Effects of Side Chain and Peptide Bond Modifications on the Targeting Properties of Stabilized Minigastrin Analogs
Abstract
1. Introduction
2. Results
2.1. Peptide Synthesis and Radiolabeling
2.2. In Vitro Characterization
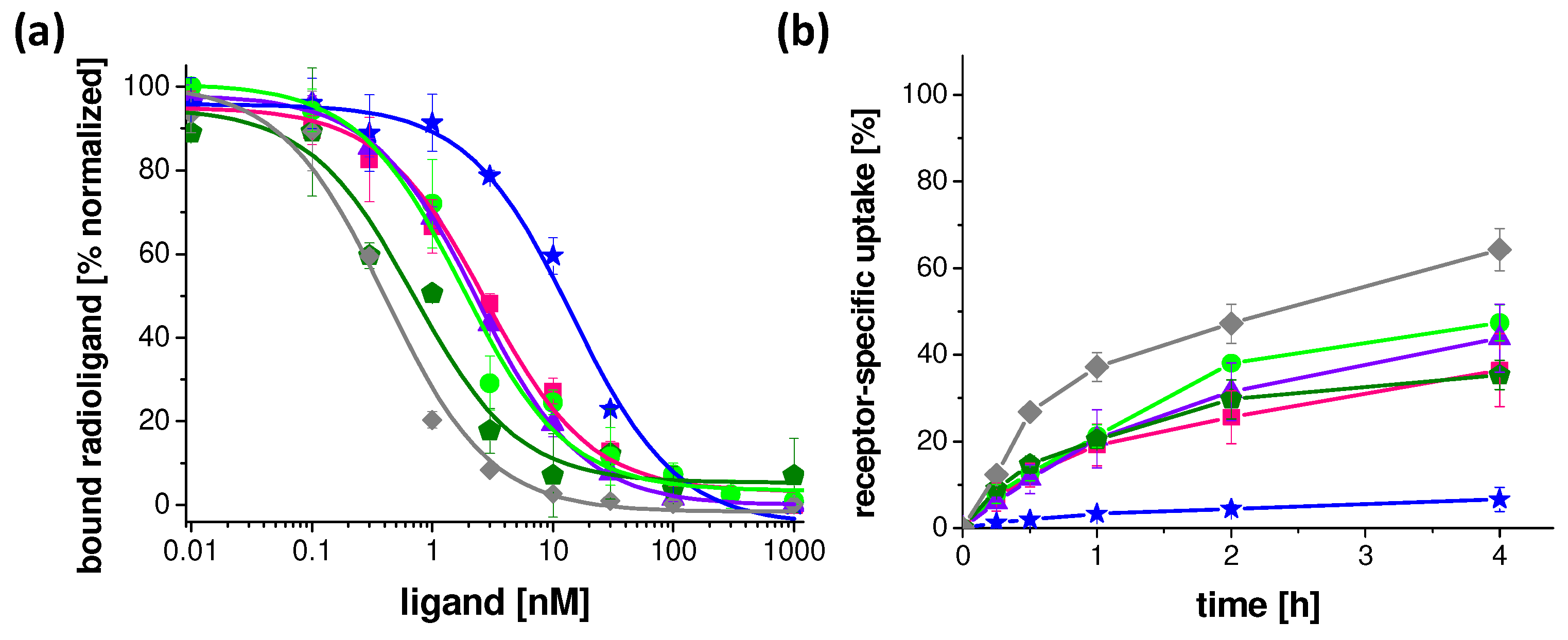
2.3. Receptor Affinity and Cell Internalization Studies
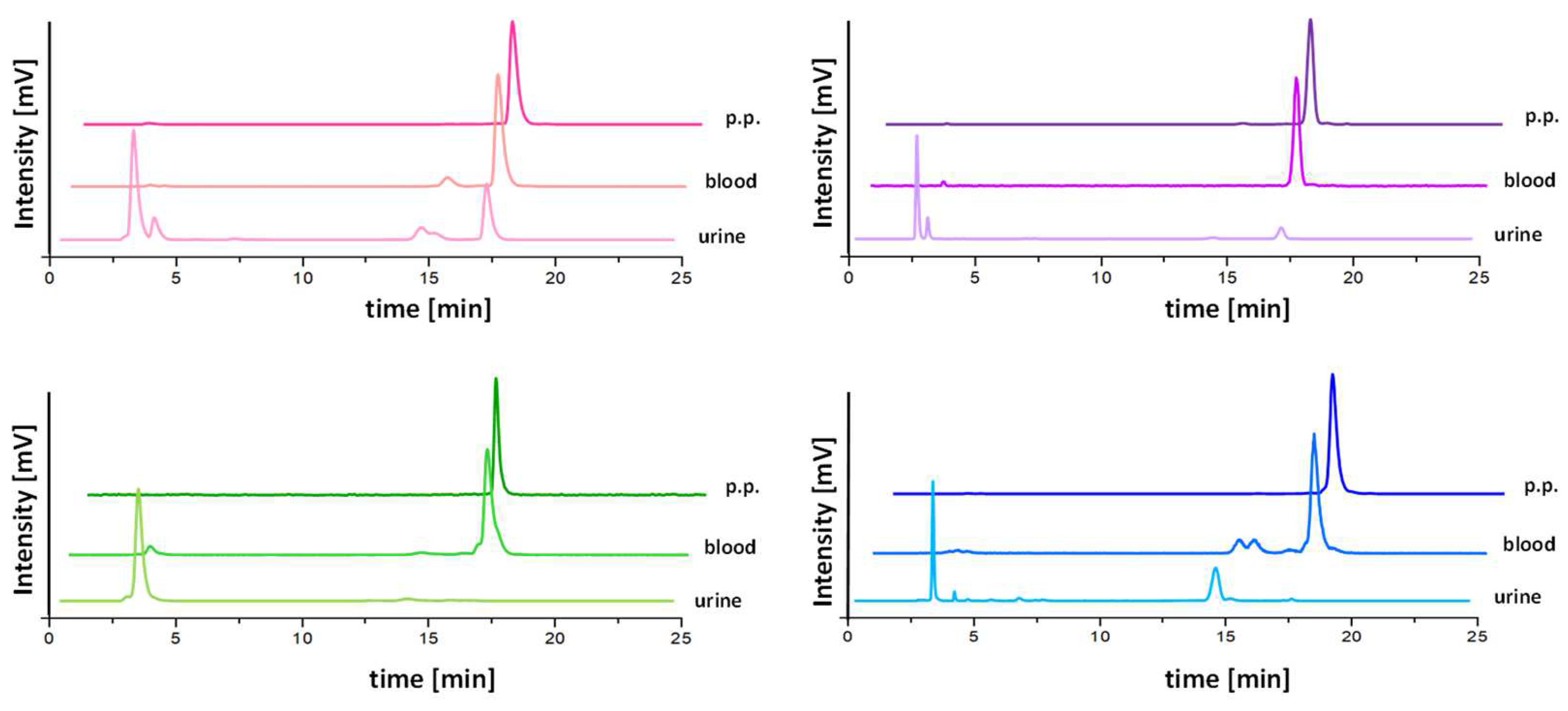
2.4. Metabolic Studies in BALB/c Mice
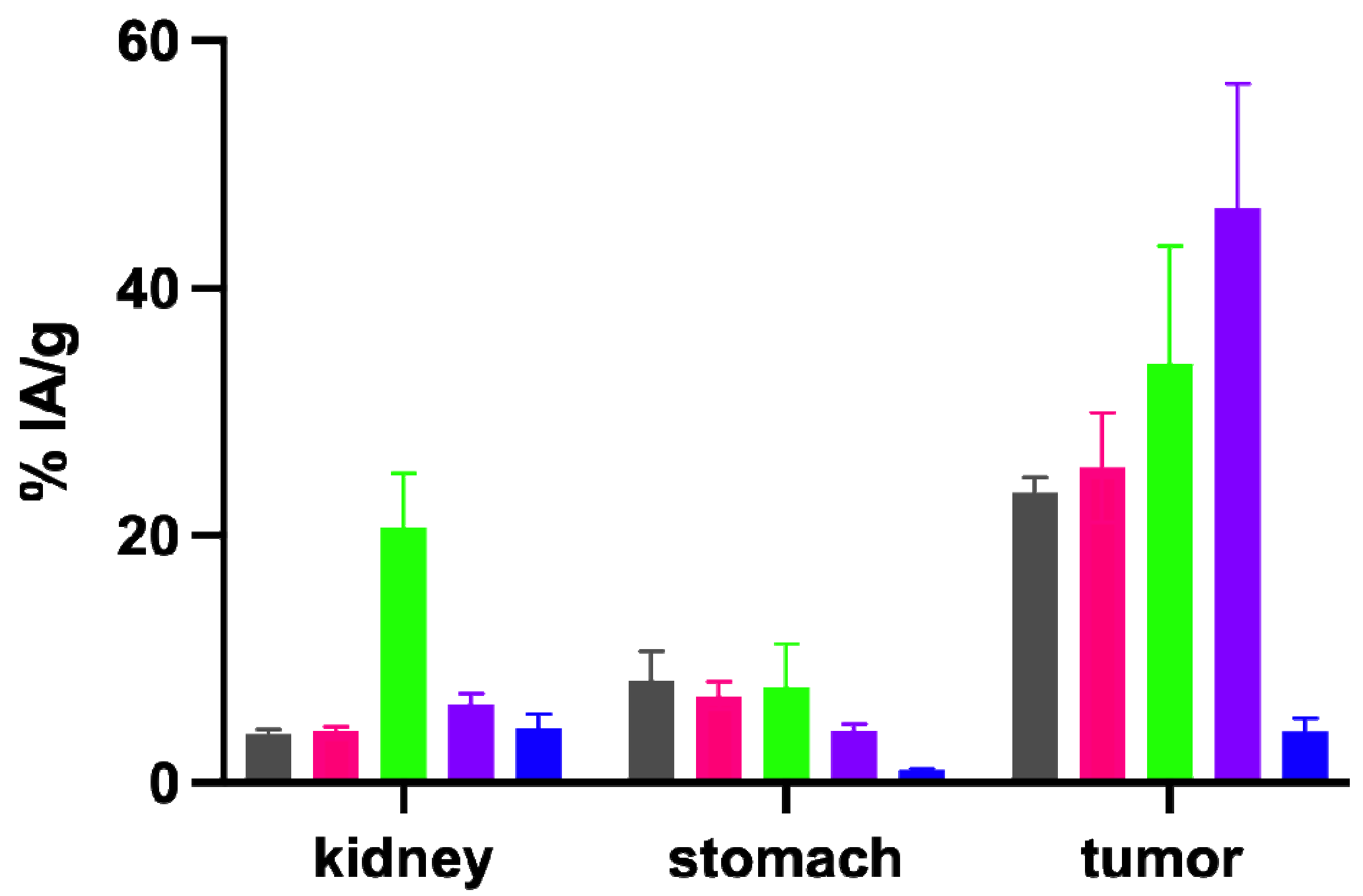
2.5. Biodistribution Studies in BALB/c Nude Mice Xenografted with A431-CCK2R and A431-Mock Cells
3. Discussion
4. Materials and Methods
4.1. Materials and Analytics
4.2. Peptide Synthesis and Radiolabeling
4.3. In Vitro Characterization
4.4. Receptor Affinity and Cell Internalization Studies
4.5. Metabolic Studies in BALB/c Mice
4.6. Biodistribution Studies in BALB/c Nude Mice Bearing A431-CCK2R/A431-Mock Xenografts
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fani, M.; Maecke, H.R. Radiopharmaceutical development of radiolabelled peptides. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 11–30. [Google Scholar] [CrossRef]
- Reubi, J.C.; Schaer, J.-C.; Waser, B. Cholecystokinin (CCK)-A and CCK-B/gastrin receptors in human tumors. Cancer Res. 1997, 57, 1377–1386. [Google Scholar]
- Reubi, J.C.; Waser, B. Unexpected high incidence of cholecystokinin-B/gastrin receptors in human medullary thyroid carcinomas. Int. J. Cancer 1996, 67, 644–647. [Google Scholar] [CrossRef]
- Bartz-Kurycki, M.A.; Oluwo, O.E.; Morris-Wiseman, L.F. Medullary thyroid carcinoma: Recent advances in identification, treatment, and prognosis. Ther. Adv. Endocrinol. Metab. 2021, 12, 20420188211049611. [Google Scholar] [CrossRef]
- Jaber, T.; Dadu, R.; Hu, M.I. Medullary thyroid carcinoma. Curr. Opin. Endocrinol. Diabetes Obes. 2021, 28, 540–546. [Google Scholar]
- Kiesewetter, B.; Riss, P.; Scheuba, C.; Raderer, M. How I treat medullary thyroid cancer. ESMO Open 2021, 6, 100183. [Google Scholar] [CrossRef] [PubMed]
