Hemodynamic and Rhythmologic Effects of Push-Dose Landiolol in Critical Care—A Retrospective Cross-Sectional Study
Abstract
1. Introduction and Literature Review
1.1. A Favourable Pharmacological Profile
1.2. Effects on Morbidity and Mortality
1.3. Landiolol and Inflammation
1.4. Bolus Application
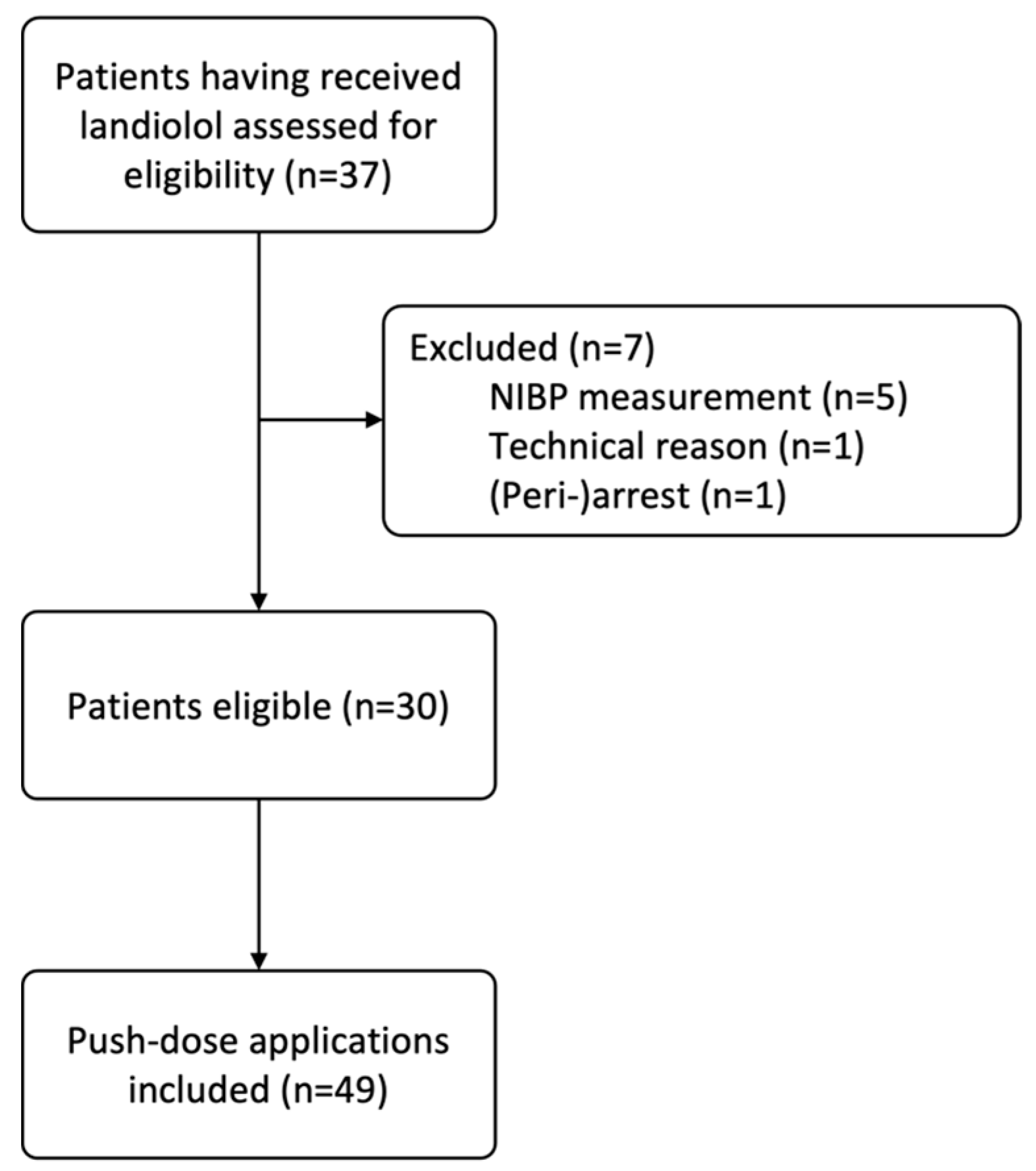
2. Materials and Methods
2.1. Study Population
2.2. Data Acquisition
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Patients and Boli
3.2. Outcomes
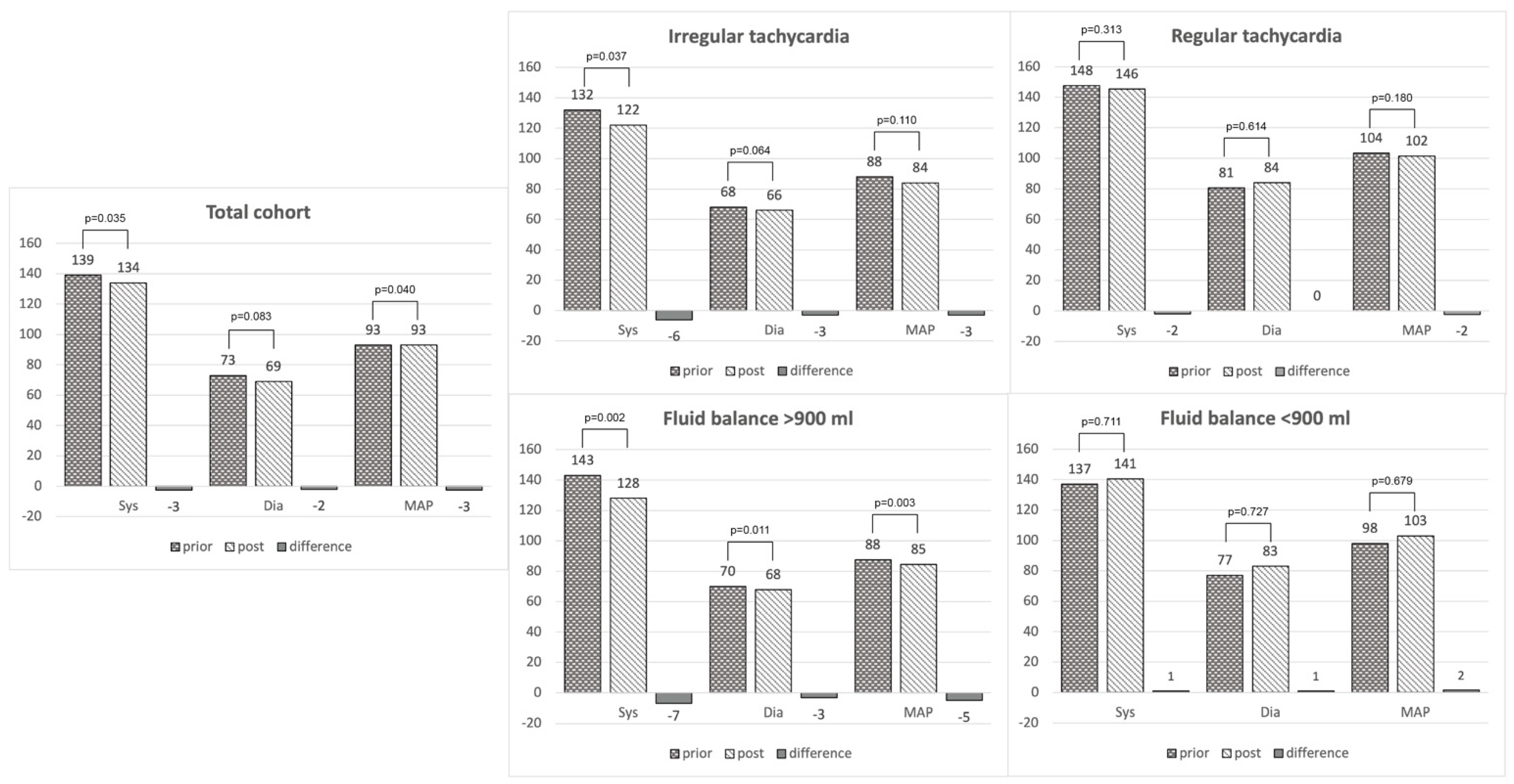
3.2.1. Heart Rate
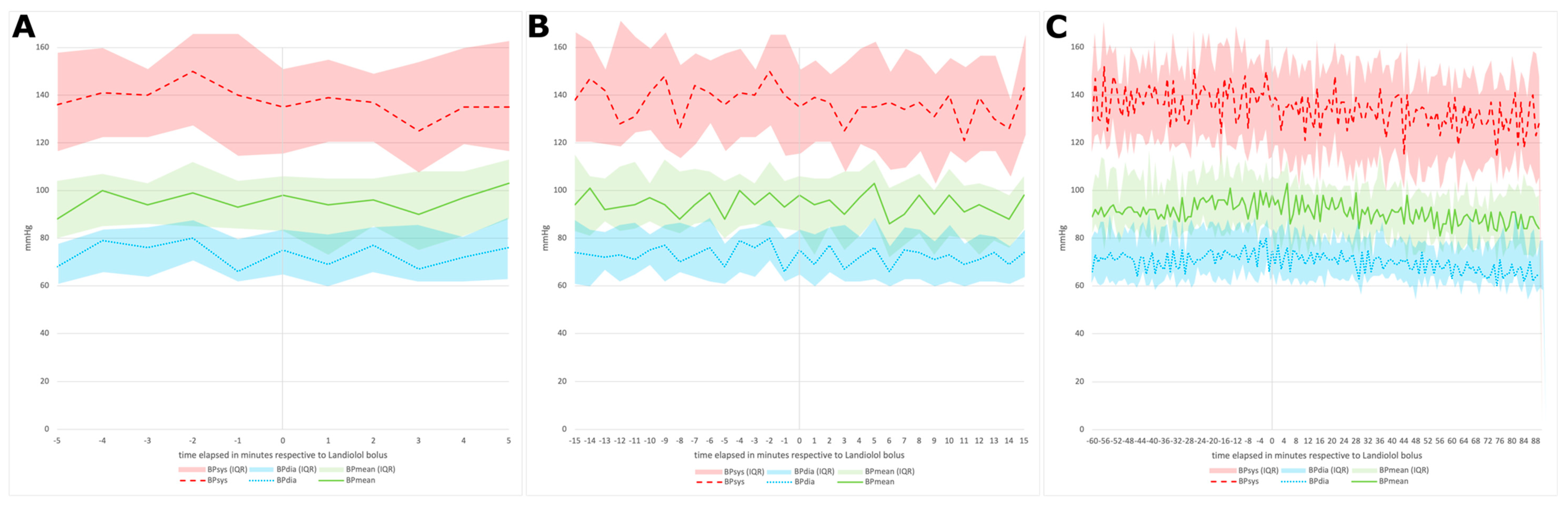
3.2.2. Blood Pressure
3.2.3. Respiration
3.2.4. Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atarashi, H.; Kuruma, A.; Yashima, M.; Saitoh, H.; Ino, T.; Endoh, Y.; Hayakawa, H. Pharmacokinetics of Landiolol hydrochloride, a new ultra-short-acting beta-blocker, in patients with cardiac arrhythmias. Clin. Pharmacol. Ther. 2000, 68, 143–150. [Google Scholar] [CrossRef]
- Krumpl, G.; Ulc, I.; Trebs, M.; Kadlecová, P.; Hodisch, J. Pharmacokinetics and pharmacodynamics of two different Landiolol formulations in a healthy Caucasian group. Eur. J. Pharm. Sci. 2016, 92, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Krumpl, G.; Ulc, I.; Trebs, M.; Kadlecová, P.; Hodisch, J.; Maurer, G.; Husch, B. Pharmacokinetics and Pharmacodynamics of Low-, Intermediate-, and High-Dose Landiolol and Esmolol During Long-Term Infusion in Healthy Whites. J. Cardiovasc. Pharmacol. 2018, 71, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, S.; Iwamura, H.; Nishizaki, M.; Hayashi, A.; Senokuchi, K.; Kobayashi, K.; Sakaki, K.; Hachiya, K.; Ichioka, Y.; Kawamura, M. Development of a highly cardioselective ultra short-acting beta-blocker, ONO-1101. Chem. Pharm. Bull. 1992, 40, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Plosker, G.L. Landiolol: A review of its use in intraoperative and postoperative tachyarrhythmias. Drugs 2013, 73, 959–977. [Google Scholar] [CrossRef]
- Hasuo, H.; Tomiyasu, S.; Hojo, M.; Fujigaki, T.; Fukusaki, M.; Sumikawa, K. Effect of ONO-1101, a novel short-acting β-blocker on hemodynamic responses to isoflurane inhalation and tracheal intubation. J. Anesth. 1998, 12, 115–118. [Google Scholar] [CrossRef]
- Summary of Product Characteristics of Rapibloc. Available online: https://mri.cts-mrp.eu/Human/Downloads/NL_H_3368_002_FinalSPC.pdf (accessed on 21 February 2022).
