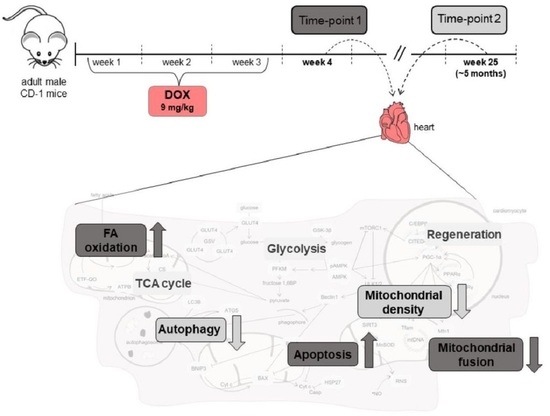
The Metabolic Fingerprint of Doxorubicin-Induced Cardiotoxicity in Male CD-1 Mice Fades Away with Time While Autophagy Increases
Abstract
:1. Introduction
2. Results
2.1. Doxorubicin Treatment Decreased Whole-Body and Heart Weight Five Months after Exposure
2.2. Doxorubicin Treatment Induced a Tendency for Decreased Albumin Concentration One Week after Exposure, and a Tendency for Decreased Content of C Reactive Protein (CRP) Five Months after Exposure on Serum
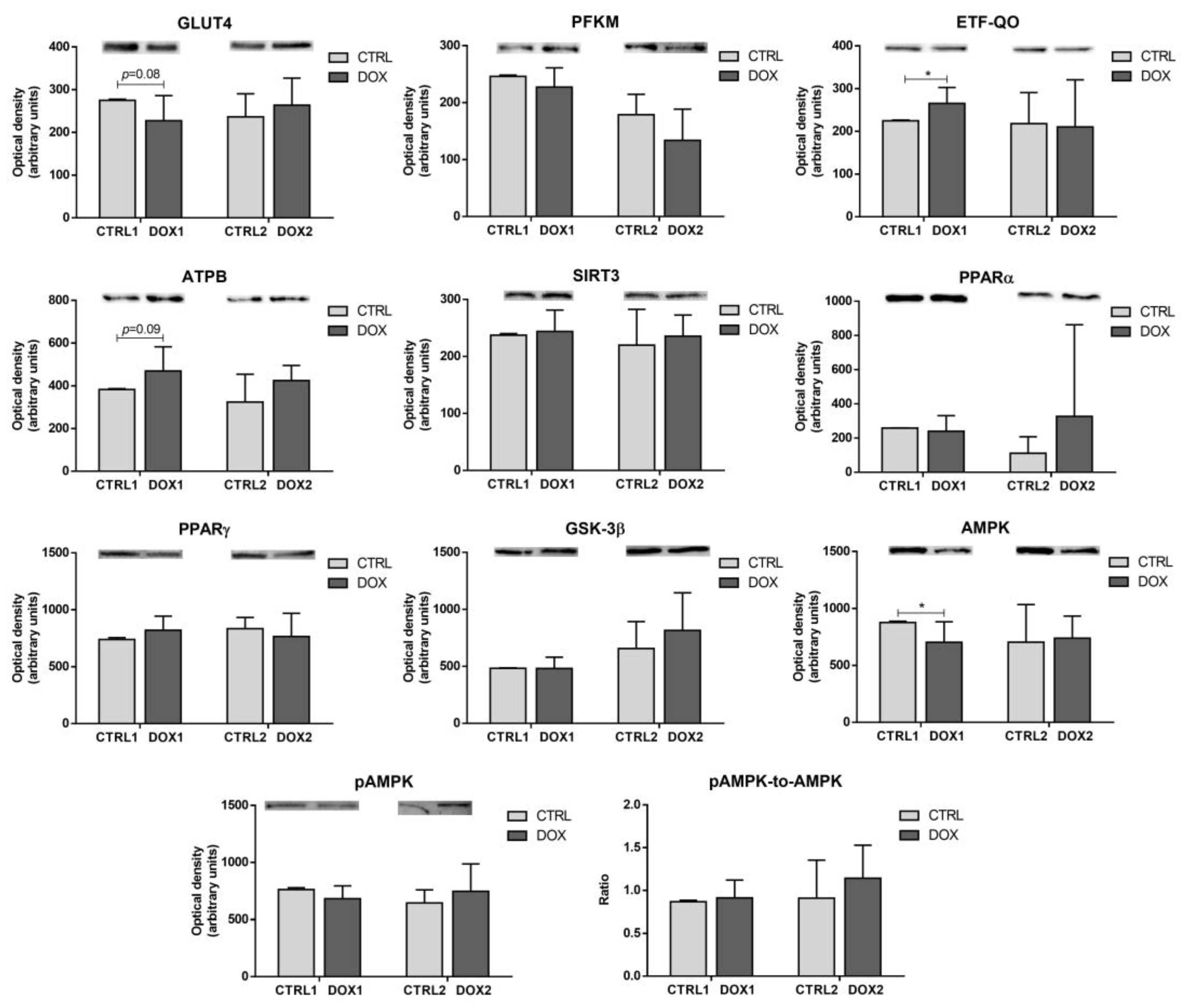
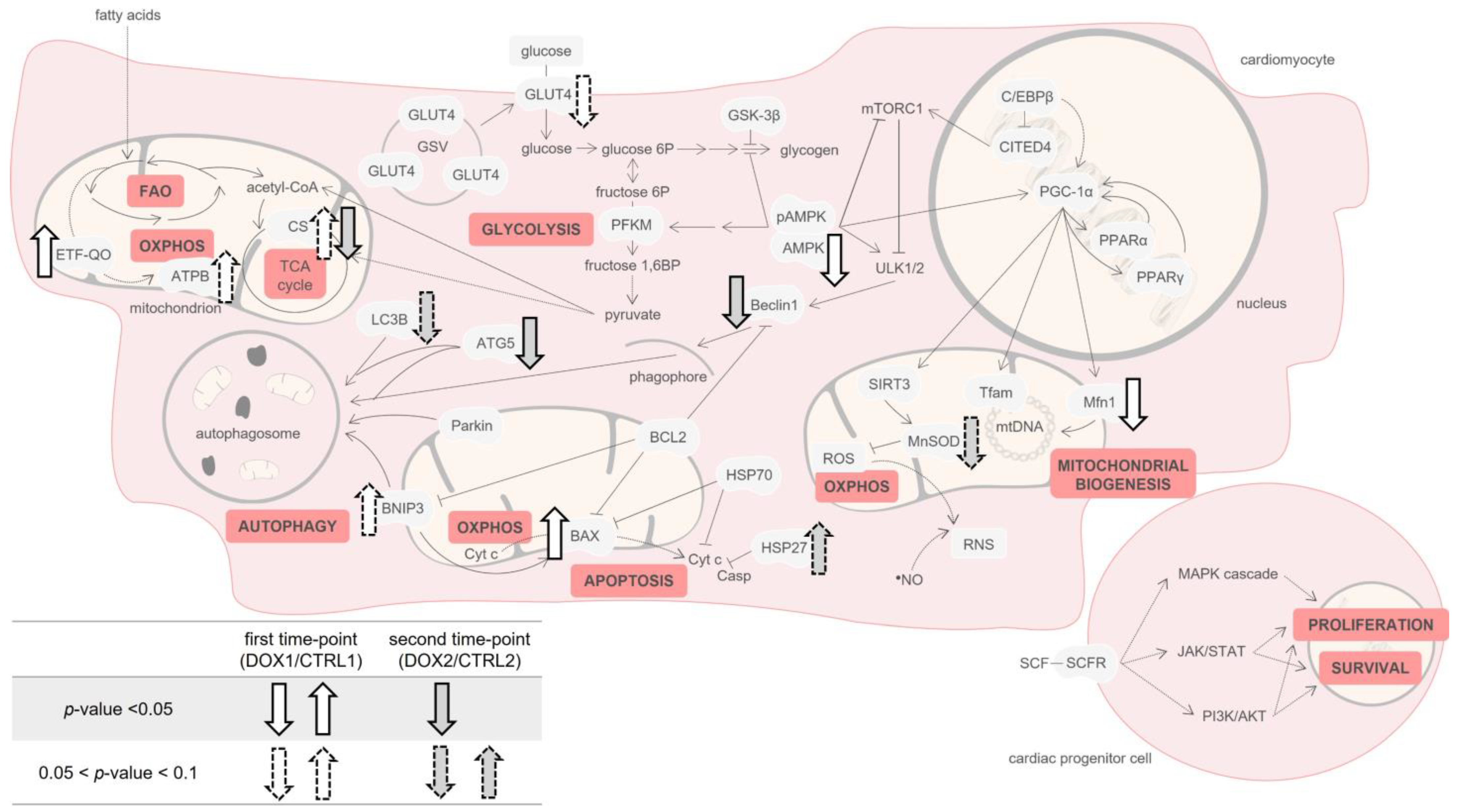
2.3. Doxorubicin Treatment Decreased the Cardiac Content of the Metabolic Regulator AMP-Activated Protein Kinase (AMPK), and Increased the Cardiac Content of Electron Transfer Flavoprotein-Ubiquinone Oxidoreductase (ETF-QO) One Week after Exposure
2.4. Doxorubicin Treatment Induced a Tendency for Decreased Cardiac Content of Manganese Superoxide Dismutase (MnSOD) Five Months after Exposure
2.5. Doxorubicin Treatment Induced a Tendency for Increased Cardiac Mitochondrial Density One Week after Exposure, While Decreasing It Five Months after Exposure
2.6. Doxorubicin Treatment Increased Cardiac Apoptosis One Week after Exposure, While Decreasing Cardiac Autophagic Markers and Increasing Heat Shock Protein 27 (HSP27) Content Five Months after Exposure
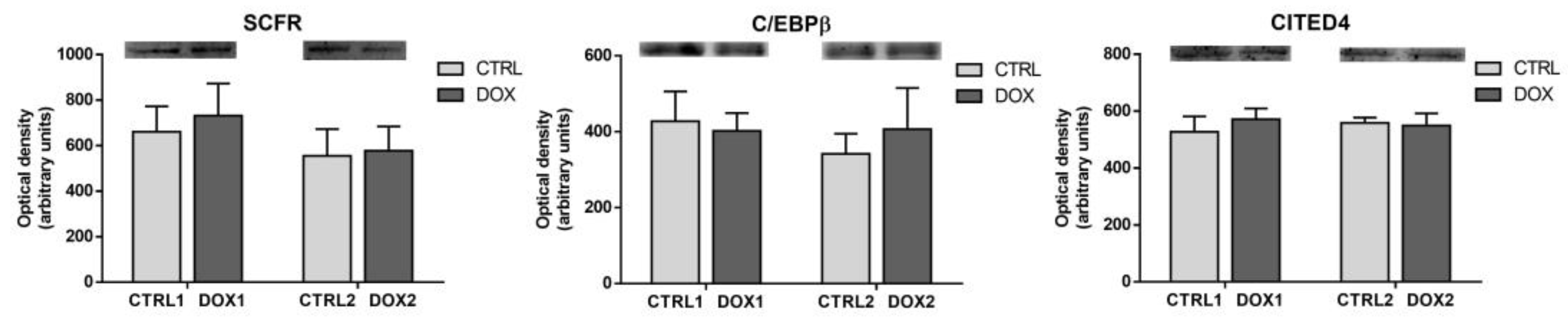
2.7. Doxorubicin Treatment Did Not Affect Cardiac Regeneration Either One Week or Five Months after Exposure
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animal Experimental Design
4.3. Blood Collection and Serum Evaluation
4.4. Heart Collection, Histological Analysis, and Homogenization
4.5. Immunoblotting Evaluation
4.6. Citrate Synthase (CS) Activity Evaluation
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Seiter, K. Toxicity of the Topoisomerase II Inhibitors. Expert Opin. Drug Saf. 2005, 4, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Herman, E.; Mhatre, R.; Lee, I.P.; Vick, J.; Waravdekar, V.S. A Comparison of the Cardiovascular Actions of Daunomycin, Adriamycin and N-Acetyldaunomycin in Hamsters and Monkeys. Pharmacology 1971, 6, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Bonadonna, G.; Monfardini, S.; de Lena, M.; Fossati-Bellani, F. Clinical Evaluation of Adriamycin, a New Antitumour Antibiotic. BMJ 1969, 3, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.; Cardinale, D. Using Cardiac Biomarkers and Treating Cardiotoxicity in Cancer. Future Cardiol. 