An Understanding of Mechanism-Based Approaches for 1,3,4-Oxadiazole Scaffolds as Cytotoxic Agents and Enzyme Inhibitors
Abstract
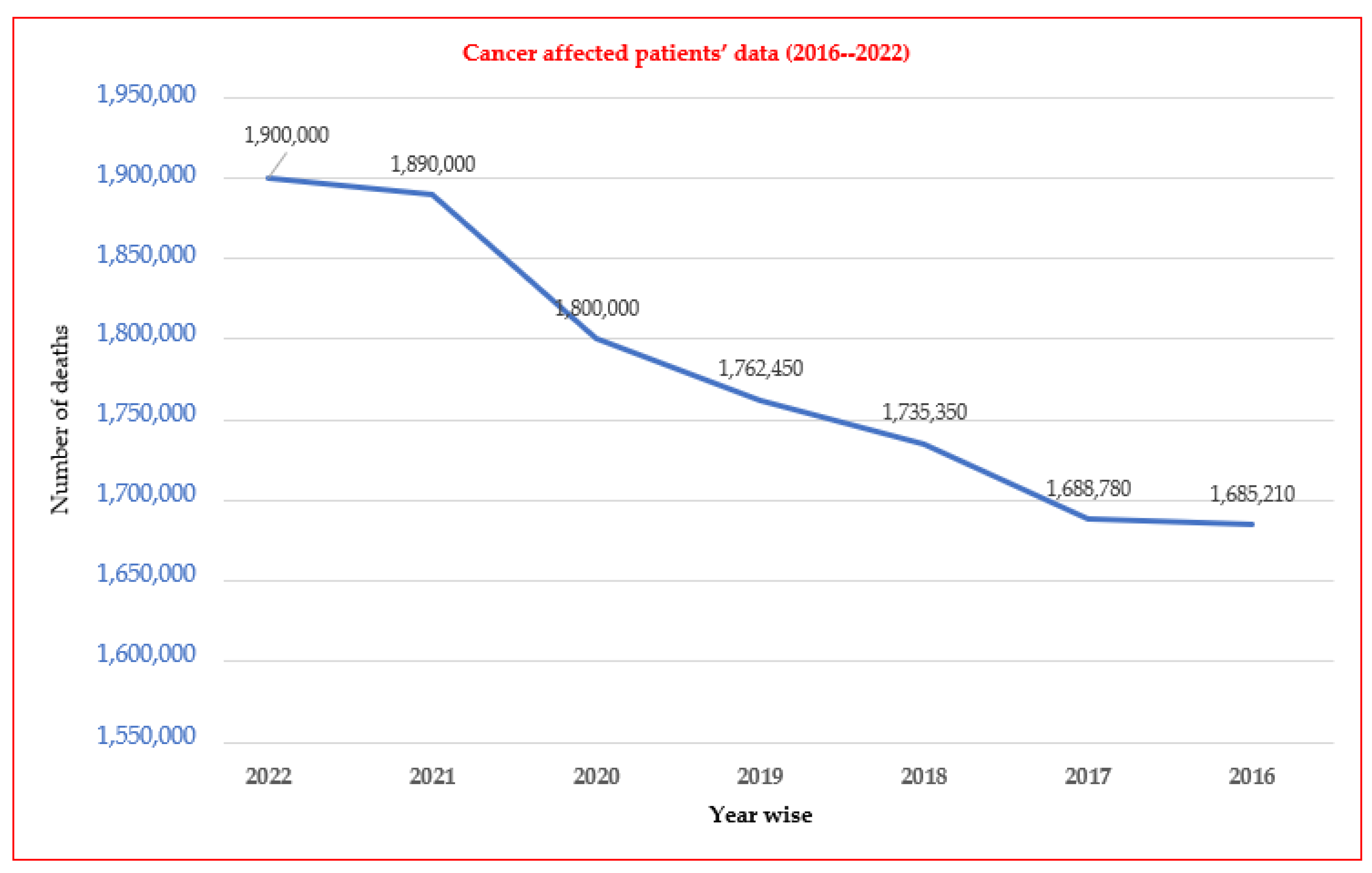
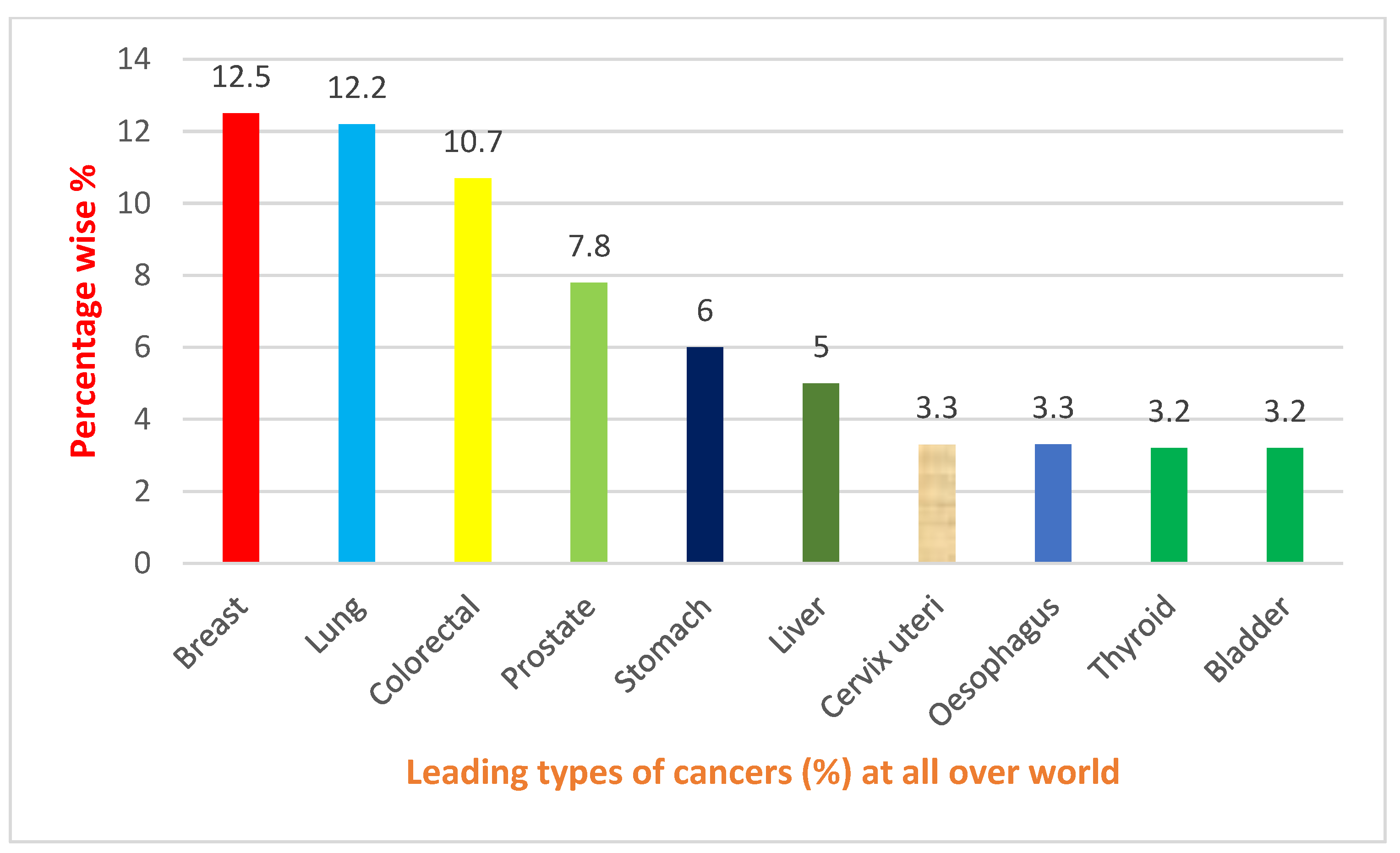
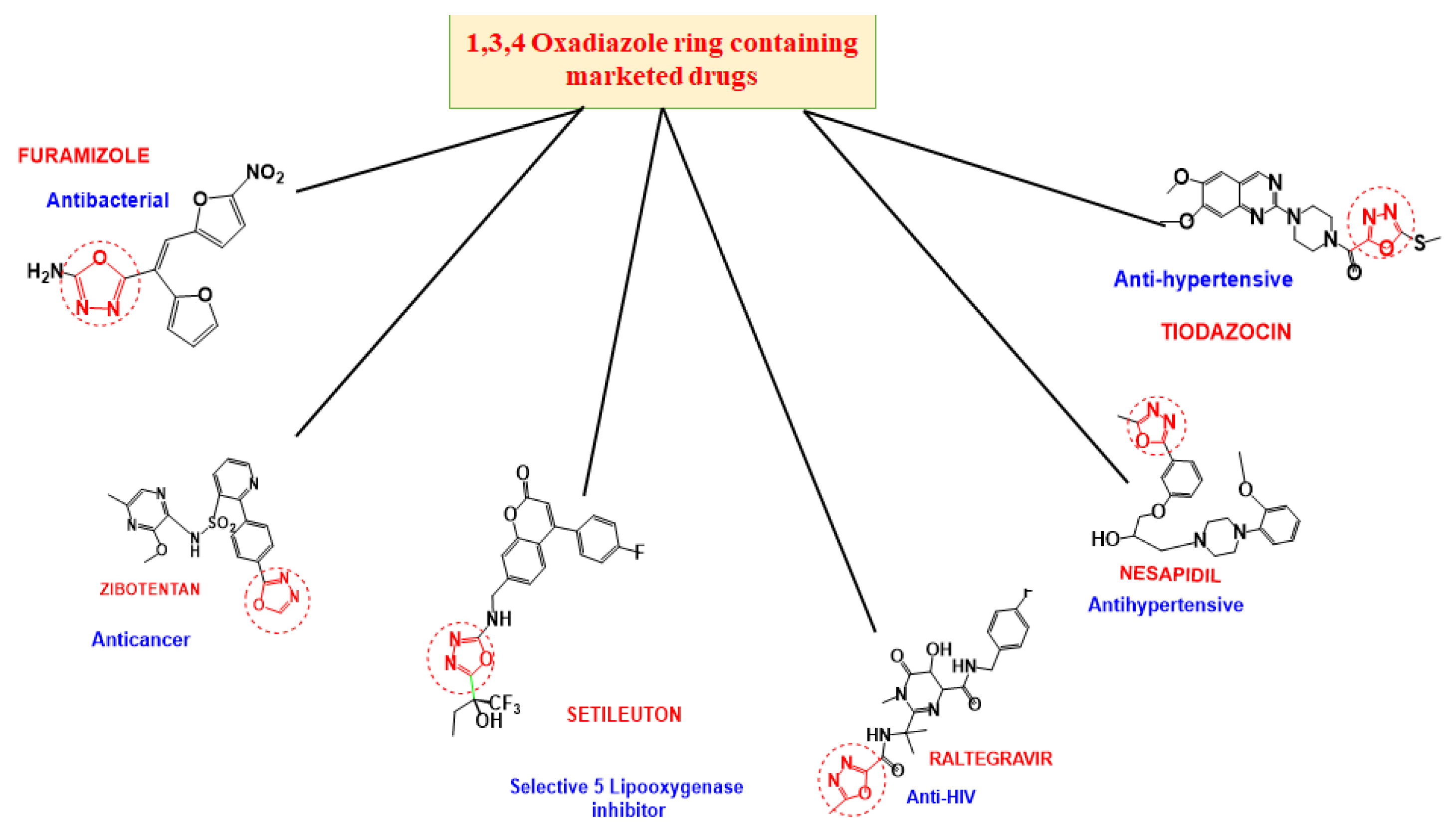
1. Introduction
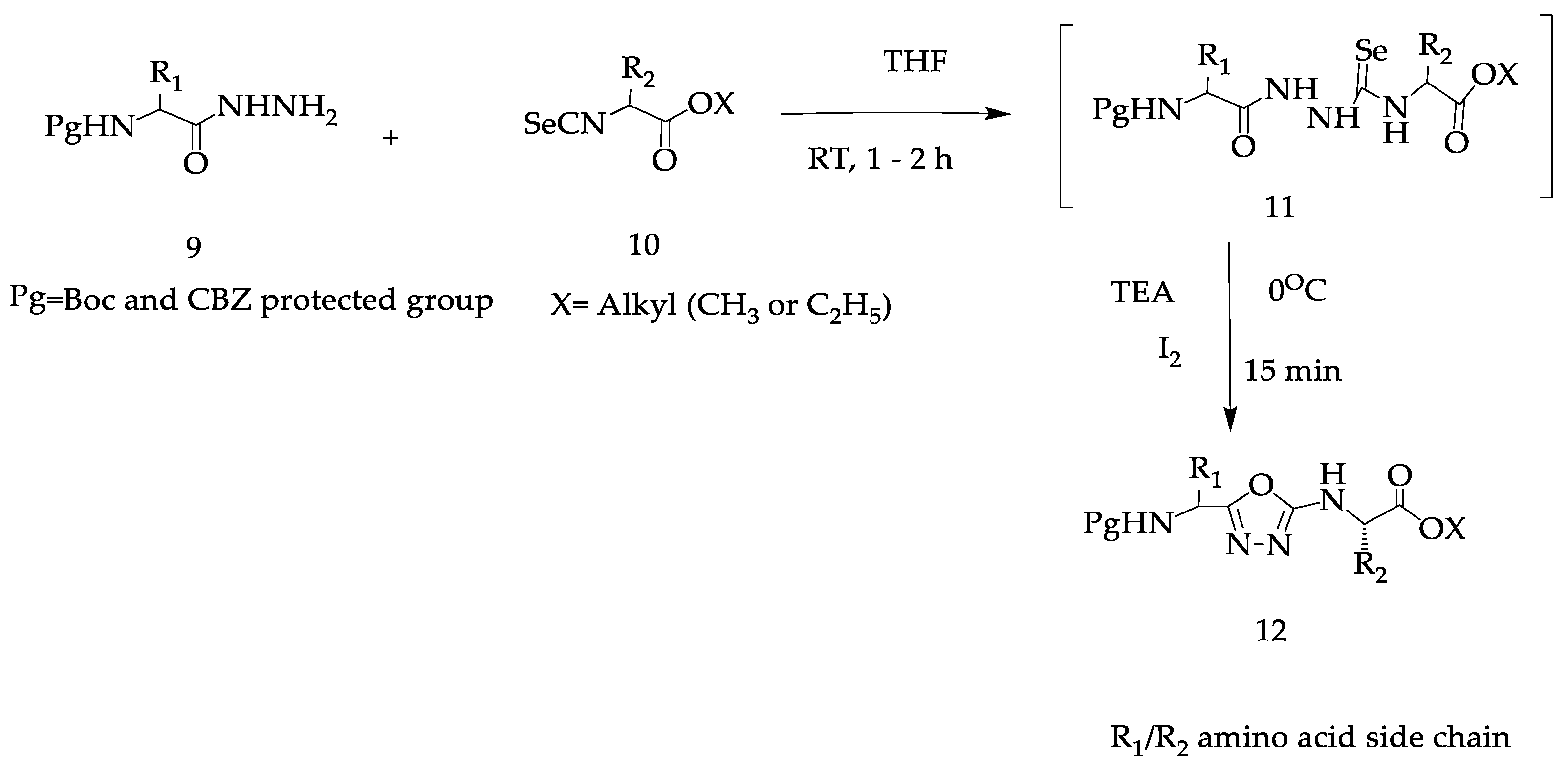
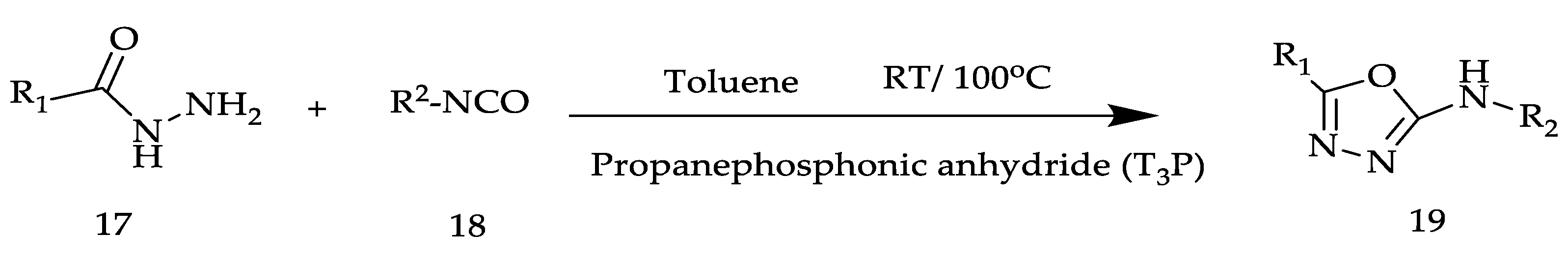
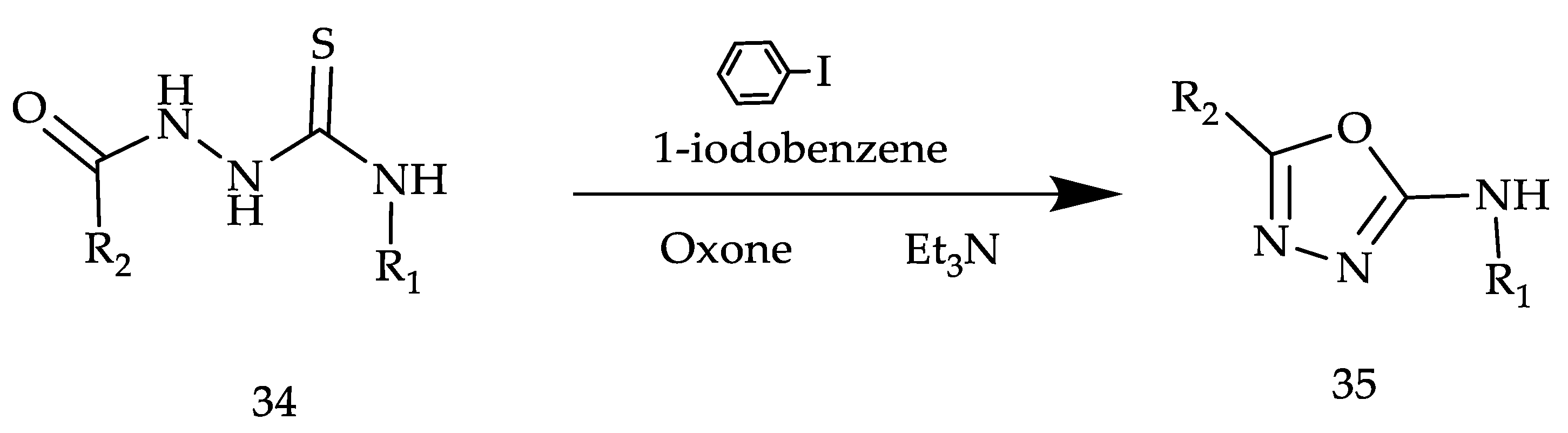
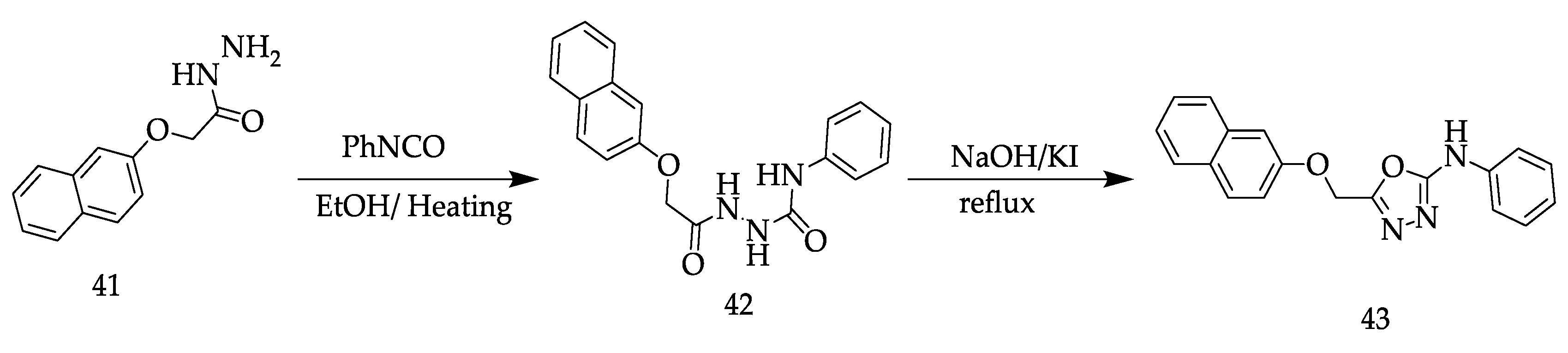
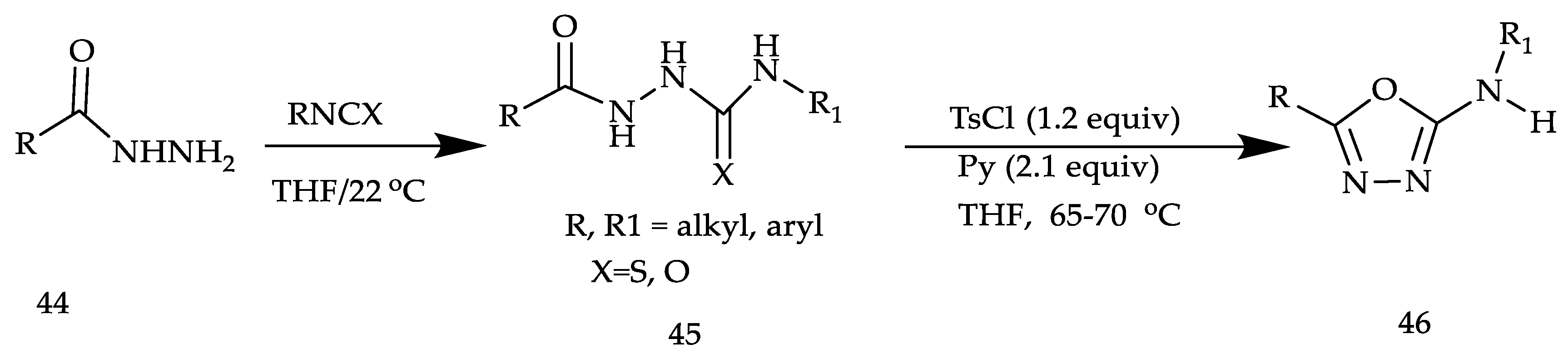
2. Various Synthetic Approaches for 1,3,4-Oxadiazole Derivatives (Figure 6)


2.1. Various Synthesis Methods for 5-Substituted-1,3,4-Oxadiazole-2-Thiols
2.2. Various Synthesis Methods for 2,5-Diaryl(alkyl/thiol)-1,3,4-Oxadiazole
3. Evidence of the Biological Potential of 1,3,4-Oxadiazole Derivatives
4. Biochemical Mechanisms Leading to Cancer
- Telomerase enzyme;
- Histone deacetylase (HDAC);
- Thymidylate synthase;
- Thymidine phosphorylase enzyme;
- Other evidence of the anticancer properties of 1,3,4-oxadiazole derivatives.
