Neural Regulation of Innate Immunity in Inflammatory Skin Diseases
Abstract
1. Introduction
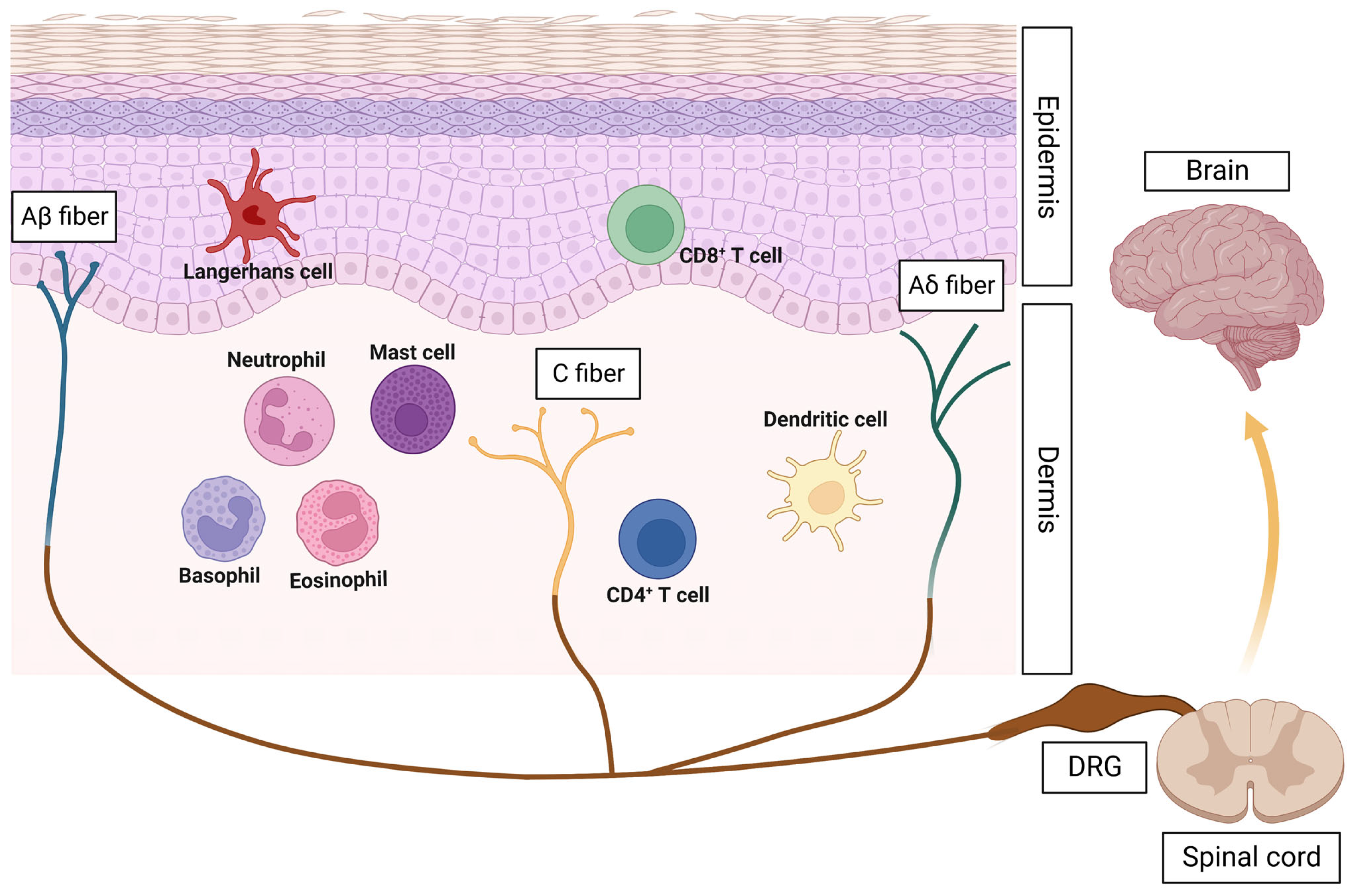
2. Neuroimmune Crosstalk: A Novel Concept in Skin Inflammation
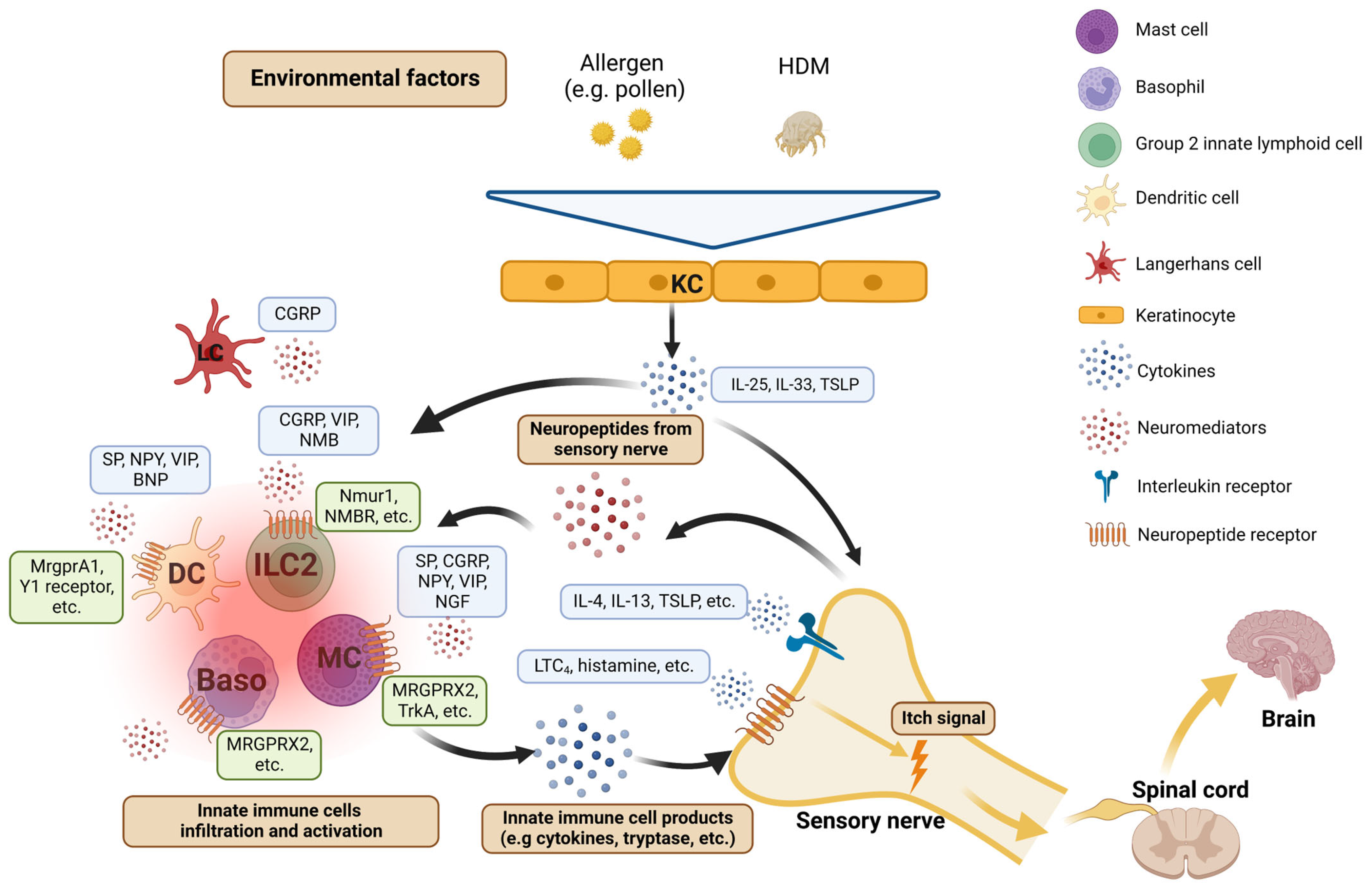
3. Atopic Dermatitis (AD): A Paradigm of Itch Neuroimmune Mechanisms
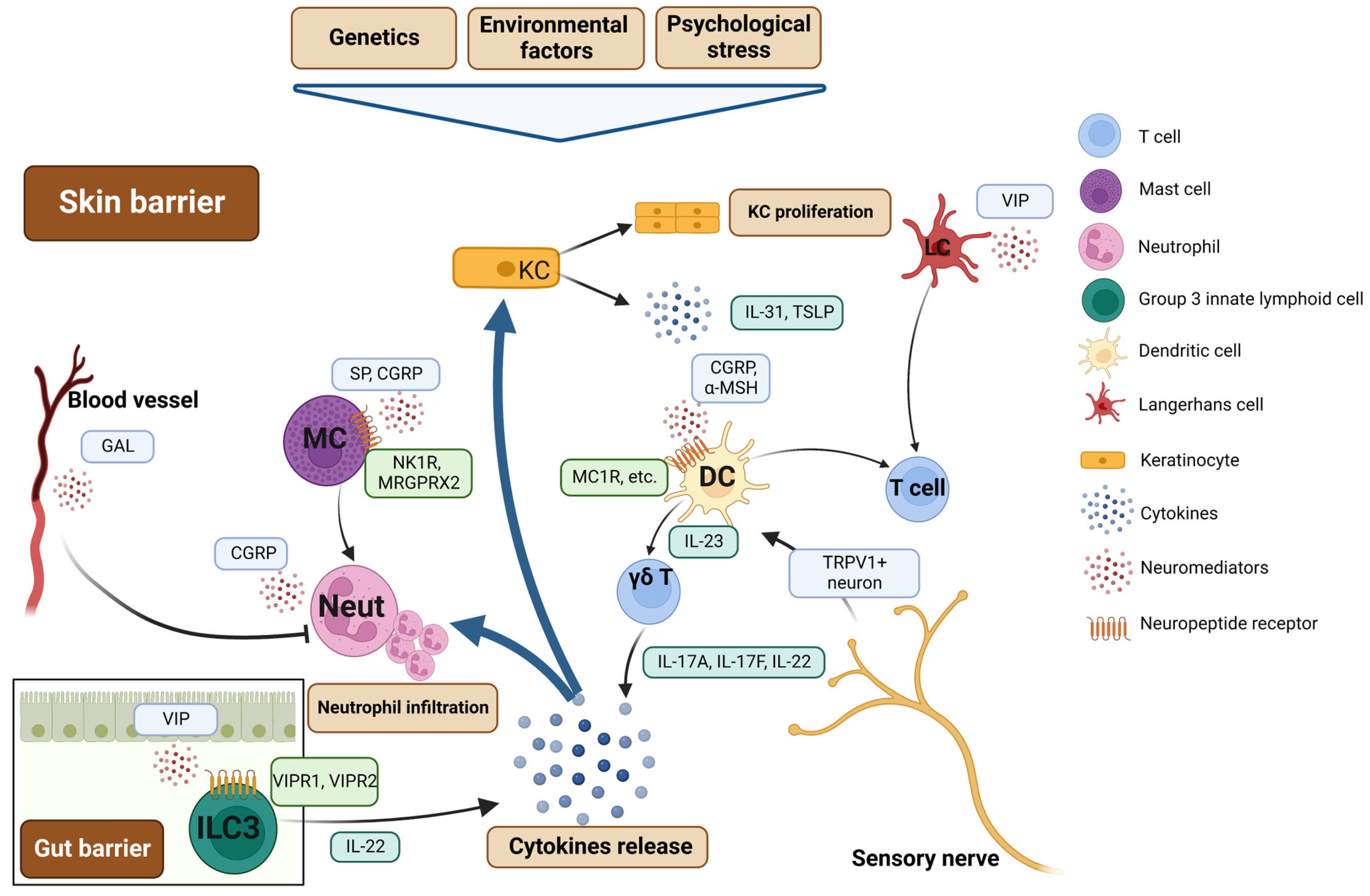
4. Psoriasis: Neurogenic Skin Inflammation
5. Other Inflammatory Skin Diseases: Neuroimmune Responses Likely Involved
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | atopic dermatitis |
| CGRP | calcitonin-gene-related peptide |
| CNS | central nervous system |
| CSU | chronic spontaneous urticaria |
| DC | dendritic cell |
| IFN-γ | interferon-γ |
| Ig | immunoglobulin |
| ILC | innate lymphoid cell |
| IL | interleukin |
| MRGPR | Mas-related G protein-coupled receptor |
| NGF | nerve growth factor |
| NK1R | neurokinin 1 receptor |
| NMB | neuromedin B |
| NMU | neuromedin U |
| NPY | neuropeptide Y |
| PNS | peripheral nervous system |
| SP | substance P |
| Th | T helper |
| TNF-α | tumor necrosis factor α |
| TSLP | thymic stromal lymphopoietin |
| VIP | vasoactive intestinal peptide |
References
- Trier, A.M.; Mack, M.R.; Kim, B.S. The Neuroimmune Axis in Skin Sensation, Inflammation, and Immunity. J. Immunol. 2019, 202, 2829–2835. [Google Scholar] [CrossRef] [PubMed]
- Abreu, D.; Kim, B.S. Innate Immune Regulation of Dermatitis. Immunol. Allergy Clin. N. Am. 2021, 41, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Kim, B.S. Itch: A Paradigm of Neuroimmune Crosstalk. Immunity 2020, 52, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Annunziato, F.; Romagnani, C.; Romagnani, S. The 3 major types of innate and adaptive cell-mediated effector immunity. J. Allergy Clin. Immunol. 2015, 135, 626–635. [Google Scholar] [CrossRef]
- Rutz, S.; Eidenschenk, C.; Ouyang, W. IL-22, not simply a Th17 cytokine. Immunol. Rev. 2013, 252, 116–132. [Google Scholar] [CrossRef]
- Harrington, L.E.; Hatton, R.D.; Mangan, P.R.; Turner, H.; Murphy, T.L.; Murphy, K.M.; Weaver, C.T. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005, 6, 1123–1132. [Google Scholar] [CrossRef]
- Oetjen, L.K.; Mack, M.R.; Feng, J.; Whelan, T.M.; Niu, H.; Guo, C.J.; Chen, S.; Trier, A.M.; Xu, A.Z.; Tripathi, S.V.; et al. Sensory Neurons Co-opt Classical Immune Signaling Pathways to Mediate Chronic Itch. Cell 2017, 171, 217–228.e213. [Google Scholar] [CrossRef]
- Cevikbas, F.; Wang, X.; Akiyama, T.; Kempkes, C.; Savinko, T.; Antal, A.; Kukova, G.; Buhl, T.; Ikoma, A.; Buddenkotte, J.; et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. J. Allergy Clin. Immunol. 2014, 133, 448–460. [Google Scholar] [CrossRef]
- Lay, M.; Dong, X. Neural Mechanisms of Itch. Annu. Rev. Neurosci. 2020, 43, 187–205. [Google Scholar] [CrossRef]
- Oleszycka, E.; Kwiecien, K.; Kwiecinska, P.; Morytko, A.; Pocalun, N.; Camacho, M.; Brzoza, P.; Zabel, B.A.; Cichy, J. Soluble mediators in the function of the epidermal-immune-neuro unit in the skin. Front. Immunol. 2022, 13, 1003970. [Google Scholar] [CrossRef]
- Zhang, S.; Edwards, T.N.; Chaudhri, V.K.; Wu, J.; Cohen, J.A.; Hirai, T.; Rittenhouse, N.; Schmitz, E.G.; Zhou, P.Y.; McNeil, B.D.; et al. Nonpeptidergic neurons suppress mast cells via glutamate to maintain skin homeostasis. Cell 2021, 184, 2151–2166.e16. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, A.S.; Rocken, M.; Ghoreschi, K. Cutaneous immunology: Basics and new concepts. Semin. Immunopathol. 2016, 38, 3–10. [Google Scholar] [CrossRef]
- Pasparakis, M.; Haase, I.; Nestle, F.O. Mechanisms regulating skin immunity and inflammation. Nat. Rev. Immunol. 2014, 14, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Kwiecien, K.; Zegar, A.; Jung, J.; Brzoza, P.; Kwitniewski, M.; Godlewska, U.; Grygier, B.; Kwiecinska, P.; Morytko, A.; Cichy, J. Architecture of antimicrobial skin defense. Cytokine Growth Factor Rev. 2019, 49, 70–84. [Google Scholar] [CrossRef]
- Abraira, V.E.; Ginty, D.D. The sensory neurons of touch. Neuron 2013, 79, 618–639. [Google Scholar] [CrossRef]
- Peters, E.M.; Liezmann, C.; Klapp, B.F.; Kruse, J. The neuroimmune connection interferes with tissue regeneration and chronic inflammatory disease in the skin. Ann. N. Y. Acad. Sci. 2012, 1262, 118–126. [Google Scholar] [CrossRef]