- Raue, F.; Frank-Raue, K. Long-Term Follow-up in Medullary Thyroid Carcinoma. Recent Results Cancer Res. 2015, 204, 207–225. [Google Scholar]
- Eychenne, R.; Bouvry, C.; Bourgeois, M.; Loyer, P.; Benoist, E.; Lepareur, N. Overview of Radiolabeled Somatostatin Analogs for Cancer Imaging and Therapy. Molecules 2020, 25, 4012. [Google Scholar] [CrossRef]
- Roosenburg, S.; Laverman, P.; van Delft, F.L.; Boerman, O.C. Radiolabeled CCK/gastrin peptides for imaging and therapy of CCK2 receptor-expressing tumors. Amino Acids 2011, 41, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Behr, T.M.; Jenner, N.; Béhé, M.; Angerstein, C.; Gratz, S.; Raue, F.; Becker, W. Radiolabeled peptides for targeting cholecystokinin-B/gastrin receptor-expressing tumors. J. Nucl. Med. 1999, 40, 1029–1044. [Google Scholar]
- Klingler, M.; Hörmann, A.A.; von Guggenberg, E. Cholecystokinin-2 receptor targeting with radiolabeled peptides: Current status and future directions. Curr. Med. Chem. 2020, 27, 7112–7132. [Google Scholar] [CrossRef] [PubMed]
- Behr, T.M.; Jenner, N.; Radetzky, S.; Béhe, M.; Gratz, S.; Yücekent, S.; Raue, F.; Becker, W. Targeting of cholecystokinin-B/gastrin receptors in vivo: Preclinical and initial clinical evaluation of the diagnostic and therapeutic potential of radiolabelled gastrin. Eur. J. Nucl. Med. 1998, 25, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Fröberg, A.C.; de Jong, M.; Nock, B.A.; Breeman, W.A.; Erion, J.L.; Maina, T.; Verdijsseldonck, M.; de Herder, W.W.; van der Lugt, A.; Kooij, P.P. Comparison of three radiolabelled peptide analogues for CCK-2 receptor scintigraphy in medullary thyroid carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1265–1272. [Google Scholar] [CrossRef]
- Vegt, E.; de Jong, M.; Wetzels, J.F.; Masereeuw, R.; Melis, M.; Oyen, W.J.; Gotthardt, M.; Boerman, O.C. Renal toxicity of radiolabeled peptides and antibody fragments: Mechanisms, impact on radionuclide therapy, and strategies for prevention. J. Nucl. Med. 2010, 51, 1049–1058. [Google Scholar] [CrossRef]
