Synthesis and Biological Evaluation of 3-Amidoquinuclidine Quaternary Ammonium Compounds as New Soft Antibacterial Agents
Abstract
1. Introduction
2. Results and Discussion
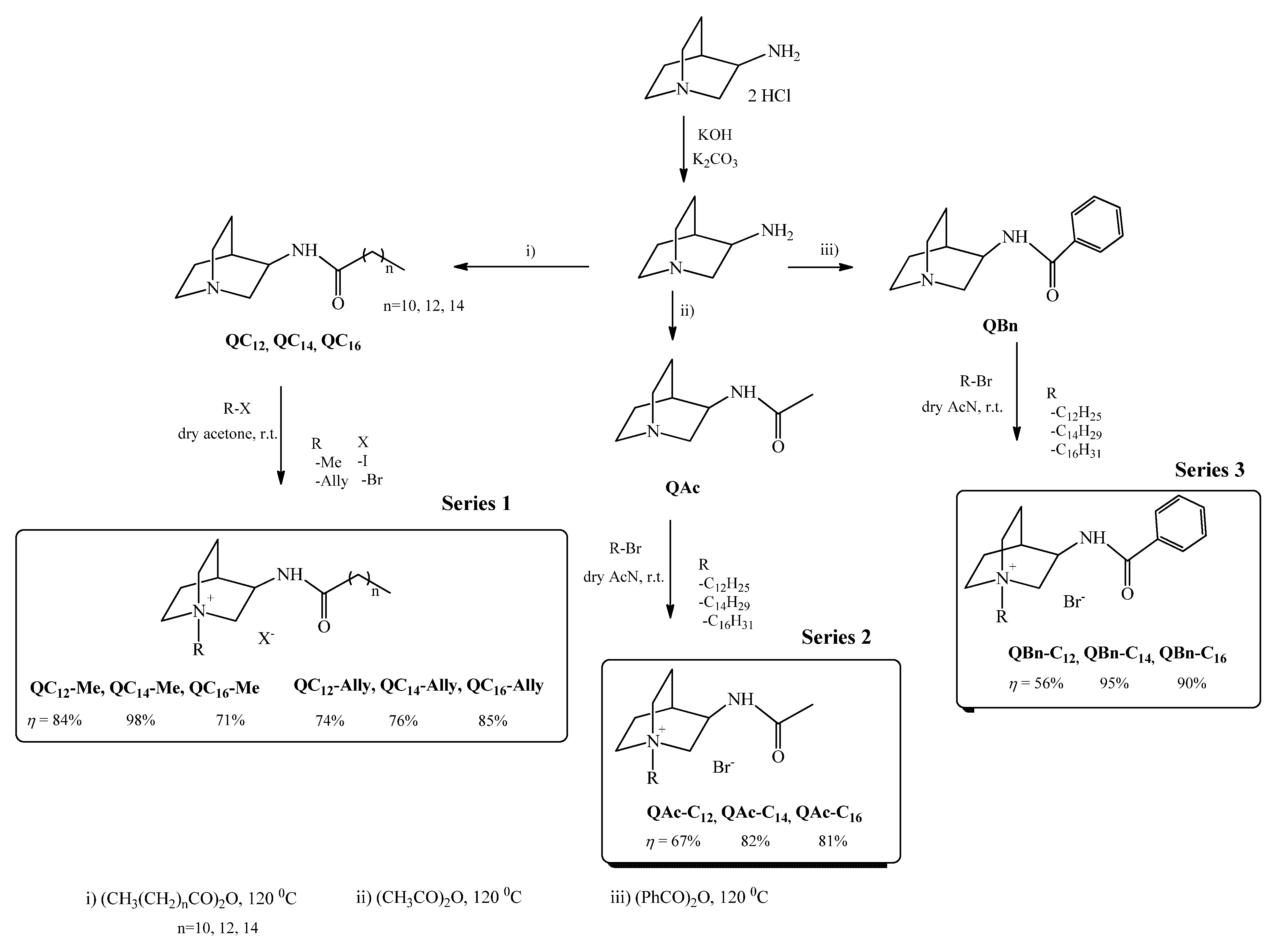
2.1. Synthesis
2.2. Antimicrobial Activity
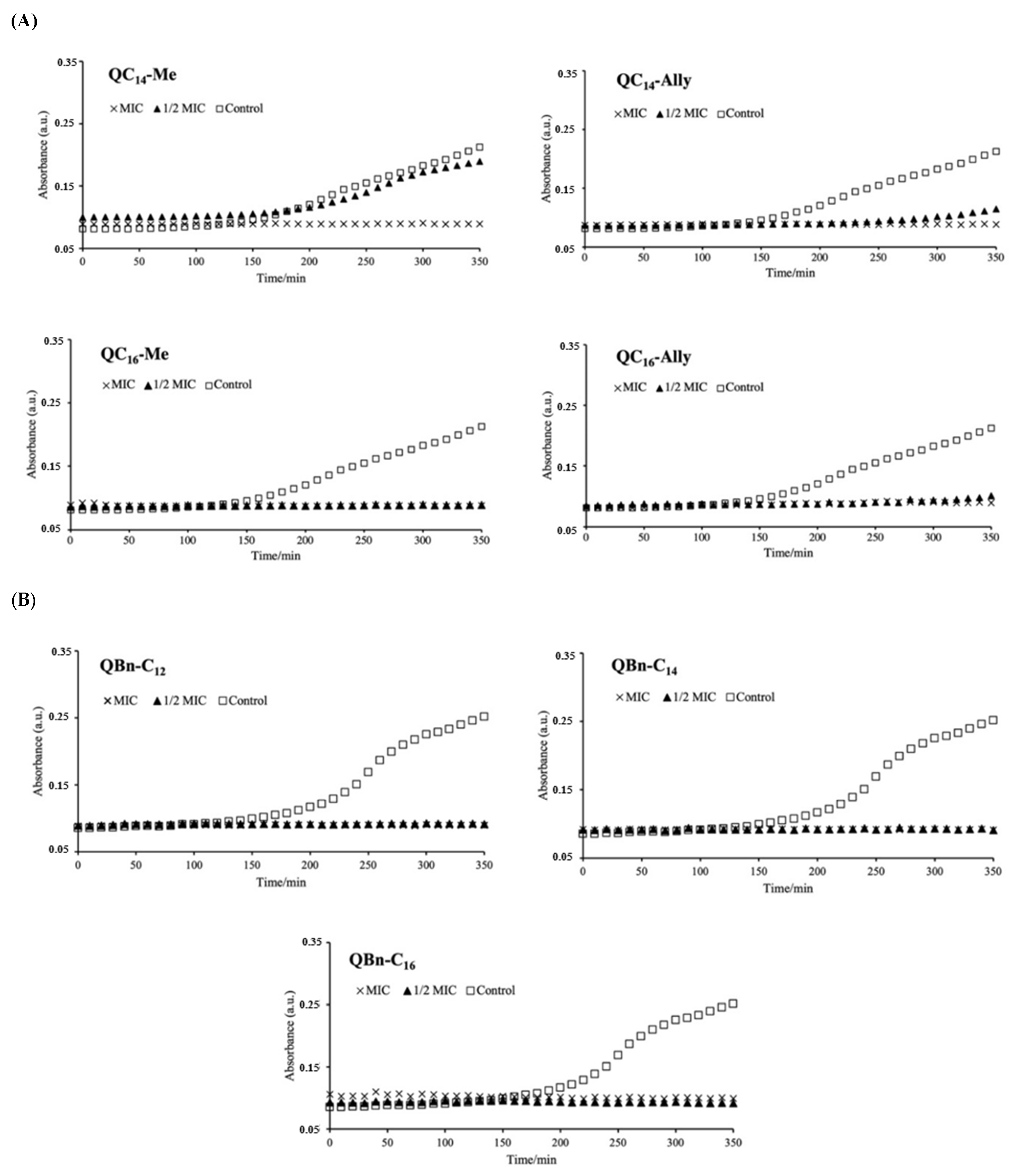
2.3. The Influence of the New 3-Amidoquinuclidine QACs on Growth Dynamics of Staphylococcus aureus ATCC 25923
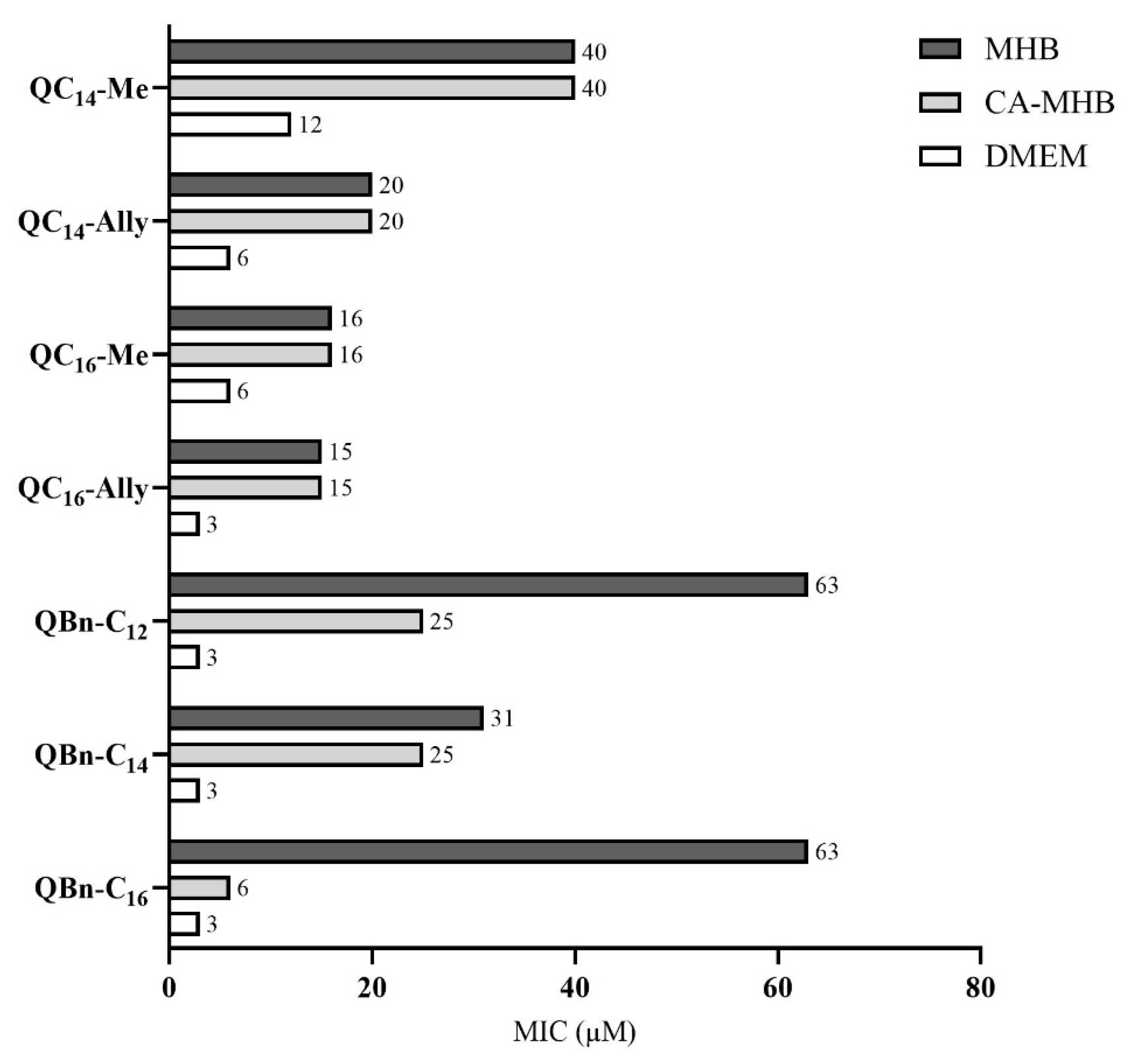
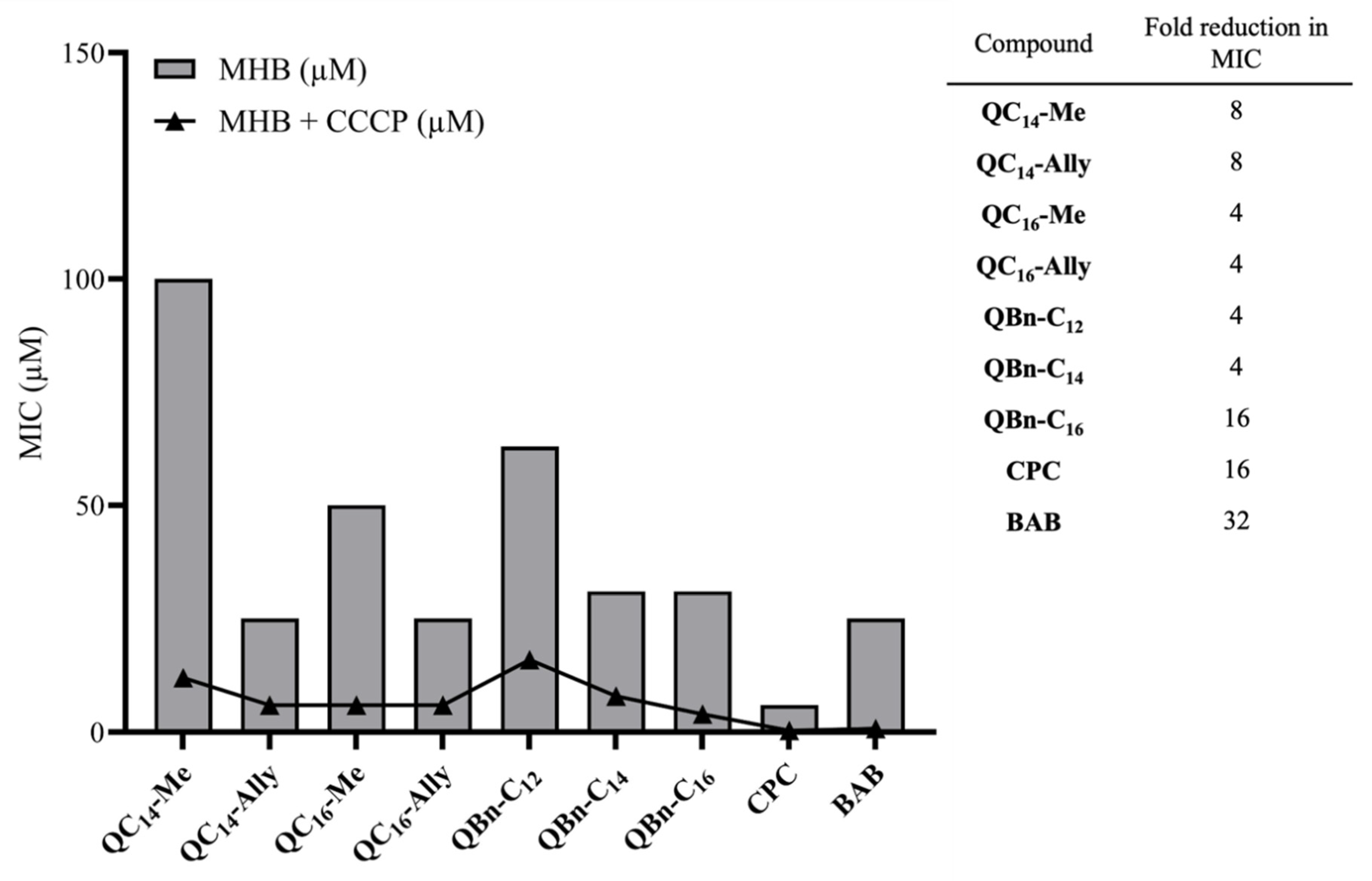
2.4. Antimicrobial Activity in Different Nutrient Media
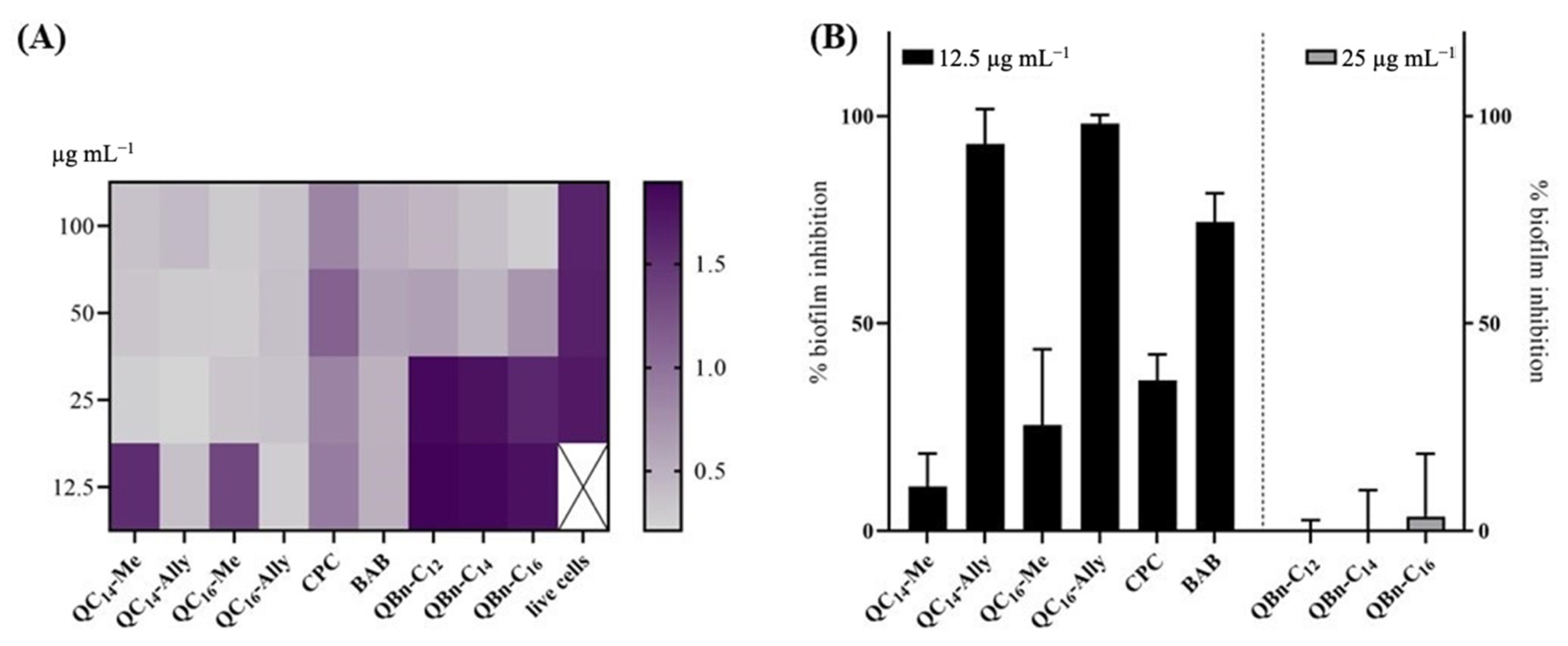
2.5. Inhibition of the Biofilm Formation of Staphylococcus aureus ATCC 25923
2.6. The Potential for Bacterial Resistance Development
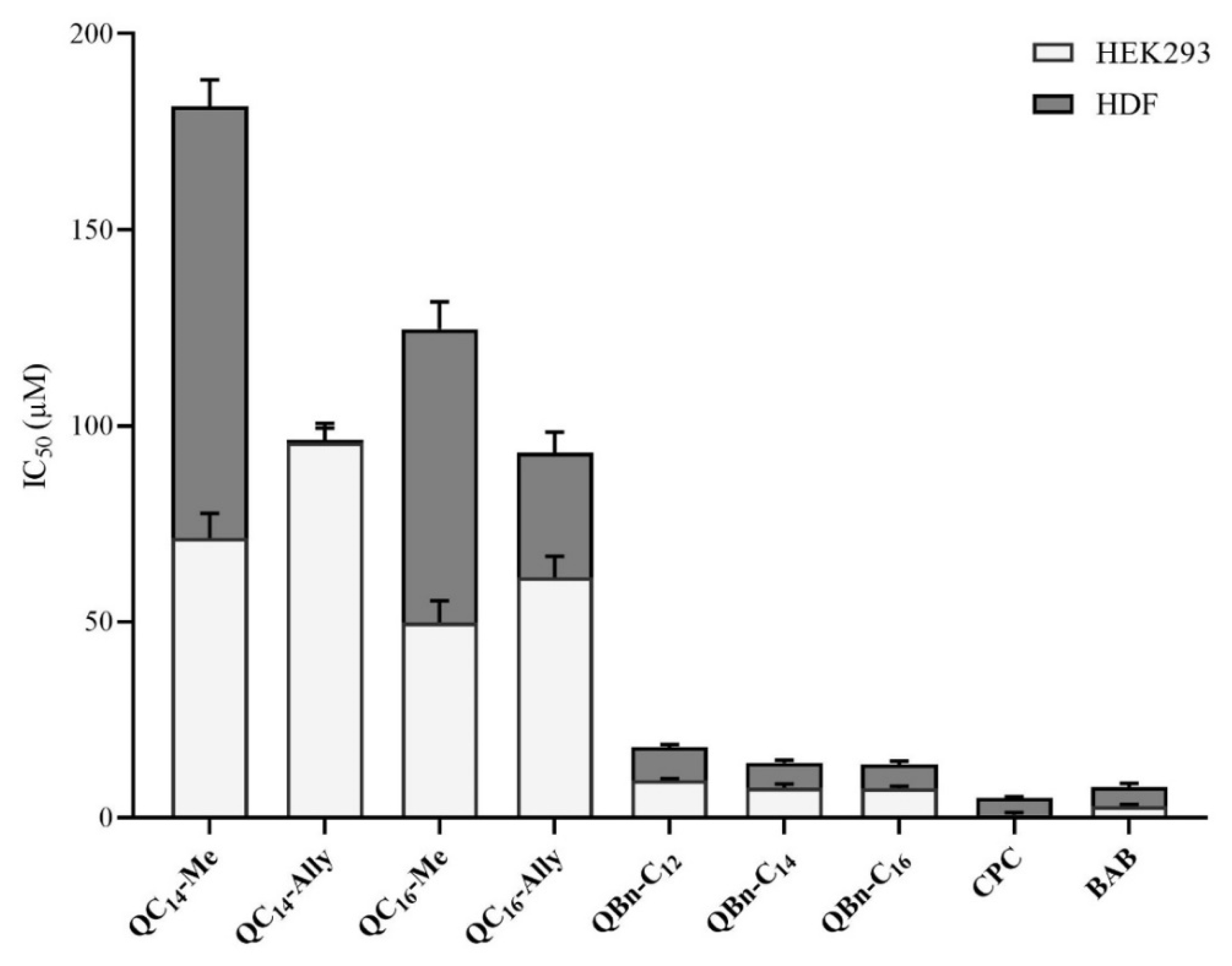
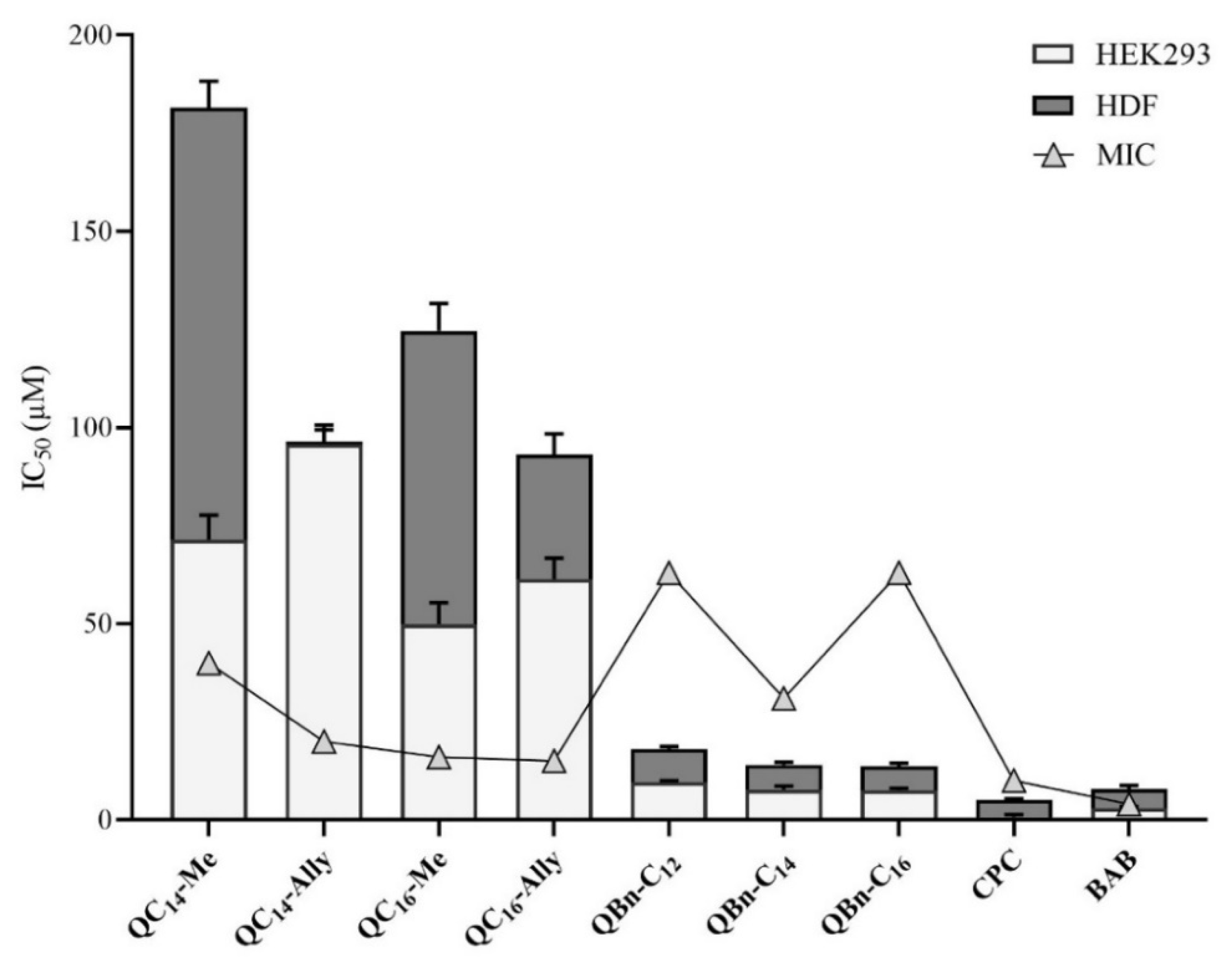
2.7. Cytotoxicity of 3-Amidoquinuclidine Quaternary Ammonium Compounds
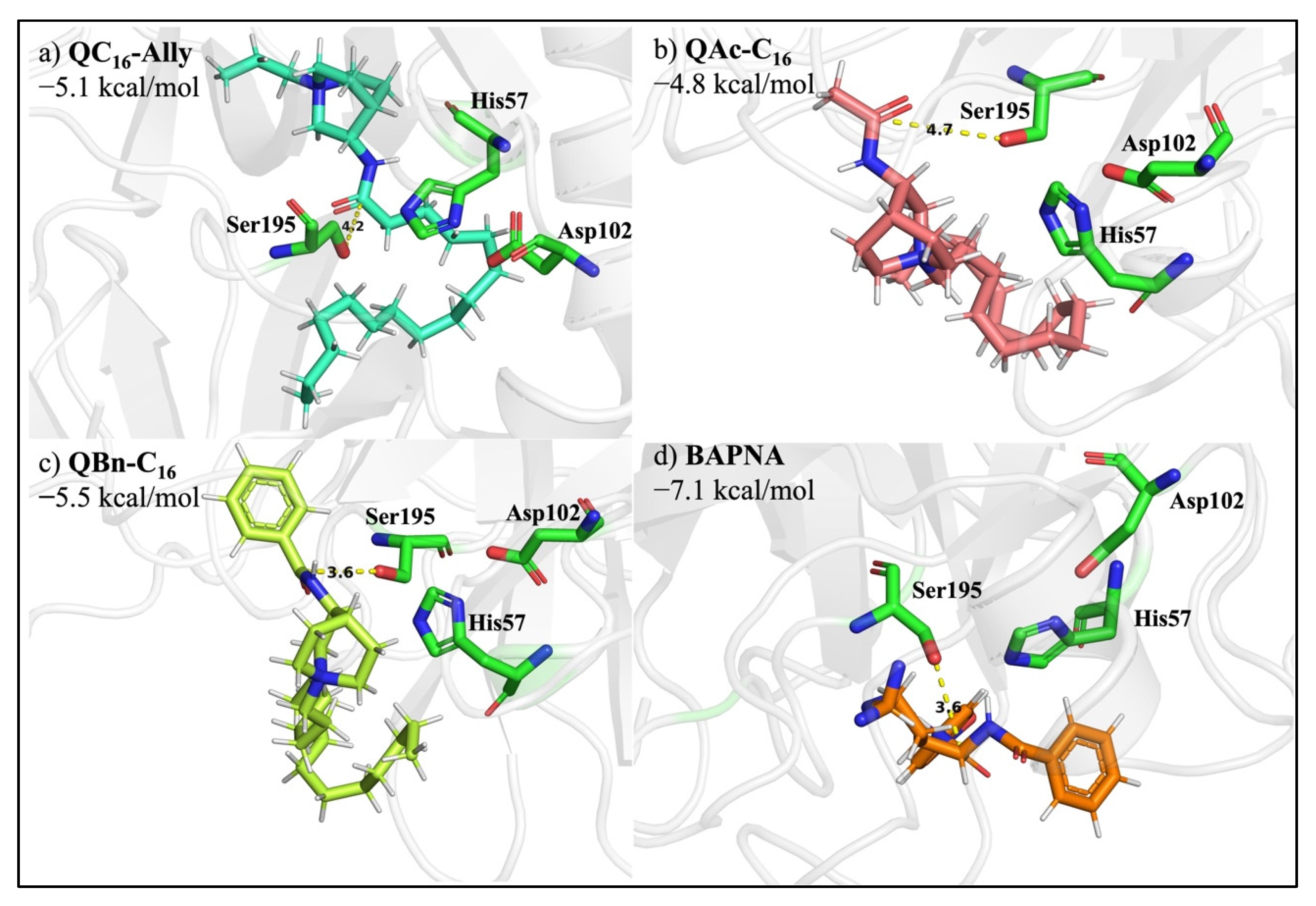
2.8. Protease Degradation of Amide Bond
3. Materials and Methods
3.1. Synthesis
3.1.1. Synthesis of (±)3-Aminoquinuclidine
3.1.2. Synthesis of (±)3-Amidoquinuclidine Precursors
3.1.3. Synthesis of Quaternary Ammonium Compounds
3.2. Broth Microdilution Assays
3.2.1. Minimum Inhibitory Concentration
3.2.2. Minimum Inhibitory Concentration in Cation-Adjusted Mueller Hinton Broth (CA-MHB)
3.2.3. Minimum Inhibitory Concentration in Dulbecco’s Modified Eagle Medium (DMEM)
3.3. Time-Resolved Growth Analysis
3.4. Biofilm Inhibition Assay
3.5. Potential of Bacterial Resistance Development
3.6. Cytotoxicity
3.7. Docking QC16-Ally to the Active Site of Serine Protease
