Exploring Mannosylpurines as Copper Chelators and Cholinesterase Inhibitors with Potential for Alzheimer’s Disease
Abstract
1. Introduction
2. Results
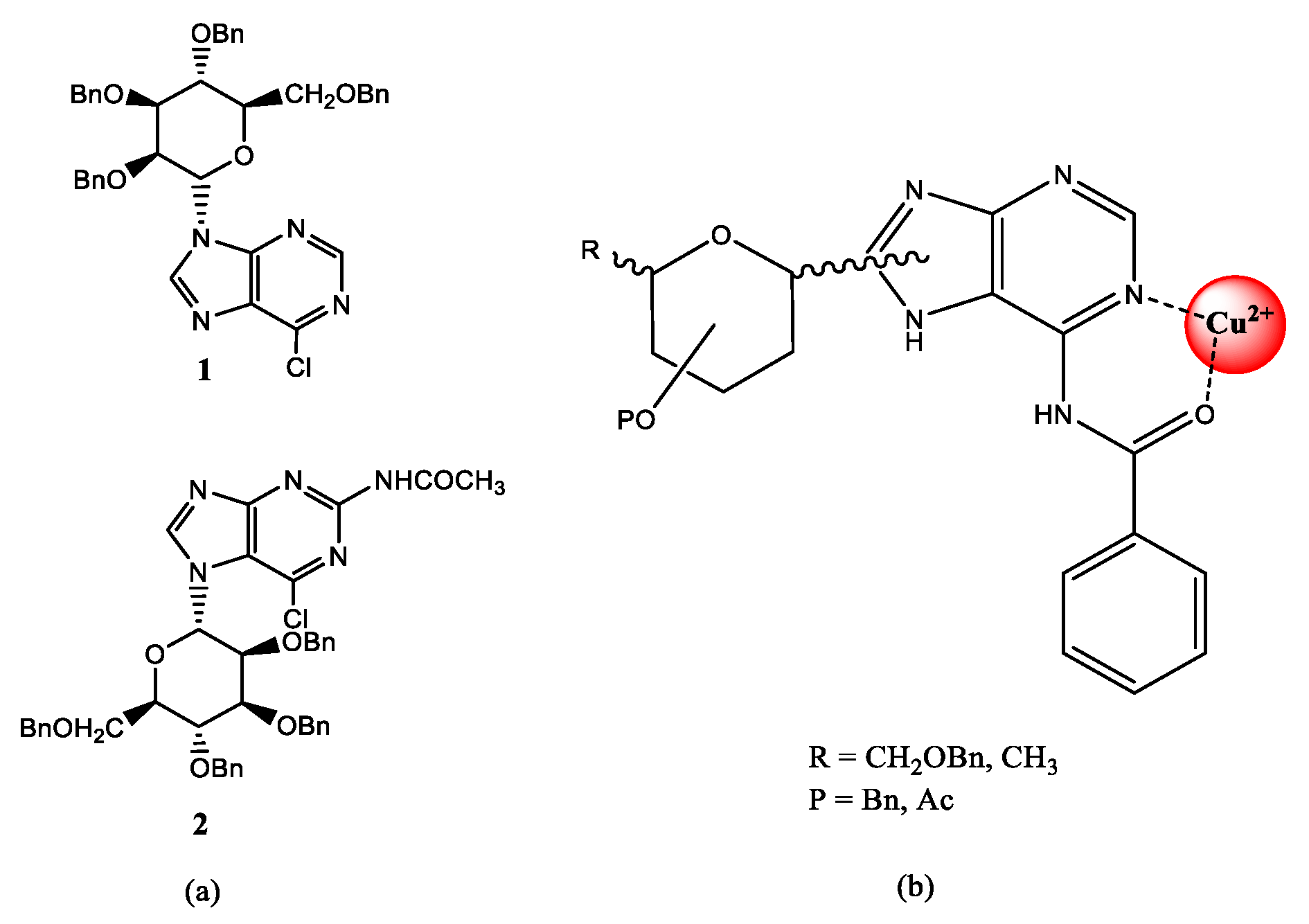
2.1. Chemistry
2.2. Anticholinesterase Activity
| Compound nr. | IC50 (μM) a | |||
|---|---|---|---|---|
| eeAChE b | hAChE c | eqBChE d | hBChE c | |
| 1 | 2.40 ± 0.30 | - | 2.80 ± 0.30 | - |
| 2 | 18.10 ± 4.80 | - | 0.05 ± 0.01 | - |
| 5 | >20 [25 ± 5] | - | >20 [11 ± 7] | - |
| 6 | 10.60 ± 1.10 | 17.00 ± 0.75 | 4.29 ± 1.70 | 4.58 ± 0.52 |
| 7 | >20 | - | 10.00 ± 1.10 | - |
| 8 | 2.61 ± 1.23 | 4.81 ± 1.24 | 4.40 ± 1.30 | 4.55 ± 1.05 |
| 9 | 4.69 ± 0.51 | >20 [25 ± 6] | >20 [28 ± 3] | 15.4 ± 1.10 |
| 10 | 15.0 ± 2.1 | 17.8 ± 2.20 | 11.7 ± 1.2 | 8.70 ± 1.35 |
| 12 | >20 [8 ± 5] | - | >20 [5 ± 3] | - |
| 13 | >20 [6 ± 3] | >20 [8 ± 5] | 5.12 ± 0.25 | >20 [25 ± 4] |
| 15 | >5 [31 ± 4] | >5 [28 ± 3] | >5 [24 ± 6] | >5 [18 ± 5] |
| 16 | >5 [18 ± 3] | >5 [16 ± 9] | >5 [26 ± 6] | >5 [30 ± 7] |
| Donepezil | 0.021± 0.020 | 0.016 ± 0.002 | 2.75 ± 0.20 | 4.80 ± 0.05 |
| Galantamine | 0.56 ± 0.12 | 5.000 ± 0.071 e | 12.0 ± 0.3 | 59.2 ± 1.7 e |
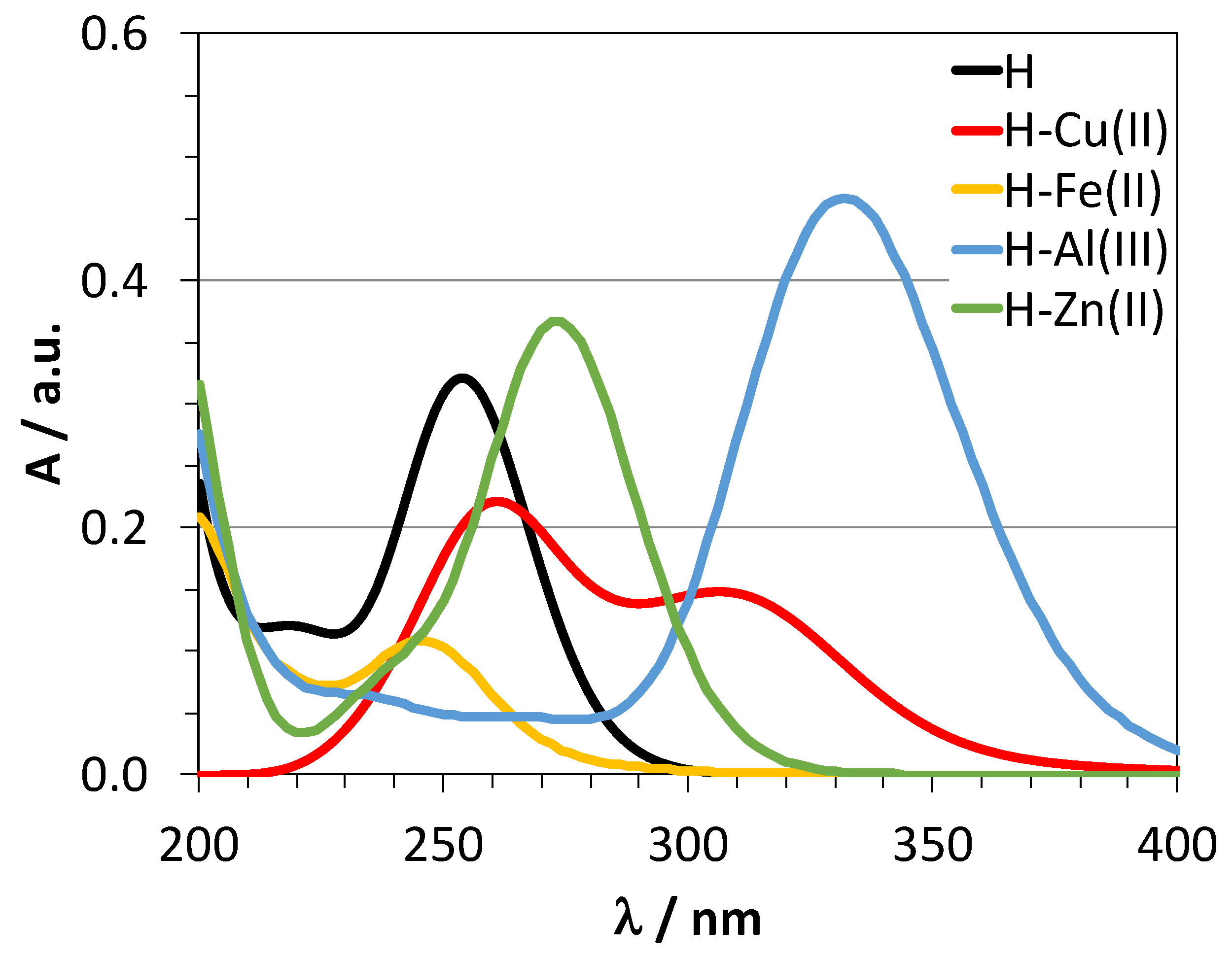
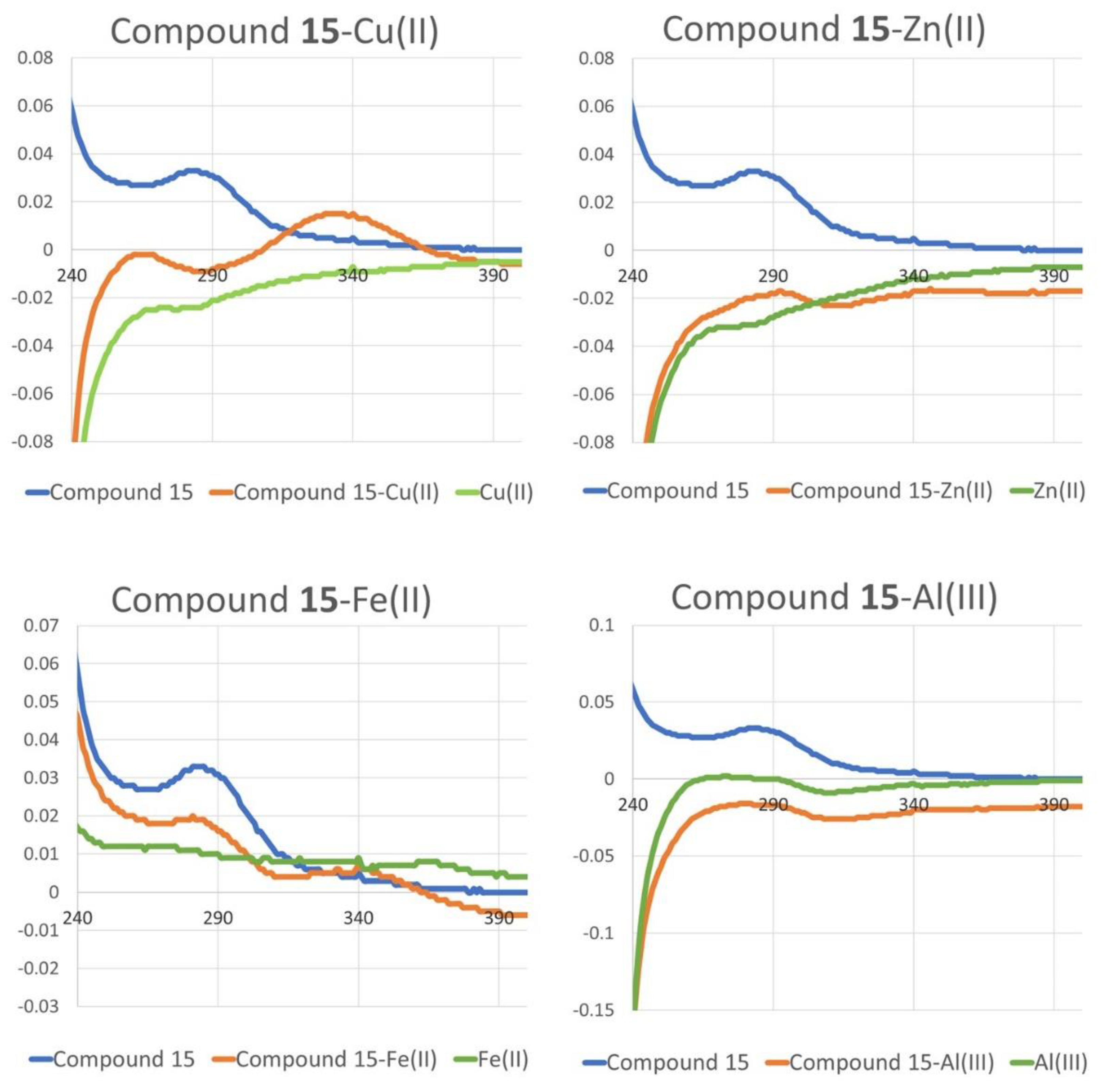
2.3. Chelating Activity
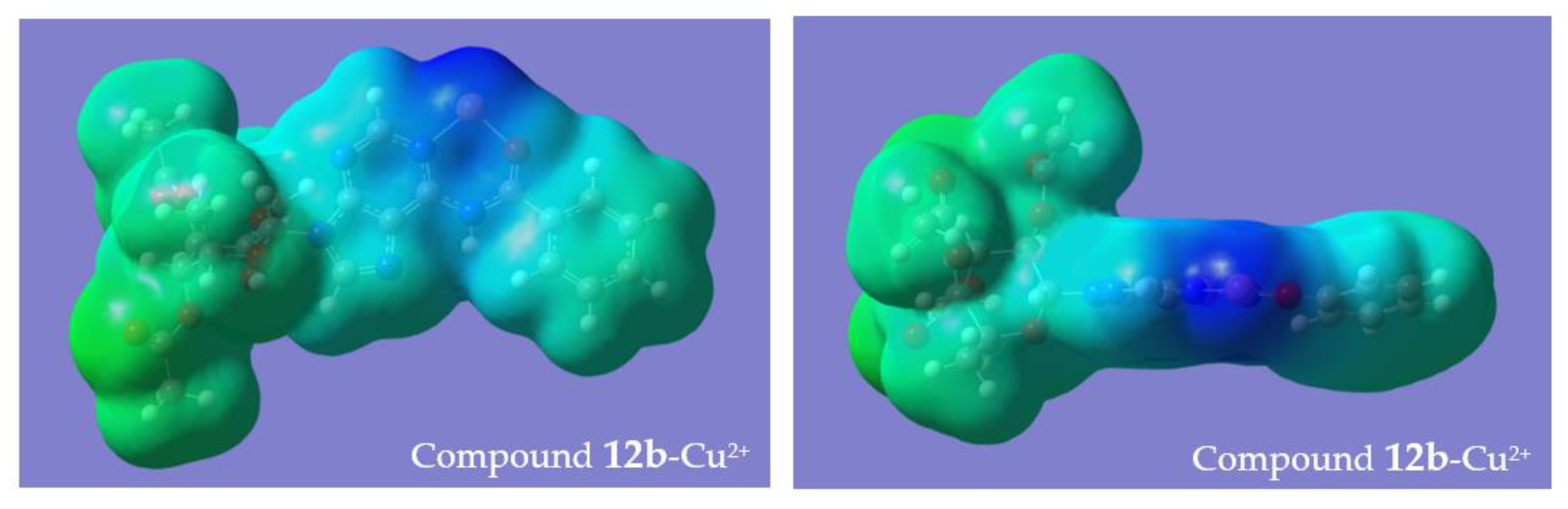
3. Discussion
4. Materials and Methods
4.1. Synthesis
4.2. Cholinesterase Inhibition Assay
