TLR4/Inflammasomes Cross-Talk and Pyroptosis Contribute to N-Acetyl Cysteine and Chlorogenic Acid Protection against Cisplatin-Induced Nephrotoxicity
Abstract
1. Introduction
2. Results
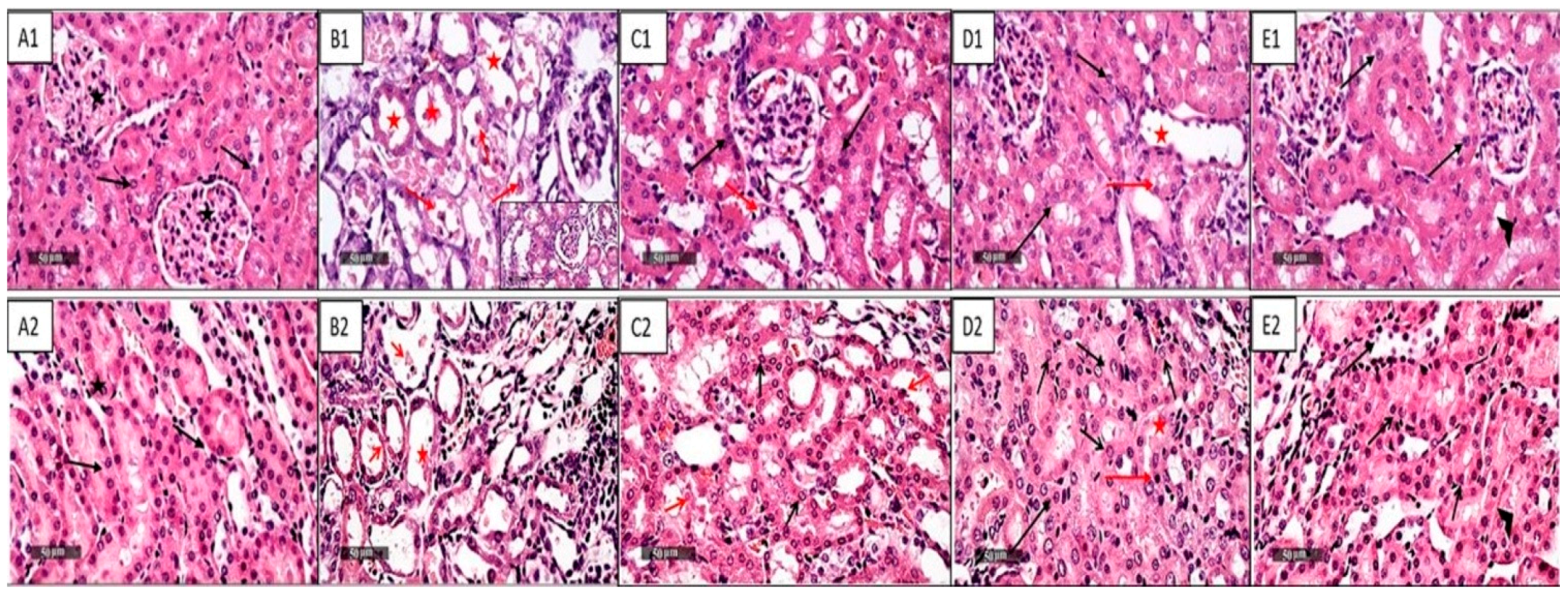
2.1. NAC and/or CGA Ameliorated Cp-Induced Nephrotoxicity
2.2. NAC and/or CGA Reduced Cp-Induced Oxidative Stress
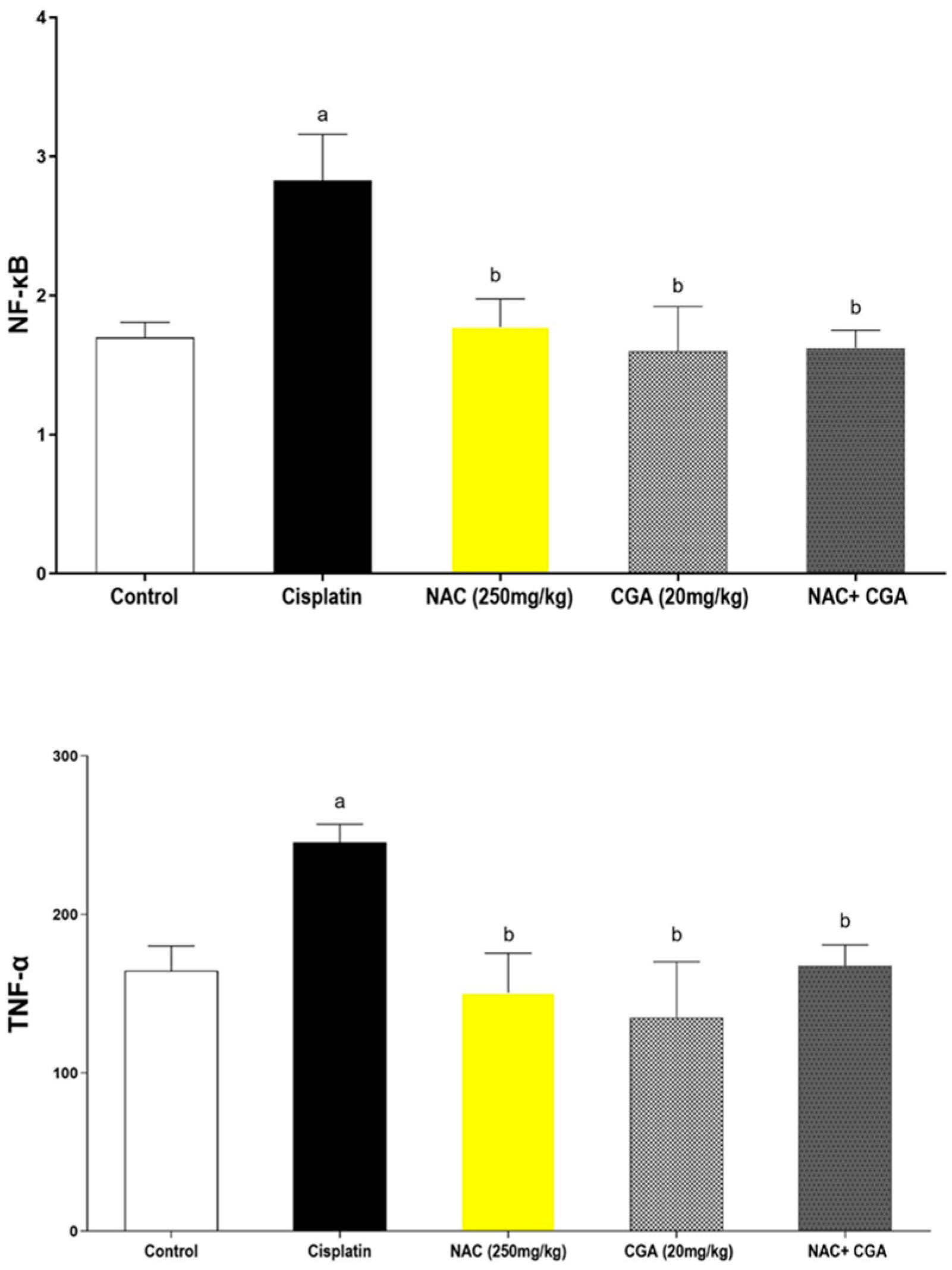
2.3. Effect of NAC and/or CGA on Cp-Induced Inflammatory Signalling Responses
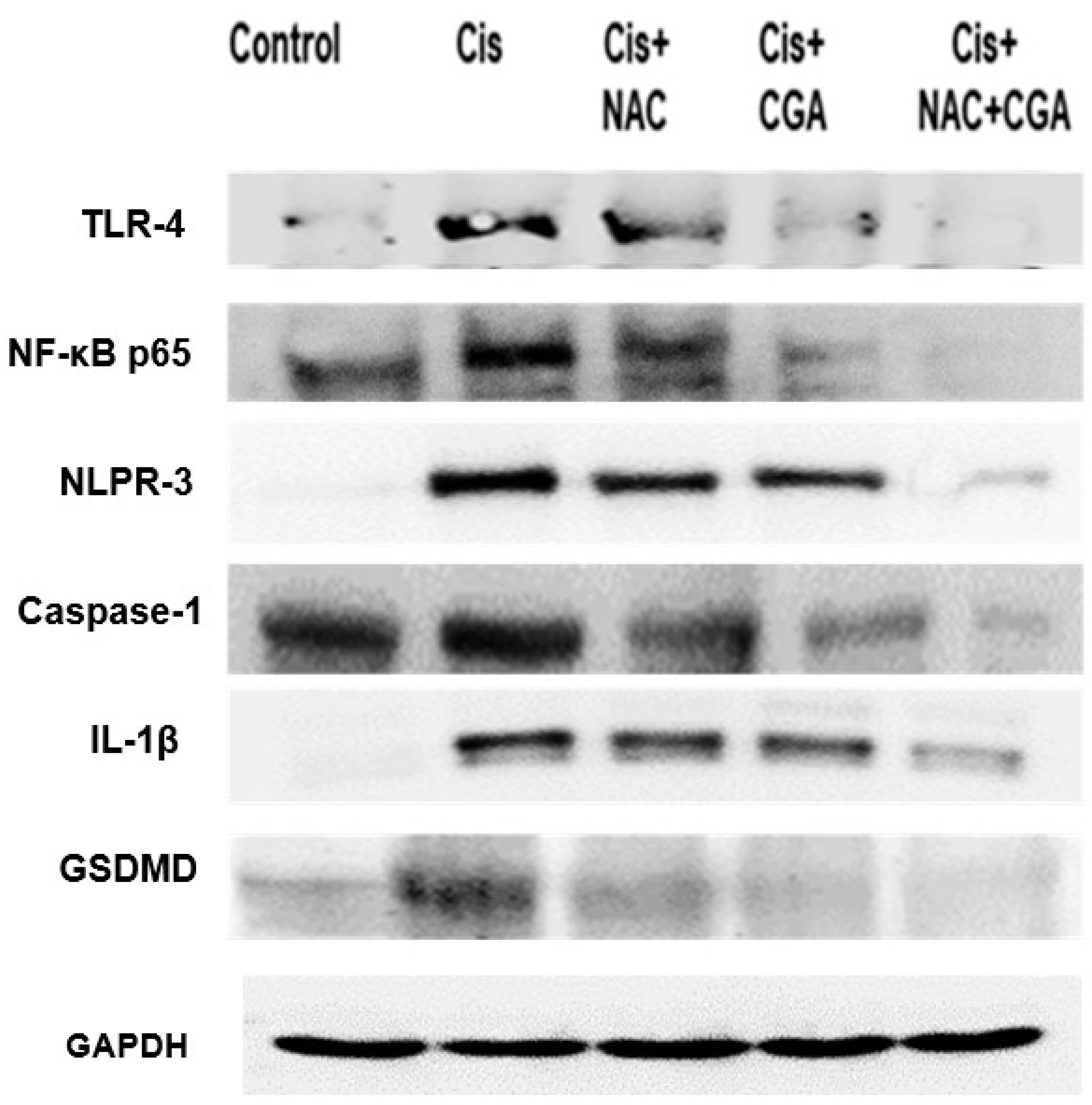
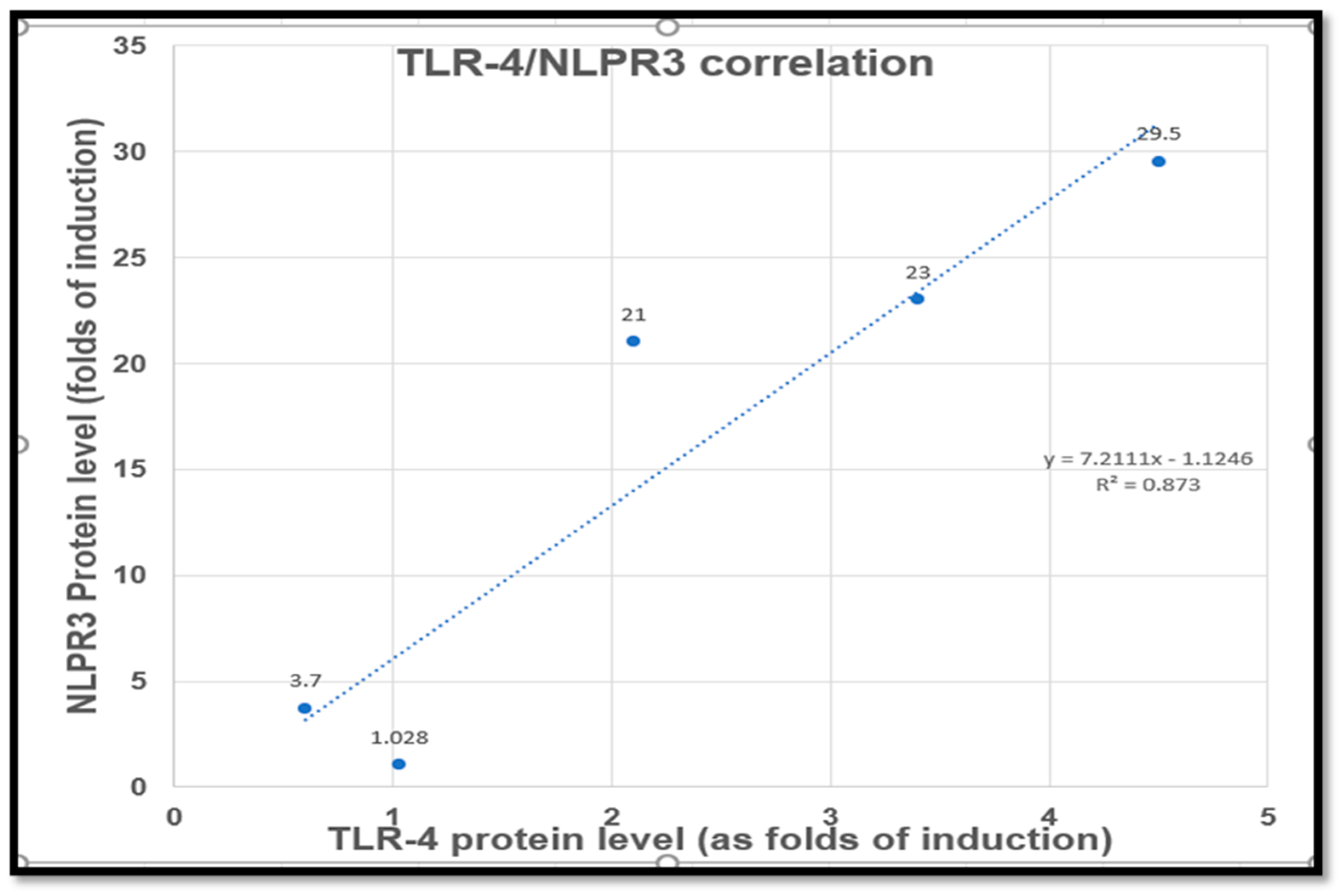
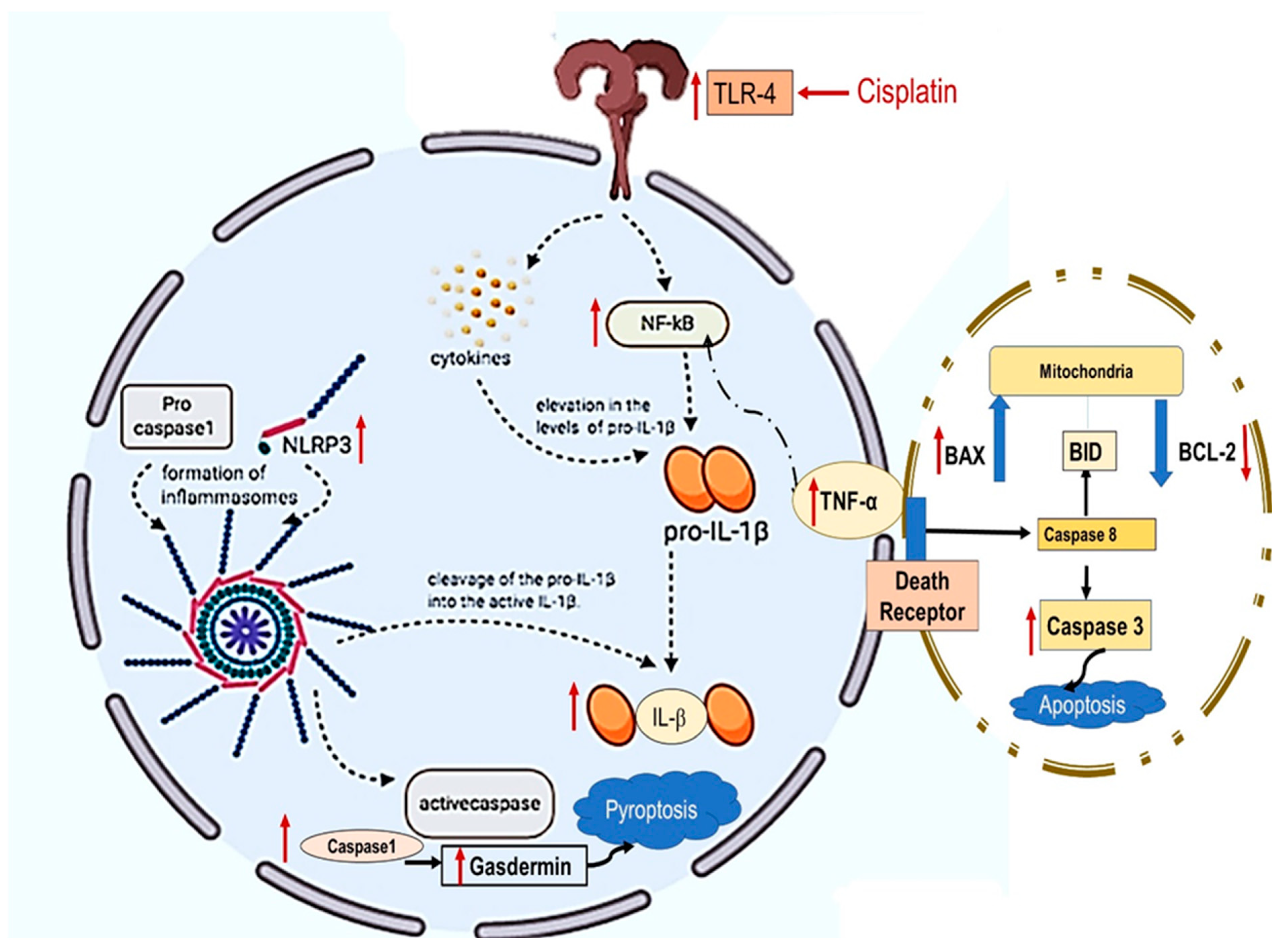
2.4. NAC and/or CGA Inhibited the TLR4/NLPR3/IL-1β and Caspase-1/GSDMD Signaling in Cp-Induced Nephrotoxicity
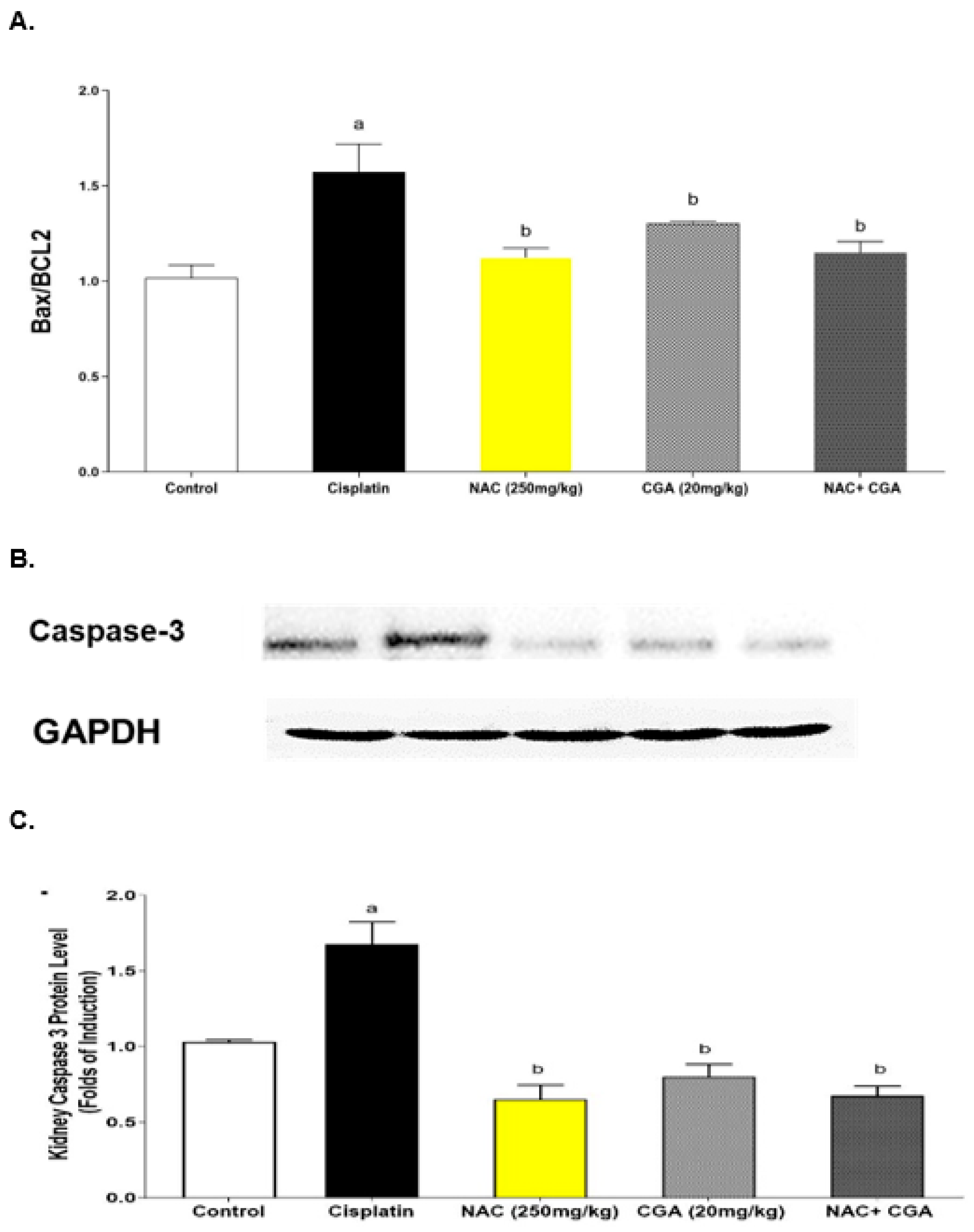
2.5. Inhibition of Apoptotic Markers by NAC’s and/or CGA’s
3. Discussion
4. Material and Methods
4.1. Materials
4.1.1. Drugs and Chemicals
4.1.2. Animals
4.2. Methods
4.2.1. Experiment Design and Sample Collection
4.2.2. Assessment of Nephrotoxicity Indices
4.2.3. Assessment of Histopathological Changes
4.2.4. Assessment of Oxidative Stress Markers in Renal Tissues
4.2.5. Assessment of Renal Expression of Inflammatory Markers (NF-κB, TNF-α) Using ELISA
4.2.6. Assessment of Renal Expression of Apoptotic Markers (Bax/Bcl-2 Ratio) Using ELISA
4.2.7. Assessment of Protein Content
4.2.8. Western Blot Analysis of TLR4, NF-κB, NLRP3, Caspase-1, IL-1β, Caspase-3, and GSDMD Protein Expression in Kidney Tissues
4.3. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Miller, R.P.; Tadagavadi, R.K.; Ramesh, G.; Reeves, W.B. Mechanisms of Cisplatin Nephrotoxicity. Toxins 2010, 2, 2490–2518. [Google Scholar] [CrossRef]
- Volarevic, V.; Djokovic, B.; Jankovic, M.G.; Harrell, C.R.; Fellabaum, C.; Djonov, V.; Arsenijevic, N. Molecular Mechanisms of Cisplatin-Induced Nephrotoxicity: A Balance on the Knife Edge between Renoprotection and Tumor Toxicity. J. Biomed. Sci. 2019, 26, 25. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Planting, A.S.; Catimel, G.; de Mulder, P.H.; de Graeff, A.; Höppener, F.; Verweij, J.; Oster, W.; Vermorken, J.B. Randomized Study of a Short Course of Weekly Cisplatin with or without Amifostine in Advanced Head and Neck Cancer. EORTC Head and Neck Cooperative Group. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 1999, 10, 693–700. [Google Scholar] [CrossRef]
- Perazella, M.A. Onco-Nephrology: Renal Toxicities of Chemotherapeutic Agents. Clin. J. Am. Soc. Nephrol. CJASN 2012, 7, 1713–1721. [Google Scholar] [CrossRef]
- Herrera-Pérez, Z.; Gretz, N.; Dweep, H. A Comprehensive Review on the Genetic Regulation of Cisplatin-Induced Nephrotoxicity. Curr. Genom. 2016, 17, 279–293. [Google Scholar] [CrossRef]
- Stathopoulos, G.P. Cisplatin: Process and Future. J. BUON Off. J. Balk. Union Oncol. 2013, 18, 564–569. [Google Scholar]
- Lee, D.W.; Faubel, S.; Edelstein, C.L. Cytokines in Acute Kidney Injury (AKI). Clin. Nephrol. 2011, 76, 165–173. [Google Scholar] [CrossRef]
- Zhai, J.; Gao, H.; Wang, S.; Zhang, S.; Qu, X.; Zhang, Y.; Tao, L.; Sun, J.; Song, Y.; Fu, L. Ginsenoside Rg3 Attenuates Cisplatin-Induced Kidney Injury through Inhibition of Apoptosis and Autophagy-Inhibited NLRP3. J. Biochem. Mol. Toxicol. 2021, 35, e22896. [Google Scholar] [CrossRef]