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, N.; Ma, S. Recent development of membrane-active molecules as antibacterial agents. Eur. J. Med. Chem. 2019, 184, 111743. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.C.; Minbiole, K.P.C.; Wuest, W.M. Quaternary Ammonium Compounds: An Antimicrobial Mainstay and Platform for Innovation to Address Bacterial Resistance. ACS Infect. Dis. 2015, 1, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Kwaśniewska, D.; Chen, Y.-L.; Wieczorek, D. Biological Activity of Quaternary Ammonium Salts and Their Derivatives. Pathogens 2020, 9, 459. [Google Scholar] [CrossRef] [PubMed]
- Hoque, J.; Akkapeddi, P.; Yarlagadda, V.; Uppu, D.S.S.M.; Kumar, P.; Haldar, J. Cleavable Cationic Antibacterial Amphiphiles: Synthesis, Mechanism of Action, and Cytotoxicities. Langmuir 2012, 28, 12225–12234. [Google Scholar] [CrossRef]
- Hora, P.I.; Pati, S.G.; McNamara, P.J.; Arnold, W.A. Increased Use of Quaternary Ammonium Compounds during the SARS-CoV-2 Pandemic and Beyond: Consideration of Environmental Implications. Environ. Sci. Technol. Lett. 2020, 7, 622–631. [Google Scholar] [CrossRef]
- Xiling, G.; Yin, C.; Ling, W.; Xiaosong, W.; Jingjing, F.; Fang, L.; Xiaoyan, Z.; Yiyue, G.; Ying, C.; Lunbiao, C.; et al. In vitro inactivation of SARS-CoV-2 by commonly used disinfection products and methods. Sci. Rep. 2021, 11, 1–9. [Google Scholar] [CrossRef]
- Bodor, N.; Kaminski, J.J. Soft drugs. 2. Soft alkylating compounds as potential antitumor agents. J. Med. Chem. 1980, 23, 566–569. [Google Scholar] [CrossRef]
- Allen, R.A.; Jennings, M.C.; Mitchell, M.A.; Al-Khalifa, S.E.; Wuest, W.M.; Minbiole, K.P. Ester- and amide-containing multiQACs: Exploring multicationic soft antimicrobial agents. Bioorganic Med. Chem. Lett. 2017, 27, 2107–2112. [Google Scholar] [CrossRef]