4.3. UV-Visible Chelating Studies
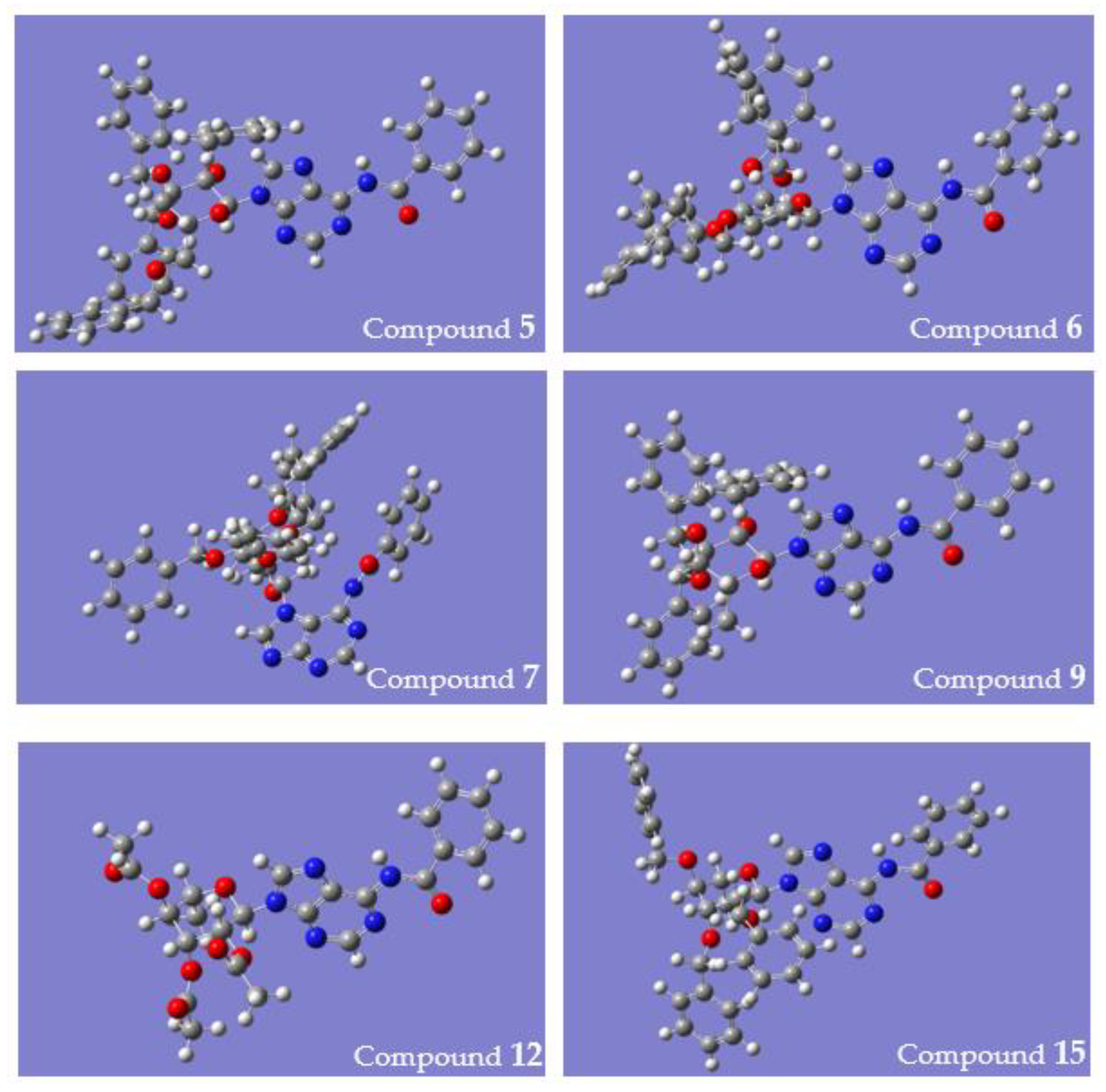
4.4. Computational Studies
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Polverino, A.; Grimaldi, M.; Sorrentino, P.; Jacini, F.; D’Ursi, A.M.; Sorrentino, G. Effects of Acetylcholine on β-Amyloid-Induced cPLA2, Activation in the TB Neuroectodermal Cell Line: Implications for the Pathogenesis of Alzheimer’s Disease. Cell Mol. Neurobiol. 2018, 38, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Bohnen, N.I.; Grothe, M.J.; Ray, N.J.; Müller, M.L.T.M.; Teipel, S.J. Recent Advances in Cholinergic Imaging and Cognitive Decline-Revisiting the Cholinergic Hypothesis of Dementia. Curr. Geriatr. Rep. 2018, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, H.; Chen, Y.; Sun, H. Recent progress in the identification of selective butyrylcholinesterase inhibitors for Alzheimer’s disease. Eur. J. Med. Chem. 2017, 132, 294–309. [Google Scholar] [CrossRef]
- Çokuğraş, A.N. Butyrylcholinesterase: Structure and Physiological Importance. Turk. J. Biochem. 2003, 28, 54–61. [Google Scholar]
- Macdonald, I.R.; Rockwood, K.; Martin, E.; Darvesh, S. Cholinesterase Inhibition in Alzheimer’s Disease: Is Specificity the Answer? J. Alzheimer’s Dis. 2014, 42, 379–384. [Google Scholar] [CrossRef]
- Sharma, K. Cholinesterase Inhibitors as Alzheimer’s Therapeutics. Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef]
