Phytofabrication and Characterisation of Zinc Oxide Nanoparticles Using Pure Curcumin
Abstract
1. Introduction
2. Results and Discussion
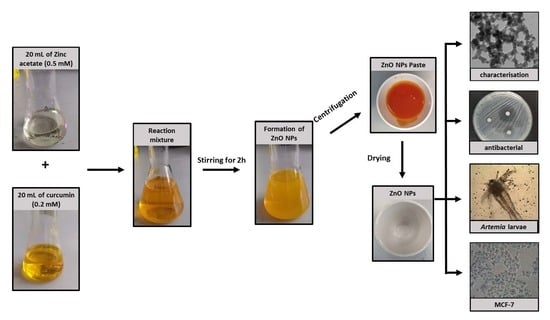
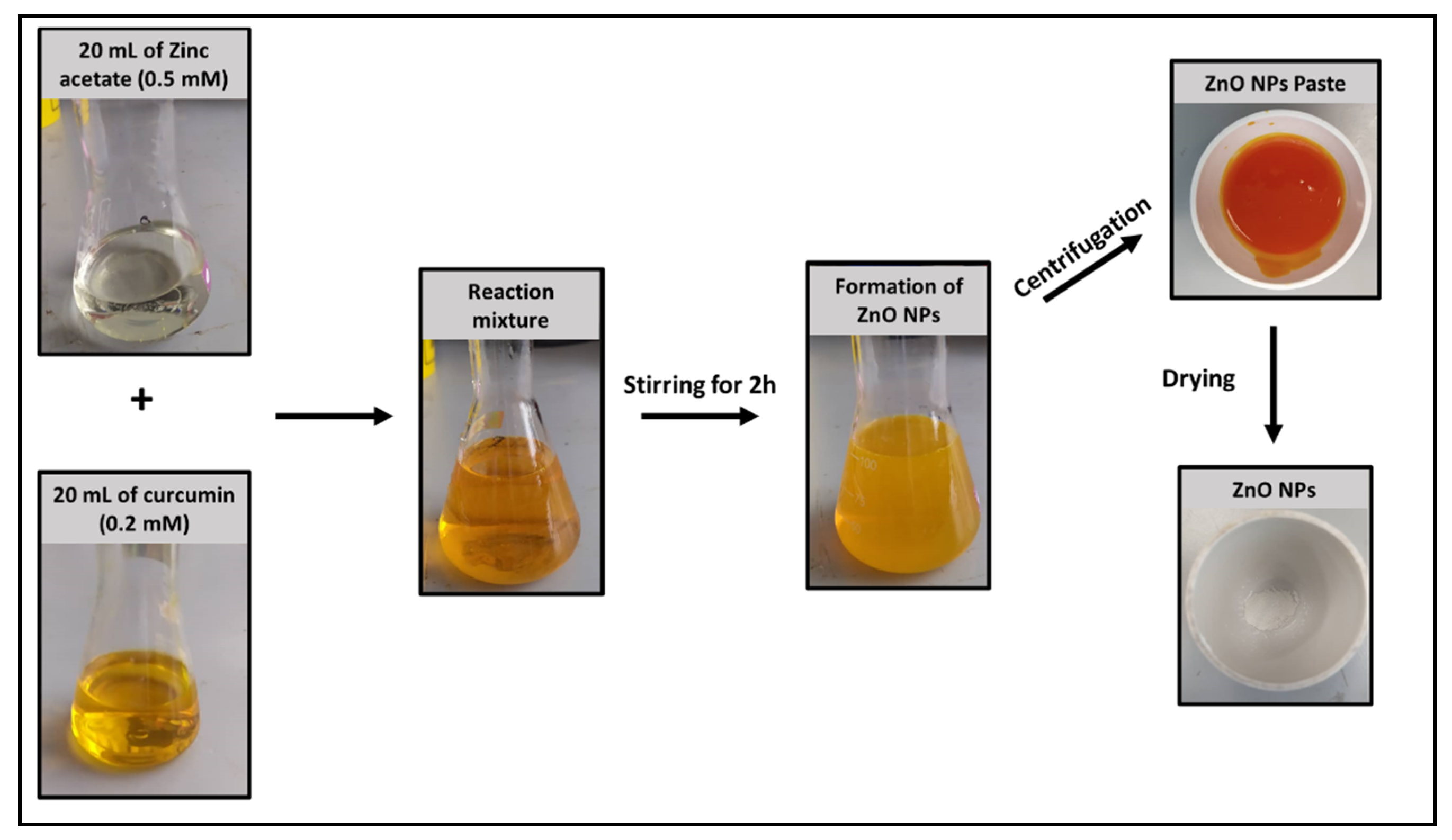
2.1. Synthesis of Green-ZnO-NPs
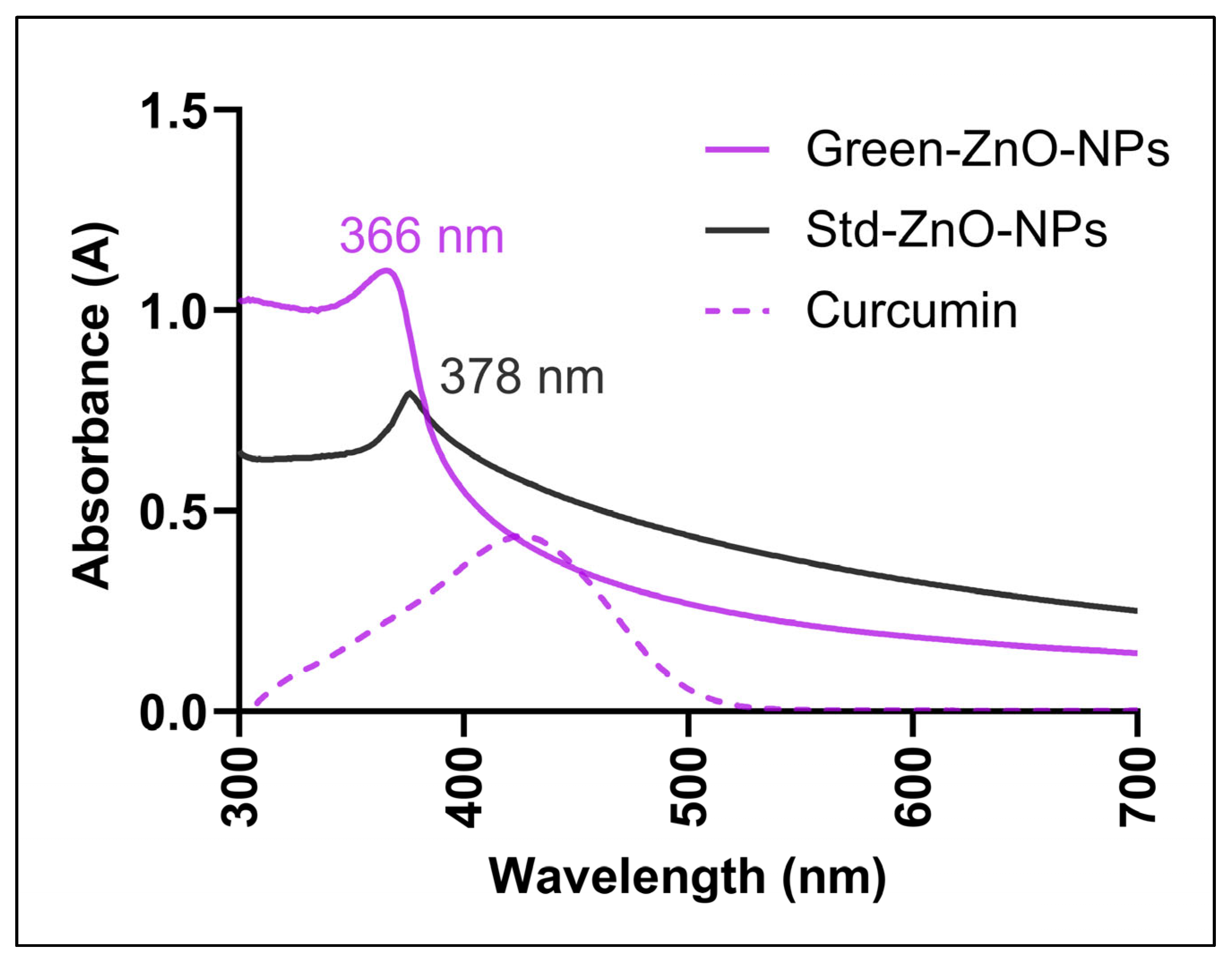
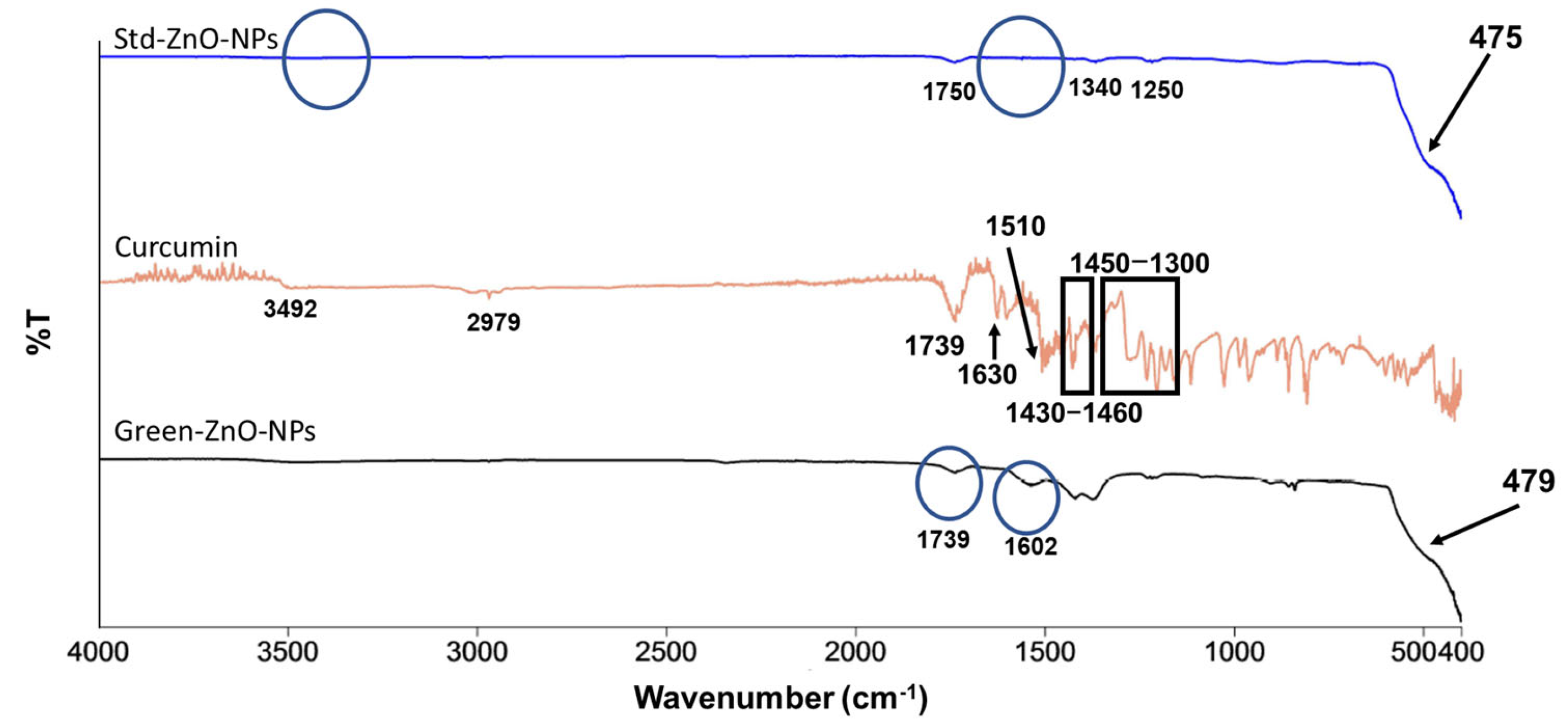
2.2. Characterisation of Green-ZnO-NPs
2.2.1. Ultraviolet–Visible (UV–Vis)
2.2.2. Attenuated Total Reflectance-Fourier-Transform Infrared (ATR-FTIR) Analysis
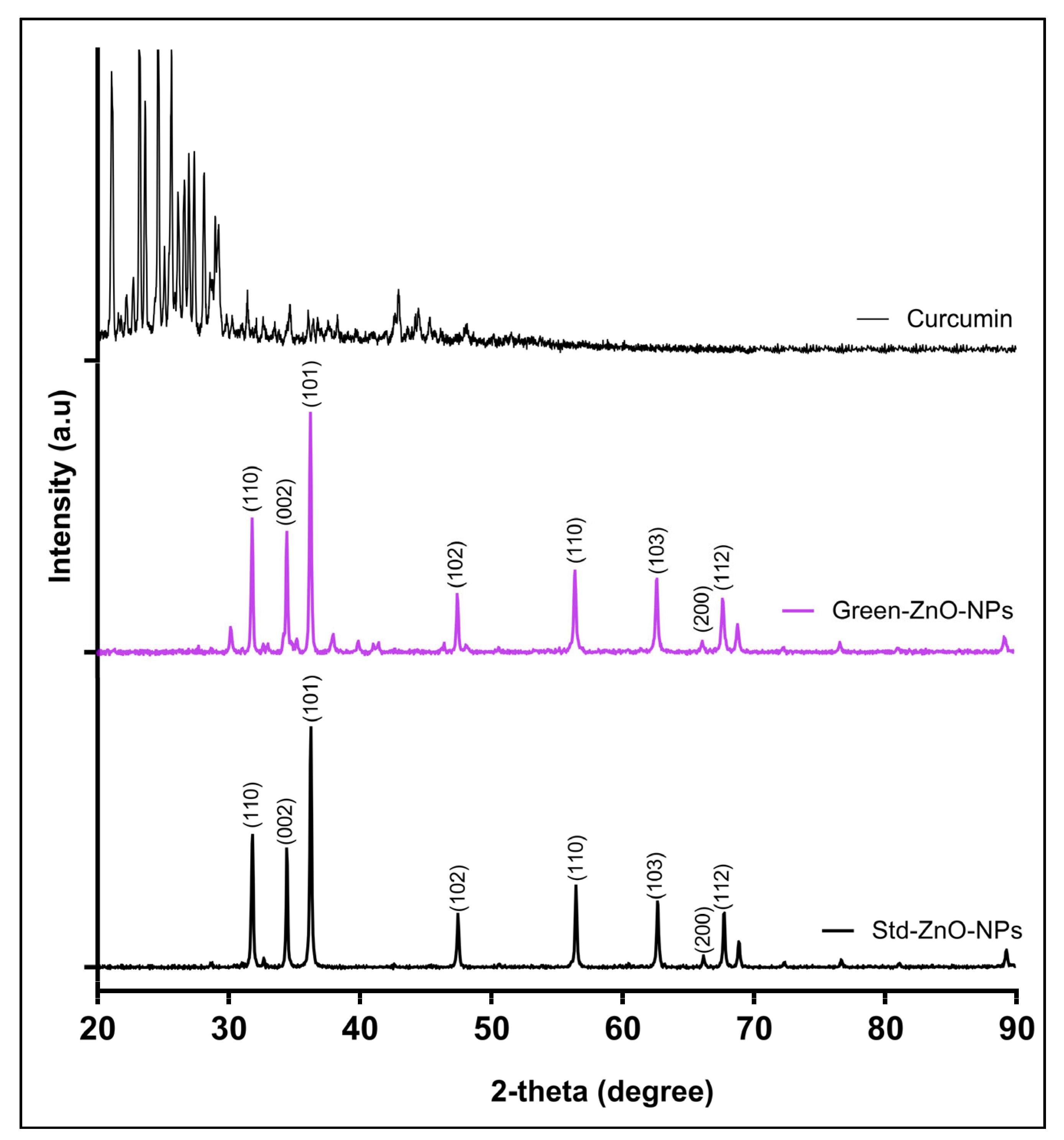
2.2.3. X-ray Powder Diffraction (XRD)
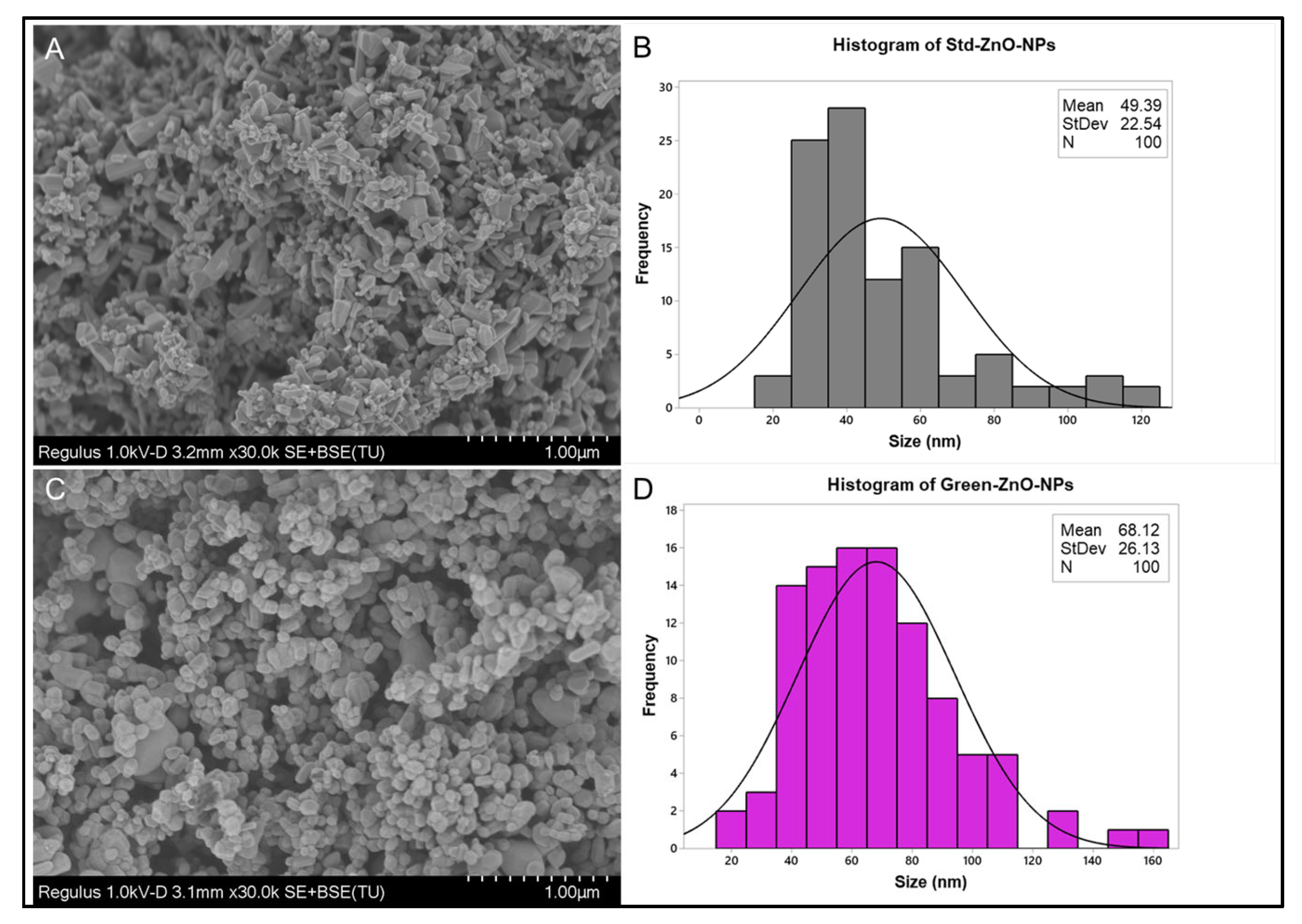
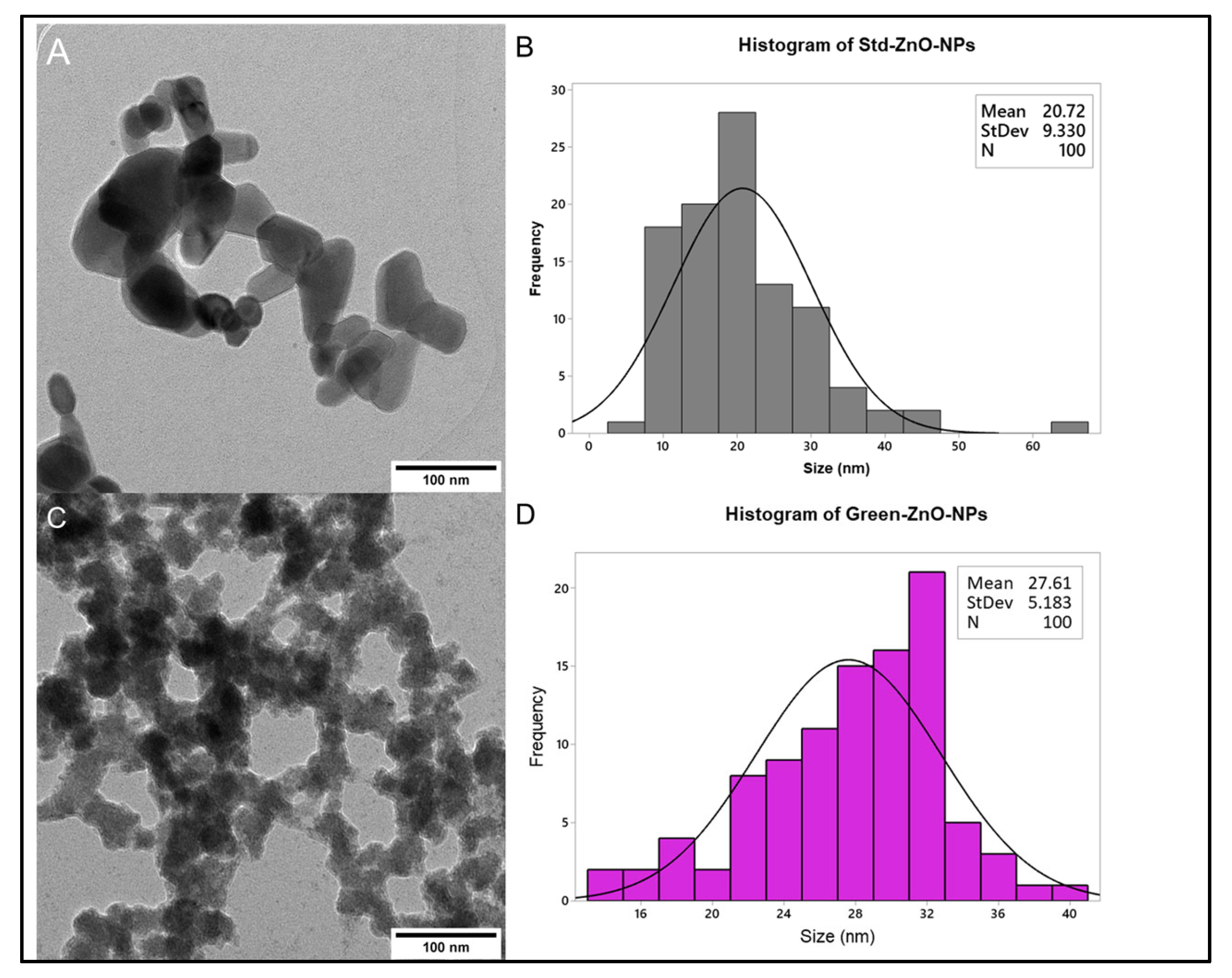
2.2.4. Surface Morphology Analysis
2.2.5. Particle Size and Zeta Potential
2.3. Antioxidant Activity
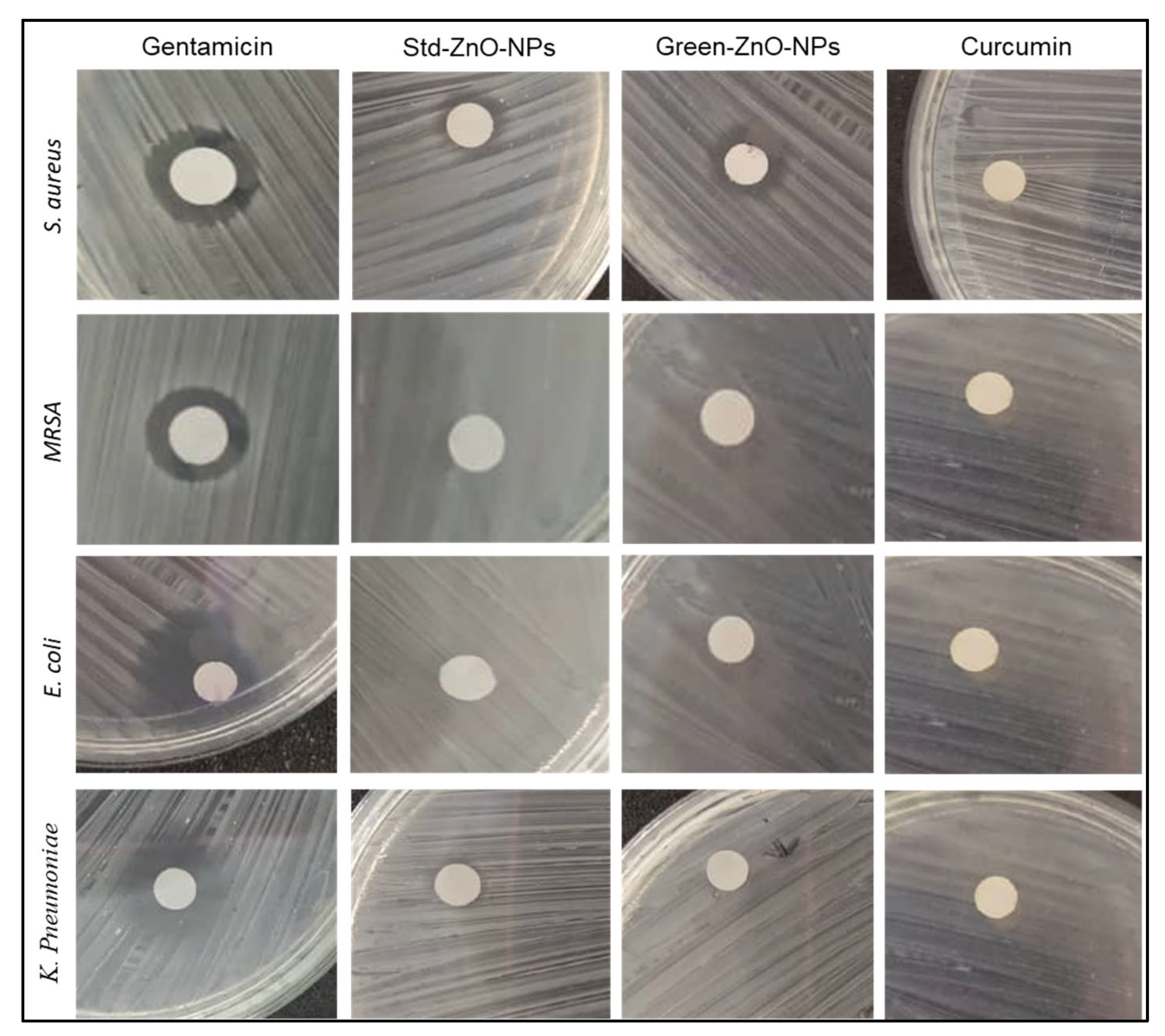
2.4. Antimicrobial Activity
2.5. Anticancer Activity
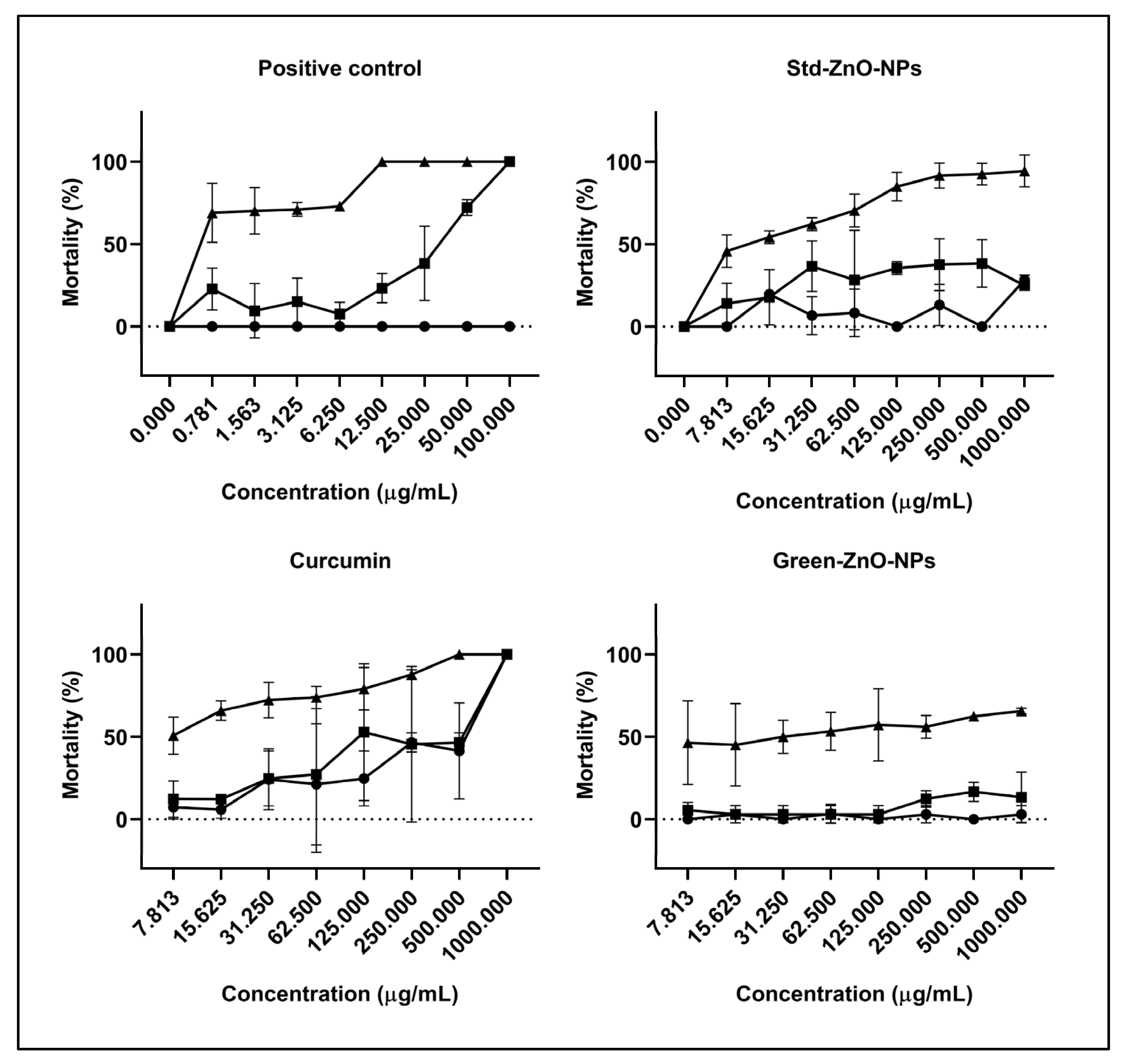
2.6. Artemia Larvae Lethality Bioassay
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Green-ZnO-NPs
3.3. Characterisation of Green-ZnO-NPs
3.3.1. UV–Vis
3.3.2. ATR-FTIR Analysis
3.3.3. XRD
3.3.4. Surface Morphology Analysis
3.3.5. Particle Size and Zeta Potential
3.4. Antioxidant Activity
3.5. Antibacterial Activity
3.5.1. Preparation of Inoculum
3.5.2. Disc Diffusion Method
3.5.3. Broth Microdilution Assay
3.6. Anticancer Activity
3.7. Artemia Larvae Lethality Bioassay
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jalal, M.Z.; John, A.; Rasheed, A.K.; Alallam, B.; Khalid, M.; Ismail, A.F.; Salleh, H. Earlier Denaturation of DNA By Using Novel Ternary Hybrid Nanoparticles. IIUM Eng. J. 2022, 23, 237–245. [Google Scholar] [CrossRef]