- Thorsteinsson, T.; Másson, M.; Kristinsson, K.G.; Hjálmarsdóttir, M.A.; Hilmarsson, H.; Loftsson, T. Soft Antimicrobial Agents: Synthesis and Activity of Labile Environmentally Friendly Long Chain Quaternary Ammonium Compounds. J. Med. Chem. 2003, 46, 4173–4181. [Google Scholar] [CrossRef]
- Haldar, J.; Kondaiah, P.; Bhattacharya, S. Synthesis and Antibacterial Properties of Novel Hydrolyzable Cationic Amphiphiles. Incorporation of Multiple Head Groups Leads to Impressive Antibacterial Activity. J. Med. Chem. 2005, 48, 3823–3831. [Google Scholar] [CrossRef]
- Bodor, N.; Buchwald, P. Soft Drug Design: General Principles and Recent Applications. Med. Res. Rev. 2000, 20, 58–101. [Google Scholar] [CrossRef]
- Colomer, A.; Pinazo, A.; Manresa, M.A.; Vinardell, M.P.; Mitjans, M.; Infante, M.R.; Pérez, L. Cationic Surfactants Derived from Lysine: Effects of Their Structure and Charge Type on Antimicrobial and Hemolytic Activities. J. Med. Chem. 2011, 54, 989–1002. [Google Scholar] [CrossRef]
- Hoque, J.; Konai, M.M.; Gonuguntla, S.; Manjunath, G.B.; Samaddar, S.; Yarlagadda, V.; Haldar, J. Membrane Active Small Molecules Show Selective Broad Spectrum Antibacterial Activity with No Detectable Resistance and Eradicate Biofilms. J. Med. Chem. 2015, 58, 5486–5500. [Google Scholar] [CrossRef]
- Bazina, L.; Maravić, A.; Krce, L.; Soldo, B.; Odžak, R.; Popović, V.B.; Aviani, I.; Primožič, I.; Šprung, M. Discovery of novel quaternary ammonium compounds based on quinuclidine-3-ol as new potential antimicrobial candidates. Eur. J. Med. Chem. 2018, 163, 626–635. [Google Scholar] [CrossRef]