- Li, D.-D.; Zhang, W.; Wang, Z.-Y.; Zhao, P. Serum Copper, Zinc, and Iron Levels in Patients with Alzheimer’s Disease: A Meta-Analysis of Case-Control Studies. Front. Aging Neurosci. 2017, 9, 300. [Google Scholar] [CrossRef]
- Exler, C.; Mold, M.J. Aluminium in human brain tissue: How much is too much? J. Biol. Inorg. Chem. 2019, 24, 1279–1282. [Google Scholar] [CrossRef]
- Weng, M.H.; Chen, S.Y.; Li, Z.Y.; Yen, G.C. Camellia oil alleviates the progression of Alzheimer’s disease in aluminum chloride-treated rats. Free Radic. Biol. Med. 2020, 152, 411–421. [Google Scholar] [CrossRef]
- Mutter, J.; Naumann, J.; Schneider, R.; Walach, H. Mercury and Alzheimer’s disease. Fortschr. Neurol. Psych. 2007, 75, 528–540. [Google Scholar] [CrossRef]
- Armstrong, R.A. Risk factors for Alzheimer’s disease. Folia Neuropathol. 2019, 57, 87–105. [Google Scholar] [CrossRef]
- Zafar, R.; Zubair, M.; Ali, S.; Shahid, K.; Waseem, W.; Naureen, H.; Haider, A.; Jan, M.S.; Ullah, F.; Sirajuddin, M.; et al. Zinc metal carboxylates as potential anti-Alzheimer’s candidate: In vitro anticholinesterase, antioxidant and molecular docking studies. J. Biomol. Struct. Dyn. 2020, 13, 1–11. [Google Scholar] [CrossRef]
- Nam, E.; Nam, G.; Lim, M.H. Synaptic Copper, Amyloid-β, and Neurotransmitters in Alzheimer’s Disease. Biochemistry 2020, 59, 15–17. [Google Scholar] [CrossRef]
