Squalene Peroxidation and Biophysical Parameters in Acne-Prone Skin: A Pilot “In Vivo” Study
Abstract
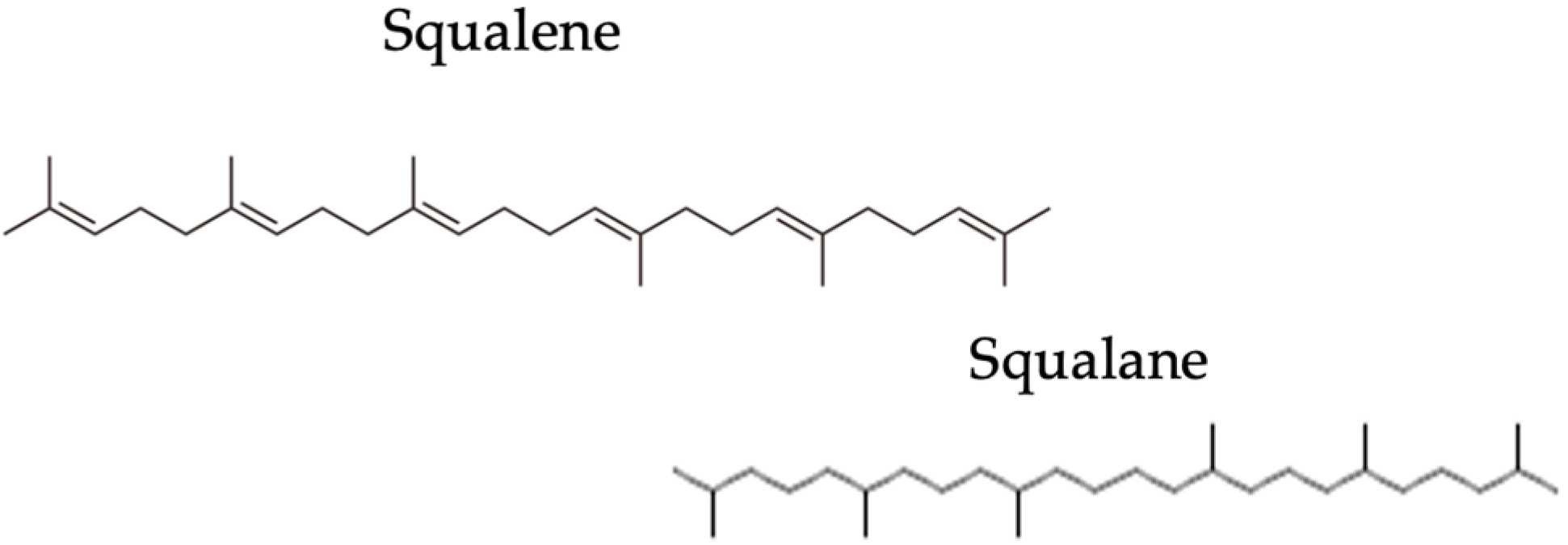
1. Introduction
2. Results
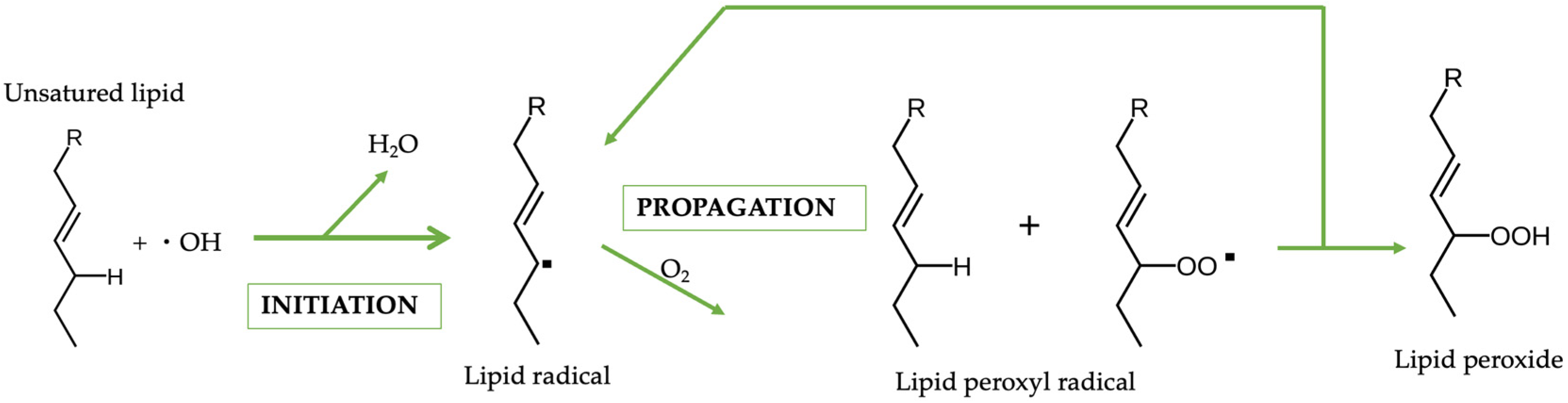
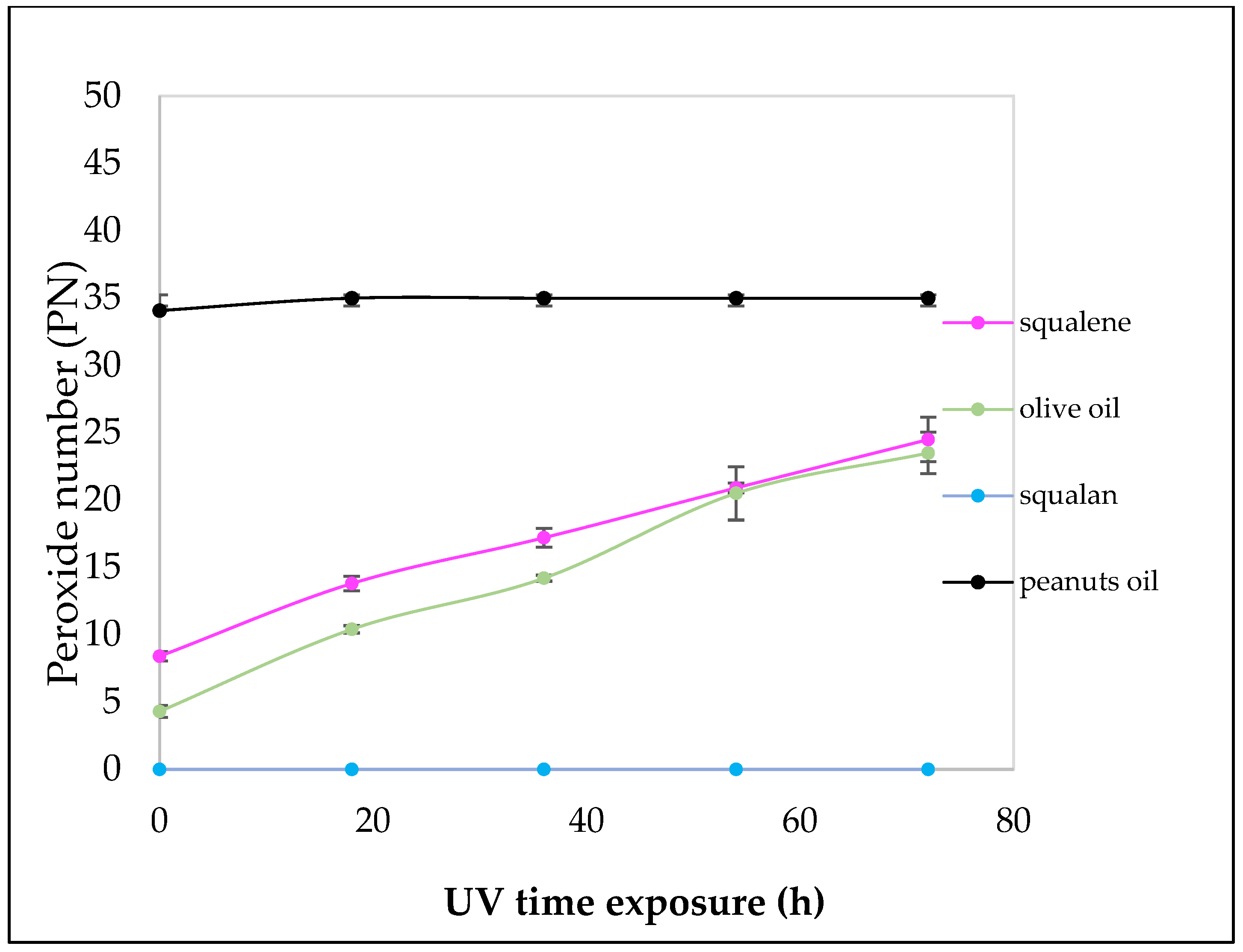
2.1. In Vitro Peroxidation Analysis
2.2. HPLC Analysis
2.3. In Vivo Experiments
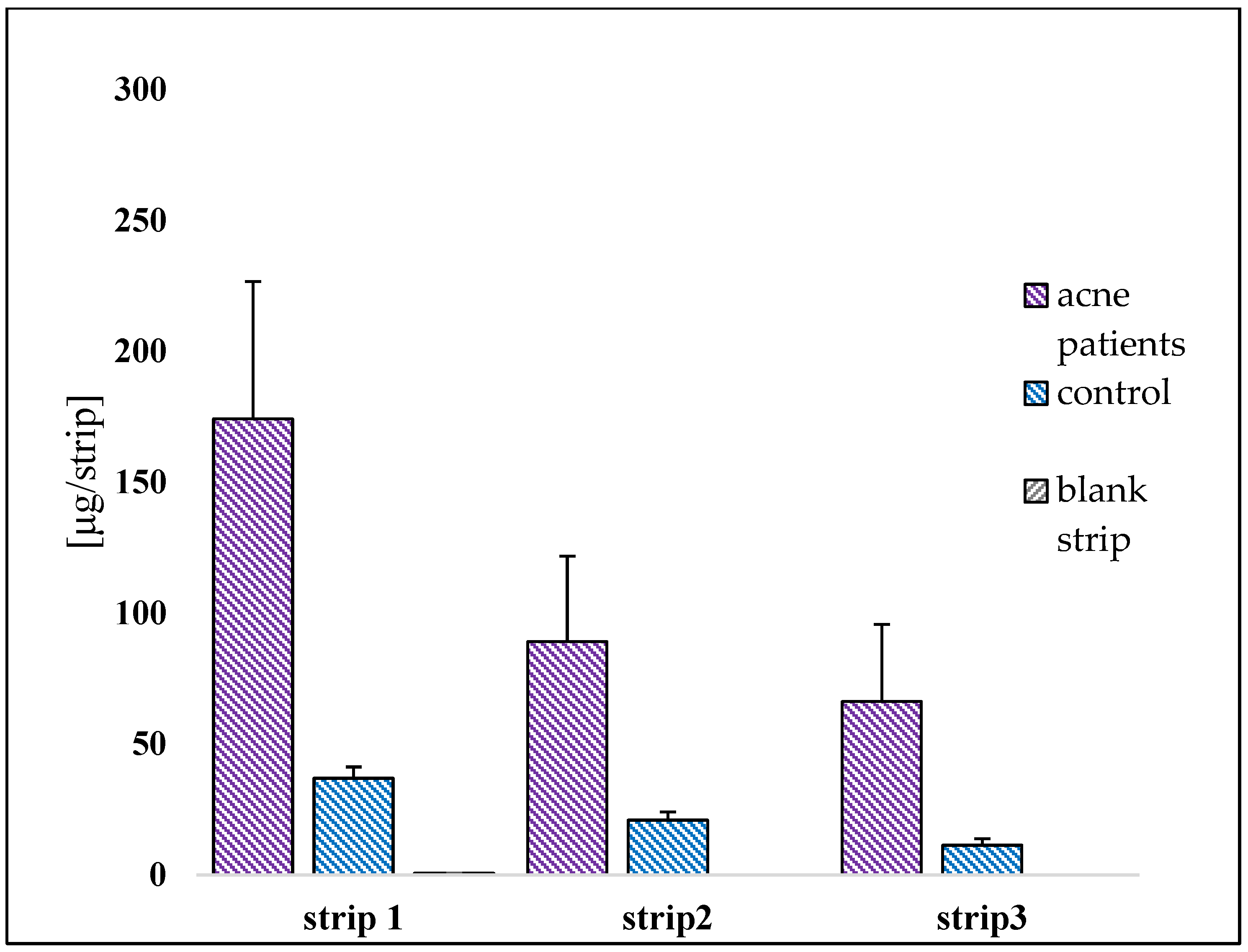
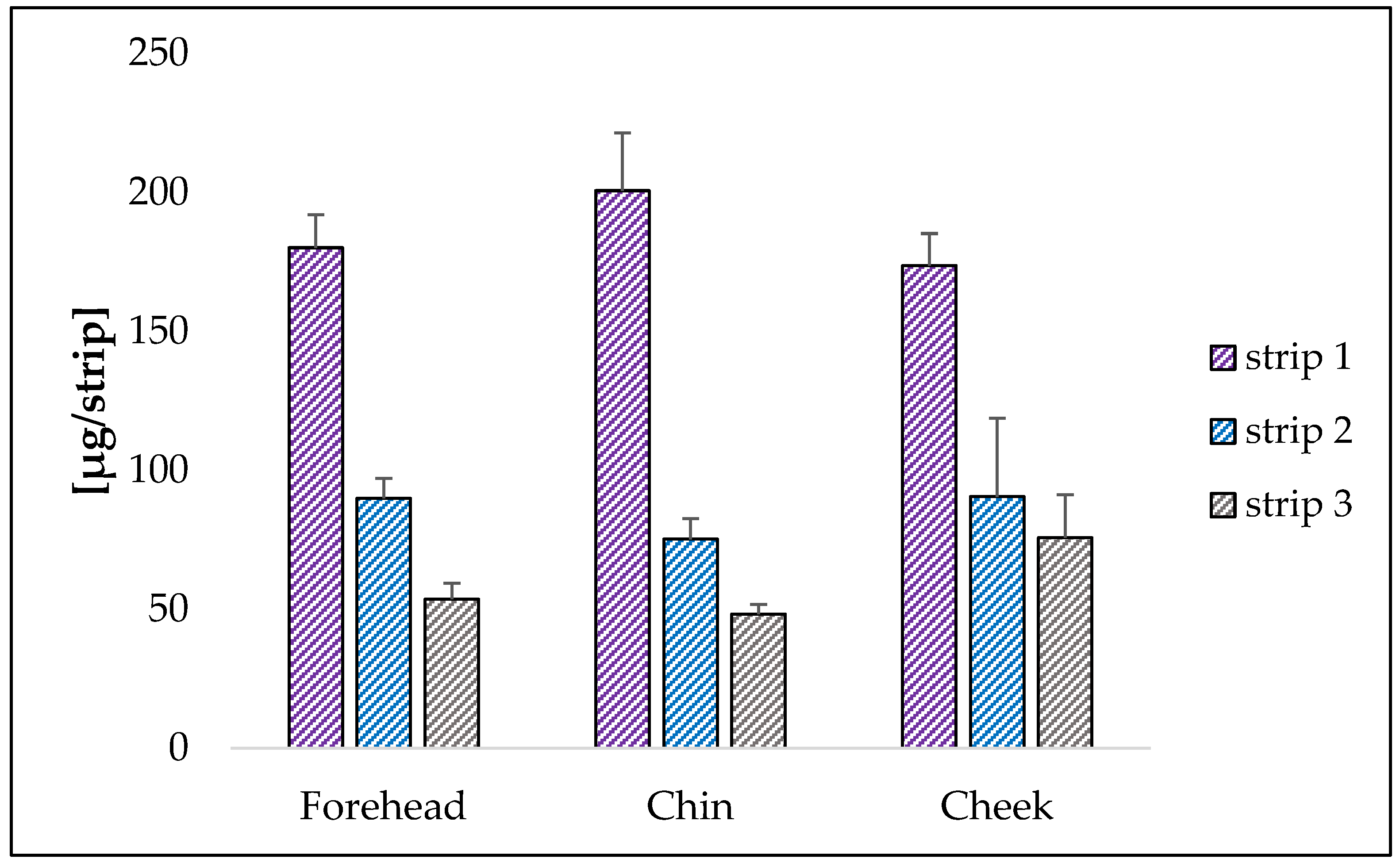