- Kastelic, A.R.; Odžak, R.; Pezdirc, I.; Sović, K.; Hrenar, T.; Gašparović, A.Č.; Skočibušić, M.; Primožič, I. New and Potent Quinuclidine-Based Antimicrobial Agents. Molecules 2019, 24, 2675. [Google Scholar] [CrossRef]
- Kontos, R.C.; Schallenhammer, S.A.; Bentley, B.S.; Morrison, K.R.; Feliciano, J.A.; Tasca, J.A.; Kaplan, A.R.; Bezpalko, M.W.; Kassel, W.S.; Wuest, W.M.; et al. An Investigation into Rigidity–Activity Relationships in BisQAC Amphiphilic Antiseptics. ChemMedChem 2018, 14, 83–87. [Google Scholar] [CrossRef]
- Leitgeb, A.J.; Feliciano, J.A.; Sanchez, H.A.; Allen, R.A.; Morrison, K.R.; Sommers, K.J.; Carden, R.G.; Wuest, W.M.; Minbiole, K.P.C. Further Investigations into Rigidity-Activity Relationships in BisQAC Amphiphilic Antiseptics. ChemMedChem 2020, 15, 667–670. [Google Scholar] [CrossRef]
- Crnčević, D.; Krce, L.; Cvitković, M.; Brkljača, Z.; Sabljić, A.; Vuko, E.; Primožič, I.; Odžak, R.; Šprung, M. New Membrane Active Antibacterial and Antiviral Amphiphiles Derived from Heterocyclic Backbone of Pyridinium-4-Aldoxime. Pharmaceuticals 2022, 15, 775. [Google Scholar] [CrossRef]
- Odžak, R.; Primožić, I.; Tomić, S. 3-Amidoquinuclidine derivatives: Synthesis and interaction with butyrylcholinesterase. Croat. Chem. Acta 2007, 80, 101–107. [Google Scholar]
- Odžak, R.; Tomić, S. 3-Amidoquinuclidine derivatives: Synthesis of compounds and inhibition of butyrylcholinesterase. Bioorganic Chem. 2006, 34, 90–98. [Google Scholar] [CrossRef]
- Mahoney, A.R.; Safaee, M.M.; Wuest, W.M.; Furst, A.L. The silent pandemic: Emergent antibiotic resistances following the global response to SARS-CoV-2. Iscience 2021, 24, 102304. [Google Scholar] [CrossRef] [PubMed]
