The Use of Triphenyl Phosphonium Cation Enhances the Mitochondrial Antiplatelet Effect of the Compound Magnolol
Abstract
1. Introduction
2. Results
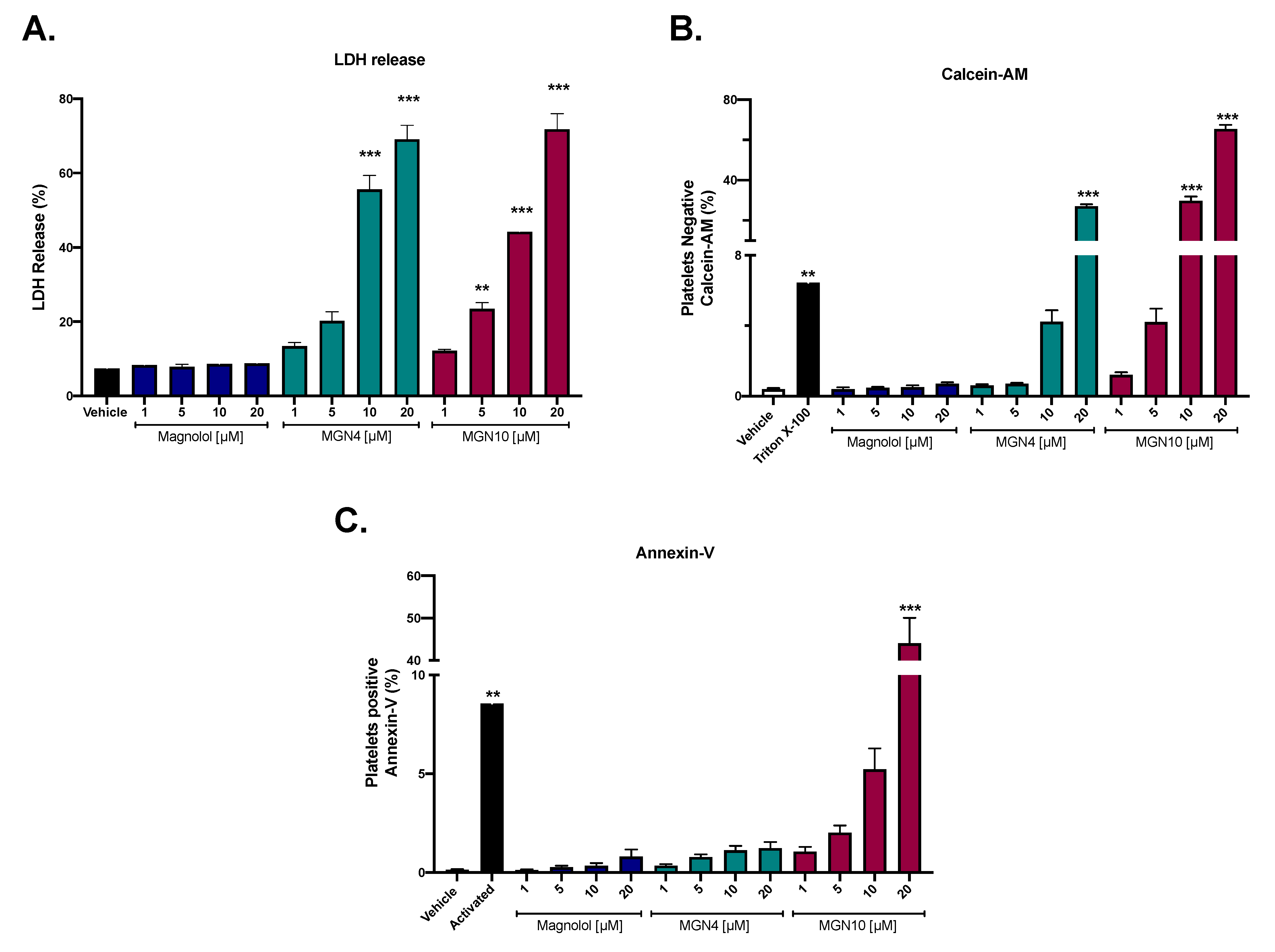
2.1. Cytotoxic Effect of Compounds
2.2. Platelet Aggregation Results
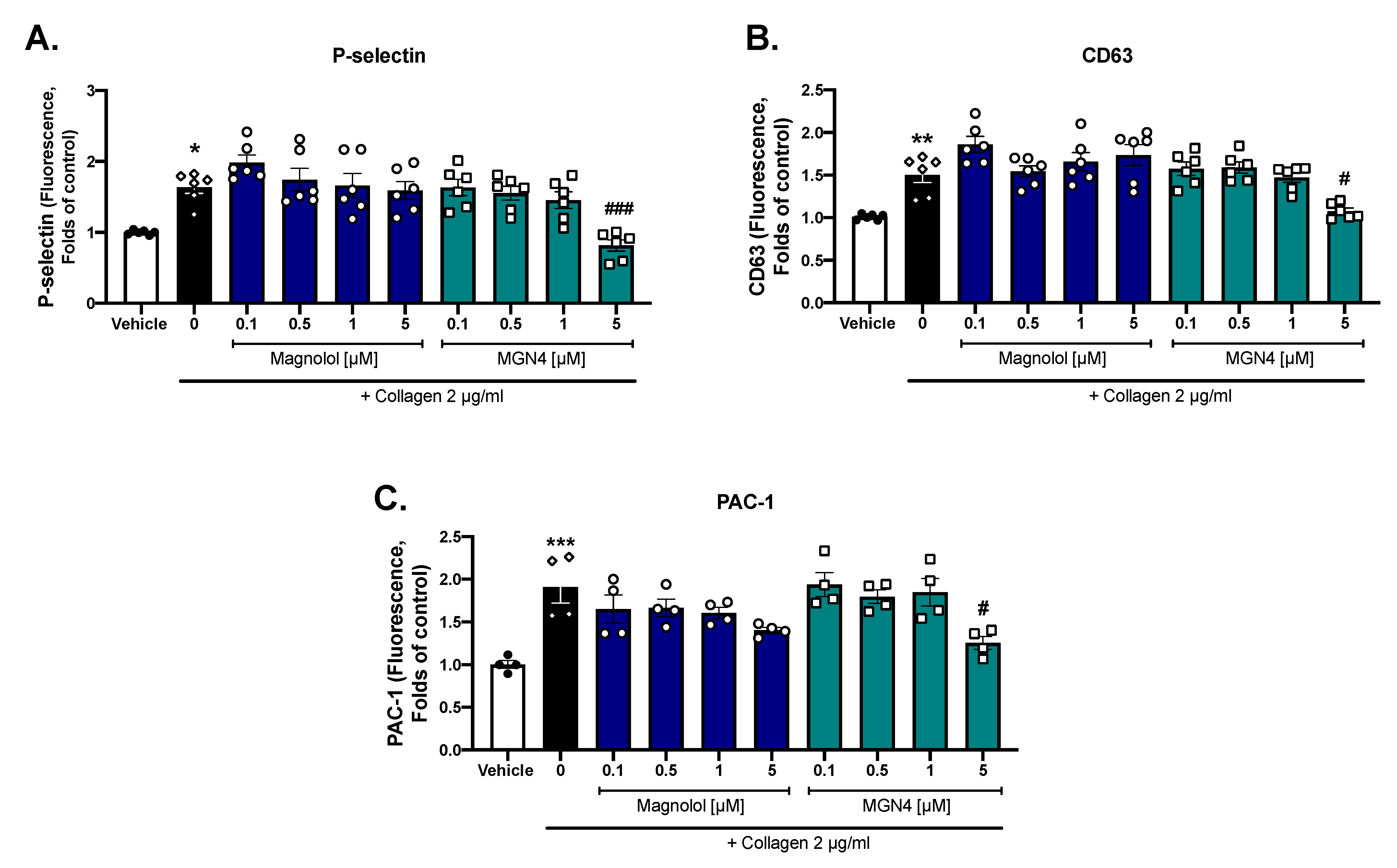
2.3. Platelet Activation Markers
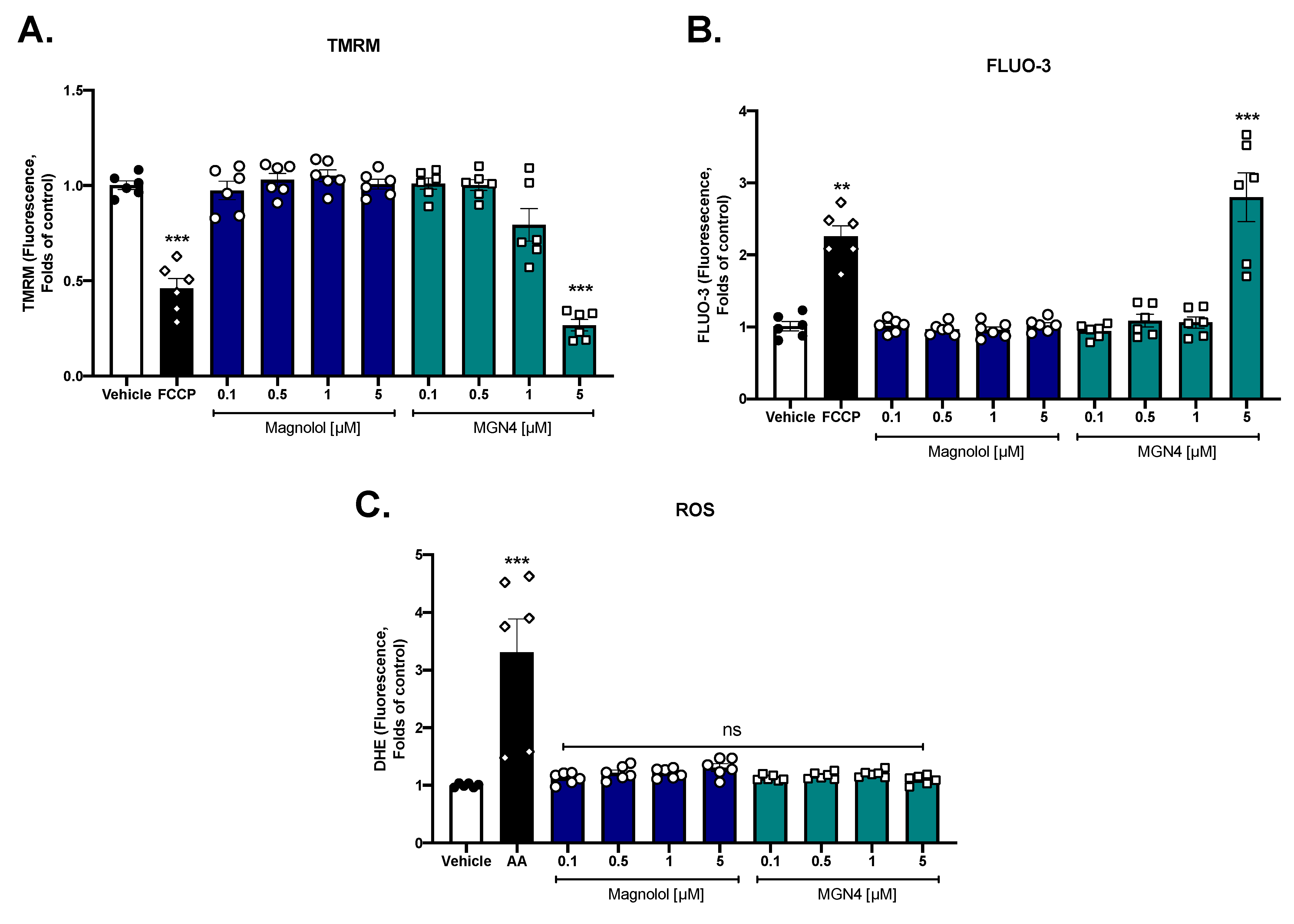
2.4. Effect on Mitochondrial Function
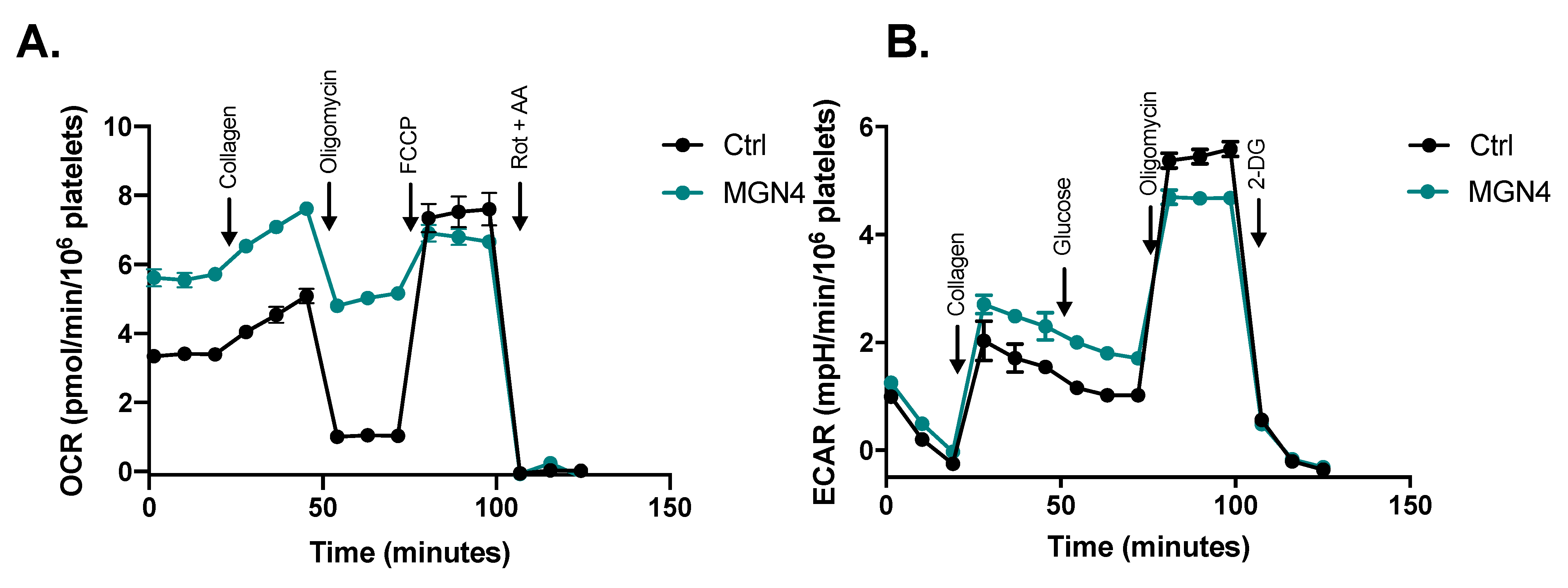
2.5. MGN4 Affects Mitochondrial Function in Platelets
3. Discussion
4. Materials and Methods
4.1. Chemical Structure of Compounds
4.2. Purification of Washed Human Platelets
4.3. Cytotoxic Activity by LDH Release
4.4. Cell Viability by Calcein-AM
4.5. Apoptosis Activity (Externalization of Phosphatidylserine)
4.6. Platelet Aggregation
4.7. Platelet Activation Markers
4.8. Mitochondrial Membrane Potential (∆Ψm)
4.9. Intraplatelet ROS Levels
4.10. Intraplatelet Calcium Levels
4.11. Oxygen Consumption Rate and Extracellular Acidification Rate Assays
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Badimon, L.; Chagas, P.; Chiva-Blanch, G. Diet and Cardiovascular Disease: Effects of Foods and Nutrients in Classical and Emerging Cardiovascular Risk Factors. Curr. Med. Chem. 2019, 26, 3639–3651. [Google Scholar] [CrossRef] [PubMed]
- Coll, P.P.; Roche, V.; Olsen, J.S.; Voit, J.H.; Bowen, E.; Kumar, M. The Prevention of Cardiovascular Disease in Older Adults. J. Am. Geriatr. Soc. 2020, 68, 1098–1106. [Google Scholar] [CrossRef]
- Tran, D.T.; Lekhak, N.; Gutierrez, K.; Moonie, S. Risk factors associated with cardiovascular disease among adult Nevadans. PLoS ONE 2021, 16, e0247105. [Google Scholar] [CrossRef] [PubMed]
- van der Meijden, P.E.J.; Heemskerk, J.W.M. Platelet biology and functions: New concepts and clinical perspectives. Nat. Rev. Cardiol. 2019, 16, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Josefsson, E.C.; Vainchenker, W.; James, C. Regulation of Platelet Production and Life Span: Role of Bcl-xL and Potential Implications for Human Platelet Diseases. Int. J. Mol. Sci. 2020, 21, 7591. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, K.; Bhattacharyya, M. Overview of platelet physiology: Its hemostatic and nonhemostatic role in disease pathogenesis. Sci. World J. 2014, 2014, 781857. [Google Scholar] [CrossRef]