- Yu, H.-J.; Zhao, W.; Zhou, Y.; Chenge, G.-J.; Sun, M.; Wang, L.; Yu, L.; Liang, S.H.; Ran, C. Salen-based bifunctional chemosensor for copper (II) ions: Inhibition of copper-induced amyloid-β aggregation. Anal. Chim. Acta 2020, 1097, 144–152. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, W.; Peng, J.; Yan, H.; Zhang, L.; Liu, X.; Zuo, Z. Design, synthesis and biological evaluation of novel copper-chelating acetylcholinesterase inhibitors with pyridine and N-benzylpiperidine fragments. Bioorg. Chem. 2019, 93, 103322. [Google Scholar] [CrossRef]
- Rana, M.; Sharma, A.K. Cu and Zn interactions with Aβ peptides:consequence of coordination on aggregation and formation of neurotoxic soluble Aβ oligomers. Metallomics 2019, 11, 64–84. [Google Scholar] [CrossRef]
- Lanza, V.; Milardi, D.; Di Natale, G.; Pappalardo, G. Repurposing of Copper (II)-chelating Drugs for the Treatment of Neurodegenerative Diseases. Curr. Med. Chem. 2018, 25, 525–539. [Google Scholar] [CrossRef]
- Gomes, L.M.F.; Bataglioli, J.C.; Storr, T. Metal complexes that bind to the amyloid-b peptide of relevance to Alzheimer’s disease. Coord. Chem. Rev. 2020, 412, 213255. [Google Scholar] [CrossRef]
- Schwarz, S.; Csuk, R.; Rauter, A.P. Microwave-assisted synthesis of novel purine nucleosides as selective cholinesterase inhibitors. Org. Biomol. Chem. 2014, 12, 2446–2456. [Google Scholar] [CrossRef]