2.3.1. Collection of Sebum
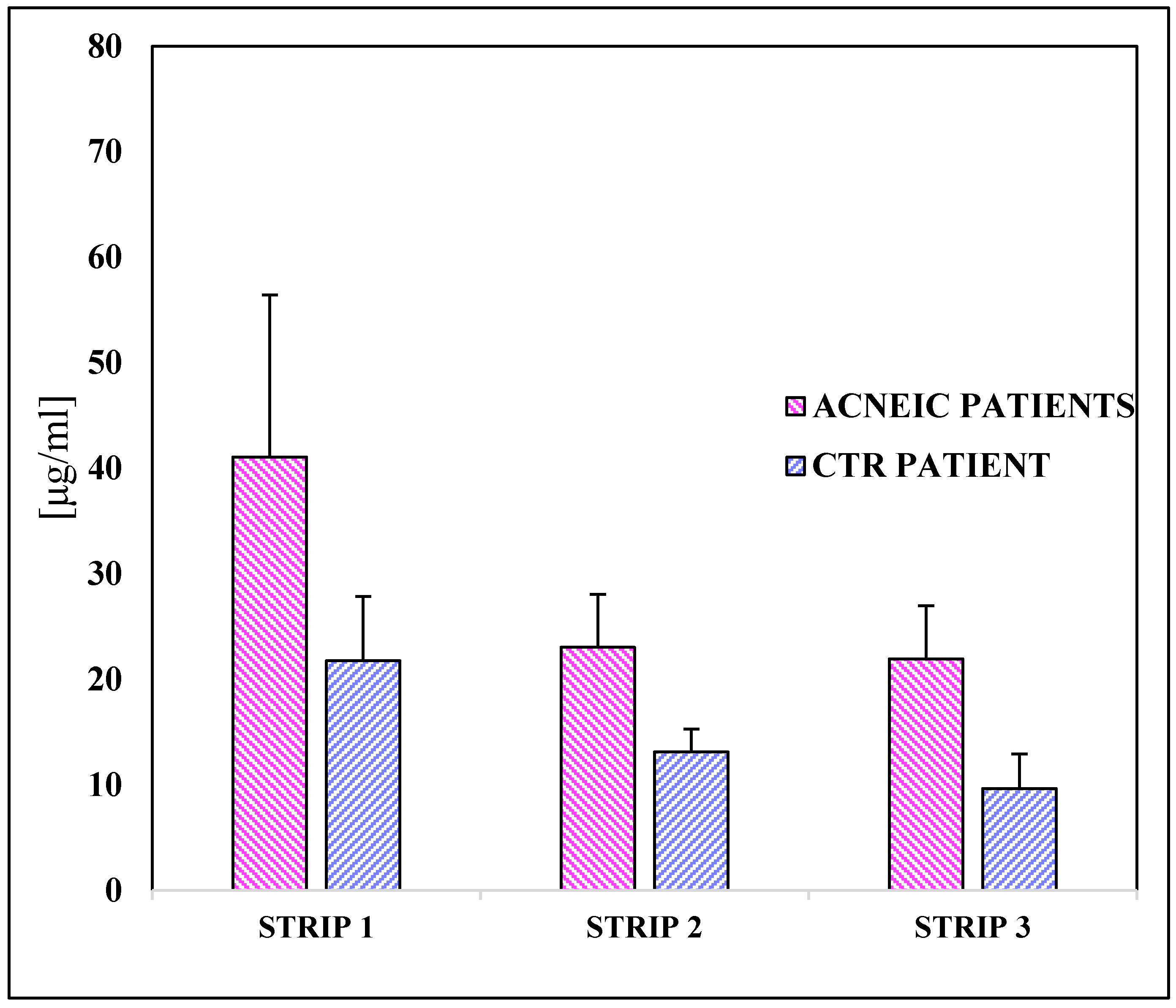
2.3.2. Squalene Quantification in the “In Vivo” Study
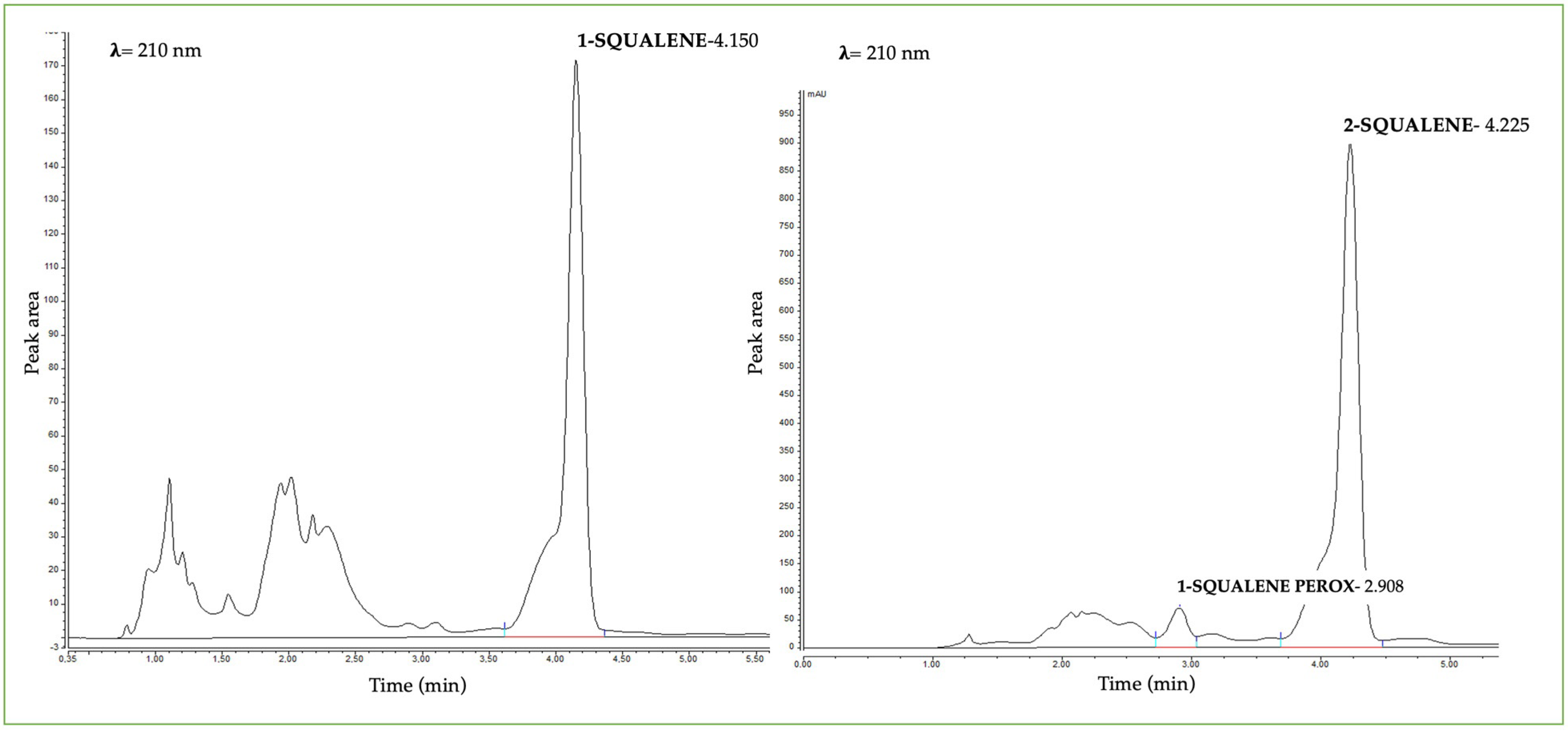
2.3.3. Example of a Chromatogram of a Healthy and an Acneic Volunteer
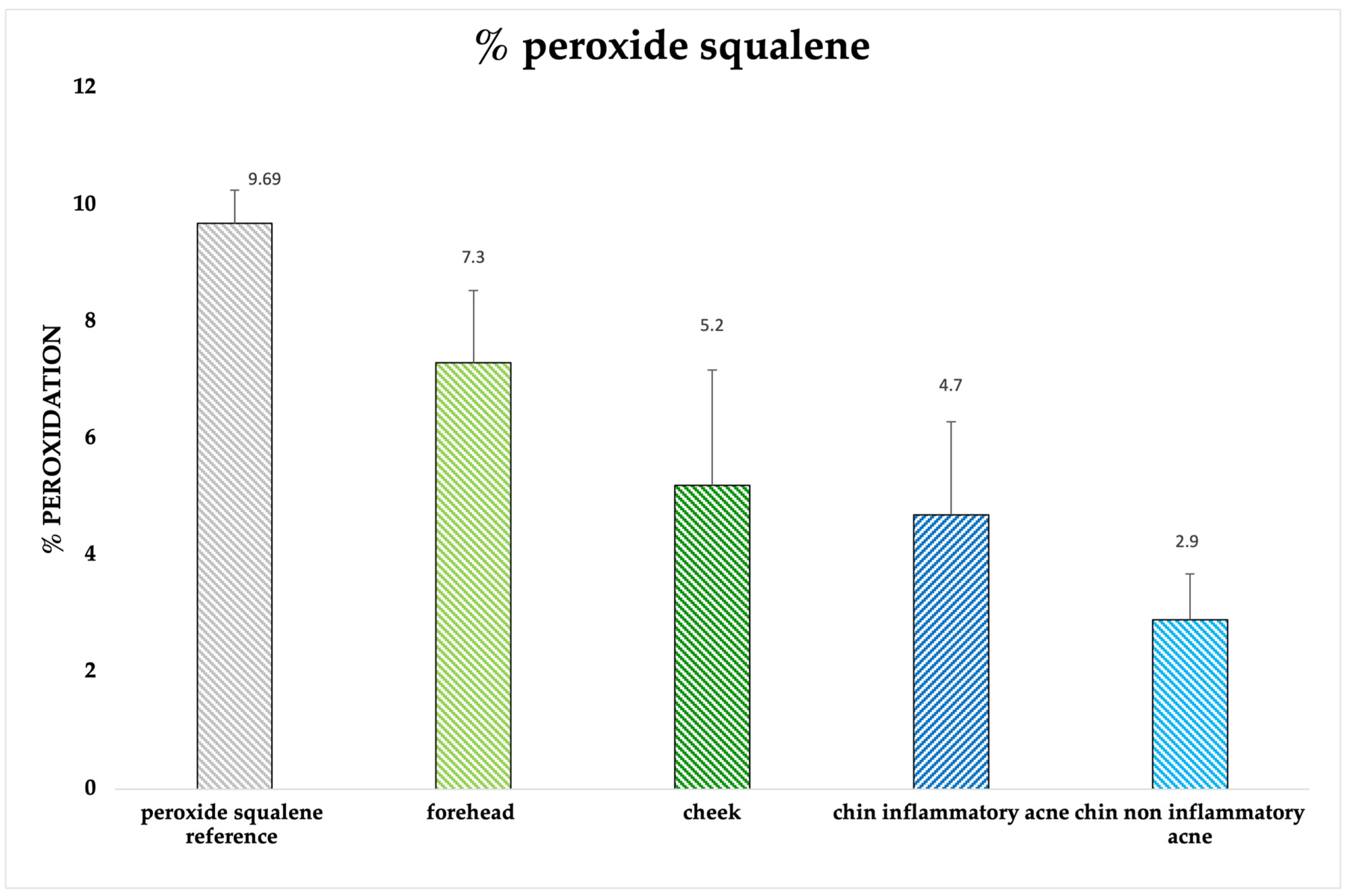
2.3.4. Quantification of Peroxide Squalene
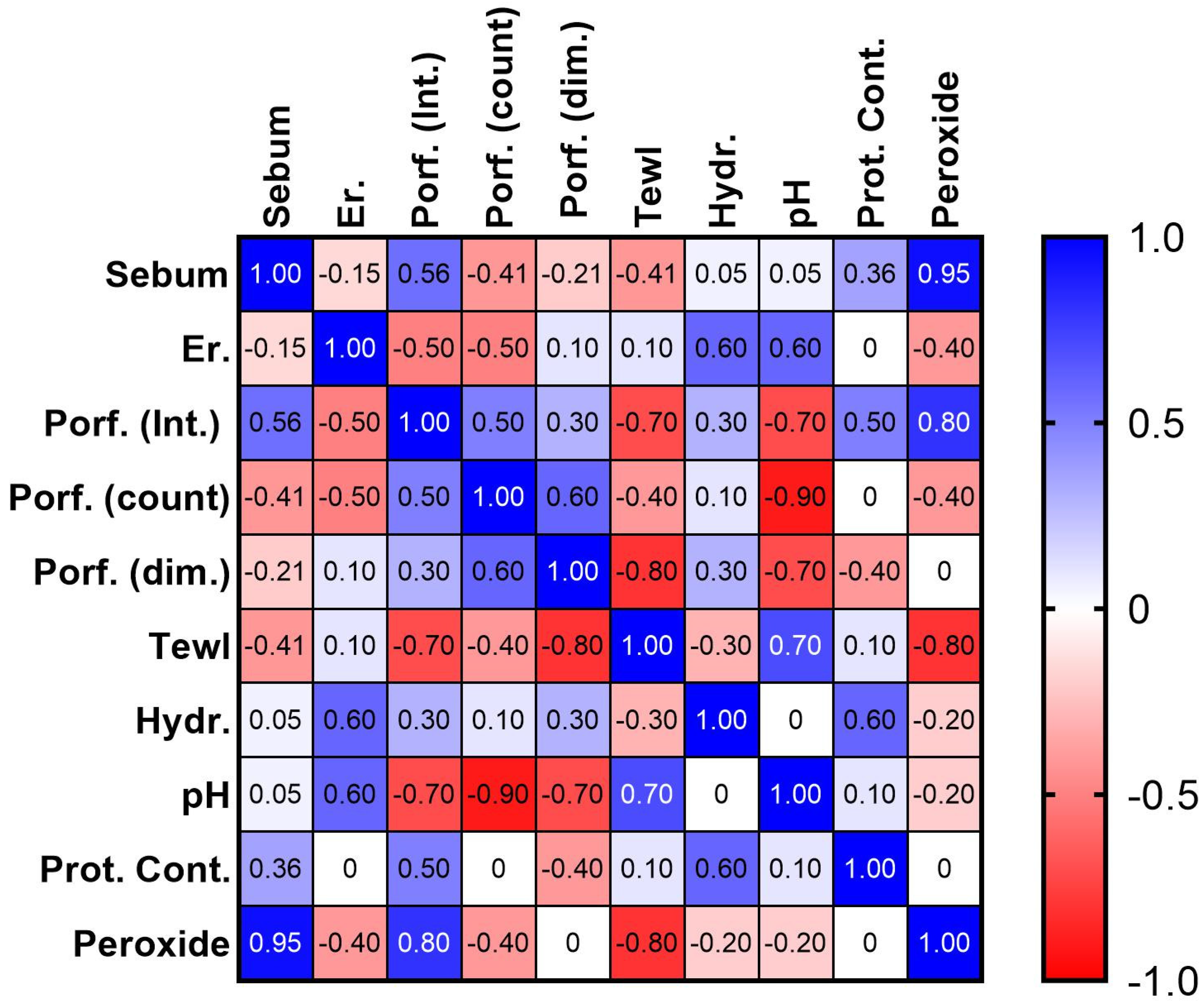
2.3.5. Skin Biophysical Parameters Acquisition
