Therapeutic Potential of Zeolites/Vitamin B12 Nanocomposite on Complete Freund’s Adjuvant-Induced Arthritis as a Bone Disorder: In Vivo Study and Bio-Molecular Investigations
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
Chemicals
2.2. Methods
2.2.1. Preparation of Nano ZT and ZT/VB12 Nanocomposite
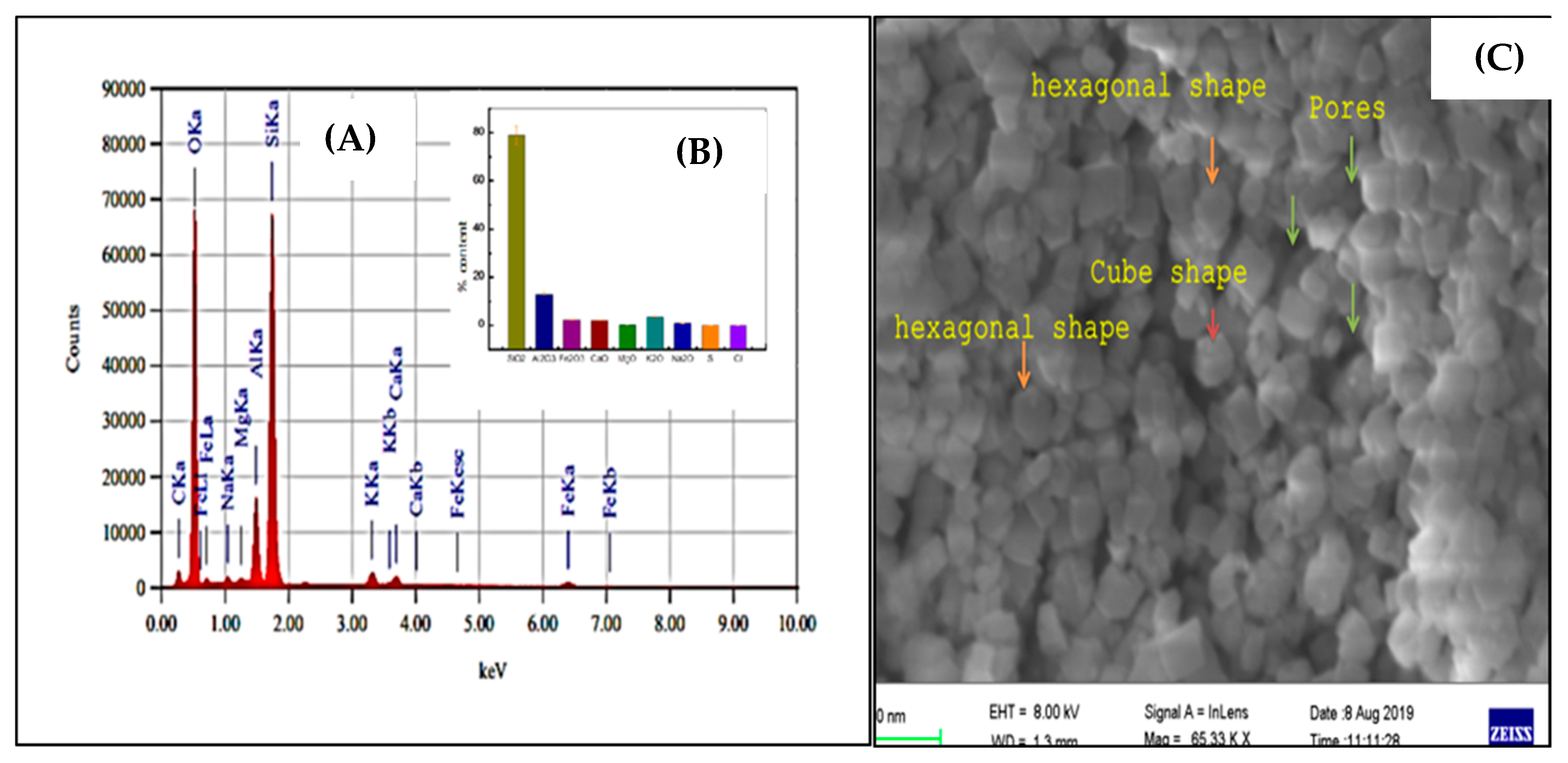
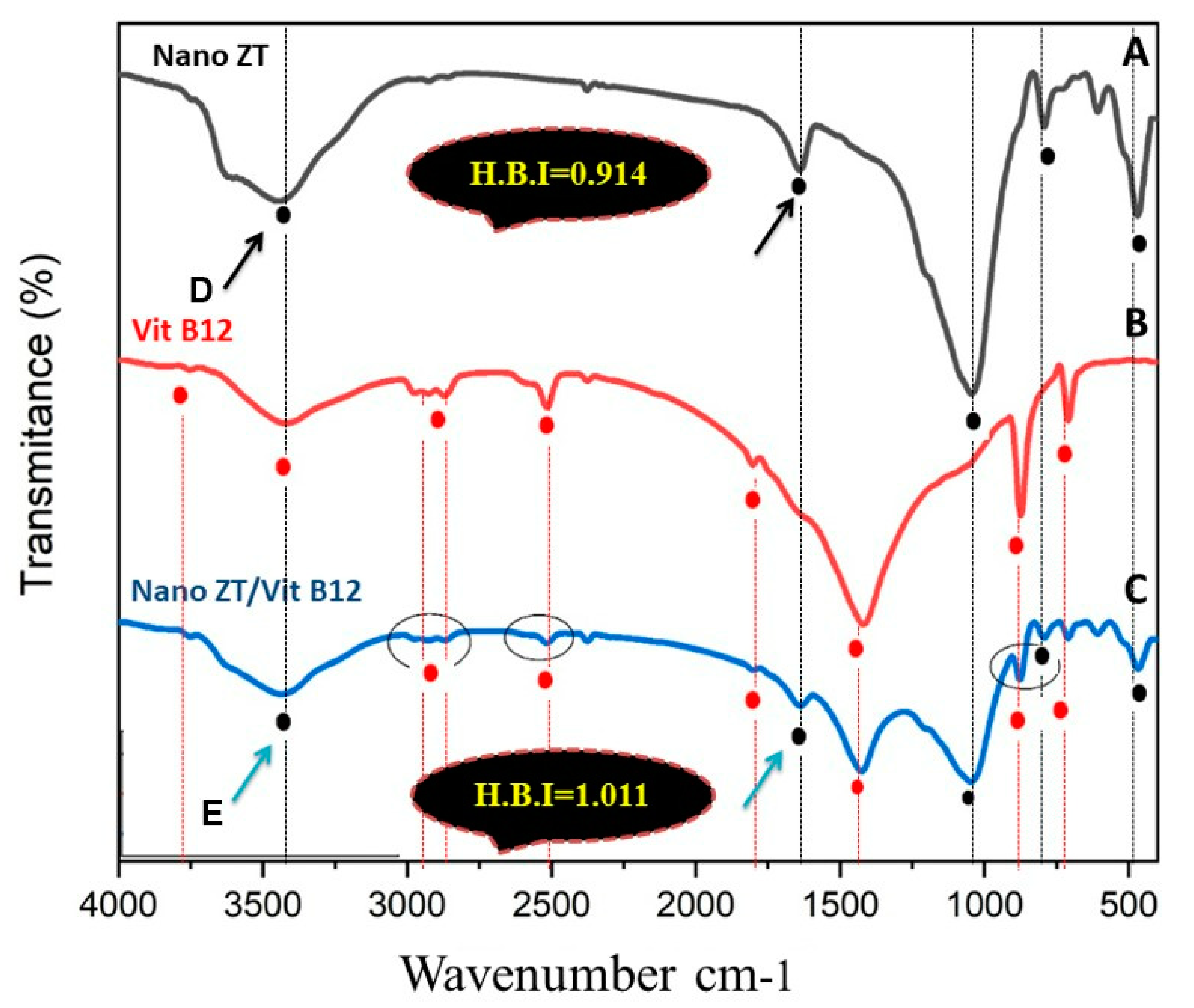
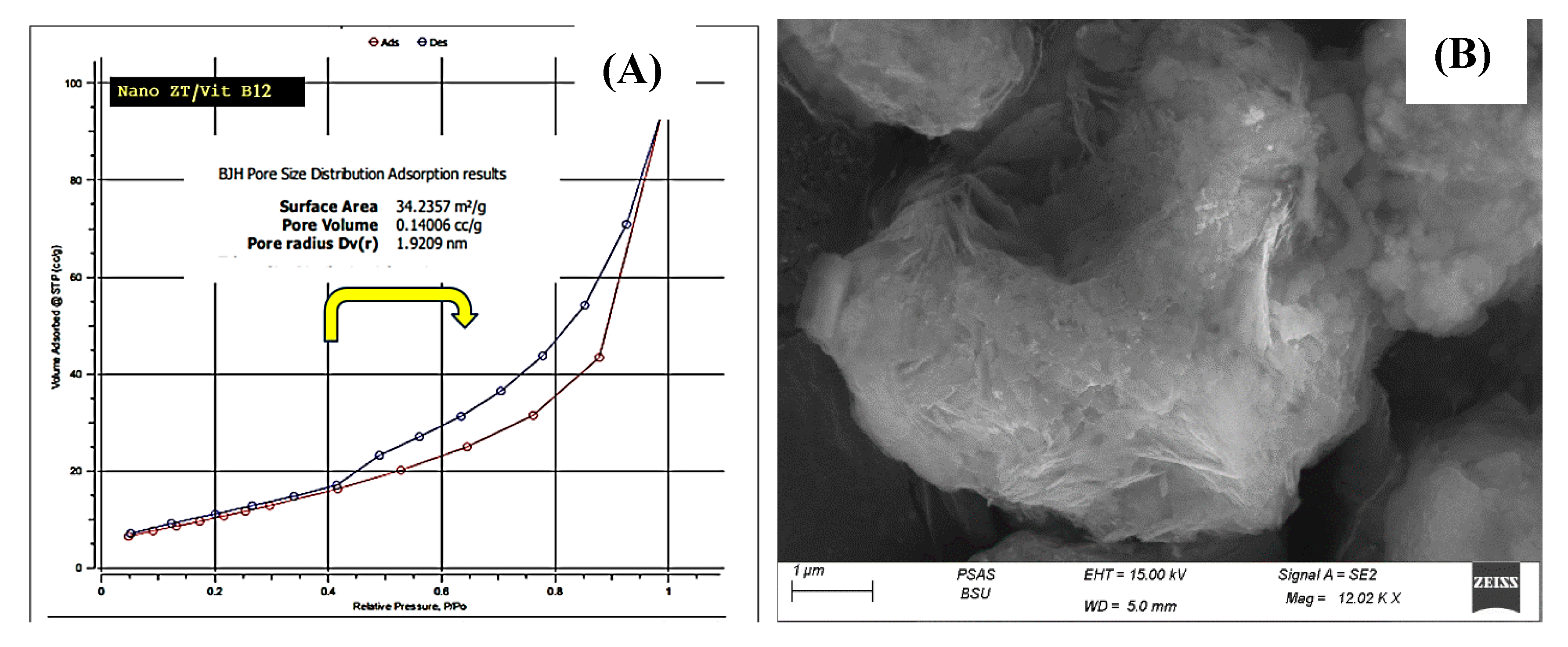
2.2.2. Nano ZT/Vit B 12 Characterization
2.3. Molecular Simulation Calculations
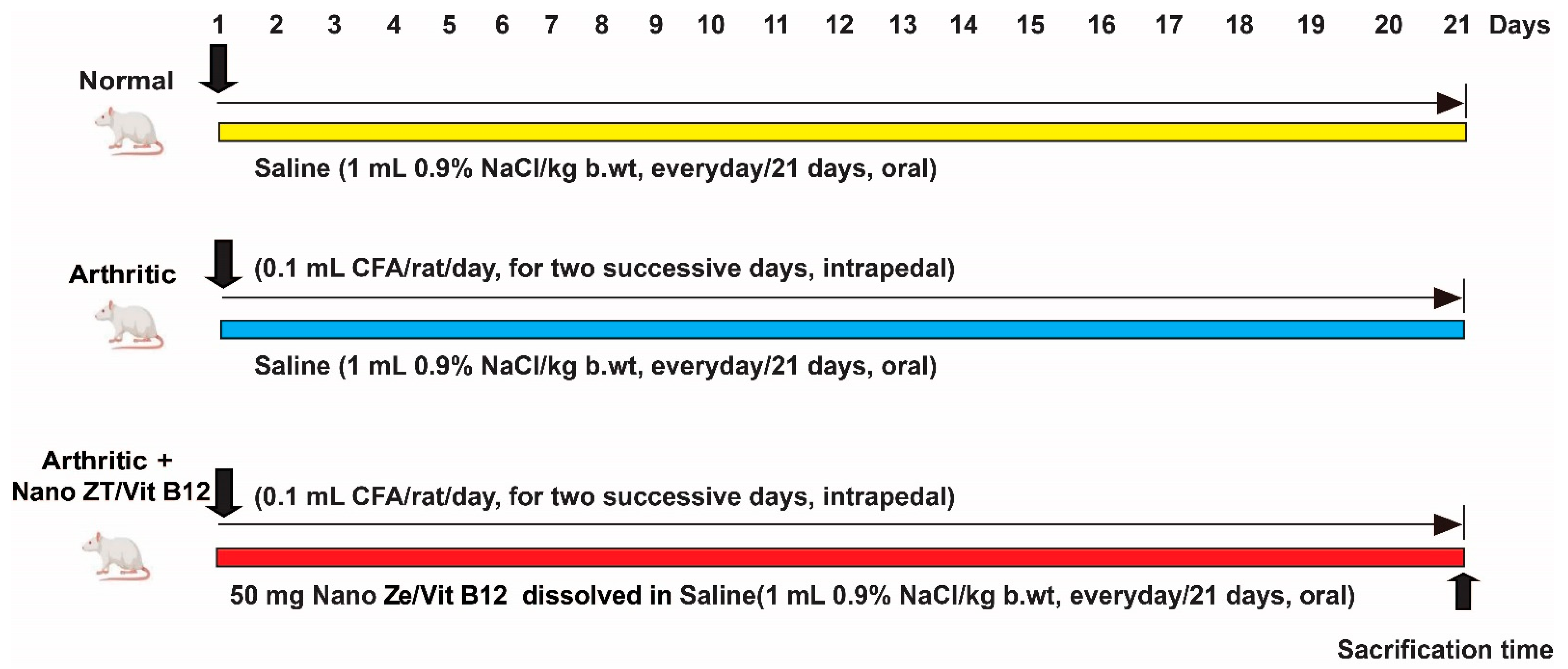
2.4. Experimental Animals
2.5. Induction of Arthritis
2.6. Animal Grouping
2.7. Assessment of Paw Edema
2.8. Histopathological Examination
2.9. Biochemical Investigations
2.10. Western Blot Analysis
2.11. q-RT-PCR Analysis
2.12. Statistical Analysis
3. Results
3.1. Nano Synthesis and Characterization
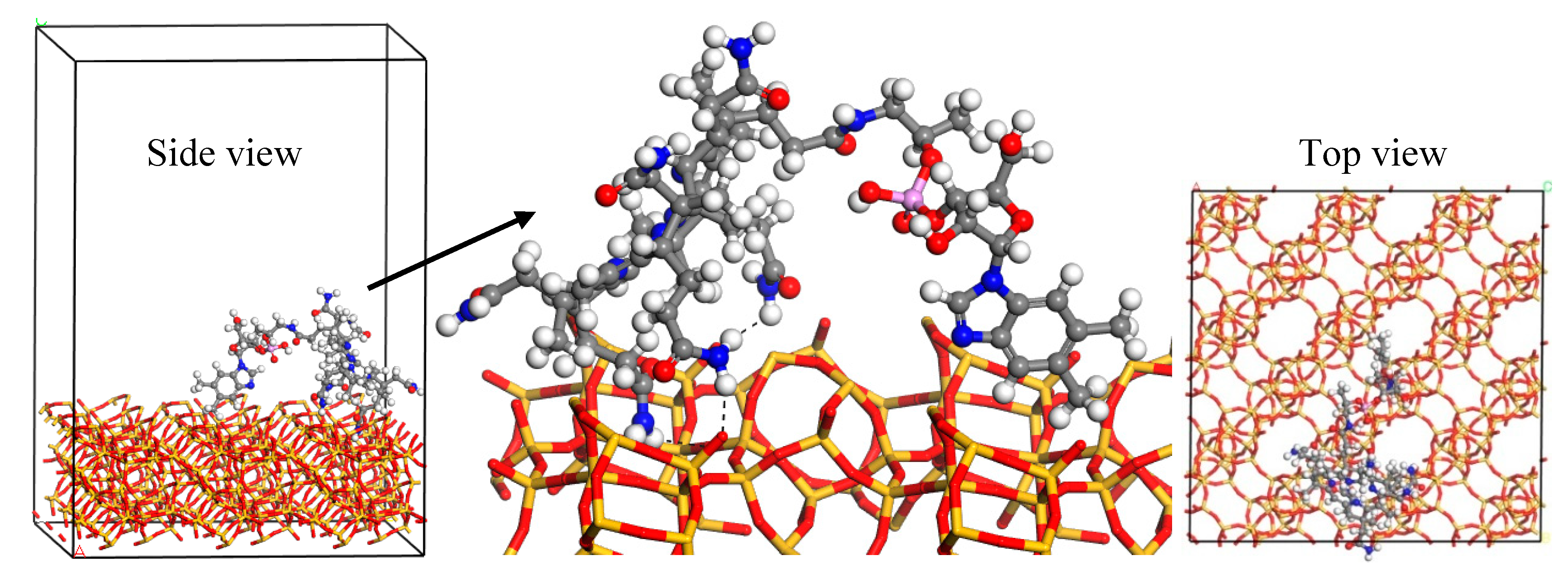
3.2. Molecular Simulations
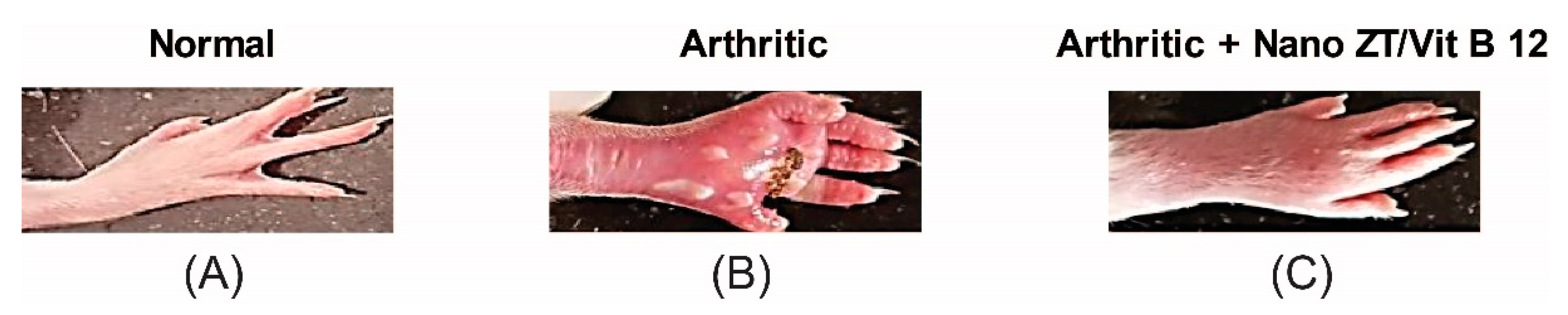
3.3. Effect of Nano ZT/Vit B12 on Gross Lesions of the Paw and Ankle Joint
3.4. Effect of Nano ZT/Vit B12 on Right Hind Paws Volume
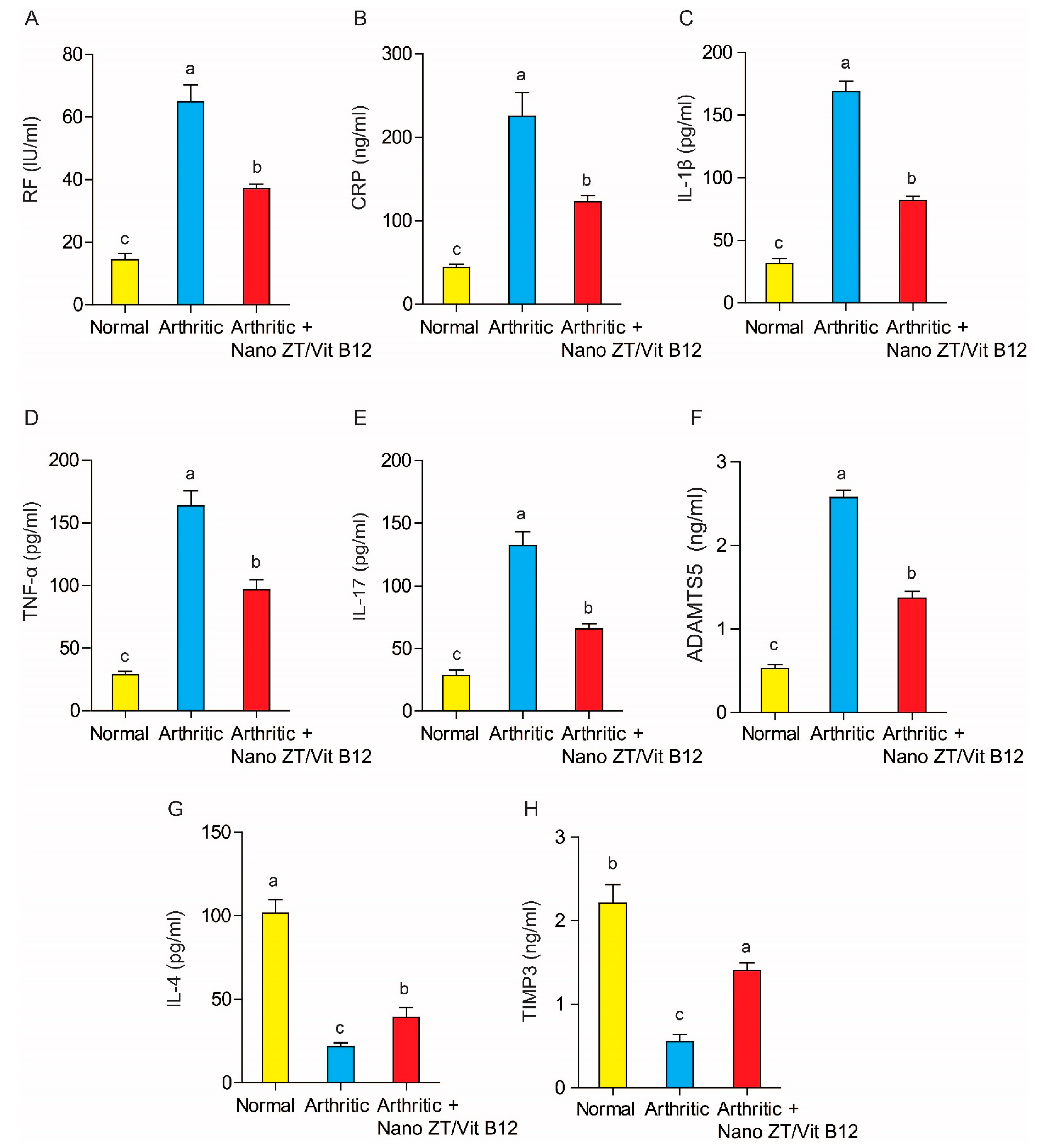
3.5. Effect of Nano ZT/Vit B12 on Serum RF, CRP, TNF-α, IL-1β, IL-17, ADAMTS-5, IL-4, and TIMP3 Levels
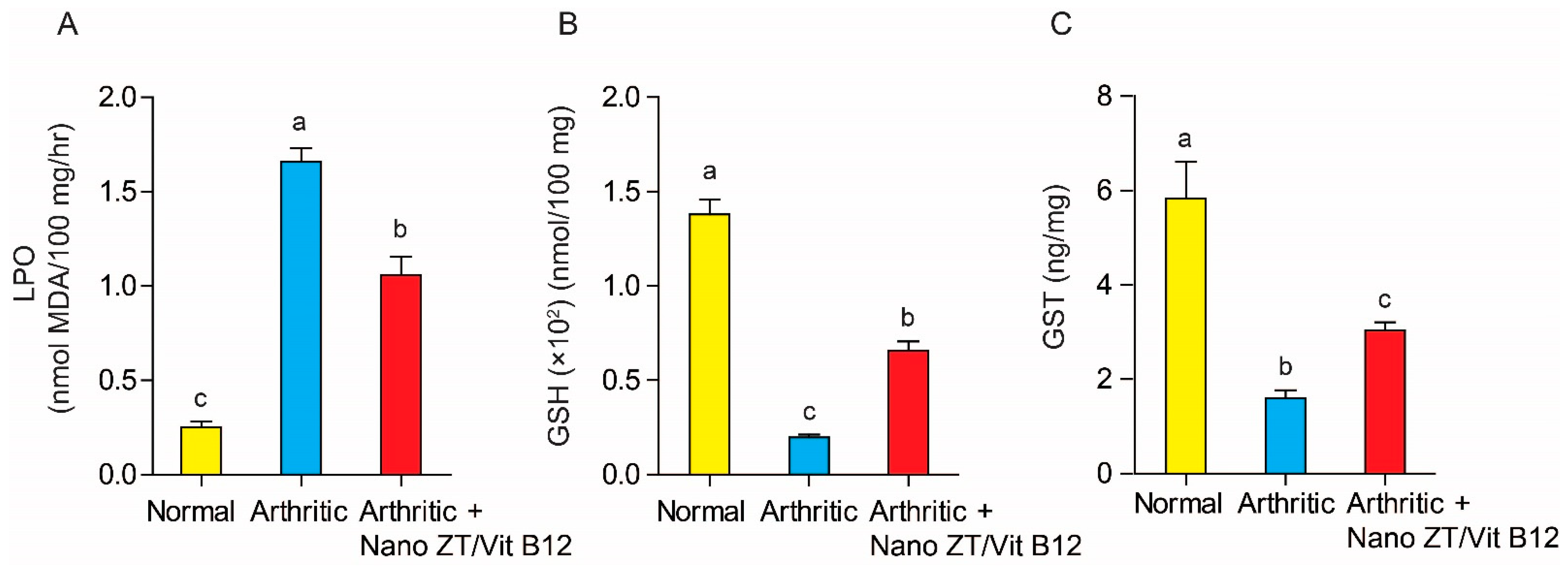
3.6. Effect of Nano ZT/Vit B12 on LPO, GSH Content, and GST Activity
3.7. Effects of Nano ZT/Vit B12 on TGF-β mRNA Expression and MMP13 Protein Levels
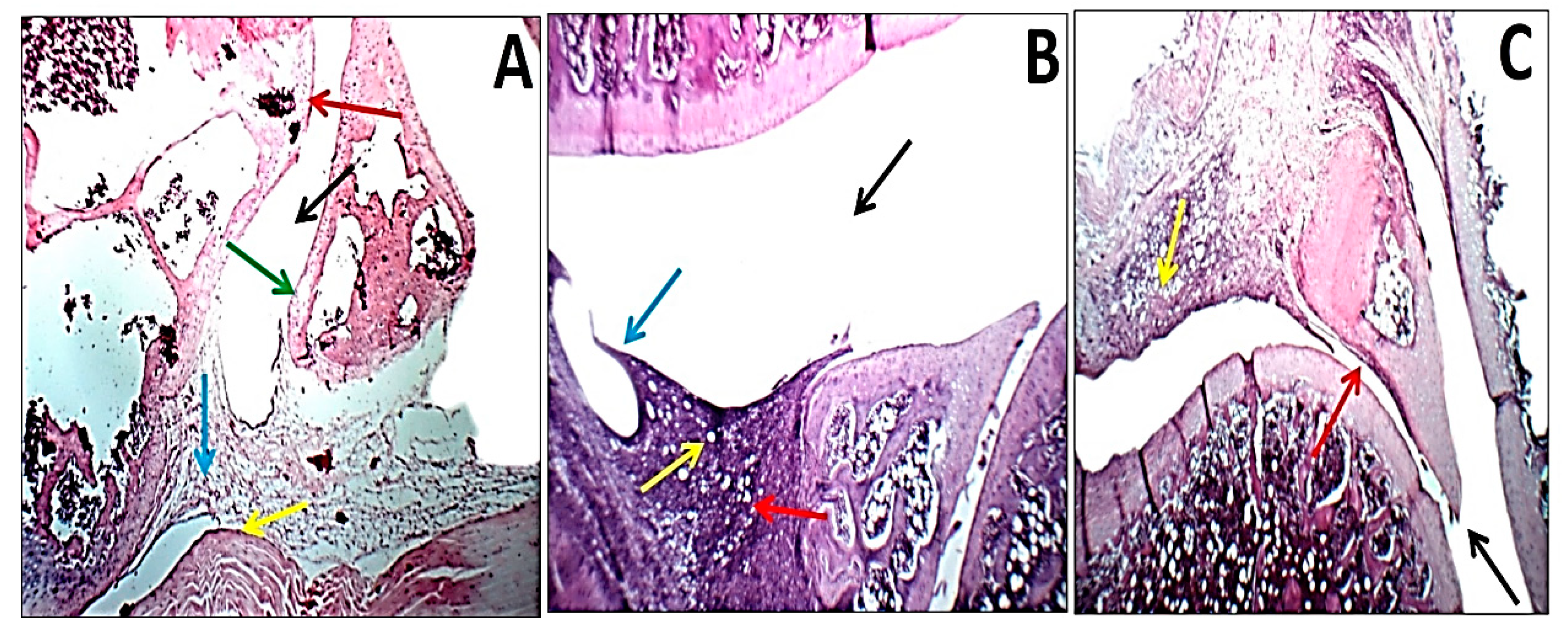
3.8. Histopathological Changes and Arthritic Score
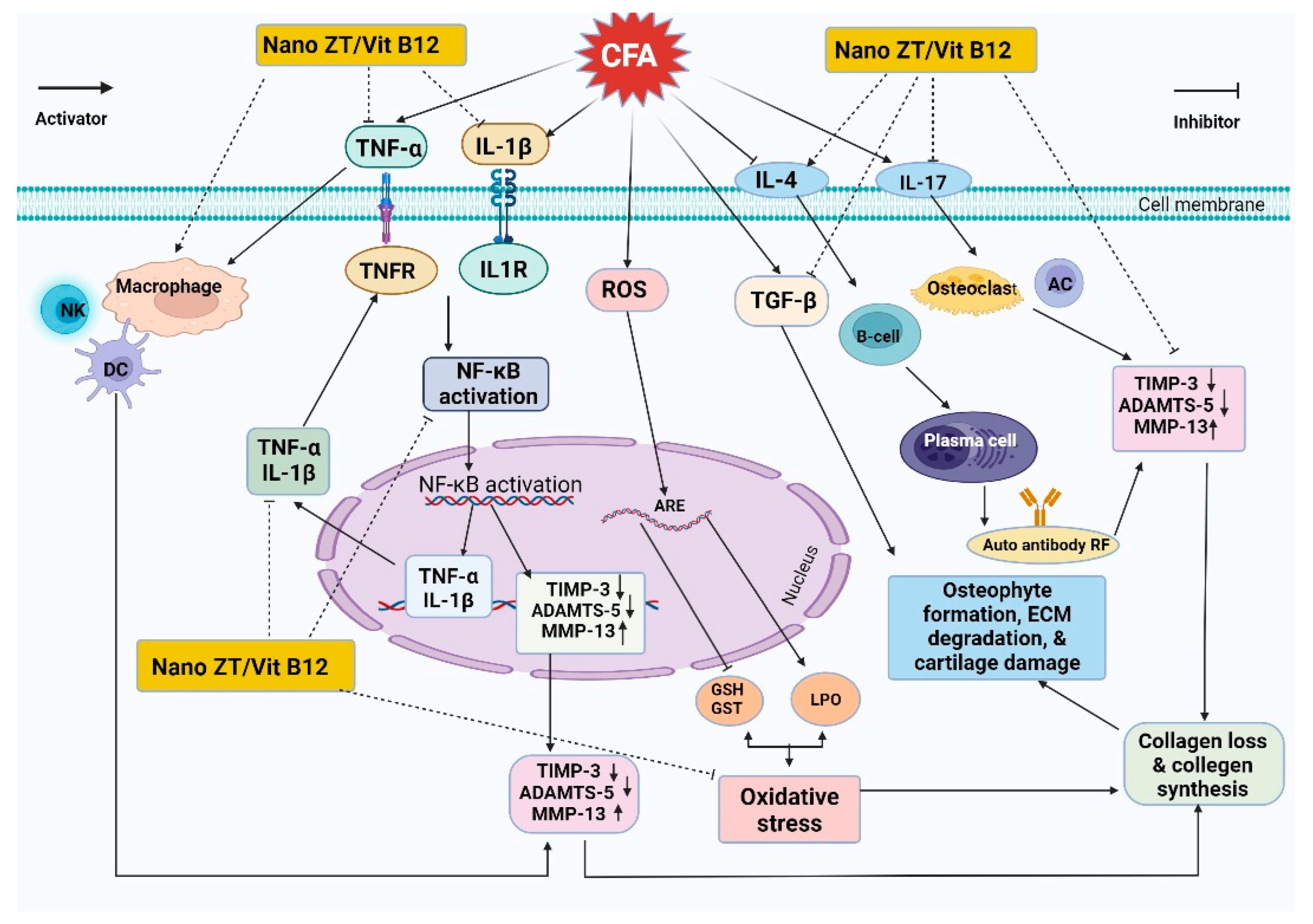
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akiyama, M.; Kaneko, Y. Pathogenesis, clinical features, and treatment strategy for rheumatoid arthritis-associated interstitial lung disease. Autoimmun. Rev. 2022, 21, 103056. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.A.; Ahmed, O.M.; Ahmed, H.I.; Fahim, T.M.; Ali, B.H.; Elesawy, M.B.; Ashour. Potency of bone marrow-derived mesenchymal stem cells and indomethacin in complete Freund’s adjuvant-induced arthritic rats: Roles of TNF-α, IL-10, iNOS, MMP-9, and TGF-β1. Stem Cells Int. 2021, 2021, 6665601. [Google Scholar] [CrossRef] [PubMed]
- Asoudeh, F.; Djafarian, K.; Akhalghi, M.; Mahmoudi, M.; Jamshidi, A.R.; Farhadi, E.; Esmaillzadeh, A. The effect of probiotic cheese consumption on inflammatory and anti-inflammatory markers, disease severity, and symptoms in patients with rheumatoid arthritis: Study protocol for a randomized, double-blind, placebo-controlled trial. Trials 2022, 23, 180. [Google Scholar] [CrossRef] [PubMed]