- Kabata, H.; Artis, D. Neuro-immune crosstalk and allergic inflammation. J. Clin. Investig. 2019, 129, 1475–1482. [Google Scholar] [CrossRef]
- Agelopoulos, K.; Pereira, M.P.; Wiegmann, H.; Stander, S. Cutaneous neuroimmune crosstalk in pruritus. Trends Mol. Med. 2022, 28, 452–462. [Google Scholar] [CrossRef]
- Steinhoff, M.; Buddenkotte, J.; Lerner, E.A. Role of mast cells and basophils in pruritus. Immunol. Rev. 2018, 282, 248–264. [Google Scholar] [CrossRef] [PubMed]
- Corbière, A.; Loste, A.; Gaudenzio, N. MRGPRX2 sensing of cationic compounds-A bridge between nociception and skin diseases? Exp. Dermatol. 2021, 30, 193–200. [Google Scholar] [CrossRef]
- Alysandratos, K.D.; Asadi, S.; Angelidou, A.; Zhang, B.; Sismanopoulos, N.; Yang, H.; Critchfield, A.; Theoharides, T.C. Neurotensin and CRH interactions augment human mast cell activation. PLoS ONE 2012, 7, e48934. [Google Scholar] [CrossRef]
- Quinlan, K.L.; Song, I.S.; Bunnett, N.W.; Letran, E.; Steinhoff, M.; Harten, B.; Olerud, J.E.; Armstrong, C.A.; Wright Caughman, S.; Ansel, J.C. Neuropeptide regulation of human dermal microvascular endothelial cell ICAM-1 expression and function. Am. J. Physiol. 1998, 275, C1580–C1590. [Google Scholar] [CrossRef] [PubMed]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef]
- Kuo, I.H.; Yoshida, T.; De Benedetto, A.; Beck, L.A. The cutaneous innate immune response in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2013, 131, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Honda, T.; Kabashima, K. Reconciling innate and acquired immunity in atopic dermatitis. J. Allergy Clin. Immunol. 2020, 145, 1136–1137. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, M.; Ahmad, F.; Pandey, A.; Datsi, A.; AlHammadi, A.; Al-Khawaga, S.; Al-Malki, A.; Meng, J.; Alam, M.; Buddenkotte, J. Neuroimmune communication regulating pruritus in atopic dermatitis. J. Allergy Clin. Immunol. 2022, 149, 1875–1898. [Google Scholar] [CrossRef]
- Wang, F.; Yang, T.B.; Kim, B.S. The Return of the Mast Cell: New Roles in Neuroimmune Itch Biology. J. Investig. Dermatol. 2020, 140, 945–951. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Jiang, B.; Tong, X.; Yan, S.; Lu, J. Current views on neuropeptides in atopic dermatitis. Exp. Dermatol. 2021, 30, 1588–1597. [Google Scholar] [CrossRef]
- Horigome, K.; Pryor, J.C.; Bullock, E.D.; Johnson, E.M., Jr. Mediator release from mast cells by nerve growth factor. Neurotrophin specificity and receptor mediation. J. Biol. Chem. 1993, 268, 14881–14887. [Google Scholar] [CrossRef]
- Nilsson, G.; Forsberg-Nilsson, K.; Xiang, Z.; Hallbook, F.; Nilsson, K.; Metcalfe, D.D. Human mast cells express functional TrkA and are a source of nerve growth factor. Eur. J. Immunol. 1997, 27, 2295–2301. [Google Scholar] [CrossRef]
- Meixiong, J.; Anderson, M.; Limjunyawong, N.; Sabbagh, M.F.; Hu, E.; Mack, M.R.; Oetjen, L.K.; Wang, F.; Kim, B.S.; Dong, X. Activation of Mast-Cell-Expressed Mas-Related G-Protein-Coupled Receptors Drives Non-histaminergic Itch. Immunity 2019, 50, 1163–1171.e1165. [Google Scholar] [CrossRef] [PubMed]
- McNeil, B.D.; Pundir, P.; Meeker, S.; Han, L.; Undem, B.J.; Kulka, M.; Dong, X. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 2015, 519, 237–241. [Google Scholar] [CrossRef]
- Kawakami, T.; Ando, T.; Kimura, M.; Wilson, B.S.; Kawakami, Y. Mast cells in atopic dermatitis. Curr. Opin. Immunol. 2009, 21, 666–678. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, J.; Di, Z.H.; Sun, W.; Kawana, S. Changes in cutaneous sensory nerve fibers induced by skin-scratching in mice. J. Dermatol. Sci. 2007, 46, 41–51. [Google Scholar] [CrossRef]
- Plum, T.; Wang, X.; Rettel, M.; Krijgsveld, J.; Feyerabend, T.B.; Rodewald, H.R. Human Mast Cell Proteome Reveals Unique Lineage, Putative Functions, and Structural Basis for Cell Ablation. Immunity 2020, 52, 404–416.e405. [Google Scholar] [CrossRef]
- Serhan, N.; Basso, L.; Sibilano, R.; Petitfils, C.; Meixiong, J.; Bonnart, C.; Reber, L.L.; Marichal, T.; Starkl, P.; Cenac, N.; et al. House dust mites activate nociceptor-mast cell clusters to drive type 2 skin inflammation. Nat. Immunol. 2019, 20, 1435–1443. [Google Scholar] [CrossRef]
- Geoghegan, J.A.; Irvine, A.D.; Foster, T.J. Staphylococcus aureus and Atopic Dermatitis: A Complex and Evolving Relationship. Trends Microbiol. 2018, 26, 484–497. [Google Scholar] [CrossRef] [PubMed]