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. In Vivo Experiment
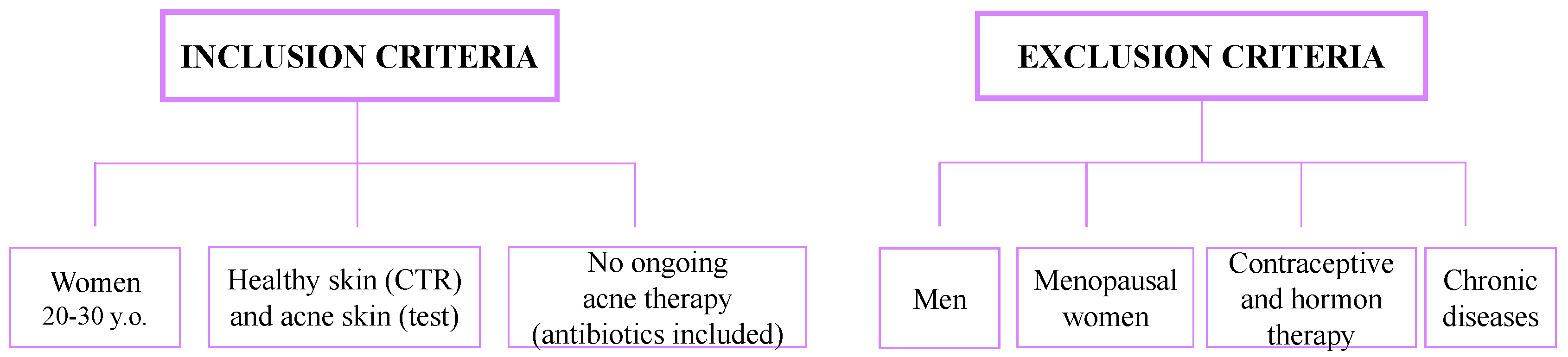
4.2.2. Selection of Acne Patients
4.2.3. Acquisition of Biophysical Parameters
4.2.4. In Vivo Squalene Analysis
Extraction Procedure
4.2.5. High-Performance Liquid Chromatography (HPLC) Analyses
HPLC Method Validation
- ▪
- For the measurement of precision, it is necessary to make repeated measurements over time and obtain similar results. For this purpose, triplicate analyses were performed on the same day, using a concentration of 90 ug/mL that corresponds to the midpoint of the calibration curve (intra-day assay). The same value was then used to perform triplicate analyses on two subsequent days (inter-day assay). Repeatability and intermediate precision results should not exceed a value of 5% [24].
- ▪
- Eight different concentrations (5–200 μg/mL) were analyzed for three consecutive days to measure linearity. The analyses were performed in triplicate, obtaining the coefficients of determination R2.
- ▪
- To assess the validity of the linear regression, the data obtained were subjected to statistical analysis with the test ANOVA one-way and significance level of α = 0.05 (95% confidence interval).
- ▪
- The accuracy was determined by recovery of known amounts of squalene reference standard added to the samples at the beginning of the process. Briefly, 50, 70 and 100 μg/mL of standard squalene was added to sample. The percentage recovery of added squalene standard was calculated. Moreover, standard deviation (SD) and the relative standard deviation (RSD%) were determined.