- Lieber, S.B.; Navarro-Millán, I.; Rajan, M.; Curtis, J.R.; Sattui, S.E.; Lui, G.; Mandl, L.A. Prevalence of frailty in ankylosing spondylitis, psoriatic arthritis, and rheumatoid arthritis: Data from a National Claims Dataset. ACR Open Rheumatol. 2022, 4, 300–305. [Google Scholar] [CrossRef]
- Qamar, T.; Mukherjee, S. Genetic approaches for the diagnosis and treatment of rheumatoid arthritis through personalized medicine. Gene Rep. 2021, 23, 101173. [Google Scholar] [CrossRef]
- Ahmed, O.M.; EL-Abd, S.F.; El Mahdi, E.A.; Abdou, E.A. Curcumin ameliorative efficacy on type 1 diabetes mellitus coexisted with rheumatoid arthritis in Wistar rats. Merit Res. J. Med. Med. Sci. 2015, 3, 256–270. [Google Scholar]
- Makuch, S.; Więcek, K.; Woźniak, M. The immunomodulatory and anti-inflammatory effect of curcumin on immune cell populations, cytokines, and in vivo models of rheumatoid arthritis. Pharmaceuticals 2021, 14, 309. [Google Scholar] [CrossRef]
- Uttra, A.M.; Shahzad, M.; Shabbir, A.; Jahan, S. Ephedra gerardiana aqueous ethanolic extract and fractions attenuate Freund Complete Adjuvant induced arthritis in Sprague Dawley rats by downregulating PGE2, COX2, IL-1β, IL-6, TNF-α, NF-kB and upregulating IL-4 and IL-10. J. Ethnopharmacol. 2018, 224, 482–496. [Google Scholar] [CrossRef]
- Mackay, I.R.; Rowley, M.J.; Bernard, C.C. Biologic Therapies for Rheumatoid Arthritis: Concepts from Considerations on Pathogenesis. In Monoclonal Antibodies, Cytokines, and Arthritis; CRC Press: Boca Raton, FL, USA, 2020; pp. 75–100. [Google Scholar]
- Veronese, N.; Cooper, C.; Bruyère, O.; Al-Daghri, N.M.; Branco, J.; Cavalier, E.; Reginster, J.Y. Multimodal multidisciplinary management of patients with moderate to severe pain in knee osteoarthritis: A need to meet patient expectations. Drugs 2022, 82, 1347–1355. [Google Scholar] [CrossRef]
- Rahman, M.; Islam, F.; Afsana Mim, S.; Khan, M.; Islam, M.; Haque, M.; Mitra, S.; Emran, T.B.; Rauf, A. Multifunctional therapeutic approach of nanomedicines against inflammation in cancer and aging. J. Nanomater. 2022, 2022, 4217529. [Google Scholar] [CrossRef]
- Servatan, M.; Zarrintaj, P.; Mahmodi, G.; Kim, S.J.; Ganjali, M.R.; Saeb, M.R.; Mozafari, M. Zeolites in drug delivery: Progress, challenges and opportunities. Drug Discov. Today 2020, 25, 642–656. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Wang, L.; Li, Y.; Zha, J.; Tian, G.; Wang, F.; Zhang, H.; Liang, J. Synthesis of zeolites by in-situ conversion of geopolymers and their performance of heavy metal ion removal in wastewater: A review. J. Clean. Prod. 2022, 349, 131441. [Google Scholar] [CrossRef]
