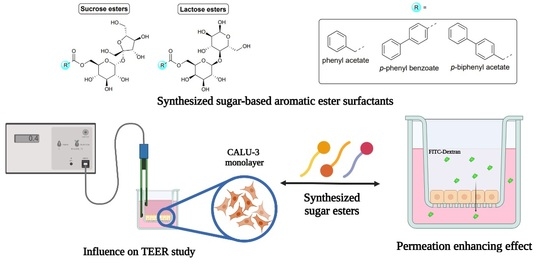
Synthesis and Properties of Sucrose- and Lactose-Based Aromatic Ester Surfactants as Potential Drugs Permeability Enhancers
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemistry
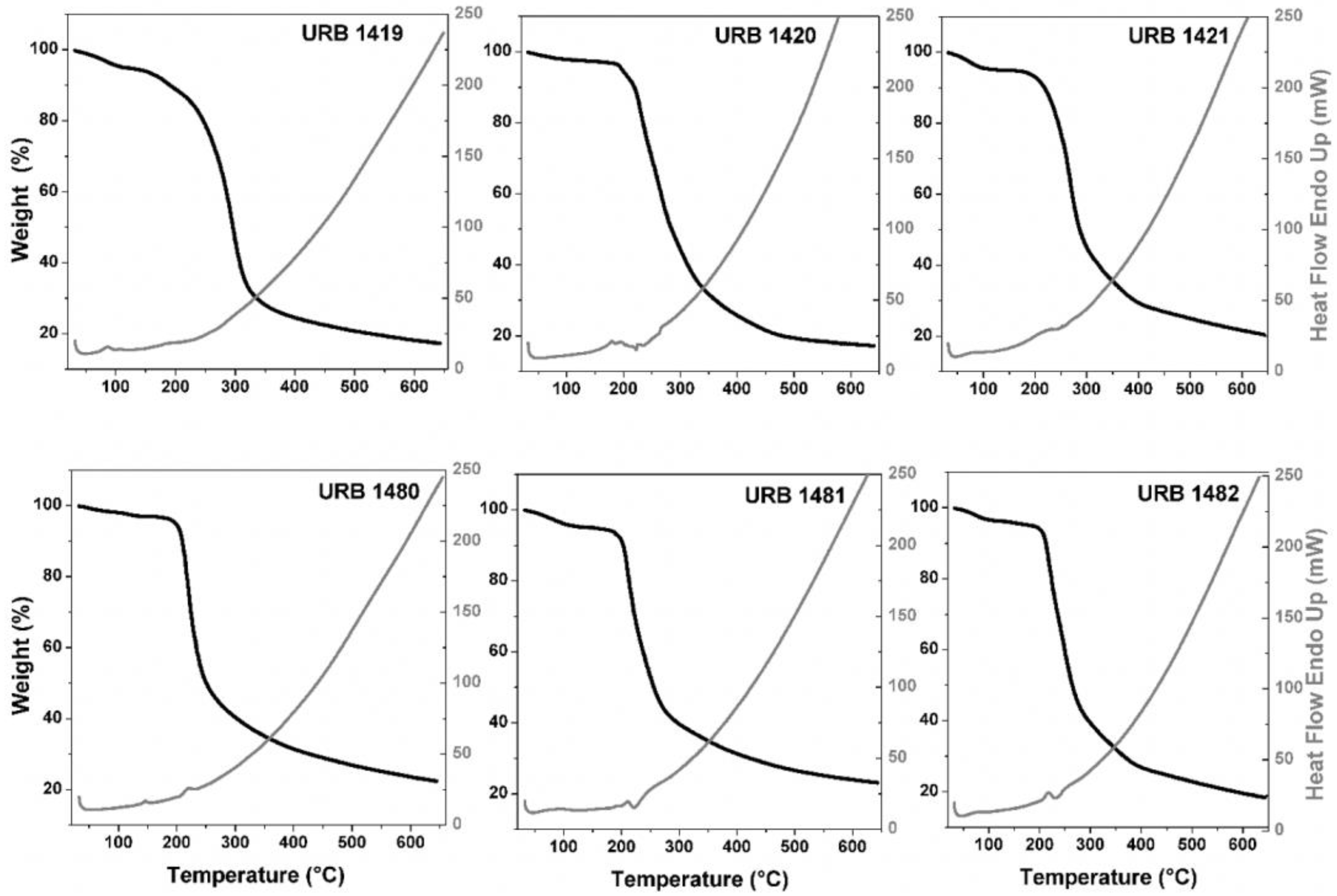
2.2. Thermogravimetric Analysis (TGA) and Differential Thermal Analysis/Scanning (DTA/DSC) Measurements
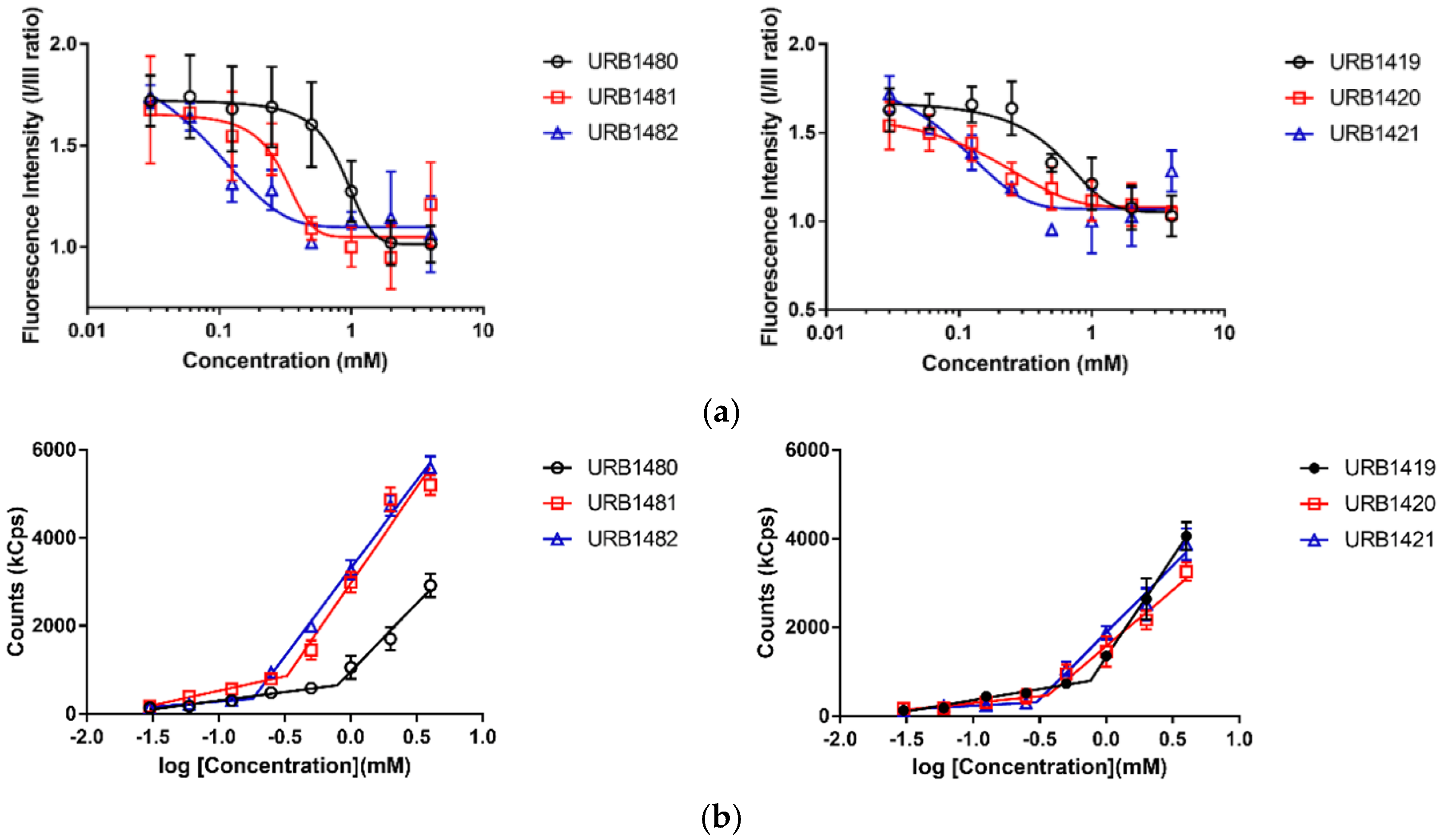
2.3. Critical Micelle Concentration (CMC) Measurements
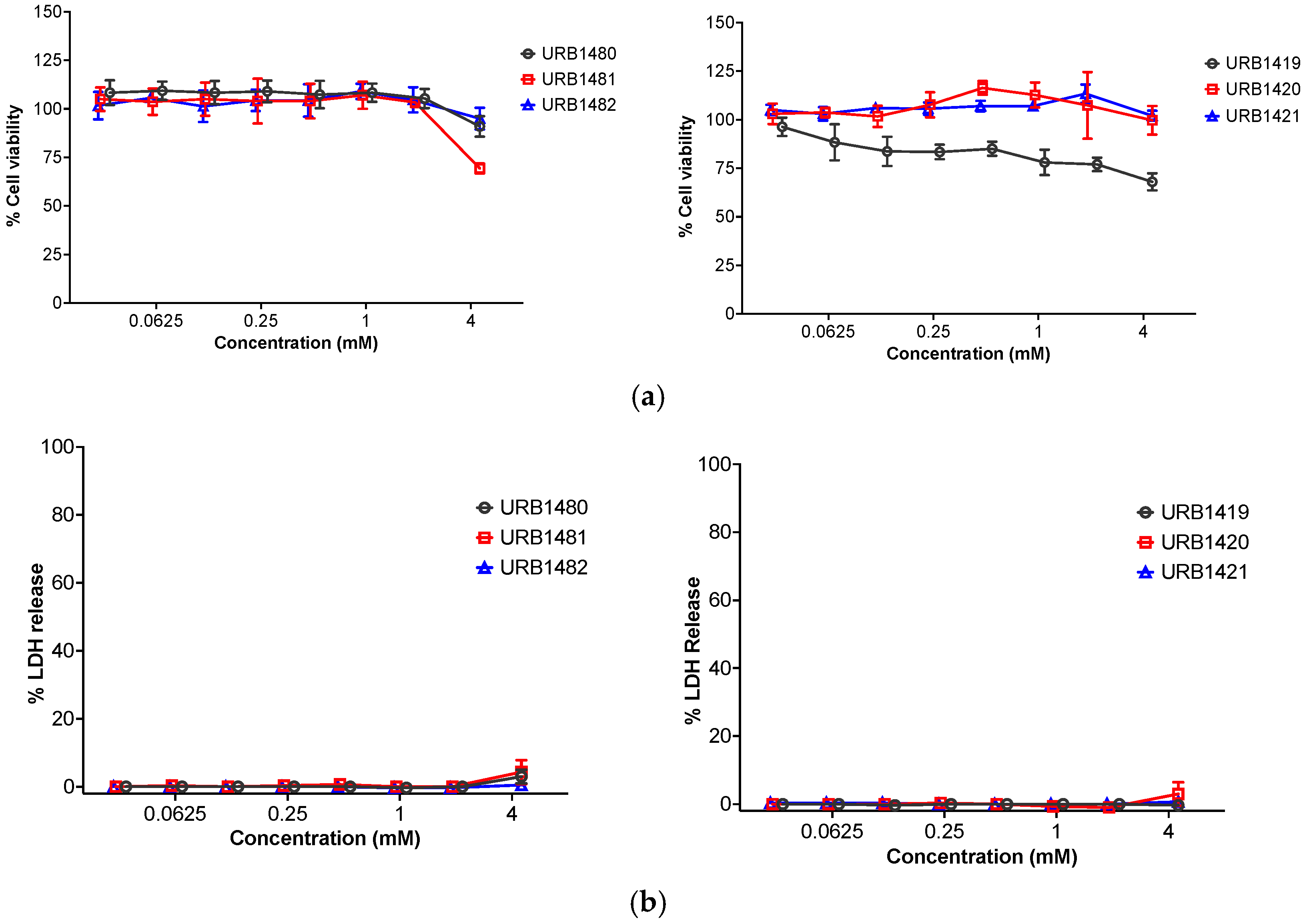
2.4. Cytotoxicity—[3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) Cell Viability and Lactate Dehydrogenase (LDH) Assays
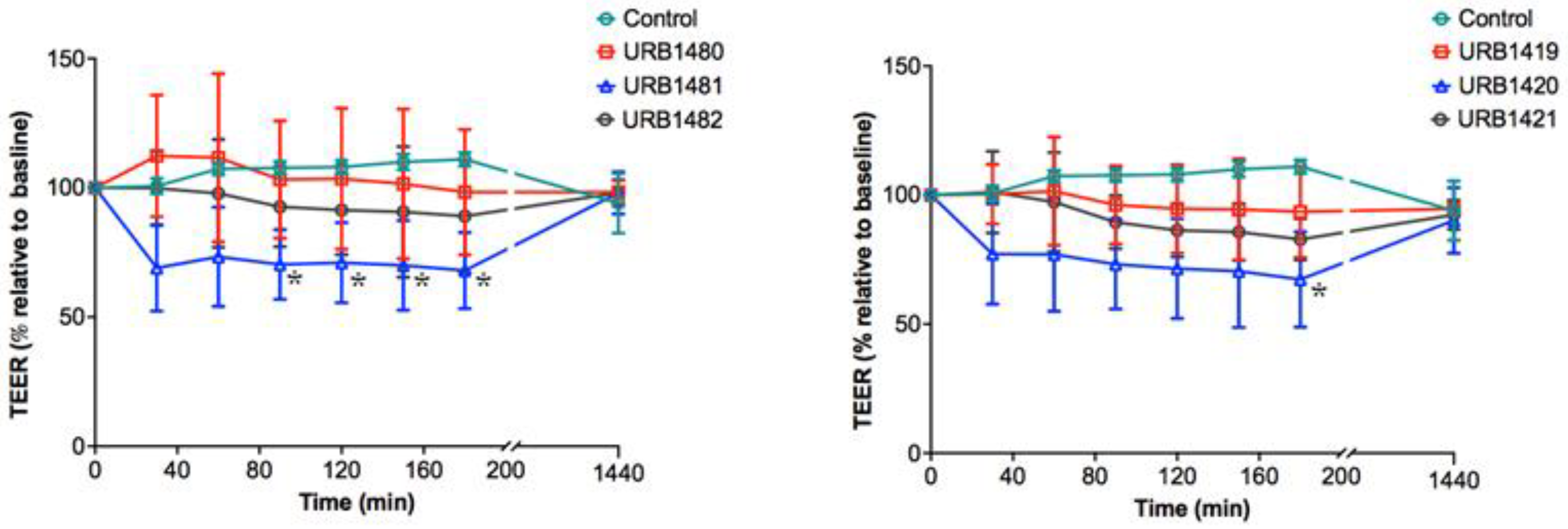
2.5. TEER Study
2.6. Permeability Study
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Sugar-Based Surfactants
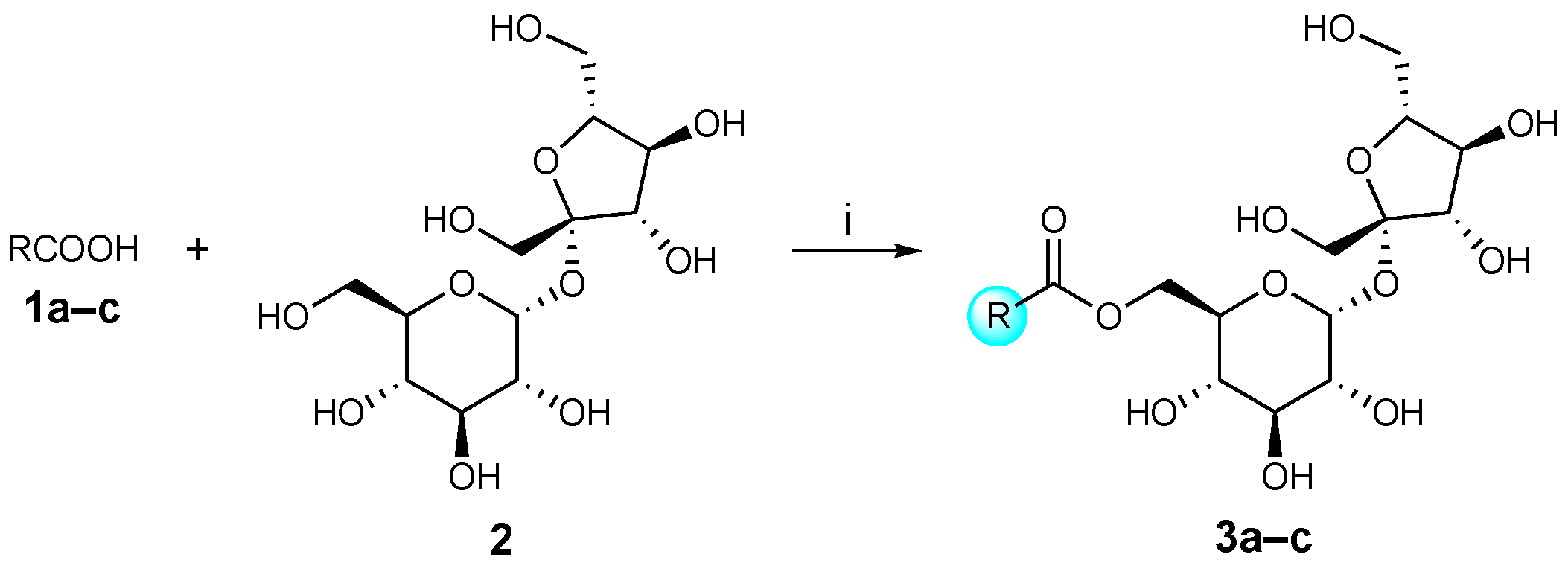
3.2.1. General Procedure for the Synthesis of Sucrose Aryl Aromatic and Aromatic Ester Surfactants (3a–c, URB1480–1482) (Scheme 1) [23]
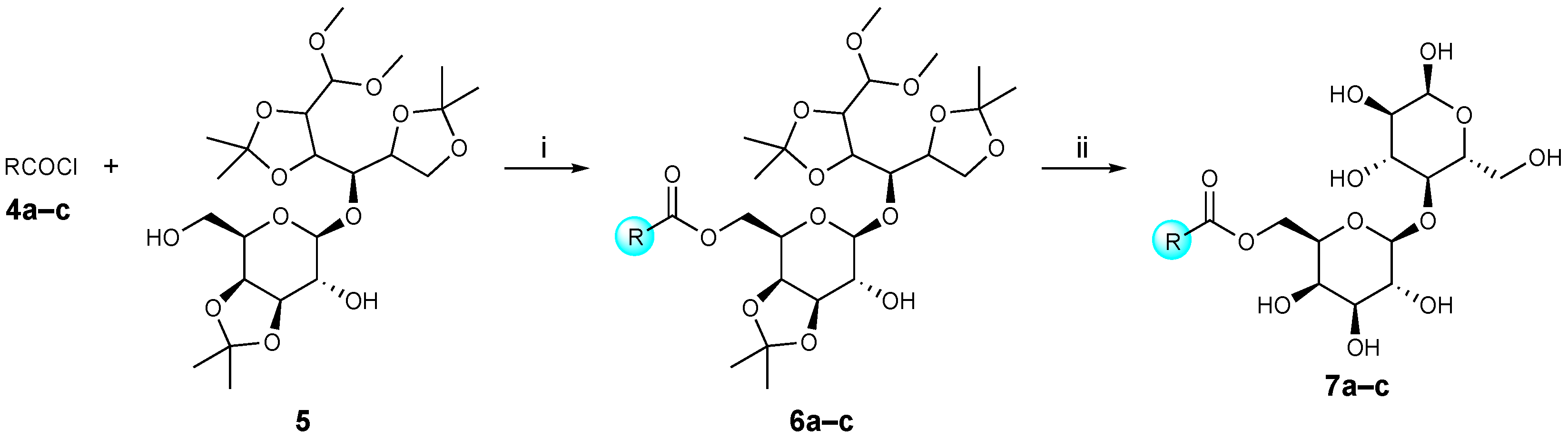
3.2.2. General Procedures for the Synthesis of Lactose Tetra Acetal Aryl Aromatic and Aromatic Esters (6a–c), and Lactose Aryl Aromatic and Aromatic Ester Surfactants (7a–c, URB1419–1421) (Scheme 2)
3.3. TGA and DTA/DSC Analyses
3.4. Fluorimetric Analysis
3.5. DLS Measurement of the CMC
3.6. Cytotoxicity Study MTT Cell Viability and LDH Release Assays
3.7. TEER Measurement
3.8. In Vitro Cell Permeability Studies
3.9. Statistics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anselmo, A.; Gokarn, Y.; Mitragotri, S. Non-invasive delivery strategies for biologics. Nat. Rev. Drug Discov. 2019, 18, 19–40. [Google Scholar] [CrossRef] [PubMed]
