Skin Toxicities Associated with Botulin Toxin Injection for Aesthetic Procedures: Data from the European Spontaneous Reporting System
Abstract
:1. Introduction
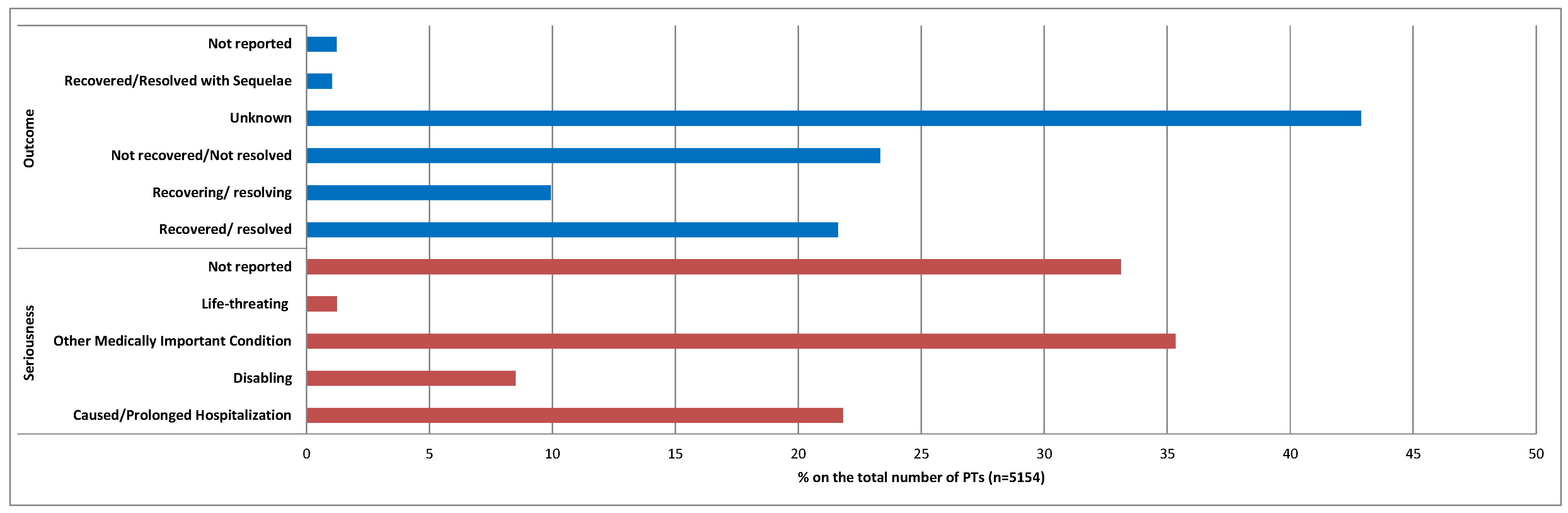
2. Results
3. Discussion
4. Material and Methods
4.1. Data Source
4.2. Descriptive Analyses
4.3. Ethical Standards
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Family Phisician. Available online: https://www.aafp.org/pubs/afp/issues/2014/0801/p168.html (accessed on 10 October 2023).
- European Medicines Agency. Nuceiva European Public Assessment Report. Available online: https://www.ema.europa.eu/en/documents/assessment-report/nuceiva-epar-public-assessment-report_en.pdf (accessed on 7 November 2023).