- Milijkovic, M. Electrostatic and Stereoelectronic Effects in Carbohydrate Chemistry; Springer: Berlin/Heidelberg, Germany, 2014; pp. 181–196. [Google Scholar]
- Purgatorio, R.; de Candia, M.; Catto, M.; Carrieri, A.; Pisani, L.; De Palma, A.; Toma, M.; Ivanova, O.A.; Voskressensky, L.G.; Altomare, C.D. Investigating 1,2,3,4,5,6-hexahydroazepino[4,3-b]indole as scaffold of butyrylcholinesterase-selective inhibitors with additional neuroprotective activities for Alzheimer’s disease. Eur. J. Med. Chem. 2019, 177, 414–424. [Google Scholar] [CrossRef]
- Purgatorio, R.; de Candia, M.; Catto, M.; Rullo, M.; Pisani, L.; Denora, N.; Carrieri, A.; Nevskaya, A.A.; Voskressensky, L.G.; Altomare, C.D. Evaluation of Water-Soluble Mannich Base Prodrugs of 2,3,4,5-Tetrahydroazepino[4,3-b]indol-1(6H)-one as Multitarget-Directed Agents for Alzheimer’s Disease. Chem. Med. Chem. 2021, 16, 589–598. [Google Scholar] [CrossRef]
- De Candia, M.; Fiorella, F.; Lopopolo, G.; Carotti, A.; Romano, M.R.; Lograno, M.D.; Martel, S.; Carrupt, P.-A.; Belviso, B.D.; Caliandro, R.; et al. Synthesis and Biological Evaluation of Direct Thrombin Inhibitors Bearing 4-(Piperidin-1-yl)pyridine at the P1 Position with Potent Anticoagulant Activity. J. Med. Chem. 2013, 56, 8696–8711. [Google Scholar] [CrossRef]