- Amit, K.G.; Ranjit, S.; Gaurav, C.; Goutam, R. Non-invasive systemic drug delivery through mucosal routes, artificial cells, nanomedicine, and biotechnology. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S2), 539–551. [Google Scholar] [CrossRef]
- Morales, J.O.; Fathe, K.R.; Brunaugh, A.; Ferrati, S.; Li, S.; Montenegro-Nicolini, M.; Mousavikhamene, Z.; McConville, J.T.; Prausnitz, M.R.; Smyth, H.D.C. Challenges and future prospects for the delivery of biologics: Oral mucosal, pulmonary, and transdermal routes. AAPS J. 2017, 19, 652–668. [Google Scholar] [CrossRef] [PubMed]
- McCartney, F.; Gleeson, J.P.; Brayden, D.J. Safety concerns over the use of intestinal permeation enhancers: A mini-review. Tissue Barriers 2016, 4, e1176822. [Google Scholar] [CrossRef] [PubMed]
- Kováčik, A.; Kopečná, M.; Vávrová, K. Permeation enhancers in transdermal drug delivery: Benefits and limitations. Exp. Opin. Drug Deliv. 2020, 17, 145–155. [Google Scholar] [CrossRef]
- Maher, S.; Brayden, D.J.; Casettari, L.; Illum, L. Application of permeation enhancers in oral delivery of macromolecules: An update. Pharmaceutics 2019, 11, 41. [Google Scholar] [CrossRef]
- Fein, K.C.; Gleeson, J.P.; Newby, A.N.; Whitehead, K.A. Intestinal permeation enhancers enable oral delivery of macromolecules up to 70 kDa in size. Eur. J. Pharm. Biopharm. 2022, 170, 70–76. [Google Scholar] [CrossRef]
