Rubia cordifolia L. Attenuates Diabetic Neuropathy by Inhibiting Apoptosis and Oxidative Stress in Rats
Abstract
:1. Introduction
2. Results
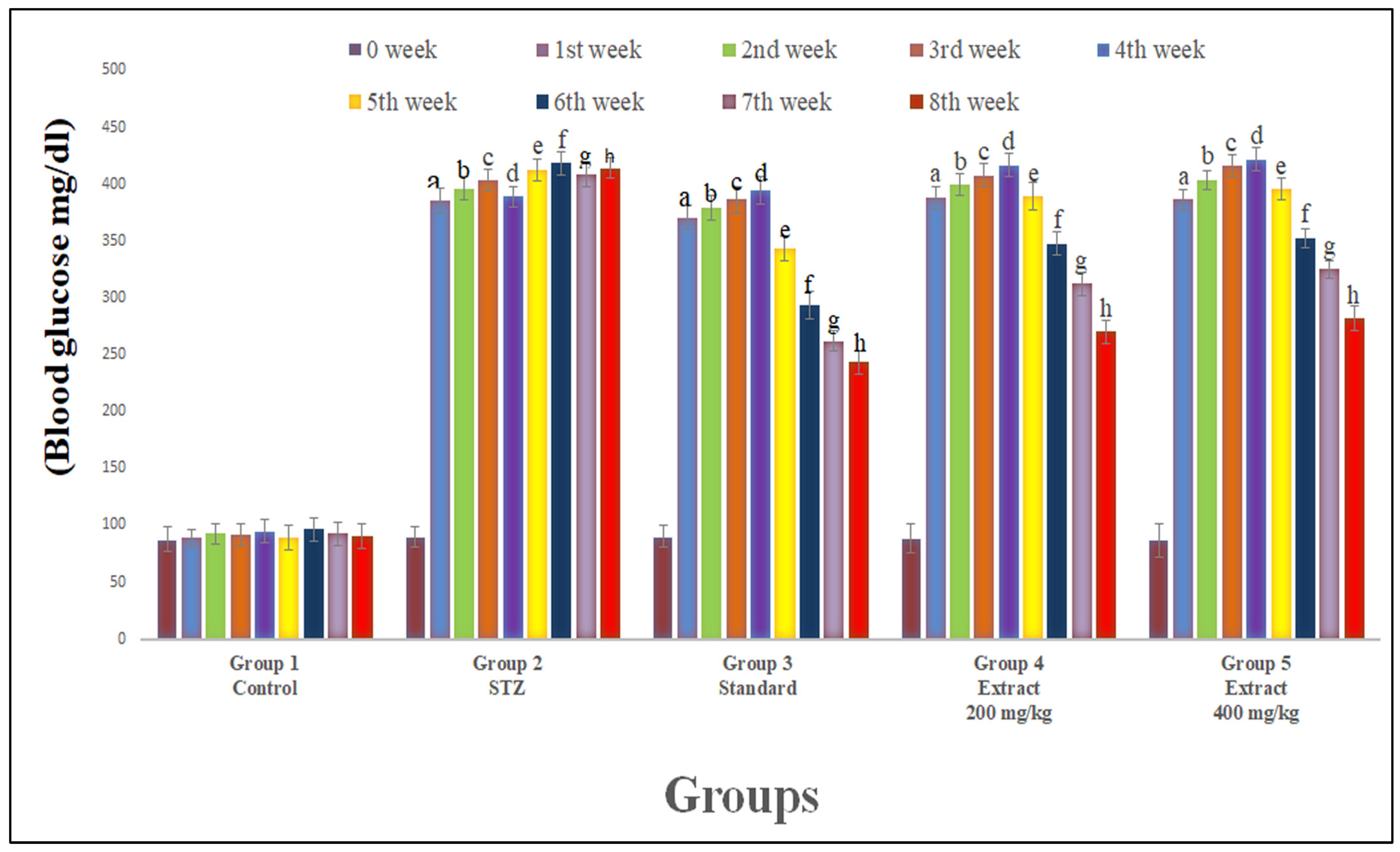
2.1. Blood Glucose and Body Weight
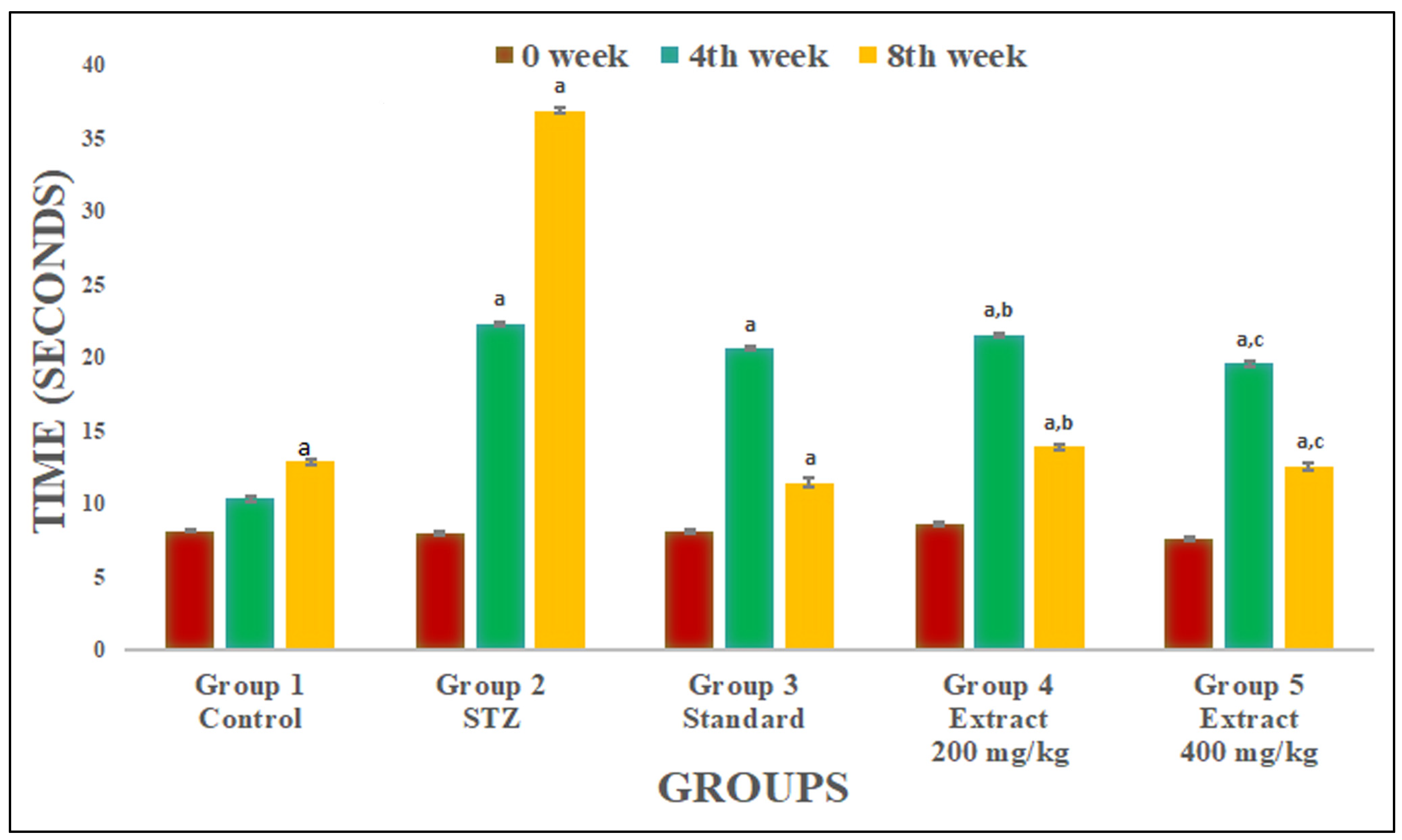
2.2. Neuropathic Pain Assessment
2.2.1. Evaluation of Paw Withdrawal Latency (PWL)
2.2.2. Evaluation of Tail Flick Latency
2.3. R. cordifolia Ameliorates Oxidative Stress
2.4. Effect of R. cordifolia on Apoptosis Factor
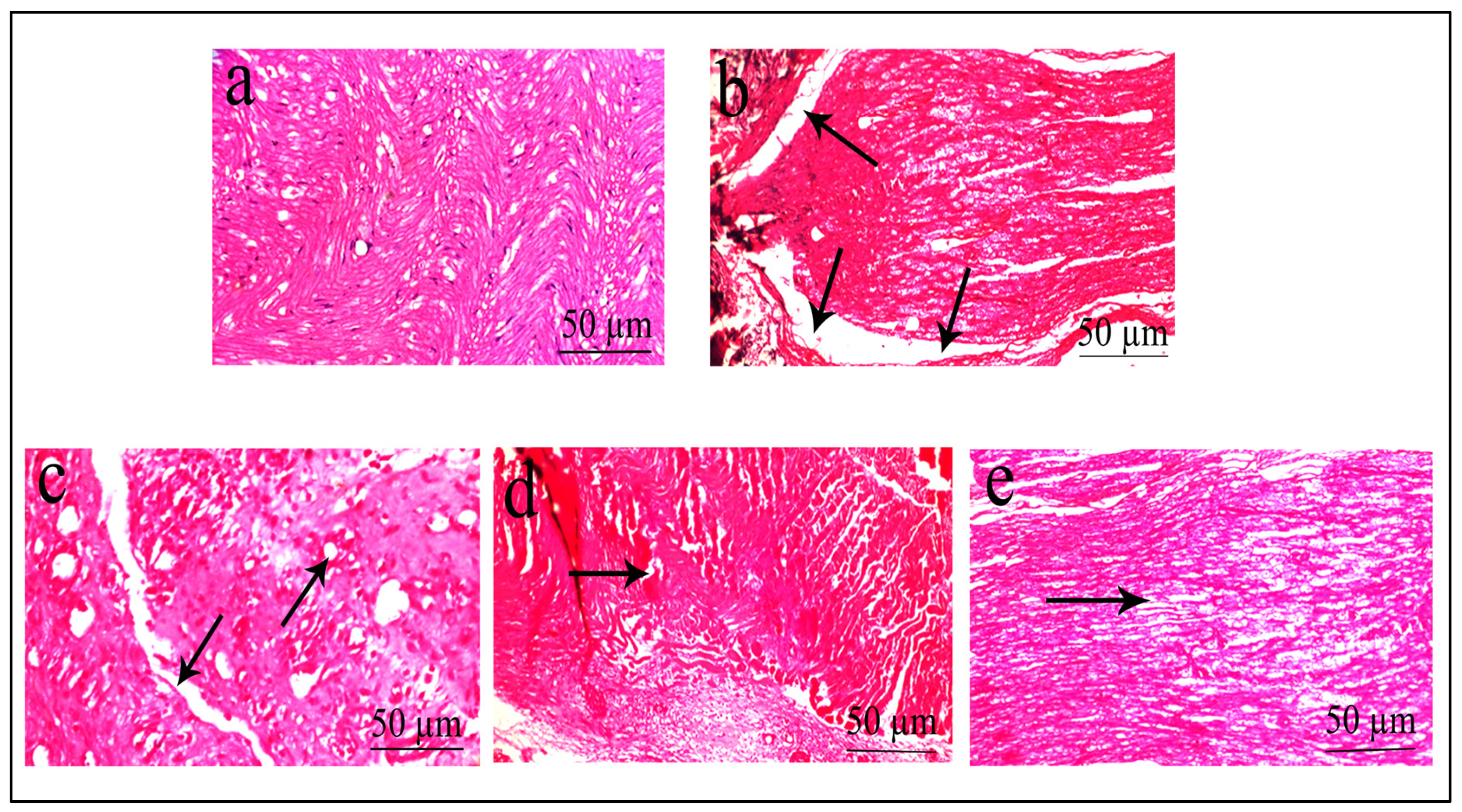
2.5. Histopathological Evaluation
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Authentication
4.3. Preparation of Plant Extract
4.4. Experimental Animals
4.5. Drug Administration and Group Design
4.6. Blood Glucose Level
4.7. Body Weight
4.8. Behavioural Experiments
4.8.1. Tail Flick Latency
4.8.2. Hot Plate Method
4.9. Biochemical Estimation
4.9.1. TBARS Level
4.9.2. GSH Level
4.9.3. CAT Activity
4.9.4. SOD Level
4.10. Western Blot Analysis
4.11. Histopathological Evaluation
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Syed, O.; Jancic, P.; Knezevic, N.N. A Review of Recent Pharmacological Advances in the Management of Diabetes-Associated Peripheral Neuropathy. Pharmaceuticals 2023, 16, 801. [Google Scholar] [CrossRef] [PubMed]