2013, 9, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Aronow, W. Cardiotoxicity of Cancer Chemotherapy in Clinical Practice. Hosp. Pract. 2019, 47, 6–15. [Google Scholar] [CrossRef]
- Steinherz, L.J.; Steinherz, P.G.; Tan, C.T.; Heller, G.; Murphy, M.L. Cardiac Toxicity 4 to 20 Years after Completing Anthracycline Therapy. JAMA 1991, 266, 1672–1677. [Google Scholar] [CrossRef]
- Silber, J.H.; Cnaan, A.; Clark, B.J.; Paridon, S.M.; Chin, A.J.; Rychik, J.; Hogarty, A.N.; Cohen, M.I.; Barber, G.; Rutkowski, M.; et al. Enalapril to Prevent Cardiac Function Decline in Long-Term Survivors of Pediatric Cancer Exposed to Anthracyclines. J. Clin. Oncol. 2004, 22, 820–828. [Google Scholar] [CrossRef]
- Brandão, S.R.; Carvalho, F.; Amado, F.; Ferreira, R.; Costa, V.M. Insights on the Molecular Targets of Cardiotoxicity Induced by Anticancer Drugs: A Systematic Review Based on Proteomic Findings. Metabolism 2022, 134, 155250. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Bawa-Khalfe, T.; Lu, L.-S.; Lyu, Y.L.; Liu, L.F.; Yeh, E.T.H. Identification of the Molecular Basis of Doxorubicin-Induced Cardiotoxicity. Nat. Med. 2012, 18, 1639–1642. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Ghanefar, M.; Bayeva, M.; Wu, R.; Khechaduri, A.; Prasad, S.V.N.; Mutharasan, R.K.; Naik, T.J.; Ardehali, H. Cardiotoxicity of Doxorubicin Is Mediated through Mitochondrial Iron Accumulation. J. Clin. Investig. 2014, 124, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Reis-Mendes, A.; Sousa, E.; de Lourdes Bastos, M.; Marisa Costa, V. The Role of the Metabolism of Anticancer Drugs in Their Induced-Cardiotoxicity. Curr. Drug Metab. 2015, 17, 75–90. [Google Scholar] [CrossRef]
- Pereira, G.C.; Pereira, S.P.; Tavares, L.C.; Carvalho, F.S.; Magalhães-Novais, S.; Barbosa, I.A.; Santos, M.S.; Bjork, J.; Moreno, A.J.; Wallace, K.B.; et al. Cardiac Cytochrome c and Cardiolipin Depletion during Anthracycline-Induced Chronic Depression of Mitochondrial Function. Mitochondrion 2016, 30, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Renu, K.; Abilash, V.G.; Tirupathi Pichiah, P.B.; Arunachalam, S. Molecular Mechanism of Doxorubicin-Induced Cardiomyopathy—An Update. Eur. J. Pharmacol. 2018, 818, 241–253. [Google Scholar] [CrossRef]