- Twarog, C.; Fattah, S.; Heade, J.; Maher, S.; Fattal, E.; Brayden, D.J. Intestinal permeation enhancers for oral delivery of macromolecules: A comparison between salcaprozate sodium (SNAC) and sodium caprate (C10). Pharmaceutics 2019, 11, 78. [Google Scholar] [CrossRef]
- Gaudin, T.; Lu, H.; Fayet, G.; Berthauld-Drelich, A.; Rotureau, P.; Pourceau, G.; Wadouachi, A.; Van Hecke, E.; Nesterenko, A.; Pezron, I. Impact of the chemical structure on amphiphilic properties of sugar-based surfactants: A literature overview. Adv. Colloid Interface Sci. 2019, 270, 87–100. [Google Scholar] [CrossRef]
- De, S.; Malik, S.; Ghosh, A.; Saha, R.; Saha, B. A review on natural surfactants. RSC Adv. 2015, 5, 65757–65767. [Google Scholar] [CrossRef]
- Mnif, I.; Ellouz-Chaambouni, S.; Ghribi, D. Glycolipid biosurfactants, main classes, functional properties and related potential applications in environmental biotechnology. J. Polym. Environ. 2018, 26, 2192–2206. [Google Scholar] [CrossRef]
- Lu, B.; Vayssade, M.; Miao, Y.; Chagnault, V.; Grand, E.; Wadouachi, A.; Postel, D.; Drelich, D.; Egles, C.; Pezron, I. Physico-chemical properties and cytotoxic effects of sugar-based surfactants: Impact of structural variations. Colloids Surf. B 2016, 145, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Staroń, J.; Dąbrowski, J.M.; Cichoń, E.; Guzik, M. Lactose esters: Synthesis and biotechnological applications. Crit. Rev. Biotechnol. 2018, 38, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Verboni, M.; Lucarini, S.; Duranti, A. 6′-O-Lactose esters surfactants as an innovative opportunity in the pharmaceutical field: From synthetic methods to biological applications. Pharmaceuticals 2021, 14, 1306. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, S.; Fagioli, L.; Campana, R.; Cole, H.; Duranti, A.; Baffone, W.; Vllasaliu, D.; Casettari, L. Unsaturated fatty acids lactose esters: Cytotoxicity, permeability enhancement and antimicrobial activity. Eur. J. Pharm. Biopharm. 2016, 107, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Perinelli, D.R.; Lucarini, S.; Fagioli, L.; Campana, R.; Vllasaliu, D.; Duranti, A.; Casettari, L. Lactose oleate as new biocompatible surfactant for pharmaceutical applications. Eur. J. Pharm. Biopharm. 2018, 124, 55–62. [Google Scholar] [CrossRef]
- McCartney, F.; Perinelli, D.R.; Tiboni, M.; Cavanagh, R.; Lucarini, S.; Palmieri, G.P.; Casettari, L.; Brayden, D.J. Permeability-enhancing effects of three laurate-disaccharide monoesters across isolated rat intestinal mucosae. Int. J. Pharm. 2021, 601, 120593. [Google Scholar] [CrossRef]
- Tiboni, M.; Elmowafy, E.; El-Derany, M.O.; Benedetti, S.; Campana, R.; Verboni, M.; Potenza, L.; Palma, F.; Citterio, B.; Sisti, M.; et al. A combination of sugar esters and chitosan to promote in vivo wound care. Int. J. Pharm. 2022, 616, 121508. [Google Scholar] [CrossRef]
- Verboni, M.; Benedetti, S.; Campana, R.; Palma, F.; Potenza, L.; Sisti, M.; Duranti, A.; Lucarini, S. synthesis and biological characterization of the new glycolipid lactose undecylenate (URB1418). Pharmaceuticals 2022, 15, 456. [Google Scholar] [CrossRef]
- Lucarini, S.; Fagioli, L.; Cavanagh, R.; Liang, W.; Perinelli, D.; Campana, M.; Stolnik, S.; Lam, J.; Casettari, L.; Duranti, A. Synthesis, structure–activity relationships and in vitro toxicity profile of lactose-based fatty acid monoesters as possible drug permeability enhancers. Pharmaceutics 2018, 10, 81. [Google Scholar] [CrossRef]