- Blake, K.J.; Baral, P.; Voisin, T.; Lubkin, A.; Pinho-Ribeiro, F.A.; Adams, K.L.; Roberson, D.P.; Ma, Y.X.C.; Otto, M.; Woolf, C.J.; et al. Staphylococcus aureus produces pain through pore-forming toxins and neuronal TRPV1 that is silenced by QX-314. Nat. Commun. 2018, 9, 37. [Google Scholar] [CrossRef]
- Arifuzzaman, M.; Mobley, Y.R.; Choi, H.W.; Bist, P.; Salinas, C.A.; Brown, Z.D.; Chen, S.L.; Staats, H.F.; Abraham, S.N. MRGPR-mediated activation of local mast cells clears cutaneous bacterial infection and protects against reinfection. Sci. Adv. 2019, 5, eaav0216. [Google Scholar] [CrossRef]
- Wang, Z.; Babina, M. MRGPRX2 signals its importance in cutaneous mast cell biology: Does MRGPRX2 connect mast cells and atopic dermatitis? Exp. Dermatol. 2020, 29, 1104–1111. [Google Scholar] [CrossRef]
- Miyake, K.; Karasuyama, H. Emerging roles of basophils in allergic inflammation. Allergol. Int. 2017, 66, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Trier, A.M.; Li, F.; Kim, S.; Chen, Z.; Chai, J.N.; Mack, M.R.; Morrison, S.A.; Hamilton, J.D.; Baek, J.; et al. A basophil-neuronal axis promotes itch. Cell 2021, 184, 422–440.e417. [Google Scholar] [CrossRef] [PubMed]
- Wedi, B.; Gehring, M.; Kapp, A. The pseudoallergen receptor MRGPRX2 on peripheral blood basophils and eosinophils: Expression and function. Allergy 2020, 75, 2229–2242. [Google Scholar] [CrossRef] [PubMed]
- Sonnenberg, G.F.; Artis, D. Innate lymphoid cell interactions with microbiota: Implications for intestinal health and disease. Immunity 2012, 37, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Monticelli, L.A.; Sonnenberg, G.F.; Artis, D. Innate lymphoid cells: Critical regulators of allergic inflammation and tissue repair in the lung. Curr. Opin. Immunol. 2012, 24, 284–289. [Google Scholar] [CrossRef]
- Soumelis, V.; Reche, P.A.; Kanzler, H.; Yuan, W.; Edward, G.; Homey, B.; Gilliet, M.; Ho, S.; Antonenko, S.; Lauerma, A.; et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002, 3, 673–680. [Google Scholar] [CrossRef]
- Deleuran, M.; Hvid, M.; Kemp, K.; Christensen, G.B.; Deleuran, B.; Vestergaard, C. IL-25 induces both inflammation and skin barrier dysfunction in atopic dermatitis. Chem. Immunol. Allergy 2012, 96, 45–49. [Google Scholar]
- Savinko, T.; Matikainen, S.; Saarialho-Kere, U.; Lehto, M.; Wang, G.; Lehtimaki, S.; Karisola, P.; Reunala, T.; Wolff, H.; Lauerma, A.; et al. IL-33 and ST2 in atopic dermatitis: Expression profiles and modulation by triggering factors. J. Investig. Dermatol. 2012, 132, 1392–1400. [Google Scholar] [CrossRef]
- Kim, B.S.; Wang, K.; Siracusa, M.C.; Saenz, S.A.; Brestoff, J.R.; Monticelli, L.A.; Noti, M.; Tait Wojno, E.D.; Fung, T.C.; Kubo, M.; et al. Basophils promote innate lymphoid cell responses in inflamed skin. J. Immunol. 2014, 193, 3717–3725. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Siracusa, M.C.; Saenz, S.A.; Noti, M.; Monticelli, L.A.; Sonnenberg, G.F.; Hepworth, M.R.; Van Voorhees, A.S.; Comeau, M.R.; Artis, D. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci. Transl. Med. 2013, 5, 170ra16. [Google Scholar] [CrossRef]
- Salimi, M.; Barlow, J.L.; Saunders, S.P.; Xue, L.; Gutowska-Owsiak, D.; Wang, X.; Huang, L.C.; Johnson, D.; Scanlon, S.T.; McKenzie, A.N.; et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J. Exp. Med. 2013, 210, 2939–2950. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Castillo, J.M.; Galand, C.; Mashiko, S.; Bissonnette, R.; McGurk, A.; Ziegler, S.F.; Dong, C.; McKenzie, A.N.J.; Sarfati, M.; Geha, R.S. ILC2 activation by keratinocyte-derived IL-25 drives IL-13 production at sites of allergic skin inflammation. J. Allergy Clin. Immunol. 2020, 145, 1606–1614.e1604. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, V.; Chesne, J.; Ribeiro, H.; Garcia-Cassani, B.; Carvalho, T.; Bouchery, T.; Shah, K.; Barbosa-Morais, N.L.; Harris, N.; Veiga-Fernandes, H. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature 2017, 549, 277–281. [Google Scholar] [CrossRef]