- Anjos, M.; Fontes-Oliveira, M.; Costa, V.M.; Santos, M.; Ferreira, R. An Update of the Molecular Mechanisms Underlying Doxorubicin plus Trastuzumab Induced Cardiotoxicity. Life Sci. 2021, 280, 119760. [Google Scholar] [CrossRef] [PubMed]
- Brandão, S.R.; Reis-Mendes, A.; Domingues, P.; Duarte, J.A.; Bastos, M.L.; Carvalho, F.; Ferreira, R.; Costa, V.M. Exploring the Aging Effect of the Anticancer Drugs Doxorubicin and Mitoxantrone on Cardiac Mitochondrial Proteome Using a Murine Model. Toxicology 2021, 459, 152852. [Google Scholar] [CrossRef]
- Abrahams, C.; Woudberg, N.J.; Kodogo, V.; Hadebe, N.; LeCour, S. Doxorubicin-Induced Cardiotoxicity Is Associated with a Change in High Density Lipoprotein Subclasses in a Mouse Breast Cancer Model. J. Mol. Cell. Cardiol. 2022, 173, 67. [Google Scholar] [CrossRef]
- Strongman, H.; Gadd, S.; Matthews, A.A.; Mansfield, K.E.; Stanway, S.; Lyon, A.R.; dos-Santos-Silva, I.; Smeeth, L.; Bhaskaran, K. Does Cardiovascular Mortality Overtake Cancer Mortality During Cancer Survivorship? JACC CardioOncol. 2022, 4, 113–123. [Google Scholar] [CrossRef]
- Reis-Mendes, A.; Ferreira, M.; Padrão, A.I.; Duarte, J.A.; Duarte-Araújo, M.; Remião, F.; Carvalho, F.; Sousa, E.; Bastos, M.L.; Costa, V.M. The Role of Nrf2 and Inflammation on the Dissimilar Cardiotoxicity of Doxorubicin in Two-Time Points: A Cardio-Oncology In Vivo Study Through Time. Inflammation 2023, in press. [Google Scholar] [CrossRef]
- Shao, D.; Tian, R. Glucose Transporters in Cardiac Metabolism and Hypertrophy. In Comprehensive Physiology; Terjung, R., Ed.; Wiley: Hoboken, NJ, USA, 2015; pp. 331–351. ISBN 978-0-470-65071-4. [Google Scholar]
- Krause, N.; Wegner, A. Fructose Metabolism in Cancer. Cells 2020, 9, 2635. [Google Scholar] [CrossRef]
- Wanders, R.J.A.; Ruiter, J.P.N.; IJlst, L.; Waterham, H.R.; Houten, S.M. The Enzymology of Mitochondrial Fatty Acid Beta-Oxidation and Its Application to Follow-up Analysis of Positive Neonatal Screening Results. J. Inherit. Metab. Dis. 2010, 33, 479–494. [Google Scholar] [CrossRef] [PubMed]