- Berry, M.G.; Stanek, J.J. Botulinum neurotoxin A: A review. J. Plast. Reconstr. Aesthetic Surg. 2012, 65, 1283–1291. [Google Scholar] [CrossRef]
- Choudhury, S.; Baker, M.R.; Chatterjee, S.; Kumar, H. Botulinum Toxin: An Update on Pharmacology and Newer Products in Development. Toxins 2021, 13, 58. [Google Scholar] [CrossRef]
- Phan, K.; Younessi, S.; Dubin, D.; Lin, M.J.; Khorasani, H. Emerging off-label esthetic uses of botulinum toxin in dermatology. Dermatol. Ther. 2022, 35, e15205. [Google Scholar] [CrossRef]
- Hong, S.O. Cosmetic Treatment Using Botulinum Toxin in the Oral and Maxillofacial Area: A Narrative Review of Esthetic Techniques. Toxins 2023, 15, 82. [Google Scholar] [CrossRef]
- Camargo, C.P.; Xia, J.; Costa, C.S.; Gemperli, R.; Tatini, M.D.; Bulsara, M.K.; Riera, R. Botulinum toxin type A for facial wrinkles. Cochrane Database Syst. Rev. 2021, 7, CD011301. [Google Scholar] [CrossRef] [PubMed]
- Frevert, J.; Dressler, D. Complexing proteins in botulinum toxin type A drugs: A help or a hindrance? Biologics 2010, 4, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.A.; Bradshaw, M. Clostridium botulinum and its neurotoxins: A metabolic and cellular perspective. Toxicon 2001, 39, 1703–1722. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, A.T.; de Boulle, K. Diffusion characteristics of botulinum neurotoxin products and their clinical significance in cosmetic applications. J. Cosmet. Laser Ther. 2007, 9 (Suppl. 1), 17–22. [Google Scholar] [CrossRef]
- Yiannakopoulou, E. Serious and long-term adverse events associated with the therapeutic and cosmetic use of botulinum toxin. Pharmacology 2015, 95, 65–69. [Google Scholar] [CrossRef]
- Witmanowski, H.; Błochowiak, K. The whole truth about botulinum toxin—A review. Postep. Dermatol. Alergol. 2020, 37, 853–861. [Google Scholar] [CrossRef]
- Non Interventional Safety Study of NUCEIVA for the Treatment of Moderate-to-Severe Glabellar Lines. Available online: https://clinicaltrials.gov/study/NCT05481931 (accessed on 9 November 2023).
- Wollina, U.; Konrad, H. Managing adverse events associated with botulinum toxin type A: A focus on cosmetic procedures. Am. J. Clin. Dermatol. 2005, 6, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.L.; Freeman, S.R. Botulinum toxins. In Cosmetic Dermatology Products & Procedures; Draelos, Z.D., Ed.; Wiley-Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Ascher, B.; Talarico, S.; Cassuto, D.; Escobar, S.; Hexsel, D.; Jaen, P.; Monheit, G.D.; Rzany, B.; Viel, M. International consensus recommendations on the aesthetic usage of botulinum toxin type A (Speywood Unit)—Part II: Wrinkles on the middle and lower face, neck and chest. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Satriyasa, B.K. Botulinum toxin (Botox) A for reducing the appearance of facial wrinkles: A literature review of clinical use and pharmacological aspect. Clin. Cosmet. Investig. Dermatol. 2019, 12, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Zhao, R.; Cao, L.; Liu, S.; Yu, D.; Wang, H. The Aesthetic Concerns of Botulinum Toxin Type A in the Treatment of Neck Wrinkles: A Systematic Review. Aesthet Surg J. 2021, 41, NP592–NP601. [Google Scholar] [CrossRef]
- Michon, A. Botulinum toxin for cosmetic treatments in young adults: An evidence-based review and survey on current practice among aesthetic practitioners. J. Cosmet. Dermatol. 2023, 22, 128–139. [Google Scholar] [CrossRef]
- Hogan, S.R.; Zachary, C.B.; Arndt, K.A. Prejuvenation: Definition of the term and evolution of the concept. Dermatol. Surg. 2021, 47, 871–872. [Google Scholar] [CrossRef]
- Haykal, D.; Nahai, F.; Cartier, H. Prejuvenation: The Global New Anti-Aging Trend. Aesthet. Surg. J. Open Forum. 2023, 5, ojad061. [Google Scholar] [CrossRef]
- Chung, K.; Orme, N.; Sherber, N. “Millennial botulinum toxin”: A retrospective age-matched cohort study with OnabotulinumtoxinA. Dermatol. Surg. 2021, 47, 882–884. [Google Scholar] [CrossRef]
- Plastic Surgery Statistics Report 2020. ASPS National Clearinghouse of Plastic Surgery Procedural Statistics. Available online: https://www.plasticsurgery.org/documents/News/Statistics/2020/plastic-surgery-statistics-full-report-2020.pdf (accessed on 12 October 2023).