- Theophel, K.; Schacht, V.J.; Schlã¼Ter, M.; Schnell, S.; Stingu, C.-S.; Schaumann, R.; Bunge, M. The importance of growth kinetic analysis in determining bacterial susceptibility against antibiotics and silver nanoparticles. Front. Microbiol. 2014, 5, 544. [Google Scholar] [CrossRef] [PubMed]
- Crnčević, D.; Krce, L.; Mastelić, L.; Maravić, A.; Soldo, B.; Aviani, I.; Primožič, I.; Odžak, R.; Šprung, M. The mode of antibacterial action of quaternary N-benzylimidazole salts against emerging opportunistic pathogens. Bioorganic Chem. 2021, 112, 104938. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, M.H.; Jennings, M.C.; Wuest, W.M. Draining the moat: Disrupting bacterial biofilms with natural products. Tetrahedron 2014, 70, 6373–6383. [Google Scholar] [CrossRef]
- DeLeo, F.R.; Chambers, H.F. Reemergence of antibiotic-resistant Staphylococcus aureus in the genomics era. J. Clin. Investig. 2009, 119, 2464–2474. [Google Scholar] [CrossRef]
- Pendleton, J.N.; Gorman, S.P.; Gilmore, B.F. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti-Infect. Ther. 2013, 11, 297–308. [Google Scholar] [CrossRef]
- Li, F.; Chai, Z.G.; Sun, M.N.; Wang, F.; Ma, S.; Zhang, L.; Fang, M.; Chen, J.H. Anti-biofilm Effect of Dental Adhesive with Cationic Monomer. J. Dent. Res. 2009, 88, 372–376. [Google Scholar] [CrossRef]
- Hoque, J.; Konai, M.M.; Samaddar, S.; Gonuguntala, S.; Manjunath, G.B.; Ghosh, C.; Haldar, J. Selective and broad spectrum amphiphilic small molecules to combat bacterial resistance and eradicate biofilms. Chem. Commun. 2015, 51, 13670–13673. [Google Scholar] [CrossRef]