- Behe, M.; Kluge, G.; Becker, W.; Gotthardt, M.; Behr, T.M. Use of polyglutamic acids to reduce uptake of radiometal-labeled minigastrin in the kidneys. J. Nucl. Med. 2005, 46, 1012–1015. [Google Scholar]
- Hubalewska-Dydejczyk, A.; Mikolajczak, R.; Decristoforo, C.; Kolenc-Peitl, P.; Erba, P.; Zaletel, K.; Maecke, H.; Maina, T.; Konijnenberg, M.; Garnuszek, P. Phase I clinical trial using a novel CCK 2 receptor-localizing radiolabelled peptide probe for personalized diagnosis and therapy of patients with progressive or metastatic medullary thyroid carcinoma-final results. Eur. J. Nucl. Med. Mol. 2019. [Google Scholar]
- Pawlak, D.; Rangger, C.; Peitl, P.K.; Garnuszek, P.; Maurin, M.; Ihli, L.; Kroselj, M.; Maina, T.; Maecke, H.; Erba, P. From preclinical development to clinical application: Kit formulation for radiolabelling the minigastrin analogue CP04 with In-111 for a first-in-human clinical trial. Eur. J. Pharm. Sci. 2016, 85, 1–9. [Google Scholar] [CrossRef]
- Rottenburger, C.; Nicolas, G.P.; McDougall, L.; Kaul, F.; Cachovan, M.; Vija, A.H.; Schibli, R.; Geistlich, S.; Schumann, A.; Rau, T. Cholecystokinin 2 receptor agonist 177Lu-PP-F11N for radionuclide therapy of medullary thyroid carcinoma: Results of the lumed phase 0a study. J. Nucl. Med. 2020, 61, 520–526. [Google Scholar] [CrossRef]
- Klingler, M.; Summer, D.; Rangger, C.; Haubner, R.; Foster, J.; Sosabowski, J.; Decristoforo, C.; Virgolini, I.; von Guggenberg, E. DOTA-MGS5, a new cholecystokinin-2 receptor-targeting peptide analog with an optimized targeting profile for theranostic use. J. Nucl. Med. 2019, 60, 1010–1016. [Google Scholar] [CrossRef]
- Hörmann, A.A.; Klingler, M.; Rangger, C.; Mair, C.; Decristoforo, C.; Uprimny, C.; Virgolini, I.J.; von Guggenberg, E. Radiopharmaceutical formulation and preclinical testing of 68Ga-labeled DOTA-MGS5 for the regulatory approval of a first exploratory clinical trial. Pharmaceuticals 2021, 14, 575. [Google Scholar] [CrossRef]