- Naumann, M.; Albanese, A.; Heinen, F.; Molenaers, G.; Relja, M. Safety and efficacy of botulinum toxin type A following long-term use. Eur. J. Neurol. 2006, 13 (Suppl. 4), 35–40. [Google Scholar] [CrossRef]
- Gostimir, M.; Liou, V.; Yoon, M.K. Safety of Botulinum Toxin A Injections for Facial Rejuvenation: A Meta-Analysis of 9,669 Patients. Ophthalmic Plast. Reconstr. Surg. 2023, 39, 13–25. [Google Scholar] [CrossRef]
- Albanese, A.; Bentivoglio, A.R.; Colosimo, C.; Galardi, G.; Maderna, L.; Tonali, P. Pretarsal injections of botulinum toxin improve blepharospasm in previously unresponsive patients. J. Neurol. Neurosurg. Psychiatry 1996, 60, 693–694. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, A.; Carruthers, J. Clinical indications and injection technique for the cosmetic use of botulinum A exotoxin. Dermatol. Surg. 1998, 24, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.W. Complications and adverse reactions with the use of botulinum toxin. Dis. Mon. 2002, 48, 336–356. [Google Scholar] [CrossRef] [PubMed]
- Kassir, M.; Gupta, M.; Galadari, H.; Kroumpouzos, G.; Katsambas, A.; Lotti, T.; Vojvodic, A.; Grabbe, S.; Juchems, E.; Goldust, M. Complications of botulinum toxin and fillers: A narrative review. J. Cosmet. Dermatol. 2020, 19, 570–573. [Google Scholar] [CrossRef]
- Coté, T.R.; Mohan, A.K.; Polder, J.A.; Walton, M.K.; Braun, M.M. Botulinum toxin type A injections: Adverse events reported to the US Food and Drug Administration in therapeutic and cosmetic cases. J. Am. Acad. Dermatol. 2005, 53, 407–415. [Google Scholar] [CrossRef]
- Moon, I.J.; Chang, S.E.; Kim, S.D. First case of anaphylaxis after botulinum toxin type A injection. Clin. Exp. Dermatol. 2017, 42, 760–762. [Google Scholar] [CrossRef]
- Li, M.; Goldberger, B.A.; Hopkins, C. Fatal case of BOTOX-related anaphylaxis? J. Forensic Sci. 2005, 50, 169–172. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.M.; Seo, K.S.; Kwon, S.M.; Row, H.S. Anaphylactic reaction after local lidocaine infiltration for retraction of retained teeth. J. Dent. Anesth. Pain. Med. 2019, 19, 175–180. [Google Scholar] [CrossRef]
- Barradas Lopes, J.; Reis Ferreira, A.; Sousa, M.J.; Cadinha, S. Anaphylactic Shock to Lidocaine: A Rare Case with Evaluation of Cross-Reactivity Between Local Anesthetics. J. Investig. Allergol. Clin. Immunol. 2021, 31, 449–450. [Google Scholar] [CrossRef]
- Small, R. Botulinum toxin injection for facial wrinkles. Am. Fam. Physician 2014, 90, 168–175. [Google Scholar]
- Pickett, A. Can botulinum toxin cause anaphylaxis after an aesthetic treatment? Clin. Exp. Dermatol. 2018, 43, 599–600. [Google Scholar] [CrossRef] [PubMed]
- Mascolo, A.; Scavone, C.; Ferrajolo, C.; Rafaniello, C.; Danesi, R.; Del Re, M.; Russo, A.; Coscioni, E.; Rossi, F.; Alfano, R.; et al. Immune Checkpoint Inhibitors and Cardiotoxicity: An Analysis of Spontaneous Reports in Eudravigilance. Drug Saf. 2021, 44, 957–971. [Google Scholar] [CrossRef] [PubMed]
- di Mauro, G.; Zinzi, A.; Scavone, C.; Mascolo, A.; Gaio, M.; Sportiello, L.; Ferrajolo, C.; Rafaniello, C.; Rossi, F.; Capuano, A. PCSK9 Inhibitors and Neurocognitive Adverse Drug Reactions: Analysis of Individual Case Safety Reports from the Eudravigilance Database. Drug Saf. 2021, 44, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Velișcu, E.M.; Liguori, V.; Anatriello, A.; Maniscalco, G.T.; Cantone, A.; Di Costanzo, L.; Stefanelli, P.; Scavone, C.; Capuano, A. Hepatobiliary Adverse Reactions during Treatment with Cladribine: Analysis of Data from the European Spontaneous Reporting System. Pharmaceuticals 2023, 16, 1071. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on Good Pharmacovigilance Practices (GVP). Available online: https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/guideline-good-pharmacovigilance-practices-gvp-module-vi-collection-management-submission-reports_en.pdf (accessed on 7 November 2023).