- Buffet-Bataillon, S.; Tattevin, P.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Emergence of resistance to antibacterial agents: The role of quaternary ammonium compounds—A critical review. Int. J. Antimicrob. Agents 2012, 39, 381–389. [Google Scholar] [CrossRef]
- Jennings, M.; Forman, M.; Duggan, S.; Minbiole, K.; Wuest, W. Efflux pumps may not be the major drivers of QAC resistance in methicillin-resistant Staphylococcus aureus. ChemMedChem 2017, 18, 1573–1577. [Google Scholar] [CrossRef]
- Paulsen, I.T.; Brown, M.H.; Littlejohn, T.G.; A Mitchell, B.; A Skurray, R. Multidrug resistance proteins QacA and QacB from Staphylococcus aureus: Membrane topology and identification of residues involved in substrate specificity. Proc. Natl. Acad. Sci. USA 1996, 93, 3630–3635. [Google Scholar] [CrossRef]
- Sekyere, J.O.; Amoako, D.G. Carbonyl Cyanide m-Chlorophenylhydrazine (CCCP) Reverses Resistance to Colistin, but Not to Carbapenems and Tigecycline in Multidrug-Resistant Enterobacteriaceae. Front. Microbiol. 2017, 8, 228. [Google Scholar] [CrossRef]
- Garrison, M.A.; Mahoney, A.R.; Wuest, W.M. Tricepyridinium-inspired QACs yield potent antimicrobials and provide insight into QAC resistance. ChemMedChem 2020, 16, 463–466. [Google Scholar] [CrossRef]
- Schumacher, M.; Miller, M.; Grkovic, S.; Brown, M.; Skurray, R.; Brennan, R. Structural basis for cooperative DNA binding by two dimers of the multidrug-binding protein QacR. EMBO J. 2002, 21, 1210–1218. [Google Scholar] [CrossRef]
- Carden, R.G.; Sommers, K.J.; Schrank, C.L.; Leitgeb, A.J.; Feliciano, J.A.; Wuest, W.M.; Minbiole, K.P.C. Advancements in the Development of Non-Nitrogen-Based Amphiphilic Antiseptics to Overcome Pathogenic Bacterial Resistance. ChemMedChem 2020, 15, 1974–1984. [Google Scholar] [CrossRef]
