Nanostructured Lipid Carriers as Robust Systems for Lupeol Delivery in the Treatment of Experimental Visceral Leishmaniasis
Abstract
:1. Introduction
2. Results
2.1. Physical–Chemical Characterization of NLC
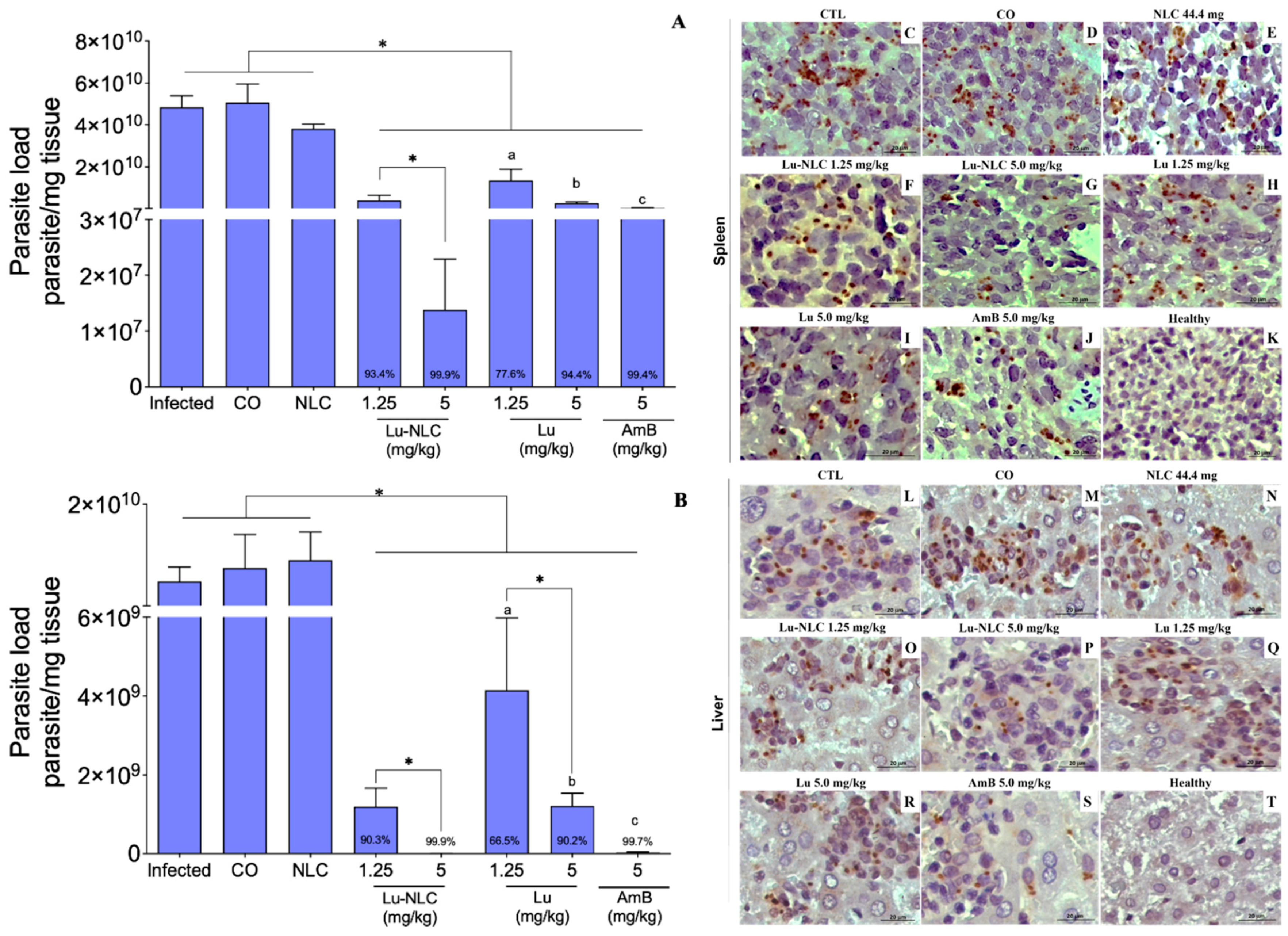
2.2. Analysis of the Therapeutic Potential of Lu-NLC
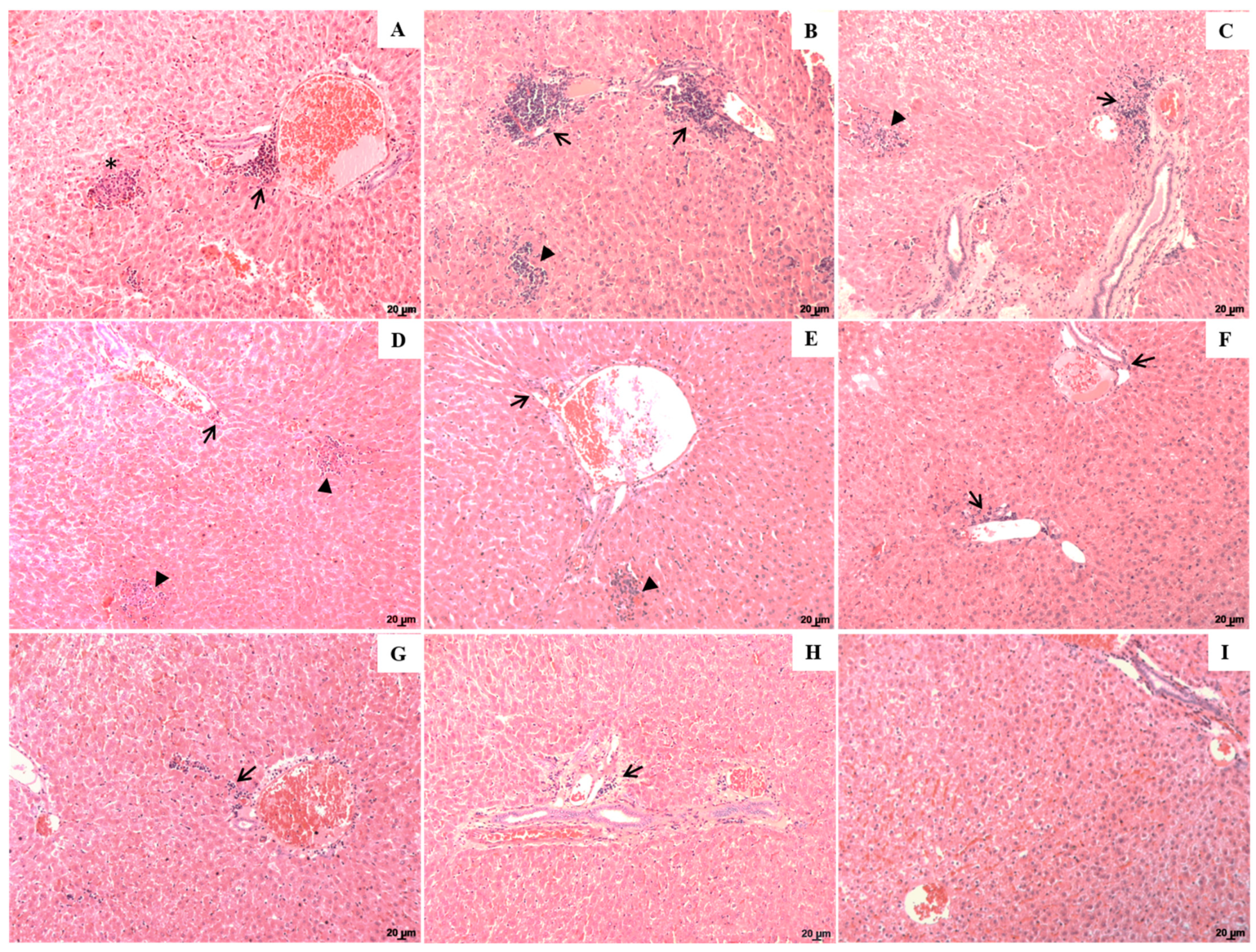
2.3. Histopathological Alterations in the Spleen and Liver of Animals Treated with Lu-NLC or Lu
2.4. Analysis of Antibody Production
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of NLC
4.3. Physical–Chemical Characterization of Nanoparticles
4.3.1. Determination of Encapsulation Efficiency
4.3.2. Determination of Size, Polydispersity, and Zeta Potential
4.3.3. Transmission Electron Microscopy of Lu-NLC
4.4. Animals
4.5. Analysis of the Efficacy of Lu-NLC in Experimental Visceral Leishmaniasis
4.5.1. Evaluation of Parasite Burden
4.5.2. Analysis of Antibody Production
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilhelm, T.J. Viszerale Leishmaniose. Der. Chir. 2019, 90, 833–837. [Google Scholar] [CrossRef]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- PAHO Leishmaniose. Available online: https://www.paho.org/pt/topicos/leishmaniose (accessed on 4 July 2023).
