Lysiphyllum strychnifolium (Craib) A. Schmitz Extracts Moderate the Expression of Drug-Metabolizing Enzymes: In Vivo Study to Clinical Propose
Abstract
1. Introduction
2. Results
2.1. Determination of Que and GA in LS Extracts
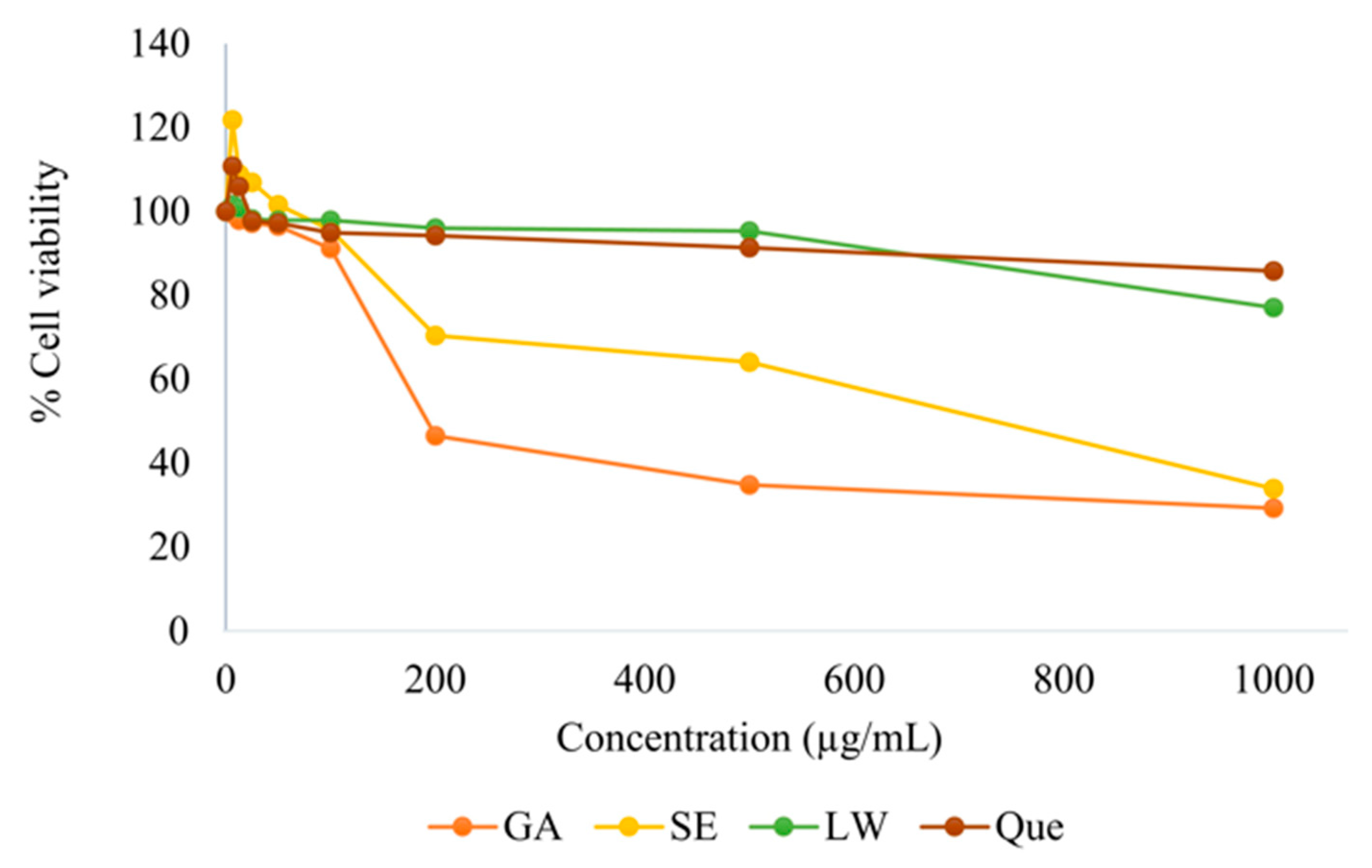
2.2. Cytotoxicity Test
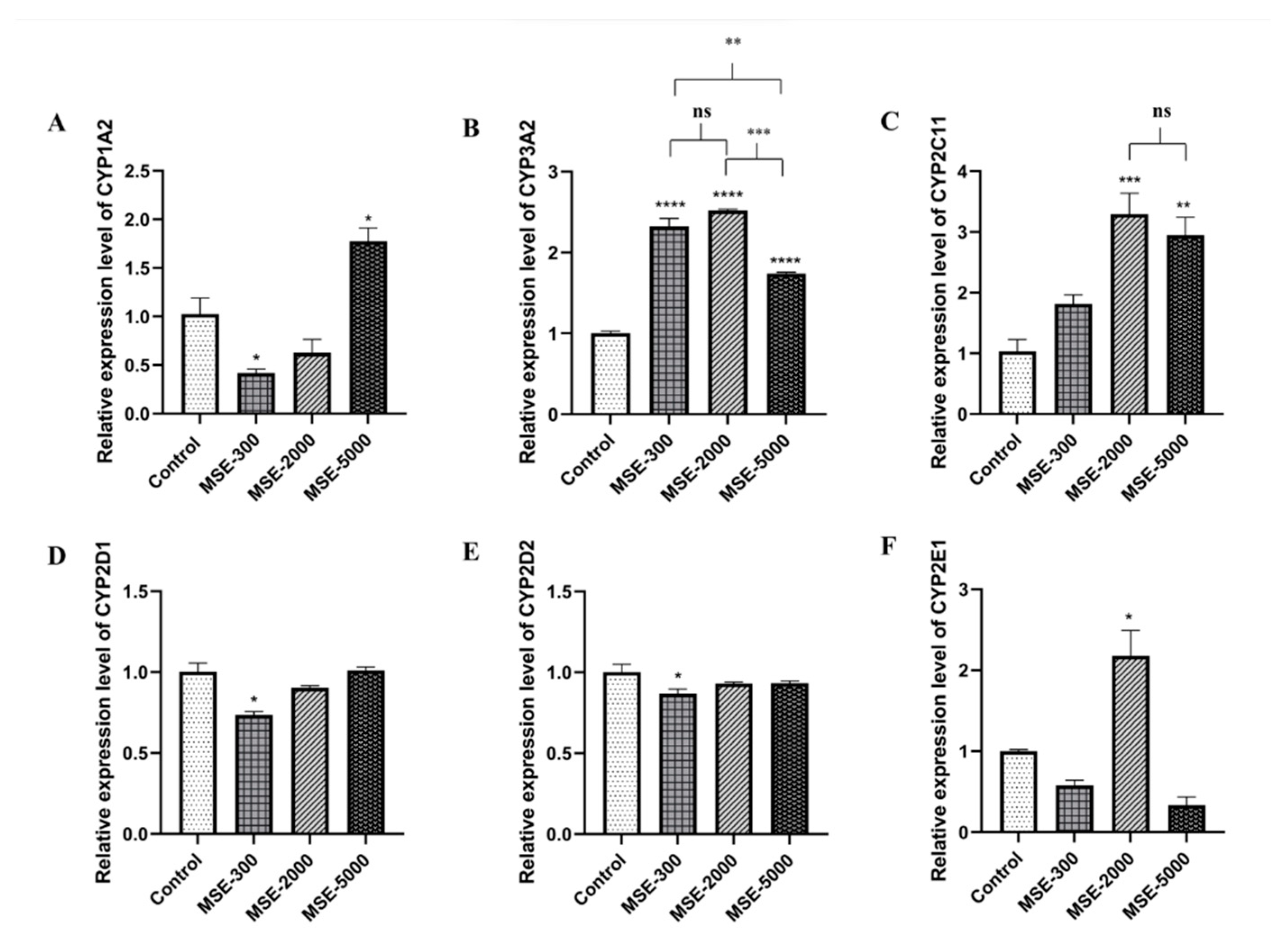
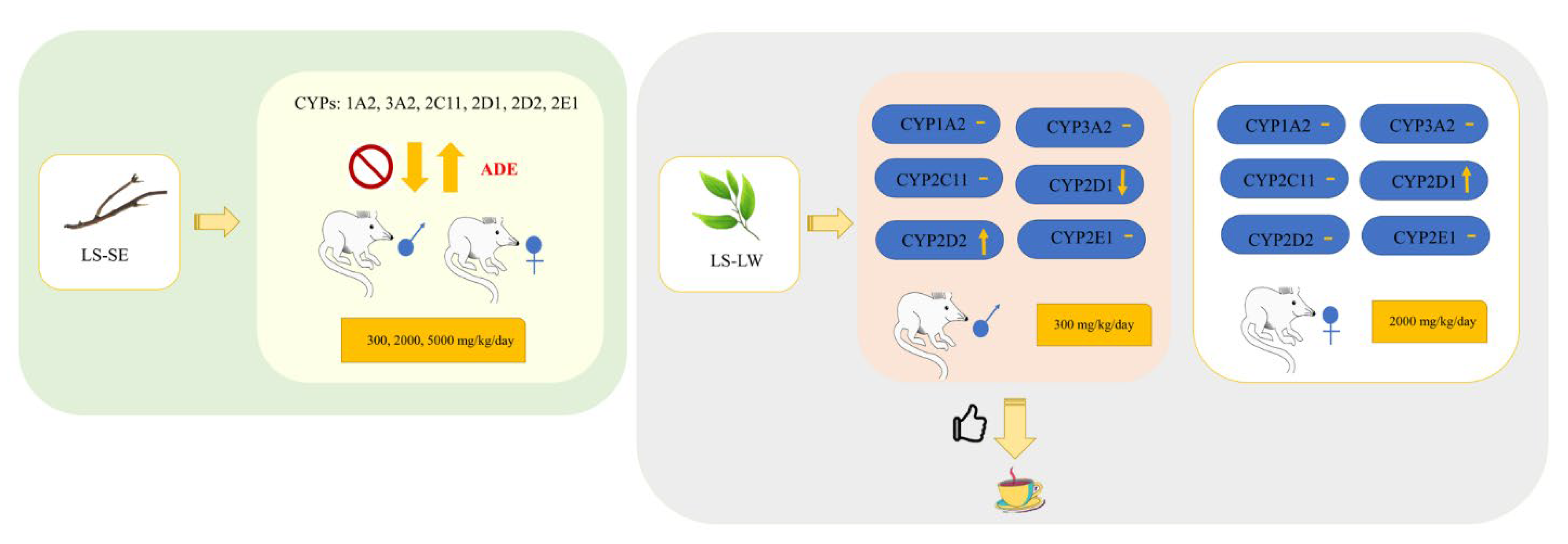
2.3. Effect of SE on Hepatic mRNA CYPs450
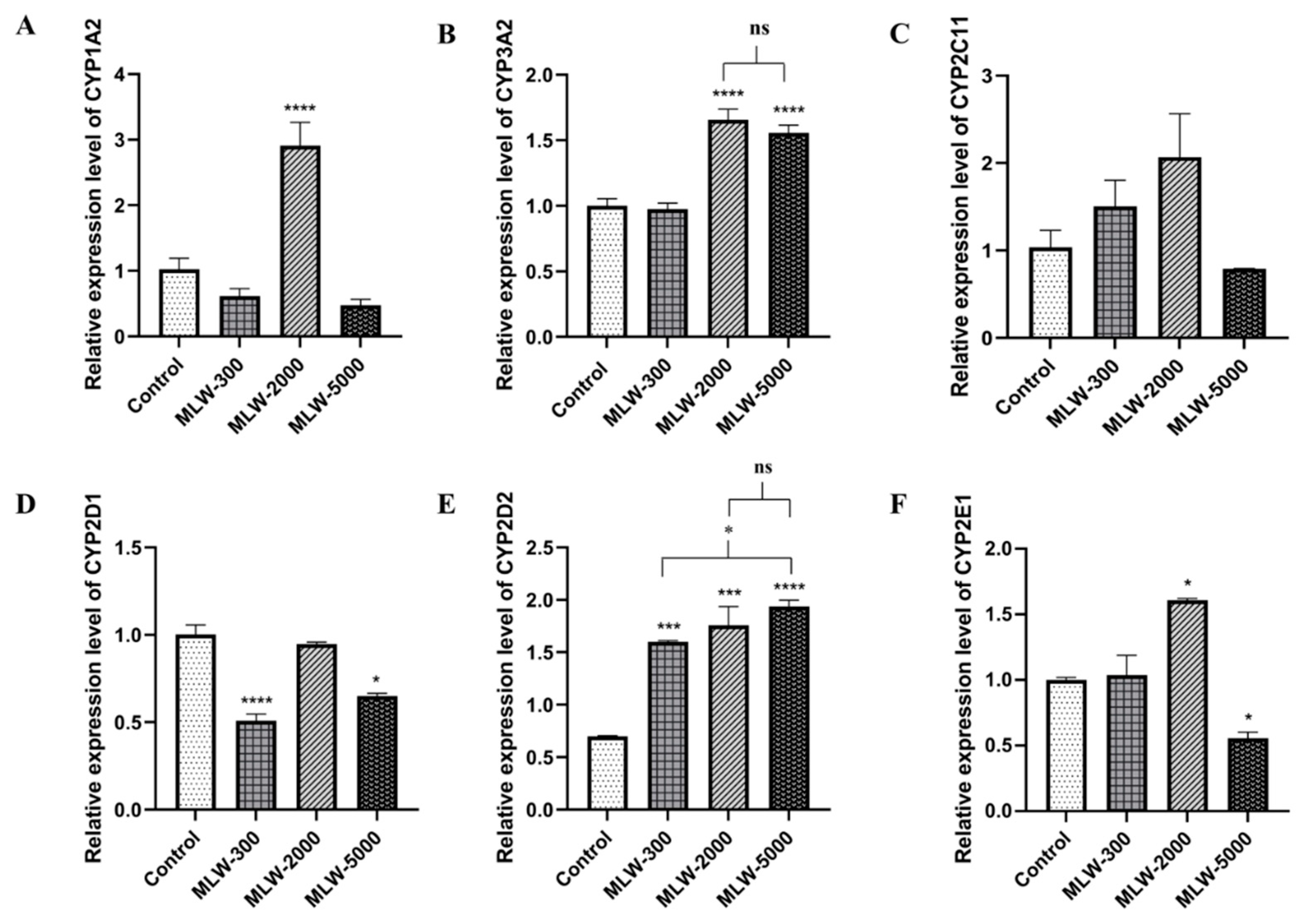
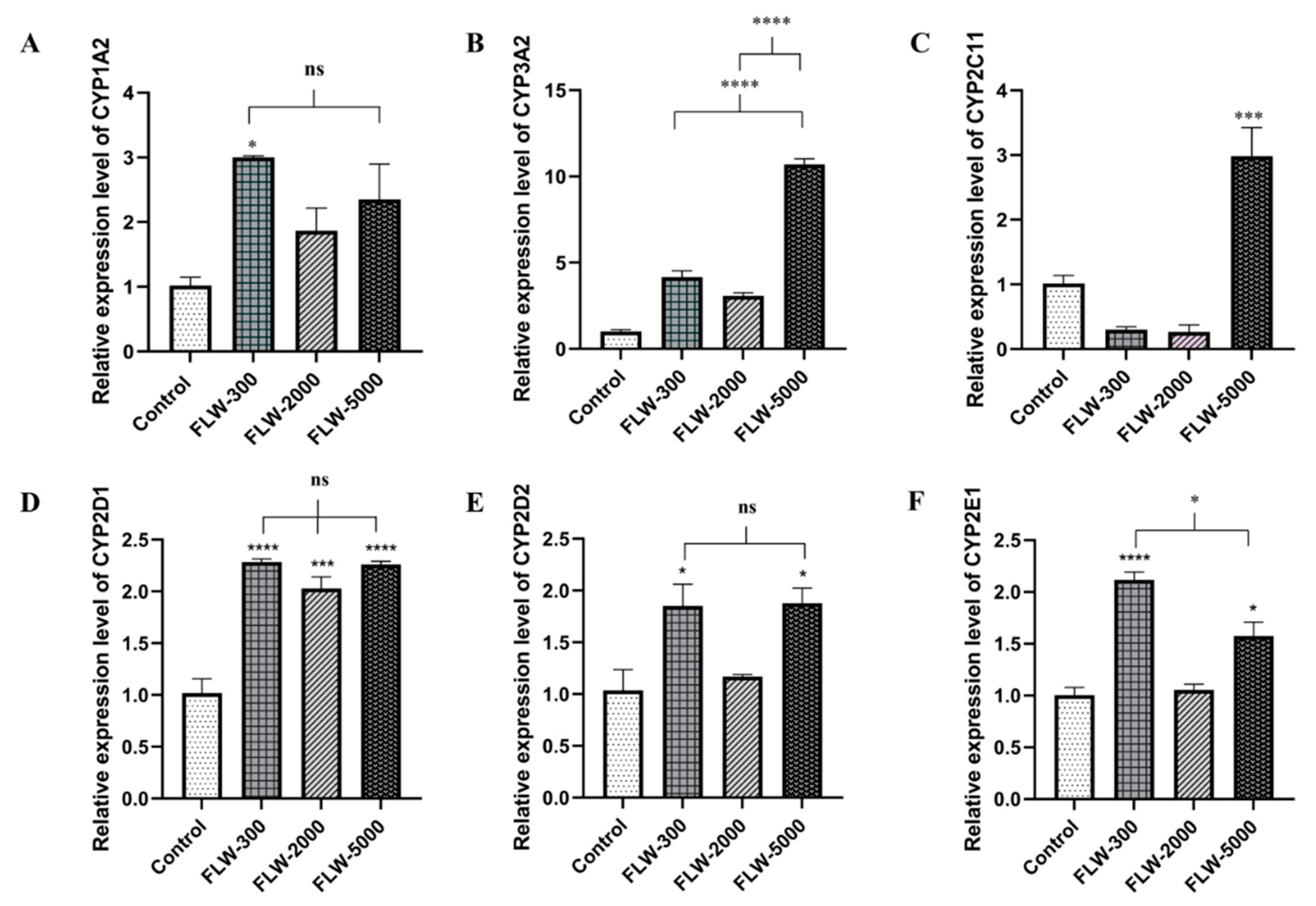
2.4. Effect of LW on Hepatic mRNA CYPs450
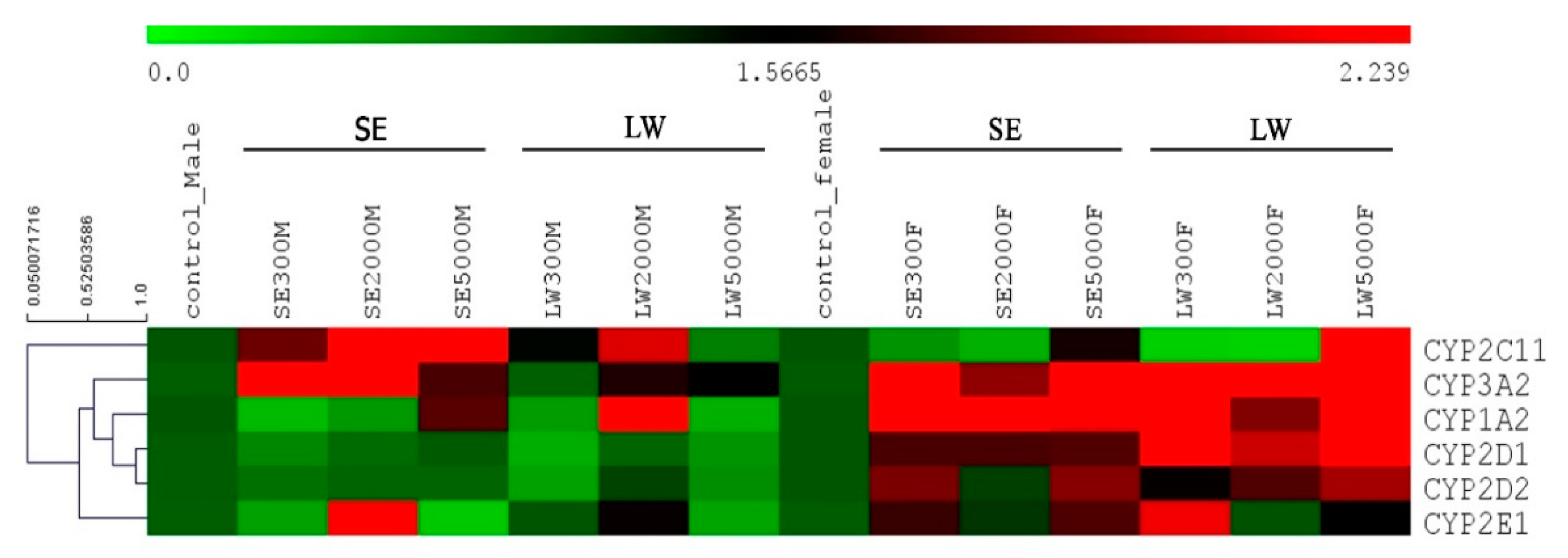
2.5. Heat Map Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Extraction
4.2. Chemicals and Reagents
4.3. HPLC Analysis
4.4. Cell Viability Assay
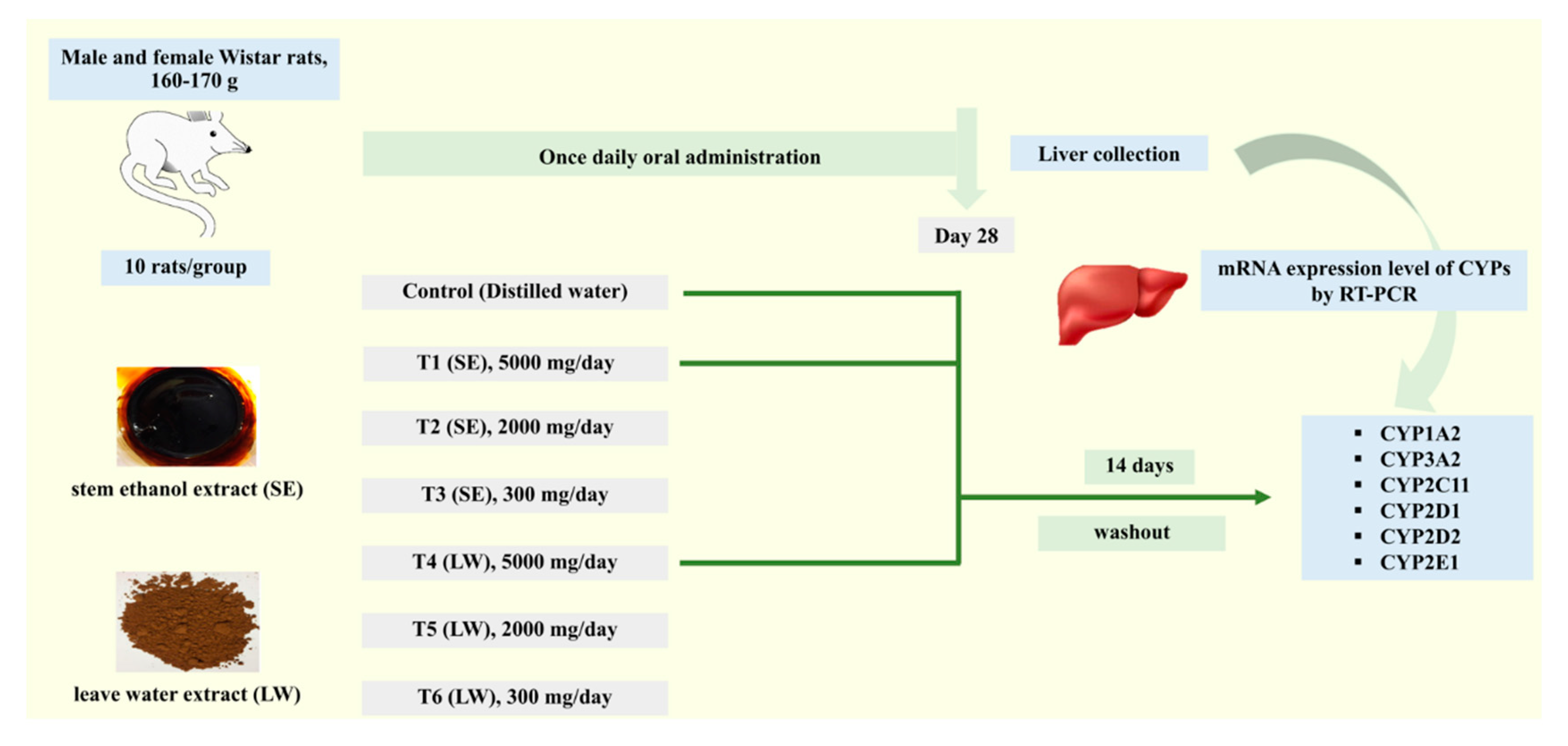
4.5. Animal Experiment
4.6. Total RNA Isolation and Quantitative RT-PCR (qRT-PCR)
4.7. Hierarchical Clustering Analysis (HCA)
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wegener, T. Patterns and trends in the use of herbal products, herbal medicine and herbal medicinal products. Int. J. Complement. Altern. Med. 2017, 9, 317. [Google Scholar] [CrossRef]
- Samuhasaneeto, S.; Yusakul, G. Modulatory effects of Benjakul extract on rat hepatic cytochrome P450 enzymes. Heliyon 2021, 7, e08498. [Google Scholar] [CrossRef]
- Sato, V.H.; Chewchinda, S.; Nuamnaichati, N.; Mangmool, S.; Sungthong, B.; Lertsatitthanakorn, P.; Ohta, S.; Sato, H. Pharmacological mechanisms of the water leaves extract of Lysiphyllum strychnifolium for its anti-inflammatory and anti-hyperuricemic actions for gout treatment. Pharmacogn. Mag. 2019, 5, 98–106. [Google Scholar] [CrossRef]
- Luengthong, N.; Lin, C.; Muangman, V.; Wongyai, S. Preliminary study of the effect of Bauhinia strychnifolia Craib on blood alcohol levels in healthy volunteers. J. Thai Tradit. Altern. Med. 2016, 14, 177–187. [Google Scholar]
- Sukprasert, S.; Deenonpoe, R.; Yimsoo, T.; Yingmema, W.; Prasopdee, S.; Krajang, A.; Kornthong, N.; Pattaraarchachai, J.; Daduang, S. Antidote activity and protective effects of Lysiphyllum strychnifolium (Craib) A. Schmitz extract against organophosphate pesticide in omethoate-treated rats. J. Tradit. Complement. Med. 2021, 11, 189–196. [Google Scholar] [CrossRef]