- Papa, S.; Martino, P.L.; Capitanio, G.; Gaballo, A.; De Rasmo, D.; Signorile, A.; Petruzzella, V. The Oxidative Phosphorylation System in Mammalian Mitochondria. In Advances in Mitochondrial Medicine; Scatena, R., Bottoni, P., Giardina, B., Eds.; Advances in Experimental Medicine and Biology; Springer: Dordrecht, The Netherlands, 2012; Volume 942, pp. 3–37. ISBN 978-94-007-2868-4. [Google Scholar]
- Larsen, S.; Nielsen, J.; Hansen, C.N.; Nielsen, L.B.; Wibrand, F.; Stride, N.; Schroder, H.D.; Boushel, R.; Helge, J.W.; Dela, F.; et al. Biomarkers of Mitochondrial Content in Skeletal Muscle of Healthy Young Human Subjects: Biomarkers of Mitochondrial Content. J. Physiol. 2012, 590, 3349–3360. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.-B.; Kalkhoran, S.B.; Hernández-Reséndiz, S.; Samangouei, P.; Ong, S.-G.; Hausenloy, D.J. Mitochondrial-Shaping Proteins in Cardiac Health and Disease—The Long and the Short of It! Cardiovasc. Drugs Ther. 2017, 31, 87–107. [Google Scholar] [CrossRef]
- Kunkel, G.H.; Chaturvedi, P.; Tyagi, S.C. Mitochondrial Pathways to Cardiac Recovery: TFAM. Heart Fail. Rev. 2016, 21, 499–517. [Google Scholar] [CrossRef]
- Pan, X.-C.; Xiong, Y.-L.; Hong, J.-H.; Liu, Y.; Cen, Y.-Y.; Liu, T.; Yang, Q.-F.; Tao, H.; Li, Y.-N.; Zhang, H.-G. Cardiomyocytic FoxP3 Is Involved in Parkin-Mediated Mitophagy during Cardiac Remodeling and the Regulatory Role of Triptolide. Theranostics 2022, 12, 2483–2501. [Google Scholar] [CrossRef]
- Dorn, G.W. Mitochondrial Pruning by Nix and BNip3: An Essential Function for Cardiac-Expressed Death Factors. J Cardiovasc. Transl. Res. 2010, 3, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Vajravelu, B.N.; Hong, K.U.; Al-Maqtari, T.; Cao, P.; Keith, M.C.L.; Wysoczynski, M.; Zhao, J.; Moore IV, J.B.; Bolli, R. C-Kit Promotes Growth and Migration of Human Cardiac Progenitor Cells via the PI3K-AKT and MEK-ERK Pathways. PLoS ONE 2015, 10, e0140798. [Google Scholar] [CrossRef]
- Nerlov, C. The C/EBP Family of Transcription Factors: A Paradigm for Interaction between Gene Expression and Proliferation Control. Trends Cell Biol. 2007, 17, 318–324. [Google Scholar] [CrossRef]