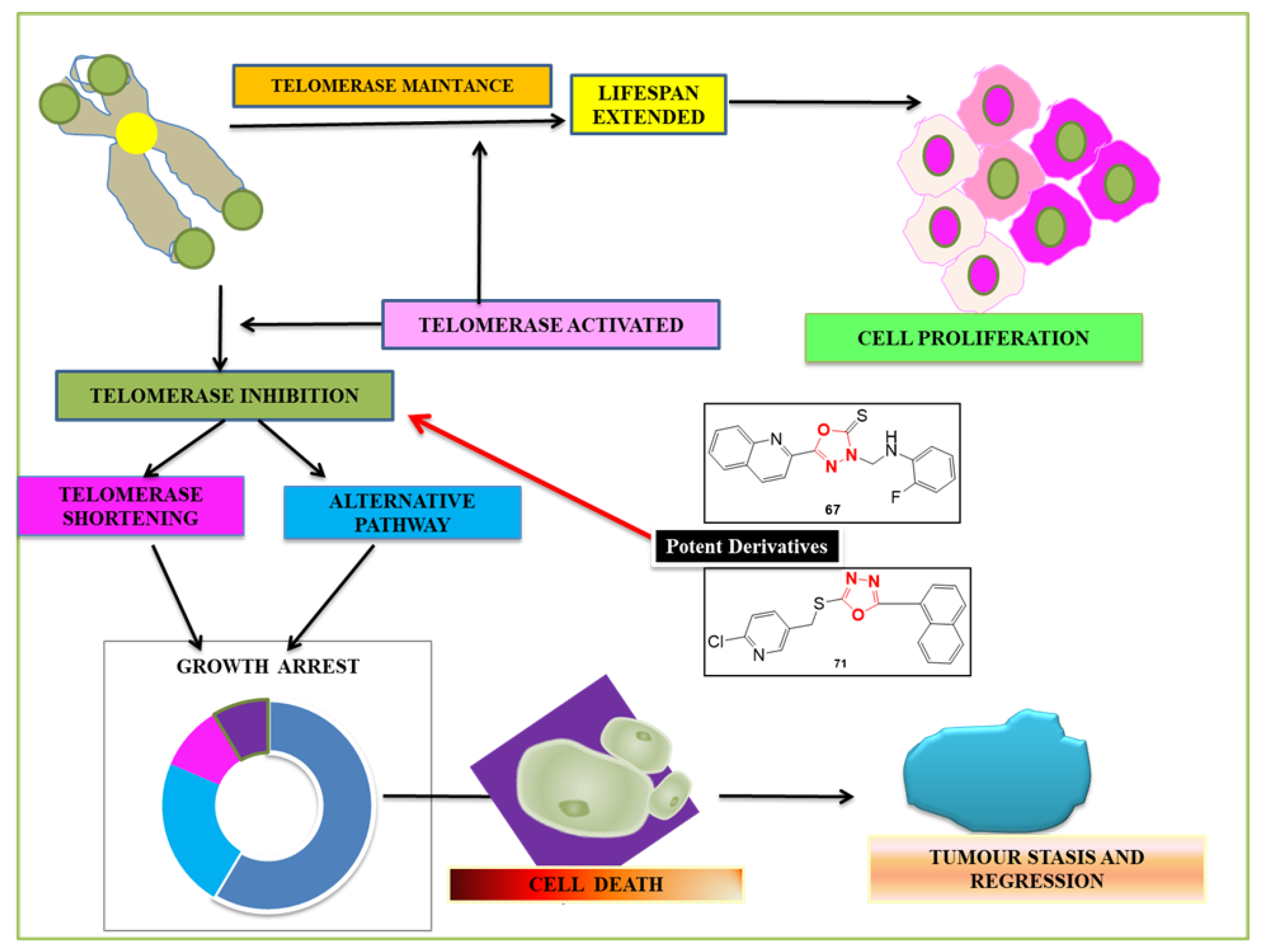
4.1. Telomerase
4.1.1. The Role of Telomerase Enzyme in Cells
4.1.2. Telomeres Association Proteins
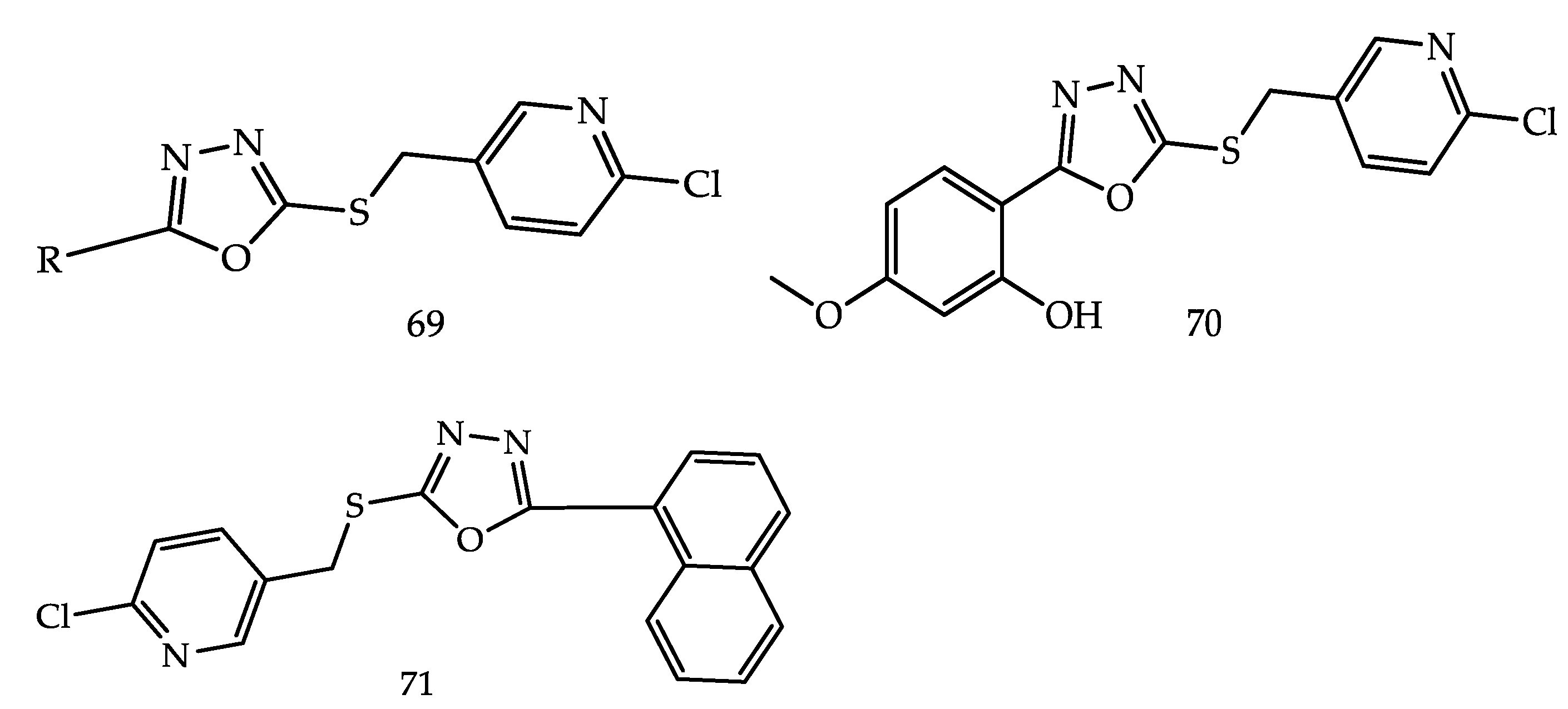
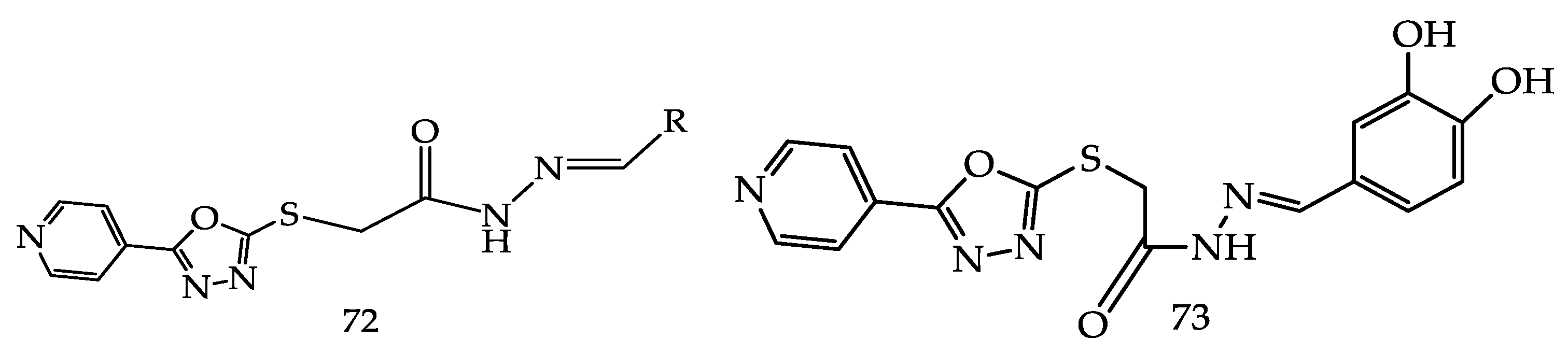
4.1.3. Telomerase Inhibitors
5. Histone Deacetylase (HDAC) Functions and Its Inhibitors
6. Thymidylate Synthase and Its Inhibitors
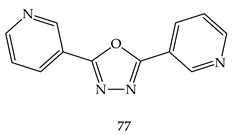
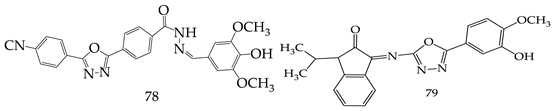


7. Miscellaneous Anticancer Properties of 1,3,4-Oxadiazole Derivatives
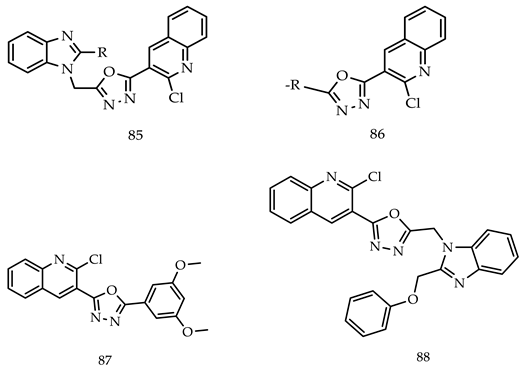
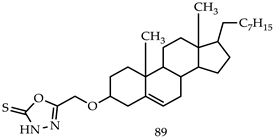




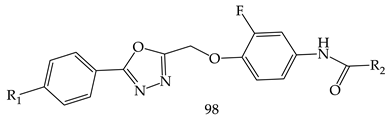
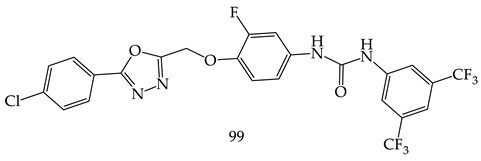
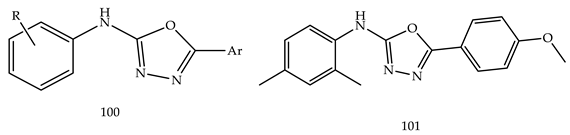
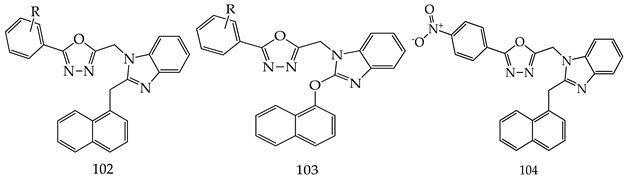


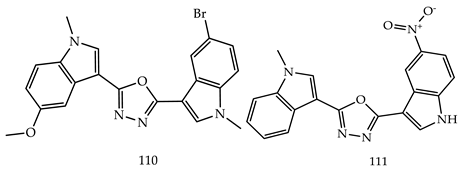

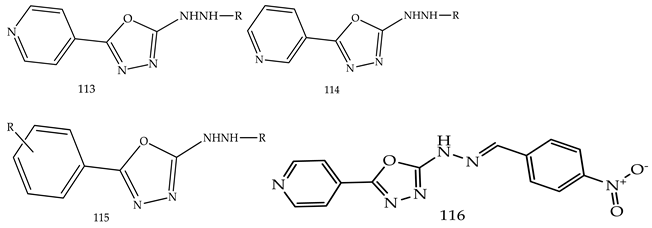
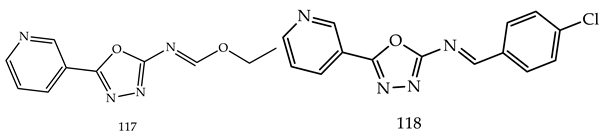
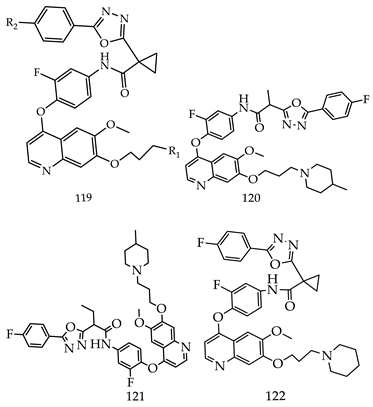

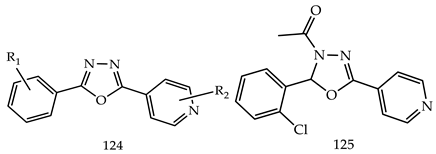
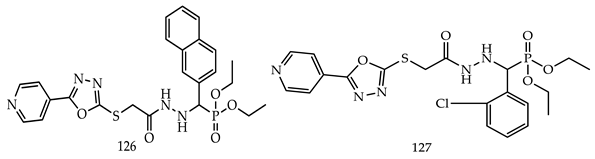
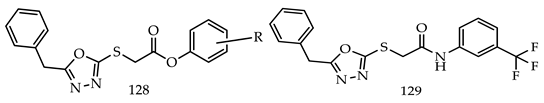


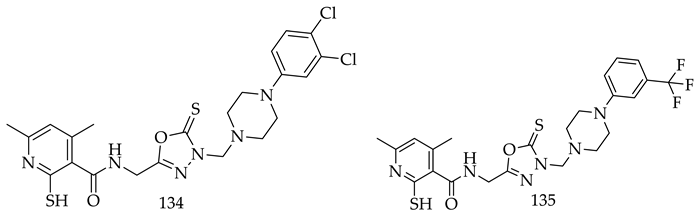

- (i)
- Compared to meta- and para-substituted derivatives, ortho substituted derivatives have remarkable potential. Therefore, derivatives 7, 9, and 10, bearing (2-OMe), (2-OH), and (2-Cl), respectively, on the phenyl ring showed the highest activity as comparison to derivatives 8 (3-COOH), 11 (3-Cl), and 13 (4-Br).