- Bunluepuech, K.; Wattanapiromsakul, C.; Madaka, F.; Tewtrakul, S. Anti-HIV-1 integrase and anti-allergic activities of Bauhinia strychnifolia. Songklanakarin J. Sci. Technol. 2013, 35, 659–664. [Google Scholar]
- Noonong, K.; Pranweerapaiboon, K.; Chaithirayanon, K.; Surayarn, K.; Ditracha, P.; Changklungmoa, N.; Kueakhai, P.; Hiransai, P.; Bunluepuech, K. Antidiabetic potential of Lysiphyllum strychnifolium (Craib) A. Schmitz compounds in human intestinal epithelial Caco-2 cells and molecular docking-based approaches. BMC Complement. Med. Ther. 2022, 22, 235. [Google Scholar] [CrossRef]
- Sukprasert, S.; Pansuksan, K.; Sriyakul, K. Lysiphyllum strychnifolium (Craib) A. Schmitz extract, a novel neuraminidase inhibitor of avian influenza virus subtype H5N1. J. Herb. Med. 2020, 20, 100330. [Google Scholar] [CrossRef]
- Sampaopan, Y.; Kitprapiumpon, N.; Kongkiatpaiboon, S.; Duangdee, N.; Wongyai, S. Isolation and HPLC Analysis of Astilbin in Lysiphyllum strychnifolium (syn. Bauhinia strychnifolia) Stems. Sci. Technol. Asia 2021, 26, 208–215. [Google Scholar]
- Kongkiatpaiboon, S.; Duangdee, N.; Tayana, N.; Schinnerl, J.; Bacher, M.; Chewchinda, S. Yanangdaengin, a dihydrochalcone glucoside galloyl ester as active antioxidative agent from leaves of Lysiphyllum strychnifolium (syn. Bauhinia strychnifolia). Chin. Herb. Med. 2020, 12, 452–455. [Google Scholar] [CrossRef]
- Guengerich, F.P.; Waterman, M.R.; Egli, M. Recent Structural Insights into Cytochrome P450 Function. Trends Pharmacol. Sci. 2016, 37, 625–640. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Liu, K. Pharmacokinetic interactions between herbal medicines and prescribed drugs: Focus on drug metabolic enzymes and transporters. Curr. Drug Metab. 2014, 15, 791–807. [Google Scholar] [CrossRef] [PubMed]
- Grimstein, M.; Huang, S.M. A regulatory science viewpoint on botanical-drug interactions. J. Food Drug Anal. 2018, 26, 12–25. [Google Scholar] [CrossRef]
