Mitigating Scar Tissue Formation in Tendon Injuries: Targeting HMGB1, AMPK Activation, and Myofibroblast Migration All at Once
Abstract
:1. Introduction
2. Results
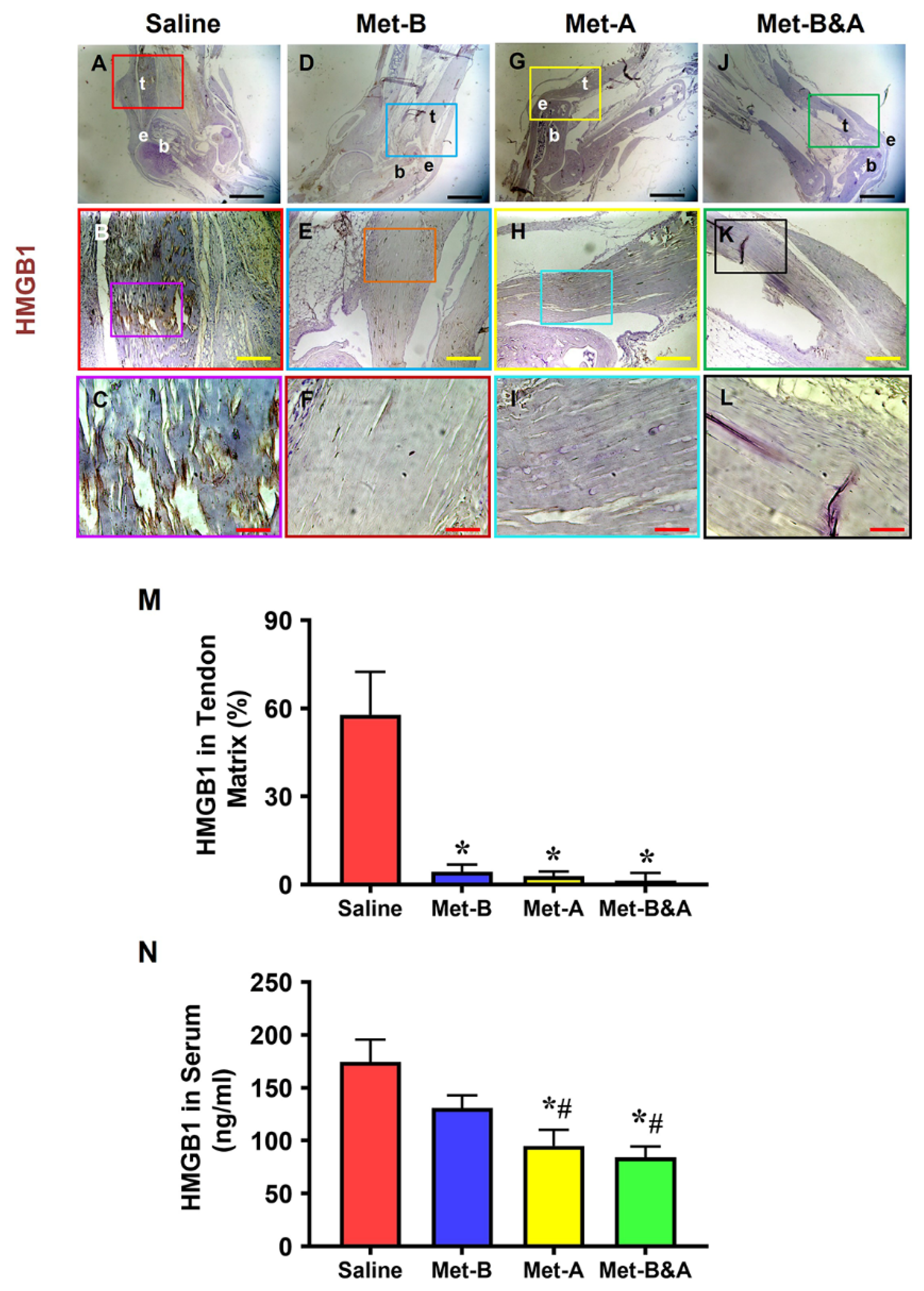
2.1. Met Injection Blocks HMGB1 Release and Reduces HMGB1 Levels
2.2. Met Injection Increases and Activates AMPK
2.3. Met Injection Reduces TGF-β1 Levels
2.4. Met Injection Inhibits α-SMA+ Cell Migration
2.5. Met Injection Improves Healing Quality of Wounded Tendons
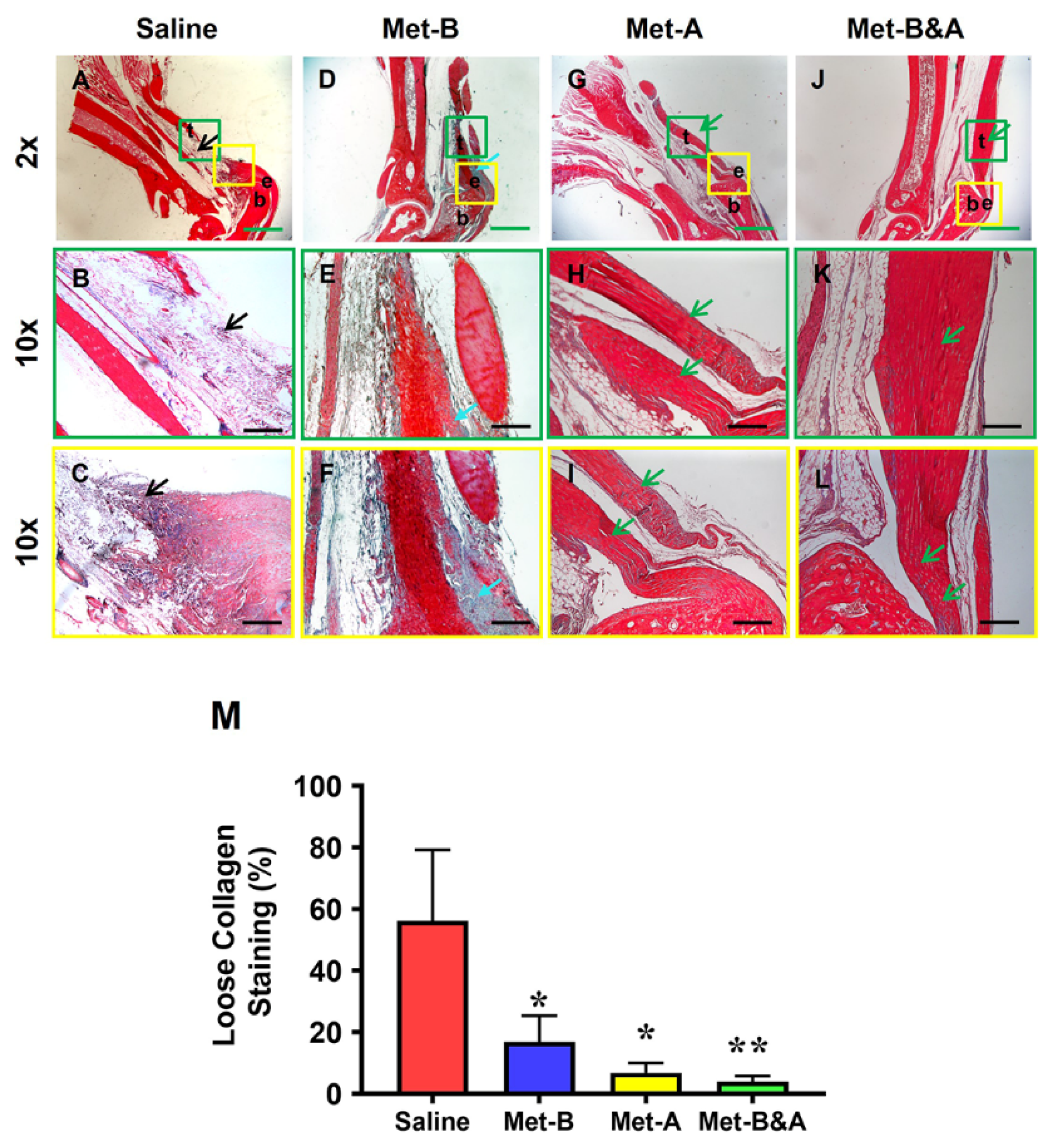
2.6. Met Injection Inhibits Scar Formation by Decreasing Loose Collagen Fibers, Collagen Type III, and Increasing Collagen Type I
3. Discussion
4. Materials and Methods
4.1. Animals
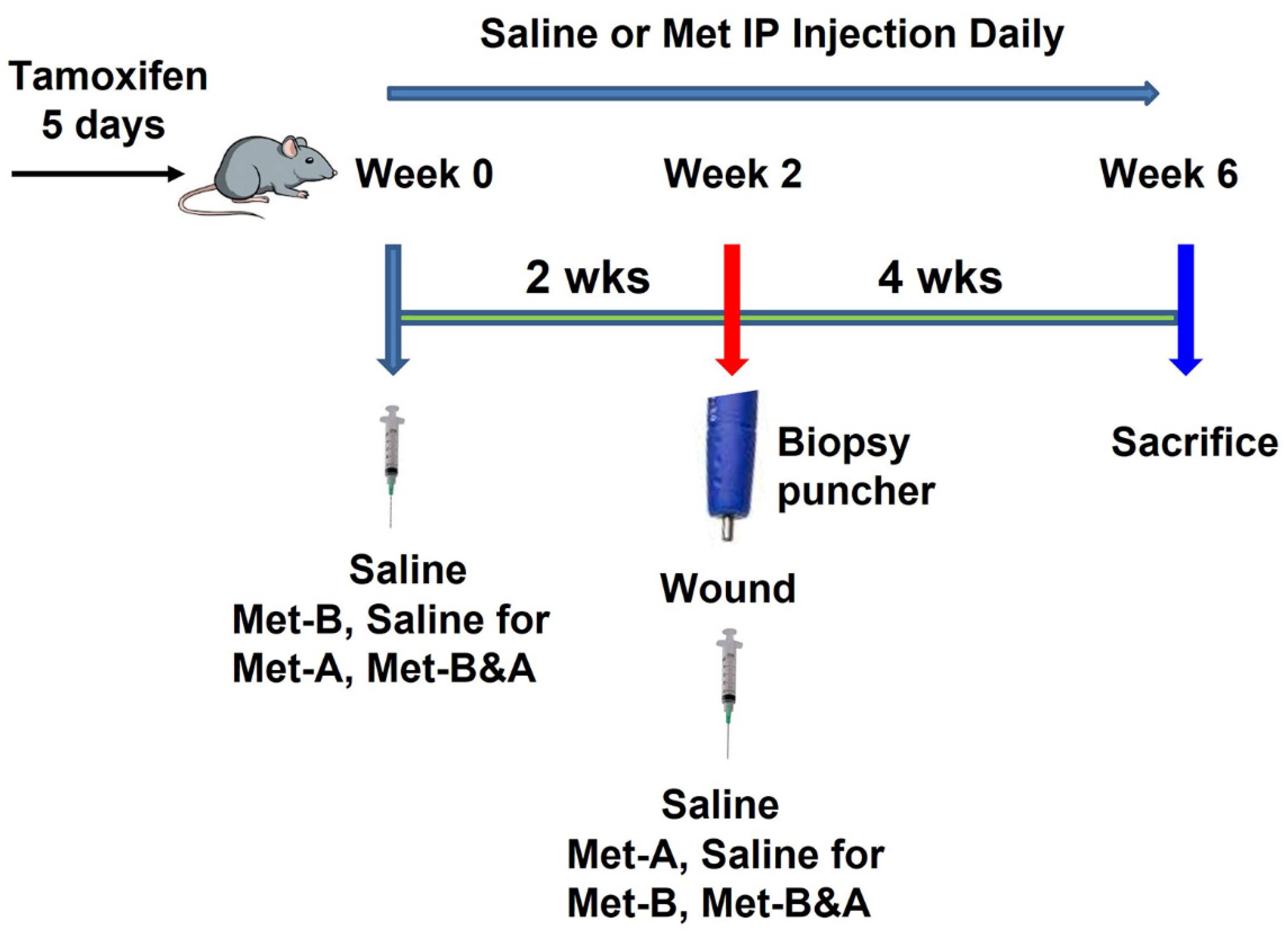
4.2. Tendon Wound Healing Model
4.3. Measurement of HMGB1 in Mouse Serum by ELISA
4.4. Histochemical Staining for the Structural Analysis of Mouse Tendon Tissues
4.5. Picro Sirius Red Staining and Polarized Light Microscopy of Mouse Tendon
4.6. Analysis of Cell Distribution on Mouse Tissue Sections Using Fluorescent Microscopy
4.7. Immunohistochemistry (IHC) Staining on Mouse Tissue Sections
4.8. Semi-Quantitative Assessment of Stained Tendon Tissue and Cells
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nichols, A.E.C.; Best, K.T.; Loiselle, A.E. The cellular basis of fibrotic tendon healing: Challenges and opportunities. Transl. Res. 2019, 209, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Nichols, A.E.C.; Oh, I.; Loiselle, A.E. Effects of Type II Diabetes Mellitus on Tendon Homeostasis and Healing. J. Orthop. Res. 2020, 38, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Butler, D.L. Evolution of functional tissue engineering for tendon and ligament repair. J. Tissue Eng. Regen. Med. 2022, 16, 1091–1108. [Google Scholar] [CrossRef] [PubMed]