- Campana, R.; Merli, A.; Verboni, M.; Biondo, F.; Favi, G.; Duranti, A.; Lucarini, S. Synthesis and evaluation of saccharide-based aliphatic and aromatic esters as antimicrobial and antibiofilm agents. Pharmaceuticals 2019, 12, 186. [Google Scholar] [CrossRef]
- Molinier, V.; Fitremann, J.; Bouchu, A.; Queneau, Y. Sucrose esterification under Mitsunobu conditions: Evidence for the formation of 6-O-acyl-3′,6′-anhydrosucrose besides mono and diesters of fatty acids. Tetrahedron Asymmetry 2004, 15, 1753–1762. [Google Scholar] [CrossRef]
- Verboni, M.; Sisti, M.; Campana, R.; Benedetti, S.; Palma, F.; Potenza, L.; Lucarini, S.; Duranti, A. Synthesis and biological evaluation of 6-O-sucrose monoester glycolipids as possible new antifungal agents. Pharmaceuticals 2023, 16, 136. [Google Scholar] [CrossRef]
- Griffin, W.C. Calculation of HLB values of non-ionic surfactants. J. Soc. Cosmet. Chem. 1954, 5, 249–256. [Google Scholar]
- Organic Chemistry Portal. Available online: https://www.organic-chemistry.org/prog/ (accessed on 19 December 2022).
- Piñeiro, L.; Novo, M.; Al-Soufi, W. Fluorescence emission of pyrene in surfactant solutions. Adv. Colloid Interface Sci. 2015, 215, 1–12. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Cespi, M.; Lorusso, N.; Palmieri, G.F.; Bonacucina, G.; Blasi, P. Surfactant self-assembling and critical micelle concentration: One approach fits all? Langmuir 2020, 36, 5745–5753. [Google Scholar] [CrossRef] [PubMed]