- Choi, Y.H.; Chin, Y.W. Multifaceted Factors Causing Conflicting Outcomes in Herb-Drug Interactions. Pharmaceutics 2021, 13, 43. [Google Scholar] [CrossRef]
- Husain, I.; Dale, O.R.; Manda, V.; Ali, Z.; Gurley, B.J.; Chittiboyina, A.G.; Khan, I.A.; Khan, S.I. Bulbine natalensis (currently Bulbine latifolia) and select bulbine knipholones modulate the activity of AhR, CYP1A2, CYP2B6, and P-gp. Planta Med. 2022, 87, 975–984. [Google Scholar] [CrossRef]
- Tarirai, C.; Viljoen, A.M.; Hamman, J.H. Herb-drug Pharmacokinetic Interactions Reviewed. Expert Opin. Drug Metab. Toxicol. 2010, 6, 1515–1538. [Google Scholar] [CrossRef]
- Hossain, S.; Yousaf, M.; Liu, Y.; Chang, D.; Zhou, X. An Overview of the Evidence and Mechanism of Drug-Herb Interactions Between Propolis and Pharmaceutical Drugs. Front. Pharmacol. 2022, 13, 876183. [Google Scholar] [CrossRef] [PubMed]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Pan, H.Y.; Wu, L.W.; Wang, P.C.; Chiu, P.H.; Wang, M.T. Real-world Evidence of the Herb-drug Interactions. J. Food Drug Anal. 2022, 15, 316–330. [Google Scholar] [CrossRef]
- Zhang, J.; Onakpoya, I.J.; Posadzki, P.; Eddouks, M. The Safety of Herbal Medicine: From Prejudice to Evidence. Evid.-Based Complement. Altern. Med. 2015, 2015, 316706. [Google Scholar] [CrossRef]
- Li, Y.; Lu, J.; Paxton, J.W. The Role of ABC and SLC Transporters in the Pharmacokinetics of Dietary and Herbal Phytochemicals and their Interactions with Xenobiotics. Curr. Drug Metab. 2012, 13, 624–639. [Google Scholar] [CrossRef]
- Tsai, H.H.; Lin, H.W.; Simon Pickard, A.; Tsai, H.Y.; Mahady, G.B. Evaluation of documented drug interactions and contraindications associated with herbs and dietary supplements: A systematic literature review. Int. J. Clin. Pract. 2012, 66, 1056–1078. [Google Scholar] [CrossRef]
- Andersson, T.B.; Bredberg, E.; Ericsson, H.; Sjoberg, H. An evaluation of the in vitro metabolism data for predicting the clearance and drug-drug interaction potential of CYP2C9 substrates. Drug Metab. Dispos. 2004, 32, 715–721. [Google Scholar] [CrossRef]