- (ii)
- In comparison with monosubstituted derivatives (139), disubstituted derivatives (140) have decreased cytotoxicity.

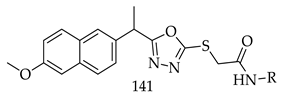
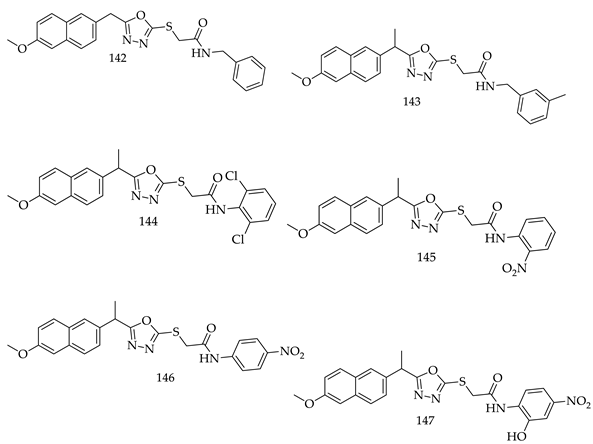
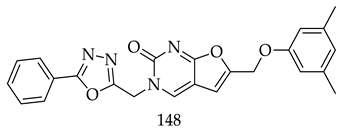




8. Patents
| S. No | Patent No | Publication Date | Title | References |
| 1. | US3065238 | 20 November 1962 | Production of 1,3,4-oxdiazoles | [136] |
| 2. | US2883391 | 21 April 1959 | Method of making 2-amino-5-substituted-1,3,4-oxadiazoles | [137] |
| 3. | 8796320 | 5 August 2014 | 1,3,4-Oxadiazole-2-carboxamide compound | [138] |
| 4. | 8846728 | 30 September 2014 | Sphingosine 1-phosphate (S1P) receptor modulators | [139] |
| 5. | 8846729 | 30 September 2014 | 2-thio-1,3,4-oxadiazoles azetidine derivatives as sphingosine-1 phosphate receptors modulators | [140] |
| 6. | WO033301 | 12 March 2015 | 1,3,4-Oxadiazole And 1,3,4-Thiadiazole Derivatives as Immunomodulators | [141] |
| 7. | 8735433 | 27 May 2014 | Sphingosine1-phosphate (S1P) receptor modulators | [142] |
| 8. | 8524751 | 3 September 2013 | 4-Oxadiazol-2-YL-indazoles as inhibitors of P13 kinases | [143] |
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kokovina, T.S.; Gadomsky, S.Y.; Terentiev, A.A.; Sanina, N.A. A Novel Approach to the Synthesis of 1,3,4-Thiadiazole-2-amine Derivatives. Molecules 2021, 26, 5159. [Google Scholar] [CrossRef]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New Approaches and Procedures for Cancer Treatment: Current Perspectives. SAGE Open Med. 2021, 9, 205031212110343. [Google Scholar] [CrossRef] [PubMed]
- Annual Cancer Facts & Figures | American Cancer Society. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures.html (accessed on 18 January 2023).
- Knight, S.R.; Shaw, C.A.; Pius, R.; Drake, T.M.; Norman, L.; Ademuyiwa, A.O.; Adisa, A.O.; Aguilera, M.L.; Al-Saqqa, S.W.; Al-Slaibi, I.; et al. Global Variation in Postoperative Mortality and Complications after Cancer Surgery: A Multicentre, Prospective Cohort Study in 82 Countries. Lancet 2021, 397, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Anthwal, T.; Nain, S. 1,3,4-Thiadiazole Scaffold: As Anti-Epileptic Agents. Front. Chem. 2022, 9, 671212. [Google Scholar] [CrossRef]
- Jain, A.K.; Sharma, S.; Vaidya, A.; Ravichandran, V.; Agrawal, R.K. 1,3,4-Thiadiazole and Its Derivatives: A Review on Recent Progress in Biological Activities. Chem. Biol. Drug Des. 2013, 81, 557–576. [Google Scholar] [CrossRef] [PubMed]
- Davinder, K.; Virender, K.; Rakesh, M.; Gajendra, S. Oxadiazole-an Important Bioactive Scaffold for Drug Discovery and Development Process against HIV and Cancer—A Review. Curr. Bioact. Compd. 2019, 15, 271–279. [Google Scholar] [CrossRef]
- de Oliveira, C.S.; Lira, B.F.; Barbosa-Filho, J.M.; Lorenzo, J.G.F.; de Athayde-Filho, P.F. Synthetic Approaches and Pharmacological Activity of 1,3,4-Oxadiazoles: A Review of the Literature from 2000–2012. Molecules 2012, 17, 10192–10231. [Google Scholar] [CrossRef]
- Nayak, S.; Gaonkar, S.L.; Musad, E.A.; Dawsar, A.M.A. 1,3,4-Oxadiazole-Containing Hybrids as Potential Anticancer Agents: Recent Developments, Mechanism of Action and Structure-Activity Relationships. J. Saudi Chem. Soc. 2021, 25, 101284. [Google Scholar] [CrossRef]
- Kashid, B.B.; Salunkhe, P.H.; Dongare, B.B.; More, K.R.; Khedkar, V.M.; Ghanwat, A.A. Synthesis of Novel of 2,5-Disubstituted 1,3,4-Oxadiazole Derivatives and Their in Vitro Anti-Inflammatory, Anti-Oxidant Evaluation, and Molecular Docking Study. Bioorg. Med. Chem. Lett. 2020, 30, 127136. [Google Scholar] [CrossRef]
- Ainsworth, C.; Hackler, R.E. AlkyI-l,3,4-Oxadiazoles. J. Org. Chem. 1966, 31, 3442–3444. [Google Scholar] [CrossRef]
- Vaidya, A.; Pathak, D.; Shah, K. 1,3,4-Oxadiazole and Its Derivatives: A Review on Recent Progress in Anticancer Activities. Chem. Biol. Drug Des. 2021, 97, 572–591. [Google Scholar] [CrossRef] [PubMed]
- Siwach, A.; Verma, P.K. Therapeutic Potential of Oxadiazole or Furadiazole Containing Compounds. BMC Chem. 2020, 14, 70. [Google Scholar] [CrossRef]
- Simon, V.; Cavalu, S.; Simon, S.; Mocuta, H.; Vanea, E.; Prinz, M.; Neumann, M. Surface functionalization of sol-gel derived aluminosilicates in simulated body fluids. Solid State Ion. 2009, 180, 764–769. [Google Scholar] [CrossRef]
- Bala, S.; Kamboj, S.; Kajal, A.; Saini, V.; Prasad, D.N. 1,3,4-Oxadiazole Derivatives: Synthesis, Characterization, Antimicrobial Potential, and Computational Studies. BioMed Res. Int. 2014, 2014, 172791. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Makawana, J.; Zhu, H.-L. 1,3,4-Oxadiazole Derivatives as Potential Biological Agents. Mini Rev. Med. Chem. 2013, 13, 1725–1743. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, S.; Asati, V.; Singh, J.; Roy, P.P. 1,3,4-Oxadiazoles: An Emerging Scaffold to Target Growth Factors, Enzymes and Kinases as Anticancer Agents. Eur. J. Med. Chem. 2015, 97, 124–141. [Google Scholar] [CrossRef]
- Salahuddin; Mazumder, A.; Yar, M.S.; Mazumder, R.; Chakraborthy, G.S.; Ahsan, M.J.; Rahman, M.U. Updates on Synthesis and Biological Activities of 1,3,4-Oxadiazole: A Review. Synth. Commun. 2017, 47, 1805–1847. [Google Scholar] [CrossRef]
- Santhosh, L.; Srinivasulu, C.; Durgamma, S.; Prabhu, G.; Sureshbabu, V.V. Facile One-Pot Synthesis of 2-Amino-1,3,4-Oxadiazole Tethered Peptidomimetics by Molecular-Iodine-Mediated Cyclodeselenization. New J. Chem. 2017, 41, 11225–11229. [Google Scholar] [CrossRef]
- Rivera, N.R.; Balsells, J.; Hansen, K.B. Synthesis of 2-Amino-5-Substituted-1,3,4-Oxadiazoles Using 1,3-Dibromo-5,5-Dimethylhydantoin as Oxidant. Tetrahedron Lett. 2006, 28, 4889–4891. [Google Scholar] [CrossRef]
- Ilangovan, A.; Saravanakumar, S.; Umesh, S. T3P as an Efficient Cyclodehydration Reagent for the One-Pot Synthesis of 2-Amino-1,3,4-Oxadiazoles. J. Chem. Sci. 2015, 127, 797–801. [Google Scholar] [CrossRef]
- Niu, P.; Kang, J.; Tian, X.; Song, L.; Liu, H.; Wu, J.; Yu, W.; Chang, J. Synthesis of 2-Amino-1,3,4-Oxadiazoles and 2-Amino-1,3,4-Thiadiazoles via Sequential Condensation and I2-Mediated Oxidative C-O/C-S Bond Formation. J. Org. Chem. 2015, 80, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. Synthesis and Biological Activity of 5-Substituted-2-Amino-1,3,4-Oxadiazole Derivatives. Turk. J. Chem. 2011, 35, 99–108. [Google Scholar] [CrossRef]
- Kapoorr, R.; Singh, S.N.; Tripathi, S.; Yadav, L.D.S. Photocatalytic Oxidative Heterocyclization of Semicarbazones: An Efficient Approach for the Synthesis of 1,3,4-Oxadiazoles. Synlett 2015, 26, 1201–1206. [Google Scholar] [CrossRef]
- Fang, T.; Tan, Q.; Ding, Z.; Liu, B.; Xu, B. Pd-Catalyzed Oxidative Annulation of Hydrazides with Isocyanides: Synthesis of 2-Amino-1,3,4-Oxadiazoles. Org. Lett. 2014, 16, 2342–2345. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.N.; Jadhav, N.C.; Jagadhane, P.B.; Telvekar, V.N. A Novel Strategy for the Construction of Azole Heterocycles via an Oxidative Desulfurization Approach Using Iodobenzene and Oxone®. Synlett 2012, 23, 1970–1972. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Vvedensky, V.; Cai, X.; Rogovoy, B.; Steel, P.J. Syntheses of 5-(2-Arylazenyl)-1,2,4-Triazoles and 2-Amino-5-Aryl-1,3,4-Oxadiazoles. Arkivoc 2002, 2002, 82–90. [Google Scholar] [CrossRef]
- Rajak, H.; Agarawal, A.; Parmar, P.; Thakur, B.S.; Veerasamy, R.; Sharma, P.C.; Kharya, M.D. 2,5-Disubstituted-1,3,4-Oxadiazoles/Thiadiazole as Surface Recognition Moiety: Design and Synthesis of Novel Hydroxamic Acid Based Histone Deacetylase Inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 5735–5738. [Google Scholar] [CrossRef]
- El-Sayed, W.A.; Ali, O.M.; Hendy, H.A.; Abdel-Rahman, A.A.H. Synthesis and Antimicrobial Activity of New 2,5-Disubstituted 1,3,4-Oxadiazoles and 1,2,4-Triazoles and Their Sugar Derivatives. Chin. J. Chem. 2012, 30, 77–83. [Google Scholar] [CrossRef]
- Dolman, S.J.; Gosselin, F.; O’Shea, P.D.; Davies, I.W. Superior Reactivity of Thiosemicarbazides in the Synthesis of 2-Amino-1,3,4-Oxadiazoles. J. Org. Chem. 2006, 71, 9548–9551. [Google Scholar] [CrossRef]
- Yang, S.J.; Lee, S.H.; Kwak, H.J.; Gong, Y.D. Regioselective Synthesis of 2-Amino-Substituted 1,3,4-Oxadiazole and 1,3,4-Thiadiazole Derivatives via Reagent-Based Cyclization of Thiosemicarbazide Intermediate. J. Org. Chem. 2013, 78, 438–444. [Google Scholar] [CrossRef]
- Koparir, M.; Çetin, A.; Cansiz, A. 5-Furan-2yl[1,3,4]Oxadiazole-2-Thiol, 5-Furan-2yl-4H [1,2,4] Triazole-3-Thiol and Their Thiol-Thione Tautomerism. Molecules 2005, 10, 475. [Google Scholar] [CrossRef]
- Suresh Kumar, G.V.; Rajendraprasad, Y.; Mallikarjuna, B.P.; Chandrashekar, S.M.; Kistayya, C. Synthesis of Some Novel 2-Substituted-5-[Isopropylthiazole] Clubbed 1,2,4-Triazole and 1,3,4-Oxadiazoles as Potential Antimicrobial and Antitubercular Agents. Eur. J. Med. Chem. 2010, 45, 2063–2074. [Google Scholar] [CrossRef]
- Dabiri, M.; Salehi, P.; Baghbanzadeh, M.; Bahramnejad, M. A Facile Procedure for the One-Pot Synthesis of Unsymmetrical 2,5-Disubstituted 1,3,4-Oxadiazoles. Tetrahedron Lett. 2006, 39, 6983–6986. [Google Scholar] [CrossRef]