- Nawaz, A.; Latif, M.S.; Alnuwaiser, M.A.; Ullah, S.; Iqbal, M.; Alfatama, M.; Lim, V. Synthesis and Characterization of Chitosan-Decorated Nanoemulsion Gel of 5-Fluorouracil for Topical Delivery. Gels 2022, 8, 412. [Google Scholar] [CrossRef] [PubMed]
- Naz, F.F.; Shah, K.U.; Niazi, Z.R.; Zaman, M.; Lim, V.; Alfatama, M. Polymeric Microparticles: Synthesis, Characterization and In Vitro Evaluation for Pulmonary Delivery of Rifampicin. Polymers 2022, 14, 2491. [Google Scholar] [CrossRef] [PubMed]
- Sabir, S.; Arshad, M.; Chaudhari, S.K. Zinc Oxide Nanoparticles for Revolutionizing Agriculture: Synthesis and Applications. Sci. World J. 2014, 2014, 925494. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, S.P.; Sengodan, K. Synthesis and Characterization of Zinc Oxide and Iron Oxide Nanoparticles Using Sesbania grandiflora Leaf Extract as Reducing Agent. J. Nanosci. 2017, 2017, 8348507. [Google Scholar] [CrossRef]
- Saleemi, M.A.; Alallam, B.; Yong, Y.K.; Lim, V. Synthesis of Zinc Oxide Nanoparticles with Bioflavonoid Rutin: Characterisation, Antioxidant and Antimicrobial Activities and In Vivo Cytotoxic Effects on Artemia Nauplii. Antioxidants 2022, 11, 1853. [Google Scholar] [CrossRef]
- Cross, S.E.; Innes, B.; Roberts, M.S.; Tsuzuki, T.; Robertson, T.A.; McCormick, P. Human Skin Penetration of Sunscreen Nanoparticles: In-vitro Assessment of a Novel Micronized Zinc Oxide Formulation. Ski. Pharmacol. Physiol. 2007, 20, 148–154. [Google Scholar] [CrossRef]