- Cho, H.J.; Yoon, I.S. Pharmacokinetic Interactions of Herbs with Cytochrome P450 and P-Glycoprotein. Evid.-Based Complement. Altern. Med. 2015, 2015, 736431. [Google Scholar] [CrossRef]
- Awortwe, C.; Makiwane, M.; Reuter, H.; Muller, C.; Louw, J.; Rosenkranz, B. Critical evaluation of causality assessment of herb-drug interactions in patients. Br. J. Clin. Pharmacol. 2018, 84, 679–693. [Google Scholar] [CrossRef]
- Clairet, A.L.; Boiteux-Jurain, M.; Curtit, E.; Jeannin, M.; Gérard, B.; Nerich, V.; Limat, S. Interaction between phytotherapy and oral anticancer agents: Prospective study and literature review. Med. Oncol. 2019, 45, 45. [Google Scholar] [CrossRef]
- Shiferaw, A.; Baye, A.M.; Amogne, W.; Feyissa, M. Herbal Medicine Use and Determinant Factors Among HIV/AIDS Patients on Antiretroviral Therapy in Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia. HIV AIDS (Auckl.) 2020, 12, 941–949. [Google Scholar] [CrossRef]
- Mangwani, N.; Singh, P.K.; Kumar, V. Medicinal plants: Adjunct treatment to tuberculosis chemotherapy to prevent hepatic damage. J. Ayurveda Integr. Med. 2020, 11, 522–528. [Google Scholar] [CrossRef]
- Kimura, Y.; Ito, H.; Ohnishi, R.; Hatano, T. Inhibitory effects of polyphenols on human cytochrome P450 3A4 and 2C9 activity. Food Chem. Toxicol. 2010, 48, 429–435. [Google Scholar] [CrossRef]
- Suroowan, S.; Abdallah, H.H.; Mahomoodally, M.F. Herb-drug interactions and toxicity: Underscoring potential mechanisms and forecasting clinically relevant interactions induced by common phytoconstituents via data mining and computational approaches. Food Chem. Toxicol. 2021, 156, 112432. [Google Scholar] [CrossRef]
- Basch, E.; Gabardi, S.; Ulbricht, C. Bitter melon (Momordica charantia): A review of efficacy and safety. Am. J. Health Syst. Pharm. 2003, 60, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Flockhart, D.A.; Oesterheld, J.R. Cytochrome P450-Mediated Drug Interactions, Child and Adolescent Psychiatric Clinics of North America. J. Psychopharmacol. 2000, 9, 43–76. [Google Scholar] [CrossRef]
