Critical Analysis and Optimization of Stoichiometric Ratio of Drug-Coformer on Cocrystal Design: Molecular Docking, In Vitro and In Vivo Assessment
Abstract
1. Introduction
2. Results and Discussion
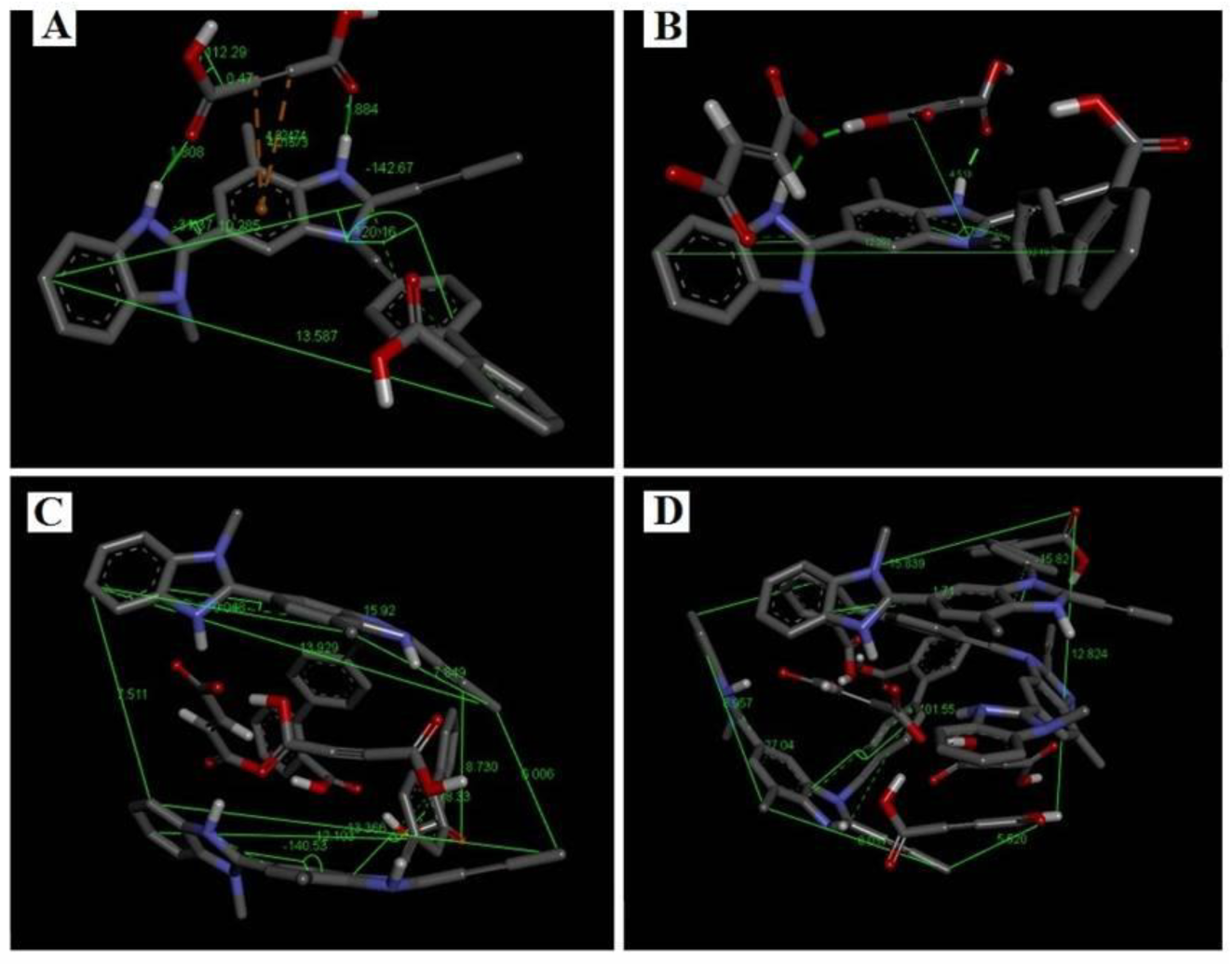
2.1. Molecular Docking
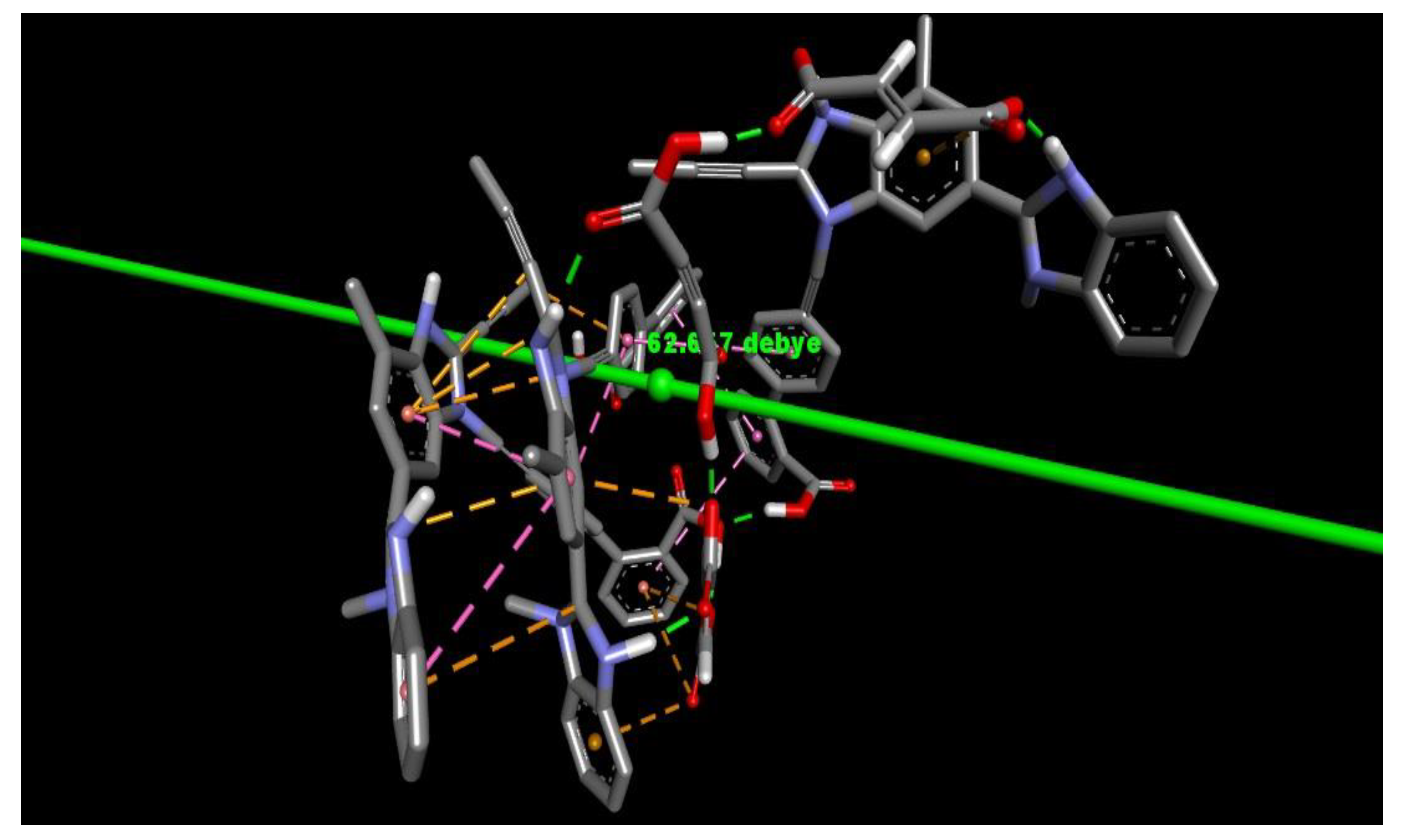
2.2. Dipole Moment Estimation
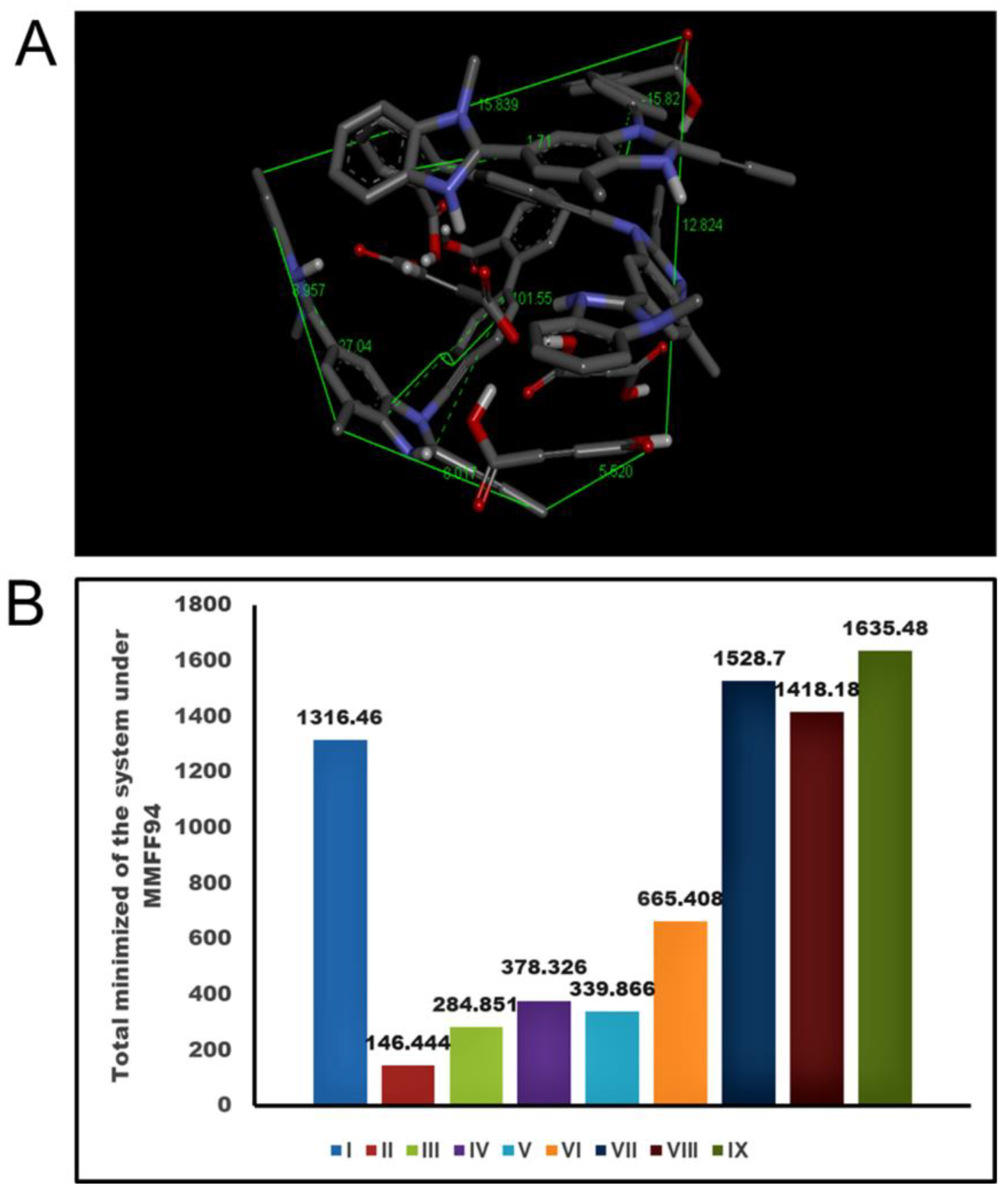
2.3. Analysis of Conformational Metrics of Drug-Coformer Repertoire
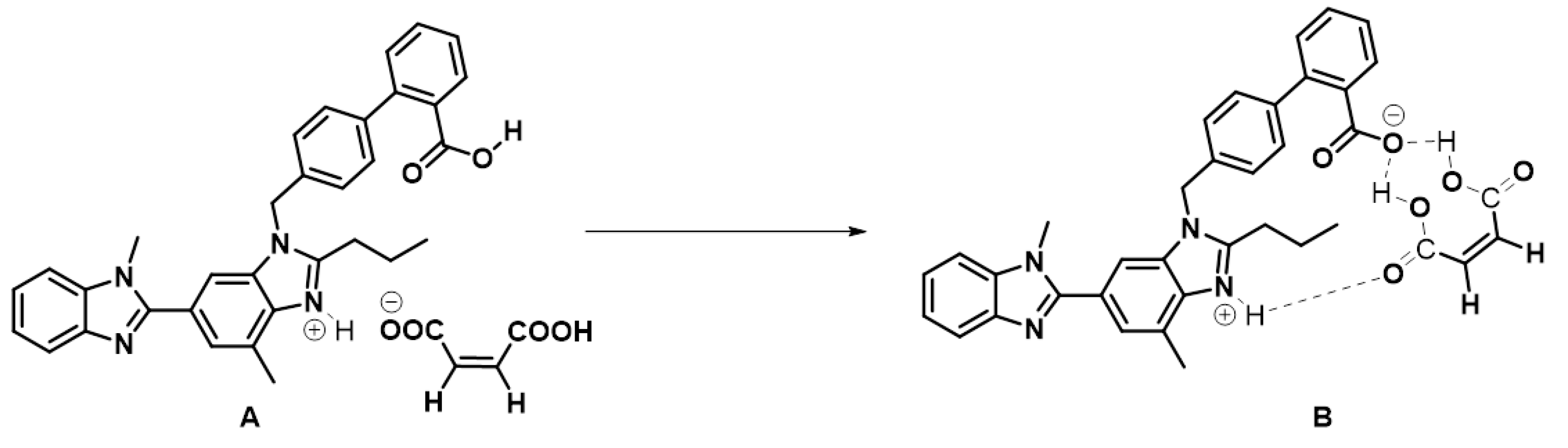
2.4. Nature of Bonding Involved in the Cocrystallization
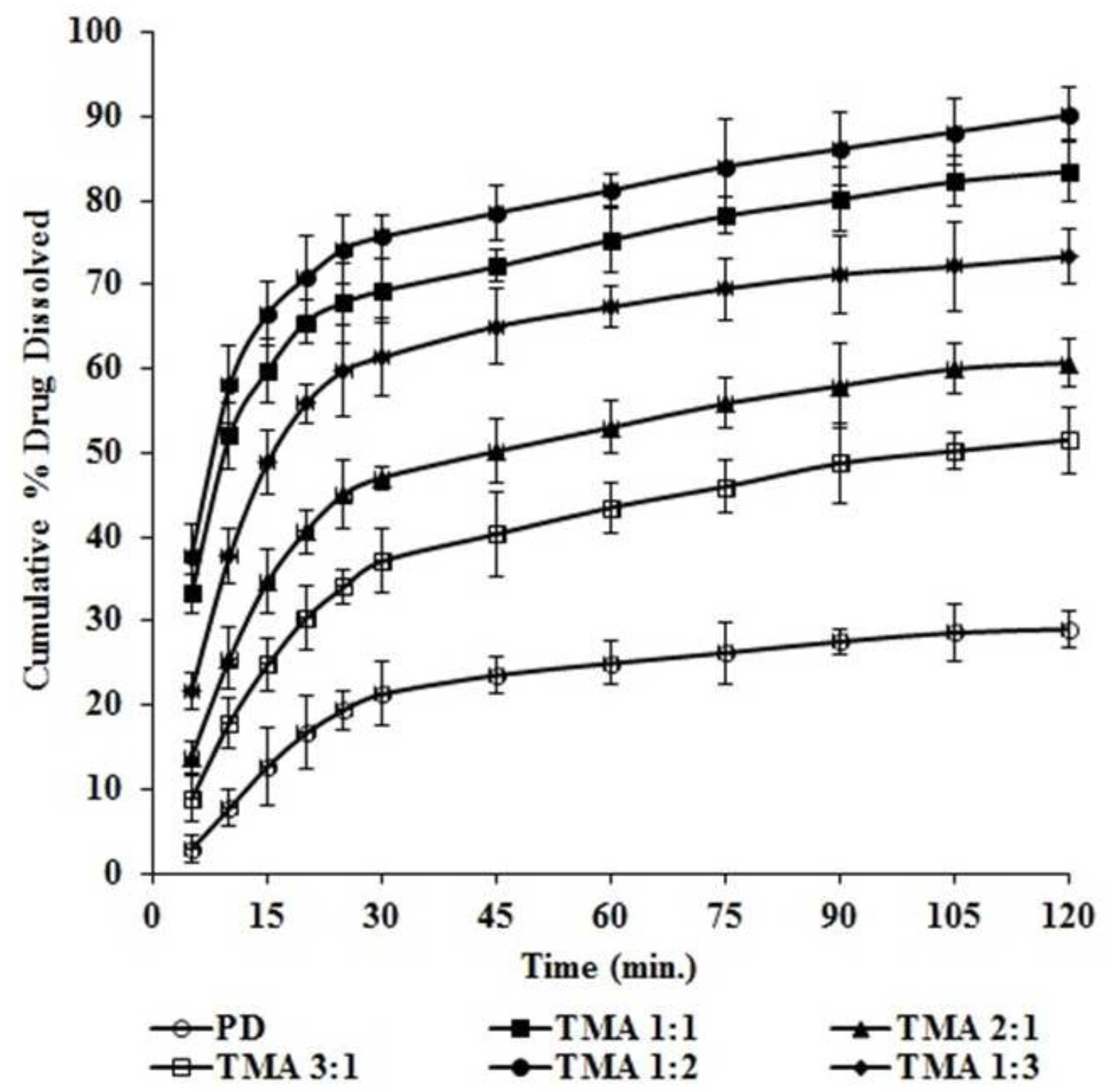
2.5. Equilibrium Solubility Analysis
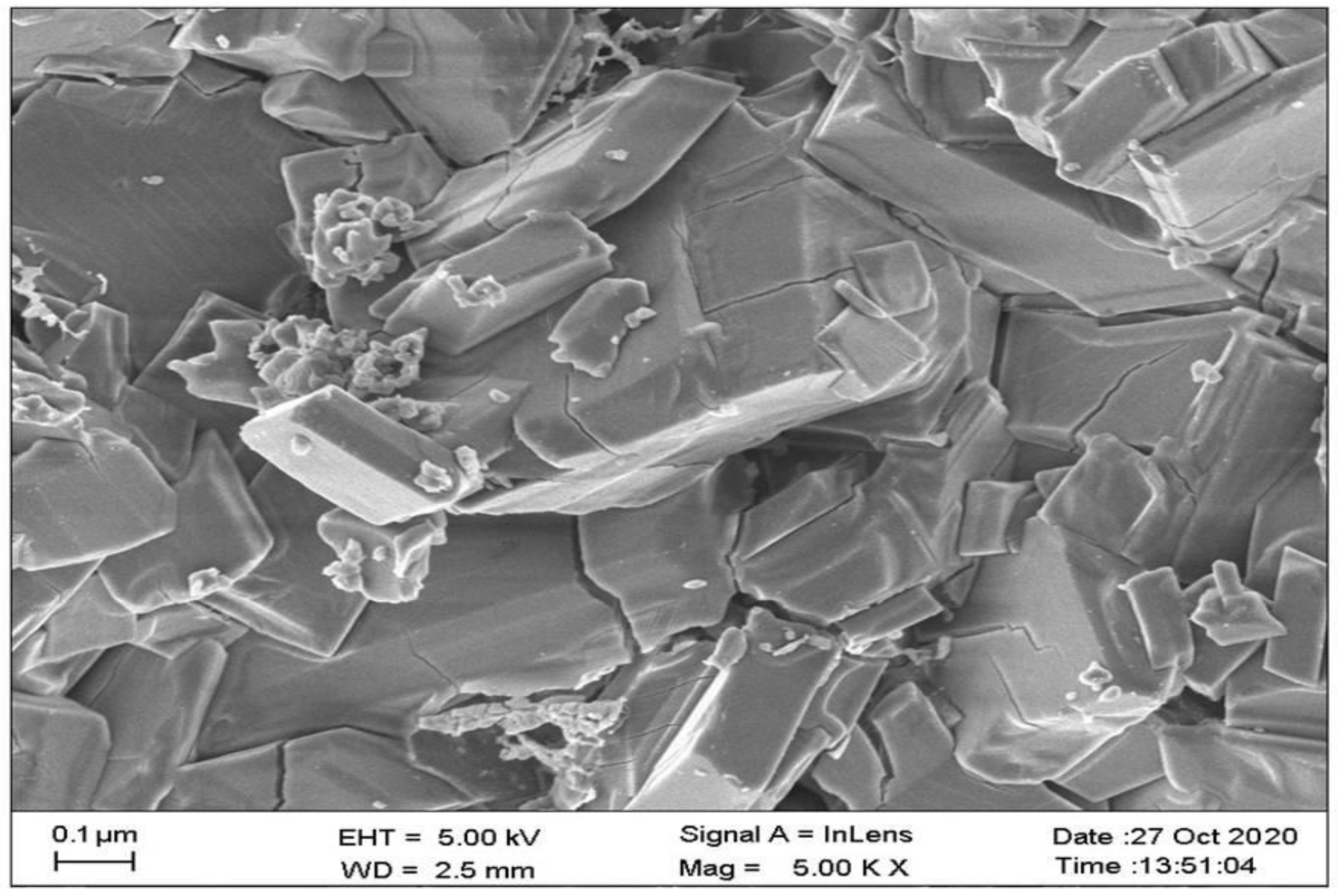
2.6. Scanning Electron Microscopic Studies
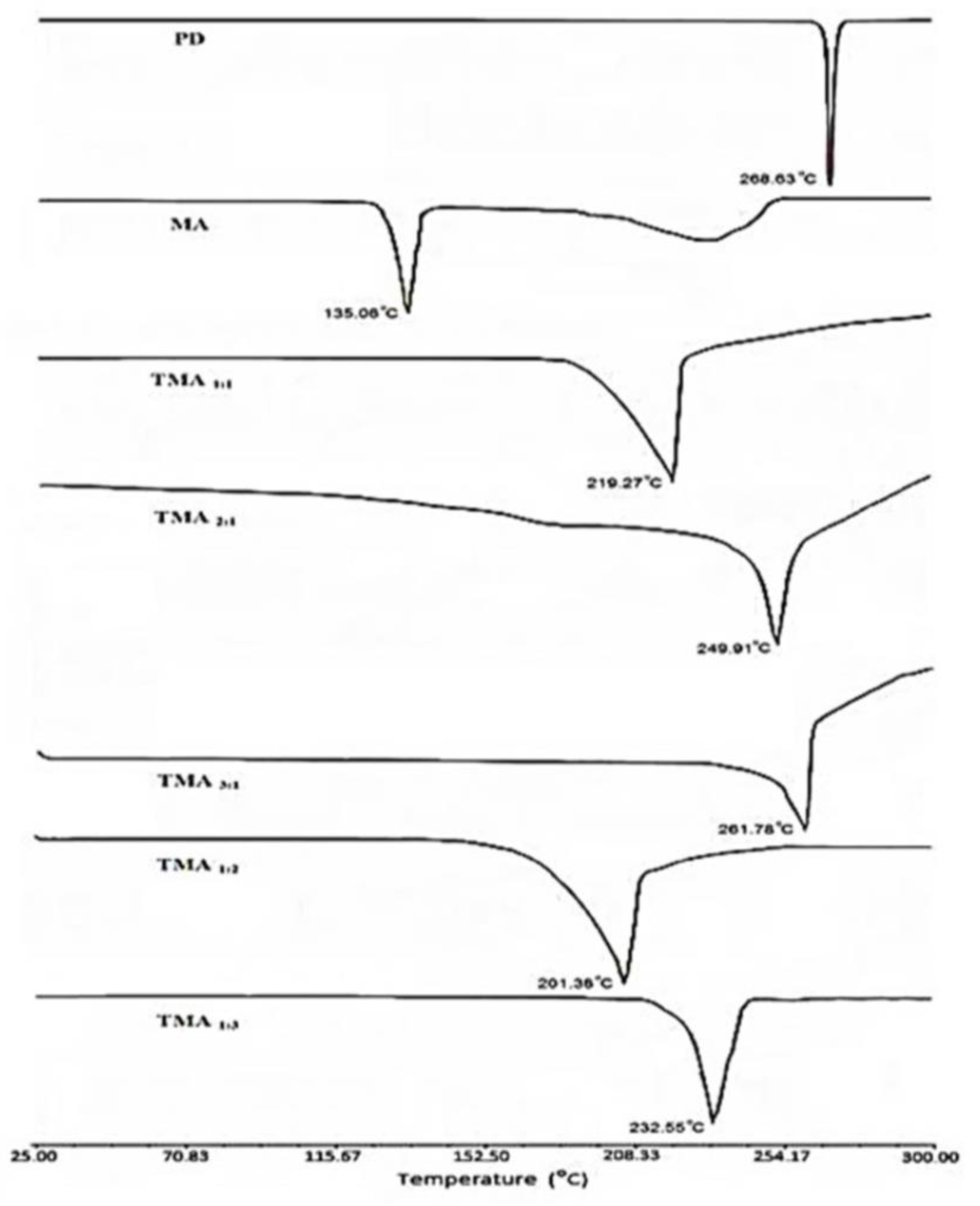
2.7. Differential Scanning Calorimetric Analysis (DSC)
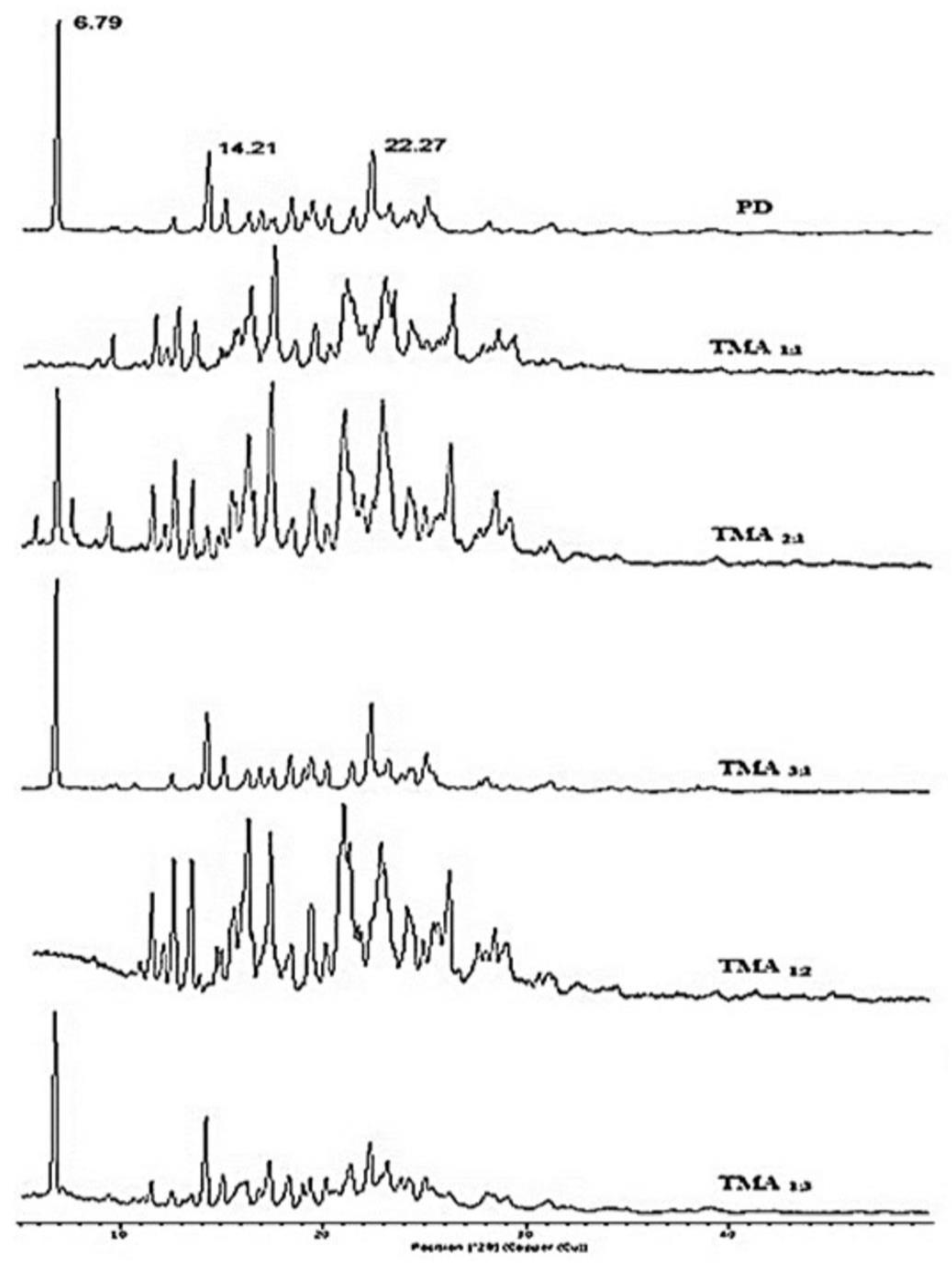
2.8. X-ray Diffraction (XRD) Studies
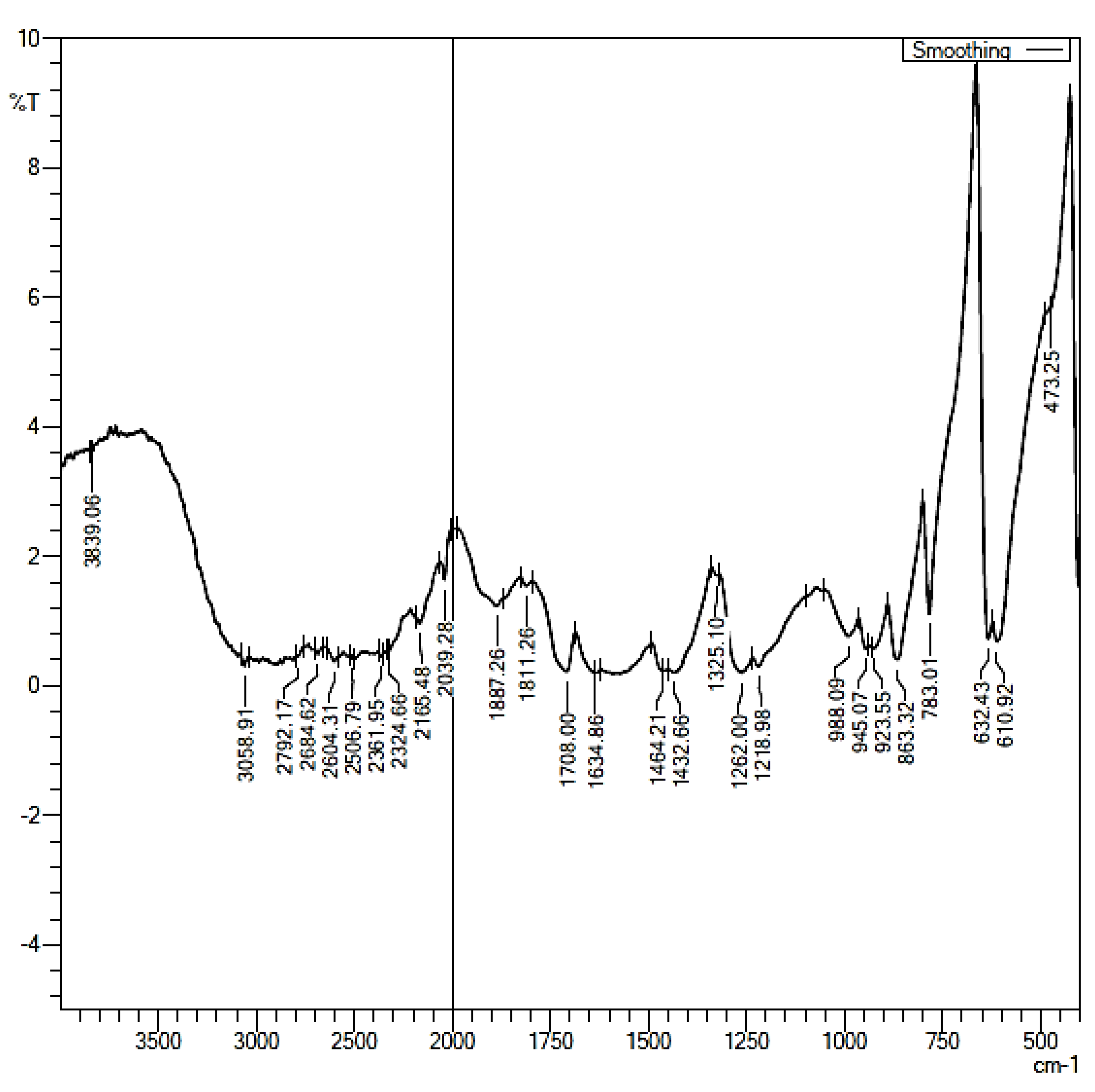
2.9. Fourier Transform Infrared Analysis (FTIR)
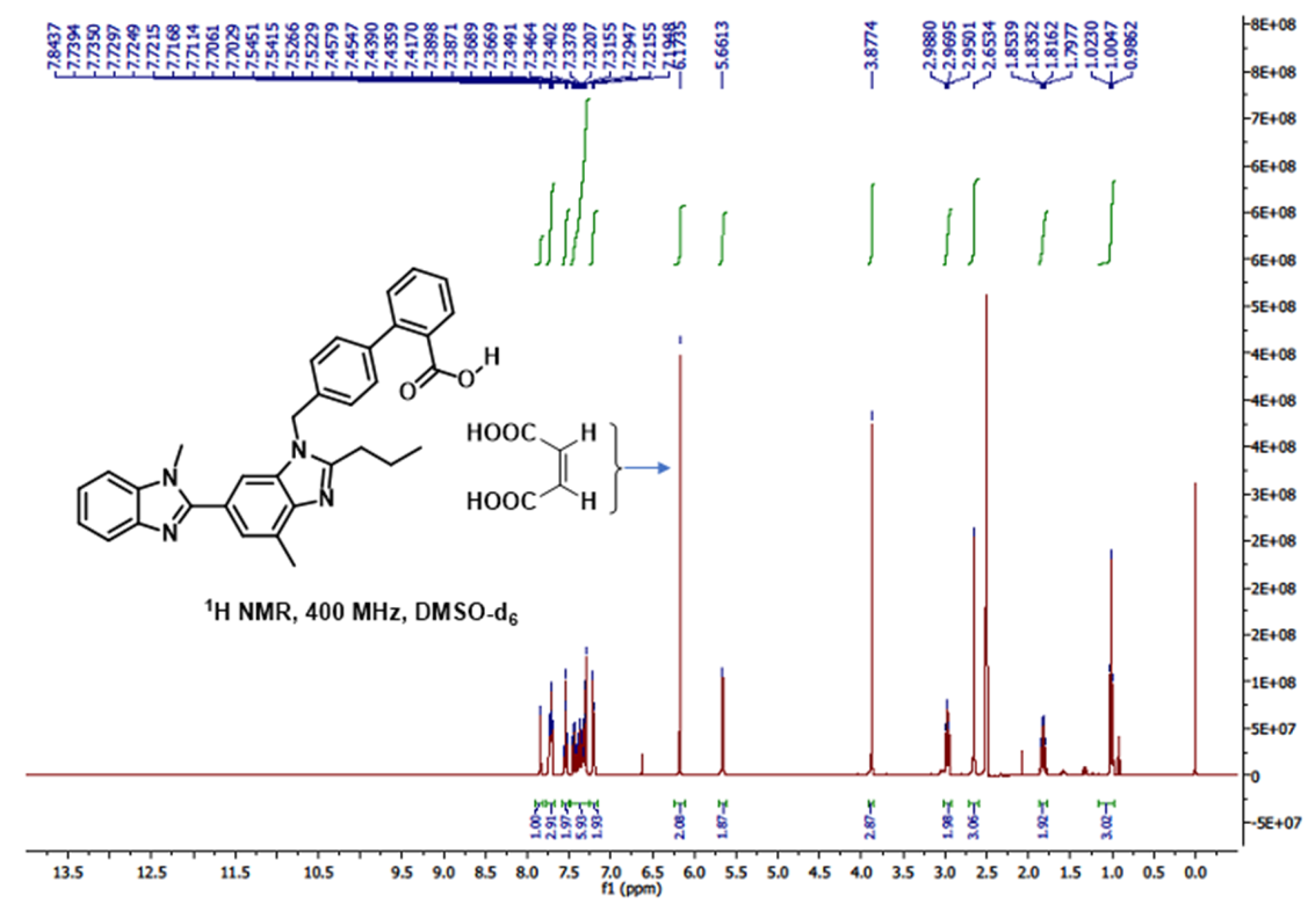
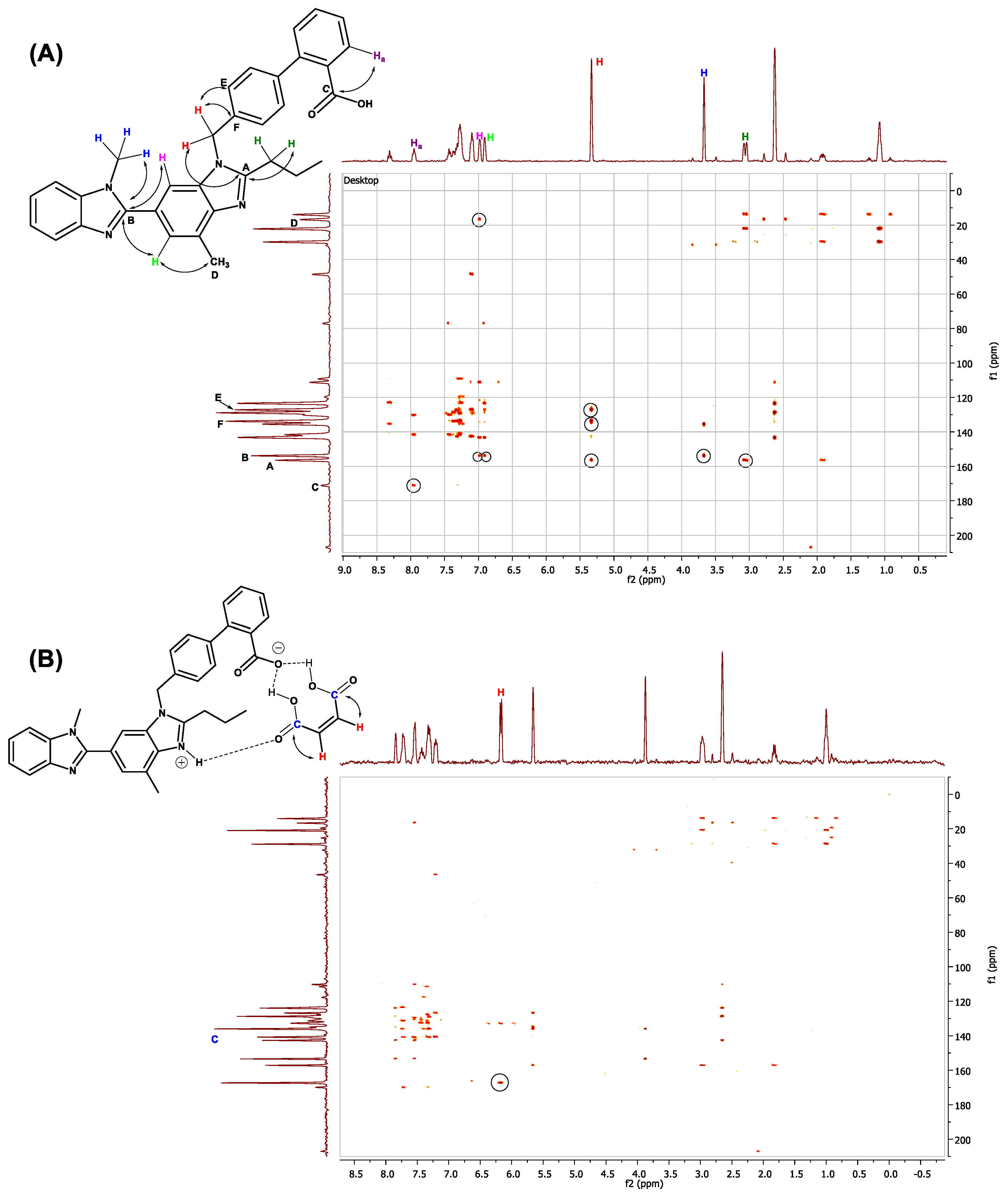
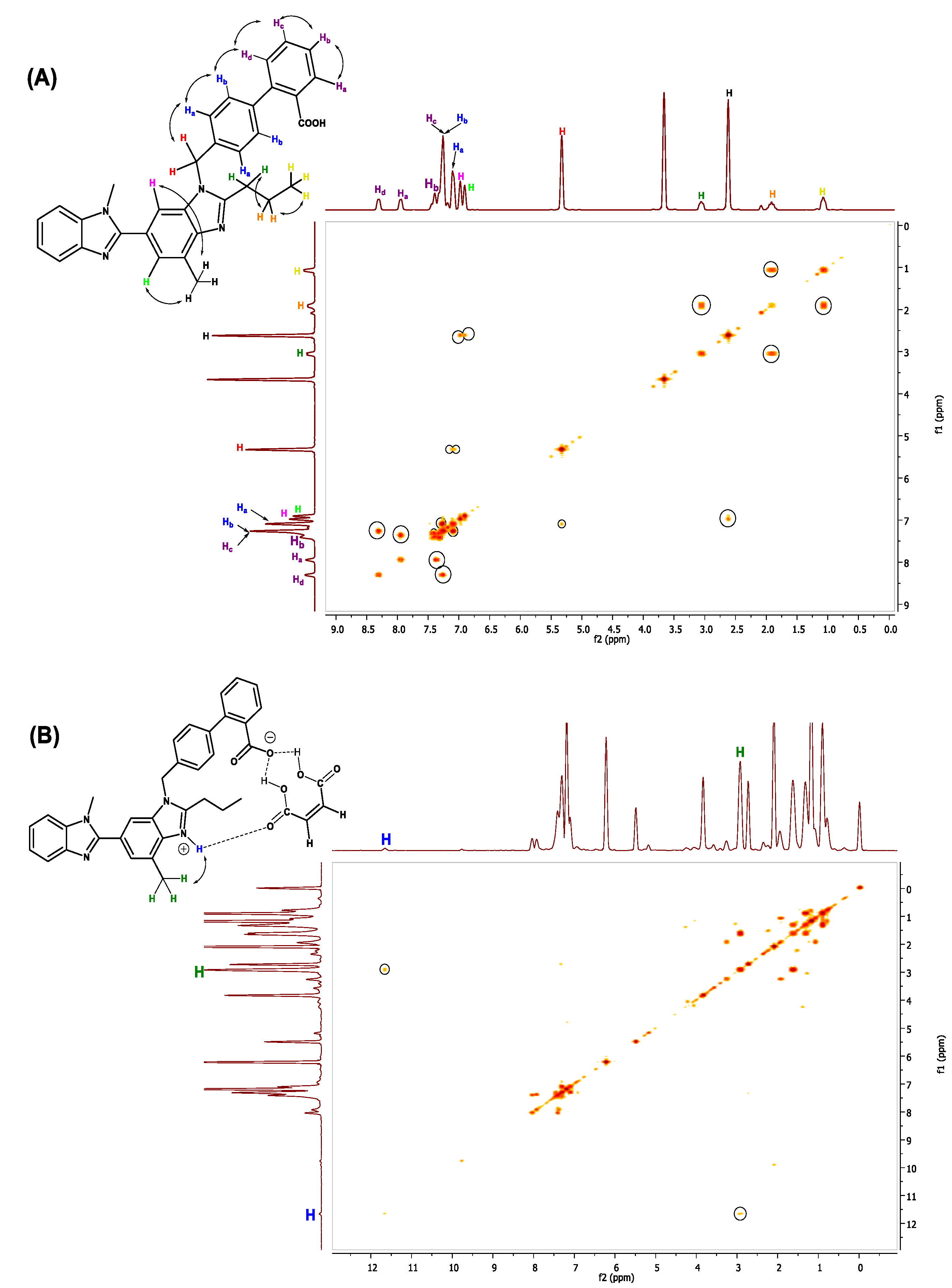
2.10. 1H, 13C and 2D-NMR Studies

2.11. Preclinical Studies
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Computational Simulation Study
3.2.2. Preparation of Structures
3.2.3. Processing of Structures for Molecular Docking
3.2.4. Molecular Docking
3.2.5. Preparation of Telmisartan Cocrystals Using Maleic Acid
3.2.6. Characterization of Pure Telmisartan and Telmisartan-Maleic Acid Cocrystal
Equilibrium Solubility Analysis
Dissolution Studies
Scanning Electron Microscopic Studies
Differential Scanning Calorimetry (DSC) Analysis