- World Health Organization Leishmaniasis. Available online: https://www.who.int/leishmaniasis/en/ (accessed on 21 October 2023).
- da Silva, T.B.F.; Silveira, F.T.; Tomokane, T.Y.; da Silva Batista, L.F.; Nunes, J.B.; da Matta, V.L.R.; Passero, L.F.D.; Laurenti, M.D. Reactivity of Purified and Axenic Amastigotes as a Source of Antigens to Be Used in Serodiagnosis of Canine Visceral Leishmaniasis. Parasitol. Int. 2020, 79, 102177. [Google Scholar] [CrossRef]
- Di Loria, A.; Dattilo, V.; Santoro, D.; Guccione, J.; De Luca, A.; Ciaramella, P.; Pirozzi, M.; Iaccino, E. Expression of Serum Exosomal MiRNA 122 and Lipoprotein Levels in Dogs Naturally Infected by Leishmania infantum: A Preliminary Study. Animals 2020, 10, 100. [Google Scholar] [CrossRef]
- Kevric, I.; Cappel, M.A.; Keeling, J.H. New World and Old World Leishmania Infections. Dermatol. Clin. 2015, 33, 579–593. [Google Scholar] [CrossRef]
- Ghorbani, M.; Farhoudi, R. Leishmaniasis in Humans: Drug or Vaccine Therapy? Drug Des. Dev. Ther. 2017, 12, 25–40. [Google Scholar] [CrossRef]
- Sundar, S.; Chakravarty, J. An Update on Pharmacotherapy for Leishmaniasis. Expert Opin. Pharmacother. 2015, 16, 237–252. [Google Scholar] [CrossRef]
- Uliana, S.R.B.; Trinconi, C.T.; Coelho, A.C. Chemotherapy of Leishmaniasis: Present Challenges. Parasitology 2018, 145, 464–480. [Google Scholar] [CrossRef] [PubMed]
- Siddique, H.R.; Saleem, M. Beneficial Health Effects of Lupeol Triterpene: A Review of Preclinical Studies. Life Sci. 2011, 88, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Rajemiarimiraho, M.; Banzouzi, J.-T.; Nicolau-Travers, M.-L.; Ramos, S.; Cheikh-Ali, Z.; Bories, C.; Rakotonandrasana, O.; Rakotonandrasana, S.; Andrianary, P.; Benoit-Vical, F. Antiprotozoal Activities of Millettia richardiana (Fabaceae) from Madagascar. Molecules 2014, 19, 4200–4211. [Google Scholar] [CrossRef]
- Al Musayeib, N.; Mothana, R.; Gamal, A.; Al-Massarani, S.; Maes, L. In Vitro Antiprotozoal Activity of Triterpenoid Constituents of Kleinia Odora Growing in Saudi Arabia. Molecules 2013, 18, 9207–9218. [Google Scholar] [CrossRef]
- Souza, A.C.; de Alves, M.M.M.; Brito, L.M.; Oliveira, L.G.D.C.; Sobrinho-Júnior, E.P.C.; Costa, I.C.G.; Freitas, S.D.L.; da Franca Rodrigues, K.A.; Chaves, M.H.; Arcanjo, D.D.R.; et al. Platonia Insignis Mart., a Brazilian Amazonian Plant: The Stem Barks Extract and Its Main Constituent Lupeol Exert Antileishmanial Effects Involving Macrophages Activation. Evid.-Based Complement. Altern. Med. 2017, 2017, 3126458. [Google Scholar] [CrossRef] [PubMed]