- ▪
- Limit of detection (LOD) and limit of quantification (LOQ) were based on standard deviation of the response and the slope. The detection limit (LOD) and the quantification limit (LOQ) may be expressed by the Formulae (1) and (2):
4.2.6. Standard Solutions and the Sample Preparation
4.2.7. In Vitro Peroxidation Analysis
Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tan, J.; Chavda, R.; Baldwin, H.; Dreno, B. Management of acne vulgaris with trifarotene. J. Cutan. Med. Surg. 2023, 27, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Condrò, G.; Guerini, M.; Castello, M.; Perugini, P. Acne vulgaris, atopic dermatitis and rosacea: The Role of the Skin Microbiota—A Review. Biomedicines 2022, 10, 2523. [Google Scholar] [CrossRef] [PubMed]
- Moradi Tuchayi, S.; Makrantonaki, E.; Ganceviciene, R.; Dessinioti, C.; Feldman, S.R.; Zouboulis, C.C. Acne vulgaris. Nat. Rev. Dis. Primers 2015, 1, 15029. [Google Scholar] [CrossRef]
- Pappas, A.; Johnsen, S.; Liu, J.-C.; Eisinger, M. Sebum analysis of individuals with and without acne. Dermato. Endocrinol. 2009, 1, 157–161. [Google Scholar] [CrossRef]
- Gopakumar, K. Therapeutic applications of squalene—A review. Fish. Technol. 2012, 49, 1–9. [Google Scholar]
- Valacchi, G.; De Luca, C.; Wertz, P.W. Lipid mediators in skin inflammation: Updates and current Views. Mediat. Inflamm. 2010, 2010, 398926. [Google Scholar] [CrossRef][Green Version]
- Ottaviani, M.; Alestas, T.; Flori, E.; Mastrofrancesco, A.; Zouboulis, C.C.; Picardo, M. Peroxidated squalene induces the production of inflammatory mediators in HaCaT keratinocytes: A possible role in acne vulgaris. J. Investig. Dermatol. 2006, 126, 2430–2437. [Google Scholar] [CrossRef]
- Assi, A.; Michael-Jubeli, R.; Jacques-Jamin, C.; Duplan, H.; Baillet-Guffroy, A.; Tfayli, A. Skin surface lipids photo-oxidation: A vibrational spectroscopy study. J. Raman Spectrosc. 2023, 54, 487–500. [Google Scholar] [CrossRef]
- Allison, A.C. Squalene and Squalane Emulsions as Adjuvants. Methods 1999, 19, 87–93. [Google Scholar] [CrossRef]
- Sarici, G.; Cinar, S.; Armutcu, F.; Altınyazar, C.; Koca, R.; Tekin, N. Oxidative stress in acne vulgaris. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 763–767. [Google Scholar] [CrossRef]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef] [PubMed]
- Piquero-Casals, J.; Morgado-Carrasco, D.; Rozas-Muñoz, E.; Mir-Bonafé, J.F.; Trullàs, C.; Jourdan, E.; Piquero-Martin, J.; Zouboulis, C.C.; Krutmann, J. Sun exposure, a relevant exposome factor in acne patients and how photoprotection can improve outcomes. J. Cosmet. Dermatol. 2023, 22, 1919–1928. [Google Scholar] [CrossRef] [PubMed]
- Shu, M.; Kuo, S.; Wang, Y.; Jiang, Y.; Liu, Y.-T.; Gallo, R.; Huang, C.-M. Porphyrin metabolisms in human skin commensal propionibacterium acnes bacteria: Potential application to monitor human radiation risk. Curr. Med. Chem. 2013, 20, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.; Kang, D.; Barnard, E.; Li, H. Strain-level differences in porphyrin production and regulation in propionibacterium acnes elucidate disease associations. mSphere 2016, 1, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zecchini, M.; Lucas, R.A.; Robertson, C.; Coban, T.; Thatti, R.; Le Gresley, A. Investigation of the Formation of Squalene Oligomers Exposed to Ultraviolet Light and Changes in the Exposed Squalene as a Potential Skin Model. Molecules 2022, 27, 3481. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.; Boussouira, B.; Moyal, D.; Nguyen, Q. Oxidization of squalene, a human skin lipid: A new and reliable marker of environmental pollution studies. Int. J. Cosmet. Sci. 2015, 37, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Perugini, P.; Grignani, C.; Condrò, G.; van der Hoeven, H.; Ratti, A.; Mondelli, A.; Colpani, A.; Bleve, M. Skin microbiota: Setting up a protocol to evaluate a correlation between the microbial flora and skin parameters. Biomedicines 2023, 11, 966. [Google Scholar] [CrossRef]
- Ishikawa, A.; Ito, J.; Shimizu, N.; Kato, S.; Kobayashi, E.; Ohnari, H.; Sakata, O.; Naru, E.; Nakagawa, K. Linoleic acid and squalene are oxidized by discrete oxidation mechanisms in human sebum. Ann. N. Y. Acad. Sci. 2021, 1500, 112–121. [Google Scholar] [CrossRef]
- Shimizu, N.; Ito, J.; Kato, S.; Eitsuka, T.; Saito, T.; Nishida, H.; Miyazawa, T.; Nakagawa, K. Evaluation of squalene oxidation mechanisms in human skin surface lipids and shark liver oil supplements. Ann. N. Y. Acad. Sci. 2019, 1457, 158–165. [Google Scholar] [CrossRef]
- Mudiyanselage, S.E.; Elsner, P.; Thiele, J.J.; Hamburger, M. Ultraviolet a induces generation of squalene monohydroperoxide isomers in human sebum and skin surface lipids in vitro and in vivo. J. Investig. Dermatol. 2003, 120, 915–922. [Google Scholar] [CrossRef]