- Kahne, D.; Still, C. Hydrolysis of a peptide bond in neutral water. J. Am. Chem. Soc. 1988, 22, 7529–7534. [Google Scholar] [CrossRef]
- Turk, B. Targeting proteases: Successes, failures and future prospects. Nat. Rev. Drug Discov. 2006, 5, 785–799. [Google Scholar] [CrossRef]
- Lee, S.-L.; Alexander, R.S.; Smallwood, A.; Trievel, R.; Mersinger, L.; Weber, P.C.; Kettner, C. New Inhibitors of Thrombin and Other Trypsin-like Proteases: Hydrogen Bonding of an Aromatic Cyano Group with a Backbone Amide of the P1 Binding Site Replaces Binding of a Basic Side Chain. Biochemistry 1997, 36, 13180–13186. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 9th ed.; CLSI document M07-A9; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
| Minimum Inhibitory Concentration (MIC)/µM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound | Gram-Positive | Gram-Negative | ||||||||
| Staphylococcus aureus ATCC25923 | Staphylococcus aureus ATCC33591 | Staphylococcus aureus Clinical/MRSA | Bacillus cereus ATCC14579 | Listeria monocytogenes ATCC7644 | Enterococcus faecalis ATCC29212 | Escherichia coli ATTC25922 | Salmonella enterica food isolate | Pseudomonas aeruginosa ATTC27853 | ||
| Series 1 | QC12-Me | 100 | >125 | 100 | 100 | 100 | 100 | 100 | >125 | >125 |
| QC12-Ally | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | |
| QC14-Me | 40 | 100 | 60 | 50 | 20 | 30 | 100 | >125 | >125 | |
| QC14-Ally | 20 | 50 | 25 | 25 | 8 | 15 | 50 | >125 | >125 | |
| QC16-Me | 16 | 25 | 31 | >100 | 8 | 100 | >100 | >125 | >125 | |
| QC16-Ally | 15 | 25 | 31 | >100 | 16 | >100 | >100 | >125 | >125 | |