- Marcelo, F.; Silva, F.V.M.; Goulart, M.; Justino, J.; Sinaÿ, P.; Bleriot, Y.; Rauter, A.P. Synthesis of novel purine nucleosides towards a selective inhibition of human butyrylcholinesterase. Bioorg. Med. Chem. 2009, 17, 5106–5116. [Google Scholar] [CrossRef]
- Rakonczay, Z. Potencies and selectivities of inhibitors of acetylcholinesterase and its molecular forms in normal and Alzheimer’s disease brain. Acta. Biol. Hung. 2003, 54, 183–189. [Google Scholar] [CrossRef]
- Yin, Q.; Chen, Q.; Lu, L.; Han, B. Sugar-based micro/mesoporous hypercross-linked polymers with in situ embedded silver nanoparticles for catalytic reduction. Beilstein J. Org. Chem. 2017, 13, 1212–1221. [Google Scholar] [CrossRef]
- Mathys, Z.K.; White, A.R. Copper and Alzheimer’s Disease. Adv. Neurobiol. 2017, 18, 199–216. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision, C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Yanai, T.; Tew, D.; Handy, N. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Scalmani, G.; Frisch, M.J. Continuous surface charge polarizable continuum models of solvation. I. General formalism. J. Chem. Phys. 2010, 132, 114110. [Google Scholar] [CrossRef]
- Lodewyk, M.W.; Siebert, M.R.; Tantillo, D.J. Computational Prediction of 1H and 13C Chemical Shifts: A Useful Tool for Natural Product, Mechanistic, and Synthetic Organic Chemistry. Chem. Rev. 2012, 112, 1839–1862. [Google Scholar] [CrossRef]
