High-Shear Granulation of Hygroscopic Probiotic-Encapsulated Skim Milk Powder: Effects of Moisture-Activation and Resistant Maltodextrin
Abstract
1. Introduction
2. Results and Discussion
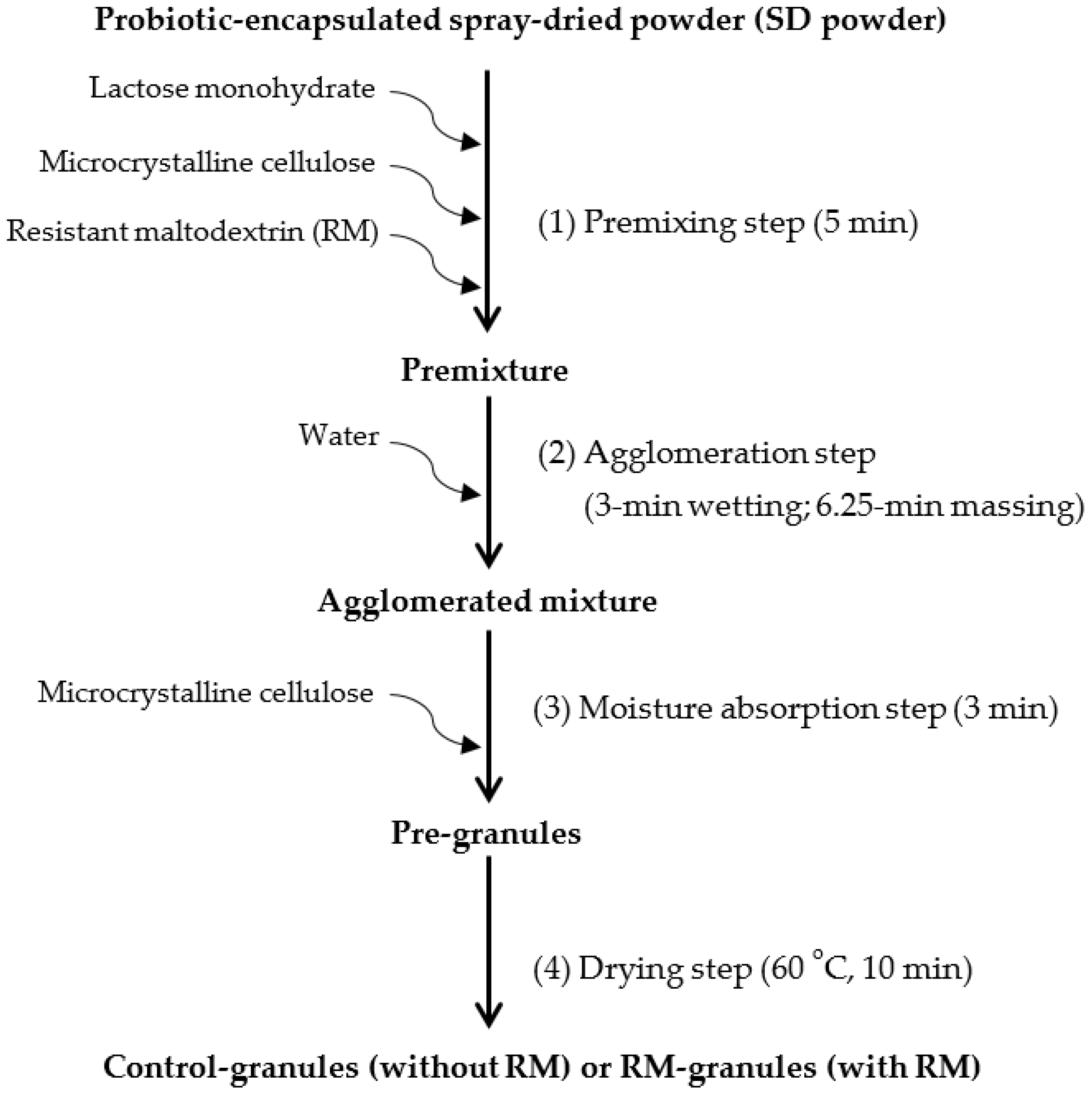
2.1. High-Shear Granulation of SD Powder
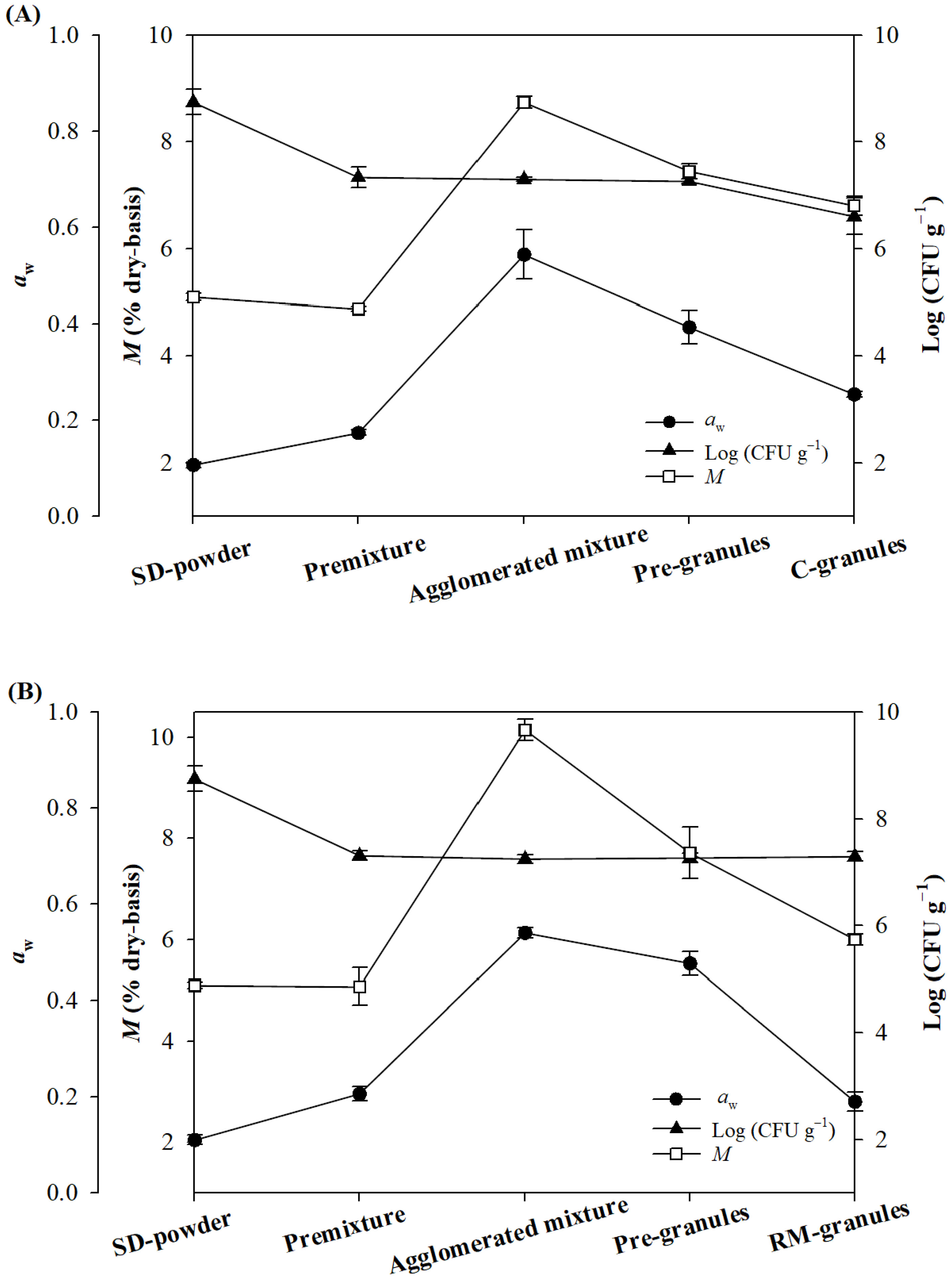
2.2. Moisture Content, Water Activity, and Viable Cells during High-Shear Granulation
2.3. Morphology, Crystallinity, and Particle Size
2.4. Density, Porosity, and Flowability
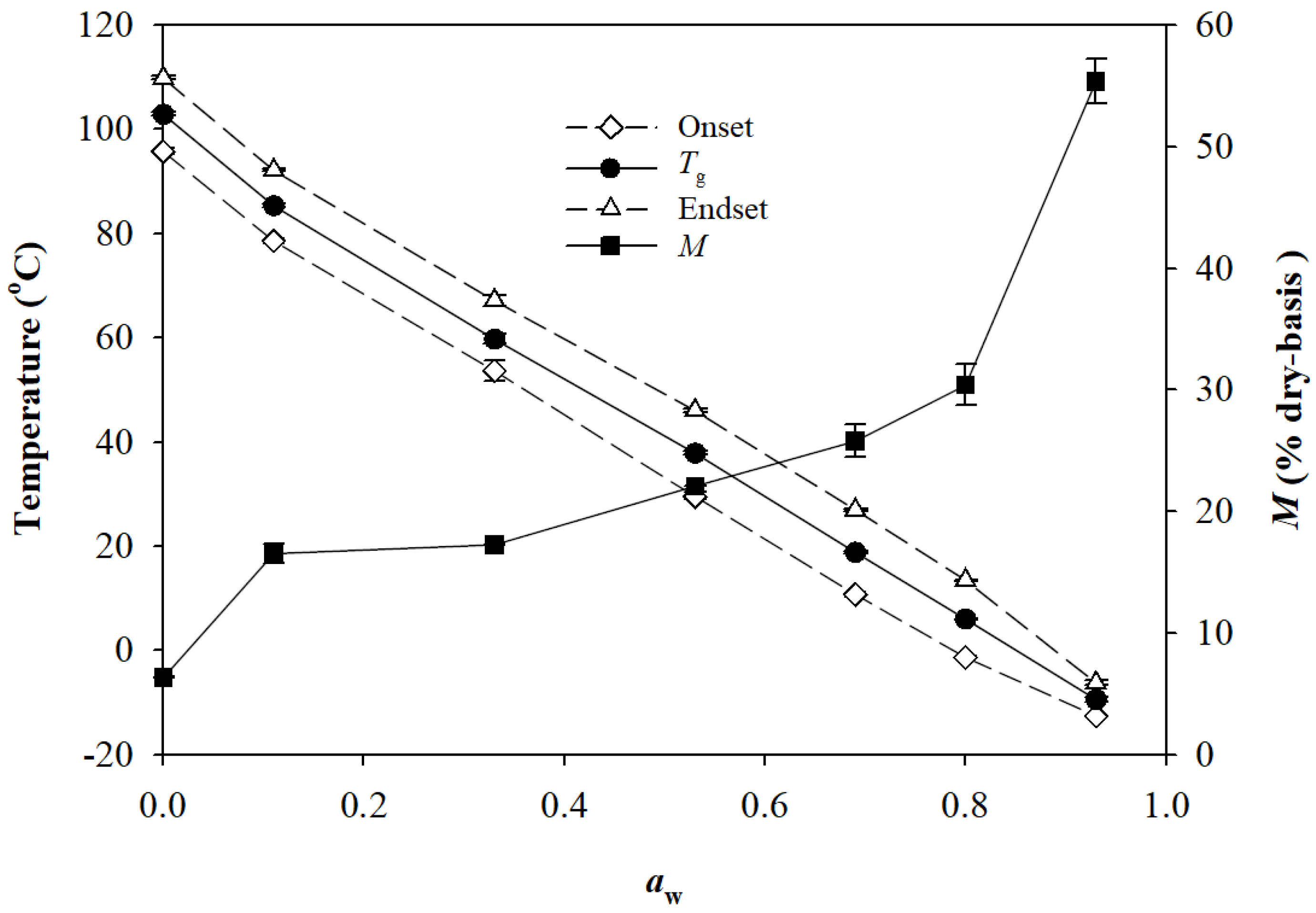
2.5. Role of RM in High-Shear Granulation
3. Materials and Methods
3.1. Materials
3.2. Preparation of LGG-Containing Spray-Dried Powder
3.3. High-Shear Granulation with Moisture-Activation
3.4. Microstructure
3.5. X-ray Diffraction Analysis
3.6. Particle Size Analysis
3.7. Determination of Density, Porosity, and Flowability
3.8. Moisture Content and Water Activity Measurements
3.9. Determination of Viable Cells
3.10. Determination of Moisture Sorption Isotherms of Lactose Monohydrate and RM
3.11. Determination of the Glass Transition of RM
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, D.-H.; Letona, A.; Lee, M.; Lim, D.; Han, N.-S.; Chung, D. Fluidized-bed granulation of probiotics-encapsulated spray-dried skim milk powder: Effects of a fluidizing aid, moisture-activation and dehydration. Foods 2021, 10, 1600. [Google Scholar] [CrossRef] [PubMed]
- Fournaise, T.; Burgain, J.; Perroud, C.; Scher, J.; Gaiani, C.; Petit, J. Impact of formulation on reconstitution and flowability of spray-dried milk powders. Powder Technol. 2020, 372, 107–116. [Google Scholar] [CrossRef]