- de Jesus, J.A.; Laurenti, M.D.; Antonangelo, L.; Faria, C.S.; Lago, J.H.G.; Passero, L.F.D. Related Pentacyclic Triterpenes Have Immunomodulatory Activity in Chronic Experimental Visceral Leishmaniasis. J. Immunol. Res. 2021, 2021, 6671287. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Chauhan, K.; Kaur, S. Lupeol Induces Immunity and Protective Efficacy in a Murine Model against Visceral Leishmaniasis. Parasitology 2019, 146, 1440–1450. [Google Scholar] [CrossRef] [PubMed]
- Jesus, J.A.; Sousa, I.M.O.; da Silva, T.N.F.; Ferreira, A.F.; Laurenti, M.D.; Antonangelo, L.; Faria, C.S.; da Costa, P.C.; de Carvalho Ferreira, D.; Passero, L.F.D. Preclinical Assessment of Ursolic Acid Loaded into Nanostructured Lipid Carriers in Experimental Visceral Leishmaniasis. Pharmaceutics 2021, 13, 908. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, F.; Farhadian, N.; Mashayekhi Goyonlo, V. Anti-Leishmaniasis Effect of Nano-Structured Lipid Carrier Containing Glucantime Drug: Preparation, Characterization, in-Vitro and in-Vivo Evaluation. J. Drug Deliv. Sci. Technol. 2023, 86, 104668. [Google Scholar] [CrossRef]
- Khan, A.S.; ud Din, F.; Ali, Z.; Bibi, M.; Zahid, F.; Zeb, A.; Mujeeb-ur-Rehman; Khan, G.M. Development, in Vitro and in Vivo Evaluation of Miltefosine Loaded Nanostructured Lipid Carriers for the Treatment of Cutaneous Leishmaniasis. Int. J. Pharm. 2021, 593, 120109. [Google Scholar] [CrossRef]
- Rebouças-Silva, J.; Tadini, M.C.; Devequi-Nunes, D.; Mansur, A.L.; Silveira-Mattos, P.S.; de Oliveira, C.I.; Formiga, F.R.; Berretta, A.A.; Marquele-Oliveira, F.; Borges, V.M. Evaluation of in Vitro and in Vivo Efficacy of a Novel Amphotericin B-Loaded Nanostructured Lipid Carrier in the Treatment of Leishmania braziliensis Infection. Int. J. Nanomed. 2020, 15, 8659–8672. [Google Scholar] [CrossRef]
- Jamal, F.; Altaf, I.; Ahmed, G.; Asad, S.; Ahmad, H.; Zia, Q.; Azhar, A.; Farheen, S.; Shafi, T.; Karim, S.; et al. Amphotericin B Nano-Assemblies Circumvent Intrinsic Toxicity and Ensure Superior Protection in Experimental Visceral Leishmaniasis with Feeble Toxic Manifestation. Vaccines 2023, 11, 100. [Google Scholar] [CrossRef]
- Vieira, A.C.; Magalhães, J.; Rocha, S.; Cardoso, M.S.; Santos, S.G.; Borges, M.; Pinheiro, M.; Reis, S. Targeted Macrophages Delivery of Rifampicin-Loaded Lipid Nanoparticles to Improve Tuberculosis Treatment. Nanomedicine 2017, 12, 2721–2736. [Google Scholar] [CrossRef]