- Chen, H.; Chen, C.; Spanos, M.; Li, G.; Lu, R.; Bei, Y.; Xiao, J. Exercise Training Maintains Cardiovascular Health: Signaling Pathways Involved and Potential Therapeutics. Signal Transduct. Target. Ther. 2022, 7, 306. [Google Scholar] [CrossRef]
- Wang, S.; Lai, X.; Deng, Y.; Song, Y. Correlation between Mouse Age and Human Age in Anti-Tumor Research: Significance and Method Establishment. Life Sci. 2020, 242, 117242. [Google Scholar] [CrossRef] [PubMed]
- Haeri, H.H.; Schunk, B.; Tomaszewski, J.; Schimm, H.; Gelos, M.J.; Hinderberger, D. Fatty Acid Binding to Human Serum Albumin in Blood Serum Characterized by EPR Spectroscopy. ChemistryOpen 2019, 8, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Reis-Mendes, A.; Padrão, A.I.; Duarte, J.A.; Gonçalves-Monteiro, S.; Duarte-Araújo, M.; Remião, F.; Carvalho, F.; Sousa, E.; Bastos, M.L.; Costa, V.M. Role of Inflammation and Redox Status on Doxorubicin-Induced Cardiotoxicity in Infant and Adult CD-1 Male Mice. Biomolecules 2021, 11, 1725. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.A.; Sousa, R.P.B.; Cadete, V.J.J.; Lopaschuk, G.D.; Palmeira, C.M.M.; Bjork, J.A.; Wallace, K.B. Metabolic Remodeling Associated with Subchronic Doxorubicin Cardiomyopathy. Toxicology 2010, 270, 92–98. [Google Scholar] [CrossRef]
- Gratia, S.; Kay, L.; Michelland, S.; Sève, M.; Schlattner, U.; Tokarska-Schlattner, M. Cardiac Phosphoproteome Reveals Cell Signaling Events Involved in Doxorubicin Cardiotoxicity. J. Proteom. 2012, 75, 4705–4716. [Google Scholar] [CrossRef]
- Kumar, S.N.; Konorev, E.A.; Aggarwal, D.; Kalyanaraman, B. Analysis of Proteome Changes in Doxorubicin-Treated Adult Rat Cardiomyocyte. J. Proteom. 2011, 74, 683–697. [Google Scholar] [CrossRef]
- Arad, M.; Seidman, C.E.; Seidman, J.G. AMP-Activated Protein Kinase in the Heart: Role During Health and Disease. Circ. Res. 2007, 100, 474–488. [Google Scholar] [CrossRef]
- Montaigne, D.; Butruille, L.; Staels, B. PPAR Control of Metabolism and Cardiovascular Functions. Nat. Rev. Cardiol. 2021, 18, 809–823. [Google Scholar] [CrossRef]
- Yoon, C.; Kim, H.; Mishchenko, N.; Vasileva, E.; Fedoreyev, S.; Stonik, V.; Han, J. Spinochrome D Attenuates Doxorubicin-Induced Cardiomyocyte Death via Improving Glutathione Metabolism and Attenuating Oxidative Stress. Mar. Drugs 2018, 17, 2. [Google Scholar] [CrossRef]