- Fournaise, T.; Burgain, J.; Perroud-Thomassin, C.; Petit, J. Impact of the whey protein/casein ratio on the reconstitution and flow properties of spray-dried dairy protein powders. Powder Technol. 2021, 391, 275–281. [Google Scholar] [CrossRef]
- Barbosa-Cánovas, G.V.; Ortega-Rivas, E.; Juliano, P.; Yan, H. Food Powders: Physical Properties, Processing, and Functionality; Kluwer/Plenum: New York, NY, USA, 2006; pp. 271–281. [Google Scholar]
- Parikh, D.M. Handbook of Pharmaceutical Granulation Technology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 129–158. [Google Scholar]
- Shanmugam, S. Granulation techniques and technologies: Recent progresses. BioImpacts 2015, 5, 55. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, W. An interdisciplinary approach to size enlargement by agglomeration. Powder Technol. 2003, 130, 8–13. [Google Scholar] [CrossRef]
- Pietsch, W.B. Agglomeration Processes: Phenomena, Technologies, Equipment; John Wiley & Sons: Weinheim, Germany, 2008; pp. 29–132. [Google Scholar]
- Morin, G.; Briens, L. A comparison of granules produced by high-shear and fluidized-bed granulation methods. Aaps. Pharmscitech. 2014, 15, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Badawy, S.I.; Narang, A.S.; LaMarche, K.; Subramanian, G.; Varia, S.A. Mechanistic basis for the effects of process parameters on quality attributes in high shear wet granulation. Int. J. Pharm. 2012, 439, 324–333. [Google Scholar] [CrossRef]
- Bansal, A.K.; Balwani, G.; Sheokand, S. Critical material attributes in wet granulation. In Handbook of Pharmaceutical Wet Granulation; Academic Press: London, UK, 2019; pp. 421–453. [Google Scholar] [CrossRef]