- Dees, C.; Chakraborty, D.; Distler, J.H.W. Cellular and molecular mechanisms in fibrosis. Exp. Dermatol. 2021, 30, 121–131. [Google Scholar] [CrossRef]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Lafyatis, R. Transforming growth factor beta—At the centre of systemic sclerosis. Nat. Rev. Rheumatol. 2014, 10, 706–719. [Google Scholar] [CrossRef]
- Li, M.; Krishnaveni, M.S.; Li, C.; Zhou, B.; Xing, Y.; Banfalvi, A.; Li, A.; Lombardi, V.; Akbari, O.; Borok, Z.; et al. Epithelium-specific deletion of TGF-beta receptor type II protects mice from bleomycin-induced pulmonary fibrosis. J. Clin. Investig. 2011, 121, 277–287. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Liang, Z.; Li, T.; Jiang, S.; Xu, J.; Di, W.; Yang, Z.; Hu, W.; Yang, Y. AMPK: A novel target for treating hepatic fibrosis. Oncotarget 2017, 8, 62780–62792. [Google Scholar] [CrossRef]
- Qi, H.; Liu, Y.; Li, S.; Chen, Y.; Li, L.; Cao, Y.; Mingyao, E.; Shi, P.; Song, C.; Li, B.; et al. Activation of AMPK Attenuated Cardiac Fibrosis by Inhibiting CDK2 via p21/p27 and miR-29 Family Pathways in Rats. Mol. Ther. Nucleic Acids 2017, 8, 277–290. [Google Scholar] [CrossRef]
- Bai, B.; Chen, H. Metformin: A Novel Weapon Against Inflammation. Front. Pharmacol. 2021, 12, 622262. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Moon, S.Y.; Kim, J.S.; Baek, C.H.; Kim, M.; Min, J.Y.; Lee, S.K. Activation of AMP-activated protein kinase inhibits ER stress and renal fibrosis. Am. J. Physiol. Renal. Physiol. 2015, 308, F226–F236. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Cool, B.L.; Laderoute, K.R.; Foretz, M.; Viollet, B.; Simonson, M.S. AMP-activated protein kinase inhibits transforming growth factor-beta-induced Smad3-dependent transcription and myofibroblast transdifferentiation. J. Biol. Chem. 2008, 283, 10461–10469. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Oh, M.A.; Kim, W.H.; Sohn, H.Y.; Park, S.I. AMP-activated protein kinase inhibits TGF-beta-induced fibrogenic responses of hepatic stellate cells by targeting transcriptional coactivator p300. J. Cell Physiol. 2012, 227, 1081–1089. [Google Scholar] [CrossRef]