- Periayah, M.H.; Halim, A.S.; Mat Saad, A.Z. Mechanism Action of Platelets and Crucial Blood Coagulation Pathways in Hemostasis. Int. J. Hematol. -Oncol. Stem Cell Res. 2017, 11, 319–327. [Google Scholar]
- Lebas, H.; Yahiaoui, K.; Martos, R.; Boulaftali, Y. Platelets Are at the Nexus of Vascular Diseases. Front. Cardiovasc. Med. 2019, 6. [Google Scholar] [CrossRef]
- Zharikov, S.; Shiva, S. Platelet mitochondrial function: From regulation of thrombosis to biomarker of disease. Biochem. Soc. Trans. 2013, 41, 118–123. [Google Scholar] [CrossRef]
- Melchinger, H.; Jain, K.; Tyagi, T.; Hwa, J. Role of Platelet Mitochondria: Life in a Nucleus-Free Zone. Front. Cardiovasc. Med. 2019, 6, 153. [Google Scholar] [CrossRef]
- Bratic, A.; Larsson, N.G. The role of mitochondria in aging. J. Clin. Investig. 2013, 123, 951–957. [Google Scholar] [CrossRef]
- Kramer, P.A.; Ravi, S.; Chacko, B.; Johnson, M.S.; Darley-Usmar, V.M. A review of the mitochondrial and glycolytic metabolism in human platelets and leukocytes: Implications for their use as bioenergetic biomarkers. Redox Biol. 2014, 2, 206–210. [Google Scholar] [CrossRef]
- Aibibula, M.; Naseem, K.M.; Sturmey, R.G. Glucose metabolism and metabolic flexibility in blood platelets. J. Thromb. Haemost. 2018, 16, 2300–2314. [Google Scholar] [CrossRef] [PubMed]
- Doery, J.C.G.; Hirsh, J.; Cooper, I. Energy Metabolism in Human Platelets: Interrelationship Between Glycolysis and Oxidative Metabolism. Blood 1970, 36, 159–168. [Google Scholar] [CrossRef]
- Kholmukhamedov, A.; Jobe, S. Platelet respiration. Blood Adv. 2019, 3, 599–602. [Google Scholar] [CrossRef]
- Smith, R.A.; Hartley, R.C.; Murphy, M.P. Mitochondria-targeted small molecule therapeutics and probes. Antioxid. Redox Signal. 2011, 15, 3021–3038. [Google Scholar] [CrossRef]
- Weinberg, F.; Hamanaka, R.; Wheaton, W.W.; Weinberg, S.; Joseph, J.; Lopez, M.; Kalyanaraman, B.; Mutlu, G.M.; Budinger, G.R.S.; Chandel, N.S. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl. Acad. Sci. USA 2010, 107, 8788–8793. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zielonka, J.; Dranka, B.P.; McAllister, D.; Mackinnon, A.C., Jr.; Joseph, J.; Kalyanaraman, B. Mitochondria-targeted drugs synergize with 2-deoxyglucose to trigger breast cancer cell death. Cancer Res. 2012, 72, 2634–2644. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zielonka, J.; McAllister, D.M.; Mackinnon, A.C., Jr.; Joseph, J.; Dwinell, M.B.; Kalyanaraman, B. Mitochondria-targeted vitamin E analogs inhibit breast cancer cell energy metabolism and promote cell death. BMC Cancer 2013, 13, 285. [Google Scholar] [CrossRef]
- Zielonka, J.; Joseph, J.; Sikora, A.; Hardy, M.; Ouari, O.; Vasquez-Vivar, J.; Cheng, G.; Lopez, M.; Kalyanaraman, B. Mitochondria-Targeted Triphenylphosphonium-Based Compounds: Syntheses, Mechanisms of Action, and Therapeutic and Diagnostic Applications. Chem. Rev. 2017, 117, 10043–10120. [Google Scholar] [CrossRef]
- Lu, P.; Bruno, B.J.; Rabenau, M.; Lim, C.S. Delivery of drugs and macromolecules to the mitochondria for cancer therapy. J. Control. Release 2016, 240, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.; Kelso, G.F.; Blaikie, F.H.; James, A.M.; Cochemé, H.M.; Filipovska, A.; Da Ros, T.; Hurd, T.R.; Smith, R.A.J.; Murphy, M.P. Lipophilic triphenylphosphonium cations as tools in mitochondrial bioenergetics and free radical biology. Biochem. Biokhimiia 2005, 70, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Millard, M.; Pathania, D.; Shabaik, Y.; Taheri, L.; Deng, J.; Neamati, N. Preclinical evaluation of novel triphenylphosphonium salts with broad-spectrum activity. PLoS ONE 2010, 5, e13131. [Google Scholar] [CrossRef]
- Fuentes, E.; Araya-Maturana, R.; Urra, F.A. Regulation of mitochondrial function as a promising target in platelet activation-related diseases. Free. Radic. Biol. Med. 2019, 136, 172–182. [Google Scholar] [CrossRef]
- Ramsey, H.; Zhang, Q.; Wu, M.X. Mitoquinone restores platelet production in irradiation-induced thrombocytopenia. Platelets 2015, 26, 459–466. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Xie, R.; Liu, R.; Lu, Y. Mitochondria-derived reactive oxygen species play an important role in Doxorubicin-induced platelet apoptosis. Int. J. Mol. Sci. 2015, 16, 11087–11100. [Google Scholar] [CrossRef]
- Xin, G.; Wei, Z.; Ji, C.; Zheng, H.; Gu, J.; Ma, L.; Huang, W.; Morris-Natschke, S.L.; Yeh, J.-L.; Zhang, R.; et al. Metformin Uniquely Prevents Thrombosis by Inhibiting Platelet Activation and mtDNA Release. Sci. Rep. 2016, 6, 36222. [Google Scholar] [CrossRef] [PubMed]