- Klose, C.S.N.; Mahlakoiv, T.; Moeller, J.B.; Rankin, L.C.; Flamar, A.L.; Kabata, H.; Monticelli, L.A.; Moriyama, S.; Putzel, G.G.; Rakhilin, N.; et al. The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature 2017, 549, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, H.; Mahlakoiv, T.; Shih, H.Y.; Davis, F.P.; Meylan, F.; Huang, Y.; Harrison, O.J.; Yao, C.; Mikami, Y.; Urban, J.F., Jr.; et al. Neuropeptide CGRP Limits Group 2 Innate Lymphoid Cell Responses and Constrains Type 2 Inflammation. Immunity 2019, 51, 682–695.e686. [Google Scholar] [CrossRef]
- Nussbaum, J.C.; Van Dyken, S.J.; von Moltke, J.; Cheng, L.E.; Mohapatra, A.; Molofsky, A.B.; Thornton, E.E.; Krummel, M.F.; Chawla, A.; Liang, H.E.; et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 2013, 502, 245–248. [Google Scholar] [CrossRef]
- Inclan-Rico, J.M.; Ponessa, J.J.; Valero-Pacheco, N.; Hernandez, C.M.; Sy, C.B.; Lemenze, A.D.; Beaulieu, A.M.; Siracusa, M.C. Basophils prime group 2 innate lymphoid cells for neuropeptide-mediated inhibition. Nat. Immunol. 2020, 21, 1181–1193. [Google Scholar] [CrossRef]
- Novak, N. An update on the role of human dendritic cells in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2012, 129, 879–886. [Google Scholar] [CrossRef]
- Tanei, R.; Hasegawa, Y. Immunohistopathological Analysis of Immunoglobulin E-Positive Epidermal Dendritic Cells with House Dust Mite Antigens in Naturally Occurring Skin Lesions of Adult and Elderly Patients with Atopic Dermatitis. Dermatopathology 2021, 8, 426–441. [Google Scholar] [CrossRef]
- Perner, C.; Flayer, C.H.; Zhu, X.; Aderhold, P.A.; Dewan, Z.N.A.; Voisin, T.; Camire, R.B.; Chow, O.A.; Chiu, I.M.; Sokol, C.L. Substance P Release by Sensory Neurons Triggers Dendritic Cell Migration and Initiates the Type-2 Immune Response to Allergens. Immunity 2020, 53, 1063–1077.e1067. [Google Scholar] [CrossRef]
- Buttari, B.; Profumo, E.; Domenici, G.; Tagliani, A.; Ippoliti, F.; Bonini, S.; Businaro, R.; Elenkov, I.; Rigano, R. Neuropeptide Y induces potent migration of human immature dendritic cells and promotes a Th2 polarization. FASEB J. 2014, 28, 3038–3049. [Google Scholar] [CrossRef] [PubMed]
- Chorny, A.; Gonzalez-Rey, E.; Delgado, M. Regulation of dendritic cell differentiation by vasoactive intestinal peptide: Therapeutic applications on autoimmunity and transplantation. Ann. N. Y. Acad. Sci. 2006, 1088, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Moriyama, M.; Feld, M.; Buddenkotte, J.; Buhl, T.; Szollosi, A.; Zhang, J.; Miller, P.; Ghetti, A.; Fischer, M.; et al. New mechanism underlying IL-31-induced atopic dermatitis. J. Allergy Clin. Immunol. 2018, 141, 1677–1689.e1678. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.H.; Chen, W.W.; Wang, J.F. Interventions in the B-type natriuretic peptide signalling pathway as a means of controlling chronic itch. Br. J. Pharmacol. 2020, 177, 1025–1040. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Stohl, L.L.; Wagner, J.A.; Granstein, R.D. Calcitonin gene-related peptide biases Langerhans cells toward Th2-type immunity. J. Immunol. 2008, 181, 6020–6026. [Google Scholar] [CrossRef]
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J.N.W.N. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef]
- Ayasse, M.T.; Buddenkotte, J.; Alam, M.; Steinhoff, M. Role of neuroimmune circuits and pruritus in psoriasis. Exp. Dermatol. 2020, 29, 414–426. [Google Scholar] [CrossRef]
- Sato, Y.; Ogawa, E.; Okuyama, R. Role of Innate Immune Cells in Psoriasis. Int. J. Mol. Sci. 2020, 21, 6604. [Google Scholar] [CrossRef]
- Nakamura, M.; Toyoda, M.; Morohashi, M. Pruritogenic mediators in psoriasis vulgaris: Comparative evaluation of itch-associated cutaneous factors. Br. J. Dermatol. 2003, 149, 718–730. [Google Scholar] [CrossRef]
- Siiskonen, H.; Harvima, I. Mast Cells and Sensory Nerves Contribute to Neurogenic Inflammation and Pruritus in Chronic Skin Inflammation. Front. Cell. Neurosci. 2019, 13, 422. [Google Scholar] [CrossRef]