- Akbar, M.; Gilchrist, D.S.; Kitson, S.M.; Nelis, B.; Crowe, L.A.N.; Garcia-Melchor, E.; Reilly, J.H.; Kerr, S.C.; Murrell, G.A.C.; McInnes, I.B.; et al. Targeting danger molecules in tendinopathy: The HMGB1/TLR4 axis. RMD Open 2017, 3, e000456. [Google Scholar] [CrossRef]
- Thankam, F.G.; Roesch, Z.K.; Dilisio, M.F.; Radwan, M.M.; Kovilam, A.; Gross, R.M.; Agrawal, D.K. Association of Inflammatory Responses and ECM Disorganization with HMGB1 Upregulation and NLRP3 Inflammasome Activation in the Injured Rotator Cuff Tendon. Sci. Rep. 2018, 8, 8918. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, J.; Nie, D.; Zhou, Y.; Li, F.; Onishi, K.; Billiar, T.; Wang, J.H. HMGB1 mediates the development of tendinopathy due to mechanical overloading. PLoS ONE 2019, 14, e0222369. [Google Scholar] [CrossRef]
- Zhang, J.; Li, F.; Nie, D.; Onishi, K.; Hogan, M.V.; Wang, J.H. Effect of Metformin on Development of Tendinopathy Due to Mechanical Overloading in an Animal Model. Foot Ankle Int. 2020, 41, 1455–1465. [Google Scholar] [CrossRef]
- Li, L.C.; Gao, J.; Li, J. Emerging role of HMGB1 in fibrotic diseases. J. Cell Mol. Med. 2014, 18, 2331–2339. [Google Scholar] [CrossRef]
- Chen, Q.; Guan, X.; Zuo, X.; Wang, J.; Yin, W. The role of high mobility group box 1 (HMGB1) in the pathogenesis of kidney diseases. Acta Pharm. Sin. B 2016, 6, 183–188. [Google Scholar] [CrossRef]
- Hamada, N.; Maeyama, T.; Kawaguchi, T.; Yoshimi, M.; Fukumoto, J.; Yamada, M.; Yamada, S.; Kuwano, K.; Nakanishi, Y. The role of high mobility group box1 in pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2008, 39, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Dardenne, A.D.; Wulff, B.C.; Wilgus, T.A. The alarmin HMGB-1 influences healing outcomes in fetal skin wounds. Wound Repair. Regen. 2013, 21, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yu, J.; Xu, Y.; Chen, L.; Zhou, F.; Zhai, Q.; Wu, J.; Shu, B.; Qi, S. Epidermal HMGB1 Activates Dermal Fibroblasts and Causes Hypertrophic Scar Formation in Reduced Hydration. J. Investig. Dermatol. 2018, 138, 2322–2332. [Google Scholar] [CrossRef] [PubMed]