- Tadros, T.F. Applied Surfactants: Principles and Applications; Wiley: Weinheim, Germany, 2005. [Google Scholar]
- Malvern Instruments Ltd. Surfactant Micelle Characterization Using Dynamic Light Scattering; Zetasizer Nano Application Note, MRK809-01; Malvern Instruments Ltd.: Malvern, UK, 2006. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Decker, T.; Lohmann-Matthes, M.L. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J. Immunol. Methods 1988, 115, 61–69. [Google Scholar] [CrossRef]
- Sarmento, B.; Andrade, F.; Da Silva, S.B.; Rodrigues, F.; Das Neves, J.; Ferreira, D. Cell-based in vitro models for predicting drug permeability. Expert Opin. Drug Metab. Toxicol. 2012, 8, 607–621. [Google Scholar] [CrossRef]
- Deli, M.A. Potential use of tight junction modulators to reversibly open membranous barriers and improve drug delivery. Biochim. Biophys. Acta 2009, 1788, 892–910. [Google Scholar] [CrossRef]
- Chen, S.; Einspanier, R.; Schoen, J. Transepithelial electrical resistance (TEER): A functional parameter to monitor the quality of oviduct epithelial cells cultured on filter supports. Histochem. Cell Biol. 2015, 144, 509. [Google Scholar] [CrossRef]
- Shubber, S.; Vllasaliu, D.; Rauch, C.; Jordan, F.; Illum, L.; Stolnik, S. Mechanism of mucosal permeability enhancement of CriticalSorb® (Solutol® HS15) investigated in vitro in cell cultures. Pharm. Res. 2015, 32, 516–527. [Google Scholar] [CrossRef] [PubMed]