- Cuq, B.; Mandato, S.; Jeantet, R.; Saleh, K.; Ruiz, T. Agglomeration/granulation in food powder production. In Handbook of Food Powders; Woodhead Publishing Limited: Philadelphia, PA, USA, 2013; pp. 150–177. [Google Scholar] [CrossRef]
- Ullah, I.; Corrao, R.; Wiley, G.; Lipper, R. Moisture activated dry granulation: A general process. Pharm. Technol. 1987, 11, 48–54. [Google Scholar]
- Ullah, I.; Wang, J.; Chang, S.-Y.; Wiley, G.J.; Jain, N.B.; Kiang, S. Moisture-activated dry granulation—Part I: A guide to excipient and equipment selection and formulation development. Pharm. Technol. 2009, 33, 62–70. [Google Scholar]
- Ullah, I.; Wang, J.; Chang, S.-Y.; Guo, H.; Kiang, S.; Jain, N.B. Moisture-activated dry granulation part II: The effects of formulation ingredients and manufacturing-process variables on granulation quality attributes. Pharm. Technol. 2009, 33, 42–51. [Google Scholar]
- Christensen, L.; Johansen, H.; Schaefer, T. Moisture-activated dry granulation in a high shear mixer. Drug Dev. Ind. Pharm. 1994, 20, 2195–2213. [Google Scholar] [CrossRef]
- Moravkar, K.K.; Ali, T.M.; Pawar, J.N.; Amin, P.D. Application of moisture activated dry granulation (MADG) process to develop high dose immediate release (IR) formulations. Adv. Powder Technol. 2017, 28, 1270–1280. [Google Scholar] [CrossRef]
- Chen, C.-M.; Alli, D.; Igga, M.R.; Czeisler, J.L. Comparison of moisture-activated dry granulation process with conventional granulation methods for sematilide hydrochloride tablets. Drug Dev. Ind. Pharm. 1990, 16, 379–394. [Google Scholar] [CrossRef]
- Takasaki, H.; Yonemochi, E.; Messerschmid, R.; Ito, M.; Wada, K.; Terada, K. Importance of excipient wettability on tablet characteristics prepared by moisture activated dry granulation (MADG). Int. J. Pharm. 2013, 456, 58–64. [Google Scholar] [CrossRef]