- Kraljević Pavelić, S.; Micek, V.; Bobinac, D.; Bazdulj, E.; Gianoncelli, A.; Krpan, D.; Žuvić, M.; Eisenwagen, S.; Stambrook, P.J.; Pavelić, K. Treatment of osteoporosis with a modified zeolite shows beneficial effects in an osteoporotic rat model and a human clinical trial. Exp. Biol. Med. 2021, 246, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Pavelić, S.K.; Krpan, D.; Žuvić, M.; Eisenwagen, S.; Pavelić, K. Clinical parameters in osteoporosis patients supplemented with PMA-zeolite at the end of 5-year double-blinded clinical trial. Front. Med. 2022, 9, 870692. [Google Scholar]
- Lamprecht, M.; Bogner, S.; Steinbauer, K.; Schuetz, B.; Greilberger, J.F.; Leber, B.; Schippinger, G. Effects of zeolite supplementation on parameters of intestinal barrier integrity, inflammation, redoxbiology and performance in aerobically trained subjects. J. Int. Soc. Sport. Nutr. 2015, 12, 40. [Google Scholar] [CrossRef]
- Watkins, K.L.; Vagnoni, D.B.; Southern, L.L. Effect of dietary sodium zeolite A and excess calcium on growth and tibia calcium and phosphorus concentration in uninfected and Eimeria acervulina-infected chicks. Poult. Sci. 1989, 68, 1236–1240. [Google Scholar] [CrossRef]
- Dai, Z.; Koh, W.P. B-vitamins and bone health—A review of the current evidence. Nutrients 2015, 7, 3322–3346. [Google Scholar] [CrossRef]
- Bolat, M.; Ciocan-Pendefunda, A.; Surlari, Z.; Bida, C.; Balcos, C.; Baciu, R.; Bosinceanu, D.G. Using shape memory effect to obtain a new polymer for the manufacture of complete dentures. IOP Conf. Ser. Mater. Sci. Eng. 2019, 572, 012014. [Google Scholar] [CrossRef]
- Elkartehi, M.E.; Mahmoud, R.; Shehata, N.; Farghali, A.; Gamil, S.; Zaher, A. LDH nanocubes synthesized with zeolite templates and their high performance as adsorbents. Nanomaterials 2021, 11, 3351. [Google Scholar] [CrossRef]
- Sun, H.; Jin, Z.; Yang, C.; Akkermans, R.L.C.; Robertson, S.H.; Spenley, N.A.; Miller, S.; Todd, S.M. COMPASS II: Extended coverage for polymer and drug-like molecule databases. J. Mol. Model. 2016, 22, 47. [Google Scholar] [CrossRef]
- Snekhalatha, U.; Anburajan, M.; Venkatraman, B.; Menaka, M. Evaluation of complete Freund’s adjuvant-induced arthritis in a Wistar rat model. Z. Rheumatol. 2013, 72, 375–382. [Google Scholar] [CrossRef]
- Thomas, J.A.; Ballantyne, B. Toxicological assessment of zeolites. J. Am. Coll. Toxicol. 1992, 11, 259–273. [Google Scholar] [CrossRef]