- Dewanjee, S.; Das, S.; Das, A.K.; Bhattacharjee, N.; Dihingia, A.; Dua, T.K.; Kalita, J.; Manna, P. Molecular Mechanism of Diabetic Neuropathy and Its Pharmacotherapeutic Targets. Eur. J. Pharmacol. 2018, 833, 472–523. [Google Scholar] [CrossRef] [PubMed]
- Martiniakova, M.; Kovacova, V.; Mondockova, V.; Svik, K.; Londzin, P.; Folwarczna, J.; Soltesova Prnova, M.; Stefek, M.; Omelka, R. The Effects of Prolonged Treatment with Cemtirestat on Bone Parameters Reflecting Bone Quality in Non-Diabetic and Streptozotocin-Induced Diabetic Rats. Pharmaceuticals 2023, 16, 628. [Google Scholar] [CrossRef]
- Chiang, J.C.; Arnold, R.; Dhanapalaratnam, R.; Markoulli, M.; Krishnan, A.V. Current and Emerging Pharmacotherapeutic Interventions for the Treatment of Peripheral Nerve Disorders. Pharmaceuticals 2022, 15, 607. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.J.M.; Vileikyte, L.; Ragnarson-Tennvall, G.; Apelqvist, J. The Global Burden of Diabetic Foot Disease. Lancet 2005, 366, 1719–1724. [Google Scholar] [CrossRef] [PubMed]
- Essmat, N.; Galambos, A.R.; Lakatos, P.P.; Karádi, D.Á.; Mohammadzadeh, A.; Abbood, S.K.; Geda, O.; Laufer, R.; Király, K.; Riba, P.; et al. Pregabalin–Tolperisone Combination to Treat Neuropathic Pain: Improved Analgesia and Reduced Side Effects in Rats. Pharmaceuticals 2023, 16, 1115. [Google Scholar]