X-ray Powder Diffraction (XRD) Studies
Fourier Transform Infrared (FTIR) Spectroscopy
1D and 2D NMR Studies
3.3. Preclinical Studies (In Vivo Studies)
3.3.1. Animals
3.3.2. Pharmacokinetic Studies in Rats
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thayer, A.M. Finding solutions. Chem. Eng. News 2010, 88, 13–18. [Google Scholar] [CrossRef]
- Ku, M.S.; Dublin, W. A biopharmaceutical classification-based right-first- time formulation approach to reduce human pharmacokinetic variability and project cycle time from first-in-human to clinical proof-of-concept. Pharm. Dev. Tech. 2010, 17, 285–302. [Google Scholar] [CrossRef] [PubMed]
- Blagden, N.; De Matas, M.; Gavan, P.T.; York, P. Crystal engineering of active pharmaceutical ingredients to improve solubility and dissolution rates. Adv. Drug Deliv. Rev. 2007, 59, 617–630. [Google Scholar] [CrossRef]
- Karimi-Jafari, M.; Padrela, L.; Walker, G.M.; Croker, D.M. Creating Cocrystals: A Review of Pharmaceutical Cocrystal Preparation Routes and Applications. Cryst. Growth Des. 2018, 18, 6370–6387. [Google Scholar] [CrossRef]
- Bikiaris, D.N. Solid dispersions, Part I: Recent evolutions and future opportunities in manufacturing methods for dissolution rate enhancement of poorly water-soluble drugs. Expert Opin. Drug Deliv. 2011, 8, 1501–1519. [Google Scholar] [CrossRef]
- Jain, S.; Patel, N.; Lin, S. Solubility and dissolution enhancement strategies: Current understanding and recent trends. Drug Dev. Ind. Pharm. 