp | 84 ± 0.16 | 83 ± 0.15 | 99 ± 0.05 | 0.59 ± 0.37 | 15 ± 0.11 | 0.78 | |
| Egypt (reddish green) seed | 50 ± 0.11 | 47 ± 0.07 | 104 ± 0.06 | 0.85 ± 0.24 | 20 ± 0.07 | 0.47 | |
| Kenya (large round red) epicarp | 67 ± 0.13 | 62 ± 0.09 | 105 ± 0.02 * | 0.70 ± 0.26 | 4 ± 0.02 | 0.56 | |
| Kenya (large round red) endocarp | 88 ± 0.13 | 87 ± 0.09 | 112 ± 0.06 * | 0.89 ± 0.21 | 1 ± 0.08 | 0.57 | |
| Kenya (large round red) mesocarp | 71 ± 0.36 | 39 ± 0.36 | 118 ± 0.07 * | 1.30 ± 0.23 | 18 ± 0.15 | 0.76 | |
| Kenya (large round red) seed | 34 ± 0.08 | 38 ± 0.11 | 98 ± 0.10 | 0.63 ± 0.27 | 33 ± 0.09 | 0.53 | |
| Sri Lanka (large yellowish green) epicarp | 75 ± 0.15 | 61 ± 0.10 | 99 ± 0.05 | 0.61 ± 0.11 ** | 12 ± 0.05 | 0.69 | |
| Sri Lanka (large yellowish green) endocarp | 82 ± 0.12 | 82 ± 0.01 | 102 ± 0.05 | 0.72 ± 0.21 | 1 ± 0.01 | 0.76 | |
| Sri Lanka (large yellowish green) mesocarp | 81 ± 0.18 | 80 ± 0.06 | 97 ± 0.02 | 0.62 ± 0.28 | 15 ± 0.03 | 0.70 | |
| Sri Lanka (large yellowish green) seed | 39 ± 0.12 | 34 ± 0.05 | 96 ± 0.02 | 0.54 ± 0.23 * | 8 ±0.02 | 0.82 | |
| Thailand (large green) epicarp | 75 ± 0.08 | 68 ± 0.17 | 119 ± 0.11 * | 0.86± 0.18 | 21 ± 0.08 | 0.88 | |
| Thailand (large green) endocarp | 80 ± 0.08 | 73 ± 0.04 | 109 ± 0.05 * | 0.42 ± 0.11 *** | 19 ± 0.03 | 0.87 | |
| Thailand (large green) mesocarp | 87 ± 0.11 | 89 ± 0.02 | 110 ± 0.05 * | 0.36 ± 0.12 ** | 41 ± 0.11 | 0.66 | |
| Thailand (large green) seed | 35 ± 0.15 | 36 ± 0.01 | 101 ± 0.07 | 0.98± 0.18 | 16 ± 0.13 | 0.54 | |
| Vietnam (large yellowish green) epicarp | 72 ± 0.20 | 70 ± 0.06 | 100 ± 0.05 | 0.99 ± 0.28 | 9 ±0.04 | 0.61 | |
| Vietnam (large yellowish green) endocarp | 84 ± 0.21 | 75 ± 0.16 | 95 ± 0.06 | 0.88 ± 0.33 | 8 ±0.03 | 0.91 | |
| Vietnam (large yellowish green) mesocarp | 87 ± 0.12 | 80 ± 0.06 | 100 ± 0.07 | 0.55± 0.28 | 28 ± 0.08 | 0.89 | |