- Jeong, W.; Yang, C.E.; Roh, T.S.; Kim, J.H.; Lee, J.H.; Lee, W.J. Scar Prevention and Enhanced Wound Healing Induced by Polydeoxyribonucleotide in a Rat Incisional Wound-Healing Model. Int. J. Mol. Sci. 2017, 18, 1698. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, T.; Sakata, N.; Narumi, Y.; Kimura, T.; Hayashi, T.; Nagano, K.; Liu, K.; Nishibori, M.; Tsukita, S.; Yamada, T.; et al. Metformin directly binds the alarmin HMGB1 and inhibits its proinflammatory activity. J. Biol. Chem. 2017, 292, 8436–8446. [Google Scholar] [CrossRef]
- Choi, S.M.; Jang, A.H.; Kim, H.; Lee, K.H.; Kim, Y.W. Metformin Reduces Bleomycin-induced Pulmonary Fibrosis in Mice. J. Korean Med. Sci. 2016, 31, 1419–1425. [Google Scholar] [CrossRef]
- Rangarajan, S.; Bone, N.B.; Zmijewska, A.A.; Jiang, S.; Park, D.W.; Bernard, K.; Locy, M.L.; Ravi, S.; Deshane, J.; Mannon, R.B.; et al. Metformin reverses established lung fibrosis in a bleomycin model. Nat. Med. 2018, 24, 1121–1127. [Google Scholar] [CrossRef]
- Gamad, N.; Malik, S.; Suchal, K.; Vasisht, S.; Tomar, A.; Arava, S.; Arya, D.S.; Bhatia, J. Metformin alleviates bleomycin-induced pulmonary fibrosis in rats: Pharmacological effects and molecular mechanisms. Biomed. Pharmacother. 2018, 97, 1544–1553. [Google Scholar] [CrossRef]
- Xiao, H.; Ma, X.; Feng, W.; Fu, Y.; Lu, Z.; Xu, M.; Shen, Q.; Zhu, Y.; Zhang, Y. Metformin attenuates cardiac fibrosis by inhibiting the TGFbeta1-Smad3 signalling pathway. Cardiovasc. Res. 2010, 87, 504–513. [Google Scholar] [CrossRef]
- Tokuda, K.; Yamanaka, Y.; Mano, Y.; Tsukamoto, M.; Tajima, T.; Suzuki, H.; Kawasaki, M.; Uchida, S.; Nakamura, E.; Wang, K.Y.; et al. Effect of metformin treatment and its time of administration on joint capsular fibrosis induced by mouse knee immobilization. Sci. Rep. 2021, 11, 17978. [Google Scholar] [CrossRef]