- Rasmussen, J.W.; Martinez, E.; Louka, P.; Wingett, D.G. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin. Drug Deliv. 2010, 7, 1063–1077. [Google Scholar] [CrossRef]
- Jamdagni, P.; Khatri, P.; Rana, J. Green synthesis of zinc oxide nanoparticles using flower extract of Nyctanthes arbor-tristis and their antifungal activity. J. King Saud Univ.-Sci. 2018, 30, 168–175. [Google Scholar] [CrossRef]
- Hasnidawani, J.; Azlina, H.; Norita, H.; Bonnia, N.; Ratim, S.; Ali, E. Synthesis of ZnO Nanostructures Using Sol-Gel Method. Procedia Chem. 2016, 19, 211–216. [Google Scholar] [CrossRef]
- Mallikarjunaswamy, C.; Ranganatha, V.L.; Ramu, R.; Udayabhanu; Nagaraju, G. Facile microwave-assisted green synthesis of ZnO nanoparticles: Application to photodegradation, antibacterial and antioxidant. J. Mater. Sci. Mater. Electron. 2019, 31, 1004–1021. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Nguyen, V.A. Synthesis, Characterization, and Photocatalytic Activity of ZnO Nanomaterials Prepared by a Green, Nonchemical Route. J. Nanomater. 2020, 2020, 1768371. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Sailo, B.L.; Banik, K.; Harsha, C.; Prasad, S.; Gupta, S.C.; Bharti, A.C.; Aggarwal, B.B. Chronic diseases, inflammation, and spices: How are they linked? J. Transl. Med. 2018, 16, 14. [Google Scholar] [CrossRef]
- Santini, A.; Tenore, G.C.; Novellino, E. Nutraceuticals: A paradigm of proactive medicine. Eur. J. Pharm. Sci. 2017, 96, 53–61. [Google Scholar] [CrossRef]
- Das, T.; Sa, G.; Saha, B.; Das, K. Multifocal signal modulation therapy of cancer: Ancient weapon, modern targets. Mol. Cell. Biochem. 2009, 336, 85–95. [Google Scholar] [CrossRef]