| Vietnam (large yellowish green) seed | 42 ± 0.17 | 39 ± 0.05 | 96 ± 0.03 | 0.72 ± 0.26 | 58 ± 0.07 | 0.47 | |
| Yemen Badami (large yellow) epicarp | 80 ± 0.21 | 74 ± 0.09 | 108 ± 0.04 * | 0.79 ± 0.19 | 14 ± 0.03 | 0.97 | |
| Yemen Badami (large yellow) endocarp | 77 ± 0.14 | 80 ± 0.07 | 114 ± 0.06 * | 0.63 ± 0.38 | 5 ± 0.03 | 0.76 | |
| Yemen Badami (large yellow) mesocarp | 90 ± 0.18 | 64 ± 0.03 | 101 ± 0.01 | 0.72± 0.40 | 15 ± 0.04 | 0.94 | |
| Yemen Badami (large yellow) seed | 42 ± 0.17 | 39 ± 0.03 | 119 ± 0.08 * | 0.62 ± 0.13 ** | 31 ± 0.07 | 0.75 | |
| Yemen Kalabathoor (large round red) epicarp | 76 ± 0.10 | 84 ± 0.13 | 99 ± 0.04 | 0.70± 0.24 | 14 ± 0.02 | 1.52 | |
| Yemen Kalabathoor (large round red) endocarp | 50 ± 0.11 | 43 ± 0.01 | 96 ± 0.05 | 0.57 ± 0.32 | 60 ± 0.05 | 0.77 | |
| Yemen Kalabathoor (large round red) mesocarp | 67 ± 0.16 | 62 ± 0.06 | 95 ± 0.04 | 0.60 ± 0.17 * | 13 ± 0.08 | 1.42 | |
| Yemen Kalabathoor (large round red) seed | 48 ± 0.13 | 40 ± 0.02 | 97 ± 0.03 | 0.77 ± 0.19 | 28 ± 0.11 | 0.81 | |
| Yemen Taimoor (large yellowish green) epicarp | 74 ± 0.17 | 57 ± 0.15 | 115 ± 0.05 ** | 0.53 ± 0.04 *** | 5 ± 0.15 | 0.95 | |
| Yemen Taimoor (large yellowish green) endocarp | 77 ± 0.12 | 84 ± 0.24 | 97 ± 0.02 | 0.63 ± 0.14 * | 12 ± 0.07 | 0.58 | |
| Yemen Taimoor (large yellowish green) mesocarp | 87 ± 0.03 | 98 ± 0.17 | 101 ± 0.03 | 0.80 ± 0.20 | 34 ± 0.13 | 0.84 | |
| Yemen Taimoor (large yellowish green) seed | 42 ± 0.21 | 38 ± 0.05 | 98 ± 0.09 | 1.25 ± 0.20 | 12 ± 0.09 | 0.82 | |
| Yemen Taimoor (small reddish green) epicarp | 73 ± 0.10 | 62 ± 0.13 | 98 ± 0.02 | 0.84 ± 0.12 | 19 ± 0.09 | 0.96 | |
| Yemen Taimoor (small reddish green) endocarp | 80 ± 0.09 | 92 ± 0.08 | 98 ± 0.02 | 0.89 ± 0.25 | 15 ± 0.01 | 1.01 | |
| Yemen Taimoor (small reddish green) mesocarp | 85 ± 0.15 | 84 ± 0.02 | 102 ± 0.07 | 0.58 ± 0.36 | 17 ± 0.10 | 0.79 | |