- Becker, D.; Rigassi, T.; Bauer-Brandl, A. Effectiveness of binders in wet granulation: A comparison using model formulations of different tabletability. Drug Dev. Ind. Pharm. 1997, 23, 791–808. [Google Scholar] [CrossRef]
- Takasaki, H.; Yonemochi, E.; Ito, M.; Wada, K.; Terada, K. The importance of binder moisture content in Metformin HCL high-dose formulations prepared by moist aqueous granulation (MAG). Results Pharma Sci. 2015, 5, 1–7. [Google Scholar] [CrossRef]
- Astina, J.; Sapwarobol, S. Resistant maltodextrin and metabolic syndrome: A review. J. Am. Coll. Nutr. 2019, 38, 380–385. [Google Scholar] [CrossRef]
- Stępień, A.; Witczak, M.; Witczak, T. Sorption properties, glass transition and state diagrams for pumpkin powders containing maltodextrins. LWT-Food Sci. Technol. 2020, 134, 110192. [Google Scholar] [CrossRef]
- Pai, D.A.; Vangala, V.R.; Ng, J.W.; Ng, W.K.; Tan, R.B.H. Resistant maltodextrin as a shell material for encapsulation of naringin: Production and physicochemical characterization. J. Food Eng. 2015, 161, 68–74. [Google Scholar] [CrossRef]
- Bronlund, J.; Paterson, T. Moisture sorption isotherms for crystalline, amorphous and predominantly crystalline lactose powders. Int. Dairy J. 2004, 14, 247–254. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in food systems: Significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef] [PubMed]