- Tung, B.T.; Nham, D.T.; Hai, N.T.; Thu, D.K. Curcuma longa, the Polyphenolic Curcumin Compound and Pharmacological Effects on Liver. In Dietary Interventions in Liver Disease: Foods, Nutrients and Dietary Supplements; Academic Press: Cambridge, MA, USA, 2019; pp. 125–134. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Hassani, A.; Mahmood, S.; Enezei, H.H.; Hussain, S.A.; Hamad, H.A.; Aldoghachi, A.F.; Hagar, A.; Doolaanea, A.A.; Ibrahim, W.N. Formulation, Characterization and Biological Activity Screening of Sodium Alginate-Gum Arabic Nanoparticles Loaded with Curcumin. Molecules 2020, 25, 2244. [Google Scholar] [CrossRef]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2007, 75, 787–809. [Google Scholar] [CrossRef]
- Verma, A.; Jain, N.; Singha, S.K.; Quraishi, M.A.; Sinha, I. Green synthesis and catalytic application of curcumin stabilized silver nanoparticles. J. Chem. Sci. 2016, 128, 1871–1878. [Google Scholar] [CrossRef]
- Khalil, M.I.; Al-Qunaibit, M.M.; Al-Zahem, A.M.; Labis, J.P. Synthesis and characterization of ZnO nanoparticles by thermal decomposition of a curcumin zinc complex. Arab. J. Chem. 2014, 7, 1178–1184. [Google Scholar] [CrossRef]
- Jayarambabu, N.; Rao, K.V.; Prabhu, Y.T. Beneficial Role of Zinc Oxide Nanoparticles on Green Crop Production Effects of Temperature, Deposition Time and Catalyst Loading on the Synthesis of Carbon Nanotubes in a Fixed Bed Reactor View Project Phytochemical Screening and Evaluation of In Vitro Antioxidant and Antimicrobial Activities of the Indigenous Medicinal Plant Albizia Odoratissima View Project. 2015. Available online: https://www.researchgate.net/publication/301541596 (accessed on 4 January 2023).