| Yemen Taimoor (small reddish green) seed | 36 ± 0.12 | 39 ± 0.07 | 97 ± 0.05 | 0.46 ± 0.05 *** | 54 ± 0.06 | 0.61 | |
| Standard | - | - | Metformin (123 ± 0.07 **) | Tannic acid (0.44 ± 0.13 ***) | - | - | |
| Descriptive statistics | |||||||
| Descriptive | HCT116 | MCF7 | Glucose uptake | GPx activity | α-Amylase inhibition | ||
| Minimum | 34 | 34 | 95 | 0.25 | 1 | ||
| Maximum | 91 | 98 | 119 | 1.30 | 60 | ||
| Mean | 68.58 | 64.81 | 102.02 | 0.72 | 18.70 | ||
| Standard deviation | 17.84 | 19.28 | 6.67 | 0.21 | 15.16 | ||
| Geographical Origin | HCT116 | MCF7 | MRC5 |
|---|---|---|---|
| Egypt (reddish green) seed | 87.54 ± 3.03 | 70.99 ± 2.74 | 96.62 ± 2.33 |
| Yemen Kalabathoor (large round red) endocarp | 96.63 ± 2.73 | 74.83 ± 1.71 | 72.86 ± 1.65 |
| Yemen Kalabathoor (large round red) seed | 76.33 ± 1.95 | 36.83 ± 1.70 | 69.91 ± 2.50 |
| Vietnam (large yellowish green) seed | 26.54 ± 1.10 | 20.17 ± 1.24 | 55.60 ± 1.07 |
| Kenya (large round red) seed | 14.44 ± 3.61 | 25.96 ± 1.08 | 42.57 ± 1.76 |
| Yemen Taimoor (large yellowish green) seed | 43.66 ± 1.92 | 34.69 ± 1.94 | 39.30 ± 2.14 |
| Egypt (reddish green) epicarp | 51.48 ± 3.72 | 33.11 ± 2.51 | 39.09 ± 3.63 |
| India Badami (large green) seed | 22.99 ± 2.00 | 26.60 ± 0.96 | 29.95 ± 2.64 |
| Yemen Badami (large yellow) seed | 28.76 ± 2.90 | 30.35 ± 1.06 | 27.85 ± 1.63 |
| Thailand (large green) seed | 20.01 ± 0.88 | 24.63 ± 2.53 | 27.47 ± 3.54 |
| Yemen Taimoor (small reddish green) seed | 18.16 ± 2.92 | 25.76 ± 2.64 | 26.00 ± 3.35 |
| Sri Lanka (large yellowish green) seed | 20.53 ± 1.56 | 17.19 ± 1.60 | 20.79 ± 1.59 |
| India Totapuri (long green) seed | 56.47 ± 2.55 | 37.44 ± 1.02 | 100.4 ± 1.43 |
| Doxorubicin | 4.19 ± 1.23 | 3.11 ± 1.34 | 6.90 ± 0.95 |
| Geographical Origin | IC50 |
|---|---|
| Yemen Taimoor (small reddish-green) seed | 190.5 ± 2.35 |
| Vietnam (large yellowish green) seed | 128.4 ± 1.25 |