- Alam, M.M.; Almalki, A.S.A.; Neamatallah, T.; Ali, N.M.; Malebari, A.M.; Nazreen, S. Synthesis of New 1, 3, 4-Oxadiazole-Incorporated 1, 2, 3-Triazole Moieties as Potential Anticancer Agents Targeting Thymidylate Synthase and Their Docking Studies. Pharmaceuticals 2020, 13, 390. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, A.; Mao, X.; Sun, X.; Gong, X. Silica-Supported Dichlorophosphate: A Recoverable Cyclodehydrant for the Eco-Friendly Synthesis of 2,5-Disubstituted 1,3,4-Oxadiazoles under Solvent-Free and Microwave Irradiation Conditions. J. Braz. Chem. Soc. 2008, 19, 1622–1626. [Google Scholar] [CrossRef]
- Guin, S.; Ghosh, T.; Rout, S.K.; Banerjee, A.; Patel, B.K. Cu(II) Catalyzed Imine C-H Functionalization Leading to Synthesis of 2,5-Substituted 1,3,4-Oxadiazoles. Org. Lett. 2011, 13, 5976–5979. [Google Scholar] [CrossRef]
- Mickevičius, V.; Vaickelionienė, R.; Sapijanskaitė, B. Synthesis of Substituted 1,3,4-Oxadiazole Derivatives. Chem. Heterocycl. Compd. 2009, 45, 215–218. [Google Scholar] [CrossRef]
- Nagendra, G.; Lamani, R.S.; Narendra, N.; Sureshbabu, V.V. A Convenient Synthesis of 1,3,4-Thiadiazole and 1,3,4-Oxadiazole Based Peptidomimetics Employing Diacylhydrazines Derived from Amino Acids. Tetrahedron Lett. 2010, 51, 6338–6341. [Google Scholar] [CrossRef]
- Stecoza, C.E.; Nitulescu, G.M.; Draghici, C.; Caproiu, M.T.; Olaru, O.T.; Bostan, M.; Mihaila, M. Synthesis and Anticancer Evaluation of New 1,3,4-Oxadiazole Derivatives. Pharmaceuticals 2021, 14, 438. [Google Scholar] [CrossRef] [PubMed]
- Mohan, C.D.; Anilkumar, N.C.; Rangappa, S.; Shanmugam, M.K.; Mishra, S.; Chinnathambi, A.; Alharbi, S.A.; Bhattacharjee, A.; Sethi, G.; Kumar, A.P.; et al. Novel 1,3,4-Oxadiazole Induces Anticancer Activity by Targeting NF-ΚB in Hepatocellular Carcinoma Cells. Front. Oncol. 2018, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Kumar, V.; Kumar, H.; Deep, A.; Kumar, R. 1,3,4-Oxadiazole as an Emerging Telomerase Inhibitor - a Prom-ising Anticancer Motif. Cancer Adv. 2022, 5, e22018. [Google Scholar] [CrossRef]
- Ningegowda, R.; Chandrashekharappa, S.; Singh, V.; Mohanlall, V.; Venugopala, K.N. Design, Synthesis and Characterization of Novel 2-(2,3-Dichlorophenyl)-5-Aryl-1,3,4-Oxadiazole Derivatives for Their Anti-Tubercular Activity against Mycobacterium Tuberculosis. Chem. Data Collect. 2020, 28, 100431. [Google Scholar] [CrossRef]
- Gholap, S.; Tambe, M.; Nawale, L.; Sarkar, D.; Sangshetti, J.; Damale, M. Design, Synthesis, and Pharmacological Evaluation of Fluorinated Azoles as Anti-Tubercular Agents. Arch. Pharm. (Weinheim) 2018, 351, 1700294. [Google Scholar] [CrossRef]
- Hamdani, S.S.; Khan, B.A.; Ahmed, M.N.; Hameed, S.; Akhter, K.; Ayub, K.; Mahmood, T. Synthesis, Crystal Structures, Computational Studies and α-Amylase Inhibition of Three Novel 1,3,4-Oxadiazole Derivatives. J. Mol. Struct. 2020, 1200, 127085. [Google Scholar] [CrossRef]
- Amir, M.; Javed, S.A.; Kumar, H. Synthesis of Some 1,3,4-Oxadiazole Derivatives as Potential Antiinflammatory Agents. ChemInform 2007, 38. [Google Scholar] [CrossRef]
- Abd-Ellah, H.S.; Abdel-Aziz, M.; Shoman, M.E.; Beshr, E.A.M.; Kaoud, T.S.; Ahmed, A.S.F.F. Novel 1,3,4-Oxadiazole/Oxime Hybrids: Synthesis, Docking Studies and Investigation of Anti-Inflammatory, Ulcerogenic Liability and Analgesic Activities. Bioorg. Chem. 2016, 69, 48–63. [Google Scholar] [CrossRef]
- Rathore, A.; Sudhakar, R.; Ahsan, M.J.; Ali, A.; Subbarao, N.; Jadav, S.S.; Umar, S.; Yar, M.S. In Vivo Anti-Inflammatory Activity and Docking Study of Newly Synthesized Benzimidazole Derivatives Bearing Oxadiazole and Morpholine Rings. Bioorg. Chem. 2017, 70, 107–117. [Google Scholar] [CrossRef]
- Abbas Tabatabai, S.; Lashkari, S.B.; Zarrindast, M.R.; Gholibeikian, M.; Shafiee, A. Design, Synthesis and Anticonvulsant Activity of 2-(2-Phenoxy) Phenyl-1,3,4-Oxadiazole Derivatives. Iran. J. Pharm. Res. 2013, 12, 105–111. [Google Scholar]
- Harish, K.; Mohana, K.; Mallesha, L.; Veeresh, B.; Reddy, B.; Kumar, N. Synthesis and Evaluation of In Vivo Anticonvulsant Activity of 2,5-Disubstituted-1,3,4-Oxadiazole Derivatives. Lett. Drug Des. Discov. 2013, 10, 783–791. [Google Scholar] [CrossRef]
- Sindhe, M.A.; Bodke, Y.D.; Kenchappa, R.; Telkar, S.; Chandrashekar, A. Synthesis of a Series of Novel 2,5-Disubstituted-1,3,4-Oxadiazole Derivatives as Potential Antioxidant and Antibacterial Agents. J. Chem. Biol. 2016, 9, 79. [Google Scholar] [CrossRef]
- Lakshmi Ranganatha, V.; Khanum, S.A. Synthesis and Evaluation of in Vitro Antioxidant Properties of Novel 2,5-Disubstituted 1,3,4-Oxadiazoles. Bioorg. Khim. 2014, 40, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Bankar, G.R.; Nandakumar, K.; Nayak, P.G.; Thakur, A.; Chamallamudi, M.R.; Nampurath, G.K. Vasorelaxant Effect in Rat Aortic Rings through Calcium Channel Blockage: A Preliminary in Vitro Assessment of a 1,3,4-Oxadiazole Derivative. Chem. Biol. Interact. 2009, 181, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zou, B.; Gunaratna, M.J.; Weerasekara, S.; Tong, Z.; Nguyen, T.D.T.; Koldas, S.; Cao, W.S.; Pascual, C.; Xie, X.S.; et al. synthesis of 1,3,4-oxadiazoles as selective t-type calcium channel inhibitors. Heterocycles 2019, 101, 145–164. [Google Scholar] [CrossRef]
- Bankar, G.R.; Nampurath, G.K.; Nayak, P.G.; Bhattacharya, S. A Possible Correlation between the Correction of Endothelial Dysfunction and Normalization of High Blood Pressure Levels by 1,3,4-Oxadiazole Derivative, an L-Type Ca2+ Channel Blocker in Deoxycorticosterone Acetate and NG-Nitro-l-Arginine Hypertensive Rats. Chem. Biol. Interact. 2010, 183, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Zabiulla; Nagesh Khadri, M.J.; Bushra Begum, A.; Sunil, M.K.; Khanum, S.A. Synthesis, Docking and Biological Evaluation of Thiadiazole and Oxadiazole Derivatives as Antimicrobial and Antioxidant Agents. Results Chem. 2020, 2, 100045. [Google Scholar] [CrossRef]
- Madhu Sekhar, M.; Nagarjuna, U.; Padmavathi, V.; Padmaja, A.; Reddy, N.V.; Vijaya, T. Synthesis and Antimicrobial Activity of Pyrimidinyl 1,3,4-Oxadiazoles, 1,3,4-Thiadiazoles and 1,2,4-Triazoles. Eur. J. Med. Chem. 2018, 145, 1–10. [Google Scholar] [CrossRef]
- Patil, A.S.; Mohite, S.K. An Overview: 1,3,4-Oxadiazole and Its Uses. Asian J. Res. Chem. 2021, 14, 389–392. [Google Scholar] [CrossRef]
- Wang, B.L.; Zhu, H.W.; Ma, Y.; Xiong, L.X.; Li, Y.Q.; Zhao, Y.; Zhang, J.F.; Chen, Y.W.; Zhou, S.; Li, Z.M. Synthesis, Insecticidal Activities, and SAR Studies of Novel Pyridylpyrazole Acid Derivatives Based on Amide Bridge Modification of Anthranilic Diamide Insecticides. J. Agric. Food. Chem. 2013, 61, 5483–5493. [Google Scholar] [CrossRef]
- Ke, S.; Li, Z.; Qian, X. 1,3,4-Oxadiazole-3(2H)-Carboxamide Derivatives as Potential Novel Class of Monoamine Oxidase (MAO) Inhibitors: Synthesis, Evaluation, and Role of Urea Moiety. Bioorg. Med. Chem. 2008, 16, 7565–7572. [Google Scholar] [CrossRef]
- Khan, M.T.H.; Choudhary, M.I.; Khan, K.M.; Rani, M.; Atta-ur-Rahman. Structure-Activity Relationships of Tyrosinase Inhibitory Combinatorial Library of 2,5-Disubstituted-1,3,4-Oxadiazole Analogues. Bioorg. Med. Chem. 2005, 13, 3385–3395. [Google Scholar] [CrossRef]
- Palmer, J.T.; Hirschbein, B.L.; Cheung, H.; McCarter, J.; Janc, J.W.; Yu, Z.W.; Wesolowski, G. Keto-1,3,4-Oxadiazoles as Cathepsin K Inhibitors. Bioorg. Med. Chem. Lett. 2006, 16, 2909–2914. [Google Scholar] [CrossRef]
- Hockemeyer, D.; Collins, K. Control of Telomerase Action at Human Telomeres. Nat. Struct. Mol. Biol. 2015, 22, 848. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, E.H. Structure and Function of Telomeres. Nature 1991, 350, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Cavalu, S.; Damian, G. Rotational correlation times of 3-carbamoyl-2,2,5,5-tetramethyl-3-pyrrolin-1-yloxy spin label with respect to heme and nonheme proteins. Biomacromolecules 2003, 6, 1630–1635. [Google Scholar] [CrossRef]
- Preston, R.J. Telomeres, Telomerase and Chromosome Stability. Radiat. Res. 1997, 147, 529–534. [Google Scholar] [CrossRef]
- Giardini, M.A.; Segatto, M.; da Silva, M.S.; Nunes, V.S.; Cano, M.I.N. Telomere and Telomerase Biology. Prog. Mol. Biol. Transl. Sci. 2014, 125, 1–40. [Google Scholar] [CrossRef]
- Grandin, N.; Charbonneau, M. Protection against Chromosome Degradation at the Telomeres. Biochimie 2008, 90, 41–59. [Google Scholar] [CrossRef]
- Gancarcíková, M.; Zemanová, Z.; Brezinová, J.; Berková, A.; Vcelíková, S.; Smigová, J.; Michalová, K. The Role of Telomeres and Telomerase Complex in Haematological Neoplasia: The Length of Telomeres as a Marker of Carcinogenesis and Prognosis of Disease. Prague Med. Rep. 2010, 111, 91–105. [Google Scholar]
- Saretzki, G. Telomerase, Mitochondria and Oxidative Stress. Exp. Gerontol. 2009, 44, 485–492. [Google Scholar] [CrossRef]