- Mendez, D.; Arauna, D.; Fuentes, F.; Araya-Maturana, R.; Palomo, I.; Alarcon, M.; Sebastián, D.; Zorzano, A.; Fuentes, E. Mitoquinone (MitoQ) Inhibits Platelet Activation Steps by Reducing ROS Levels. Int. J. Mol. Sci. 2020, 21, 6192. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, G.; Pan, J.; Zielonka, J.; Xiong, D.; Myers, C.R.; Feng, L.; Shin, S.S.; Kim, Y.H.; Bui, D.; et al. Magnolia extract is effective for the chemoprevention of oral cancer through its ability to inhibit mitochondrial respiration at complex I. Cell Commun. Signal. 2020, 18, 58. [Google Scholar] [CrossRef]
- Cheng, G.; Hardy, M.; Zielonka, J.; Weh, K.; Zielonka, M.; Boyle, K.A.; Abu Eid, M.; McAllister, D.; Bennett, B.; Kresty, L.A.; et al. Mitochondria-targeted magnolol inhibits OXPHOS, proliferation, and tumor growth via modulation of energetics and autophagy in melanoma cells. Cancer Treat. Res. Commun. 2020, 25, 100210. [Google Scholar] [CrossRef]
- Sarrica, A.; Kirika, N.; Romeo, M.; Salmona, M.; Diomede, L. Safety and Toxicology of Magnolol and Honokiol. Planta Med. 2018, 84, 1151–1164. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-J.; Lee, Y.M.; Lee, C.-K.; Jung, J.K.; Han, S.B.; Hong, J.T. Therapeutic applications of compounds in the Magnolia family. Pharmacol. Ther. 2011, 130, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Park, H.J.; Chung, H.J.; Min, H.Y.; Park, E.J.; Lee, M.A.; Shin, Y.; Lee, S.K. Wnt/beta-catenin signaling mediates the antitumor activity of magnolol in colorectal cancer cells. Mol. Pharmacol. 2012, 82, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Szczepanski, M.J.; Lee, Y.J. Magnolol induces apoptosis via inhibiting the EGFR/PI3K/Akt signaling pathway in human prostate cancer cells. J. Cell. Biochem. 2009, 106, 1113–1122. [Google Scholar] [CrossRef]
- Fong, W.F.; Tse, A.K.; Poon, K.H.; Wang, C. Magnolol and honokiol enhance HL-60 human leukemia cell differentiation induced by 1,25-dihydroxyvitamin D3 and retinoic acid. Int. J. Biochem. Cell Biol. 2005, 37, 427–441. [Google Scholar] [CrossRef]
- Ou, H.C.; Chou, F.P.; Sheu, W.H.; Hsu, S.L.; Lee, W.J. Protective effects of magnolol against oxidized LDL-induced apoptosis in endothelial cells. Arch. Toxicol. 2007, 81, 421–432. [Google Scholar] [CrossRef]