2014, 41, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Vishweshwar, P.; McMahon, J.A.; Bis, J.A.; Zaworotko, M.J. Pharmaceutical co-crystals. J. Pharm. Sci. 2006, 95, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Corti, G.; Capasso, G.; Maestrelli, F.; Cirri, M.; Mura, P. Physical–chemical characterization of binary systems of metformin hydrochloride with triacetyl-β-cyclodextrin. J. Pharm. Biomed. Anal. 2007, 45, 480–486. [Google Scholar] [CrossRef]
- Jones, W.; Motherwell, W.S.; Trask, A.V. Pharmaceutical Cocrystals: An Emerging Approach to Physical Property Enhancement. MRS Bull. 2006, 31, 875–879. [Google Scholar] [CrossRef]
- Bhogala, B.R.; Nangia, A. Ternary and quaternary co-crystals of 1,3-cis,5-cis- cyclohexane tricarboxylic acid and 4,4-bipyridines. N. J. Chem. 2008, 32, 800–807. [Google Scholar] [CrossRef]
- Schultheiss, N.; Newman, A. Pharmaceutical Cocrystals and Their Physicochemical Properties. Cryst. Growth Des. 2009, 9, 2950–2967. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.J.; Steed, J.W. Pharmaceutical Cocrystals, Salts and Multicomponent Systems; Intermolecular Interactions and Property Based Design. Adv. Drug Deliv. Rev. 2017, 117, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Aakeröy, C.B.; Sinha, A.S. Co-crystals. Preparation, Characterization and Applications. In Royal Society of Chemistry; Monographs in Supramolecular Chemistry No. 24, Hardcover; Royal Society of Chemistry: London, UK, 2018; p. 342. [Google Scholar]