- Gabbay, K.H. Aldose Reductase Inhibition in the Treatment of Diabetic Neuropathy: Where Are We in 2004? Curr. Diab. Rep. 2004, 4, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Magadmi, R.; Borouk, K.; Youssef, D.T.A.; Shaala, L.A.; Alrafiah, A.R.; Shaik, R.A.; Alharthi, S.E. Neuroprotective Effect of Red Sea Marine Sponge Xestospongia Testudinaria Extract Using In Vitro and In Vivo Diabetic Peripheral Neuropathy Models. Pharmaceuticals 2022, 15, 1309. [Google Scholar] [CrossRef]
- Fowler, M.J. Microvascular and macrovascular complications of diabetes. Clin. Diab. 2008, 26, 77–82. [Google Scholar] [CrossRef]
- Russell, J.W.; Golovoy, D.; Vincent, A.M.; Mahendru, P.; Olzmann, J.A.; Mentzer, A.; Feldman, E.L. High Glucose-Induced Oxidative Stress and Mitochondrial Dysfunction in Neurons. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2002, 16, 1738–1748. [Google Scholar] [CrossRef] [PubMed]
- Cederberg, J.; Basu, S.; Eriksson, U.J. Increased Rate of Lipid Peroxidation and Protein Carbonylation in Experimental Diabetic Pregnancy. Diabetologia 2001, 44, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Ostovar, M.; Akbari, A.; Anbardar, M.H.; Iraji, A.; Salmanpour, M.; Hafez Ghoran, S.; Heydari, M.; Shams, M. Effects of Citrullus colocynthis L. in a Rat Model of Diabetic Neuropathy. J. Integr. Med. 2020, 18, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Papanas, N.; Ziegler, D. Efficacy of α-Lipoic Acid in Diabetic Neuropathy. Expert Opin. Pharmacother. 2014, 15, 2721–2731. [Google Scholar] [CrossRef]