- Chen, S.C.; Chang, Y.L.; Wang, D.L.; Cheng, J.J. Herbal remedy magnolol suppresses IL-6-induced STAT3 activation and gene expression in endothelial cells. Br. J. Pharmacol. 2006, 148, 226–232. [Google Scholar] [CrossRef]
- Poivre, M.; Duez, P. Biological activity and toxicity of the Chinese herb Magnolia officinalis Rehder & E. Wilson (Houpo) and its constituents. J. Zhejiang Univ. Sci. B. 2017, 18, 194–214. [Google Scholar]
- Zhao, X.; Li, F.; Sun, W.; Gao, L.; Kim, K.S.; Kim, K.T.; Cai, L.; Zhang, Z.; Zheng, Y. Extracts of Magnolia Species-Induced Prevention of Diabetic Complications: A Brief Review. Int. J. Mol. Sci. 2016, 17, 1629. [Google Scholar] [CrossRef]
- Cheng, J.; Dong, S.; Yi, L.; Geng, D.; Liu, Q. Magnolol abrogates chronic mild stress-induced depressive-like behaviors by inhibiting neuroinflammation and oxidative stress in the prefrontal cortex of mice. Int. Immunopharmacol. 2018, 59, 61–67. [Google Scholar] [CrossRef]
- Ho, J.H.; Hong, C.Y. Cardiovascular protection of magnolol: Cell-type specificity and dose-related effects. J. Biomed. Sci. 2012, 19, 70. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Li, Y.; Zeng, Y.; Tian, B.; Qu, X.; Yuan, Q.; Song, Y. Pharmacology, Toxicity, Bioavailability, and Formulation of Magnolol: An Update. Front. Pharmacol. 2021, 12, 632767. [Google Scholar] [CrossRef] [PubMed]
- Montecino-Garrido, H.; Mendez, D.; Araya-Maturana, R.; Millas-Vargas, J.P.; Wehinger, S.; Fuentes, E. In Vitro Effect of Mitochondria-Targeted Triphenylphosphonium-Based Compounds (Honokiol, Lonidamine, and Atovaquone) on the Platelet Function and Cytotoxic Activity. Front. Pharmacol. 2022, 13, 893873. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Li, K.; Fan, D.; Gao, X.; Ma, X.; Zhao, Y.; Zhao, D.; Liang, Y.; Ji, Q.; Chen, Y.; et al. Water-Soluble Tomato Concentrate, a Potential Antioxidant Supplement, Can Attenuate Platelet Apoptosis and Oxidative Stress in Healthy Middle-Aged and Elderly Adults: A Randomized, Double-Blinded, Crossover Clinical Trial. Nutrients 2022, 14, 3374. [Google Scholar] [CrossRef]
- Wu, L.; Zou, H.; Xia, W.; Dong, Q.; Wang, L. Role of magnolol in the proliferation of vascular smooth muscle cells. Herz 2015, 40, 542–548. [Google Scholar] [CrossRef]