- Vesterlund, S.; Salminen, K.; Salminen, S. Water activity in dry foods containing live probiotic bacteria should be carefully considered: A case study with Lactobacillus rhamnosus GG in flaxseed. Int. J. Food Microbiol. 2012, 157, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Chávez, B.; Ledeboer, A. Drying of probiotics: Optimization of formulation and process to enhance storage survival. Drying Technol. 2007, 25, 1193–1201. [Google Scholar] [CrossRef]
- Neffe-Skocińska, K.; Rzepkowska, A.; Szydłowska, A.; Kołożyn-Krajewska, D. Trends and possibilities of the use of probiotics in food production. In Alternative and Replacement Foods; Academic Press: London, UK, 2018; pp. 65–94. [Google Scholar] [CrossRef]
- Bates, S.; Zografi, G.; Engers, D.; Morris, K.; Crowley, K.; Newman, A. Analysis of amorphous and nanocrystalline solids from their X-ray diffraction patterns. Pharm. Res. 2006, 23, 2333–2349. [Google Scholar] [CrossRef]
- Atalar, I.; Yazici, F. Effect of different binders on reconstitution behaviors and physical, structural, and morphological properties of fluidized bed agglomerated yoghurt powder. Drying Technol. 2019, 37, 1656–1664. [Google Scholar] [CrossRef]
- Jinapong, N.; Suphantharika, M.; Jamnong, P. Production of instant soymilk powders by ultrafiltration, spray drying and fluidized bed agglomeration. J. Food Eng. 2008, 84, 194–205. [Google Scholar] [CrossRef]
- USP. U.P. 35–NF 30. In Proceedings of the United States Pharmacopeial Convention; United States Pharmacopeial Convention: Rockville, MD, USA, 2012. [Google Scholar]
- Sperling, L.H. Introduction to Physical Polymer Science, 4th ed.; John Wiley & Sons: New York, NY, USA, 2006; pp. 355–361. [Google Scholar]