- El-Kattan, N.; Emam, A.N.; Mansour, A.S.; Ibrahim, M.A.; El-Razik, A.B.A.; Allam, K.A.M.; Riad, N.Y.; Ibrahim, S.A. Curcumin assisted green synthesis of silver and zinc oxide nanostructures and their antibacterial activity against some clinical pathogenic multi-drug resistant bacteria. RSC Adv. 2022, 12, 18022–18038. [Google Scholar] [CrossRef] [PubMed]
- Perera, W.P.T.D.; Dissanayake, R.K.; Ranatunga, U.I.; Hettiarachchi, N.M.; Perera, K.D.C.; Unagolla, J.M.; De Silva, R.T.; Pahalagedara, L.R. Curcumin loaded zinc oxide nanoparticles for activity-enhanced antibacterial and anticancer applications. RSC Adv. 2020, 10, 30785–30795. [Google Scholar] [CrossRef] [PubMed]
- Facile Synthesis of Silver-Zinc Oxide Nanocomposites Using Curcuma Longa Extract and Its In Vitro Antimicrobial Efficacy against Multi-Drug Resistant Pathogens of Public Health Importance—Google Search. Available online: https://www.google.com/search?q=Facile+synthesis+of+silver-zinc+oxide+nanocomposites+using+Curcuma+longa+extract+and+its+in+vitro+antimicrobial+efficacy+against+multi-drug+resistant+pathogens+of+public+health+importance&oq=Facile+synthesis+of+silver-zinc+oxide+nanocomposites+using+Curcuma+longa+extract+and+its+in+vitro+antimicrobial+efficacy+against+multi-drug+resistant+pathogens+of+public+health+importance&aqs=chrome..69i57j69i61l3.201j0j4&sourceid=chrome&ie=UTF-8 (accessed on 4 January 2023).