- Qu, X.; Gao, H.; Tao, L.; Zhang, Y.; Zhai, J.; Song, Y.; Zhang, S. Autophagy Inhibition-Enhanced Assembly of the NLRP3 Inflammasome Is Associated with Cisplatin-Induced Acute Injury to the Liver and Kidneys in Rats. J. Biochem. Mol. Toxicol. 2018, 33, e22208. [Google Scholar] [CrossRef]
- Xiang, H.; Zhu, F.; Xu, Z.; Xiong, J. Role of Inflammasomes in Kidney Diseases via Both Canonical and Non-Canonical Pathways. Front. Cell Dev. Biol. 2020, 8, 106. [Google Scholar] [CrossRef]
- Hassan, H.M.; Mahran, Y.F.; Ghanim, A.M.H. Ganoderma Lucidum Ameliorates the Diabetic Nephropathy via Down-Regulatory Effect on TGFβ-1 and TLR-4/NFκB Signalling Pathways. J. Pharm. Pharmacol. 2021, 73, 1250–1261. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, H.; Li, X.; Yi, B.; Huang, L.; Hu, Z.; Li, A.; Du, J.; Li, Y.; Zhang, W. Vitamin D/VDR Attenuate Cisplatin-Induced AKI by down-Regulating NLRP3/Caspase-1/GSDMD Pyroptosis Pathway. J. Steroid Biochem. Mol. Biol. 2021, 206, 105789. [Google Scholar] [CrossRef]
- Faubel, S.; Lewis, E.C.; Reznikov, L.; Ljubanovic, D.; Hoke, T.S.; Somerset, H.; Oh, D.-J.; Lu, L.; Klein, C.L.; Dinarello, C.A.; et al. Cisplatin-Induced Acute Renal Failure Is Associated with an Increase in the Cytokines Interleukin (IL)-1beta, IL-18, IL-6, and Neutrophil Infiltration in the Kidney. J. Pharmacol. Exp. Ther. 2007, 322, 8–15. [Google Scholar] [CrossRef]
- Eisa, N.H.; El-Sherbiny, M.; Abo El-Magd, N.F. Betulin Alleviates Cisplatin-Induced Hepatic Injury in Rats: Targeting Apoptosis and Nek7-Independent NLRP3 Inflammasome Pathways. Int. Immunopharmacol. 2021, 99, 107925. [Google Scholar] [CrossRef]
- Fang, Y.; Tian, S.; Pan, Y.; Li, W.; Wang, Q.; Tang, Y.; Yu, T.; Wu, X.; Shi, Y.; Ma, P.; et al. Pyroptosis: A New Frontier in Cancer. Biomed. Pharmacother. 2020, 121, 109595. [Google Scholar] [CrossRef]
- Li, Y.; Xia, W.; Wu, M.; Yin, J.; Wang, Q.; Li, S.; Zhang, A.; Huang, S.; Zhang, Y.; Jia, Z. Activation of GSDMD Contributes to Acute Kidney Injury Induced by Cisplatin. Am. J. Physiol. Renal Physiol. 2020, 318, F96–F106. [Google Scholar] [CrossRef]
- Zhang, K.; Wu, Q.; Jiang, S.; Ding, L.; Liu, C.; Xu, M.; Wang, Y.; Zhou, Y.; Li, L. Pyroptosis: A New Frontier in Kidney Diseases. Oxid. Med. Cell. Longev. 2021, 2021, 6686617. [Google Scholar] [CrossRef]
- Tajima, T.; Yoshifuji, A.; Matsui, A.; Itoh, T.; Uchiyama, K.; Kanda, T.; Tokuyama, H.; Wakino, S.; Itoh, H. β-Hydroxybutyrate Attenuates Renal Ischemia-Reperfusion Injury through Its Anti-Pyroptotic Effects. Kidney Int. 2019, 95, 1120–1137. [Google Scholar] [CrossRef]
- Yang, J.-R.; Yao, F.-H.; Zhang, J.-G.; Ji, Z.-Y.; Li, K.-L.; Zhan, J.; Tong, Y.-N.; Lin, L.-R.; He, Y.-N. Ischemia-Reperfusion Induces Renal Tubule Pyroptosis via the CHOP-Caspase-11 Pathway. Am. J. Physiol. Renal Physiol. 2014, 306, F75–F84. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, X.; Yuan, S.; Wen, S.; Liu, X.; Wang, C.; Qu, Z.; Li, J.; Liu, H.; Sun, L.; et al. TLR4/NF-ΚB Signaling Induces GSDMD-Related Pyroptosis in Tubular Cells in Diabetic Kidney Disease. Front. Endocrinol. 2019, 10, 603. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, Y.; Huang, Z.-X.; Chen, H.; Lan, R.; Wang, Z.; Lai, K.; Chen, H.; Chen, Z.; Zou, Z.; et al. GSDME-Mediated Pyroptosis Promotes Inflammation and Fibrosis in Obstructive Nephropathy. Cell Death Differ. 2021, 28, 2333–2350. [Google Scholar] [CrossRef]