- Sancho, D.; Gómez, M.; Viedma, F.; Esplugues, E.; Gordón-Alonso, M.; García-López, M.A.; Sánchez-Madrid, F. CD69 downregulates autoimmune reactivity through active transforming growth factor-β production in collagen-induced arthritis. J. Clin. Investig. 2003, 112, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Sancho, D.; Gómez, M.; Viedma, F.; Esplugues, E.; Gordón-Alonso, M.; García-López, M.; Sánchez-Madrid, F. Comparative effects of chromium, vanadium and Gymnema sylvestre on sugar-induced blood pressure elevations in SHR. J. Am. Coll. Nutr. 1998, 17, 116–123. [Google Scholar]
- Beutler, E. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Matkovics, B.; Kotorman, M.; Varga, I.S.; Hai, D.Q.; Varga, C.S. Oxidative stress in experimental diabetes induced by streptozotocin. Acta Physiol. Hung. 1997, 85, 29–38. [Google Scholar]
- Mannervik, B.; Guthenberg, C. [28] Glutathione transferase (human placenta). Methods Enzymol. 1981, 77, 231–235. [Google Scholar]
- Mahmoud, A.N.; Barakat, A.F.; Elgendy, A.Y.; Schneibel, E.; Mentias, A.; Abuzaid, A.; Elgendy, I.Y. Long-term efficacy and safety of everolimus-eluting bioresorbable vascular scaffolds versus everolimus-eluting metallic stents: A meta-analysis of randomized trials, Circulation. Cardiovasc. Interv. 2017, 10, e005286. [Google Scholar] [CrossRef]
- Wen, X.; Yang, F.; Ke, Q.F.; Xie, X.T.; Guo, Y.P. Hollow mesoporous ZSM-5 zeolite/chitosan ellipsoids loaded with doxorubicin as pH-responsive drug delivery systems against osteosarcoma. J. Mater. Chem. B 2017, 5, 7866–7875. [Google Scholar] [CrossRef] [PubMed]
- Elkartehi, M.E.; Mahmoud, R.; Shehata, N.; Farghali, A.; Gamil, S.; Zaher, A. LDH nanocubes synthesized with zeolite templates and their high performance as adsorbents. Nanomaterials 2021, 11, 3315. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhou, X.; Zhao, W.; Peng, C.; Wu, H.; Chen, H. Pt/Fe co-loaded mesoporous zeolite beta for CO oxidation with high catalytic activity and water resistance. RSC Adv. 2019, 9, 28089–28094. [Google Scholar] [CrossRef] [PubMed]
- Slovák, L.; Poništ, S.; Kuncírová, V.; Mihalová, D.; Fedorova, T.; Bauerová, K. Evaluation of the effect of carnosine, its novel derivative trolox-carnosine and trolox in a pre-clinical study focussing on the regulation of immunity. Eur. Pharm. J. 2016, 63, 16–19. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Ashour, M.B.; Fahim, H.I.; Ahmed, N.A. (The role of Th1/Th2/Th17 cytokines and antioxidant defense system in mediating the effects of lemon and grapefruit peel hydroethanolic extracts on adjuvant-induced arthritis in rats. J. Appl. Pharm. Sci. 2018, 8, 69–081. [Google Scholar]