- He, X.; Sun, J.; Huang, X. Expression of caspase-3, Bax and Bcl-2 in hippocampus of rats with diabetes and subarachnoid hemorrhage. Exp. Ther. Med. 2018, 15, 873–877. [Google Scholar] [CrossRef]
- Chung, Y.C.; Lim, J.H.; Oh, H.M.; Kim, H.W.; Kim, M.Y.; Kim, E.N.; Kim, Y.; Chang, Y.S.; Kim, H.W.; Park, C.W. Calcimimetic Restores Diabetic Peripheral Neuropathy by Ameliorating Apoptosis and Improving Autophagy. Cell Death Dis. 2018, 9, 1163. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, G.; Shukla, M.; Kaul, G.; Rekha, K.; Chopra, S.; Pandey, R. Characterization and Antimicrobial Evaluation of Anthraquinones and Triterpenes from Rubia cordifolia. J. Asian Nat. Prod. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, Y.B.; Sharma, M. Comparison of the Antioxidant Action of the Alcoholic Extract of Rubia cordifolia with Rubiadin. Indian J. Biochem. Biophys. 1998, 35, 313–316. [Google Scholar] [PubMed]
- Baskar, R.; Bhakshu, L.M.; Vijaya Bharathi, G.; Sreenivasa Reddy, S.; Karuna, R.; Kesava Reddy, G.; Saralakumari, D. Antihyperglycemic Activity of Aqueous Root Extract of Rubia cordifolia. in Streptozotocin-Induced Diabetic Rats. Pharm. Biol. 2006, 44, 475–479. [Google Scholar] [CrossRef]
- Chinese Pharmacopeia Commission. Pharmacopoeia of the People’s Republic of China; Chinese Medical Science Press: Beijing, China, 2015; Volume 1, pp. 234–235.