- AOP Orphan Pharmaceuticals AG Announces European “Approvable” Opinion of Its Ultra-Short Acting Beta Blocker Rapibloc® (Landiolol). Available online: https://www.aop-health.com/global_en/our-company/newsroom-archive/aop-orphan-pharmaceuticals-ag-announces-european-approvable-opinion-of-its-ultra-short-acting-beta-blocker-rapibloc-r-Landiolol (accessed on 21 February 2022).
- Nasrollahi-Shirazi, S.; Sucic, S.; Yang, Q.; Freissmuth, M.; Nanoff, C. Comparison of the β-Adrenergic Receptor Antagonists Landiolol and Esmolol: Receptor Selectivity, Partial Agonism, and Pharmacochaperoning Actions. J. Pharmacol. Exp. Ther. 2016, 359, 73–81. [Google Scholar] [CrossRef]
- Sasao, J.; Tarver, S.D.; Kindscher, J.D.; Taneyama, C.; Benson, K.T.; Goto, H. In rabbits, Landiolol, a new ultra-short-acting beta-blocker, exerts a more potent negative chronotropic effect and less effect on blood pressure than esmolol. Can. J. Anaesth. 2001, 48, 985–989. [Google Scholar] [CrossRef]
- Frishman, W.H. Beta-adrenergic receptor blockers. Adverse effects and drug interactions. Hypertension 1988, 11, Ii21–Ii29. [Google Scholar] [CrossRef]
- Poirier, L.; Tobe, S.W. Contemporary Use of β-Blockers: Clinical Relevance of Subclassification. Can. J. Cardiol. 2014, 30, S9–S15. [Google Scholar] [CrossRef]
- Kakuta, N.; Kawano, T.; Tanaka, K.; Oshita, S. A comparison of Landiolol and esmolol for attenuation of cardiovascular response and plasma renin activity against tracheal intubation with laryngoscopy. Anesthesiology 2005, 103, A433. [Google Scholar]
- Krumpl, G.; Ulč, I.; Trebs, M.; Kadlecová, P.; Hodisch, J. Pharmacodynamic and pharmacokinetic behavior of Landiolol during dobutamine challenge in healthy adults. BMC Pharmacol. Toxicol. 2020, 21, 82. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Okamoto, Y.; Endo, S.; Ono, K. Direct effects of esmolol and Landiolol on cardiac function, coronary vasoactivity, and ventricular electrophysiology in guinea-pig hearts. J. Pharmacol. Sci. 2012, 118, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Gibb, S.; Rehberg, S. The role of the ultra short-acting β1-adrenoreceptor antagonist Landiolol in the treatment of atrial fibrillation: Pharmacology, clinical application and current evidence in anaesthesiology, intensive care and emergency medicine. Anästhesiologie Intensivmed. 2018, 59, 407–421. [Google Scholar]
- Krumpl, G.; Ulc, I.; Trebs, M.; Kadlecová, P.; Hodisch, J. Bolus application of Landiolol and esmolol: Comparison of the pharmacokinetic and pharmacodynamic profiles in a healthy Caucasian group. Eur. J. Clin. Pharmacol. 2017, 73, 417–428. [Google Scholar] [CrossRef]
- Patel, P.A.; Tilley, D.G.; Rockman, H.A. Beta-arrestin-mediated signaling in the heart. Circ. J. 2008, 72, 1725–1729. [Google Scholar] [CrossRef]
- Whalen, E.J.; Rajagopal, S.; Lefkowitz, R.J. Therapeutic potential of β-arrestin- and G protein-biased agonists. Trends Mol. Med. 2011, 17, 126–139. [Google Scholar] [CrossRef]
- Lips, D.J.; Bueno, O.F.; Wilkins, B.J.; Purcell, N.H.; Kaiser, R.A.; Lorenz, J.N.; Voisin, L.; Saba-El-Leil, M.K.; Meloche, S.; Pouysségur, J.; et al. MEK1-ERK2 signaling pathway protects myocardium from ischemic injury in vivo. Circulation 2004, 109, 1938–1941. [Google Scholar] [CrossRef]
- Kovacs, K.; Hanto, K.; Bognar, Z.; Tapodi, A.; Bognar, E.; Kiss, G.N.; Szabo, A.; Rappai, G.; Kiss, T.; Sumegi, B.; et al. Prevalent role of Akt and ERK activation in cardioprotective effect of Ca2+ channel- and beta-adrenergic receptor blockers. Mol. Cell. Biochem. 2009, 321, 155–164. [Google Scholar] [CrossRef]
- Domanovits, H.; Wolzt, M.; Stix, G. Landiolol: Pharmacology and its use for rate control in atrial fibrillation in an emergency setting. Eur. Heart J. Suppl. 2018, 20, A1–A32018. [Google Scholar] [CrossRef]
- Matsuishi, Y.; Mathis, B.J.; Shimojo, N.; Kawano, S.; Inoue, Y. Evaluating the Therapeutic Efficacy and Safety of Landiolol Hydrochloride for Management of Arrhythmia in Critical Settings: Review of the Literature. Vasc. Health Risk Manag. 2020, 16, 111–123. [Google Scholar] [CrossRef]