- Li, J.; Tao, L.; Dali, M.; Buckley, D.; Gao, J.; Hubert, M. The effect of the physical states of binders on high-shear wet granulation and granule properties: A mechanistic approach toward understanding high-shear wet granulation process. Part I. Physical characterization of binders. J. Pharm. Sci. 2011, 100, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tao, L.; Dali, M.; Buckley, D.; Gao, J.; Hubert, M. The effect of the physical states of binders on high-shear wet granulation and granule properties: A mechanistic approach toward understanding high-shear wet granulation process. Part II. Granulation and granule properties. J. Pharm. Sci. 2011, 100, 294–310. [Google Scholar] [CrossRef]
- AOAC. Method 930.15. In Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Rockville, MD, USA, 2000. [Google Scholar]
- Yu, D.; Kwon, G.; An, J.; Kim, H.J.; Chung, D. Glass transition and stickiness characteristics of sea tangle powder fermented with Lactobacillus brevis. J. Food Process Preserv. 2021, 45, e15574. [Google Scholar] [CrossRef]


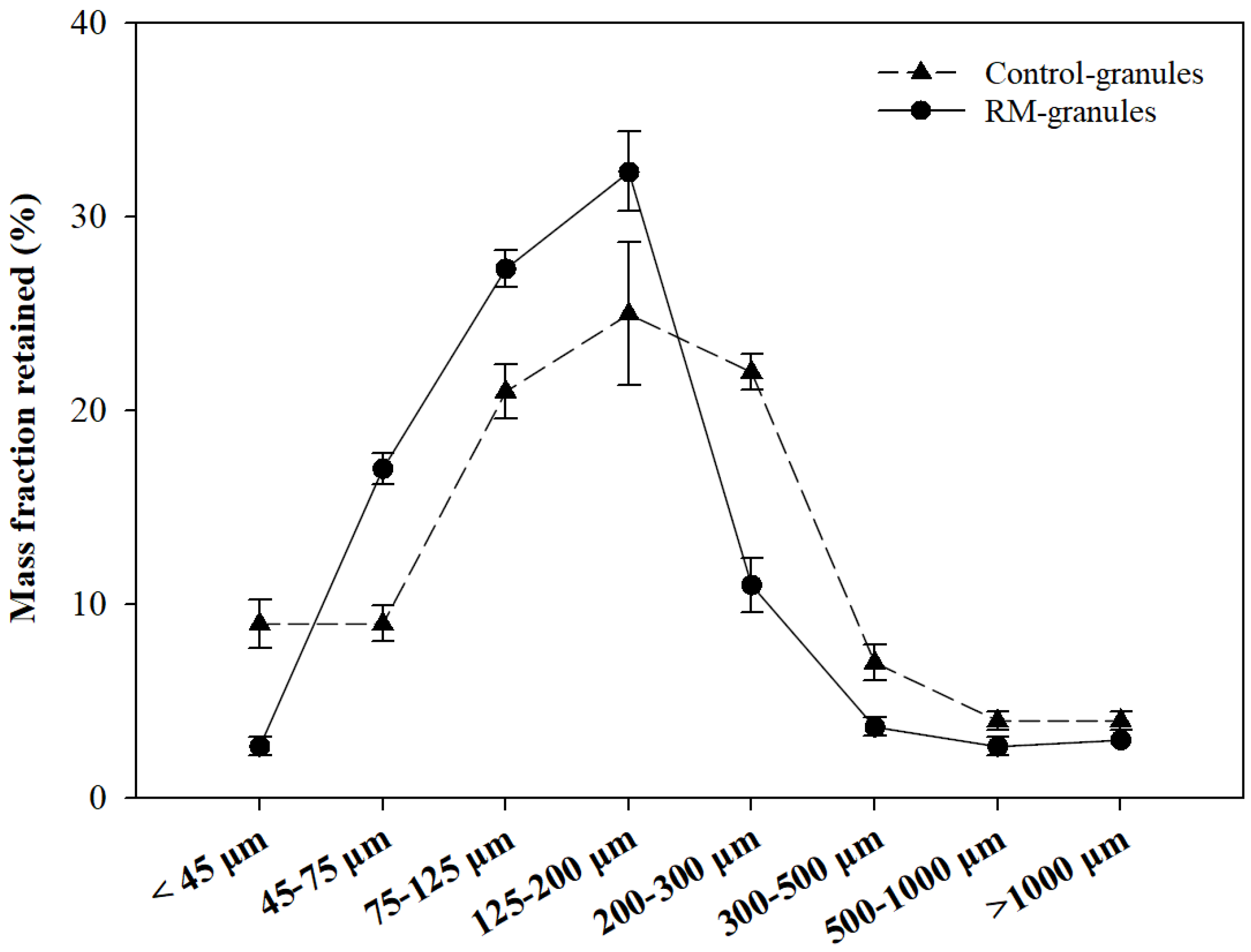
| Size Parameter | Control-Granules | RM-Granules |
|---|---|---|
| dm (µm) | 204.20 ± 7.65 | 161.98 ± 3.15 |
| d10 (µm) | 34.33 ± 2.05 | 57.67 ± 0.47 |
| d50 (µm) | 162.00 ± 3.56 | 131.33 ± 2.05 |
| d90 (µm) | 436.67 ± 15.33 | 294.67 ± 4.64 |
| Span | 2.48 ± 0.10 | 1.81 ± 0.05 |
| ρtrue (g cm−3) | ρlb (g cm−3) | ρtb (g cm−3) | ε (%) | CI (%) | HR | |
|---|---|---|---|---|---|---|
| SD powder | 1.46 ± 0.00 c | 0.54 ± 0.00 a | 0.73 ± 0.00 a | 50.1 ± 0.2 c | 26.02 ± 0.52 a | 1.35 ± 0.01 a |
| Control-granules | 1.52 ± 0.01 a | 0.48 ± 0.00 c | 0.57 ± 0.01 b | 62.7 ± 0.8 a | 14.40 ± 1.16 b | 1.17 ± 0.02 b |
| RM-granules | 1.50 ± 0.00 b | 0.52 ± 0.00 b | 0.58 ± 0.00 b | 61.2 ± 0.2 b | 10.79 ± 0.56 c | 1.12 ± 0.01 c |