- Klingler, M.; Decristoforo, C.; Rangger, C.; Summer, D.; Foster, J.; Sosabowski, J.K.; von Guggenberg, E. Site-specific stabilization of minigastrin analogs against enzymatic degradation for enhanced cholecystokinin-2 receptor targeting. Theranostics 2018, 8, 2896. [Google Scholar] [CrossRef] [PubMed]
- Klingler, M.; Hörmann, A.A.; Rangger, C.; Desrues, L.; Castel, H.l.n.; Gandolfo, P.; von Guggenberg, E. Stabilization strategies for linear minigastrin analogues: Further improvements via the inclusion of proline into the peptide sequence. J. Med. Chem. 2020, 63, 14668–14679. [Google Scholar] [CrossRef] [PubMed]
- Hörmann, A.A.; Klingler, M.; Rezaeianpour, M.; Hörmann, N.; Gust, R.; Shahhosseini, S.; Guggenberg, E.V. Initial in vitro and in vivo evaluation of a novel CCK2R targeting peptide analog labeled with lutetium-177. Molecules 2020, 25, 4585. [Google Scholar] [CrossRef] [PubMed]
- Hörmann, A.A.; Klingler, M.; Rangger, C.; Mair, C.; Joosten, L.; Franssen, G.M.; Laverman, P.; von Guggenberg, E. Effect of Peptide Length on in Vitro and in Vivo Properties of 177Lu-labeled Peptide Analogs Targeting CCK2R. Preprints 2022, 2022090198. [Google Scholar]
- Behe, M.; Behr, T.M. Cholecystokinin—B (CCK-B)/gastrin receptor targeting peptides for staging and therapy of medullary thyroid cancer and other CCK-B receptor expressing malignancies. J. Pept. Sci. 2002, 66, 399–418. [Google Scholar]
- Ocak, M.; Helbok, A.; Rangger, C.; Kolenc-Peitl, P.; Nock, B.A.; Morelli, G.; Eek, A.; Sosabowski, J.K.; Breeman, W.A.; Reubi, J.C. Comparison of biological stability and metabolism of CCK2 receptor targeting peptides, a collaborative project under COST BM0607. Eur. J. Pharm. Sci. 2011, 38, 1426–1435. [Google Scholar] [CrossRef]
- Klingler, M.; Rangger, C.; Summer, D.; Kaeopookum, P.; Decristoforo, C.; von Guggenberg, E. Cholecystokinin-2 receptor targeting with novel c-terminally stabilized HYNIC-Minigastrin analogs radiolabeled with technetium-99m. Pharmaceuticals 2019, 12, 13. [Google Scholar] [CrossRef]