- Kakihana, Y.; Nishida, O.; Taniguchi, T.; Okajima, M.; Morimatsu, H.; Ogura, H.; Yamada, Y.; Nagano, T.; Morishima, E.; Matsuda, N. Efficacy and safety of Landiolol, an ultra-short-acting β1-selective antagonist, for treatment of sepsis-related tachyarrhythmia (J-Land 3S): A multicentre, open-label, randomised controlled trial. Lancet Respir. Med. 2020, 8, 863–872. [Google Scholar] [CrossRef]
- Suzuki, K.; Numaguchi, A.; Adachi, Y.U.; Obata, Y.; Hatano, T.; Ejima, T.; Sato, S.; Matsuda, N. Continuous administration of Landiolol reduced QT dispersion in postoperative patients. J. Clin. Anesth. 2014, 26, 438–442. [Google Scholar] [CrossRef]
- Karle, C.A.; Zitron, E.; Zhang, W.; Kathöfer, S.; Schoels, W.; Kiehn, J. Rapid component IKr of the guinea-pig cardiac delayed rectifier K+ current is inhibited by β1-adrenoreceptor activation, via cAMP/protein kinase A-dependent pathways. Cardiovasc. Res. 2002, 53, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Matsura, M.; Fujiwara, Y.; Ito, H.; Kandatsu, N.; Kato, N.; Harada, J.; Komatsu, T. Prolongation of QT Interval Induced by Electroconvulsive Therapy is Attenuated by Landiolol. J. ECT 2010, 26, 37–40. [Google Scholar] [CrossRef]
- Rehberg, S.; Joannidis, M.; Whitehouse, T.; Morelli, A. Landiolol for managing atrial fibrillation in intensive care. Eur. Heart J. Suppl. 2018, 20, A15–A18. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Terajima, K.; Sato, C.; Akada, S.; Miyagi, Y.; Hongo, T.; Takeda, S.; Tanaka, K.; Sakamoto, A. Clinical role and efficacy of Landiolol in the intensive care unit. J. Anesth. 2008, 22, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Okajima, M.; Takamura, M.; Taniguchi, T. Landiolol, an ultra-short-acting β1-blocker, is useful for managing supraventricular tachyarrhythmias in sepsis. World J. Crit. Care Med. 2015, 4, 251–257. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2020, 42, 373–498. [Google Scholar] [CrossRef]
- Yamashita, T.; Nakasu, Y.; Mizutani, H.; Sumitani, K. A prospective observational survey on Landiolol in atrial fibrillation/atrial flutter patients with chronic heart failure—AF-CHF Landiolol survey. J. Cardiol. 2019, 74, 418–425. [Google Scholar] [CrossRef]
- Nagai, R.; Kinugawa, K.; Inoue, H.; Atarashi, H.; Seino, Y.; Yamashita, T.; Shimizu, W.; Aiba, T.; Kitakaze, M.; Sakamoto, A.; et al. Urgent management of rapid heart rate in patients with atrial fibrillation/flutter and left ventricular dysfunction: Comparison of the ultra-short-acting β1-selective blocker Landiolol with digoxin (J-Land Study). Circ. J. 2013, 77, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ai, Q.; Lin, L.; Ge, P.; Yang, C.; Zhang, L. Efficacy and safety of Landiolol for prevention of atrial fibrillation after cardiac surgery: A meta-analysis of randomized controlled trials. Int. J. Clin. Exp. Med. 2015, 8, 10265–10273. [Google Scholar] [PubMed]
- Kinugawa, K.; Nagai, R.; Inoue, H.; Atarashi, H.; Seino, Y.; Yamashita, T.; Shimizu, W.; Aiba, T.; Kitakaze, M.; Sakamoto, A.; et al. Impacts of Patient Characteristics on the Effectiveness of Landiolol in AF/AFL Patients Complicated with LV Dysfunction: Subgroup Analysis of the J-Land Study. Adv. Ther. 2014, 31, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Syed, Y.Y. Landiolol: A Review in Tachyarrhythmias. Drugs 2018, 78, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Shiga, T.; Ikeda, T.; Shimizu, W.; Kinugawa, K.; Sakamoto, A.; Nagai, R.; Daimon, T.; Oki, K.; Okamoto, H.; Yamashita, T. Efficacy and Safety of Landiolol in Patients with Ventricular Tachyarrhythmias with or without Renal Impairment—Subanalysis of the J-Land II Study. Circ. Rep. 2020, 2, 440–445. [Google Scholar] [CrossRef]
- Takahata, T.; Yasui-Furukori, N.; Sakamoto, J.; Suto, K.; Suto, T.; Tateishi, T.; Munakata, A. Influence of hepatic impairment on the pharmacokinetics and pharmacodynamics of Landiolol hydrochloride, an ultra-short-acting beta1-blocker. Drugs R D 2005, 6, 385–394. [Google Scholar] [CrossRef]