- Passero, L.F.D.; dos Brunelli, E.S.; Sauini, T.; Amorim Pavani, T.F.; Jesus, J.A.; Rodrigues, E. The Potential of Traditional Knowledge to Develop Effective Medicines for the Treatment of Leishmaniasis. Front. Pharmacol. 2021, 12, 690432. [Google Scholar] [CrossRef]
- Riaz, A.; Hendricks, S.; Elbrink, K.; Guy, C.; Maes, L.; Ahmed, N.; Kiekens, F.; Khan, G.M. Preparation and Characterization of Nanostructured Lipid Carriers for Improved Topical Drug Delivery: Evaluation in Cutaneous Leishmaniasis and Vaginal Candidiasis Animal Models. AAPS PharmSciTech 2020, 21, 185. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Jawed, J.J.; Das, M.C.; Sandhu, P.; De, U.C.; Dinda, B.; Akhter, Y.; Bhattacharjee, S. Antileishmanial and Immunomodulatory Activities of Lupeol, a Triterpene Compound Isolated from Sterculia villosa. Int. J. Antimicrob. Agents 2017, 50, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Jawed, J.J.; Das, M.C.; Parveen, S.; Ghosh, C.; Majumdar, S.; Saha, B.; Bhattacharjee, S. Lupeol and Amphotericin B Mediate Synergistic Anti-Leishmanial Immunomodulatory Effects in Leishmania donovani-Infected BALB/c Mice. Cytokine 2021, 137, 155319. [Google Scholar] [CrossRef] [PubMed]
- Madane, R.G.; Mahajan, H.S. Curcumin-Loaded Nanostructured Lipid Carriers (NLCs) for Nasal Administration: Design, Characterization, and in Vivo Study. Drug Deliv. 2016, 23, 1326–1334. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The Effect of Nanoparticle Size on in Vivo Pharmacokinetics and Cellular Interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.N.M.; Breitkreitz, M.C.; Guilherme, V.A.; da Silva, G.H.R.; Couto, V.M.; Castro, S.R.; de Paula, B.O.; Machado, D.; de Paula, E. Natural Lipids-Based NLC Containing Lidocaine: From Pre-Formulation to in Vivo Studies. Eur. J. Pharm. Sci. 2017, 106, 102–112. [Google Scholar] [CrossRef]
- de Ribeiro, L.N.M.; de Paula, E.; Rossi, D.A.; Martins, F.A.; de Melo, R.T.; Monteiro, G.P.; Breitkreitz, M.C.; Goulart, L.R.; Fonseca, B.B. Nanocarriers From Natural Lipids With In Vitro Activity Against Campylobacter Jejuni. Front. Cell. Infect. Microbiol. 2021, 10, 571040. [Google Scholar] [CrossRef]
- Negi, L.M.; Jaggi, M.; Talegaonkar, S. Development of Protocol for Screening the Formulation Components and the Assessment of Common Quality Problems of Nano-Structured Lipid Carriers. Int. J. Pharm. 2014, 461, 403–410. [Google Scholar] [CrossRef]
- Sakellari, G.I.; Zafeiri, I.; Batchelor, H.; Spyropoulos, F. Formulation Design, Production and Characterisation of Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) for the Encapsulation of a Model Hydrophobic Active. Food Hydrocoll. Health 2021, 1, 100024. [Google Scholar] [CrossRef]