- Chiba, K.; Yoshizawa, K.; Makino, I.; Kawakami, K.; Onoue, M. Comedogenicity of squalene monohydroperoxide in the skin after topical application. J. Toxicol. Sci. 2000, 25, 77–83. [Google Scholar] [CrossRef]
- Bavisetty, S.C.B.; Narayan, B. An improved RP-HPLC method for simultaneous analyses of squalene and cholesterol especially in aquatic foods. J. Food Sci. Technol. 2015, 52, 6083–6089. [Google Scholar] [CrossRef]
- Auffray, B. Protection against singlet oxygen, the main actor of sebum squalene peroxidation during sun exposure, using Commiphora myrrha essential oil. Int. J. Cosmet. Sci. 2007, 29, 23–29. [Google Scholar] [CrossRef]
- Zaenglein, A.L.; Pathy, A.L.; Schlosser, B.J.; Alikhan, A.; Baldwin, H.E.; Berson, D.S.; Bowe, W.P.; Graber, E.M.; Harper, J.C.; Kang, S.; et al. Guidelines of care for the management of acne vulgaris. J. Am. Acad. Dermatol. 2016, 74, 945–973.e33. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association declaration of Helsinki ethical principles for medical research involving human subjects. JAMA 2013, 310, 20. [Google Scholar]
- Delgado-Arias, S.; Zapata-Valencia, S.; Cano-Agudelo, Y.; Osorio-Arias, J.; Vega-Castro, O. Evaluation of the antioxidant and physical properties of an exfoliating cream developed from coffee grounds. J. Food Process. Eng. 2020, 43, 1–10. [Google Scholar] [CrossRef]
- Verdier-Sévrain, S.; Bonté, F. Skin hydration: A review on its molecular mechanisms. J. Cosmet. Dermatol. 2007, 6, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Pinnagoda, J.; Tupkek, R.A.; Agner, T.; Serup, J. Guidelines for transepidermal water loss (TEWL) measurement. Contact Dermat. 1990, 22, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Chavez, J.J.; Merino-Sanjuán, V.; López-Cervantes, M.; Urban-Morlan, Z.; Piñón-Segundo, E.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. The tape-stripping technique as a method for drug quantification in skin. J. Pharm. Pharm. Sci. 2008, 11, 104–130. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Validation of Analytical Procedures: Text and Methodology; ICH Guideline Q2(R1); European Medicines Agency: Geneva, Switzerland, 2005. [Google Scholar]
- Sander, C.S.; Chang, H.; Salzmann, S.; Müller, C.S.L.; Ekanayake-Mudiyanselage, S.; Elsner, P.; Thiele, J.J. Photoaging is associated with protein oxidation in human skin in vivo. J. Investig. Dermatol. 2002, 118, 618–625. [Google Scholar] [CrossRef]
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef] [PubMed]
| Parameters | |
|---|---|
| Regression equation | y = 0.7973x + 1.4809 |
| Correlation equation | R2 = 0.9994 |
| Range [ug/mL] | 5–200 |
| LOD [ug/mL] | 9.72 |
| LOQ [ug/mL] | 29.45 |
| PARAMETERS | ACNE SKIN | HEALTHY SKIN | Variation Acneic vs. Healthy Skin (%) | ||
|---|---|---|---|---|---|
| TEWL (g/m2 h) | 12.16 ± 2.56 | 9.85 ± 1.09 | 23.45 | ||
| Protein content (µg/cm2) | STRIP 1 | 13.68 ± 1.92 | STRIP 1 | 8.90 ± 2.60 | 53.71 |
| STRIP 2 | 11.82 ± 1.69 | STRIP 2 | 8.70 ± 1.70 | 35.86 | |
| STRIP 3 | 10.34 ± 1.95 | STRIP 3 | 8.30 ± 1.35 | 24.58 | |
| pH | 5.40 ± 0.22 | 5.18 ± 0.37 | 4.25 | ||
| Sebum levels (µg/cm2) | 172.2 ± 55.90 | 81.70 ± 36.35 | 110.77 | ||
| Parameters | |
|---|---|
| Column | CAPCELL PAK (Shiseido) C18 UG 120 Å 5 µm, 250 mm × 4.6 mm |
| Flow | 1.5 mL/min |
| Column temperature | 25 °C |
| Injection volume | 50 μL |
| Wavelength | 210 nm |
| Time course | 10 min |
| Mobile phase | Ethanol/Acetonitrile 70:30 Isocratic elution |
| Retention time | 4.096 min for squalene |
| Coefficient Value | Strength Interpretation | |
|---|---|---|
| +1 | −1 | Perfect positive or negative correlation |
| +0.9–0.7 | −0.9–0.7 | Very strong correlation |
| +0.6–0.4 | −0.6–0.4 | Strong correlation |
| +0.3 | −0.3 | Moderate correlation |
| +0.2 | −0.2 | Weak correlation |
| +0.1 | −0.1 | Negligible correlation |
| 0 | 0 | No correlation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Condrò, G.; Sciortino, R.; Perugini, P. Squalene Peroxidation and Biophysical Parameters in Acne-Prone Skin: A Pilot “In Vivo” Study. Pharmaceuticals 2023, 16, 1704. https://doi.org/10.3390/ph16121704
Condrò G, Sciortino R, Perugini P. Squalene Peroxidation and Biophysical Parameters in Acne-Prone Skin: A Pilot “In Vivo” Study. Pharmaceuticals. 2023; 16(12):1704. https://doi.org/10.3390/ph16121704
Chicago/Turabian StyleCondrò, Giorgia, Roberta Sciortino, and Paola Perugini. 2023. "Squalene Peroxidation and Biophysical Parameters in Acne-Prone Skin: A Pilot “In Vivo” Study" Pharmaceuticals 16, no. 12: 1704. https://doi.org/10.3390/ph16121704
APA StyleCondrò, G., Sciortino, R., & Perugini, P. (2023). Squalene Peroxidation and Biophysical Parameters in Acne-Prone Skin: A Pilot “In Vivo” Study. Pharmaceuticals, 16(12), 1704. https://doi.org/10.3390/ph16121704