- Yan, H.; Luo, B.; Wu, X.; Guan, F.; Yu, X.; Zhao, L.; Ke, X.; Wu, J.; Yuan, J. Cisplatin Induces Pyroptosis via Activation of MEG3/NLRP3/Caspase-1/GSDMD Pathway in Triple-Negative Breast Cancer. Int. J. Biol. Sci. 2021, 17, 2606–2621. [Google Scholar] [CrossRef]
- Priante, G.; Gianesello, L.; Ceol, M.; Del Prete, D.; Anglani, F. Cell Death in the Kidney. Int. J. Mol. Sci. 2019, 20, 3598. [Google Scholar] [CrossRef]
- Havasi, A.; Borkan, S.C. Apoptosis and Acute Kidney Injury. Kidney Int. 2011, 80, 29–40. [Google Scholar] [CrossRef]
- McSweeney, K.R.; Gadanec, L.K.; Qaradakhi, T.; Ali, B.A.; Zulli, A.; Apostolopoulos, V. Mechanisms of Cisplatin-Induced Acute Kidney Injury: Pathological Mechanisms, Pharmacological Interventions, and Genetic Mitigations. Cancers 2021, 13, 1572. [Google Scholar] [CrossRef]
- Badr, A.; Fouad, D.; Attia, H. Insights Into Protective Mechanisms of Dandelion Leaf Extract Against Cisplatin-Induced Nephrotoxicity in Rats: Role of Inhibitory Effect on Inflammatory and Apoptotic Pathways. Dose-Response 2019, 17, 155932581987489. [Google Scholar] [CrossRef]
- Morsy, M.A.; Heeba, G.H. Nebivolol Ameliorates Cisplatin-Induced Nephrotoxicity in Rats. Basic Clin. Pharmacol. Toxicol. 2016, 118, 449–455. [Google Scholar] [CrossRef]
- Abouzed, T.K.; Sherif, E.A.E.; Barakat, M.E.S.; Sadek, K.M.; Aldhahrani, A.; Nasr, N.E.; Eldomany, E.; Khailo, K.; Dorghamm, D.A. Assessment of Gentamicin and Cisplatin-Induced Kidney Damage Mediated via Necrotic and Apoptosis Genes in Albino Rats. BMC Vet. Res. 2021, 17, 350. [Google Scholar] [CrossRef]
- Ewees, M.G.E.-D.; Abdel-Bakky, M.S.; Bayoumi, A.M.A.; Abo-Saif, A.A.; Altowayan, W.M.; Alharbi, K.S.; Messiha, B.A.S. Dabigatran Mitigates Cisplatin-Mediated Nephrotoxicity through down Regulation of Thrombin Pathway. J. Adv. Res. 2021, 31, 127–136. [Google Scholar] [CrossRef]
- Fang, C.; Lou, D.; Zhou, L.; Wang, J.; Yang, B.; He, Q.; Wang, J.; Weng, Q. Natural Products: Potential Treatments for Cisplatin-Induced Nephrotoxicity. Acta Pharmacol. Sin. 2021, 42, 1951–1969. [Google Scholar] [CrossRef]
- Dludla, P.V.; Dias, S.C.; Obonye, N.; Johnson, R.; Louw, J.; Nkambule, B.B. A Systematic Review on the Protective Effect of N-Acetyl Cysteine Against Diabetes-Associated Cardiovascular Complications. Am. J. Cardiovasc. Drugs Drugs Devices Interv. 2018, 18, 283–298. [Google Scholar] [CrossRef]
- El-Maddawy, Z.K.; El-Sayed, Y.S. Comparative Analysis of the Protective Effects of Curcumin and N-Acetyl Cysteine against Paracetamol-Induced Hepatic, Renal, and Testicular Toxicity in Wistar Rats. Environ. Sci. Pollut. Res. Int. 2018, 25, 3468–3479. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, S.; Chen, L.; Zhang, L.; Meng, F.; Sha, S.; Ai, C.; Tai, J. Chlorogenic Acid Ameliorates Lead-Induced Renal Damage in Mice. Biol. Trace Elem. Res. 2019, 189, 109–117. [Google Scholar] [CrossRef]
- Miao, M.; Xiang, L. Pharmacological Action and Potential Targets of Chlorogenic Acid. Adv. Pharmacol. San Diego Calif 2020, 87, 71–88. [Google Scholar] [CrossRef]