- Matsuda, N.; Nishida, O.; Taniguchi, T.; Okajima, M.; Morimatsu, H.; Ogura, H.; Yamada, Y.; Nagano, T.; Ichikawa, A.; Kakihana, Y. Impact of patient characteristics on the efficacy and safety of Landiolol in patients with sepsis-related tachyarrhythmia: Subanalysis of the J-Land 3S randomised controlled study. eClinicalMedicine 2020, 28, 100571. [Google Scholar] [CrossRef]
- Tamura, T.; Yatabe, T.; Yokoyama, M. Prevention of atrial fibrillation after cardiac surgery using low-dose Landiolol: A systematic review and meta-analysis. J. Clin. Anesth. 2017, 42, 1–6. [Google Scholar] [CrossRef]
- Hao, J.; Zhou, J.; Xu, W.; Chen, C.; Zhang, J.; Peng, H.; Liu, L. Beta-Blocker Landiolol Hydrochloride in Preventing Atrial Fibrillation Following Cardiothoracic Surgery: A Systematic Review and Meta-Analysis. Ann. Thorac. Cardiovasc. Surg. 2022, 28, 18–31. [Google Scholar] [CrossRef]
- Ikeda, T.; Shiga, T.; Shimizu, W.; Kinugawa, K.; Sakamoto, A.; Nagai, R.; Daimon, T.; Oki, K.; Okamoto, H.; Yamashita, T. Efficacy and Safety of the Ultra-Short-Acting β1-Selective Blocker Landiolol in Patients With Recurrent Hemodynamically Unstable Ventricular Tachyarrhymias—Outcomes of J-Land II Study. Circ. J. 2019, 83, 1456–1462. [Google Scholar] [CrossRef]
- Ditali, V.; Garatti, L.; Morici, N.; Villanova, L.; Colombo, C.; Oliva, F.; Sacco, A. Effect of Landiolol in patients with tachyarrhythmias and acute decompensated heart failure (ADHF): A case series. ESC Heart Fail. 2022, 9, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, S.; Iwasaka, H.; Maeda, H.; Noguchi, T. Landiolol, an ultrashort-acting beta1-adrenoceptor antagonist, has protective effects in an LPS-induced systemic inflammation model. Shock 2009, 31, 515–520. [Google Scholar] [CrossRef]
- Hasegawa, D.; Sato, R.; Prasitlumkum, N.; Nishida, K.; Takahashi, K.; Yatabe, T.; Nishida, O. Effect of Ultrashort-Acting β-Blockers on Mortality in Patients With Sepsis With Persistent Tachycardia Despite Initial Resuscitation: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Chest 2021, 159, 2289–2300. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Suzuki, Y.; Okuda, J.; Kurazumi, T.; Suhara, T.; Ueda, T.; Nagata, H.; Morisaki, H. Sepsis-induced cardiac dysfunction and β-adrenergic blockade therapy for sepsis. J. Intensive Care 2017, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Harasawa, R.; Hayashi, Y.; Iwasaki, M.; Kamibayashi, T.; Mashimo, T. Bolus administration of Landiolol, a short-acting, selective beta1-blocker, to treat tachycardia during anesthesia: A dose-dependent study. J. Cardiothorac. Vasc. Anesth. 2006, 20, 793–795. [Google Scholar] [CrossRef]
- Kinoshita, H.; Kakutani, T.; Mizumoto, K.; Hatano, Y. Effectiveness of bolus Landiolol on paroxysmal atrial tachycardia. Can. J. Anaesth. 2005, 52, 999–1000. [Google Scholar] [CrossRef]
- Osawa, K.; Miyoshi, T.; Sato, S.; Akagi, N.; Morimitsu, Y.; Nakamura, K.; Kohno, K.; Kusano, K.; Kanazawa, S.; Ito, H. Safety and efficacy of a bolus injection of Landiolol hydrochloride as a premedication for multidetector-row computed tomography coronary angiography. Circ. J. 2013, 77, 146–152. [Google Scholar] [CrossRef]
- Stix, G.; Wolzt, M.; Domanovits, H.; Kadlecová, P.; Husch, B.; Trebs, M.; Hodisch, J.; Unger, M.; Krumpl, G. Open-Label Two-Dose Pilot Study of Landiolol for the Treatment of Atrial Fibrillation/Atrial Flutter in Caucasian Patients. Circ. J. 2019, 84, 33–42. [Google Scholar] [CrossRef]
- Joannidis, M.; Druml, W.; Forni, L.G.; Groeneveld, A.B.J.; Honore, P.M.; Hoste, E.; Ostermann, M.; Oudemans-van Straaten, H.M.; Schetz, M. Prevention of acute kidney injury and protection of renal function in the intensive care unit: Update 2017: Expert opinion of the Working Group on Prevention, AKI section, European Society of Intensive Care Medicine. Intensive Care Med. 2017, 43, 730–749. [Google Scholar] [CrossRef]
- Salahuddin, N.; Sammani, M.; Hamdan, A.; Joseph, M.; Al-Nemary, Y.; Alquaiz, R.; Dahli, R.; Maghrabi, K. Fluid overload is an independent risk factor for acute kidney injury in critically Ill patients: Results of a cohort study. BMC Nephrol. 2017, 18, 45. [Google Scholar] [CrossRef]