- Kaloudi, A.; Nock, B.A.; Lymperis, E.; Krenning, E.P.; de Jong, M.; Maina, T. Improving the in vivo profile of minigastrin radiotracers: A comparative study involving the neutral endopeptidase inhibitor phosphoramidon. Cancer Biother. Radiopharm. 2016, 31, 20–28. [Google Scholar] [CrossRef]
- Sauter, A.W.; Mansi, R.; Hassiepen, U.; Muller, L.; Panigada, T.; Wiehr, S.; Wild, A.-M.; Geistlich, S.; Béhé, M.; Rottenburger, C. Targeting of the cholecystokinin-2 receptor with the minigastrin analog 177Lu-DOTA-PP-F11N: Does the use of protease inhibitors further improve in vivo distribution? J. Nucl. Med. 2019, 60, 393–399. [Google Scholar] [CrossRef]
- Laverman, P.; Joosten, L.; Eek, A.; Roosenburg, S.; Kolenc-Peitl, P.; Maina, T.; Mäcke, H.; Aloj, L.; von Guggenberg, E.; Sosabowski, J.K. Comparative biodistribution of 12 111In-labelled gastrin/CCK2 receptor-targeting peptides. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1410–1416. [Google Scholar] [CrossRef]
- Maina, T.; Konijnenberg, M.W.; Kolenc-Peitl, P.; Garnuszek, P.; Nock, B.A.; Kaloudi, A.; Kroselj, M.; Zaletel, K.; Maecke, H.; Mansi, R.; et al. Preclinical pharmacokinetics, biodistribution, radiation dosimetry and toxicity studies required for regulatory approval of a phase I clinical trial with (111)In-CP04 in medullary thyroid carcinoma patients. Eur. J. Pharm. Sci. 2016, 91, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, J.; Gilon, C.; Hoffman, A.; Kessler, H. N-methylation of peptides: A new perspective in medicinal chemistry. Acc. Chem. Res. 2008, 41, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Aloj, L.; Caracò, C.; Panico, M.; Zannetti, A.; Del Vecchio, S.; Tesauro, D.; De Luca, S.; Arra, C.; Pedone, C.; Morelli, G.; et al. In vitro and in vivo evaluation of 111In-DTPAGlu-G-CCK8 for cholecystokinin-B receptor imaging. J. Nucl. Med. 2004, 45, 485–494. [Google Scholar] [PubMed]
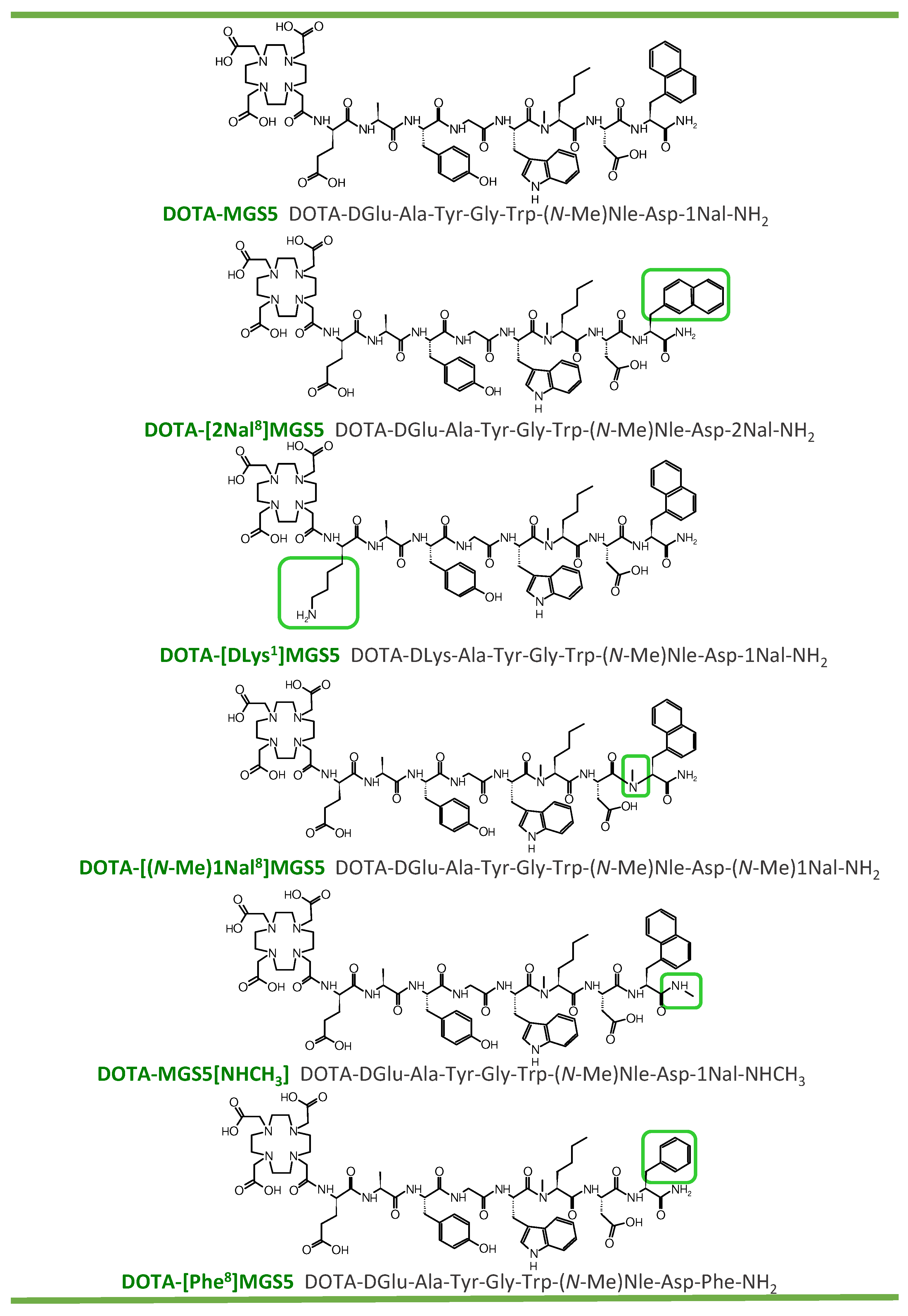

| Compound | Calculated Mass (m/z [M+H]+) | Found Mass (m/z [M+H]+) | Binding to Serum Proteins (%) * n = 2 | LogD n = 8 | Intact Radiopeptide in Human Serum (%) * n = 2 |