| Series 2 | QAc-C12 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| QAc-C14 | >125 | >125 | >125 | >125 | 63 | 63 | >125 | >125 | >125 | |
| QAc-C16 | >125 | >125 | >125 | >125 | 8 | 31 | >125 | >125 | >125 | |
| Series 3 | QBn-C12 | 63 | 63 | 63 | 63 | 31 | 31 | 63 | 63 | >125 |
| QBn-C14 | 31 | 31 | 31 | 63 | 4 | 8 | 31 | 31 | 63 | |
| QBn-C16 | 63 | 31 | 31 | 63 | 4 | 4 | 31 | 63 | >125 | |
| Commercial QACs | CPC | 4 | 6 | 8 | 16 | 8 | 8 | 16 | 63 | 250 |
| BAB | 10 | 25 | 25 | 13 | 10 | 15 | 63 | 50 | >125 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odžak, R.; Crnčević, D.; Sabljić, A.; Primožič, I.; Šprung, M. Synthesis and Biological Evaluation of 3-Amidoquinuclidine Quaternary Ammonium Compounds as New Soft Antibacterial Agents. Pharmaceuticals 2023, 16, 187. https://doi.org/10.3390/ph16020187
Odžak R, Crnčević D, Sabljić A, Primožič I, Šprung M. Synthesis and Biological Evaluation of 3-Amidoquinuclidine Quaternary Ammonium Compounds as New Soft Antibacterial Agents. Pharmaceuticals. 2023; 16(2):187. https://doi.org/10.3390/ph16020187
Chicago/Turabian StyleOdžak, Renata, Doris Crnčević, Antonio Sabljić, Ines Primožič, and Matilda Šprung. 2023. "Synthesis and Biological Evaluation of 3-Amidoquinuclidine Quaternary Ammonium Compounds as New Soft Antibacterial Agents" Pharmaceuticals 16, no. 2: 187. https://doi.org/10.3390/ph16020187
APA StyleOdžak, R., Crnčević, D., Sabljić, A., Primožič, I., & Šprung, M. (2023). Synthesis and Biological Evaluation of 3-Amidoquinuclidine Quaternary Ammonium Compounds as New Soft Antibacterial Agents. Pharmaceuticals, 16(2), 187. https://doi.org/10.3390/ph16020187