| Yemen Kalabathoor (large round red) endocarp | 108.8 ± 0.70 |
| India Badami (large green) seed | 202.5 ± 2.80 |
| Acarbose | 78.41 ± 0.67 |
| Geographical Origin | Fruit Part | HCT116 | MCF7 | Glucose Uptake | GPx Activity | α-Amylase Activity | MG Amount | |
|---|---|---|---|---|---|---|---|---|
| Fruit part | 0.000 1.000 | 1 | ||||||
| HCT116 | −0.044 0.764 | −0.550 0.000 | 1 | |||||
| MCF7 | −0.060 0.686 | −0.442 0.002 | 0.883 0.000 | 1 | ||||
| Glucose uptake | 0.074 0.615 | −0.182 0.216 | 0.198 0.177 | 0.058 0.693 | 1 | |||
| GPx activity | −0.160 0.277 | −0.032 0.828 | −0.079 0.591 | −0.153 0.299 | 0.108 0.466 | 1 | ||
| α-amylase activity | −0.089 0.548 | 0.315 0.029 | −0.430 0.002 | −0.373 0.009 | −0.079 0.592 | −0.109 0.460 | 1 | |
| MG amount | −0.603 0.000 | −0.237 0.104 | 0.280 0.054 | 0.248 0.089 | 0.047 0.751 | 0.069 0.639 | −0.198 0.177 | 1 |
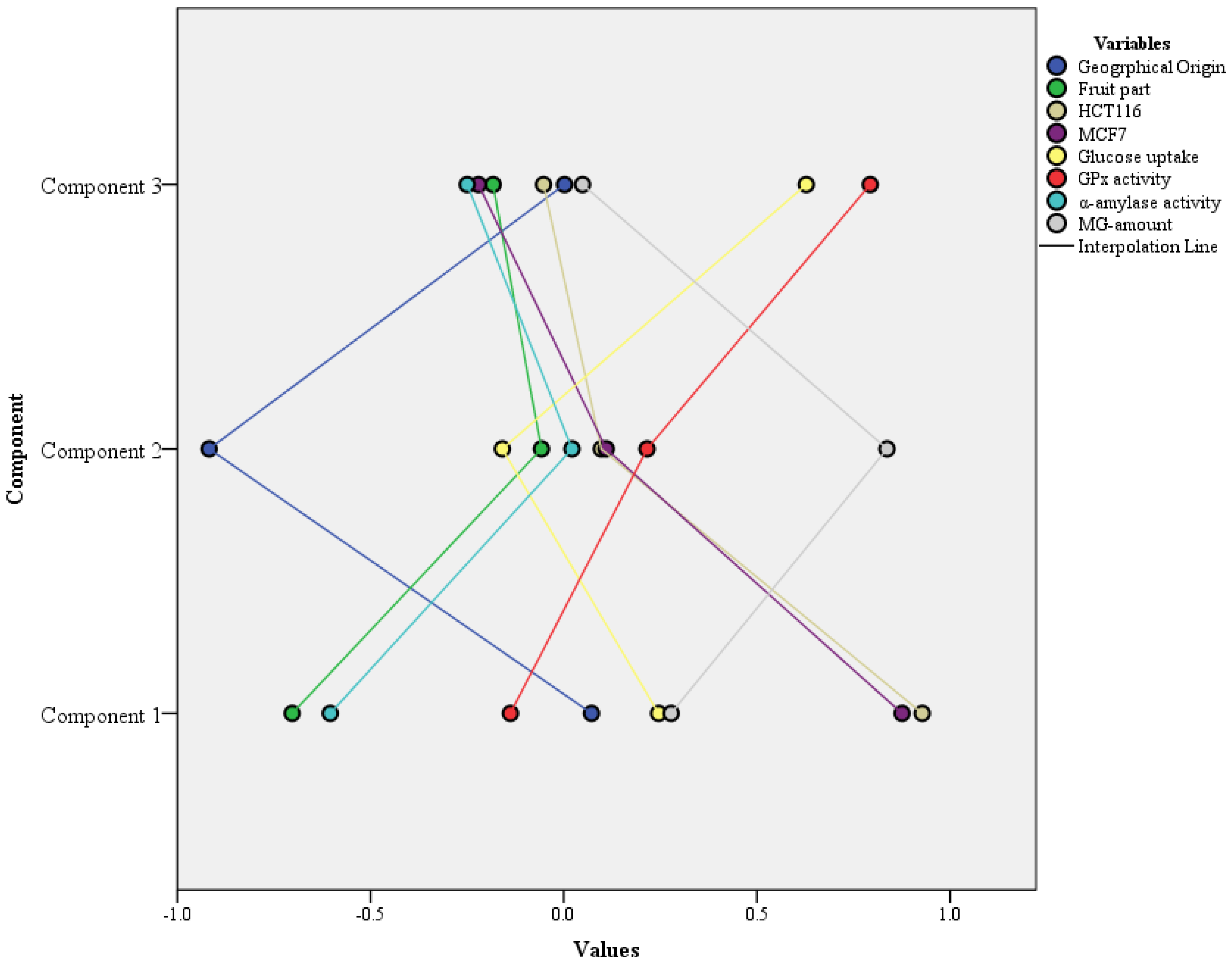
| Factors | PC1 | PC2 | PC3 | Kaiser-Meyer-Olkin and Bartlett’s Test | ||
|---|---|---|---|---|---|---|
| Geographical origin | 0.072 | −0.918 | 0.002 | KMO Measure of Sampling Adequacy | 0.60 | |
| Fruit part | −0.703 | −0.058 | −0.183 | Bartlett’s Test of Sphericity | Approx. Chi-Square | 129.78 |
| HCT116 | 0.928 | 0.097 | −0.053 | df | 28 | |
| MCF7 | 0.875 | 0.110 | −0.221 | Sig. | 0.00 | |
| Glucose uptake | 0.245 | −0.159 | 0.627 | |||
| GPx activity | −0.138 | 0.216 | 0.793 | |||
| α-amylase activity | −0.604 | 0.021 | −0.250 | |||
| MG amount | 0.278 | 0.837 | 0.049 | |||
| Individual variance (%) | 33.092 | 20.487 | 14.653 | |||
| Cumulative variance (%) | 33.092 | 53.579 | 68.232 | |||
| Geographical Origin | Fruit Part | HCT116 | MCF7 | Glucose Uptake | GPx Activity | α-Amylase Activity | |
|---|---|---|---|---|---|---|---|
| Fruit part | 0.000 | ||||||
| HCT116 | −0.044 | −0.550 | |||||
| MCF7 | −0.060 | −0.442 | 0.883 | ||||