- Volpe, E.; Pattarini, L.; Martinez-Cingolani, C.; Meller, S.; Donnadieu, M.H.; Bogiatzi, S.I.; Fernandez, M.I.; Touzot, M.; Bichet, J.C.; Reyal, F.; et al. Thymic stromal lymphopoietin links keratinocytes and dendritic cell-derived IL-23 in patients with psoriasis. J. Allergy Clin. Immunol. 2014, 134, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Narbutt, J.; Olejniczak, I.; Sobolewska-Sztychny, D.; Sysa-Jedrzejowska, A.; Slowik-Kwiatkowska, I.; Hawro, T.; Lesiak, A. Narrow band ultraviolet B irradiations cause alteration in interleukin-31 serum level in psoriatic patients. Arch. Dermatol. Res. 2013, 305, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Nattkemper, L.A.; Tey, H.L.; Valdes-Rodriguez, R.; Lee, H.; Mollanazar, N.K.; Albornoz, C.; Sanders, K.M.; Yosipovitch, G. The Genetics of Chronic Itch: Gene Expression in the Skin of Patients with Atopic Dermatitis and Psoriasis with Severe Itch. J. Investig. Dermatol. 2018, 138, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Szepietowski, J.C.; Reich, A.; Wisnicka, B. Pruritus and psoriasis. Br. J. Dermatol. 2004, 151, 1284. [Google Scholar] [CrossRef]
- Chang, S.E.; Han, S.S.; Jung, H.J.; Choi, J.H. Neuropeptides and their receptors in psoriatic skin in relation to pruritus. Br. J. Dermatol. 2007, 156, 1272–1277. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Talamantes, A.K.; Gomez-Gonzalez, B.A.; Uriarte-Mayorga, D.F.; Martinez-Guzman, M.A.; Wheber-Hidalgo, K.A.; Alvarado-Navarro, A. Neurotransmitters, neuropeptides and their receptors interact with immune response in healthy and psoriatic skin. Neuropeptides 2020, 79, 102004. [Google Scholar] [CrossRef]
- Takahashi, T.; Yamasaki, K. Psoriasis and Antimicrobial Peptides. Int. J. Mol. Sci. 2020, 21, 6791. [Google Scholar] [CrossRef]
- Smith, C.H.; Barker, J.N.; Morris, R.W.; MacDonald, D.M.; Lee, T.H. Neuropeptides induce rapid expression of endothelial cell adhesion molecules and elicit granulocytic infiltration in human skin. J. Immunol. 1993, 151, 3274–3282. [Google Scholar] [CrossRef]
- Locker, F.; Vidali, S.; Holub, B.S.; Stockinger, J.; Brunner, S.M.; Ebner, S.; Koller, A.; Trost, A.; Reitsamer, H.A.; Schwarzenbacher, D.; et al. Lack of Galanin Receptor 3 Alleviates Psoriasis by Altering Vascularization, Immune Cell Infiltration, and Cytokine Expression. J. Investig. Dermatol. 2018, 138, 199–207. [Google Scholar] [CrossRef]
- Raychaudhuri, S.K.; Raychaudhuri, S.P. NGF and its receptor system: A new dimension in the pathogenesis of psoriasis and psoriatic arthritis. Ann. N. Y. Acad. Sci. 2009, 1173, 470–477. [Google Scholar] [CrossRef]
- Riol-Blanco, L.; Ordovas-Montanes, J.; Perro, M.; Naval, E.; Thiriot, A.; Alvarez, D.; Paust, S.; Wood, J.N.; von Andrian, U.H. Nociceptive sensory neurons drive interleukin-23-mediated psoriasiform skin inflammation. Nature 2014, 510, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Spits, H.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.; Mebius, R.E.; et al. Innate lymphoid cells—A proposal for uniform nomenclature. Nat. Rev. Immunol. 2013, 13, 145–149. [Google Scholar] [CrossRef]
- Teunissen, M.B.M.; Munneke, J.M.; Bernink, J.H.; Spuls, P.I.; Res, P.C.M.; Te Velde, A.; Cheuk, S.; Brouwer, M.W.D.; Menting, S.P.; Eidsmo, L.; et al. Composition of innate lymphoid cell subsets in the human skin: Enrichment of NCR(+) ILC3 in lesional skin and blood of psoriasis patients. J. Investig. Dermatol. 2014, 134, 2351–2360. [Google Scholar] [CrossRef] [PubMed]
- Seillet, C.; Luong, K.; Tellier, J.; Jacquelot, N.; Shen, R.D.; Hickey, P.; Wimmer, V.C.; Whitehead, L.; Rogers, K.; Smyth, G.K.; et al. The neuropeptide VIP confers anticipatory mucosal immunity by regulating ILC3 activity. Nat. Immunol. 2020, 21, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Talbot, J.; Hahn, P.; Kroehling, L.; Nguyen, H.; Li, D.; Littman, D.R. Feeding-dependent VIP neuron-ILC3 circuit regulates the intestinal barrier. Nature 2020, 579, 575–580. [Google Scholar] [CrossRef]
- Yu, H.B.; Yang, H.; Allaire, J.M.; Ma, C.; Graef, F.A.; Mortha, A.; Liang, Q.; Bosman, E.S.; Reid, G.S.; Waschek, J.A.; et al. Vasoactive intestinal peptide promotes host defense against enteric pathogens by modulating the recruitment of group 3 innate lymphoid cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2106634118. [Google Scholar] [CrossRef]
- Nakajima, K.; Kataoka, S.; Sato, K.; Takaishi, M.; Yamamoto, M.; Nakajima, H.; Sano, S. Stat3 activation in epidermal keratinocytes induces Langerhans cell activation to form an essential circuit for psoriasis via IL-23 production. J. Dermatol. Sci. 2019, 93, 82–91. [Google Scholar] [CrossRef]
- Martini, E.; Wiken, M.; Cheuk, S.; Gallais Serezal, I.; Baharom, F.; Stahle, M.; Smed-Sorensen, A.; Eidsmo, L. Dynamic Changes in Resident and Infiltrating Epidermal Dendritic Cells in Active and Resolved Psoriasis. J. Investig. Dermatol. 2017, 137, 865–873. [Google Scholar] [CrossRef]