|---|---|---|---|---|---|
| DOTA-MGS5 | 1449.7 | 1449.3 | 44.3 ± 0.3 | −2.0 ± 0.1 | 97.2 ± 0.6 |
| DOTA-[2Nal8]MGS5 | 1449.7 | 1447.7 | 34.6 ± 0.3 | −1.7 ± 0.2 | 92.0 ± 1.2 |
| DOTA-[DLys1]MGS5 | 1448.7 | 1450.8 | 27.8 ± 0.4 | −1.3 ± 0.1 | 98.1 ± 1.1 |
| DOTA-[(N-Me)1Nal8]MGS5 | 1463.7 | 1463.7 | 39.8 ± 4.2 | −2.4 ± 0.3 | 99.6 ± 0.1 |
| DOTA-MGS5[NHCH3] | 1463.7 | 1463.8 | 40.9 ± 3.1 | −2.3 ± 0.2 | 98.1 ± 0.4 |
| DOTA-[Phe8]MGS5 | 1399.7 | 1399.6 | 20.2 ± 1.6 | −3.2 ± 0.1 | 54.0 ± 0.8 |
| Peptide | IC50 (nM) | EC50 (nM) |
|---|---|---|
| DOTA-MGS5 | 0.4 ± 0.2 [19] | 7.9 ± 1.9 |
| DOTA-[2Nal8]MGS5 | 1.7 ± 0.1 | 12.8 ± 2.4 |
| DOTA-[DLys1]MGS5 | 0.9 ± 0.8 | 43.5 ± 14.1 |
| DOTA-[(N-Me)1Nal8]MGS5 | 2.7 ± 0.3 | 63.4 ± 19.9 |
| DOTA-MGS5[NHCH3] | 13.2 ± 1.5 | 41.0 ± 14.7 |
| DOTA-[Phe8]MGS5 | 0.7 ± 0.1 | 6.3 ± 3.6 |
| [111In]In-DOTA-MGS5 | [111In]In-DOTA-[2Nal8]MGS5 | [111In]In-DOTA-[DLys1]MGS5 | [111In]In-DOTA- [(N-Me)1Nal8]MGS5 | [111In]In-DOTA-MGS5[NHCH3] | |
|---|---|---|---|---|---|
| blood | 82.7 ± 3.3 | 88.4 ± 0.4 | 93.9 ± 1.2 | 98.4 ± 0.1 | 71.7 ± 6.2 |
| liver | 87.8 ± 2.1 | 83.9 ± 0.1 | 82.5 ± 2.4 | 94.9 ± 2.8 | 78.1 ± 3.7 |
| kidney | 21.5 ± 1.7 | 24.7 ± 0.3 | 20.6 ± 3.8 | 40.2 ± 4.1 | 7.4 ± 8.6 |
| urine | 11.5 ± 2.5 | 28.2 ± 1.0 | 5.8 ± 0.2 | 15.9 ± 3.4 | 1.3 ± 0.2 |
| %IA/g | [111In]In-DOTA-MGS5 | [111In]In-DOTA-[2Nal8]MGS5 | [111In]In-DOTA-[DLys1]MGS5 | [111In]In-DOTA- [(N-Me)1Nal8]MGS5 | [111In]In-DOTA-MGS5[NHCH3] |
|---|---|---|---|---|---|
| blood | 0.05 ± 0.03 | 0.06 ± 0.003 | 0.20 ± 0.09 * | 0.19 ± 0.07 * | 0.08 ± 0.07 |
| lung | 0.09 ± 0.05 | 0.13 ± 0.03 | 0.30 ± 0.11 * | 0.21 ± 0.07 * | 0.07 ± 0.01 |
| heart | 0.05 ± 0.02 | 0.08 ± 0.01 * | 0.22 ± 0.06 * | 0.14 ± 0.03 * | 0.06 ± 0.02 |
| muscle | 0.26 ± 0.28 | 0.04 ± 0.01 | 0.13 ± 0.05 | 0.12 ± 0.05 | 0.04 ± 0.02 |
| spleen | 0.16 ± 0.04 | 0.18 ± 0.05 | 0.54 ± 0.12 * | 0.32 ± 0.05 * | 0.13 ± 0.02 |
| intestine | 1.15 ± 0.31 | 0.95 ± 0.26 | 1.96 ± 0.87 | 0.81 ± 0.10 | 0.59 ± 0.46 |