- Lee, J.; de Louw, E.; Niemi, M.; Nelson, R.; Mark, R.G.; Celi, L.A.; Mukamal, K.J.; Danziger, J. Association between fluid balance and survival in critically ill patients. J. Intern. Med. 2015, 277, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-J.; Pai, K.-C.; Huang, C.-T.; Wong, L.-T.; Wang, M.-S.; Lai, C.-M.; Chen, C.-H.; Wu, C.-L.; Chao, W.-C. A Positive Fluid Balance in the First Week Was Associated With Increased Long-Term Mortality in Critically Ill Patients: A Retrospective Cohort Study. Front. Med. 2022, 9, 727103. [Google Scholar] [CrossRef]
- Cordemans, C.; De laet, I.; Van Regenmortel, N.; Schoonheydt, K.; Dits, H.; Huber, W.; Malbrain, M.L.N.G. Fluid management in critically ill patients: The role of extravascular lung water, abdominal hypertension, capillary leak, and fluid balance. Ann. Intensive Care 2012, 2, S12012. [Google Scholar] [CrossRef]
- Anesi, G.L.; Liu, V.X.; Chowdhury, M.; Small, D.S.; Wang, W.; Delgado, M.K.; Bayes, B.; Dress, E.; Escobar, G.J.; Halpern, S.D. Association of ICU Admission and Outcomes in Sepsis and Acute Respiratory Failure. Am. J. Respir. Crit. Care Med. 2022, 205, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Tseng, K.L.; Ho, C.H.; Chiang, S.R.; Chen, C.M.; Chan, K.S.; Chao, C.M.; Hsing, S.C.; Cheng, K.C. Prognosis of patients with acute respiratory failure and prolonged intensive care unit stay. J. Thorac. Dis. 2019, 11, 2051–2057. [Google Scholar] [CrossRef] [PubMed]
- Coppola, S.; Froio, S.; Chiumello, D. β-blockers in critically ill patients: From physiology to clinical evidence. Crit. Care 2015, 19, 119. [Google Scholar] [CrossRef]
- Kargin, F.; Takir, H.B.; Salturk, C.; Goksenoglu, N.C.; Karabay, C.Y.; Mocin, O.Y.; Adiguzel, N.; Gungor, G.; Balci, M.K.; Yalcinsoy, M.; et al. The safety of beta-blocker use in chronic obstructive pulmonary disease patients with respiratory failure in the intensive care unit. Multidiscip. Respir. Med. 2014, 9, 8. [Google Scholar] [CrossRef]
- Salpeter, S.R.; Ormiston, T.M.; Salpeter, E.E.; Poole, P.J.; Cates, C.J. Cardioselective beta-blockers for chronic obstructive pulmonary disease: A meta-analysis. Respir. Med. 2003, 97, 1094–1101. [Google Scholar] [CrossRef]
- Aboab, J.; Sebille, V.; Jourdain, M.; Mangalaboyi, J.; Gharbi, M.; Mansart, A.; Annane, D. Effects of esmolol on systemic and pulmonary hemodynamics and on oxygenation in pigs with hypodynamic endotoxin shock. Intensive Care Med. 2011, 37, 1344–1351. [Google Scholar] [CrossRef]
- Walter, E.; Heringlake, M. Cost-Effectiveness Analysis of Landiolol, an Ultrashort-Acting Beta-Blocker, for Prevention of Postoperative Atrial Fibrillation for the Germany Health Care System. J. Cardiothorac. Vasc. Anesth. 2020, 34, 888–897. [Google Scholar] [CrossRef]
- Brugada, J.; Katritsis, D.G.; Arbelo, E.; Arribas, F.; Bax, J.J.; Blomström-Lundqvist, C.; Calkins, H.; Corrado, D.; Deftereos, S.G.; Diller, G.-P.; et al. 2019 ESC Guidelines for the management of patients with supraventricular tachycardiaThe Task Force for the management of patients with supraventricular tachycardia of the European Society of Cardiology (ESC): Developed in collaboration with the Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2019, 41, 655–720. [Google Scholar] [CrossRef]
| (n = Patients) | Total | Male | Female | p-Value | RT | IRT | p-Value | Fluid Balance < 900 mL | Fluid Balance > 900 mL | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| N (% of total) | 30 | 17 (56.7) | 13 (43.3) | 16 (53.3) | 14 (46.7) | 14 (46.7) | 15 (50.0) | |||
| Male sex, n (%) | 17 (56.7) | 11 (68.8) | 6 (42.9) | 0.153 | 9 (64.3) | 7 (46.7) | 0.340 | |||
| Age, years (IQR) | 67 (55–72) | 67 (55–71) | 67 (55–77) | 0.630 | 66 (53–73) | 70 (58–72) | 0.492 | 59 (44–71) | 69 (62–77) | 0.066 |
| BMI, kg/m2 (IQR) | 29 (23–31) | 24.2 (22.7–30.1) | 30.5 (29.4–36.2) | 0.038 | 28.9 (23.1–35.3) | 29.3 (22.5–30.6) | 0.406 | 28.3 (23.2–37.7) | 29.4 (22.7–30.9) | 0.458 |