- Zhang, A.; Qian, F.; Li, Y.; Li, B.; Yang, F.; Hu, C.; Sun, W.; Huang, Y. Research progress of metformin in the treatment of liver fibrosis. Int. Immunopharmacol. 2023, 116, 109738. [Google Scholar] [CrossRef] [PubMed]
- Saisho, Y. Metformin and Inflammation: Its Potential Beyond Glucose-lowering Effect. Endocr. Metab. Immune Disord. Drug Targets 2015, 15, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Jiang, Z.; Li, J.; Lin, H.; Xu, B.; Liao, X.; Fu, Z.; Ao, H.; Guo, G.; Liu, M. Metformin Improves Burn Wound Healing by Modulating Microenvironmental Fibroblasts and Macrophages. Cells 2022, 11, 4094. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Yoo, H.; Kim, J.Y.; Oh, S.H.; Kang, J.W.; Yoo, B.R.; Han, S.Y.; Kim, C.S.; Choi, W.H.; Lee, E.J.; et al. Metformin Alleviates Radiation-Induced Skin Fibrosis via the Downregulation of FOXO3. Cell Physiol. Biochem. 2018, 48, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Chogan, F.; Mirmajidi, T.; Rezayan, A.H.; Sharifi, A.M.; Ghahary, A.; Nourmohammadi, J.; Kamali, A.; Rahaie, M. Design, fabrication, and optimization of a dual function three-layer scaffold for controlled release of metformin hydrochloride to alleviate fibrosis and accelerate wound healing. Acta Biomater. 2020, 113, 144–163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Brown, R.; Hogan, M.V.; Onishi, K.; Wang, J.H. Metformin improves tendon degeneration by blocking translocation of HMGB1 and suppressing tendon inflammation and senescence in aging mice. J. Orthop. Res. 2023, 41, 1162–1176. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Song, J.; Zhang, Y.; Chen, S.; Ruan, H.; Fan, C. Metformin prevents peritendinous fibrosis by inhibiting transforming growth factor-beta signaling. Oncotarget 2017, 8, 101784–101794. [Google Scholar] [CrossRef]