- Kim, G.D.; Oh, J.; Park, H.J.; Bae, K.; Lee, S.K. Magnolol inhibits angiogenesis by regulating ROS-mediated apoptosis and the PI3K/AKT/mTOR signaling pathway in mES/EB-derived endothelial-like cells. Int. J. Oncol. 2013, 43, 600–610. [Google Scholar] [CrossRef]
- Teng, C.M.; Chen, C.C.; Ko, F.N.; Lee, L.G.; Huang, T.F.; Chen, Y.-P.; Hsu, H.-Y. Two antiplatelet agents from Magnolia officinalis. Thromb. Res. 1988, 50, 757–765. [Google Scholar] [CrossRef]
- Shih, C.Y.; Chou, T.C. The antiplatelet activity of magnolol is mediated by PPAR-beta/gamma. Biochem. Pharmacol. 2012, 84, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.H.; Tsai, W.J.; Chou, C.J.; Chen, C.F. Magnolol inhibits collagen-induced platelet serotonin release. Thromb. Res. 1995, 78, 265–270. [Google Scholar] [CrossRef]
- Chen, S.; Shen, J.; Zhao, J.; Wang, J.; Shan, T.; Li, J.; Xu, M.; Chen, X.; Liu, Y.; Cao, G. Magnolol Suppresses Pancreatic Cancer Development In Vivo and In Vitro via Negatively Regulating TGF-beta/Smad Signaling. Front. Oncol. 2020, 10, 597672. [Google Scholar] [CrossRef]
- Gao, T.; Xu, H.; Jia, S.; Cai, Z.; Chen, B.; Fan, G.; Zhang, Z.; Chen, G. Magnolol induces human Ewing sarcoma SK-ES-1 cell apoptosis via the mitochondrial and death receptor pathways. Am. J. Transl. Res. 2020, 12, 1672–1682. [Google Scholar] [PubMed]
- Li, M.; Zhang, F.; Wang, X.; Wu, X.; Zhang, B.; Zhang, N.; Wu, W.; Wang, Z.; Weng, H.; Liu, S.; et al. Magnolol inhibits growth of gallbladder cancer cells through the p53 pathway. Cancer Sci. 2015, 106, 1341–1350. [Google Scholar]
- Tao, C.; Chen, J.; Huang, X.; Chen, Z.; Li, X.; Li, Y.; Xu, Y.; Ma, M.; Wu, Z. CT1-3, a novel magnolol-sulforaphane hybrid suppresses tumorigenesis through inducing mitochondria-mediated apoptosis and inhibiting epithelial mesenchymal transition. Eur. J. Med. Chem. 2020, 199, 112441. [Google Scholar] [CrossRef] [PubMed]
- Ouseph, M.M.; Huang, Y.; Banerjee, M.; Joshi, S.; MacDonald, L.; Zhong, Y.; Liu, H.; Li, X.; Xiang, B.; Zhang, G.; et al. Autophagy is induced upon platelet activation and is essential for hemostasis and thrombosis. Blood 2015, 126, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Schwertz, H.; Middleton, E.A. Autophagy and its consequences for platelet biology. Thromb. Res. 2022. [Google Scholar] [CrossRef]