- Sibinovska, N.; Žakelj, S.; Roškar, R.; Kristan, K. Suitability and functional characterization of two Calu-3 cell models for prediction of drug permeability across the airway epithelial barrier. Int. J. Pharm. 2020, 585, 119484. [Google Scholar] [CrossRef] [PubMed]
- Kreft, M.E.; Jerman, U.D.; Lasič, E.; Hevir-Kene, N.; Rižner, T.L.; Peternel, L.; Kristan, K. The characterization of the human cell line Calu-3 under different culture conditions and its use as an optimized in vitro model to investigate bronchial epithelial function. Eur. J. Pharm. Sci. 2015, 69, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Furuya, T.; Ushiyama, M.; Asada, Y.; Yoshikawa, T.; Orihara, Y. Biotransformation of phenylacetic acid and 2-phenyl-propionic acid in suspension culture of Coffea arabica. Phytochemistry 1988, 27, 803–807. [Google Scholar] [CrossRef]
- Sousa, F.; Castro, P. Cell-based in vitro models for nasal permeability studies. In Concepts and Models for Drug Permeability Studies; Cell and Tissue Based In Vitro Culture Models; Woodhead Publishing: Sawston, UK, 2016; pp. 83–100. [Google Scholar] [CrossRef]


| Sugar Ester | MW | HLB a | clogP b | ||
|---|---|---|---|---|---|
| 3a | URB1480 | Sucrose phenyl acetate | 460.4 | 12.9 | −2.05 |
| 3b | URB1481 | Sucrose p-phenyl benzoate | 522.5 | 11.4 | −0.32 |
| 3c | URB1482 | Sucrose p-biphenyl acetate | 536.5 | 11.1 | −0.37 |
| 7a | URB1419 | Lactose phenyl acetate | 460.4 | 12.9 | −2.05 |
| 7b | URB1420 | Lactose p-phenyl benzoate | 522.5 | 11.4 | −0.32 |
| 7c | URB1421 | Lactose p-biphenyl acetate | 536.5 | 11.1 | −0.37 |
| Sugar Ester | Fluorescence Spectroscopy CMC (mM) | Dynamic Light Scattering CMC (mM) | |
|---|---|---|---|