| Height, cm (IQR) | 171 (165–180) | 180 (171–182) | 165 (160–170) | <0.001 | 177 (169–182) | 169 (162–180) | 0.086 | 178 (165–182) | 170 (164–180) | 0.282 |
| Weight, kg (IQR) | 80 (70–96) | 80 (70.0–97.5) | 85 (71.5–95.0) | 0.629 | 87.5 (71.3–107.5) | 78.5 (64.8–90.0) | 0.095 | 85.0 (70.0–111.8) | 80 (75–90) | 0.405 |
| Comorbidities | ||||||||||
| AHTN, n (%) | 12 (40) | 5 (29.4) | 7 (53.8) | 0.176 | 7 (43.8) | 5 (35.7) | 0.654 | 6 (42.9) | 6 (40.0) | 0.876 |
| HLP, n (%) | 3 (10) | 2 (11.8) | 1 (7.7) | 0.713 | 3 (18.8) | 0 (0) | 0.228 | 2 (14.3) | 1 (6.7) | 0.598 |
| DM II, n (%) | 10 (33.3) | 4 (23.5) | 6 (46.2) | 0.255 | 6 (37.5) | 4 (28.6) | 0.709 | 5 (35.7) | 5 (33.3) | 1.000 |
| CAD, n (%) | 6 (20.0) | 3 (17.6) | 3 (23.1) | 1.000 | 3 (18.8 | 3 (21.4) | 1.000 | 2 (14.3) | 4 (26.7) | 0.651 |
| CKI, n (%) | 4 (13.3) | 1 (5.9) | 3 (23.1) | 0.290 | 1 (6.3) | 3 (21.4) | 0.315 | 3 (21.4) | 1 (6.7) | 0.330 |
| PAD, n (%) | 2 (6.7) | 0 (0) | 2 (15.4) | 0.179 | 1 (6.3) | 1 (7.1) | 1.000 | 1 (7.1) | 1 (6.7) | 1.000 |
| Thyroid disease, n (%) | 8 (26.7) | 3 (17.6) | 5 (38.5) | 0.242 | 4 (25.0) | 4 (28.6) | 1.000 | 4 (28.6) | 4 (26.7) | 1.000 |
| AF, n (%) | 13 (43.3) | 5 (29.4) | 8 (61.5) | 0.078 | 3 (18.8) | 10 (71.4) | 0.004 | 5 (35.7) | 8 (53.3) | 0.340 |
| Paroxysmal, n (%) | 12 (40.0) | 5 (29.4) | 7 (53.8) | 0.176 | 3 (18.8) | 9 (64.3) | 0.011 | 5 (35.7) | 7 (46.7) | 0.550 |
| Permanent, n (%) | 1 (3.3) | 0 (0) | 1 (7.7) | 0.433 | 0 (0) | 1 (7.1) | 0.467 | 0 (0) | 1 (6.7) | 1.000 |
| AFL, n (%) | 2 (6.7) | 1 (7.7) | 1 (7.7) | 1.000 | 1 (6.3) | 1 (7.1) | 1.000 | 2 (14.3) | 0 (0) | 0.224 |
| Reason for admission | ||||||||||
| Neurological reason, n (%) | 1 (3.3) | 1 (5.9) | 0 (0) | 1.000 | 0 (0) | 1 (7.1) | 0.467 | 0 (0) | 1 (6.7) | 1.000 |
| Dysrhythmia, n (%) | 1 (3.3) | 1 (5.9) | 0 (0) | 1.000 | 0 (0) | 1 (7.1) | 0.467 | 1 (7.1) | 0 (0) | 0.483 |
| Respiratory failure, n (%) | 10 (33.3) | 6 (35.3) | 4 (30.8) | 1.000 | 5 (31.3) | 5 (35.7) | 1.000 | 5 (35.7) | 4 (26.7) | 0.700 |
| St.p. CPR, n (%) | 3 (10) | 1 (5.9) | 2 (15.4) | 0.565 | 1 (6.3) | 2 (14.3) | 0.586 | 0 (0) | 3 (20.0) | 0.224 |
| Weaning post-COVID, n (%) | 11 (36.7) | 6 (35.3) | 5 (38.5) | 1.000 | 8 (50) | 3 (21.4) | 0.105 | 7 (50) | 4 (26.7) | 0.196 |
| Sepsis, n (%) | 2 (6.7) | 1 (5.9) | 1 (7.7) | 1.000 | 1 (6.3) | 1 (7.1) | 1.000 | 0 (0) | 2 (13.3) | 0.483 |
| Other reasons, n (%) | 2 (6.7) | 1 (5.9) | 1 (7.7) | 1.000 | 1 (6.3) | 1 (7.1) | 1.000 | 1 (7.1) | 1 (6.7) | 1.000 |
| Admission from... | ||||||||||
| home, n (%) | 7 (23.3) | 3 (17.6) | 4 (30.8) | 0.666 | 3 (18.8) | 4 (28.6) | 0.675 | 2 (14.3) | 4 (26.7) | 0.651 |
| General ward, n (%) | 8 (26.7) | 4 (23.5) | 4 (30.8) | 0.698 | 2 (12.5) | 6 (42.9) | 0.101 | 1 (7.1) | 7 (46.7) | 0.035 |
| ICU, n (%) | 15 (50) | 10 (58.8) | 5 (38.5) | 0.269 | 11 (68.8) | 4 (28.6) | 0.028 | 11 (78.6) | 4 (26.7) | 0.005 |
| (n = Bolus Applications) | Total | Male | Female | p-Value | RT | IRT | p-Value | Fluid Balance < 900 mL | Fluid Balance > 900 mL | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| N (% of total) | 49 | 26 (53.1) | 23 (46.9) | 22 (44.9) | 27 (55.1) | 19 (38.8) | 28 (57.1) | |||
| Length of stay until Landiolol bolus, days (IQR) | 13 (6–22) | 14 (6–25) | 11 (5–22) | 0.569 | 13 (8–25) | 14 (4–22) | 0.656 | 14 (7–20) | 16 (4–26) | 0.712 |
| Dose of Landiolol, mg (IQR) | 7.0 (6.5–13.0) | 7 (6–13) | 7 (7–13) | 0.430 | 7 (6.8–10.8) | 7 (6–14) | 0.551 | 7 (7–14) | 7 (7–11.5) | 0.404 |
| Irregular tachycardia, n (%) | 27 (55.1) | 11 (42.3) | 16 (69.6) | 0.056 | 7 (36.8) | 19 (67.9) | 0.038 | |||
| HR prior bolus, bpm (IQR) | 145 (130–150) | 145 (129–153) | 150 (130–150) | 0.569 | 130 (125–150) | 150 (140–155) | 0.004 | 150 (135–160) | 143 (126–150) | 0.080 |
| HR post bolus, bpm (IQR) | 105 (100–125) | 113 (100–125) | 100 (95–130) | 0.385 | 105 (99–121) | 105 (100–130) | 0.859 | 115 (95–130) | 105 (100–125) | 0.472 |
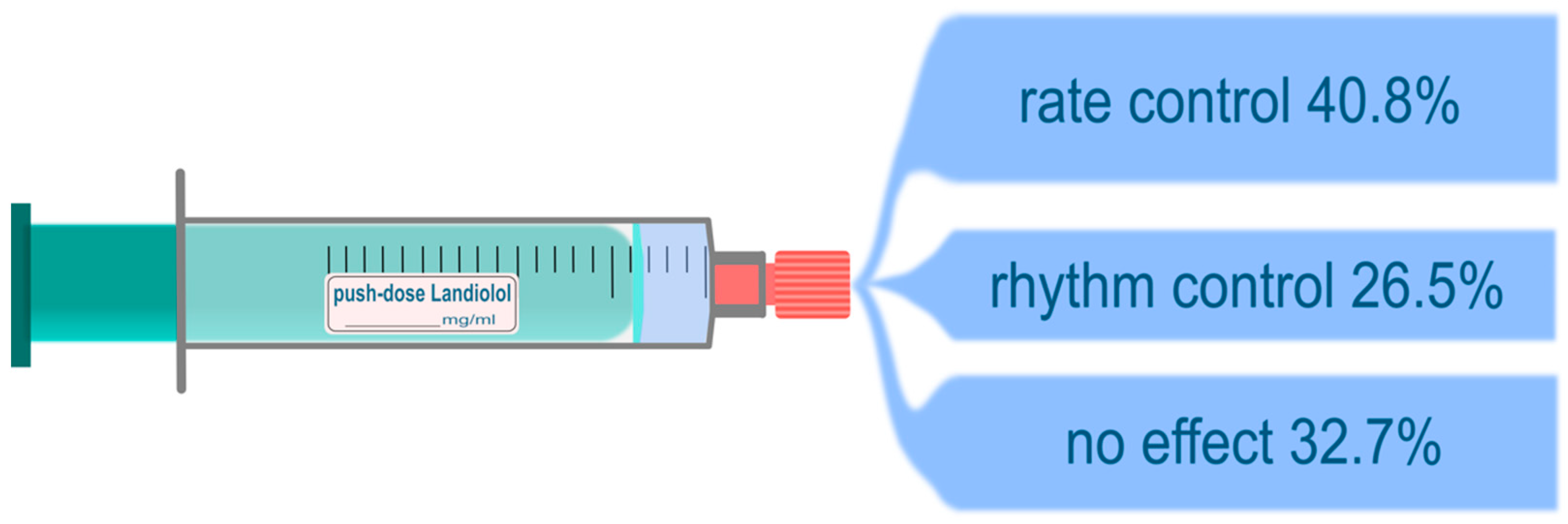