- Chiricozzi, A.; Romanelli, P.; Volpe, E.; Borsellino, G.; Romanelli, M. Scanning the Immunopathogenesis of Psoriasis. Int. J. Mol. Sci. 2018, 19, 179. [Google Scholar] [CrossRef]
- Ostrowski, S.M.; Belkadi, A.; Loyd, C.M.; Diaconu, D.; Ward, N.L. Cutaneous denervation of psoriasiform mouse skin improves acanthosis and inflammation in a sensory neuropeptide-dependent manner. J. Investig. Dermatol. 2011, 131, 1530–1538. [Google Scholar] [CrossRef]
- Auriemma, M.; Brzoska, T.; Klenner, L.; Kupas, V.; Goerge, T.; Voskort, M.; Zhao, Z.; Sparwasser, T.; Luger, T.A.; Loser, K. α-MSH-stimulated tolerogenic dendritic cells induce functional regulatory T cells and ameliorate ongoing skin inflammation. J. Investig. Dermatol. 2012, 132, 1814–1824. [Google Scholar] [CrossRef]
- Ding, W.; Manni, M.; Stohl, L.L.; Zhou, X.K.; Wagner, J.A.; Granstein, R.D. Pituitary adenylate cyclase-activating peptide and vasoactive intestinal polypeptide bias Langerhans cell Ag presentation toward Th17 cells. Eur. J. Immunol. 2012, 42, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Borici-Mazi, R.; Kouridakis, S.; Kontou-Fili, K. Cutaneous responses to substance P and calcitonin gene-related peptide in chronic urticaria: The effect of cetirizine and dimethindene. Allergy 1999, 54, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.H.; Atkinson, B.; Morris, R.W.; Hayes, N.; Foreman, J.C.; Lee, T.H. Cutaneous responses to vasoactive intestinal polypeptide in chronic idiopathic urticaria. Lancet 1992, 339, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, D.; Kashiwakura, J.; Kita, H.; Kikukawa, Y.; Fujitani, Y.; Sasaki-Sakamoto, T.; Kuroda, K.; Nunomura, S.; Hayama, K.; Terui, T.; et al. Expression of Mas-related gene X2 on mast cells is upregulated in the skin of patients with severe chronic urticaria. J. Allergy Clin. Immunol. 2014, 134, 622. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, J.; Zhu, W.; Xu, C.; He, S. Upregulated expression of substance P in basophils of the patients with chronic spontaneous urticaria: Induction of histamine release and basophil accumulation by substance P. Cell Biol. Toxicol. 2016, 32, 217–228. [Google Scholar] [CrossRef]
- Hashimoto, T.; Kursewicz, C.D.; Fayne, R.A.; Nanda, S.; Shah, S.M.; Nattkemper, L.; Yokozeki, H.; Yosipovitch, G. Pathophysiologic mechanisms of itch in bullous pemphigoid. J. Am. Acad. Dermatol. 2020, 83, 53–62. [Google Scholar] [CrossRef]
- Lonndahl, L.; Holst, M.; Bradley, M.; Killasli, H.; Heilborn, J.; Hall, M.A.; Theodorsson, E.; Holmberg, J.; Nordlind, K. Substance P Antagonist Aprepitant Shows no Additive Effect Compared with Standardized Topical Treatment Alone in Patients with Atopic Dermatitis. Acta Derm. Venereol. 2018, 98, 324–328. [Google Scholar] [CrossRef]
- Ohanyan, T.; Schoepke, N.; Eirefelt, S.; Hoey, G.; Koopman, W.; Hawro, T.; Maurer, M.; Metz, M. Role of Substance P and Its Receptor Neurokinin 1 in Chronic Prurigo: A Randomized, Proof-of-Concept, Controlled Trial with Topical Aprepitant. Acta Derm. Venereol. 2018, 98, 26–31. [Google Scholar] [CrossRef]
- Pojawa-Golab, M.; Jaworecka, K.; Reich, A. NK-1 Receptor Antagonists and Pruritus: Review of Current Literature. Dermatol. Ther. 2019, 9, 391–405. [Google Scholar] [CrossRef]
- Yosipovitch, G.; Stander, S.; Kerby, M.B.; Larrick, J.W.; Perlman, A.J.; Schnipper, E.F.; Zhang, X.; Tang, J.Y.; Luger, T.; Steinhoff, M. Serlopitant for psoriatic pruritus: A phase 2 randomized, double-blind, placebo-controlled clinical trial. J. Am. Acad. Dermatol. 2020, 82, 1314–1320. [Google Scholar]
- Heitman, A.; Xiao, C.F.; Cho, Y.; Polymeropoulos, C.; Birznieks, G.; Polymeropoulos, M. Serlopitant reduced pruritus in patients with prurigo nodularis in a phase 2, randomized, placebo-controlled trial. J. Am. Acad. Dermatol. 2019, 80, 1395–1402. [Google Scholar]
- Welsh, S.E.; Xiao, C.; Kaden, A.R.; Brzezynski, J.L.; Mohrman, M.A.; Wang, J.; Smieszek, S.P.; Przychodzen, B.; Stander, S.; Polymeropoulos, C.; et al. Serlopitant for the treatment of chronic pruritus: Results of a randomized, multicenter, placebo-controlled phase 2 clinical trial. J. Am. Acad. Dermatol. 2018, 78, 882. [Google Scholar]