- Salvador, M.R.; Monteiro, C.; Pereira, L.; Duarte, A.P. Quality of Spontaneous Reports of Adverse Drug Reactions Sent to a Regional Pharmacovigilance Unit. Int. J. Env. Res. Public Health 2022, 19, 3754. [Google Scholar] [CrossRef]
- Bandekar, M.S.; Anwikar, S.R.; Kshirsagar, N.A. Quality check of spontaneous adverse drug reaction reporting forms of different countries. Pharmacoepidemiol. Drug Saf. 2010, 19, 1181–1185. [Google Scholar] [CrossRef]
- Durrieu, G.; Jacquot, J.; Mège, M.; Bondon-Guitton, E.; Rousseau, V.; Montastruc, F.; Montastruc, J.L. Completeness of Spontaneous Adverse Drug Reaction Reports Sent by General Practitioners to a Regional Pharmacovigilance Centre: A Descriptive Study. Drug Saf. 2016, 39, 1189–1195. [Google Scholar] [CrossRef]
- Inácio, P.; Cavaco, A.; Airaksinen, M. The value of patient reporting to the pharmacovigilance system: A systematic review. Br. J. Clin. Pharmacol. 2017, 83, 227–246. [Google Scholar] [CrossRef]
- Scavone, C.; Di Mauro, C.; Ruggiero, R.; Bernardi, F.F.; Trama, U.; Aiezza, M.L.; Rafaniello, C.; Capuano, A. Severe Cutaneous Adverse Drug Reactions Associated with Allopurinol: An Analysis of Spontaneous Reporting System in Southern Italy. Drugs Real. World Outcomes 2020, 7, 41–51. [Google Scholar] [CrossRef]
- ICH Topic E 2 A Clinical Safety Data Management: Definitions and Standards for Expedited Reporting. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-15.pdf (accessed on 7 November 2023).
| Variable | Level | All ICSRs (n = 718), n (%) |
|---|---|---|
| Age group | 18–64 years | 479 (67) |
| 65–85 years | 44 (6) | |
| Not specified | 195 (27) | |
| Gender | F | 662 (92.1) |
| M | 40 (5.6) | |
| Missing | 16 (2.3) | |
| Primary Source Qualification | Healthcare Professional | 602 (83.9) |
| Non-Healthcare Professional | 115 (16.0) | |
| Not specified | 1 (0.1) | |
| Primary Source Country for Regulatory Purposes | European Economic Area | 378 (52.6) |
| Non-European Economic Area | 340 (47.4) | |
| Suspected drug(s) other than botulin toxin type A | 0 | 390 (54.3) |
| 1 | 164 (22.8) | |
| 2 | 117 (16.4) | |
| 3 | 27 (3.8) | |
| 4 | 20 (2.8) | |
| Concomitant drug(s) | 0 | 512 (71.3) |
| 1 | 92 (12.8) | |
| 2 | 49 (6.8) | |
| 3 | 25 (3.5) | |
| 4 | 16 (2.2) | |
| ≥5 | 24 (3.5) |
| Case No. | Age Group | Sex | Preferred Terms (PTs) | Suspect Drug(s) Other Than Botulin Toxin Type A | Concomitant Medication |
|---|---|---|---|---|---|
| 1 | NA | F | Anaphylactic reaction, Dyspnea, Pruritus, Swelling face, Swollen tongue | - | - |
| 2 | 18–64 Years | F | Anaphylactic reaction, Erythema, Rash pruritic | - | - |
| 3 | NA | F | Anaphylactic shock, Chest discomfort, Facial paresis, Hypersensitivity, Nausea, Seborrhea | - | Levothyroxine |
| 4 | 18–64 Years | F | Acne, Asthenia, Coma, Dyspnea, Erythema, Fatigue, Hemorrhage, Influenza like illness, Off-label use, Pneumonia, Pseudomonas infection, Staphylococcal infection, White blood cell count abnormal | Hyaluronic acid | Alprazolam, albutamol |