| Successful Landiolol response, n (%) | 33 (67.3) | 18 (69,2) | 15 (65.2) | 0.765 | 16 (27.7) | 17 (63.0) | 0.468 | 12 (63.2) | 19 (67.9) | 0.739 |
| Rate control, n (%) | 20 (40.8) | 11 (42.3) | 9 (39.1) | 0.821 | 12 (54.5) | 8 (29.6) | 0.078 | 7 (36.8) | 13 (46.4) | 0.541 |
| Rhythm control, n (%) | 13 (26.5) | 7 (26.9) | 6 (26.1) | 0.947 | 4 (18.2) | 9 (33.3) | 0.232 | 5 (26.3) | 6 (21.4) | 0.737 |
| No effect, n (%) | 16 (32.7) | 8 (30.8) | 8 (34.8) | 0.765 | 6 (27.3) | 10 (37.0) | 0.468 | 7 (36.8) | 9 (32.1) | 0.739 |
| Switch to perfusor, n (%) | 11 (22.4) | 8 (30.8) | 3 (13.0) | 0.138 | 2 (9.1) | 9 (33.3) | 0.043 | 3 (15.8) | 7 (25.0) | 0.718 |
| Offset | ||||||||||
| Electrical cardioversion, n (%) | 2 (4.1) | 1 (3.8) | 1 (4.3) | 0.929 | 0 (0) | 2 (7.4) | 0.495 | 2 (10.5) | 0 (0) | 0.158 |
| Pharmacological cardioversion, n (%) | 28 (57.1) | 14 (53.8) | 14 (60.9) | 0.620 | 12 (54.5) | 16 (59.3) | 0.779 | 12 (63.2) | 14 (50) | 0.373 |
| Spontaneous, n (%) | 19 (38.8) | 10 (38.5) | 9 (39.1) | 0.962 | 9 (40.9) | 10 (37.0) | 0.783 | 5 (26.3) | 14 (50) | 0.104 |
| Fluid balance, ml (IQR) | 1340 (60–1979) | 1210 (138–1727) | 1540 (−240–2590) | 0.371 | 480 (−150–1835) | 1520 (630–2297) | 0.089 | n.a. | n.a. | n.a. |
| Ventilation | ||||||||||
| Mechanical ventilation, n (%) | 46 (93.9) | 23 (88.5) | 23 (100) | 0.237 | 20 (90.9) | 26 (96.3) | 0.581 | 17 (89.5) | 27 (96.4) | 0.338 |
| NIV, n (%) | 5 (10.2) | 3 (11.5) | 2 (8.7) | 1.000 | 2 (13.6) | 2 (7.4) | 0.474 | 1 (5.3) | 3 (10.7) | 0.638 |
| Invasive, n (%) | 31 (63.3) | 20 (76.9) | 11 (47.8) | 0.035 | 14 (63.6) | 17 (63.0) | 0.961 | 11 (57.9) | 19 (67.9) | 0.485 |
| NHF, n (%) | 10 (20.4) | 0 (0) | 10 (43.5) | <0.001 | 3 (13.6) | 7 (25.9) | 0.242 | 5 (26.3) | 5 (17.9) | 0.366 |
| Medication | ||||||||||
| Catecholamine support, n (%) | 19 (38.8) | 9 (34.6) | 10 (43.5) | 0.569 | 4 (18.2) | 15 (55.6) | 0.008 | 4 (21.1) | 14 (50) | 0.045 |
| Corticosteroids, n (%) | 21 (42.9) | 11 (42.3) | 10 (43.5) | 0.934 | 11 (50) | 10 (37.0) | 0.362 | 8 (42.1) | 11 (39.3) | 0.847 |
| Antibiotics, n (%) | 40 (81.6) | 22 (84.6) | 18 (78.3) | 0.716 | 17 (77.3) | 23 (85.2) | 0.713 | 15 (78.9) | 24 (85.7) | 0.697 |
| Chronic oral ß-blocker, n (%) | 13 (26.5) | 8 (30.8) | 5 (21.7) | 0.607 | 7 (31.8) | 6 (22.2) | 0.672 | 5 (26.3) | 7 (25.0) | 0.987 |
| Blood Pressure MAP 15 min Post | ||||
|---|---|---|---|---|
| Predictors | Coefficient | SE | CI | p-Value |
| Constant | 14.008 | 13.750 | −14.114–42.130 | 0.317 |
| FiO2 prior | −0.188 | 0.096 | −0.384–0.007 | 0.058 |
| Mean MAP 15 min prior | 0.912 | 0.108 | 0.690–1.134 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schnaubelt, S.; Eibensteiner, F.; Oppenauer, J.; Tihanyi, D.; Neymayer, M.; Brock, R.; Kornfehl, A.; Veigl, C.; Al Jalali, V.; Anders, S.; et al. Hemodynamic and Rhythmologic Effects of Push-Dose Landiolol in Critical Care—A Retrospective Cross-Sectional Study. Pharmaceuticals 2023, 16, 134. https://doi.org/10.3390/ph16020134
Schnaubelt S, Eibensteiner F, Oppenauer J, Tihanyi D, Neymayer M, Brock R, Kornfehl A, Veigl C, Al Jalali V, Anders S, et al. Hemodynamic and Rhythmologic Effects of Push-Dose Landiolol in Critical Care—A Retrospective Cross-Sectional Study. Pharmaceuticals. 2023; 16(2):134. https://doi.org/10.3390/ph16020134
Chicago/Turabian StyleSchnaubelt, Sebastian, Felix Eibensteiner, Julia Oppenauer, Daniel Tihanyi, Marco Neymayer, Roman Brock, Andrea Kornfehl, Christoph Veigl, Valentin Al Jalali, Sonja Anders, and et al. 2023. "Hemodynamic and Rhythmologic Effects of Push-Dose Landiolol in Critical Care—A Retrospective Cross-Sectional Study" Pharmaceuticals 16, no. 2: 134. https://doi.org/10.3390/ph16020134
APA StyleSchnaubelt, S., Eibensteiner, F., Oppenauer, J., Tihanyi, D., Neymayer, M., Brock, R., Kornfehl, A., Veigl, C., Al Jalali, V., Anders, S., Steinlechner, B., Domanovits, H., & Sulzgruber, P. (2023). Hemodynamic and Rhythmologic Effects of Push-Dose Landiolol in Critical Care—A Retrospective Cross-Sectional Study. Pharmaceuticals, 16(2), 134. https://doi.org/10.3390/ph16020134