- Roblin, D.; Yosipovitch, G.; Boyce, B.; Robinson, J.; Sandy, J.; Mainero, V.; Wickramasinghe, R.; Anand, U.; Anand, P. Tradipitant improves worst itch and disease severity in patients with chronic pruritus related to atopic dermatitis. J. Am. Acad. Dermatol. 2018, 79, Ab300. [Google Scholar]
- Lee, Y.W.; Won, C.H.; Jung, K.; Nam, H.J.; Choi, G.; Park, Y.H.; Park, M.; Kim, B. Neurokinin-1 receptor antagonist tradipitant has mixed effects on itch in atopic dermatitis: Results from EPIONE, a randomized clinical trial. J. Eur. Acad. Dermatol. Venereol. 2021, 35, E338–E340. [Google Scholar]
- Roblin, D.; Yosipovitch, G.; Boyce, B.; Robinson, J.; Sandy, J.; Mainero, V.; Wickramasinghe, R.; Anand, U.; Anand, P. Topical TrkA Kinase Inhibitor CT327 is an Effective, Novel Therapy for the Treatment of Pruritus due to Psoriasis: Results from Experimental Studies, and Efficacy and Safety of CT327 in a Phase 2b Clinical Trial in Patients with Psoriasis. Acta Derm. Venereol. 2015, 95, 542–548. [Google Scholar] [CrossRef]
- Lee, Y.W.; Won, C.H.; Jung, K.; Nam, H.J.; Choi, G.; Park, Y.H.; Park, M.; Kim, B. Efficacy and safety of PAC-14028 cream—A novel, topical, nonsteroidal, selective TRPV1 antagonist in patients with mild-to-moderate atopic dermatitis: A phase IIb randomized trial. Br. J. Dermatol. 2019, 180, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.M.; Park, Y.H. Development of PAC-14028, a Novel Transient Receptor Potential Vanilloid Type 1 (TRPV1) Channel Antagonist as a New Drug for Refractory Skin Diseases. Arch. Pharmacal Res. 2012, 35, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, A.; Blauvelt, A.; Guttman-Yassky, E.; Worm, M.; Lynde, C.; Lacour, J.P.; Spelman, L.; Katoh, N.; Saeki, H.; Poulin, Y.; et al. Tralokinumab for moderate-to-severe atopic dermatitis: Results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br. J. Dermatol. 2021, 184, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Blauvelt, A.; Eichenfield, L.F.; Paller, A.S.; Armstrong, A.W.; Drew, J.; Gopalan, R.; Simpson, E.L. Efficacy and Safety of Lebrikizumab, a High-Affinity Interleukin 13 Inhibitor, in Adults With Moderate to Severe Atopic Dermatitis: A Phase 2b Randomized Clinical Trial. JAMA Dermatol. 2020, 156, 411–420. [Google Scholar] [CrossRef]
- Chen, Y.L.; Gutowska-Owsiak, D.; Hardman, C.S.; Westmoreland, M.; MacKenzie, T.; Cifuentes, L.; Waithe, D.; Lloyd-Lavery, A.; Marquette, A.; Londei, M.; et al. Proof-of-concept clinical trial of etokimab shows a key role for IL-33 in atopic dermatitis pathogenesis. Sci. Transl. Med. 2019, 11, eaax2945. [Google Scholar] [CrossRef]
- Simpson, E.L.; Parnes, J.R.; She, D.; Crouch, S.; Rees, W.; Mo, M.; van der Merwe, R. Tezepelumab, an anti-thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: A randomized phase 2a clinical trial. J. Am. Acad. Dermatol. 2019, 80, 1013–1021. [Google Scholar] [CrossRef]
- Napolitano, M.; Fabbrocini, G.; Scalvenzi, M.; Nistico, S.P.; Dastoli, S.; Patruno, C. Effectiveness of Dupilumab for the Treatment of Generalized Prurigo Nodularis Phenotype of Adult Atopic Dermatitis. Dermatitis 2020, 31, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Calugareanu, A.; Jachiet, M.; Tauber, M.; Nosbaum, A.; Aubin, F.; Misery, L.; Droitcourt, C.; Barbarot, S.; Debarbieux, S.; Saussine, A.; et al. Effectiveness and safety of dupilumab for the treatment of prurigo nodularis in a French multicenter adult cohort of 16 patients. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e74–e76. [Google Scholar] [CrossRef] [PubMed]
- Giura, M.T.; Viola, R.; Fierro, M.T.; Ribero, S.; Ortoncelli, M. Efficacy of dupilumab in prurigo nodularis in elderly patient. Dermatol. Ther. 2020, 33, e13201. [Google Scholar] [CrossRef] [PubMed]
- Tanis, R.; Ferenczi, K.; Payette, M. Dupilumab Treatment for Prurigo Nodularis and Pruritis. J. Drugs Dermatol. 2019, 18, 940–942. [Google Scholar] [PubMed]
- Zhai, L.L.; Savage, K.T.; Qiu, C.C.; Jin, A.; Valdes-Rodriguez, R.; Mollanazar, N.K. Chronic Pruritus Responding to Dupilumab—A Case Series. Medicines 2019, 6, 72. [Google Scholar] [CrossRef]
- Kabashima, K.; Furue, M.; Hanifin, J.M.; Pulka, G.; Wollenberg, A.; Galus, R.; Etoh, T.; Mihara, R.; Nakano, M.; Ruzicka, T. Nemolizumab in patients with moderate-to-severe atopic dermatitis: Randomized, phase II, long-term extension study. J. Allergy Clin. Immunol. 2018, 142, 1121–1130.e7. [Google Scholar] [CrossRef]