- Khalil, M.I.; Al-Zahem, A.; Qunaibit, M.M. Synthesis, characterization, and antitumor activity of binuclear curcumin-metal(II) hydroxo complexes. Med. Chem. Res. 2014, 23, 1683–1689. [Google Scholar] [CrossRef]
- Rajapriya, M.; Sharmili, S.A.; Baskar, R.; Balaji, R.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Alanzi, K.F.; Vaseeharan, B. Synthesis and Characterization of Zinc Oxide Nanoparticles Using Cynara scolymus Leaves: Enhanced Hemolytic, Antimicrobial, Antiproliferative, and Photocatalytic Activity. J. Clust. Sci. 2019, 31, 791–801. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Pan, M.-H.; Cheng, A.-L.; Lin, L.-I.; Ho, Y.-S.; Hsieh, C.-Y.; Lin, J.-K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Payton, F.; Sandusky, P.; Alworth, W.L. NMR study of the solution structure of curcumin. J. Nat. Prod. 2007, 70, 143–146. [Google Scholar] [CrossRef]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar] [CrossRef]
- Jovanovic, S.V.; Steenken, S.; Boone, C.W.; Simic, M.G. H-Atom Transfer Is a Preferred Antioxidant Mechanism of Curcumin. J. Am. Chem. Soc. 1999, 121, 9677–9681. [Google Scholar] [CrossRef]
- Dobrucka, R.; Dlugaszewska, J.; Kaczmarek, M. Cytotoxic and antimicrobial effects of biosynthesized ZnO nanoparticles using of Chelidonium majus extract. Biomed. Microdevices 2017, 20, 5. [Google Scholar] [CrossRef]
- Zak, A.K.; Majid, W.A.; Mahmoudian, M.; Darroudi, M.; Yousefi, R. Starch-stabilized synthesis of ZnO nanopowders at low temperature and optical properties study. Adv. Powder Technol. 2013, 24, 618–624. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, D.J.; Karthick, S.N.; Hemalatha, K.V.; Raj, C.J.; Ok, S.; Choe, Y. Curcumin Dye Extracted from Curcuma longa L. Used as Sensitizers for Efficient Dye-Sensitized Solar Cells. Int. J. Electrochem. Sci. 2013, 8, 8320–8328. [Google Scholar]
- Das, R.K.; Pachapur, V.L.; Lonappan, L.; Naghdi, M.; Pulicharla, R.; Maiti, S.; Cledon, M.; Dalila, L.M.A.; Sarma, S.J.; Brar, S.K. Biological synthesis of metallic nanoparticles: Plants, animals and microbial aspects. Nanotechnol. Environ. Eng. 2017, 2, 18. [Google Scholar] [CrossRef]
- Xiong, H.-M.; Ma, R.-Z.; Wang, S.-F.; Xia, Y.-Y. Photoluminescent ZnO nanoparticles synthesized at the interface between air and triethylene glycol. J. Mater. Chem. 2011, 21, 3178–3182. [Google Scholar] [CrossRef]
- Umar, A.; Rahman, M.; Vaseem, M.; Hahn, Y.-B. Ultra-sensitive cholesterol biosensor based on low-temperature grown ZnO nanoparticles. Electrochem. Commun. 2009, 11, 118–121. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Kim, K.H.; Lim, C.S.; Shim, K.B. Characterization of ZnO nanopowders synthesized by the polymerized complex method via an organochemical route. J. Ceram. Process. Res. 2002, 3, 146–149. [Google Scholar]
- Kolev, T.M.; Velcheva, E.A.; Stamboliyska, B.A.; Spiteller, M. DFT and experimental studies of the structure and vibrational spectra of curcumin. Int. J. Quantum Chem. 2005, 102, 1069–1079. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef]
- Ciszewski, A.; Milczarek, G.; Lewandowska, B.; Krutowski, K. Electrocatalytic Properties of Electropolymerized Ni(II)curcumin Complex. Electroanalysis 2003, 15, 518–523. [Google Scholar] [CrossRef]
- Tayyari, S.F.; Rahemi, H.; Nekoei, A.R.; Zahedi-Tabrizi, M.; Wang, Y.A. Vibrational assignment and structure of dibenzoylmethane. A density functional theoretical study. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2007, 66, 394–404. [Google Scholar] [CrossRef]
- Krishnankutty, K.; John, V.D. Synthesis, Characterization, and Antitumour Studies of Metal Chelates of Some Synthetic Curcuminoids. Synth. React. Inorganic, Met. Nano-Metal Chem. 2003, 33, 343–358. [Google Scholar] [CrossRef]
- Rajalaxshmi, A.; Clara Jeyageetha, J. Green Syntheses and Characterization of Zinc Oxide and Cerium Ion Doped Zinc Oxide Nanoparticles Assisted by Mangifera Indica. Eur. J. Pharm. Med. Res. 2018, 4, 712–717. [Google Scholar]
- Nasrallah, O.; El Kurdi, R.; Mouslmani, M.; Patra, D. Doping of ZnO Nanoparticles with Curcumin: pH Dependent Release and DPPH Scavenging Activity of Curcumin in the Nanocomposites. Curr. Nanomater. 2019, 3, 147–152. [Google Scholar] [CrossRef]
- Joseph, E.; Singhvi, G. Multifunctional nanocrystals for cancer therapy: A potential nanocarrier. In Nanomaterials for Drug Delivery and Therapy; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 91–116. [Google Scholar]
- An, S.S.A.; Kim, K.; Choi, M.; Lee, J.-K.; Jeong, J.; Kim, Y.-R.; Kim, M.-K.; Paek, S.-M.; Shin, J.-H. Physicochemical properties of surface charge-modified ZnO nanoparticles with different particle sizes. Int. J. Nanomed. 2014, 9, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Jacob, V.; P, R. In vitro analysis: The antimicrobial and antioxidant activity of zinc oxide nanoparticles from curcuma longa. Asian J. Pharm. Clin. Res. 2019, 12, 200–204. [Google Scholar] [CrossRef]
- Somu, P.; Paul, S. A biomolecule-assisted one-pot synthesis of zinc oxide nanoparticles and its bioconjugate with curcumin for potential multifaceted therapeutic applications. New J. Chem. 2019, 43, 11934–11948. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Kumar, V.; Mohan, S.; Singh, D.K.; Verma, D.K.; Singh, V.K.; Hasan, S.H. Photo-mediated optimized synthesis of silver nanoparticles for the selective detection of Iron(III), antibacterial and antioxidant activity. Mater. Sci. Eng. C 2017, 71, 1004–1019. [Google Scholar] [CrossRef]
- Yakimovich, N.O.; Ezhevskii, A.A.; Guseinov, D.V.; Smirnova, L.A.; Gracheva, T.A.; Klychkov, K.S. Antioxidant properties of gold nanoparticles studied by ESR spectroscopy. Russ. Chem. Bull. 2008, 57, 520–523. [Google Scholar] [CrossRef]
- Stan, M.; Popa, A.; Toloman, D.; Silipas, T.-D.; Vodnar, D.C. Antibacterial and Antioxidant Activities of ZnO Nanoparticles Synthesized Using Extracts of Allium sativum, Rosmarinus officinalis and Ocimum basilicum. Acta Metall. Sin. 2016, 29, 228–236. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J. Nanoparticle Res. 2007, 9, 479–489. [Google Scholar] [CrossRef]