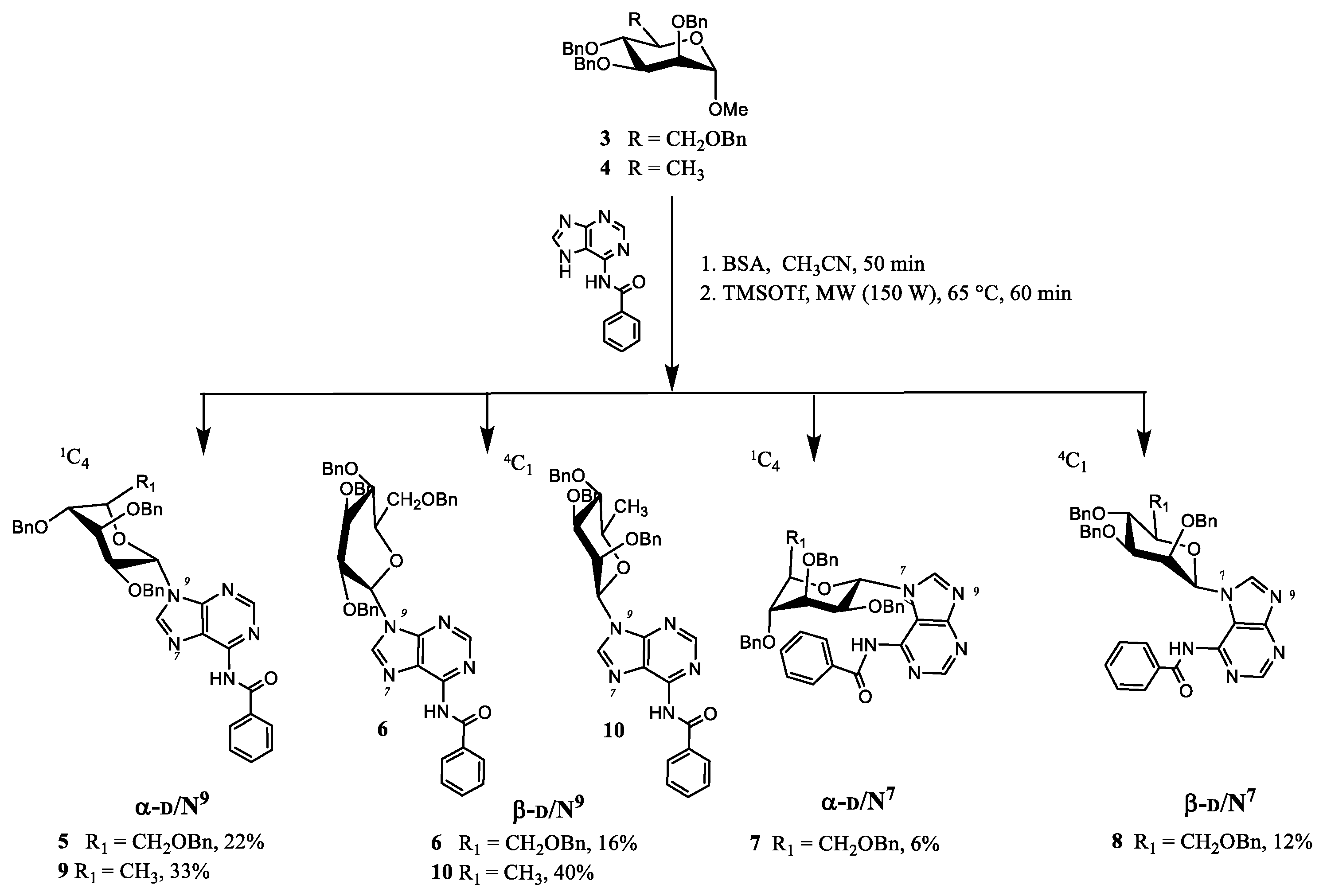

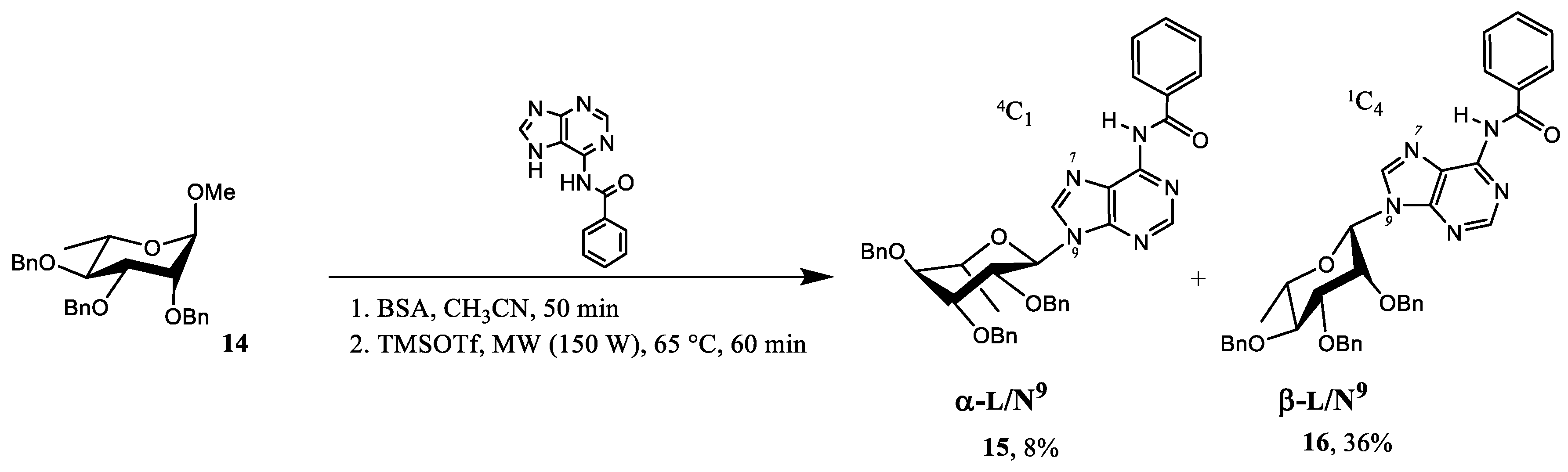




| Compound nr. | N7/N9 Purine Ligation | α,β Anomer | Glycone | Isolated Yield (%) |
|---|---|---|---|---|
| 5 | N9 | α | 2,3,4,6-Tetra-O-benzyl-α-d-mannosyl | 22 |
| 6 | N9 | β | 2,3,4,6-Tetra-O-benzyl-β-d-mannosyl | 16 |
| 7 | N7 | α | 2,3,4,6-Tetra-O-benzyl-α-d-mannosyl | 6 |
| 8 | N7 | β | 2,3,4,6-Tetra-O-benzyl-β-d-mannosyl | 12 |
| 9 | N9 | α | 2,3,4-Tri-O-benzyl-6-deoxy-α-d-mannosyl | 33 |
| 10 | N9 | β | 2,3,4-Tri-O-benzyl-6-deoxy-β-d-mannosyl | 40 |
| 12 | N9 | α | 2,3,4-tri-O-acetyl-6-deoxy-α-l-mannosyl | 63 |
| 13 | N9 | α | 2,3,4-tri-O-benzyl-6-deoxy-α-l-mannosyl a | 15 |
| 15 | N9 | α | 2,3,4-tri-O-benzyl-6-deoxy-α-l-mannosyl | 8 |
| 16 | N9 | β | 2,3,4-tri-O-benzyl-6-deoxy-β-l-mannosyl | 36 |
| Anomer | Compounds 12 (α Anomer) and 12a (β Anomer) | ||
|---|---|---|---|
| E/kcal·mol−1 | ΔE (α-β) | Boltzmann Population | |
| α | −1,132,494.89 | −6.30 | 1.00 |
| β | −1,132,488.59 | 0.00 | |
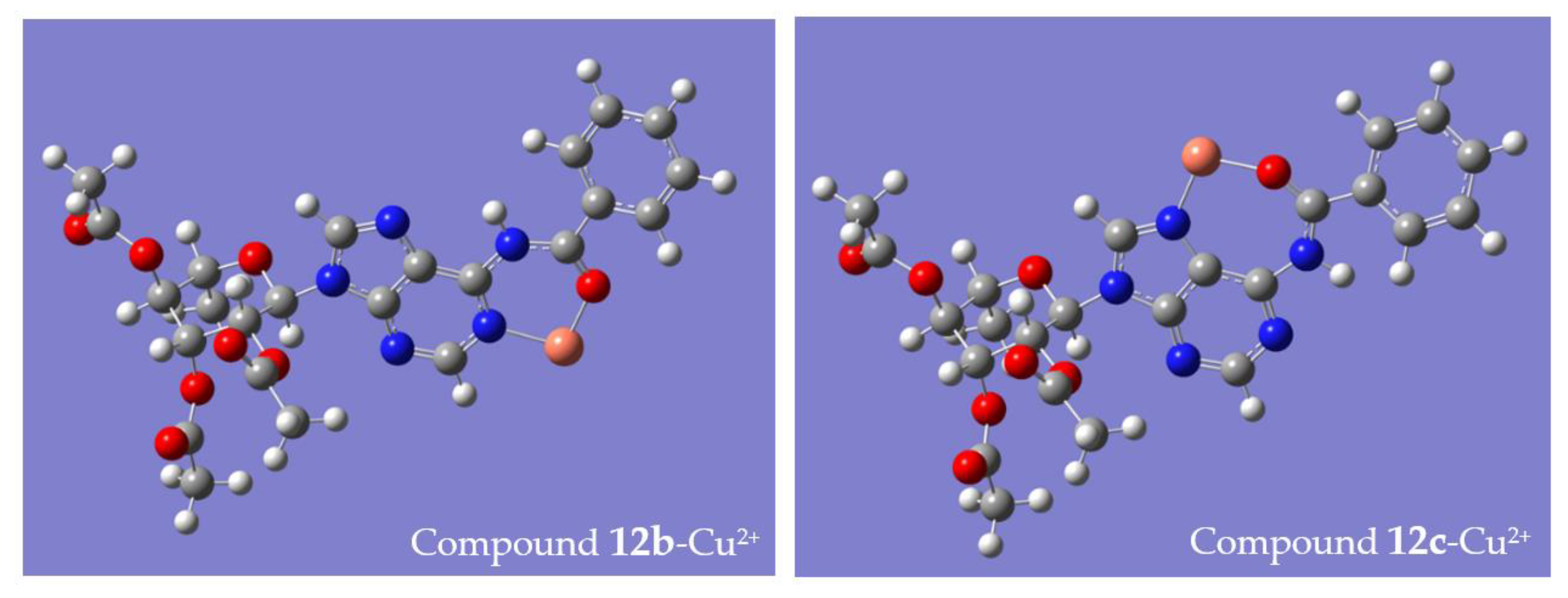
| Conformer-Cu2+ | Complex 12-Cu2+ | ||
|---|---|---|---|
| E/kcal·mol−1 | ΔE(12a–12b)/kcal·mol−1 | Boltzmann Population | |
| 12b-Cu2+ | −2,161,780.22 | −0.99 | 0.84 |