- Jesus, J.A.; Fragoso, T.N.; Yamamoto, E.S.; Laurenti, M.D.; Silva, M.S.; Ferreira, A.F.; Lago, J.H.G.; Santos-Gomes, G.; Passero, L.F.D. Therapeutic Effect of Ursolic Acid in Experimental Visceral Leishmaniasis. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 1–11, Erratum in Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 250. [Google Scholar] [CrossRef]
- Monteiro, L.M.; Löbenberg, R.; Barbosa, E.J.; de Araujo, G.L.B.; Sato, P.K.; Kanashiro, E.; de Araujo Eliodoro, R.H.; Rocha, M.; de Freitas, V.L.T.; Fotaki, N.; et al. Oral Administration of Buparvaquone Nanostructured Lipid Carrier Enables in Vivo Activity against Leishmania infantum. Eur. J. Pharm. Sci. 2022, 169, 106097. [Google Scholar] [CrossRef] [PubMed]
- Kar, N.; Chakraborty, S.; De, A.K.; Ghosh, S.; Bera, T. Development and Evaluation of a Cedrol-Loaded Nanostructured Lipid Carrier System for in Vitro and in Vivo Susceptibilities of Wild and Drug Resistant Leishmania donovani Amastigotes. Eur. J. Pharm. Sci. 2017, 104, 196–211. [Google Scholar] [CrossRef]
- Üner, M.; Karaman, E.; Aydoğmuş, Z. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers of Loratadine for Topical Application: Physicochemical Stability and Drug Penetration through Rat Skin. Trop. J. Pharm. Res. 2014, 13, 653. [Google Scholar] [CrossRef]
- Galvão, J.G.; Santos, R.L.; Silva, A.R.S.T.; Santos, J.S.; Costa, A.M.B.; Chandasana, H.; Andrade-Neto, V.V.; Torres-Santos, E.C.; Lira, A.A.M.; Dolabella, S.; et al. Carvacrol loaded nanostructured lipid carriers as a promising parenteral formulation for leishmaniasis treatment. Eur. J. Pharm. Sci. 2020, 150, 105335. [Google Scholar] [CrossRef]
- Riça-Capela, M.; Cortes, S.; Leandro, C.; Peleteiro, M.; Santos-Gomes, G.; Campino, L. Immunological and Histopathological Studies in a Rodent Model Infected with Leishmania infantum Promastigotes or Amastigotes. Parasitol. Res. 2003, 89, 163–169. [Google Scholar] [CrossRef]
- Vianna, V.; Takiya, C.; de Brito-Gitirana, L. Histopathologic Analysis of Hamster Hepatocytes Submitted to Experimental Infection with Leishmania donovani. Parasitol. Res. 2002, 88, 829–836. [Google Scholar] [CrossRef]