- Schiraldi, M.; Raucci, A.; Munoz, L.M.; Livoti, E.; Celona, B.; Venereau, E.; Apuzzo, T.; De Marchis, F.; Pedotti, M.; Bachi, A.; et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J. Exp. Med. 2012, 209, 551–563. [Google Scholar] [CrossRef]
- Venereau, E.; Schiraldi, M.; Uguccioni, M.; Bianchi, M.E. HMGB1 and leukocyte migration during trauma and sterile inflammation. Mol. Immunol. 2013, 55, 76–82. [Google Scholar] [CrossRef]
- Magna, M.; Pisetsky, D.S. The role of HMGB1 in the pathogenesis of inflammatory and autoimmune diseases. Mol. Med. 2014, 20, 138–146. [Google Scholar] [CrossRef]
- Yoshizaki, A.; Komura, K.; Iwata, Y.; Ogawa, F.; Hara, T.; Muroi, E.; Takenaka, M.; Shimizu, K.; Hasegawa, M.; Fujimoto, M.; et al. Clinical significance of serum HMGB-1 and sRAGE levels in systemic sclerosis: Association with disease severity. J. Clin. Immunol. 2009, 29, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.N.; Yu, T.Y.; Zhou, J.C.; Li, M.; Gao, H.K.; Zhao, C.; Dong, R.Q.; Peng, D.; Hu, Z.W.; Zhang, X.W.; et al. Targeting HMGB1 ameliorates cardiac fibrosis through restoring TLR2-mediated autophagy suppression in myocardial fibroblasts. Int. J. Cardiol. 2018, 267, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Shan, W.; Gao, L.; Gao, D.; Hu, Y.; Wang, G.; Zhang, N.; Li, Z.; Tian, X.; Xu, W.; et al. Inhibition of HMGB1 release via salvianolic acid B-mediated SIRT1 up-regulation protects rats against non-alcoholic fatty liver disease. Sci. Rep. 2015, 5, 16013. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Li, T.; Yang, Z.; Yi, W.; Di, S.; Sun, Y.; Wang, D.; Yang, Y. AMPK orchestrates an elaborate cascade protecting tissue from fibrosis and aging. Ageing Res. Rev. 2017, 38, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Hasanvand, A. The role of AMPK-dependent pathways in cellular and molecular mechanisms of metformin: A new perspective for treatment and prevention of diseases. Inflammopharmacology 2022, 30, 775–788. [Google Scholar] [CrossRef]