- Gong, X.-P.; Sun, Y.-Y.; Chen, W.; Guo, X.; Guan, J.-K.; Li, D.-Y.; Du, G. Anti-Diarrheal and Anti-Inflammatory Activities of Aqueous Extract of the Aerial Part of Rubia cordifolia. BMC Complement. Altern. Med. 2017, 17, 20. [Google Scholar] [CrossRef]
- Rawal, A.K.; Muddeshwar, M.G.; Biswas, S.K. Rubia cordifolia, Fagonia Cretica Linn and Tinospora Cordifolia Exert Neuroprotection by Modulating the Antioxidant System in Rat Hippocampal Slices Subjected to Oxygen Glucose Deprivation. BMC Complement. Altern. Med. 2004, 4, 11. [Google Scholar] [CrossRef]
- Itokawa, H.; Mihara, K.; Takeya, K. Studies on a novel anthraquinone and its glycosides isolated from Rubia cordifolia and R. akane. Chem. Pharm. Bull. 1983, 31, 2353–2358. [Google Scholar] [CrossRef]
- Itokawa, H.; Qiao, Y.F.; Takeya, K. Anthraquinones and naphthohydroquinones from Rubia cordifolia. Phytochemistry 1989, 28, 3465–3468. [Google Scholar] [CrossRef]
- Singh, R. Isolation and synthesis of anthraquinones and related compounds of Rubia cordifolia. J. Serbian Chem. Soc. 2005, 70, 937–942. [Google Scholar] [CrossRef]
- Patil, R.A.; Kasture, S.B. Protective effect of Rubia cordifolia on reserpine-induced orofacial dyskinesia. Nat. Prod. Res. 2012, 26, 2159–2161. [Google Scholar] [CrossRef] [PubMed]
- Javed, S.; Petropoulos, I.N.; Alam, U.; Malik, R.A. Treatment of Painful Diabetic Neuropathy. Ther. Adv. Chronic Dis. 2015, 6, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Gul, H.; Yildiz, O.; Dogrul, A.; Yesilyurt, O.; Isimer, A. The Interaction between IL-1beta and Morphine: Possible Mechanism of the Deficiency of Morphine-Induced Analgesia in Diabetic Mice. Pain 2000, 89, 39–45. [Google Scholar] [CrossRef]
- Calcutt, N.A.; Chaplan, S.R. Spinal Pharmacology of Tactile Allodynia in Diabetic Rats. Br. J. Pharmacol. 1997, 122, 1478–1482. [Google Scholar] [CrossRef]
- Kamboj, S.S.; Vasishta, R.K.; Sandhir, R. N-Acetylcysteine Inhibits Hyperglycemia-Induced Oxidative Stress and Apoptosis Markers in Diabetic Neuropathy. J. Neurochem. 2010, 112, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Ghosh, A.; Hazra, B. Evaluation of the Antibacterial Activity of Ventilago Madraspatana Gaertn., Rubia cordifolia Linn. and Lantana Camara Linn.: Isolation of Emodin and Physcion as Active Antibacterial Agents. Phytother. Res. 2005, 19, 888–894. [Google Scholar] [CrossRef]
- Afrazi, S.; Esmaeili-Mahani, S.; Sheibani, V.; Abbasnejad, M. Neurosteroid Allopregnanolone Attenuates High Glucose-Induced Apoptosis and Prevents Experimental Diabetic Neuropathic Pain: In Vitro and in Vivo Studies. J. Steroid Biochem. Mol. Biol. 2014, 139, 98–103. [Google Scholar] [CrossRef]
- Shen, C.-H.; Liu, C.-T.; Song, X.-J.; Zeng, W.-Y.; Lu, X.-Y.; Zheng, Z.-L.; Pan, J.; Zhan, R.-T.; Yan, P. Evaluation of Analgesic and Anti-Inflammatory Activities of Rubia cordifolia L. by Spectrum-Effect Relationships. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1090, 73–80. [Google Scholar] [CrossRef]