- Ding, Q.; Qi, Y.; Tsang, S.-Y. Mitochondrial Biogenesis, Mitochondrial Dynamics, and Mitophagy in the Maturation of Cardiomyocytes. Cells 2021, 10, 2463. [Google Scholar] [CrossRef]
- Willis, M.S.; Parry, T.L.; Brown, D.I.; Mota, R.I.; Huang, W.; Beak, J.Y.; Sola, M.; Zhou, C.; Hicks, S.T.; Caughey, M.C.; et al. Doxorubicin Exposure Causes Subacute Cardiac Atrophy Dependent on the Striated Muscle–Specific Ubiquitin Ligase MuRF1. Circ. Heart Fail. 2019, 12, e005234. [Google Scholar] [CrossRef]
- Hancock, J.T. Oxygen Is Instrumental for Biological Signaling: An Overview. Oxygen 2021, 1, 3–15. [Google Scholar] [CrossRef]
- Meng, Y.-Y.; Yuan, Y.-P.; Zhang, X.; Kong, C.-Y.; Song, P.; Ma, Z.-G.; Tang, Q.-Z. Protection against Doxorubicin-Induced Cytotoxicity by Geniposide Involves AMPK α Signaling Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 7901735. [Google Scholar] [CrossRef] [PubMed]
- Gerber, L.; Clow, K.A.; Katan, T.; Emam, M.; Leeuwis, R.H.J.; Parrish, C.C.; Gamperl, A.K. Cardiac Mitochondrial Function, Nitric Oxide Sensitivity and Lipid Composition Following Hypoxia Acclimation in Sablefish. J. Exp. Biol. 2019, 222, jeb.208074. [Google Scholar] [CrossRef]
- Tang, S.-W.; Ducroux, A.; Jeang, K.-T.; Neuveut, C. Impact of Cellular Autophagy on Viruses: Insights from Hepatitis B Virus and Human Retroviruses. J. Biomed. Sci. 2012, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Russo, M.; Pirozzi, F.; Tocchetti, C.G.; Ghigo, A. Autophagy and Cancer Therapy Cardiotoxicity: From Molecular Mechanisms to Therapeutic Opportunities. Biochim. Biophys. Acta BBA Mol. Cell Res. 2020, 1867, 118493. [Google Scholar] [CrossRef]
- Rodríguez, M.E.; Arévalo, D.E.; Sanabria, L.M.; Carrión, F.D.C.; Fanelli, M.A.; Rivarola, V.A. Heat Shock Protein 27 Modulates Autophagy and Promotes Cell Survival after Photodynamic Therapy. Photochem. Photobiol. Sci. 2019, 18, 546–554. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose Translation from Animal to Human Studies Revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Brandão, S.R.; Reis-Mendes, A.; Araújo, M.D.; Neuparth, M.J.; Rocha, H.; Carvalho, F.; Ferreira, R.; Costa, V.M. Cardiac Molecular Remodeling by Anticancer Drugs: Doxorubicin Affects More Metabolism While Mitoxantrone Impacts More Autophagy in Adult CD-1 Male Mice. Biomolecules 2023, 13, 921. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Coore, H.G.; Denton, R.M.; Martin, B.R.; Randle, P.J. Regulation of Adipose Tissue Pyruvate Dehydrogenase by Insulin and Other Hormones. Biochem. J. 1971, 125, 115–127. [Google Scholar] [CrossRef] [PubMed]





| Morphometric Parameter | CTRL1 | DOX1 | CTRL2 | DOX2 |
|---|---|---|---|---|
| Whole-body weight (g) | 42.671 ± 3.450 | 40.557 ± 2.348 | 47.525 ± 2.416 | 43.167 ± 4.373 * |
| Heart weight (g) | 0.228 ± 0.048 | 0.216 ± 0.028 | 0.246 ± 0.017 | 0.220 ± 0.025 * |
| Tibial length (cm) | 1.90 ± 0.08 | 1.90 ± 0.13 | 2.02 ± 0.05 | 1.92 ± 0.06 ** |
| Heart weight to whole-body weight (mg/g) | 5.30 ± 0.81 | 5.33 ± 0.61 | 5.20 ± 0.55 | 5.11 ± 0.42 |
| Heart weight to tibial length (g/cm) | 0.120 ± 0.025 | 0.114 ± 0.015 | 0.122 ± 0.007 | 0.115 ± 0.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandão, S.R.; Reis-Mendes, A.; Neuparth, M.J.; Carvalho, F.; Ferreira, R.; Costa, V.M. The Metabolic Fingerprint of Doxorubicin-Induced Cardiotoxicity in Male CD-1 Mice Fades Away with Time While Autophagy Increases. Pharmaceuticals 2023, 16, 1613. https://doi.org/10.3390/ph16111613
Brandão SR, Reis-Mendes A, Neuparth MJ, Carvalho F, Ferreira R, Costa VM. The Metabolic Fingerprint of Doxorubicin-Induced Cardiotoxicity in Male CD-1 Mice Fades Away with Time While Autophagy Increases. Pharmaceuticals. 2023; 16(11):1613. https://doi.org/10.3390/ph16111613
Chicago/Turabian StyleBrandão, Sofia Reis, Ana Reis-Mendes, Maria João Neuparth, Félix Carvalho, Rita Ferreira, and Vera Marisa Costa. 2023. "The Metabolic Fingerprint of Doxorubicin-Induced Cardiotoxicity in Male CD-1 Mice Fades Away with Time While Autophagy Increases" Pharmaceuticals 16, no. 11: 1613. https://doi.org/10.3390/ph16111613
APA StyleBrandão, S. R., Reis-Mendes, A., Neuparth, M. J., Carvalho, F., Ferreira, R., & Costa, V. M. (2023). The Metabolic Fingerprint of Doxorubicin-Induced Cardiotoxicity in Male CD-1 Mice Fades Away with Time While Autophagy Increases. Pharmaceuticals, 16(11), 1613. https://doi.org/10.3390/ph16111613