- Gong, X.; Duan, Y.; Zheng, J.; Wang, Y.; Wang, G.; Norgren, S.; Hei, T.K. Nephroprotective Effects of N-Acetylcysteine Amide against Contrast-Induced Nephropathy through Upregulating Thioredoxin-1, Inhibiting ASK1/P38MAPK Pathway, and Suppressing Oxidative Stress and Apoptosis in Rats. Oxid. Med. Cell. Longev. 2016, 2016, 8715185. [Google Scholar] [CrossRef]
- Dobrek, L.; Nalik-Iwaniak, K.; Fic, K.; Arent, Z. The Effect of Acetylcysteine on Renal Function in Experimental Models of Cyclophosphamide-and Ifosfamide-Induced Cystitis. Curr. Urol. 2020, 14, 150–162. [Google Scholar] [CrossRef]
- Arfian, N.; Wahyudi, D.A.P.; Zulfatina, I.B.; Citta, A.N.; Anggorowati, N.; Multazam, A.; Romi, M.M.; Sari, D.C.R. Chlorogenic Acid Attenuates Kidney Ischemic/Reperfusion Injury via Reducing Inflammation, Tubular Injury, and Myofibroblast Formation. BioMed Res. Int. 2019, 2019, 5423703. [Google Scholar] [CrossRef]
- Al-Megrin, W.A.; Metwally, D.M.; Habotta, O.A.; Amin, H.K.; Abdel Moneim, A.E.; El-Khadragy, M. Nephroprotective Effects of Chlorogenic Acid against Sodium Arsenite-Induced Oxidative Stress, Inflammation, and Apoptosis. J. Sci. Food Agric. 2020, 100, 5162–5170. [Google Scholar] [CrossRef]
- Shi, A.; Shi, H.; Wang, Y.; Liu, X.; Cheng, Y.; Li, H.; Zhao, H.; Wang, S.; Dong, L. Activation of Nrf2 Pathway and Inhibition of NLRP3 Inflammasome Activation Contribute to the Protective Effect of Chlorogenic Acid on Acute Liver Injury. Int. Immunopharmacol. 2018, 54, 125–130. [Google Scholar] [CrossRef]
- Li, B.; Zhang, X.; Huo, S.; Zhang, J.; Du, J.; Xiao, B.; Song, M.; Shao, B.; Li, Y. Aluminum Activates NLRP3 Inflammasome-Mediated Pyroptosis via Reactive Oxygen Species to Induce Liver Injury in Mice. Chem. Biol. Interact. 2022, 368, 110229. [Google Scholar] [CrossRef]
- Ozkok, A.; Edelstein, C.L. Pathophysiology of Cisplatin-Induced Acute Kidney Injury. BioMed Res. Int. 2014, 2014, 967826. [Google Scholar] [CrossRef]
- Bafna, P.A.; Balaraman, R. Antioxidant Activity of Pepticare, a Herbomineral Formulation, in Experimentally Induced Renal and Cardiac Damage. J. Herb. Pharmacother. 2006, 6, 1–12. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, M.-H.; Dong, Z. Differential Gender Differences in Ischemic and Nephrotoxic Acute Renal Failure. Am. J. Nephrol. 2005, 25, 491–499. [Google Scholar] [CrossRef]
- Mahran, Y.F.; Hassan, H.M. Ganoderma Lucidum Prevents Cisplatin-Induced Nephrotoxicity through Inhibition of Epidermal Growth Factor Receptor Signaling and Autophagy-Mediated Apoptosis. Oxid. Med. Cell. Longev. 2020, 2020, 4932587. [Google Scholar] [CrossRef]
- Mahran, Y.F. New Insights into the Protection of Growth Hormone in Cisplatin-Induced Nephrotoxicity: The Impact of IGF-1 on the Keap1-Nrf2/HO-1 Signaling. Life Sci. 2020, 253, 117581. [Google Scholar] [CrossRef]
- Dobrek, L.; Nalik-Iwaniak, K.; Arent, Z. The Effectiveness of N-Acetylcysteine in Alleviating Kidney Dysfunction in Ifosfamide-Treated Rats. Open Urol. Nephrol. J. 2020, 13, 21–31. [Google Scholar] [CrossRef]
- Nagai, N.; Okuda, R.; Kinoshita, M.; Ogata, H. Decomposition Kinetics of Cisplatin in Human Biological Fluids. J. Pharm. Pharmacol. 1996, 48, 918–924. [Google Scholar] [CrossRef]