- Li, L.; Huang, W.; Li, K.; Zhang, K.; Lin, C.; Han, R.; Lu, C.; Wang, Y.; Chen, H.; Sun, F.; et al. Metformin attenuates gefitinib-induced exacerbation of pulmonary fibrosis by inhibition of TGF-beta signaling pathway. Oncotarget 2015, 6, 43605–43619. [Google Scholar] [CrossRef]
- Park, C.S.; Bang, B.R.; Kwon, H.S.; Moon, K.A.; Kim, T.B.; Lee, K.Y.; Moon, H.B.; Cho, Y.S. Metformin reduces airway inflammation and remodeling via activation of AMP-activated protein kinase. Biochem. Pharmacol. 2012, 84, 1660–1670. [Google Scholar] [CrossRef]
- Sato, N.; Takasaka, N.; Yoshida, M.; Tsubouchi, K.; Minagawa, S.; Araya, J.; Saito, N.; Fujita, Y.; Kurita, Y.; Kobayashi, K.; et al. Metformin attenuates lung fibrosis development via NOX4 suppression. Respir. Res. 2016, 17, 107. [Google Scholar] [CrossRef]
- Takenouchi, Y.; Kitakaze, K.; Tsuboi, K.; Okamoto, Y. Growth differentiation factor 15 facilitates lung fibrosis by activating macrophages and fibroblasts. Exp. Cell Res. 2020, 391, 112010. [Google Scholar] [CrossRef]
- Hinz, B. Myofibroblasts. Exp. Eye Res. 2016, 142, 56–70. [Google Scholar] [CrossRef]
- Wipff, P.J.; Rifkin, D.B.; Meister, J.J.; Hinz, B. Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J. Cell Biol. 2007, 179, 1311–1323. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Viswanadhapalli, S.; Kopp, J.B.; Shi, Q.; Barnes, J.L.; Block, K.; Gorin, Y.; Abboud, H.E. Activation of AMP-activated protein kinase prevents TGF-beta1-induced epithelial-mesenchymal transition and myofibroblast activation. Am. J. Pathol. 2015, 185, 2168–2180. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Xu, Q.; Wang, Y.; Li, G.; Sun, W.; Ma, D.; Zhou, S.; Liu, Y.; Han, L.; Ni, C. Metformin attenuates silica-induced pulmonary fibrosis via AMPK signaling. J. Transl. Med. 2021, 19, 349. [Google Scholar] [CrossRef] [PubMed]
- Disser, N.P.; Yu, J.S.; Yao, V.J.H.; Rodeo, S.A. Pharmacological Therapies for Connective Tissue Fibrosis in Orthopaedics. Am. J. Sports Med. 2023, 51, 2766–2773. [Google Scholar] [CrossRef]