- Jin, S.E.; Ha, H.; Seo, C.S.; Shin, H.K.; Jeong, S.J. Expression of Cytochrome P450s in the Liver of Rats Administered with Socheongryong-tang, a Traditional Herbal Formula. Pharmacogn. Mag. 2016, 12, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Cui, M.; Zhao, L.; Wang, D.; Tang, T.; Wang, W.; Wang, S.; Huang, H.; Qiu, X. Potential Metabolic Drug-Drug Interaction of Citrus aurantium L. (Rutaceae) Evaluating by Its Effect on 3 CYP450. Front. Pharmacol. 2018, 9, 895. [Google Scholar] [CrossRef] [PubMed]
- Goli, A.S.; Leanpolchareanchai, J.; Chewchinda, S.; Yahuafai, J.; Nontakham, J.; Sato, H.; Sato, V.H. Microencapsulation of Lysiphyllum strychnifolium extract using pectin as a carrier matrix and its characterization. Med. Biomed. 2022, 6, 1–12. [Google Scholar] [CrossRef]
- Jiang, B.; Meng, L.; Zhang, F.; Jin, X.; Zhang, G. Enzyme-inducing effects of berberine on cytochrome P450 1A2 in vitro and in vivo. Life Sci. 2017, 15, 1–7. [Google Scholar] [CrossRef]
- Kapoor, N.; Pant, A.B.; Dhawan, A.; Dwievedi, U.N.; Seth, P.K.; Parmar, D. Cytochrome P450 1A isoenzymes in brain cells: Expression and inducibility in cultured rat brain neuronal and glial cells. Life Sci. 2006, 79, 2387–2394. [Google Scholar] [CrossRef]
- Sheng, C.; Shi, X.; Ding, Z.; Chen, Y.; Shi, X.; Wu, Y.; Zhang, W.; Chen, W. Effects of mulberry leaf extracts on activity and mRNA expression of five cytochrome P450 enzymes in rat. Braz. J. Pharm. Sci. 2021, 57, 1–15. [Google Scholar] [CrossRef]
- Picking, D.; Chambers, B.; Barker, J.; Shah, I.; Porter, R.; Naughton, D.P.; Delgoda, R. Inhibition of Cytochrome P450 Activities by Extracts of Hyptis verticillata Jacq.: Assessment for Potential HERB-Drug Interactions. Molecules 2018, 23, 430. [Google Scholar] [CrossRef]
- Xiao, J.; Huang, W.H.; Peng, J.B.; Tan, Z.R.; Ou-Yang, D.S.; Hu, D.L.; Zhang, W.; Chen, Y. Quercetin Significantly Inhibits the Metabolism of Caffeine, a Substrate of Cytochrome P450 1A2 Unrelated to CYP1A2*1C (−2964G>A) and *1F (734C>A) Gene Polymorphisms. Biomed. Res. Int. 2014, 2014, 405071. [Google Scholar] [CrossRef]