- Saretzki, G. Extra-Telomeric Functions of Human Telomerase: Cancer, Mitochondria and Oxidative Stress. Curr. Pharm. Des. 2014, 20, 6386–6403. [Google Scholar] [CrossRef]
- Ahmed, S.; Passos, J.F.; Birket, M.J.; Beckmann, T.; Brings, S.; Peters, H.; Birch-Machin, M.A.; von Zglinicki, T.; Saretzki, G. Telomerase Does Not Counteract Telomere Shortening but Protects Mitochondrial Function under Oxidative Stress. J. Cell Sci. 2008, 121, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Makpol, S.; Zainuddin, A.; Rahim, N.A.; Yusof, Y.A.M.; Ngah, W.Z.W. Alpha-Tocopherol Modulates Hydrogen Peroxide-Induced DNA Damage and Telomere Shortening of Human Skin Fibroblasts Derived from Differently Aged Individuals. Planta Med. 2010, 76, 869–875. [Google Scholar] [CrossRef]
- Cinta-Pinzaru, S.; Cavalu, S.; Leopold, N.; Petry, R.; Kiefer, W. Raman and surface-enhanced Raman spectroscopy of tempyo spin labelled ovalbumin. J. Mol. Struct. 2001, 565, 225–229. [Google Scholar] [CrossRef]
- Shay, J.W. Telomeres and Aging. Curr. Opin. Cell Biol. 2018, 52, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yu, H.; Ding, L.; Wu, Z.; de Los Santos, F.G.; Liu, J.; Ullenbruch, M.; Hu, B.; Martins, V.; Phan, S.H. Conditional Knockout of Telomerase Reverse Transcriptase in Mesenchymal Cells Impairs Mouse Pulmonary Fibrosis. PLoS ONE 2015, 10, e0142547. [Google Scholar] [CrossRef]
- Mukherjee, S.; Firpo, E.J.; Wang, Y.; Roberts, J.M. Separation of Telomerase Functions by Reverse Genetics. Proc. Natl. Acad. Sci. USA 2011, 108, E1363–E1371. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.L.; Coller, H.A.; Roberts, J.M. Telomerase Modulates Expression of Growth-Controlling Genes and Enhances Cell Proliferation. Nat. Cell Biol. 2003, 5, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Oshimura, M.; Barrett, J.C. Multiple Pathways to Cellular Senescence: Role of Telomerase Repressors. Eur. J. Cancer 1997, 33, 710–715. [Google Scholar] [CrossRef]
- de Vitis, M.; Berardinelli, F.; Sgura, A. Telomere Length Maintenance in Cancer: At the Crossroad between Telomerase and Alternative Lengthening of Telomeres (ALT). Int. J. Mol. Sci. 2018, 19, 606. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, F.; Liu, L. Alternative Lengthening of Telomeres (ALT) in Tumors and Pluripotent Stem Cells. Genes 2019, 10, 1030. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Y.; Liu, Y.; Xing, Y.; Miao, C.; Zhao, Y.; Chang, X.; Zhang, Q. The Role of Oxidative Stress and Natural Antioxidants in Ovarian Aging. Front. Pharmacol. 2021, 11, 2364. [Google Scholar] [CrossRef]
- Rashid, M.; Husain, A.; Mishra, R.; Karim, S.; Khan, S.; Ahmad, M.; Al-wabel, N.; Husain, A.; Ahmad, A.; Khan, S.A. Design and Synthesis of Benzimidazoles Containing Substituted Oxadiazole, Thiadiazole and Triazolo-Thiadiazines as a Source of New Anticancer Agents. Arab. J. Chem. 2019, 12, 3202–3224. [Google Scholar] [CrossRef]
- Zhang, X.M.; Qiu, M.; Sun, J.; Zhang, Y.B.; Yang, Y.S.; Wang, X.L.; Tang, J.F.; Zhu, H.L. Synthesis, Biological Evaluation, and Molecular Docking Studies of 1,3,4-Oxadiazole Derivatives Possessing 1,4-Benzodioxan Moiety as Potential Anticancer Agents. Bioorg. Med. Chem. 2011, 19, 6518–6524. [Google Scholar] [CrossRef]
- Sun, J.; Zhu, H.; Yang, Z.M.; Zhu, H.L. Synthesis, Molecular Modeling and Biological Evaluation of 2-Aminomethyl-5-(Quinolin-2-Yl)-1,3,4-Oxadiazole-2(3H)-Thione Quinolone Derivatives as Novel Anticancer Agent. Eur. J. Med. Chem. 2013, 60, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.Z.; Zhang, X.M.; Xu, Y.; Cheng, K.; Jiao, Q.C.; Zhu, H.L. Synthesis, Biological Evaluation, and Molecular Docking Studies of 2-Chloropyridine Derivatives Possessing 1,3,4-Oxadiazole Moiety as Potential Antitumor Agents. Bioorg. Med. Chem. 2010, 18, 7836–7841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, X.L.; Shi, J.; Wang, S.F.; Yin, Y.; Yang, Y.S.; Zhang, W.M.; Zhu, H.L. Synthesis, Molecular Modeling and Biological Evaluation of N-Benzylidene-2-((5-(Pyridin-4-Yl)-1,3,4-Oxadiazol-2-Yl)Thio)Acetohydrazide Derivatives as Potential Anticancer Agents. Bioorg. Med. Chem. 2014, 22, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Clayton, A.L.; Hazzalin, C.A.; Mahadevan, L.C. Enhanced Histone Acetylation and Transcription: A Dynamic Perspective. Mol. Cell 2006, 23, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Salahuddin; Sharma, V.; Kumar, R.; Joshi, S.; Kumari, S.; Saxena, S.; Mazumder, A.; Yar, M.S.; Ahsan, M.J. 1,3,4-Oxadiazoles as Potential Pharmacophore for Cytotoxic Potentiality: A Comprehensive Review. Curr. Top. Med. Chem. 2021, 21, 1377–1397. [Google Scholar] [CrossRef]
- Gujral, P.; Mahajan, V.; Lissaman, A.C.; Ponnampalam, A.P. Histone Acetylation and the Role of Histone Deacetylases in Normal Cyclic Endometrium. Reprod. Biol. Endocrinol. 2020, 18, 84. [Google Scholar] [CrossRef]
- Valente, S.; Trisciuoglio, D.; de Luca, T.; Nebbioso, A.; Labella, D.; Lenoci, A.; Bigogno, C.; Dondio, G.; Miceli, M.; Brosch, G.; et al. 1,3,4-Oxadiazole-Containing Histone Deacetylase Inhibitors: Anticancer Activities in Cancer Cells. J. Med. Chem. 2014, 57, 6259–6265. [Google Scholar] [CrossRef]
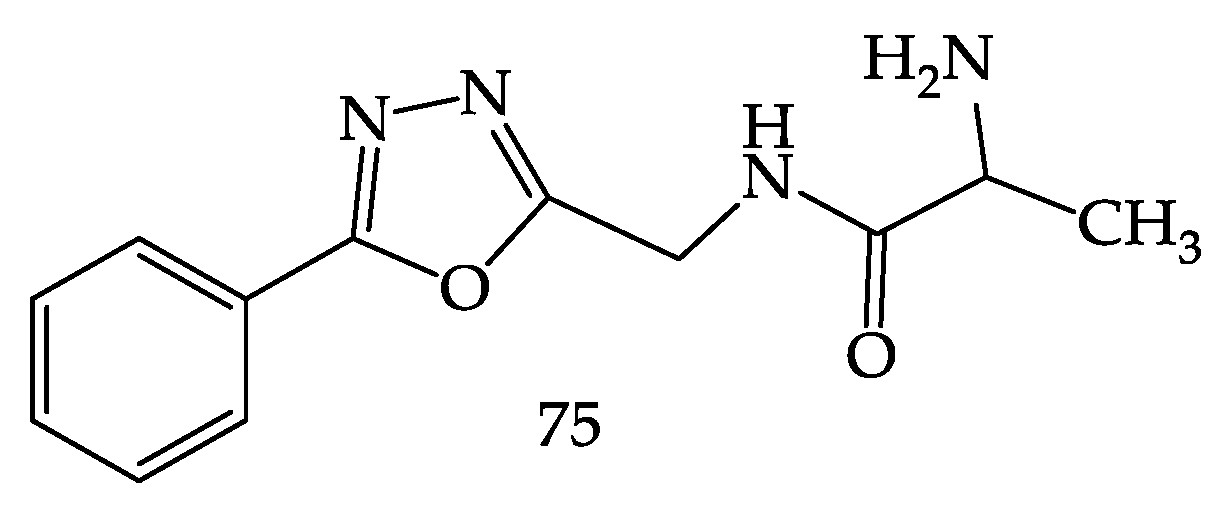
- Pidugu, V.R.; Yarla, N.S.; Bishayee, A.; Kalle, A.M.; Satya, A.K. Novel Histone Deacetylase 8-Selective Inhibitor 1,3,4-Oxadiazole-Alanine Hybrid Induces Apoptosis in Breast Cancer Cells. Apoptosis 2017, 22, 1394–1403. [Google Scholar] [CrossRef]
- Pidugu, V.R.; Yarla, N.S.; Pedada, S.R.; Kalle, A.M.; Satya, A.K. Design and Synthesis of Novel HDAC8 Inhibitory 2,5-Disubstituted-1,3,4-Oxadiazoles Containing Glycine and Alanine Hybrids with Anti Cancer Activity. Bioorg. Med. Chem. 2016, 24, 5611–5617. [Google Scholar] [CrossRef] [PubMed]
- Alzhrani, Z.M.M.; Alam, M.M.; Neamatallah, T.; Nazreen, S. Design, Synthesis and in Vitro Antiproliferative Activity of New Thiazolidinedione-1,3,4-Oxadiazole Hybrids as Thymidylate Synthase Inhibitors. J. Enzyme Inhib Med. Chem. 2020, 35, 1116–1123. [Google Scholar] [CrossRef]
- Li, X.-Y.; Wang, D.-P.; Lu, G.-Q.; Liu, K.-L.; Zhang, T.-J.; Li, S.; Mohamed, O.K.; Xue, W.-H.; Qian, X.-H.; Meng, F.-H. Development of a Novel Thymidylate Synthase (TS) Inhibitor Capable of up-Regulating P53 Expression and Inhibiting Angiogenesis in NSCLC. J. Adv. Res. 2020, 26, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.R.; Li, D.D.; Pi, Y.Z.; Li, J.R.; Sun, J.; Fang, F.; Zhong, W.Q.; Gong, H.B.; Zhu, H.L. Novel 1,3,4-Oxadiazole Thioether Derivatives Targeting Thymidylate Synthase as Dual Anticancer/Antimicrobial Agents. Bioorg. Med. Chem. 2013, 21, 2286–2297. [Google Scholar] [CrossRef]
- Nakajima, Y.; Madhyastha, R.; Maruyama, M. 2-Deoxy-D-Ribose, a Downstream Mediator of Thymidine Phosphorylase, Regulates Tumor Angiogenesis and Progression. Anticancer Agents Med. Chem. 2009, 9, 239–245. [Google Scholar] [CrossRef]
- Takebayashi, Y.; Yamada, K.; Miyadera, K.; Sumizawa, T.; Furukawa, T.; Kinoshita, F.; Aoki, D.; Okumura, H.; Yamada, Y.; Akiyama, S.I.; et al. The Activity and Expression of Thymidine Phosphorylase in Human Solid Tumours. Eur. J. Cancer 1996, 32, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Tabata, S.; Yamamoto, M.; Soga, T.; Goto, H.; Hirayama, A.; Ohishi, M.; Kuramoto, T.; Mitsuhashi, A.; Ikeda, R.; Haraguchi, M.; et al. Thymidine Catabolism as a Metabolic Strategy for Cancer Survival. Cell Rep. 2017, 19, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Glomb, T.; Szymankiewicz, K.; Świątek, P. Anti-Cancer Activity of Derivatives of 1,3,4-Oxadiazole. Molecules 2018, 23, 3361. [Google Scholar] [CrossRef]
- Khan, K.M.; Rani, M.; Ambreen, N.; Ali, M.; Hussain, S.; Perveen, S.; Choudhary, M.I. 2,5-Disubstituted-1,3,4-Oxadiazoles: Thymidine Phosphorylase Inhibitors. Med. Chem. Res. 2013, 22, 6022–6028. [Google Scholar] [CrossRef]
- Javid, M.T.; Rahim, F.; Taha, M.; Nawaz, M.; Wadood, A.; Ali, M.; Mosaddik, A.; Shah, S.A.A.; Farooq, R.K. Synthesis, SAR Elucidations and Molecular Docking Study of Newly Designed Isatin Based Oxadiazole Analogs as Potent Inhibitors of Thymidine Phosphorylase. Bioorg. Chem. 2018, 79, 323–333. [Google Scholar] [CrossRef]
- Bajaj, S.; Roy, P.P.; Singh, J. Synthesis, Thymidine Phosphorylase Inhibitory and Computational Study of Novel 1,3,4-Oxadiazole-2-Thione Derivatives as Potential Anticancer Agents. Comput. Biol. Chem. 2018, 76, 151–160. [Google Scholar] [CrossRef]
- Taha, M.; Rashid, U.; Imran, S.; Ali, M. Rational Design of Bis-Indolylmethane-Oxadiazole Hybrids as Inhibitors of Thymidine Phosphorylase. Bioorg. Med. Chem. 2018, 26, 3654–3663. [Google Scholar] [CrossRef]
- Salahuddin; Mazumder, A.; Shaharyar, M. Synthesis, Antibacterial and Anticancer Evaluation of 5-Substituted (1,3,4-Oxadiazol-2-Yl)Quinoline. Med. Chem. Res. 2015, 6, 2514–2528. [Google Scholar] [CrossRef]
- Shamsuzzaman; Siddiqui, T.; Alam, M.G.; Dar, A.M. Original Article. J. Saudi Chem. Soc. 2015, 4, 387–391. [Google Scholar] [CrossRef]