- Xie, X.; van Delft, M.A.; Shuweihdi, F.; Kingsbury, S.R.; Trouw, L.A.; Doody, G.M.; Ponchel, F. Auto-antibodies to post-translationally modified proteins in osteoarthritis. Osteoarthr. Cartil. 2021, 29, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.M.; Balach, V.; Sasikala, K.; Manikantan, P.; Arun, M.; Krishnan, B.B.; Sudha, S. Elevated rheumatoid factor (RF) from peripheral blood of patients with rheumatoid arthritis (RA) has altered chromosomes in Coimbatore population, South India. J. Clin. Med. Res. 2010, 2, 167–174. [Google Scholar]
- Shaaban, S.; Fayez, A.M.; Abdelaziz, M.; Abou El-ezz, D. Amelioration of Autoimmunity and Inflammation by Zinc Oxide Nanoparticles in Experimental Rheumatoid Arthritis. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 1975–1981. [Google Scholar]
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted physiological roles of adiponectin in inflammation and diseases. Int. J. Mol. Sci. 2020, 21, 1219. [Google Scholar] [CrossRef]
- Shaaban, H.H.; Hozayen, W.G.; Khaliefa, A.K.; El-Kenawy, A.E.; Ali, T.M.; Ahmed, O.M. Diosmin and Trolox Have Anti-Arthritic, Anti-Inflammatory and Antioxidant Potencies in Complete Freund’s Adjuvant-Induced Arthritic Male Wistar Rats: Roles of NF-κB, iNOS, Nrf2 and MMPs. Antioxidants 2022, 11, 1721. [Google Scholar] [CrossRef]
- Chen, Z.; Bozec, A.; Ramming, A.; Schett, G. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat. Rev. Rheumatol. 2019, 15, 9–17. [Google Scholar] [CrossRef]
- van Delft, M.A.; Huizinga, T.W. An overview of autoantibodies in rheumatoid arthritis. J. Autoimmun. 2020, 110, 102392. [Google Scholar] [CrossRef]
- Liu, S.; Deng, Z.; Chen, K.; Jian, S.; Zhou, F.; Yang, Y.; Zhu, W. Cartilage tissue engineering: From proinflammatory and anti-inflammatory cytokines to osteoarthritis treatments. Mol. Med. Rep. 2022, 25, 99. [Google Scholar] [CrossRef]
- Mateen, S.; Moin, S.; Shahzad, S.; Khan, A.Q. Level of inflammatory cytokines in rheumatoid arthritis patients: Correlation with 25-hydroxy vitamin D and reactive oxygen species. PLoS ONE 2017, 12, e0178879. [Google Scholar] [CrossRef] [PubMed]
- Mateen, S.; Zafar, A.; Moin, S.; Khan, A.Q.; Zubair, S. Understanding the role of cytokines in the pathogenesis of rheumatoid arthritis. Clin. Chim. Acta 2016, 455, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Chomarat, P.; Vannier, E.; Dechanet, J.; Rissoan, M.C.; Banchereau, J.; Dinarello, C.A.; Miossec, P. Balance of IL-1 receptor antagonist/IL-1 beta in rheumatoid synovium and its regulation by IL-4 and IL-10. J. Immunol. 1995, 154, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Finnegan, A.; Grusby, M.J.; Kaplan, C.D.; O’Neill, S.K.; Eibel, H.; Koreny, T.; Zhang, J. IL-4 and IL-12 regulate proteoglycan-induced arthritis through Stat-dependent mechanisms. J. Immunol. 2002, 169, 3345–3352. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and biological attributes of matrix metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar] [PubMed]