- Lu, J.; Shao, Y.; Qin, X.; Liu, D.; Chen, A.; Li, D.; Liu, M.; Wang, X. CRISPR knockout rat cytochrome P450 3A1/2 model for advancing drug metabolism and pharmacokinetics research. Sci. Rep. 2017, 7, 42922. [Google Scholar] [CrossRef]
- Pekthonga, D.; Blanchardc, N.; Abadiec, C.; Bonet, A.; Heydd, B.; Mantiond, G.; Berthelot, A.; Richert, L.; Martina, H. Effects of Andrographis paniculata extract and Andrographolide on hepatic cytochrome P450 mRNA expression and monooxygenase activities after in vivo administration to rats and in vitro in rat and human hepatocyte cultures. Chem.-Biol. Interact. 2009, 179, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Van der Logt, E.M.J. Induction of rat hepatic and intestinal UDP-glucuronosyltransferases by naturally occurring dietary anticarcinogens. Carcinogenesis 2003, 24, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Hofman, J.; Vagiannis, D.; Chen, S.; Guo, L. Roles of CYP3A4, CYP3A5 and CYP2C8 drug-metabolizing enzymes in cellular cytostatic resistance. Chem.-Biol. Interact. 2021, 340, 109448. [Google Scholar] [CrossRef]
- Yan, J.; He, X.; Feng, S.; Zhai, Y.; Ma, Y.; Liang, S.; Jin, C. Up-regulation on cytochromes P450 in rat mediated by total alkaloid extract from Corydalis yanhusuo. BMC Complement. Altern. Med. 2014, 14, 306. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Sui, D.; Liu, W.; Yuan, Y.; Ouyang, Z.; Wei, Y. Effects of CYP2C11 gene knockout on the pharmacokinetics and pharmacodynamics of warfarin in rats. Xenobiotica 2019, 49, 1478–1484. [Google Scholar] [CrossRef]
- Daly, A.K.; Rettie, A.E.; Fowler, D.M.; Miners, J.O. Pharmacogenomics of CYP2C9: Functional and Clinical Considerations. J. Pers. Med. 2017, 8, 1. [Google Scholar] [CrossRef]
- Appiah-Opong, R.; Commandeur, J.N.M.; Axson, C.; Vermeulen, N.P.E. Interactions between cytochromes P450, glutathione S-transferases and Ghanaian medicinal plants. Food Chem. Toxicol. 2008, 46, 3598–3603. [Google Scholar] [CrossRef]
- Ingelman-Sundberg, M. Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): Clinical consequences, evolutionary aspects and functional diversity. Pharm. J. 2005, 5, 6–13. [Google Scholar] [CrossRef]