- Teague, T.T.; Payne, S.R.; Kelly, B.T.; Dempsey, T.M.; McCoy, R.G.; Sangaralingham, L.R.; Limper, A.H. Evaluation for clinical benefit of metformin in patients with idiopathic pulmonary fibrosis and type 2 diabetes mellitus: A national claims-based cohort analysis. Respir. Res. 2022, 23, 91. [Google Scholar] [CrossRef]
- Korcari, A.; Muscat, S.; McGinn, E.; Buckley, M.R.; Loiselle, A.E. Depletion of Scleraxis-lineage cells during tendon healing transiently impairs multi-scale restoration of tendon structure during early healing. PLoS ONE 2022, 17, e0274227. [Google Scholar] [CrossRef]
- Zhang, J.; Li, F.; Williamson, K.M.; Tan, S.; Scott, D.; Onishi, K.; Hogan, M.V.; Wang, J.H. Characterization of the structure, vascularity, and stem/progenitor cell populations in porcine Achilles tendon (PAT). Cell Tissue Res. 2021, 384, 367–387. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Brown, R.; Hogan, M.V.; Wang, J.H.-C. Mitigating Scar Tissue Formation in Tendon Injuries: Targeting HMGB1, AMPK Activation, and Myofibroblast Migration All at Once. Pharmaceuticals 2023, 16, 1739. https://doi.org/10.3390/ph16121739
Zhang J, Brown R, Hogan MV, Wang JH-C. Mitigating Scar Tissue Formation in Tendon Injuries: Targeting HMGB1, AMPK Activation, and Myofibroblast Migration All at Once. Pharmaceuticals. 2023; 16(12):1739. https://doi.org/10.3390/ph16121739
Chicago/Turabian StyleZhang, Jianying, Roshawn Brown, MaCalus V. Hogan, and James H-C. Wang. 2023. "Mitigating Scar Tissue Formation in Tendon Injuries: Targeting HMGB1, AMPK Activation, and Myofibroblast Migration All at Once" Pharmaceuticals 16, no. 12: 1739. https://doi.org/10.3390/ph16121739
APA StyleZhang, J., Brown, R., Hogan, M. V., & Wang, J. H.-C. (2023). Mitigating Scar Tissue Formation in Tendon Injuries: Targeting HMGB1, AMPK Activation, and Myofibroblast Migration All at Once. Pharmaceuticals, 16(12), 1739. https://doi.org/10.3390/ph16121739