- Marullo, R.; Werner, E.; Degtyareva, N.; Moore, B.; Altavilla, G.; Ramalingam, S.S.; Doetsch, P.W. Cisplatin Induces a Mitochondrial-ROS Response That Contributes to Cytotoxicity Depending on Mitochondrial Redox Status and Bioenergetic Functions. PLoS ONE 2013, 8, e81162. [Google Scholar] [CrossRef]
- Luo, J.; Tsuji, T.; Yasuda, H.; Sun, Y.; Fujigaki, Y.; Hishida, A. The Molecular Mechanisms of the Attenuation of Cisplatin-Induced Acute Renal Failure by N-Acetylcysteine in Rats. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2008, 23, 2198–2205. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chen, C.-H.; Hsu, Y.-H.; Chen, T.-H.; Sue, Y.-M.; Cheng, C.-Y.; Chen, T.-W. Leptin Reduces Gentamicin-Induced Apoptosis in Rat Renal Tubular Cells via the PI3K-Akt Signaling Pathway. Eur. J. Pharmacol. 2011, 658, 213–218. [Google Scholar] [CrossRef]
- Li, W.; Yang, Y.; Li, Y.; Zhao, Y.; Jiang, H. Sirt5 Attenuates Cisplatin-Induced Acute Kidney Injury through Regulation of Nrf2/HO-1 and Bcl-2. BioMed Res. Int. 2019, 2019, 4745132. [Google Scholar] [CrossRef]
- Chien, L.-H.; Wu, C.-T.; Deng, J.-S.; Jiang, W.-P.; Huang, W.-C.; Huang, G.-J. Salvianolic Acid C Protects against Cisplatin-Induced Acute Kidney Injury through Attenuation of Inflammation, Oxidative Stress and Apoptotic Effects and Activation of the CaMKK-AMPK-Sirt1-Associated Signaling Pathway in Mouse Models. Antioxid. Basel Switz. 2021, 10, 1620. [Google Scholar] [CrossRef]
- Richter, C.; Gogvadze, V.; Laffranchi, R.; Schlapbach, R.; Schweizer, M.; Suter, M.; Walter, P.; Yaffee, M. Oxidants in Mitochondria: From Physiology to Diseases. Biochim. Biophys. Acta BBA Mol. Basis Dis. 1995, 1271, 67–74. [Google Scholar] [CrossRef]
- Ramesh, G.; Reeves, W.B. Salicylate Reduces Cisplatin Nephrotoxicity by Inhibition of Tumor Necrosis Factor-Alpha. Kidney Int. 2004, 65, 490–499. [Google Scholar] [CrossRef]
- Cai, Y.; Feng, Z.; Jia, Q.; Guo, J.; Zhang, P.; Zhao, Q.; Wang, Y.X.; Liu, Y.N.; Liu, W.J. Cordyceps Cicadae Ameliorates Renal Hypertensive Injury and Fibrosis Through the Regulation of SIRT1-Mediated Autophagy. Front. Pharmacol. 2022, 12, 801094. [Google Scholar] [CrossRef]
- Shaw, J.; Media, J.; Chen, B.; Valeriote, F. The Small-Molecule TNF-α Inhibitor, UTL-5g, Delays Deaths and Increases Survival Rates for Mice Treated with High Doses of Cisplatin. Cancer Chemother. Pharmacol. 2013, 72, 703–707. [Google Scholar] [CrossRef]
- Omar, H.A.; Mohamed, W.R.; Arab, H.H.; Arafa, E.-S.A. Tangeretin Alleviates Cisplatin-Induced Acute Hepatic Injury in Rats: Targeting MAPKs and Apoptosis. PLoS ONE 2016, 11, e0151649. [Google Scholar] [CrossRef]
- Kim, D.-U.; Kim, D.-G.; Choi, J.-W.; Shin, J.Y.; Kweon, B.; Zhou, Z.; Lee, H.-S.; Song, H.-J.; Bae, G.-S.; Park, S.-J. Loganin Attenuates the Severity of Acute Kidney Injury Induced by Cisplatin through the Inhibition of ERK Activation in Mice. Int. J. Mol. Sci. 2021, 22, 1421. [Google Scholar] [CrossRef]
- Zheng, R.; Tan, Y.; Gu, M.; Kang, T.; Zhang, H.; Guo, L. N-Acetyl Cysteine Inhibits Lipopolysaccharide-Mediated Synthesis of Interleukin-1β and Tumor Necrosis Factor-α in Human Periodontal Ligament Fibroblast Cells through Nuclear Factor-Kappa B Signaling. Medicine 2019, 98, e17126. [Google Scholar] [CrossRef]