- Dickmann, L.J.; Tay, S.; Senn, T.D.; Zhang, H.; Visone, A.; Unadkat, J.D.; Hebert, M.F.; Isoherranen, N. Changes in maternal liver Cyp2c and Cyp2d expression and activity during rat pregnancy. Biochem. Pharmacol. 2008, 75, 1677–1687. [Google Scholar] [CrossRef]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef] [PubMed]
- Taşçıoğlu, N.; Saatçi, Ç.; Emekli, R.; Tuncel, G.; Eşel, E.; Dundar, M. Investigation of cytochrome p450 CYP1A2, CYP2D6, CYP2E1 and CYP3A4 gene expressions and polymorphisms in alcohol withdrawal. J. Clin. Psychiatry 2021, 24, 298–306. [Google Scholar] [CrossRef]
- Oneta, C.M.; Lieber, C.S.; Li, J.; Rüttimann, S.; Schmid, B.; Lattmann, J.; Rosman, A.S.; Seitz, H.K. Dynamics of cytochrome P4502E1 activity in man: Induction by ethanol and disappearance during withdrawal phase. J. Hepatol. 2002, 36, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Caro, A.A.; Cederbaum, A.I. Oxidative stress, toxicology, and pharmacology of CYP2E1. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 27–42. [Google Scholar] [CrossRef]
- Karakaş, D.; Ari, F.; Ulukaya, E. The MTT viability assay yields strikingly false-positive viabilities although the cells are killed by some plant extracts. Turk. J. Biol. 2017, 18, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for cell viability assays. Food Front. 2020, 1, 332–349. [Google Scholar] [CrossRef]
- Woottisin, N.; Sukprasert, S.; Kulsirirat, T.; Tharavanij, T.; Sathirakul, K. Evaluation of the Intestinal Permeability of Rosmarinic Acid from Thunbergia laurifolia Leaf Water Extract in a Caco-2 Cell Model. Molecules 2022, 27, 3884. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sumner, L.W.; Mendes, P.; Dixon, R.A. Plant metabolomics: Large-scale phytochemistry in the functional genomics era. Phytochemistry 2003, 62, 817–836. [Google Scholar] [CrossRef]
- Saeed, A.I.; Sharov, V.; White, J.; Li, J.; Liang, W.; Bhagabati, N.; Braisted, J.; Klapa, M.; Currier, T.; Thiagarajan, M.; et al. TM4: A free, open-source system for microarray data management and analysis. Biotechniques 2003, 34, 374–378. [Google Scholar] [CrossRef]