| 5 | 18–64 Years | F | Blood pressure increased, Erythema, Histamine level increased, Hypotension, Localized edema, Tachycardia, Urticaria | - | - |
| 6 | 18–64 Years | F | Angioedema | Tozinameran, Hyaluronic Acid, Lidocaine | Perindopril Tert-Butylamine, Indapamide |
| 7 | 18–64 Years | F | Anxiety, Dyspnea, Eye pain, Eye pruritus, Eye swelling, Headache, Hypersensitivity, Incorrect route of product administration, Lacrimation increased, Malaise, Paresthesia oral, Rash, Rash vesicular, Skin discoloration, Urticaria, Vision blurred, Wheezing | - | - |
| 8 | 18–64 Years | F | Angioedema, Injection site hematoma, Injection site hemorrhage, Injection site swelling, Off label use | - | Lisinopril |
| 9 | 18–64 Years | F | Angioedema, Face edema | - | - |
| List of PTs Reported in Retrieved ICSRs | |
|---|---|
| N (%) | |
| Off-label use | 179 (3.5%) |
| Erythema | 128 (2.5%) |
| Headache | 124 (2.4%) |
| Rash | 118 (2.3%) |
| Pruritus | 103 (2.0%) |
| Urticaria | 85 (1.6%) |
| Swelling face | 80 (1.5%) |
| Brow ptosis | 76 (1.5%) |
| Dyspnea | 76 (1.5%) |
| Hypersensitivity | 73 (1.4%) |
| Dizziness | 63 (1.2%) |
| Eyelid ptosis | 60 (1.2%) |
| Injection site pain | 58 (1.1%) |
| Nausea | 55 (1.1%) |
| Fatigue | 54 (1.0%) |
| Angioedema | 54 (1.0%) |
| Pain | 53 (1.0%) |
| Vision blurred | 52 (1.0%) |
| Hypoesthesia | 50 (0.9%) |
| Malaise | 50 (0.9%) |
| Paresthesia | 49 (0.9%) |
| Muscular weakness | 48 (0.9%) |
| Anxiety | 45 (0.9%) |
| Swelling | 43 (0.8%) |
| Product preparation error | 43 (0.8%) |
| Dysphagia | 42 (0.8%) |
| Drug ineffective | 42 (0.8%) |
| Injection site swelling | 39 (0.8%) |
| Asthenia | 38 (0.7%) |
| Hyperhidrosis | 38 (0.7%) |
| Skin tightness | 34 (0.7%) |
| Eye swelling | 33 (0.6%) |
| Insomnia | 32 (0.6%) |
| Palpitations | 32 (0.6%) |
| Dry mouth | 30 (0.6%) |
| Facial paresis | 29 (0.6%) |
| Influenza like illness | 28 (0.5%) |
| Face oedema | 28 (0.5%) |
| Visual impairment | 28 (0.5%) |
| Dry eye | 27 (0.5%) |
| Facial pain | 27 (0.5%) |
| Tremor | 27 (0.5%) |
| Vomiting | 27 (0.5%) |
| Eye pain | 26 (0.5%) |
| Swelling of eyelid | 26 (0.5%) |
| Diarrhoea | 26 (0.5%) |
| Alopecia | 25 (0.5%) |
| Facial paralysis | 24 (0.5%) |
| Injection site erythema | 24 (0.5%) |
| Feeling abnormal | 23 (0.4%) |
| Neck pain | 22 (0.4%) |
| Pyrexia | 22 (0.4%) |
| Migraine | 21 (0.4%) |
| Periorbital swelling | 21 (0.4%) |
| Skin disorder | 20 (0.4%) |
| Muscle spasms | 20 (0.4%) |
| Skin wrinkling | 20 (0.4%) |
| Arthralgia | 19 (0.4%) |
| Skin burning sensation | 19 (0.4%) |
| Weight decreased | 19 (0.4%) |
| Feeling hot | 19 (0.4%) |
| Overdose | 19 (0.4%) |
| Burning sensation | 17 (0.3%) |
| Botulism | 17 (0.3%) |
| Depression | 16 (0.3%) |
| Dry skin | 16 (0.3%) |
| Head discomfort | 16 (0.3%) |
| Swollen tongue | 16 (0.3%) |
| Diplopia | 16 (0.3%) |
| Skin discolouration | 16 (0.3%) |
| Musculoskeletal stiffness | 15 (0.3%) |
| Myalgia | 15 (0.3%) |
| Eye irritation | 15 (0.3%) |
| Muscle twitching | 15 (0.3%) |
| Contusion | 15 (0.3%) |
| Drug hypersensitivity | 15 (0.3%) |