- Almarsson, Ö.; Peterson, M.L.; Zaworotko, M. The A to Z of Pharmaceutical Cocrystals: A Decade of Fast-Moving New Science and Patents. Pharm. Pat. Anal. 2012, 1, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.S.; Ghosh, S.; Mehta, G.N. Efficient and improved synthesis of Telmisartan. Beilstein J. Org. Chem. 2010, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Cagigal, E.; Gonzalez, L.; Alonso, R.M.; Jiménez, R.M. pK(a) determination of angiotensin II receptor antagonists (ARA II) by spectrofluorimetry. J. Pharm. Biomed. Anal. 2001, 26, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.H.L.; Tran, H.T.T.; Lee, B.-J. Modulation of microenvironmental pH and crystallinity of ionizable telmisartan using alkalizers in solid dispersions for controlled release. J. Control. Release 2008, 129, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Stangier, J.; Su, C.; Roth, W. Pharmacokinetics of Orally and Intravenously Administered Telmisartan in Healthy Young and Elderly Volunteers and in Hypertensive Patients. J. Int. Med. Res. 2000, 28, 149–167. [Google Scholar] [CrossRef]
- Cao, Y.; Shi, L.-L.; Cao, Q.-R.; Yang, M.; Cui, J.-H. In-Vitro Characterization and Oral Bioavailability of Organic Solvent-free Solid Dispersions Containing Telmisartan. Iran J. Pharm. Res. 2016, 15, 385–394. [Google Scholar] [CrossRef]
- Zhong, L.; Zhu, X.; Yu, B.; Su, W. Influence of alkalizers on dissolution properties of telmisartan in solid dispersions prepared by co-grinding. Drug Dev. Ind. Pharm. 2014, 40, 1660–1669. [Google Scholar] [CrossRef]
- Zhong, L.; Zhu, X.; Luo, X.; Su, W. Dissolution Properties and Physical Characterization of Telmisartan–Chitosan Solid Dispersions Prepared by Mechanochemical Activation. AAPS PharmSciTech 2013, 14, 541–550. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, T.; Zhang, Q.; Wang, S. Inclusion of telmisartan in mesocellular foam nanoparticles: Drug loading and release property. Eur. J. Pharm. Biopharm. 2010, 76, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Sekar, V.; Chellan, V.R. Immediate release tablets of telmisartan using super disintegrant formulation, evaluation and stability studies. Chem. Pharm. Bull. 2008, 56, 575–577. [Google Scholar] [CrossRef]
- Lepek, P.; Sawicki, W.; Wlodarski, K.; Wojnarowska, Z.; Paluch, M.; Guzik, L. Effect of amorphization method on telmisartan solubility and the tableting process. Eur. J. Pharm. Biopharm. 2013, 83, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhi, Z.; Jiang, T.; Zhang, J.; Wang, Z.; Wang, S. Spherical mesoporous silica nanoparticles for loading and release of the poorly water-soluble drug telmisartan. J. Control. Release 2010, 145, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Kevin, G.; Patel, A.; Raval, M.; Sheth, N. Design and development of a self-nanoemulsifying drug delivery system for telmisartan for oral drug delivery. Int. J. Pharm. Investig. 2011, 1, 112–118. [Google Scholar] [CrossRef]
- Kundu, S.; Kumari, N.; Soni, S.R.; Ranjan, S.; Kumar, R.; Sharon, A.; Ghosh, A. Enhanced Solubility of Telmisartan Phthalic Acid Cocrystals within the pH Range of a Systemic Absorption Site. ACS Omega 2018, 3, 15380–15388. [Google Scholar] [CrossRef]
- Arora, P.; Kaur, A.; Haneef, J.; Chadha, R. Solubility improvement of telmisartan by co-crystallization with citric acid. Int. J. Pharm. Sci. Res. 2017, 8, 3768–3775. [Google Scholar]