- Diwane, C.; Patil, R.; Vyavahare, P.; Bhambar, R. Protective Effect of Rubia cordifolia in Paclitaxel-Induced Neuropathic Pain in Experimental Animals. Indian J. Pain 2015, 29, 150–154. [Google Scholar] [CrossRef]
- Figueroa-Romero, C.; Sadidi, M.; Feldman, E.L. Mechanisms of Disease: The Oxidative Stress Theory of Diabetic Neuropathy. Rev. Endocr. Metab. Disord. 2008, 9, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Bonnefont-Rousselot, D. Glucose and Reactive Oxygen Species. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Kaeidi, A.; Esmaeili-Mahani, S.; Abbasnejad, M.; Sheibani, V.; Rasoulian, B.; Hajializadeh, Z.; Pasban-Aliabadi, H. Satureja Khuzestanica Attenuates Apoptosis in Hyperglycemic PC12 Cells and Spinal Cord of Diabetic Rats. J. Nat. Med. 2013, 67, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.; Kumar, N.; Kumar Saxena, P.; Pratap Singh, A.; Bana, S. Pitavastatin Attenuates Neuropathic Pain Induced by Partial Sciatic Nerve in Wistar Rats. J. Pharm. Pharmacol. 2023, 75, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Orrenius, S.; Gogvadze, V.; Zhivotovsky, B. Mitochondrial Oxidative Stress: Implications for Cell Death. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 143–183. [Google Scholar] [CrossRef]
- Edwards, J.L.; Vincent, A.M.; Cheng, H.T.; Feldman, E.L. Diabetic Neuropathy: Mechanisms to Management. Pharmacol. Ther. 2008, 120, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Hajializadeh, Z.; Nasri, S.; Kaeidi, A.; Sheibani, V.; Rasoulian, B.; Esmaeili-Mahani, S. Inhibitory Effect of Thymus Caramanicus Jalas on Hyperglycemia-Induced Apoptosis in in Vitro and in Vivo Models of Diabetic Neuropathic Pain. J. Ethnopharmacol. 2014, 153, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Rashidipour, M.; Hajializadeh, Z.; Esmaeili-Mahani, S.; Kaeidi, A. The Effect of Rosmarinus Officinalis L Extract on the Inhibition of High Glucose-Induced Neurotoxicity in PC12 Cells: An In Vitro Model of Diabetic Neuropathy. Herb. Med. J. Summer 2017, 2, 114–135. [Google Scholar]
- Sharifi, A.M.; Eslami, H.; Larijani, B.; Davoodi, J. Involvement of Caspase-8, -9, and -3 in High Glucose-Induced Apoptosis in PC12 Cells. Neurosci. Lett. 2009, 459, 47–51. [Google Scholar] [CrossRef]
- Jensen, T.S.; Finnerup, N.B. Allodynia and Hyperalgesia in Neuropathic Pain: Clinical Manifestations and Mechanisms. Lancet. Neurol. 2014, 13, 924–935. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Nandakumar, N.; Rengarajan, T.; Palaniswami, R.; Gnanadhas, E.N.; Lakshminarasaiah, U.; Gopas, J.; Nishigaki, I. Antioxidants and Human Diseases. Clin. Chim. Acta 2014, 436, 332–347. [Google Scholar] [CrossRef]
- Patel, A.; Patel, T.; Macwan, C.; Patel, M.; Chauhan, K.; Patel, J. Evaluation of Anti Inflammatory and Analgesic Activity of Roots of Rubia cordifolia in Rats. J. Pharm. Sci. Res. 2010, 2, 809–813. [Google Scholar]
- Wen, M.; Chen, Q.; Chen, W.; Yang, J.; Zhou, X.; Zhang, C.; Wu, A.; Lai, J.; Chen, J.; Mei, Q.; et al. A Comprehensive Review of Rubia cordifolia L.: Traditional Uses, Phytochemistry, Pharmacological Activities, and Clinical Applications. Front. Pharmacol. 2022, 13, 965390. [Google Scholar] [CrossRef]