| Glucose uptake | 0.074 | −0.182 | 0.198 | 0.058 | |||
| GPx activity | −0.160 | −0.032 | −0.079 | −0.153 | 0.108 | ||
| α-amylase activity | −0.089 | 0.315 | −0.430 | −0.373 | −0.079 | −0.109 | |
| MG amount | −0.603 | −0.237 | 0.280 | 0.248 | 0.047 | 0.069 | −0.198 |
| ANOVA table | |||||||
| Sum of Squares | Mean Square | F | Sig | ||||
| Between groups | 6634.99 | 141.17 | 627.81 | 0.00 | |||
| Within groups | Between Items | 525,689.83 | 75,098.54 | ||||
| Residual | 39,354.55 | 119.61 | |||||
| Total | 565,044.39 | 1681.68 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, R.; Alqathama, A.; Aldholmi, M.; Riaz, M.; Abdalla, A.N.; Aljishi, F.; Althomali, E.; Amir, M.; Abdullah, O.; Alamer, M.A.; et al. Antidiabetic and Anticancer Potentials of Mangifera indica L. from Different Geographical Origins. Pharmaceuticals 2023, 16, 350. https://doi.org/10.3390/ph16030350
Ahmad R, Alqathama A, Aldholmi M, Riaz M, Abdalla AN, Aljishi F, Althomali E, Amir M, Abdullah O, Alamer MA, et al. Antidiabetic and Anticancer Potentials of Mangifera indica L. from Different Geographical Origins. Pharmaceuticals. 2023; 16(3):350. https://doi.org/10.3390/ph16030350
Chicago/Turabian StyleAhmad, Rizwan, Aljawharah Alqathama, Mohammed Aldholmi, Muhammad Riaz, Ashraf N. Abdalla, Fatema Aljishi, Ebtihal Althomali, Mohd Amir, Omeima Abdullah, Muntathir Ali Alamer, and et al. 2023. "Antidiabetic and Anticancer Potentials of Mangifera indica L. from Different Geographical Origins" Pharmaceuticals 16, no. 3: 350. https://doi.org/10.3390/ph16030350
APA StyleAhmad, R., Alqathama, A., Aldholmi, M., Riaz, M., Abdalla, A. N., Aljishi, F., Althomali, E., Amir, M., Abdullah, O., Alamer, M. A., Alaswad, D., Alsulais, W., & Alsulays, A. (2023). Antidiabetic and Anticancer Potentials of Mangifera indica L. from Different Geographical Origins. Pharmaceuticals, 16(3), 350. https://doi.org/10.3390/ph16030350