| 12c-Cu2+ | −2,161,779.23 | 0.16 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schino, I.; Cantore, M.; de Candia, M.; Altomare, C.D.; Maria, C.; Barros, J.; Cachatra, V.; Calado, P.; Shimizu, K.; Freitas, A.A.; et al. Exploring Mannosylpurines as Copper Chelators and Cholinesterase Inhibitors with Potential for Alzheimer’s Disease. Pharmaceuticals 2023, 16, 54. https://doi.org/10.3390/ph16010054
Schino I, Cantore M, de Candia M, Altomare CD, Maria C, Barros J, Cachatra V, Calado P, Shimizu K, Freitas AA, et al. Exploring Mannosylpurines as Copper Chelators and Cholinesterase Inhibitors with Potential for Alzheimer’s Disease. Pharmaceuticals. 2023; 16(1):54. https://doi.org/10.3390/ph16010054
Chicago/Turabian StyleSchino, Ignazio, Mariangela Cantore, Modesto de Candia, Cosimo D. Altomare, Catarina Maria, João Barros, Vasco Cachatra, Patrícia Calado, Karina Shimizu, Adilson A. Freitas, and et al. 2023. "Exploring Mannosylpurines as Copper Chelators and Cholinesterase Inhibitors with Potential for Alzheimer’s Disease" Pharmaceuticals 16, no. 1: 54. https://doi.org/10.3390/ph16010054
APA StyleSchino, I., Cantore, M., de Candia, M., Altomare, C. D., Maria, C., Barros, J., Cachatra, V., Calado, P., Shimizu, K., Freitas, A. A., Oliveira, M. C., Ferreira, M. J., Lopes, J. N. C., Colabufo, N. A., & Rauter, A. P. (2023). Exploring Mannosylpurines as Copper Chelators and Cholinesterase Inhibitors with Potential for Alzheimer’s Disease. Pharmaceuticals, 16(1), 54. https://doi.org/10.3390/ph16010054