- Yamamoto, O. Influence of particle size on the antibacterial activity of zinc oxide. Int. J. Inorg. Mater. 2001, 3, 643–646. [Google Scholar] [CrossRef]
- Peng, X.; Palma, S.; Fisher, N.S.; Wong, S.S. Effect of morphology of ZnO nanostructures on their toxicity to marine algae. Aquat. Toxicol. 2011, 102, 186–196. [Google Scholar] [CrossRef]
- Reddy, K.M.; Feris, K.; Bell, J.; Wingett, D.G.; Hanley, C.; Punnoose, A. Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Appl. Phys. Lett. 2007, 90, 213902–2139023. [Google Scholar] [CrossRef]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced bioactivity of ZnO nanoparticles—An antimicrobial study. Sci. Technol. Adv. Mater. 2008, 9, 035004. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.; Cui, F.; Kim, T.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Brayner, R.; Ferrari-Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, M.F.; Fiévet, F. Toxicological Impact Studies Based on Escherichia coli Bacteria in Ultrafine ZnO Nanoparticles Colloidal Medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef]
- Adams, L.K.; Lyon, D.Y.; Alvarez, P.J. Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res. 2006, 40, 3527–3532. [Google Scholar] [CrossRef]
- Li, M.; Zhu, L.; Lin, D. Toxicity of ZnO Nanoparticles toEscherichia coli: Mechanism and the Influence of Medium Components. Environ. Sci. Technol. 2011, 45, 1977–1983. [Google Scholar] [CrossRef]
- Brunner, T.J.; Wick, P.; Manser, P.; Spohn, P.; Grass, R.N.; Limbach, L.K.; Bruinink, A.; Stark, W.J. In Vitro Cytotoxicity of Oxide Nanoparticles: Comparison to Asbestos, Silica, and the Effect of Particle Solubility. Environ. Sci. Technol. 2006, 40, 4374–4381. [Google Scholar] [CrossRef] [PubMed]
- Kasemets, K.; Ivask, A.; Dubourguier, H.-C.; Kahru, A. Toxicity of nanoparticles of ZnO, CuO and TiO2 to yeast Saccharomyces cerevisiae. Toxicol. Vitr. 2009, 23, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Lipovsky, A.; Nitzan, Y.; Gedanken, A.; Lubart, R. Antifungal activity of ZnO nanoparticles—The role of ROS mediated cell injury. Nanotechnology 2011, 22, 105101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ding, Y.; Povey, M.; York, D. ZnO nanofluids—A potential antibacterial agent. Prog. Nat. Sci. 2008, 18, 939–944. [Google Scholar] [CrossRef]
- Jalal, R.; Goharshadi, E.K.; Abareshi, M.; Moosavi, M.; Yousefi, A.; Nancarrow, P. ZnO nanofluids: Green synthesis, characterization, and antibacterial activity. Mater. Chem. Phys. 2010, 121, 198–201. [Google Scholar] [CrossRef]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef]
- Feris, K.; Otto, C.; Tinker, J.; Wingett, D.; Punnoose, A.; Thurber, A.; Kongara, M.; Sabetian, M.; Quinn, B.; Hanna, C.; et al. Electrostatic Interactions Affect Nanoparticle-Mediated Toxicity to Gram-Negative Bacterium Pseudomonas aeruginosa PAO1. Langmuir 2009, 26, 4429–4436. [Google Scholar] [CrossRef]
- Shah, A.; Manikandan, E.; Ahamed, M.B.; Mir, D.A.; Mir, S. Antibacterial and Blue shift investigations in sol–gel synthesized CrxZn1−xO Nanostructures. J. Lumin. 2014, 145, 944–950. [Google Scholar] [CrossRef]
- Da Silva, B.L.; Abuçafy, M.P.; Manaia, E.B.; Junior, J.A.O.; Chiari-Andréo, B.G.; Pietro, R.C.L.R.; Chiavacci, L.A. Relationship Between Structure And Antimicrobial Activity Of Zinc Oxide Nanoparticles: An Overview. Int. J. Nanomed. 2019, 14, 9395. [Google Scholar] [CrossRef]
- Yıldırım, Ö.A.; Unalan, H.E.; Durucan, C. Highly Efficient Room Temperature Synthesis of Silver-Doped Zinc Oxide (ZnO:Ag) Nanoparticles: Structural, Optical, and Photocatalytic Properties. J. Am. Ceram. Soc. 2013, 96, 766–773. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, L.; Yan, X.; Yang, Y.; Lei, Y.; Zhou, J.; Huang, Y.; Gu, Y.; Zhang, Y. Structure and photocatalytic activity of Ni-doped ZnO nanorods. Mater. Res. Bull. 2011, 46, 1207–1210. [Google Scholar] [CrossRef]
- Ma, J.; Liu, J.; Bao, Y.; Zhu, Z.; Wang, X.; Zhang, J. Synthesis of large-scale uniform mulberry-like ZnO particles with microwave hydrothermal method and its antibacterial property. Ceram. Int. 2013, 39, 2803–2810. [Google Scholar] [CrossRef]
- Talebian, N.; Amininezhad, S.M.; Doudi, M. Controllable synthesis of ZnO nanoparticles and their morphology-dependent antibacterial and optical properties. J. Photochem. Photobiol. B Biol. 2013, 120, 66–73. [Google Scholar] [CrossRef]
- Stanković, A.; Dimitrijević, S.; Uskoković, D. Influence of size scale and morphology on antibacterial properties of ZnO powders hydrothemally synthesized using different surface stabilizing agents. Colloids Surfaces B Biointerfaces 2013, 102, 21–28. [Google Scholar] [CrossRef]
- Vennilaraj, R.; Palanisamy, K.; Arthanareeswari, M.; Bitragunta, S. Green synthesis of silver nanoparticles from Cleistanthus Collinus leaf extract and their biological effects. Int. J. Chem. 2022, 34, 1103–1107. [Google Scholar]
- Tong, G.-X.; Du, F.-F.; Liang, Y.; Hu, Q.; Wu, R.-N.; Guan, J.-G.; Hu, X. Polymorphous ZnO complex architectures: Selective synthesis, mechanism, surface area and Zn-polar plane-codetermining antibacterial activity. J. Mater. Chem. B 2012, 1, 454–463. [Google Scholar] [CrossRef]
- Kurita, T.; Makino, Y. Novel curcumin oral delivery systems. Anticancer Res. 2013, 33, 2807–2822. [Google Scholar]
- Kundu, M.; Sadhukhan, P.; Ghosh, N.; Chatterjee, S.; Manna, P.; Das, J.; Sil, P. pH-responsive and targeted delivery of curcumin via phenylboronic acid-functionalized ZnO nanoparticles for breast cancer therapy. J. Adv. Res. 2019, 18, 161–172. [Google Scholar] [CrossRef]
- Bansal, S.S.; Goel, M.; Aqil, F.; Vadhanam, M.V.; Gupta, R.C. Advanced Drug Delivery Systems of Curcumin for Cancer Chemoprevention. Cancer Prev. Res. 2011, 4, 1158–1171. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Liu, Q.; Yang, L.; Zhu, R.; Yu, C.; Wang, S. Rational Design of Multifunctional Dendritic Mesoporous Silica Nanoparticles to Load Curcumin and Enhance Efficacy for Breast Cancer Therapy. ACS Appl. Mater. Interfaces 2016, 8, 26511–26523. [Google Scholar] [CrossRef]
- Moghaddam, A.B.; Moniri, M.; Azizi, S.; Rahim, R.A.; Bin Ariff, A.; Navaderi, M.; Mohamad, R. Eco-Friendly Formulated Zinc Oxide Nanoparticles: Induction of Cell Cycle Arrest and Apoptosis in the MCF-7 Cancer Cell Line. Genes 2017, 8, 281. [Google Scholar] [CrossRef]