- Chadha, R.; Bhandari, S.; Haneef, J.; Khullar, S.; Mandal, S. Cocrystals of telmisartan: Characterization, structure elucidation, in vivo and toxicity studies. Crystengcomm 2014, 16, 8375–8389. [Google Scholar] [CrossRef]
- Thipparaboina, R.; Kumar, D.; Chavan, R.B.; Shastri, N.R. Multidrug co-crystals: Towards the development of effective therapeutic hybrids. Drug Discov. Today 2016, 21, 481–490. [Google Scholar] [CrossRef]
- Haneef, J.; Arora, P.; Chadha, R. Implication of Coformer Structural Diversity on Cocrystallization Outcomes of Telmisartan with Improved Biopharmaceutical Performance. AAPS PharmSciTech 2019, 21, 10. [Google Scholar] [CrossRef]
- Fukte, S.R.; Wagh, M.P.; Rawat, S. Coformer Selection: An Important Tool in Cocrystal Formation. Int. J. Pharm. Pharm. Sci. 2014, 6, 9–14. [Google Scholar]
- Bis, J.A.; Zaworotko, M.J. The 2-Aminopyridinium-carboxylate Supramolecular Heterosynthon: A Robust Motif for Generation of Multiple-Component Crystals. Cryst. Growth Des. 2005, 5, 1169–1179. [Google Scholar] [CrossRef]
- Aakeroy, C. Is There Any Point in Making Co-Crystals? Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2015, 71, 387–391. [Google Scholar] [CrossRef]
- Fornari, F.; Montisci, F.; Bianchi, F.; Cocchi, M.; Carraro, C.; Cavaliere, F.; Cozzini, P.; Peccati, F.; Mazzeo, P.P.; Riboni, N.; et al. Chemometric-Assisted Cocrystallization: Supervised Pattern Recognition for Predicting the Formation of New Functional Cocrystals. Chemom. Intell. Lab. Syst. 2022, 226, 104580. [Google Scholar] [CrossRef]
- Corpinot, M.K.; Bučar, D.K. A Practical Guide to the Design of Molecular Crystals. Cryst. Growth Des. 2019, 19, 1426–1453. [Google Scholar] [CrossRef]