- Leandro, C.; Santos-Gomes, G.; Campino, L.; Romão, P.; Cortes, S.; Rolão, N.; Gomes-Pereira, S.; Riça Capela, M.; Abranches, P. Cell Mediated Immunity and Specific IgG1 and IgG2 Antibody Response in Natural and Experimental Canine Leishmaniosis. Vet. Immunol. Immunopathol. 2001, 79, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Todolí, F.; Rodríguez-Cortés, A.; del Núñez, M.C.; Laurenti, M.D.; Gómez-Sebastián, S.; Rodríguez, F.; Pérez-Martín, E.; Escribano, J.M.; Alberola, J. Head-to-Head Comparison of Three Vaccination Strategies Based on DNA and Raw Insect-Derived Recombinant Proteins against Leishmania. PLoS ONE 2012, 7, e51181. [Google Scholar] [CrossRef] [PubMed]
- Rostamian, M.; Akya, A.; Niknam, H.M. Heterogeneity of Humoral Immune Response to Leishmania tropica in an Experimental Model. Parasitol. Res. 2019, 118, 1231–1237. [Google Scholar] [CrossRef]
- Volpedo, G.; Costa, L.; Ryan, N.; Halsey, G.; Satoskar, A.; Oghumu, S. Nanoparticulate Drug Delivery Systems for the Treatment of Neglected Tropical Protozoan Diseases. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019, 25, e144118. [Google Scholar] [CrossRef]
- de Eloy, J.O.; de Oliveira, E.C.V.; Marotta-Oliveira, S.S.; Saraiva, J.; Marchetti, J.M. Desenvolvimento e Validação de Um Método Analítico Por CLAE Para Quantificação de Ácido Ursólico Em Dispersões Sólidas. Quim. Nova 2012, 35, 1036–1040. [Google Scholar] [CrossRef]
- Taralkar, S.V.; Chattopadhyay, S. A HPLC Method for Determination of Ursolic Acid and Betulinic Acids from Their Methanolic Extracts of Vitex Negundo Linn. J. Anal. Bioanal. Tech. 2012, 3, 134. [Google Scholar] [CrossRef]
- Das, S.; Ng, W.K.; Tan, R.B.H. Sucrose Ester Stabilized Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: I. Effect of Formulation Variables on the Physicochemical Properties, Drug Release and Stability of Clotrimazole-Loaded Nanoparticles. Nanotechnology 2014, 25, 105101. [Google Scholar] [CrossRef] [PubMed]
- Corral, M.J.; Serrano, D.R.; Moreno, I.; Torrado, J.J.; Dominguez, M.; Alunda, J.M. Efficacy of Low Doses of Amphotericin B plus Allicin against Experimental Visceral Leishmaniasis. J. Antimicrob. Chemother. 2014, 69, 3268–3274. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.K.; Poon, R.T.P.; Wo, J.Y.; Ma, S.; Guan, X.-Y.; Myers, J.N.; Altevogt, P.; Yuen, A.P.W. Lupeol Suppresses Cisplatin-Induced Nuclear Factor-KappaB Activation in Head and Neck Squamous Cell Carcinoma and Inhibits Local Invasion and Nodal Metastasis in an Orthotopic Nude Mouse Model. Cancer Res. 2007, 67, 8800–8809. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, I.; Saleem, M.; Adhami, V.M.; Hafeez, B.B.; Mukhtar, H. Suppression of CFLIP by Lupeol, a Dietary Triterpene, Is Sufficient to Overcome Resistance to TRAIL-Mediated Apoptosis in Chemoresistant Human Pancreatic Cancer Cells. Cancer Res. 2009, 69, 1156–1165. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Kaur, S.; Kweon, M.-H.; Adhami, V.M.; Afaq, F.; Mukhtar, H. Lupeol, a Fruit and Vegetable Based Triterpene, Induces Apoptotic Death of Human Pancreatic Adenocarcinoma Cells via Inhibition of Ras Signaling Pathway. Carcinogenesis 2005, 26, 1956–1964. [Google Scholar] [CrossRef]
- Laurenti, M.D.; Passero, L.F.D.; Tomokane, T.Y.; de Francesquini, F.C.; Rocha, M.C.; de Castro Gomes, C.M.; Corbett, C.E.P.; Silveira, F.T. Dynamic of the Cellular Immune Response at the Dermal Site of Leishmania (L.) amazonensis and Leishmania (V.) braziliensis Infection in Sapajus Apella Primate. Biomed Res. Int. 2014, 2014, 134236. [Google Scholar] [CrossRef]
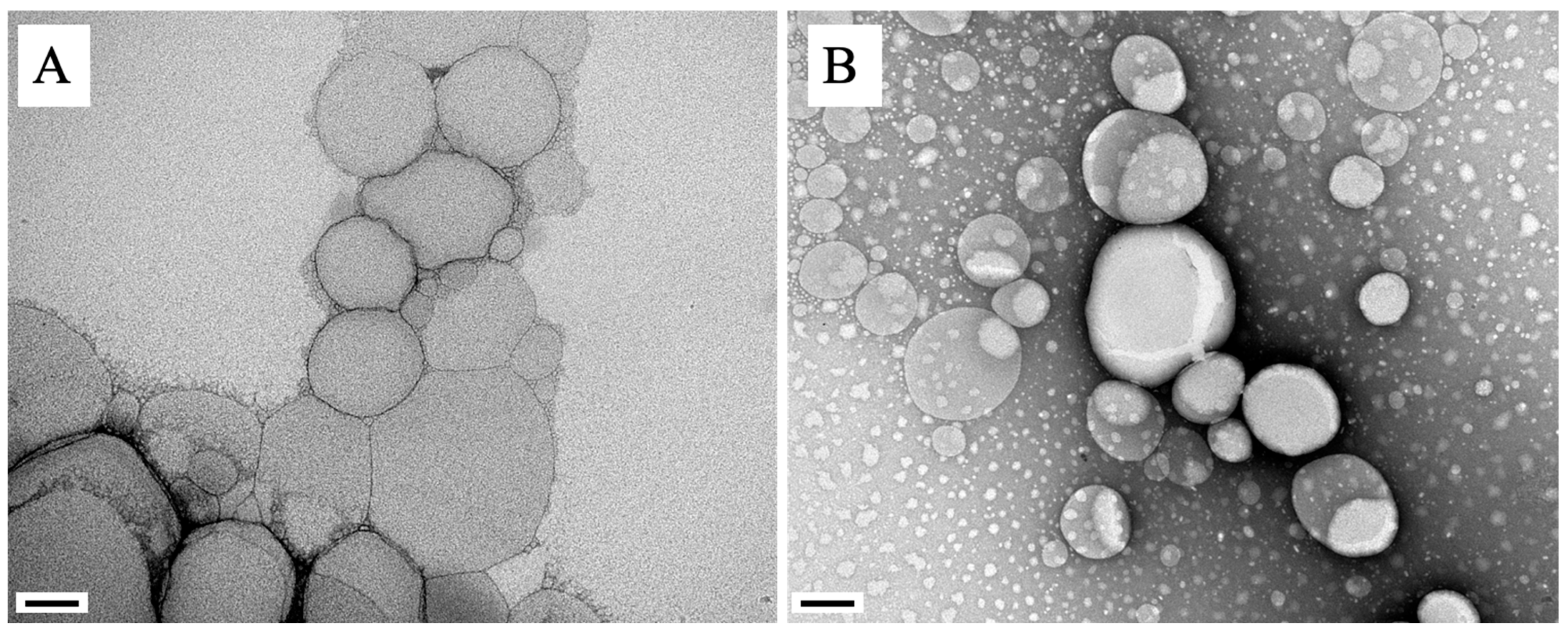


| Nanoparticle | PS (nm) | PDI | ZP (mV) | EE (%) |
|---|---|---|---|---|
| NLC | 266.3 ± 2.6 | 0.16 ± 0.013 | −26.5 ± 1.18 | |
| Lu-NLC | 265.3 ± 4.6 | 0.21 ± 0.011 | −37.2 ± 0.84 | 84.04 ± 0.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jesus, J.A.; da Silva, T.N.F.; Sousa, I.M.O.; Ferreira, A.F.; Laurenti, M.D.; da Costa, P.C.; de Carvalho Ferreira, D.; Passero, L.F.D. Nanostructured Lipid Carriers as Robust Systems for Lupeol Delivery in the Treatment of Experimental Visceral Leishmaniasis. Pharmaceuticals 2023, 16, 1646. https://doi.org/10.3390/ph16121646
Jesus JA, da Silva TNF, Sousa IMO, Ferreira AF, Laurenti MD, da Costa PC, de Carvalho Ferreira D, Passero LFD. Nanostructured Lipid Carriers as Robust Systems for Lupeol Delivery in the Treatment of Experimental Visceral Leishmaniasis. Pharmaceuticals. 2023; 16(12):1646. https://doi.org/10.3390/ph16121646
Chicago/Turabian StyleJesus, Jéssica Adriana, Thays Nicolli Fragoso da Silva, Ilza Maria Oliveira Sousa, Aurea Favero Ferreira, Márcia Dalastra Laurenti, Paulo Cardoso da Costa, Domingos de Carvalho Ferreira, and Luiz Felipe Domingues Passero. 2023. "Nanostructured Lipid Carriers as Robust Systems for Lupeol Delivery in the Treatment of Experimental Visceral Leishmaniasis" Pharmaceuticals 16, no. 12: 1646. https://doi.org/10.3390/ph16121646
APA StyleJesus, J. A., da Silva, T. N. F., Sousa, I. M. O., Ferreira, A. F., Laurenti, M. D., da Costa, P. C., de Carvalho Ferreira, D., & Passero, L. F. D. (2023). Nanostructured Lipid Carriers as Robust Systems for Lupeol Delivery in the Treatment of Experimental Visceral Leishmaniasis. Pharmaceuticals, 16(12), 1646. https://doi.org/10.3390/ph16121646