| Acne | 15 (0.3%) |
| Ocular hyperaemia | 14 (0.3%) |
| Chest pain | 14 (0.3%) |
| Photophobia | 14 (0.3%) |
| Dysphonia | 13 (0.2%) |
| Oedema | 13 (0.2%) |
| Rash erythematous | 13 (0.2%) |
| Pain in extremity | 13 (0.2%) |
| Discomfort | 13 (0.2%) |
| Abdominal pain upper | 13 (0.2%) |
| Facial asymmetry | 13 (0.2%) |
| Dysarthria | 12 (0.2%) |
| Syncope | 12 (0.2%) |
| Lip swelling | 12 (0.2%) |
| Injection site pruritus | 12 (0.2%) |
| Throat tightness | 12 (0.2%) |
| Photosensitivity reaction | 12 (0.2%) |
| Tachycardia | 12 (0.2%) |
| Papule | 12 (0.2%) |
| Back pain | 11 (0.2%) |
| Therapeutic response decreased | 11 (0.2%) |
| Confusional state | 11 (0.2%) |
| Night sweats | 11 (0.2%) |
| Flushing | 11 (0.2%) |
| Hypoaesthesia oral | 11 (0.2%) |
| Chest discomfort | 11 (0.2%) |
| Inflammation | 11 (0.2%) |
| Intentional product use issue | 11 (0.2%) |
| Neuralgia | 10 (0.2%) |
| Chills | 10 (0.2%) |
| Skin lesion | 10 (0.2%) |
| Ear discomfort | 10 (0.2%) |
| Tinnitus | 10 (0.2%) |
| Loss of consciousness | 10 (0.2%) |
| Skin exfoliation | 10 (0.2%) |
| Madarosis | 10 (0.2%) |
| Injection site mass | 10 (0.2%) |
| Lymphadenopathy | 10 (0.2%) |
| Skin mass | 10 (0.2%) |
| Incorrect route of product administration | 10 (0.2%) |
| Nodule | 10 (0.2%) |
| Condition aggravated | 10 (0.2%) |
| Neuromuscular toxicity | 10 (0.2%) |
| Other PTs | 1626 (31.5%) |
| Total PTs recorded in 718 retrieved ICSRs | 5154 (100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicoletti, M.M.; Anatriello, A.; Liguori, V.; Cantone, A.; di Mauro, G.; Izzo, I.; Lettera, N.; Della Ragione, J.M.; Campitiello, M.R.; Cosenza, V.; et al. Skin Toxicities Associated with Botulin Toxin Injection for Aesthetic Procedures: Data from the European Spontaneous Reporting System. Pharmaceuticals 2023, 16, 1611. https://doi.org/10.3390/ph16111611
Nicoletti MM, Anatriello A, Liguori V, Cantone A, di Mauro G, Izzo I, Lettera N, Della Ragione JM, Campitiello MR, Cosenza V, et al. Skin Toxicities Associated with Botulin Toxin Injection for Aesthetic Procedures: Data from the European Spontaneous Reporting System. Pharmaceuticals. 2023; 16(11):1611. https://doi.org/10.3390/ph16111611
Chicago/Turabian StyleNicoletti, Maria Maddalena, Antonietta Anatriello, Valerio Liguori, Andrea Cantone, Gabriella di Mauro, Imma Izzo, Nicoletta Lettera, Joao Marcos Della Ragione, Maria Rosaria Campitiello, Vincenzo Cosenza, and et al. 2023. "Skin Toxicities Associated with Botulin Toxin Injection for Aesthetic Procedures: Data from the European Spontaneous Reporting System" Pharmaceuticals 16, no. 11: 1611. https://doi.org/10.3390/ph16111611
APA StyleNicoletti, M. M., Anatriello, A., Liguori, V., Cantone, A., di Mauro, G., Izzo, I., Lettera, N., Della Ragione, J. M., Campitiello, M. R., Cosenza, V., & Scavone, C. (2023). Skin Toxicities Associated with Botulin Toxin Injection for Aesthetic Procedures: Data from the European Spontaneous Reporting System. Pharmaceuticals, 16(11), 1611. https://doi.org/10.3390/ph16111611