- Roca-Paixão, L.; Correia, N.T.; Affouard, F. Affinity Prediction Computations and Mechanosynthesis of Carbamazepine Based Cocrystals. CrystEngComm 2019, 21, 6991–7001. [Google Scholar] [CrossRef]
- Musumeci, D.; Hunter, C.A.; Prohens, R.; Scuderi, S.; McCabe, J.F. Virtual cocrystal screening. Chem. Sci. 2011, 2, 883–890. [Google Scholar] [CrossRef]
- Bolla, G.; Nangia, A. Pharmaceutical cocrystals: Walking the talk. Chem. Commun. 2016, 52, 8342–8360. [Google Scholar] [CrossRef]
- I Corrigan, O.; Stanley, C.T. Mechanism of drug dissolution rate enhancement from β-cyclodextrin-drug systems. J. Pharm. Pharmacol. 1982, 34, 621–626. [Google Scholar] [CrossRef]
- Dhibar, M.; Basak, S. Assessment of Effects of Solvents on Cocrystallization by Computational Simulation Approach. Curr. Drug Deliv. 2021, 18, 44–53. [Google Scholar] [CrossRef]
- Alatas, F.; Ratih, H.; Soewandhi, S.N. Enhancement of solubility and dissolution rate of telmisartan by telmisartan-oxalic acid co-crystal formation. Int. J. Pharm. Pharm. Sci. 2015, 7, 423–426. [Google Scholar]
- Tong, P.; Taylor, L.S.; Zografi, G. Influence of alkali metal counterions on the glass transition temperature of amorphous indomethacin salts. Pharm. Res. 2002, 19, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.K.; Vanwert, A.; Bogner, R.H. Formation of Physically Stable Amorphous Drugs by Milling with Neusilin. J. Pharm. Sci. 2003, 92, 536–551. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Indian Pharmacopoeia; The Indian Pharmacopoeia Commission: Ghaziabad, India, 2014; pp. 2830–2832.