- Sun, S.; Bay-Jensen, A.C.; Karsdal, M.A.; Siebuhr, A.S.; Zheng, Q.; Maksymowych, W.P.; Henriksen, K. The active form of MMP-3 is a marker of synovial inflammation and cartilage turnover in inflammatory joint diseases. BMC Musculoskelet. Disord. 2014, 15, 93. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, F.F.; Smookler, D.S.; Khokha, R. Metalloproteinases, inflammation, and rheumatoid arthritis. Ann. Rheum. Dis. 2003, 62, 43–47. [Google Scholar] [CrossRef]
- Ciregia, F.; Deroyer, C.; Cobraiville, G.; Plener, Z.; Malaise, O.; Gillet, P.; de Seny, D. Modulation of αVβ6 integrin in osteoarthritis-related synovitis and the interaction with VTN (381–397 aa) competing for TGF-β1 activation. Exp. Mol. Med. 2021, 53, 210–222. [Google Scholar] [CrossRef]
- Budi, E.H.; Schaub, J.R.; Decaris, M.; Turner, S.; Derynck, R. TGF-β as a driver of fibrosis: Physiological roles and therapeutic opportunities. J. Pathol. 2021, 254, 358–373. [Google Scholar] [CrossRef]
- Bira, Y.; Tani, K.; Nishioka, Y.; Miyata, J.; Sato, K.; Hayashi, A.; Sone, S. Transforming growth factor β stimulates rheumatoid synovial fibroblasts via the type II receptor. Mod. Rheumatol. 2005, 15, 108–113. [Google Scholar] [CrossRef] [PubMed]
- KATİCA, M.; TEPEKOY, F. The effect of Calcitriol 1, 25 (OH) 2-D3 on osteoblast-like cell proliferation during in vitro cultivation. Vet. J. Mehmet Akif Ersoy Univ. 2020, 5, 11–17. [Google Scholar]
- Keeting, P.E.; Oursler, M.J.; Wiegand, K.E.; Bonde, S.K.; Spelsberg, T.C.; Riggs, B.L. Zeolite a increases proliferation, differentiation, and transforming growth factor β production in normal adult human osteoblast-like cells in vitro. J. Bone Miner. Res. 1992, 7, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Szeremeta, A.; Jura-Półtorak, A.; Zoń-Giebel, A.; Kopeć-Mędrek, M.; Kucharz, E.J.; Olczyk, K. Aggrecan turnover in women with rheumatoid arthritis treated with TNF-α inhibitors. J. Clin. Med. 2020, 9, 1377. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Lin, J.; Zhao, S.; Wu, J.; Jin, Y.; Yu, L.; Lin, M. ADAMTS5 in Osteoarthritis: Biological Functions, Regulatory Network, and Potential Targeting Therapies. Front. Mol. Biosci. 2021, 8, 703110. [Google Scholar] [CrossRef] [PubMed]
- Bailey, R.L.; van Wijngaarden, J.P. The role of B-vitamins in bone health and disease in older adults. Curr. Osteoporos. Rep. 2015, 13, 256–261. [Google Scholar] [CrossRef]