- Shivarama Holla, B.; Poojary, N.; Bhat, S.; Ashok, M.; Poojary, B. Synthesis and Anticancer Activity Studies on Some 2-Chloro-1,4-Bis-(5-Substituted-1,3,4-Oxadiazol-2-Ylmethyleneoxy) Phenylene Derivatives. Indian J. Chem. 2005, 44, 1669–1673. [Google Scholar]
- Ragab, F.A.F.; Abou-Seri, S.M.; Abdel-Aziz, S.A.; Alfayomy, A.M.; Aboelmagd, M. Design, Synthesis and Anticancer Activity of New Monastrol Analogues Bearing 1,3,4-Oxadiazole Moiety. Eur. J. Med. Chem. 2017, 138, 140–151. [Google Scholar] [CrossRef]
- Mansour, A.K.; Eid, M.M.; Khalil, N.S.A.M. Synthesis and Reactions of Some New Heterocyclic Carbohydrazides and Related Compounds as Potential Anticancer Agents. Molecules 2003, 8, 744–755. [Google Scholar] [CrossRef]
- Gamal El-Din, M.M.; El-Gamal, M.I.; Abdel-Maksoud, M.S.; Yoo, K.H.; Oh, C.H. Synthesis and in Vitro Antiproliferative Activity of New 1,3,4-Oxadiazole Derivatives Possessing Sulfonamide Moiety. Eur. J. Med. Chem. 2015, 90, 45–52. [Google Scholar] [CrossRef]
- Gamal El-Din, M.M.; El-Gamal, M.I.; Abdel-Maksoud, M.S.; Yoo, K.H.; Baek, D.; Choi, J.; Lee, H.; Oh, C.H. Design, Synthesis, and in Vitro Antiproliferative and Kinase Inhibitory Effects of Pyrimidinylpyrazole Derivatives Terminating with Arylsulfonamido or Cyclic Sulfamide Substituents. J. Enzyme Inhib Med. Chem. 2016, 31, 111–122. [Google Scholar] [CrossRef]
- Ahsan, M.J.; Sharma, J.; Singh, M.; Jadav, S.S.; Yasmin, S. Synthesis and Anticancer Activity of N-Aryl-5-substituted-1,3,4-oxadiazol-2-amine Analogues. BioMed Res. Int. 2014, 2014, 814984. [Google Scholar] [CrossRef]
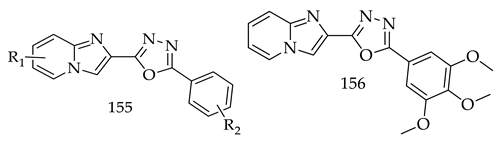
- Salahuddin; Shaharyar, M.; Mazumder, A.; Ahsan, M.J. Synthesis, Characterization and Anticancer Evaluation of 2-(Naphthalen-1-Ylmethyl/Naphthalen-2-Yloxymethyl)-1-[5-(Substituted Phenyl)-[1,3,4]Oxadiazol-2-Ylmethyl]-1H-Benzimidazole. Arab. J. Chem. 2014, 7, 418–424. [Google Scholar] [CrossRef]
- Jawed Ahsan, M.; Pratap Singh Rathod, V.; Singh, M.; Sharma, R.; Singh Jadav, S.; Yasmin, S.; Kumar, P. Synthesis, Anticancer and Molecular Docking Studies of 2-(4-Chlorophenyl)-5-aryl-1,3,4-oxadiazole Analogues. Med. Chem. 2013, 3, 294–297. [Google Scholar] [CrossRef]
- Caneschi, W.; Enes, K.B.; Carvalho de Mendonça, C.; de Souza Fernandes, F.; Miguel, F.B.; da Silva Martins, J.; le Hyaric, M.; Pinho, R.R.; Duarte, L.M.; Leal de Oliveira, M.A.; et al. Synthesis and Anticancer Evaluation of New Lipophilic 1,2,4 and 1,3,4-Oxadiazoles. Eur. J. Med. Chem. 2019, 165, 18–30. [Google Scholar] [CrossRef]
- Sreenivasulu, R.; Durgesh, R.; Jadav, S.S.; Sujitha, P.; Ganesh Kumar, C.; Raju, R.R. Synthesis, Anticancer Evaluation and Molecular Docking Studies of Bis(Indolyl) Triazinones, Nortopsentin Analogs. Chem. Pap. 2018, 72, 1369–1378. [Google Scholar] [CrossRef]
- Fathi, M.A.A.; Abd El-Hafeez, A.A.; Abdelhamid, D.; Abbas, S.H.; Montano, M.M.; Abdel-Aziz, M. 1,3,4-Oxadiazole/Chalcone Hybrids: Design, Synthesis, and Inhibition of Leukemia Cell Growth and EGFR, Src, IL-6 and STAT3 Activities. Bioorg. Chem. 2019, 84, 150–163. [Google Scholar] [CrossRef]
- El-Sayed, N.A.; Nour, M.S.; Salem, M.A.; Arafa, R.K. New Oxadiazoles with Selective-COX-2 and EGFR Dual Inhibitory Activity: Design, Synthesis, Cytotoxicity Evaluation and in Silico Studies. Eur. J. Med. Chem. 2019, 183, 111693. [Google Scholar] [CrossRef]
- Xu, C.; Han, Y.; Xu, S.; Wang, R.; Yue, M.; Tian, Y.; Li, X.; Zhao, Y.; Gong, P. Design, Synthesis and Biological Evaluation of New Axl Kinase Inhibitors Containing 1,3,4-Oxadiazole Acetamide Moiety as Novel Linker. Eur. J. Med. Chem. 2020, 186, 111867. [Google Scholar] [CrossRef]
- Dhawan, S.; Kerru, N.; Awolade, P.; Singh-Pillay, A.; Saha, S.T.; Kaur, M.; Jonnalagadda, S.B.; Singh, P. Synthesis, Computational Studies and Antiproliferative Activities of Coumarin-Tagged 1,3,4-Oxadiazole Conjugates against MDA-MB-231 and MCF-7 Human Breast Cancer Cells. Bioorg. Med. Chem. 2018, 26, 5612–5623. [Google Scholar] [CrossRef]
- Sankhe, N.M.; Durgashivaprasad, E.; Gopalan Kutty, N.; Venkata Rao, J.; Narayanan, K.; Kumar, N.; Jain, P.; Udupa, N.; Vasanth Raj, P. Novel 2,5-Disubstituted-1,3,4-Oxadiazole Derivatives Induce Apoptosis in HepG2 Cells through P53 Mediated Intrinsic Pathway. Arab. J. Chem. 2019, 12, 2548–2555. [Google Scholar] [CrossRef]
- Ewies, E.F.; El-Hussieny, M.; El-Sayed, N.F.; Fouad, M.A. Design, Synthesis and Biological Evaluation of Novel α-Aminophosphonate Oxadiazoles via Optimized Iron Triflate Catalyzed Reaction as Apoptotic Inducers. Eur. J. Med. Chem. 2019, 180, 310–320. [Google Scholar] [CrossRef]
- Iqbal, Z.; Ashraf, Z.; Hassan, M.; Abbas, Q.; Jabeen, E. Substituted Phenyl[(5-benzyl-1,3,4-oxadiazol-2-yl)sulfanyl]acetates/acetamides as Alkaline Phosphatase Inhibitors: Synthesis, Computational Studies, Enzyme Inhibitory Kinetics and DNA Binding Studies. Bioorg. Chem. 2019, 90, 103108. [Google Scholar] [CrossRef]
- Çevik, U.A.; Celik, I.; Mella, J.; Mellado, M.; Özkay, Y.; Kaplanclkll, Z.A. Design, Synthesis, and Molecular Modeling Studies of a Novel Benzimidazole as an Aromatase Inhibitor. ACS Omega 2022, 7, 16152. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Nazreen, S.; Almalki, A.S.A.; Elhenawy, A.A.; Alsenani, N.I.; Elbehairi, S.E.I.; Malebari, A.M.; Alfaifi, M.Y.; Alsharif, M.A.; Alfaifi, S.Y.M. Naproxen Based 1,3,4-Oxadiazole Derivatives as EGFR Inhibitors: Design, Synthesis, Anticancer, and Computational Studies. Pharmaceuticals 2021, 14, 870. [Google Scholar] [CrossRef]
- Pham, E.C.; Truong, T.N.; Dong, N.H.; Vo, D.D.; Hong Do, T.T. Synthesis of a Series of Novel 2-Amino-5-substituted 1,3,4-Oxadiazole and 1,3,4-Thiadiazole Derivatives as Potential Anticancer, Antifungal and Antibacterial Agents. Med. Chem. 2022, 18, 558–573. [Google Scholar] [CrossRef]
- Świątek, P.; Glomb, T.; Dobosz, A.; Gębarowski, T.; Wojtkowiak, K.; Jezierska, A.; Panek, J.J.; Świątek, M.; Strzelecka, M. Biological Evaluation and Molecular Docking Studies of Novel 1,3,4-Oxadiazole Derivatives of 4,6-Dimethyl-2-sulfanylpyridine-3-carboxamide. Int. J. Mol. Sci. 2022, 23, 549. [Google Scholar] [CrossRef]
- Almalki, A.S.A.; Nazreen, S.; Malebari, A.M.; Ali, N.M.; Elhenawy, A.A.; Alghamdi, A.A.A.; Ahmad, A.; Alfaifi, S.Y.M.; Alsharif, M.A.; Alam, M.M. Synthesis and Biological Evaluation of 1,2,3-Triazole Tethered Thymol-1,3,4-oxadiazole Derivatives as Anticancer and Antimicrobial Agents. Pharmaceuticals 2021, 14, 866. [Google Scholar] [CrossRef]
- Hagras, M.; Saleh, M.A.; EzzEldin, R.R.; Abuelkhir, A.A.; Khidr, E.G.; El-Husseiny, A.A.; El-Mahdy, H.A.; Elkaeed, E.B.; Eissa, I.H. 1,3,4-Oxadiazole-Naphthalene Hybrids as Potential VEGFR-2 Inhibitors: Design, Synthesis, Antiproliferative Activity, Apoptotic Effect, and in Silico Studies. J. Enzyme Inhib. Med. Chem. 2022, 37, 380–396. [Google Scholar] [CrossRef] [PubMed]
- El Mansouri, A.E.; Oubella, A.; Dânoun, K.; Ahmad, M.; Neyts, J.; Jochmans, D.; Snoeck, R.; Andrei, G.; Morjani, H.; Zahouily, M.; et al. Discovery of Novel Furo[2,3-d]pyrimidin-2-one–1,3,4-oxadiazole Hybrid Derivatives as Dual Antiviral and Anticancer Agents That Induce Apoptosis. Arch. Pharm. (Weinheim) 2021, 354, 2100146. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, S.; Kumar, M.S.; Tinwala, H.; YC, M. Design, Synthesis, Modelling Studies and Biological Evaluation of 1,3,4-Oxadiazole Derivatives as Potent Anticancer Agents Targeting Thymidine Phosphorylase Enzyme. Bioorg. Chem. 2021, 111, 104873. [Google Scholar] [CrossRef]
- El Mansouri, A.E.; Oubella, A.; Mehdi, A.; AitItto, M.Y.; Zahouily, M.; Morjani, H.; Lazrek, H.B. Design, Synthesis, Biological Evaluation and Molecular Docking of New 1,3,4-Oxadiazole Homonucleosides and Their Double-Headed Analogs as Antitumor Agents. Bioorg. Chem. 2021, 108, 104558. [Google Scholar] [CrossRef]
- Shaikh, A.S.; Kiranmai, G.; Parimala Devi, G.; Makhal, P.N.; Sigalapalli, D.K.; Tokala, R.; Kaki, V.R.; Shankaraiah, N.; Nagesh, N.; Babu, B.N.; et al. Exploration of Mercaptoacetamide-Linked Pyrimidine-1,3,4-Oxadiazole Derivatives as DNA Intercalative Topo II Inhibitors: Cytotoxicity and Apoptosis Induction. Bioorg. Med. Chem. Lett. 2022, 65, 128697. [Google Scholar] [CrossRef]
- Sigalapalli, D.K.; Kiranmai, G.; Parimala Devi, G.; Tokala, R.; Sana, S.; Tripura, C.; Jadhav, G.S.; Kadagathur, M.; Shankaraiah, N.; Nagesh, N.; et al. Synthesis and Biological Evaluation of Novel Imidazo[1,2-a]Pyridine-Oxadiazole Hybrids as Anti-Proliferative Agents: Study of Microtubule Polymerization Inhibition and DNA Binding. Bioorg. Med. Chem. 2021, 43, 116277. [Google Scholar] [CrossRef]
- Nazreen, S.; Almalki, A.S.A.; Elbehairi, S.E.I.; Shati, A.A.; Alfaifi, M.Y.; Elhenawy, A.A.; Alsenani, N.I.; Alfarsi, A.; Alhadhrami, A.; Alqurashi, E.A.; et al. Cell Cycle Arrest and Apoptosis-Inducing Ability of Benzimidazole Derivatives: Design, Synthesis, Docking, and Biological Evaluation. Molecules 2022, 27, 6899. [Google Scholar] [CrossRef]
- Production of 1,3,4-Oxdiazoles. Google Patents US3065238A, 20 November 1962. Available online: https://patents.google.com/patent/US3065238A/en (accessed on 27 July 2022).