- Seelam, R.R.; Chandiran, S.; Divi, K.R.; Jayaveera, K.N. Development and validation of high-performance liquid chromatography-tandem mass spectrometric method for simultaneous quantification of telmisartan in human plasma. Int. J. Pharm. Sci. Drug. Res. 2010, 2, 188–192. [Google Scholar]


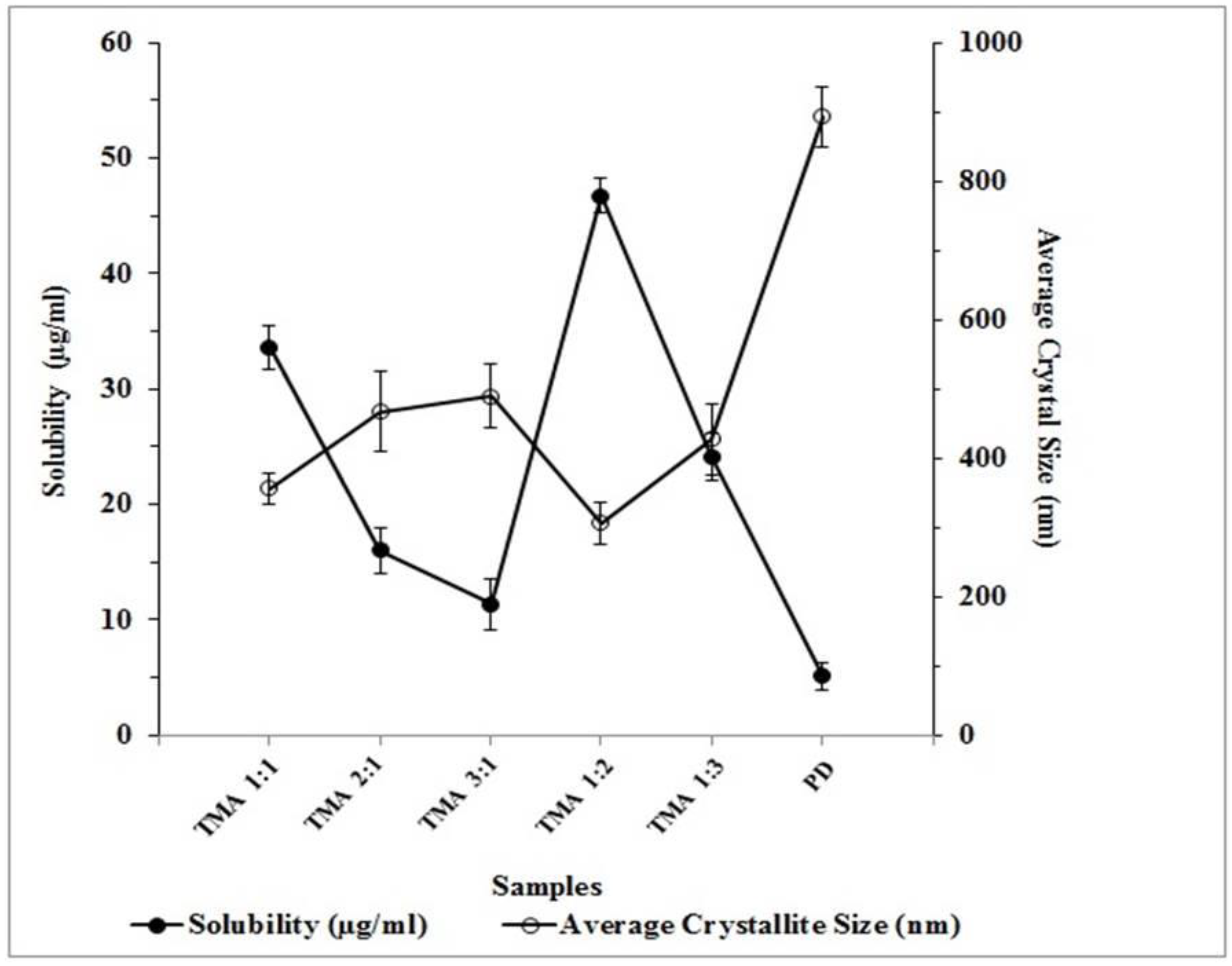



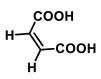
| Formulation | (Drug:Coformer) (Molar Ratio) | Telmisartan (mg) | Maleic Acid (mg) | Solubility (µg/mL; at 25 °C) |
|---|---|---|---|---|
| TMA 1:1 | 1:1 | 514 | 116 | 33.61 ± 1.93 |
| TMA 2:1 | 2:1 | 1028 | 116 | 16.01 ± 1.99 |
| TMA 3:1 | 3:1 | 1542 | 116 | 11.38 ± 2.23 |
| TMA 1:2 | 1:2 | 514 | 232 | 46.78 ± 1.48 |
| TMA 1:3 | 1:3 | 514 | 348 | 24.15 ± 2.07 |
| PD | - | - | - | 5.15 ± 1.16 |
| Pharmacokinetic Parameters | PD | TMA 1:2 |
|---|---|---|
| Cmax (ng/mL) | 945.31 ± 27.92 | 1900.43 ± 56.33 |
| AUC0-∞ (ng/mL × hr) | 4184.17 ± 87.82 | 10,956.32 ± 103.27 |
| KE (h−1) | 0.108 ± 0.036 | 0.184 ± 0.019 |
| Relative bioavailability | - | 2.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhibar, M.; Chakraborty, S.; Basak, S.; Pattanayak, P.; Chatterjee, T.; Ghosh, B.; Raafat, M.; Abourehab, M.A.S. Critical Analysis and Optimization of Stoichiometric Ratio of Drug-Coformer on Cocrystal Design: Molecular Docking, In Vitro and In Vivo Assessment. Pharmaceuticals 2023, 16, 284. https://doi.org/10.3390/ph16020284
Dhibar M, Chakraborty S, Basak S, Pattanayak P, Chatterjee T, Ghosh B, Raafat M, Abourehab MAS. Critical Analysis and Optimization of Stoichiometric Ratio of Drug-Coformer on Cocrystal Design: Molecular Docking, In Vitro and In Vivo Assessment. Pharmaceuticals. 2023; 16(2):284. https://doi.org/10.3390/ph16020284
Chicago/Turabian StyleDhibar, Manami, Santanu Chakraborty, Souvik Basak, Paramita Pattanayak, Tanmay Chatterjee, Balaram Ghosh, Mohamed Raafat, and Mohammed A. S. Abourehab. 2023. "Critical Analysis and Optimization of Stoichiometric Ratio of Drug-Coformer on Cocrystal Design: Molecular Docking, In Vitro and In Vivo Assessment" Pharmaceuticals 16, no. 2: 284. https://doi.org/10.3390/ph16020284
APA StyleDhibar, M., Chakraborty, S., Basak, S., Pattanayak, P., Chatterjee, T., Ghosh, B., Raafat, M., & Abourehab, M. A. S. (2023). Critical Analysis and Optimization of Stoichiometric Ratio of Drug-Coformer on Cocrystal Design: Molecular Docking, In Vitro and In Vivo Assessment. Pharmaceuticals, 16(2), 284. https://doi.org/10.3390/ph16020284