| liver | 0.46 ± 0.03 | 0.66 ± 0.07 * | 1.94 ± 0.52 * | 1.39 ± 0.16 * | 0.36 ± 0.11 |
| kidneys | 3.88 ± 0.45 | 4.21 ± 0.29 | 20.67 ± 4.37 * | 6.33 ± 0.89 * | 4.42 ± 1.15 |
| pancreas | 1.6. ± 0.50 | 1.84 ± 0.52 | 2.27 ± 1.20 | 1.37 ± 0.22 | 0.40 ± 0.04 * |
| stomach | 8.24 ± 2.40 | 7.03 ± 1.10 | 7.73 ± 3.49 | 4.20 ± 0.51 * | 0.96 ± 0.17 * |
| A431-mock xenograft | 0.3 ± 0.12 | 0.17 ± 0.04 | 0.56 ± 0.27 | 0.38 ± 0.08 | 0.19 ± 0.08 |
| A431-CCK2R xenograft | 23.49 ± 1.25 | 25.45 ± 4.45 | 33.94 ± 9.53 | 48.10 ± 9.15 * | 4.16 ± 1.01 * |
| tumor-to-organ ratio | |||||
| stomach | 3.08 ± 1.07 | 3.66 ± 0.64 | 4.69 ± 1.19 | 11.46 ± 1.82 * | 4.33 ± 0.56 |
| kidney | 6.11 ± 0.64 | 6.04 ± 0.87 | 1.62 ± 0.15 * | 7.55 ± 0.48 * | 0.96 ± 0.20 * |
| blood | 572 ± 243 | 398.77 ± 89.59 | 175.77 ± 31.88 * | 280.85 ± 88.51 | 69.87 ± 38.31 * |
| DOTA-[(N-Me)1Nal8]MGS5 Radiolabeled with | DOTA-MGS5 Radiolabeled with | |||||
|---|---|---|---|---|---|---|
| % IA/g | indium-111 | gallium-68 | lutetium-177 | indium-111 | gallium-68 | lutetium-177 |
| blood | 0.19 ± 0.07 * | 2.82 ± 0.88 | 0.13 ± 0.04 | 0.05 ± 0.03 | 1.47 ± 0.82 | 0.11 ± 0.11 |
| lung | 0.21 ± 0.07 * | 1.61 ± 0.42 | 0.20 ± 0.04 | 0.09 ± 0.05 | 1.35 ± 0.64 | 0.17 ± 0.14 |
| heart | 0.14 ± 0.03 * | 0.95 ± 0.32 | 0.15 ± 0.02 | 0.05 ± 0.02 | 0.82 ± 0.51 | 0.10 ± 0.06 |
| muscle | 0.12 ± 0.05 | 0.45 ± 0.08 | 0.15 ± 0.06 | 0.26 ± 0.28 | 0.22 ± 0.17 | 0.10 ± 0.02 |
| spleen | 0.32 ± 0.05 * | 1.19 ± 0.25 | 0.27 ± 0.04 | 0.16 ± 0.04 | 0.68 ± 0.38 | 0.27 ± 0.18 |
| intestine | 0.81 ± 0.10 | 0.82 ± 0.18 | 1.47 ± 0.26 | 1.15 ± 0.31 | 0.79 ± 0.35 | 1.02 ± 0.23 |
| liver | 1.39 ± 0.16 * | 2.25 ± 0.55 * | 1.05 ± 0.15 | 0.46 ± 0.03 | 1.02 ± 0.52 | 1.02 ± 0.80 |
| kidneys | 6.33 ± 0.89 * | 7.70 ± 3.10 | 4.69 ± 0.68 | 3.88 ± 0.45 | 5.71 ± 1.38 | 3.45 ± 0.91 |
| pancreas | 1.37 ± 0.22 | 0.87 ± 0.29 * | 0.60 ± 0.16 * | 1.6. ± 0.50 | 1.83 ± 0.42 | 1.91 ± 0.91 |
| stomach | 4.20 ± 0.51 * | 1.63 ± 0.41 * | 2.37 ± 0.33 | 8.24 ± 2.40 | 5.12 ± 1.13 | 6.26 ± 4.28 |