- Nemoto, O.; Furue, M.; Nakagawa, H.; Shiramoto, M.; Hanada, R.; Matsuki, S.; Imayama, S.; Kato, M.; Hasebe, I.; Taira, K.; et al. The first trial of CIM331, a humanized antihuman interleukin-31 receptor A antibody, in healthy volunteers and patients with atopic dermatitis to evaluate safety, tolerability and pharmacokinetics of a single dose in a randomized, double-blind, placebo-controlled study. Br. J. Dermatol. 2016, 174, 296–304. [Google Scholar]
- Ruzicka, T.; Hanifin, J.M.; Furue, M.; Pulka, G.; Mlynarczyk, I.; Wollenberg, A.; Galus, R.; Etoh, T.; Mihara, R.; Yoshida, H.; et al. Anti-Interleukin-31 Receptor A Antibody for Atopic Dermatitis. N. Engl. J. Med. 2017, 376, 826–835. [Google Scholar] [CrossRef]
- Stander, S.; Yosipovitch, G.; Legat, F.J.; Lacour, J.P.; Paul, C.; Narbutt, J.; Bieber, T.; Misery, L.; Wollenberg, A.; Reich, A.; et al. Trial of Nemolizumab in Moderate-to-Severe Prurigo Nodularis. N. Engl. J. Med. 2020, 382, 706–716. [Google Scholar] [CrossRef] [PubMed]
| Atopic Dermatitis or Type 2 Inflammation | Psoriasis or Type 1/17 Inflammation | |
|---|---|---|
| Predominant inflammatory factors | IL-4, IL-13, IL-31, IL-25, IL-33, TSLP | IFN-γ, IL-17A, IL-22, IL-23, TNF-α |
| Neuropeptide mechanism | ||
| Substance P | Promotes mast cell activation and degranulation; promotes migration of CD301b+ DCs | Promotes mast cell degranulation |
| Calcitonin-gene-related peptide | Promotes mast cell degranulation; limits ILC2 proliferation and IL-13 production; enhances LC function | Promotes mast cell degranulation; promotes infiltration of DCs and T cells |
| Vasoactive intestinal peptide | Promotes mast cell degranulation; promotes IL-5 release from ILC2s; induces Th2 polarization | Promotes mast cell degranulation; promotes ILC3 recruitment; influences IL-22 production from ILC3s; enhances LC function |
| Brain natriuretic peptide | Stimulates inflammatory factors from DCs | Unknown |
| Neuropeptide Y | Promotes mast cell degranulation; induces migration of human immature DCs | Unknown |
| Neuromedin B | Limits type 2 inflammation | Unknown |
| Neuromedin U | Induces ILC2 proliferation and activation | Unknown |
| Nerve growth factor | Promotes mast cell degranulation | Enhances basophil function |
| Galanin | Unknown | Induces neo-vascularization, neutrophil infiltration, and cytokine release |
| α-Melanocyte-stimulating hormone | Unknown | Induces tolerogenic DCs; leads to Treg proliferation; inhibits Th17 activities |
| Target | Therapeutic Agents | Mode | Indications | References/NCT Number |
|---|---|---|---|---|
| NK-1R | Aprepitant | Antagonist | AD; chronic prurigo | [98,99] |
| Serlopitant | Antagonist | AD; psoriasis; PN; chronic refractory pruritus | [100,101,102,103]; NCT02975206; NCT03343639; NCT03546816; NCT01951274 | |
| Tradipitant | Antagonist | AD | [104,105]; NCT02004041; NCT02651714 | |
| TrkA | Pegcantratinib | Inhibitor | Psoriasis | [106]; NCT03448081 |
| KOR/MOR | Nalbuphine | Agonist of KOR/antagonist of MOR | Chronic prurigo | NCT02174419; NCT02174432 |
| TRPV1 | Asivatrep | Antagonist | AD | [107,108] |
| IL-13 | Tralokinumab | mAb | AD | [109]; NCT03363854 |
| Lebrikizumab | mAb | AD | [110]; NCT04146363; NCT04178967; NCT04392154; NCT04250350; NCT04250337 | |
| IL-33 | Etokimab | mAb | AD | [111]; NCT03533751 |
| TSLP | Tezepelumab | mAb | AD | [112]; NCT02525094; NCT03809663 |
| IL-4Rα | Dupilumab | mAb | AD; PN; chronic pruritus | [113,114,115,116,117] |
| IL-31RA | Nemolizumab | mAb | AD; PN | [118,119,120,121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Li, F.; Wang, F. Neural Regulation of Innate Immunity in Inflammatory Skin Diseases. Pharmaceuticals 2023, 16, 246. https://doi.org/10.3390/ph16020246
Huang X, Li F, Wang F. Neural Regulation of Innate Immunity in Inflammatory Skin Diseases. Pharmaceuticals. 2023; 16(2):246. https://doi.org/10.3390/ph16020246
Chicago/Turabian StyleHuang, Xiaobao, Fengxian Li, and Fang Wang. 2023. "Neural Regulation of Innate Immunity in Inflammatory Skin Diseases" Pharmaceuticals 16, no. 2: 246. https://doi.org/10.3390/ph16020246
APA StyleHuang, X., Li, F., & Wang, F. (2023). Neural Regulation of Innate Immunity in Inflammatory Skin Diseases. Pharmaceuticals, 16(2), 246. https://doi.org/10.3390/ph16020246