- Rodríguez, L.; Badimon, L.; Méndez, D.; Padró, T.; Vilahur, G.; Peña, E.; Carrasco, B.; Vogel, H.; Palomo, I.; Fuentes, E. Antiplatelet Activity of Isorhamnetin via Mitochondrial Regulation. Antioxidants 2021, 10, 666. [Google Scholar] [CrossRef]
- Méndez, D.; Urra, F.A.; Millas-Vargas, J.P.; Alarcón, M.; Rodríguez-Lavado, J.; Palomo, I.; Trostchansky, A.; Araya-Maturana, R.; Fuentes, E. Synthesis of antiplatelet ortho-carbonyl hydroquinones with differential action on platelet aggregation stimulated by collagen or TRAP-6. Eur. J. Med. Chem. 2020, 192, 112187. [Google Scholar] [CrossRef]
- Mendez, D.; Donoso-Bustamante, V.; Pablo Millas-Vargas, J.; Pessoa-Mahana, H.; Araya-Maturana, R.; Fuentes, E. Synthesis and pharmacological evaluation of acylhydroquinone derivatives as potent antiplatelet agents. Biochem. Pharmacol. 2021, 183, 114341. [Google Scholar] [CrossRef]
- Monroy-Cardenas, M.; Mendez, D.; Trostchansky, A.; Martinez-Cifuentes, M.; Araya-Maturana, R.; Fuentes, E. Synthesis and Biological Evaluation of Thio-Derivatives of 2-Hydroxy-1,4-Naphthoquinone (Lawsone) as Novel Antiplatelet Agents. Front. Chem. 2020, 8, 533. [Google Scholar] [CrossRef]
- Venturini, W.; Olate-Briones, A.; Valenzuela, C.; Méndez, D.; Fuentes, E.; Cayo, A.; Mancilla, D.; Segovia, R.; Brown, N.E.; Moore-Carrasco, R. Platelet Activation Is Triggered by Factors Secreted by Senescent Endothelial HMEC-1 Cells In Vitro. Int. J. Mol. Sci. 2020, 21, 3287. [Google Scholar] [CrossRef]
- Gaspar, R.S.; Mansilla, S.; Vieira, V.A.; da Silva, L.B.; Gibbins, J.M.; Castro, L.; Trostchansky, A.; Paes, A.M.D.A. The protein disulphide isomerase inhibitor CxxCpep modulates oxidative burst and mitochondrial function in platelets. Free. Radic. Biol. Med. 2021, 172, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D.; Nicholls, D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011, 435, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Braganza, A.; Annarapu, G.K.; Shiva, S. Blood-based bioenergetics: An emerging translational and clinical tool. Mol. Asp. Med. 2020, 71, 100835. [Google Scholar] [CrossRef] [PubMed]


| Compound | IC50 Collagen [μM] | IC50 TRAP-6 [μM] |