- Bisht, A.; Dickens, M.; Rutherfurd-Markwick, K.; Thota, R.; Mutukumira, A.N.; Singh, H. Chlorogenic Acid Potentiates the Anti-Inflammatory Activity of Curcumin in LPS-Stimulated THP-1 Cells. Nutrients 2020, 12, 2706. [Google Scholar] [CrossRef]
- Meng, Q.; Bi, P.; Zhang, G.; Li, Y.; Chen, S.; Nie, K. Forsythiae Fructus Aqueous Extract Attenuates Cisplatin-Induced Kaolin Consumption (Pica) by Inhibiting NLRP3 Inflammasome Activation in Rats. Biosci. Biotechnol. Biochem. 2021, 85, 2054–2064. [Google Scholar] [CrossRef]
- Michel, H.E.; Menze, E.T. Tetramethylpyrazine Guards against Cisplatin-Induced Nephrotoxicity in Rats through Inhibiting HMGB1/TLR4/NF-ΚB and Activating Nrf2 and PPAR-γ Signaling Pathways. Eur. J. Pharmacol. 2019, 857, 172422. [Google Scholar] [CrossRef]
- Yang, J.; Wise, L.; Fukuchi, K. TLR4 Cross-Talk With NLRP3 Inflammasome and Complement Signaling Pathways in Alzheimer’s Disease. Front. Immunol. 2020, 11, 724. [Google Scholar] [CrossRef]
- Kim, J. Poly(ADP-Ribose) Polymerase Activation Induces High Mobility Group Box 1 Release from Proximal Tubular Cells during Cisplatin Nephrotoxicity. Physiol. Res. 2016, 65, 333–340. [Google Scholar] [CrossRef]
- Klune, J.R.; Dhupar, R.; Cardinal, J.; Billiar, T.R.; Tsung, A. HMGB1: Endogenous Danger Signaling. Mol. Med. 2008, 14, 476–484. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P.-Y. The NLRP3 Inflammasome: Molecular Activation and Regulation to Therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of Assembly, Regulation and Signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef]
- Babolmorad, G.; Latif, A.; Domingo, I.K.; Pollock, N.M.; Delyea, C.; Rieger, A.M.; Allison, W.T.; Bhavsar, A.P. Toll-like Receptor 4 Is Activated by Platinum and Contributes to Cisplatin-induced Ototoxicity. EMBO Rep. 2021, 22, e51280. [Google Scholar] [CrossRef]
- Qu, X.; Gao, H.; Tao, L.; Zhang, Y.; Zhai, J.; Sun, J.; Song, Y.; Zhang, S. Astragaloside IV Protects against Cisplatin-Induced Liver and Kidney Injury via Autophagy-Mediated Inhibition of NLRP3 in Rats. J. Toxicol. Sci. 2019, 44, 167–175. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, P.; Song, W.; Yao, Q.; Li, Y.; Liu, L.; Li, Y.; Zhou, S. GSDME Enhances Cisplatin Sensitivity to Regress Non-Small Cell Lung Carcinoma by Mediating Pyroptosis to Trigger Antitumor Immunocyte Infiltration. Signal Transduct. Target. Ther. 2020, 5, 159. [Google Scholar] [CrossRef]
- Mai, F.-Y.; He, P.; Ye, J.-Z.; Xu, L.-H.; Ouyang, D.-Y.; Li, C.-G.; Zeng, Q.-Z.; Zeng, C.-Y.; Zhang, C.-C.; He, X.-H.; et al. Caspase-3-Mediated GSDME Activation Contributes to Cisplatin- and Doxorubicin-Induced Secondary Necrosis in Mouse Macrophages. Cell Prolif. 2019, 52, e12663. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, B.; Chu, Z.; Jiang, F.; Han, J. Wogonin Alleviates Cisplatin-Induced Cardiotoxicity in Mice Via Inhibiting Gasdermin D-Mediated Pyroptosis. J. Cardiovasc. Pharmacol. 2021, 78, 597–603. [Google Scholar] [CrossRef]
- Yu, W.; Zong, S.; Zhou, P.; Wei, J.; Wang, E.; Ming, R.; Xiao, H. Cochlear Marginal Cell Pyroptosis Is Induced by Cisplatin via NLRP3 Inflammasome Activation. Front. Immunol. 2022, 13, 823439. [Google Scholar] [CrossRef]