| A431-mock xenograft | 0.38 ± 0.08 | 1.51 ± 0.98 | 0.28 ± 0.04 * | 0.3 ± 0.12 | 0.78 ± 0.26 | 0.19 ± 0.03 |
| A431-CCK2R xenograft | 48.10 ± 9.15 * | 15.67 ± 2.21 * | 35.13 ± 6.32 * | 23.49 ± 1.25 | 23.25 ± 4.70 | 22.89 ± 4.67 |
| tumor-to-organ ratio | indium-111 | gallium-68 | lutetium-177 | indium-111 | gallium-68 | lutetium-177 |
| stomach | 11.5 ± 1.82 * | 10.2 ± 3.50 * | 14.9 ± 2.63 * | 3.08 ± 1.07 | 4.56 ± 0.80 | 4.76 ± 2.62 |
| kidney | 7.55 ± 0.48 * | 2.36 ± 1.14 * | 7.72 ± 2.26 | 6.11 ± 0.64 | 4.10 ± 0.26 | 6.96 ± 2.23 |
| blood | 281 ± 89 | 6.03 ± 2.26 * | 296 ± 141 | 572 ± 243 | 18.93 ± 7.51 | 463 ± 396 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zavvar, T.S.; Hörmann, A.A.; Klingler, M.; Summer, D.; Rangger, C.; Desrues, L.; Castel, H.; Gandolfo, P.; von Guggenberg, E. Effects of Side Chain and Peptide Bond Modifications on the Targeting Properties of Stabilized Minigastrin Analogs. Pharmaceuticals 2023, 16, 278. https://doi.org/10.3390/ph16020278
Zavvar TS, Hörmann AA, Klingler M, Summer D, Rangger C, Desrues L, Castel H, Gandolfo P, von Guggenberg E. Effects of Side Chain and Peptide Bond Modifications on the Targeting Properties of Stabilized Minigastrin Analogs. Pharmaceuticals. 2023; 16(2):278. https://doi.org/10.3390/ph16020278
Chicago/Turabian StyleZavvar, Taraneh Sadat, Anton Amadeus Hörmann, Maximilian Klingler, Dominik Summer, Christine Rangger, Laurence Desrues, Hélène Castel, Pierrick Gandolfo, and Elisabeth von Guggenberg. 2023. "Effects of Side Chain and Peptide Bond Modifications on the Targeting Properties of Stabilized Minigastrin Analogs" Pharmaceuticals 16, no. 2: 278. https://doi.org/10.3390/ph16020278
APA StyleZavvar, T. S., Hörmann, A. A., Klingler, M., Summer, D., Rangger, C., Desrues, L., Castel, H., Gandolfo, P., & von Guggenberg, E. (2023). Effects of Side Chain and Peptide Bond Modifications on the Targeting Properties of Stabilized Minigastrin Analogs. Pharmaceuticals, 16(2), 278. https://doi.org/10.3390/ph16020278