| Gene | Primer Sequences (5’ ➔ 3’) | Size (bp) | Accession Number |
|---|---|---|---|
| CYP1A2 | F: CGCCCAGAGCGGTTTCTTA | 81 | NM_012541.3 |
| R: TCCCAAGCCGAAGAGCATC | |||
| CYP3A2 | F: GGACTTAATTGACTGCTCTTGATG | 222 | NM_153312.2 |
| R: GGACGAGGACATGGTTACTATC | |||
| CYP2C11 | F: AGCTCTTGTTGATCTAGGAG | 426 | XM_008760364.2 |
| R: GGGAAGTAATCAATAATGGC | |||
| CYP42D1 | F: ACCCATGGCTTCTTTGCTTTTC | 98 | NM_153313.2 |
| R: CCTGTAGACTGGACTGGAA | |||
| CYP2D2 | F: CCAGGGCAACTTTGTGAAGC | 193 | NM_012730.2 |
| CYP2E1 | R: TGGAGTAACTGGCAATGCGT F: GCTGTCAAGGAGGTGCTAC R: GCCTCATTACCCTGTTTCC | 182 | NM_031543.1 |
| GAPDH | F: TTCAACGGCACAGTCAAG | 116 | XM_017593963.1 |
| R: TACTCAGCACCAGCATCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuendee, N.; Naladta, A.; Kulsirirat, T.; Yimsoo, T.; Yingmema, W.; Pansuksan, K.; Sathirakul, K.; Sukprasert, S. Lysiphyllum strychnifolium (Craib) A. Schmitz Extracts Moderate the Expression of Drug-Metabolizing Enzymes: In Vivo Study to Clinical Propose. Pharmaceuticals 2023, 16, 237. https://doi.org/10.3390/ph16020237
Kuendee N, Naladta A, Kulsirirat T, Yimsoo T, Yingmema W, Pansuksan K, Sathirakul K, Sukprasert S. Lysiphyllum strychnifolium (Craib) A. Schmitz Extracts Moderate the Expression of Drug-Metabolizing Enzymes: In Vivo Study to Clinical Propose. Pharmaceuticals. 2023; 16(2):237. https://doi.org/10.3390/ph16020237
Chicago/Turabian StyleKuendee, Natthaporn, Alisa Naladta, Thitianan Kulsirirat, Thunyatorn Yimsoo, Werayut Yingmema, Kanoktip Pansuksan, Korbtham Sathirakul, and Sophida Sukprasert. 2023. "Lysiphyllum strychnifolium (Craib) A. Schmitz Extracts Moderate the Expression of Drug-Metabolizing Enzymes: In Vivo Study to Clinical Propose" Pharmaceuticals 16, no. 2: 237. https://doi.org/10.3390/ph16020237
APA StyleKuendee, N., Naladta, A., Kulsirirat, T., Yimsoo, T., Yingmema, W., Pansuksan, K., Sathirakul, K., & Sukprasert, S. (2023). Lysiphyllum strychnifolium (Craib) A. Schmitz Extracts Moderate the Expression of Drug-Metabolizing Enzymes: In Vivo Study to Clinical Propose. Pharmaceuticals, 16(2), 237. https://doi.org/10.3390/ph16020237