- Visacri, M.B.; Quintanilha, J.C.F.; de Sousa, V.M.; Amaral, L.S.; de F. L. Ambrósio, R.; Calonga, L.; Curi, S.F.B.B.; de T. Leme, M.F.; Chone, C.T.; Altemani, J.M.C.; et al. Can Acetylcysteine Ameliorate Cisplatin-Induced Toxicities and Oxidative Stress without Decreasing Antitumor Efficacy? A Randomized, Double-Blind, Placebo-Controlled Trial Involving Patients with Head and Neck Cancer. Cancer Med. 2019, 8, 2020–2030. [Google Scholar] [CrossRef]
- Wu, Y.J.; Muldoon, L.L.; Neuwelt, E.A. The Chemoprotective Agent N-Acetylcysteine Blocks Cisplatin-Induced Apoptosis through Caspase Signaling Pathway. J. Pharmacol. Exp. Ther. 2005, 312, 424–431. [Google Scholar] [CrossRef]
- Suberu, J.O.; Romero-Canelón, I.; Sullivan, N.; Lapkin, A.A.; Barker, G.C. Comparative Cytotoxicity of Artemisinin and Cisplatin and Their Interactions with Chlorogenic Acids in MCF7 Breast Cancer Cells. ChemMedChem 2014, 9, 2791–2797. [Google Scholar] [CrossRef]
- Catanzaro, D.; Filippini, R.; Vianello, C.; Carrara, M.; Ragazzi, E.; Montopoli, M. Chlorogenic Acid Interaction with Cisplatin and Oxaliplatin: Studies in Cervical Carcinoma Cells. Nat. Prod. Commun. 2016, 11, 499–502. [Google Scholar] [CrossRef]
- Badr, A.; Fouad, D. Anti-Apoptotic and Anti-Inflammatory Effects of Olive Leaf Extract Against Cisplatin-Induced Nephrotoxicity in Male Rats. Int. J. Pharmacol. 2016, 12, 675–688. [Google Scholar] [CrossRef]
- Nishi; Ahad, A.; Kumar, P. Protective Effect of Chlorogenic Acid against Diabetic Nephropathy in High Fat Diet/Streptozotocin Induced Type-2 Diabetic Rats. Int. J. Pharm. Pharm. Sci. 2013, 5, 489–495. [Google Scholar]
- Palygin, O.; Spires, D.; Levchenko, V.; Bohovyk, R.; Fedoriuk, M.; Klemens, C.A.; Sykes, O.; Bukowy, J.D.; Cowley, A.W.; Lazar, J.; et al. Progression of Diabetic Kidney Disease in T2DN Rats. Am. J. Physiol. Ren. Physiol. 2019, 317, F1450–F1461. [Google Scholar] [CrossRef]
- Kishi, S.; Yamakawa, K.; Nakano-Narusawa, Y.; Kanie, S.; Hashimoto, N.; Saoo, K.; Yokohira, M.; Imaida, K.; Matsuda, Y. Preexisting Diabetes Mellitus Had No Effect on the No-Observed-Adverse-Effect-Level of Acetaminophen in Rats. J. Toxicol. Sci. 2020, 45, 151–162. [Google Scholar] [CrossRef]
- Schirmeister, J.; Willmann, H.; Kiefer, H. Kritische Beurteilung des Plasmakreatinins als Test des Glomerulusfiltrates; Bergmann-Verlag: Munich, Germany, 1964; pp. 678–681. [Google Scholar]
- Chaney, A.L.; Marbach, E.P. Modified Reagents for Determination of Urea and Ammonia. Clin. Chem. 1962, 8, 130–132. [Google Scholar] [CrossRef]
- El-Nabarawy, N.A.; Gouda, A.S.; Khattab, M.A.; Rashed, L.A. Effects of Nitrite Graded Doses on Hepatotoxicity and Nephrotoxicity, Histopathological Alterations, and Activation of Apoptosis in Adult Rats. Environ. Sci. Pollut. Res. Int. 2020, 27, 14019–14032. [Google Scholar] [CrossRef]
- Moon, D.; Padanilam, B.J.; Jang, H.-S.; Kim, J. 2-Mercaptoethanol Protects against DNA Double-Strand Breaks after Kidney Ischemia and Reperfusion Injury through GPX4 Upregulation. Pharmacol. Rep. PR 2022, 74, 1041–1053. [Google Scholar] [CrossRef]
- Ellman, G.L.; Lysko, H. Disulfide and Sulfhydryl Compounds in TCA Extracts of Human Blood and Plasma. J. Lab. Clin. Med. 1967, 70, 518–527. [Google Scholar]