| Steps | Ingredients Added | Composition (%, w/w) | |
|---|---|---|---|
| Control-Granules | RM-Granules | ||
| Premixing | SD powder | 25.0 | 25.0 |
| Lactose monohydrate | 46.0 | 36.0 | |
| Microcrystalline cellulose | 17.5 | 17.5 | |
| Resistant maltodextrin (RM) | - | 10.0 | |
| Agglomeration | Water | 4.0 | 4.0 |
| Moisture absorption | Microcrystalline cellulose | 7.5 | 7.5 |
| Total | 100 | 100 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Letona, A.; Ahn, S.; An, S.; Yun, D.; Kim, Y.-R.; Muralles, M.; Chung, D. High-Shear Granulation of Hygroscopic Probiotic-Encapsulated Skim Milk Powder: Effects of Moisture-Activation and Resistant Maltodextrin. Pharmaceuticals 2023, 16, 217. https://doi.org/10.3390/ph16020217
Letona A, Ahn S, An S, Yun D, Kim Y-R, Muralles M, Chung D. High-Shear Granulation of Hygroscopic Probiotic-Encapsulated Skim Milk Powder: Effects of Moisture-Activation and Resistant Maltodextrin. Pharmaceuticals. 2023; 16(2):217. https://doi.org/10.3390/ph16020217
Chicago/Turabian StyleLetona, Andres, Sungahm Ahn, Suyeon An, Daebeom Yun, Young-Rok Kim, Mario Muralles, and Donghwa Chung. 2023. "High-Shear Granulation of Hygroscopic Probiotic-Encapsulated Skim Milk Powder: Effects of Moisture-Activation and Resistant Maltodextrin" Pharmaceuticals 16, no. 2: 217. https://doi.org/10.3390/ph16020217
APA StyleLetona, A., Ahn, S., An, S., Yun, D., Kim, Y.-R., Muralles, M., & Chung, D. (2023). High-Shear Granulation of Hygroscopic Probiotic-Encapsulated Skim Milk Powder: Effects of Moisture-Activation and Resistant Maltodextrin. Pharmaceuticals, 16(2), 217. https://doi.org/10.3390/ph16020217