- Al Mutairi, F. Hyperhomocysteinemia: Clinical insights. J. Cent. Nerv. Syst. Dis. 2020, 12, 2230. [Google Scholar] [CrossRef]
- Chen, X.; Andresen, B.T.; Hill, M.; Zhang, J.; Booth, F.; Zhang, C. Role of reactive oxygen species in tumor necrosis factor-alpha induced endothelial dysfunction. Curr. Hypertens. Rev. 2008, 4, 245–255. [Google Scholar] [CrossRef]
- Holley, A.; Cheeseman, K. Measuring free radical reactions in vivo. Br. Med. Bull. 1993, 49, 494–505. [Google Scholar] [CrossRef]
- Ali, E.A.B.; Barakat, M.; Hassan, R. Antioxidant and angiostatic effect of Spirulina platensis suspension in complete Freund’s adjuvant-induced arthritis in rats. PLoS ONE 2015, 10, e0121523. [Google Scholar] [CrossRef]
- Ma, Q. Transcriptional responses to oxidative stress: Pathological and toxicological implications. Pharmacol. Ther. 2010, 125, 376–393. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T. Signal transduction by mitochondrial oxidants. J. Biol. Chem. 2012, 287, 4434–4440. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Bossy-Wetzel, E.; Schwarzenbacher, R.; Lipton, S.A. Molecular pathways to neurodegeneration. Nat. Med. 2004, 10, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Bayrak, B.B.; Yilmaz, S.; Hacihasanoglu Cakmak, N.; Yanardag, R. The effects of edaravone, a free-radical scavenger in lung injury induced by valproic acid demonstrated via different biochemical parameters. J. Biochem. Mol. Toxicol. 2021, 35, e22847. [Google Scholar] [CrossRef]
- Choy, E.H.; Panayi, G.S. Cytokine pathways and joint inflammation in rheumatoid arthritis. N. Engl. J. Med. 2001, 344, 907–916. [Google Scholar] [CrossRef]
- Kumar, V.; Bhatt, P.C.; Sharma, K.; Rahman, M.; Patel, D.K.; Sethi, N.; Verma, A. Melastoma malabathricum Linn attenuates complete freund’s adjuvant-induced chronic inflammation in Wistar rats via inflammation response. BMC Complement. Altern. Med. 2016, 16, 516. [Google Scholar] [CrossRef]
- Ahmed, R.H.; Galaly, S.R.; Moustafa, N.; Ahmed, R.R.; Ali, T.M.; Elesawy, B.H.; Abdul-Hamid, M. Curcumin and mesenchymal stem cells ameliorate ankle, testis, and ovary deleterious histological changes in arthritic rats via suppression of oxidative stress and inflammation. Stem Cells Int. 2021, 2021, 3516834. [Google Scholar] [CrossRef]



| Genes. | GenBank Accession Number | Sequence (5′–3′) |
|---|---|---|
| TGF-β | XM_032894155.1 | F: GACTCTCCACCTGCAAGACC R: GGACTGGCGAGCCTTAGTTT |
| GAPDH | XM_017592435.1 | F: CACCCTGTTGCTGTAGCCATATTC R: GACATCAAGAAGGTGGTGAAGCAG |
| Cavity | Synovial Lining | Inflammatory Infiltrate | Blood Vessels | Pannus | Articular Cartilage | Menisci | |