| 3a | URB1480 | 0.861 ± 0.076 | 0.791 ± 0.045 |
| 3b | URB1481 | 0.305 ± 0.091 | 0.329 ± 0.023 |
| 3c | URB1482 | 0.095 ± 0.015 | 0.181 ± 0.026 |
| 7a | URB1419 | 0.753 ± 0.084 | 0.729 ± 0.075 |
| 7b | URB1420 | 0.270 ± 0.015 | 0.344 ± 0.034 |
| 7c | URB1421 | 0.110 ± 0.017 | 0.259 ± 0.063 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verboni, M.; Perinelli, D.R.; Qiu, C.Y.; Tiboni, M.; Aluigi, A.; Lucarini, S.; Lam, J.K.W.; Duranti, A. Synthesis and Properties of Sucrose- and Lactose-Based Aromatic Ester Surfactants as Potential Drugs Permeability Enhancers. Pharmaceuticals 2023, 16, 223. https://doi.org/10.3390/ph16020223
Verboni M, Perinelli DR, Qiu CY, Tiboni M, Aluigi A, Lucarini S, Lam JKW, Duranti A. Synthesis and Properties of Sucrose- and Lactose-Based Aromatic Ester Surfactants as Potential Drugs Permeability Enhancers. Pharmaceuticals. 2023; 16(2):223. https://doi.org/10.3390/ph16020223
Chicago/Turabian StyleVerboni, Michele, Diego Romano Perinelli, Carol Yingshan Qiu, Mattia Tiboni, Annalisa Aluigi, Simone Lucarini, Jenny K. W. Lam, and Andrea Duranti. 2023. "Synthesis and Properties of Sucrose- and Lactose-Based Aromatic Ester Surfactants as Potential Drugs Permeability Enhancers" Pharmaceuticals 16, no. 2: 223. https://doi.org/10.3390/ph16020223
APA StyleVerboni, M., Perinelli, D. R., Qiu, C. Y., Tiboni, M., Aluigi, A., Lucarini, S., Lam, J. K. W., & Duranti, A. (2023). Synthesis and Properties of Sucrose- and Lactose-Based Aromatic Ester Surfactants as Potential Drugs Permeability Enhancers. Pharmaceuticals, 16(2), 223. https://doi.org/10.3390/ph16020223