|---|---|---|
| Magnolol | 1.78 ± 0.6 | >20 * |
| MGN4 | 0.59 ± 0.3 | 13.94 ± 6.65 * |
| MGN10 | >5 * | >20 * |
| Rate | Control | MGN4 (5 µM) |
|---|---|---|
| Basal (OCR/106 platelets) | 3.4 (0.2) | 5.6 (0.6) **** |
| Collagen (OCR/106 platelets) | 4.6 (0.3) | 7.1 (0.3) **** |
| Activation (OCRCollagen—OCRBasal) | 1.2 (0.4) | 1.5 (0.3) |
| ATP-indep (OCR/106 platelets) | 1.0 (0.2) | 5.0 (0.4) **** |
| ATP-dep (OCR/106 platelets) | 2.4 (0.1) | 0.6 (0.4) **** |
| Maximum (OCR/106 platelets) | 7.5 (0.8) | 6.8 (0.6) |
| Spare (OCRMaximum—OCRBasal) | 4.1 (1.0) | 1.2 (0.8) **** |
| Non-mito (OCR/106 platelets) | 3 (0.4) | 0.9 (0.5) **** |
| Coupling efficiency ((OCRBasal—OCRATP-indep)/OCRBasal) | 0.70 (0.03) | 0.11 (0.06) ** |
| Glycolysis (mpH/min/106 platelets) | 1.1 (0.1) | 1.8 (0.2) *** |
| Glycolytic capacity (mpH/min/106 platelets) | 5.5 (0.2) | 4.7 (0.1) *** |
| Non-glycolytic acidification (mpH/min /106 platelets) | 1.3 (0.1) | 1.5 (0.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tellería, F.; Mansilla, S.; Méndez, D.; Sepúlveda, M.; Araya-Maturana, R.; Castro, L.; Trostchansky, A.; Fuentes, E. The Use of Triphenyl Phosphonium Cation Enhances the Mitochondrial Antiplatelet Effect of the Compound Magnolol. Pharmaceuticals 2023, 16, 210. https://doi.org/10.3390/ph16020210
Tellería F, Mansilla S, Méndez D, Sepúlveda M, Araya-Maturana R, Castro L, Trostchansky A, Fuentes E. The Use of Triphenyl Phosphonium Cation Enhances the Mitochondrial Antiplatelet Effect of the Compound Magnolol. Pharmaceuticals. 2023; 16(2):210. https://doi.org/10.3390/ph16020210
Chicago/Turabian StyleTellería, Francisca, Santiago Mansilla, Diego Méndez, Magdalena Sepúlveda, Ramiro Araya-Maturana, Laura Castro, Andrés Trostchansky, and Eduardo Fuentes. 2023. "The Use of Triphenyl Phosphonium Cation Enhances the Mitochondrial Antiplatelet Effect of the Compound Magnolol" Pharmaceuticals 16, no. 2: 210. https://doi.org/10.3390/ph16020210
APA StyleTellería, F., Mansilla, S., Méndez, D., Sepúlveda, M., Araya-Maturana, R., Castro, L., Trostchansky, A., & Fuentes, E. (2023). The Use of Triphenyl Phosphonium Cation Enhances the Mitochondrial Antiplatelet Effect of the Compound Magnolol. Pharmaceuticals, 16(2), 210. https://doi.org/10.3390/ph16020210