- Punnoose, A.; Dodge, K.; Rasmussen, J.W.; Chess, J.; Wingett, D.; Anders, C. Cytotoxicity of ZnO Nanoparticles Can Be Tailored by Modifying Their Surface Structure: A Green Chemistry Approach for Safer Nanomaterials. ACS Sustain. Chem. Eng. 2014, 2, 1666–1673. [Google Scholar] [CrossRef] [PubMed]
- Sindhura, K.S.; Prasad, T.N.V.K.V.; Selvam, P.P.; Hussain, O.M. Synthesis, characterization and evaluation of effect of phytogenic zinc nanoparticles on soil exo-enzymes. Appl. Nanosci. 2013, 4, 819–827. [Google Scholar] [CrossRef]
- Bharathi, D.; Bhuvaneshwari, V. Synthesis of zinc oxide nanoparticles (ZnO NPs) using pure bioflavonoid rutin and their biomedical applications: Antibacterial, antioxidant and cytotoxic activities. Res. Chem. Intermed. 2019, 45, 2065–2078. [Google Scholar] [CrossRef]
- Bisht, G.; Rayamajhi, S. ZnO Nanoparticles: A Promising Anticancer Agent. Nanobiomedicine 2016, 3, 9. [Google Scholar] [CrossRef]
- Mortimer, M.; Kasemets, K.; Kahru, A. Toxicity of ZnO and CuO nanoparticles to ciliated protozoa Tetrahymena thermophila. Toxicology 2010, 269, 182–189. [Google Scholar] [CrossRef]
- Franklin, N.M.; Rogers, N.J.; Apte, S.C.; Batley, G.E.; Gadd, G.E.; Casey, P.S. Comparative Toxicity of Nanoparticulate ZnO, Bulk ZnO, and ZnCl2 to a Freshwater Microalga (Pseudokirchneriella subcapitata): The Importance of Particle Solubility. Environ. Sci. Technol. 2007, 41, 8484–8490. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, J.; Zhang, X.; Chang, Y.; Chen, Y. The impact of ZnO nanoparticle aggregates on the embryonic development of zebrafish (Danio rerio). Nanotechnology 2009, 20, 195103. [Google Scholar] [CrossRef]
- Poynton, H.C.; Lazorchak, J.M.; Impellitteri, C.A.; Smith, M.E.; Rogers, K.; Patra, M.; Hammer, K.A.; Allen, H.J.; Vulpe, C.D. Differential Gene Expression in Daphnia magna Suggests Distinct Modes of Action and Bioavailability for ZnO Nanoparticles and Zn Ions. Environ. Sci. Technol. 2010, 45, 762–768. [Google Scholar] [CrossRef]
- Wang, H.; Wick, R.L.; Xing, B. Toxicity of nanoparticulate and bulk ZnO, Al2O3 and TiO2 to the nematode Caenorhabditis elegans. Environ. Pollut. 2009, 157, 1171–1177. [Google Scholar] [CrossRef]
- Abinaya, C.; Devi, R.M.; Suresh, P.; Balasubramanian, N.; Muthaiya, N.; Kannan, N.D.; Annaraj, J.; Shanmugaiah, V.; Pearce, J.M.; Shanmugapriya, P.; et al. Antibacterial and anticancer activity of hydrothermally-synthesized zinc oxide nanomaterials using natural extracts of neem, pepper and turmeric as solvent media. Nano Express 2020, 1, 010029. [Google Scholar] [CrossRef]
- Oo, M.K.; Alallam, B.; Doolaanea, A.A.; Khatib, A.; Mohamed, F.; Chatterjee, B. Exploring the Effect of Glycerol and Hydrochloric Acid on Mesoporous Silica Synthesis: Application in Insulin Loading. ACS Omega 2022, 7, 27126–27134. [Google Scholar] [CrossRef]
- Rahim, R.A.; Jayusman, P.A.; Lim, V.; Ahmad, N.H.; Hamid, Z.A.A.; Mohamed, S.; Muhammad, N.; Ahmad, F.; Mokhtar, N.; Mohamed, N.; et al. Phytochemical Analysis, Antioxidant and Bone Anabolic Effects of Blainvillea acmella (L.) Philipson. Front. Pharmacol. 2022, 12, 796509. [Google Scholar] [CrossRef]
- Chiu, H.I.; Mood, C.N.A.C.; Zain, N.N.M.; Ramachandran, M.R.; Yahaya, N.; Kamal, N.N.S.N.M.; Tung, W.H.; Yong, Y.K.; Lee, C.K.; Lim, V. Biogenic Silver Nanoparticles of Clinacanthus nutans as Antioxidant with Antimicrobial and Cytotoxic Effects. Bioinorg. Chem. Appl. 2021, 2021, 9920890. [Google Scholar] [CrossRef]
- Yaseen, M.R.; Faisal, G.G.; Fuaat, A.A.; Affandi, K.A.; Alallam, B.; Nasir, M.H.M. Preparation of Euyrycoma Longifolia Jack (E.L) Tongkat Ali (Ta) Root Extract Hydrogel for Wound Application. Pharmacogn. J. 2021, 13, 1456–1463. [Google Scholar] [CrossRef]
- Ates, M.; Daniels, J.; Arslan, Z.; Farah, I.O.; Rivera, H.F. Comparative evaluation of impact of Zn and ZnO nanoparticles on brine shrimp (Artemia salina) larvae: Effects of particle size and solubility on toxicity. Environ. Sci. Process. Impacts 2012, 15, 225–233. [Google Scholar] [CrossRef]

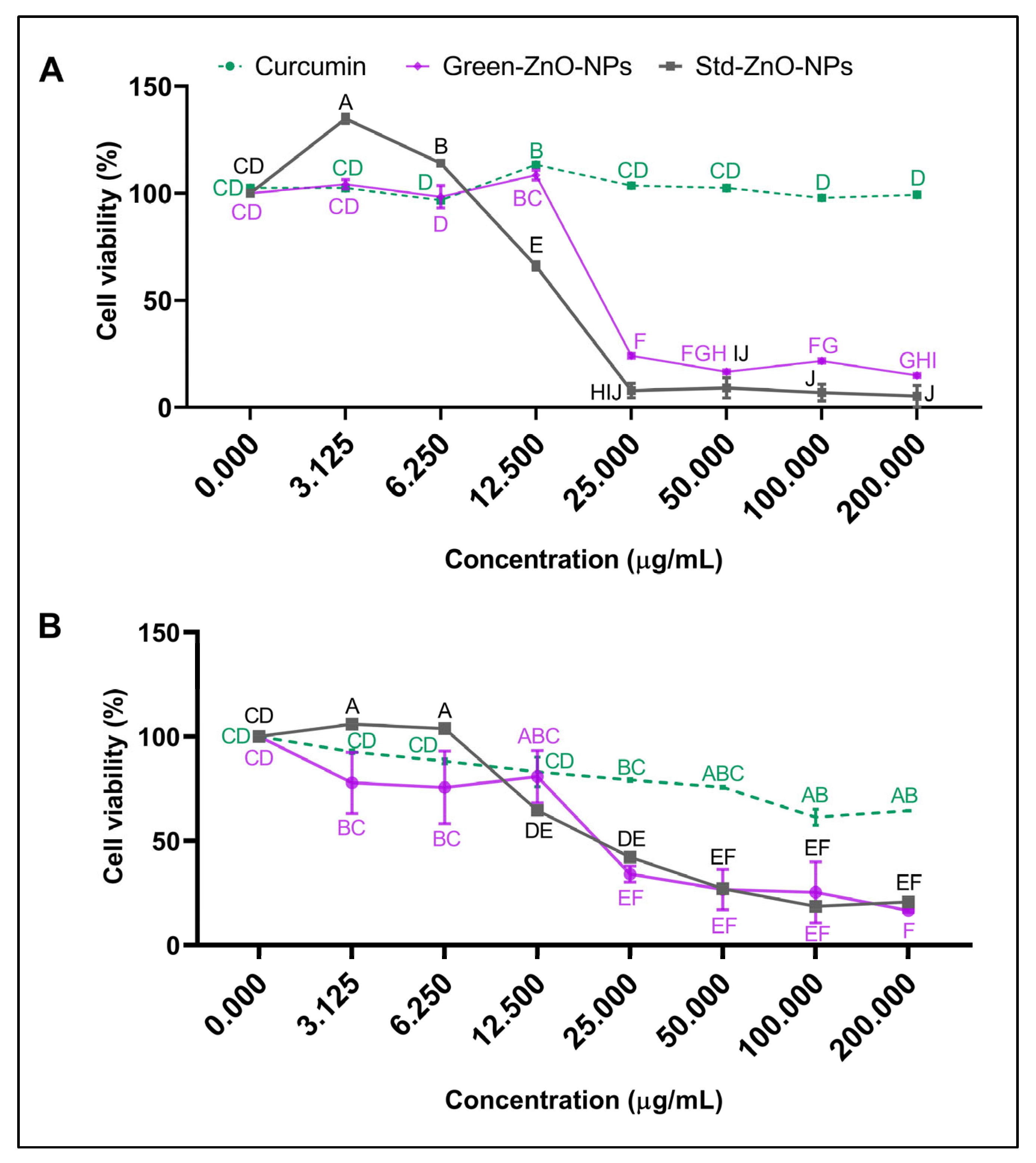
 ), 8 (
), 8 ( ), and 24 h (
), and 24 h ( ).
).
 ), 8 (
), 8 ( ), and 24 h (
), and 24 h ( ).
).

| Zeta Potential (mV) | PDI | Particle Size (nm) | |||
|---|---|---|---|---|---|
| DLS | SEM | TEM | |||
| Std-ZnO-NPs | +2.76 ± 0.20 a | 0.412 ± 0.039 a | 909 ± 65.18 a | 48.98 ± 24.51 a | 49.39 ± 22.54 a |
| Green-ZnO-NPs | −16.90 ± 0.26 b | 0.698 ± 0.271a | 171.67 ± 45.83 b | 68.12 ± 26.13 a | 27.61 ± 5.18 a |
| Curcumin | −3.82 ± 0.31 c | - | - | - | - |
| Microorganism | Gentamicin | Std-ZnO-NPs | Green-ZnO-NPs | Curcumin |
|---|---|---|---|---|
| ZOI (mm) | ||||
| E. coli | 22.87 ± 0.30 a | - | - | - |
| K. pneumonia | 11.63 ± 0.10 b | - | - | - |
| MRSA | 11.20 ± 0.06 c | - | - | - |
| S. aureus | 15.7 ± 0.04 d | 10.40 ± 0.15 e | 10.60 ± 0.10 e | - |
| MIC (µg/mL) | ||||
| E. coli | 1.95 | - | 500 | - |
| K. pneumonia | 15.63 | - | - | - |
| MRSA | 7.81 | - | 500 | - |
| S. aureus | 0.98 | - | 500 | - |
| MBC (µg/mL) | ||||
| E. coli | 7.81 | - | - | - |
| K. pneumonia | 31.25 | - | - | - |
| MRSA | 31.25 | - | - | - |
| S. aureus | 1.95 | - | - | - |
| Indicates no activity | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alallam, B.; Doolaanea, A.A.; Alfatama, M.; Lim, V. Phytofabrication and Characterisation of Zinc Oxide Nanoparticles Using Pure Curcumin. Pharmaceuticals 2023, 16, 269. https://doi.org/10.3390/ph16020269
Alallam B, Doolaanea AA, Alfatama M, Lim V. Phytofabrication and Characterisation of Zinc Oxide Nanoparticles Using Pure Curcumin. Pharmaceuticals. 2023; 16(2):269. https://doi.org/10.3390/ph16020269
Chicago/Turabian StyleAlallam, Batoul, Abd Almonem Doolaanea, Mulham Alfatama, and Vuanghao Lim. 2023. "Phytofabrication and Characterisation of Zinc Oxide Nanoparticles Using Pure Curcumin" Pharmaceuticals 16, no. 2: 269. https://doi.org/10.3390/ph16020269
APA StyleAlallam, B., Doolaanea, A. A., Alfatama, M., & Lim, V. (2023). Phytofabrication and Characterisation of Zinc Oxide Nanoparticles Using Pure Curcumin. Pharmaceuticals, 16(2), 269. https://doi.org/10.3390/ph16020269