| Normal | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Arthritic | 0 | ++ | ++ | 0 | ++ | ++ | ++ |
| Arthritic + Nano ZT/Vit B12 | 0 | 0 | + | 0 | + | 0 | 0 |
| Cavity: | |||||||
| 0: Average +: Narrow ++: Very narrow | |||||||
| Synovial lining: | |||||||
| 0: Average/intact +: Thickened/hyperplastic ++: Necrotic/ulcerated | |||||||
| Inflammatory infiltrate: | |||||||
| 0: No +: Scattered/mild ++: Moderate/marked/with excess fibroblasts | |||||||
| Blood vessels: | |||||||
| 0: Average +: Mildly dilated/congested ++: Markedly dilated/congested | |||||||
| Pannus: | |||||||
| 0: No +: Small/large non-destructing ++: Large destructing/with fibrous bands | |||||||
| Articular cartilage: | |||||||
| 0: Average +: Mildly destructed/thickened ++: Markedly destructed | |||||||
| Menisci: | |||||||
| 0: Average +: Mildly destructed ++: Markedly destructed | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belal, A.; Mahmoud, R.; Taha, M.; Halfaya, F.M.; Hassaballa, A.; Elbanna, E.S.; Khaled, E.; Farghali, A.; Abo El-Ela, F.I.; Mahgoub, S.M.; et al. Therapeutic Potential of Zeolites/Vitamin B12 Nanocomposite on Complete Freund’s Adjuvant-Induced Arthritis as a Bone Disorder: In Vivo Study and Bio-Molecular Investigations. Pharmaceuticals 2023, 16, 285. https://doi.org/10.3390/ph16020285
Belal A, Mahmoud R, Taha M, Halfaya FM, Hassaballa A, Elbanna ES, Khaled E, Farghali A, Abo El-Ela FI, Mahgoub SM, et al. Therapeutic Potential of Zeolites/Vitamin B12 Nanocomposite on Complete Freund’s Adjuvant-Induced Arthritis as a Bone Disorder: In Vivo Study and Bio-Molecular Investigations. Pharmaceuticals. 2023; 16(2):285. https://doi.org/10.3390/ph16020285
Chicago/Turabian StyleBelal, Amany, Rehab Mahmoud, Mohamed Taha, Fatma Mohamed Halfaya, Ahmed Hassaballa, Esraa Salah Elbanna, Esraa Khaled, Ahmed Farghali, Fatma I. Abo El-Ela, Samar M. Mahgoub, and et al. 2023. "Therapeutic Potential of Zeolites/Vitamin B12 Nanocomposite on Complete Freund’s Adjuvant-Induced Arthritis as a Bone Disorder: In Vivo Study and Bio-Molecular Investigations" Pharmaceuticals 16, no. 2: 285. https://doi.org/10.3390/ph16020285
APA StyleBelal, A., Mahmoud, R., Taha, M., Halfaya, F. M., Hassaballa, A., Elbanna, E. S., Khaled, E., Farghali, A., Abo El-Ela, F. I., Mahgoub, S. M., Ghoneim, M. M., & Zaky, M. Y. (2023). Therapeutic Potential of Zeolites/Vitamin B12 Nanocomposite on Complete Freund’s Adjuvant-Induced Arthritis as a Bone Disorder: In Vivo Study and Bio-Molecular Investigations. Pharmaceuticals, 16(2), 285. https://doi.org/10.3390/ph16020285