- Mihara, M.; Uchiyama, M. Determination of Malonaldehyde Precursor in Tissues by Thiobarbituric Acid Test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Al-Khrashi, L.A.; Badr, A.M.; Al-Amin, M.A.; Mahran, Y.F. Thymol Ameliorates 5-Fluorouracil-Induced Intestinal Mucositis: Evidence of down-Regulatory Effect on TGF-β/MAPK Pathways through NF-ΚB. J. Biochem. Mol. Toxicol. 2022, 36, e22932. [Google Scholar] [CrossRef]

| Treated Groups | Kidney Function Parameters | Oxidative Stress Markers | |||||
|---|---|---|---|---|---|---|---|
| Kidney Index (%) | BUN (mg/dL) | Creatinine (mg/dL) | Lipid Peroxidation (nmol/mg Protein) | Total Antioxidant (mM) | Catalase (U/mg Protein) | Glutathione Peroxidase (mU/mL) | |
| Control | 0.96 ± 0.03 | 30.56 ± 3.250 | 0.31 ± 0.03 | 1.35 ± 0.18 | 2.18 ± 0.14 | 7.55 ± 0.61 | 2.52 ± 0.50 |
| Cisplatin | 1.36 a ± 0.16 | 238.90 a ± 29.09 | 2.89 a ± 0.45 | 2.72 a ± 0.24 | 1.24 a ± 0.07 | 1.45 a ± 0.57 | 37.00 a ± 6.15 |
| NAC (250 mg/kg) | 1.17 ± 0.11 | 62.76 b ± 6.45 | 1.26 b ± 0.32 | 1.29 b ± 0.06 | 2.11 b ± 0.29 | 3.60 a,b ± 0.57 | 11.60 b ± 1.96 |
| CGA (20 mg/kg) | 1.31 ± 0.14 | 82.31 b ± 11.07 | 1.49 b ± 0.20 | 1.69 b ± 0.14 | 1.70 b ± 0.12 | 2.43 a,b ± 0.09 | 23 a ± 3.48 |
| NAC + CGA | 1.12 ± 0.12 | 43.75 b ± 7.03 | 1.13 b ± 0.14 | 1.35 b ± 0.17 | 2.75 b ± 0.48 | 3.25 a,b ± 0.32 | 26 a ± 2.27 |
| F, df, p value | 6, 0.048 # | 23.77, 4, 0.0001 | 9.5, 4, 0.0003 | 12.22, 4, 0.0001 | 5.23, 4, 0.0069 | 29.14, 4, 0.0001 | 18.20, 4, 0.001 |
| Control | Cisplatin | Cisplatin + NAC | Cisplatin + CGA | Cisplatin + NAC + CGA | |
|---|---|---|---|---|---|
| tubular degenerative changes | - | ++++ | ++ | + | - |
| tubular dilatation | - | +++ | ++ | + | + |
| intraluminal casts | - | +++ | - | - | - |
| inflammatory cells infiltrates | - | ++ | - | - | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badr, A.M.; Al-Kharashi, L.A.; Attia, H.; Alshehri, S.; Alajami, H.N.; Ali, R.A.; Mahran, Y.F. TLR4/Inflammasomes Cross-Talk and Pyroptosis Contribute to N-Acetyl Cysteine and Chlorogenic Acid Protection against Cisplatin-Induced Nephrotoxicity. Pharmaceuticals 2023, 16, 337. https://doi.org/10.3390/ph16030337
Badr AM, Al-Kharashi LA, Attia H, Alshehri S, Alajami HN, Ali RA, Mahran YF. TLR4/Inflammasomes Cross-Talk and Pyroptosis Contribute to N-Acetyl Cysteine and Chlorogenic Acid Protection against Cisplatin-Induced Nephrotoxicity. Pharmaceuticals. 2023; 16(3):337. https://doi.org/10.3390/ph16030337
Chicago/Turabian StyleBadr, Amira M., Layla A. Al-Kharashi, Hala Attia, Samiyah Alshehri, Hanaa N. Alajami, Rehab A. Ali, and Yasmen F. Mahran. 2023. "TLR4/Inflammasomes Cross-Talk and Pyroptosis Contribute to N-Acetyl Cysteine and Chlorogenic Acid Protection against Cisplatin-Induced Nephrotoxicity" Pharmaceuticals 16, no. 3: 337. https://doi.org/10.3390/ph16030337
APA StyleBadr, A. M., Al-Kharashi, L. A., Attia, H., Alshehri, S., Alajami, H. N., Ali, R. A., & Mahran, Y. F. (2023). TLR4/Inflammasomes Cross-Talk and Pyroptosis Contribute to N-Acetyl Cysteine and Chlorogenic Acid Protection against Cisplatin-Induced Nephrotoxicity. Pharmaceuticals, 16(3), 337. https://doi.org/10.3390/ph16030337