- Method of Making 2-Amino-5-substituted-1,3,4-oxadiazoles. Google Patents US2883391A, 21 April 1959. Available online: https://patents.google.com/patent/US2883391A/en (accessed on 27 July 2022).
- 1,3,4-Oxadiazole-2-carboxamide Compound. Google Patents EP2520575B1, 30 November 2016. Available online: https://patents.google.com/patent/EP2520575B1/ja (accessed on 27 July 2022).
- Oxadiazole Derivatives as Sphingosine 1-Phosphate (s1p)Receptor Modulators. Google Patents EP2646021, 5 November 2014. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=EP95317828 (accessed on 27 July 2022).
- 2-Thio-1,3,4-oxadiazoles Azetidine Derivatives as Sphingosine-1 Phosphate Receptors Modulators. Google Patents WO2014078200, 22 May 2014. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2014078200 (accessed on 27 July 2022).
- WO2015033301A1-1,3,4-Oxadiazole and 1,3,4-Thiadiazole Derivatives as Immunomodulators-Google Patents. Available online: https://patents.google.com/patent/WO2015033301A1/en (accessed on 24 January 2023).
- Aryl Oxadiazole Derivatives as Sphingosine 1-Phosphate (s1p) receptor Modulators. Google Patents WO2014078208, 14 November 2011. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2014078208 (accessed on 27 July 2022).
- 4-Oxadiazol-2-yl-indazoles as Inhibitors of P13 Kinases. Google Patents WO2010102958A1, 16 September 2010. Available online: https://patents.google.com/patent/WO2010102958A1/en (accessed on 27 July 2022).






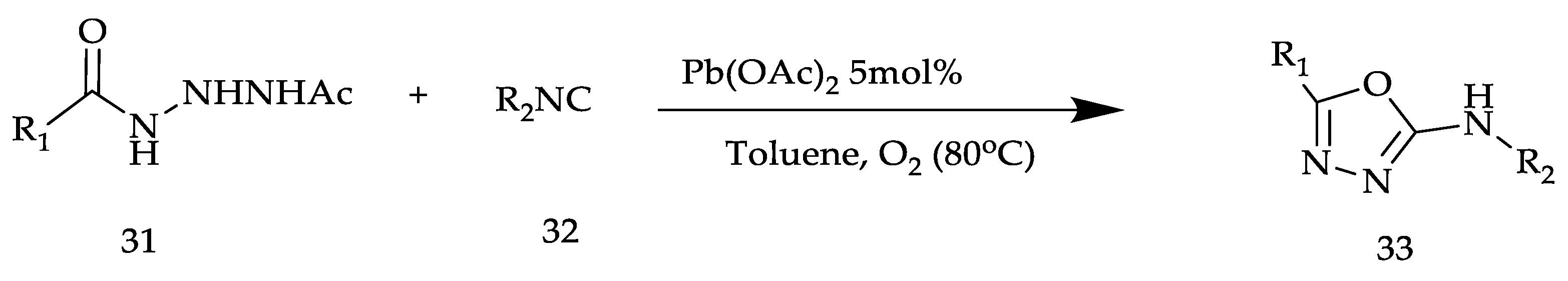


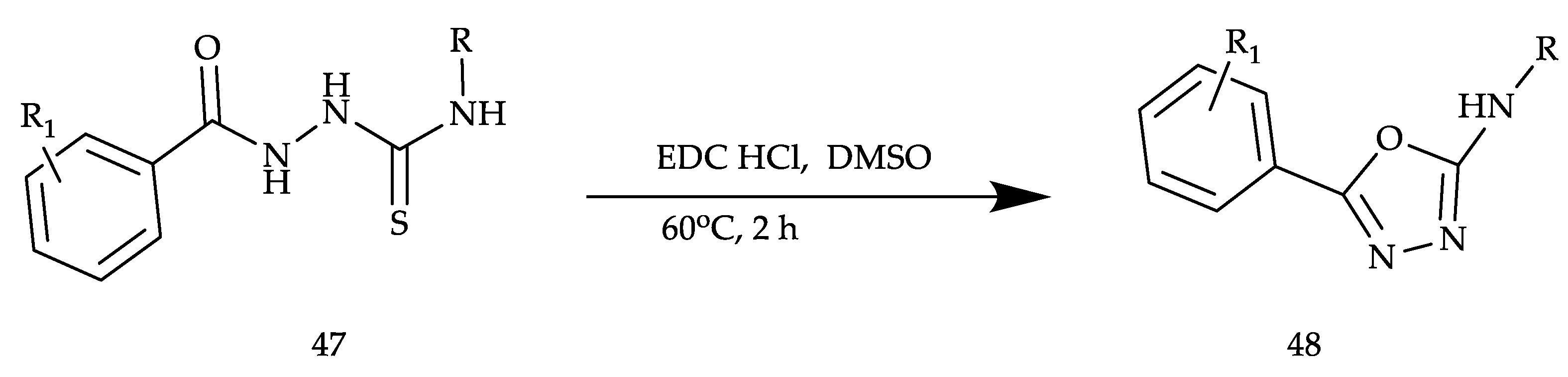



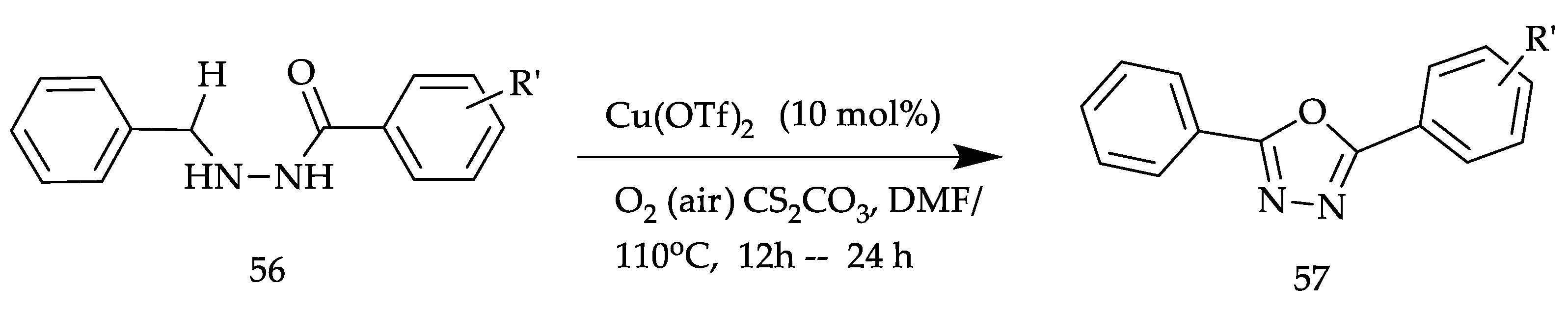















| Cell Line | Type of Cancer (s) | IC50 (µM) | IC50 (µM) Sorafenib |
|---|---|---|---|
| PC-3 | Prostate | 0.67 | 1.99 |
| HCT-116 | Colon | 0.80 | 1.58 |
| ACHN | Renal | 0.87 | 2.51 |
| Cell Lines | K-562 (Leukemia) | MDA-MB-435 (Melanoma) | HCT-15 (Colon Cancer) | T-47D (Breast Cancer) |
|---|---|---|---|---|
| Growth percent (GP) | 18.22 | 15.43 | 39.77 | 34.27 |
| Compd. No. | IC50 (µM) |
|---|---|
| 116 | 0.4123 ± 0.022 |
| 117 | 0.2757 ± 0.013 |
| 118 | 1.1289 ± 0.045 |
| Erlotinib (Standard) | 0.4178 ± 0.014 |
| Comp. No. | IC50 on Axl (μM) |
|---|---|
| 120 | 0.036 |
| 121 | 0.077 |
| 122 | 0.010 |
| Foretinib (Standard) | 0.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, D.; Aggarwal, N.; Deep, A.; Kumar, H.; Chopra, H.; Marwaha, R.K.; Cavalu, S. An Understanding of Mechanism-Based Approaches for 1,3,4-Oxadiazole Scaffolds as Cytotoxic Agents and Enzyme Inhibitors. Pharmaceuticals 2023, 16, 254. https://doi.org/10.3390/ph16020254
Kumar D, Aggarwal N, Deep A, Kumar H, Chopra H, Marwaha RK, Cavalu S. An Understanding of Mechanism-Based Approaches for 1,3,4-Oxadiazole Scaffolds as Cytotoxic Agents and Enzyme Inhibitors. Pharmaceuticals. 2023; 16(2):254. https://doi.org/10.3390/ph16020254
Chicago/Turabian StyleKumar, Davinder, Navidha Aggarwal, Aakash Deep, Harsh Kumar, Hitesh Chopra, Rakesh Kumar Marwaha, and Simona Cavalu. 2023. "An Understanding of Mechanism-Based Approaches for 1,3,4-Oxadiazole Scaffolds as Cytotoxic Agents and Enzyme Inhibitors" Pharmaceuticals 16, no. 2: 254. https://doi.org/10.3390/ph16020254
APA StyleKumar, D., Aggarwal, N., Deep, A., Kumar, H., Chopra, H., Marwaha, R. K., & Cavalu, S. (2023). An Understanding of Mechanism-Based Approaches for 1,3,4-Oxadiazole Scaffolds as Cytotoxic Agents and Enzyme Inhibitors. Pharmaceuticals, 16(2), 254. https://doi.org/10.3390/ph16020254