- Patil, R.A.; Jagdale, S.C.; Kasture, S.B. Antihyperglycemic, Antistress and Nootropic Activity of Roots of Rubia cordifolia Linn. Indian J. Exp. Biol. 2006, 44, 987–992. [Google Scholar] [PubMed]
- Tripathi, Y.B.; Shukla, S.; Sharma, M.; Shukla, V.K. Antioxidant Property of Rubia cordifolia Extract and Its Comparison with Vitamin E and Parabenzoquinone. Phyther. Res. 1995, 9, 440–443. [Google Scholar] [CrossRef]
- Joharapurkar, A.A.; Deode, N.M.; Zambad, S.P.; Umathe, S.N. Immunomodulatory activity of alcoholic extract of Rubiacordifolia Linn. Ind. Drugs 2003, 40, 179–181. [Google Scholar]
- Gao, J.; Wang, Z.; Ye, Z. Madder (Rubia cordifolia L.) Alleviates Myocardial Ischemia-Reperfusion Injury by Protecting Endothelial Cells from Apoptosis and Inflammation. Mediators Inflamm. 2023, 2023, 5015039. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, B.S.; Prabhakara, S.; Mohan, T.; Shabeer, D.; Bhandare, B.; Nalini, M.; Sharmila, P.S.; Meghana, D.L.; Reddy, B.K.; Rao, H.M.H.; et al. Characterization of Rubia cordifolia L. root extract and its evaluation of cardioprotective effect in Wistar rat model. Ind. J. Pharmacol. 2018, 50, 12–21. [Google Scholar] [CrossRef]
- Divakar, K.; Pawar, A.T.; Chandrasekhar, S.B.; Dighe, S.B.; Divakar, G. Protective Effect of the Hydro-Alcoholic Extract of Rubia cordifolia Roots against Ethylene Glycol Induced Urolithiasis in Rats. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2010, 48, 1013–1018. [Google Scholar] [CrossRef]
- Solanki, N.D.; Bhavsar, S.K. An Evaluation of the Protective Role of Ficus Racemosa Linn. in Streptozotocin-Induced Diabetic Neuropathy with Neurodegeneration. Indian J. Pharmacol. 2015, 47, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Soliman, G.A.; Abdel-Gawad, S.A.; Ansari, M.N.; Altamimi, A.M.S.; El-Banna, H.A.; Elzorba, H.Y.; Hassan, N.F. The Potential Cardioprotective Effect of Matricaria Chamomilla Extract against Diabetes-Induced Oxidative Stress in Rats. Farmacia 2020, 68, 269–279. [Google Scholar] [CrossRef]
- Jain, D.; Bansal, M.K.; Dalvi, R.; Upganlawar, A.; Somani, R. Protective Effect of Diosmin against Diabetic Neuropathy in Experimental Rats. J. Integr. Med. 2014, 12, 35–41. [Google Scholar] [CrossRef]
- Kanaan, S.A.; Saadé, N.E.; Haddad, J.J.; Abdelnoor, A.M.; Atweh, S.F.; Jabbur, S.J.; Safieh-Garabedian, B. Endotoxin-Induced Local Inflammation and Hyperalgesia in Rats and Mice: A New Model for Inflammatory Pain. Pain 1996, 66, 373–379. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Sedlak, J.; Lindsay, R.H. Estimation of Total, Protein-Bound, and Nonprotein Sulfhydryl Groups in Tissue with Ellman’s Reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Claiborne, A. Catalase Activity. In CRC Hand Book of Methods in Oxygen Radical Research; Claiborne, A., Ed.; CRC Press: Greenwald, MN, USA, 1985; pp. 283–284. [Google Scholar]
- Marklund, S.; Marklund, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Hao, J.; Cheng, M.; Zhang, C.; Huo, C.; Liu, Y.; Du, W.; Zhang, X. Hyperglycemia-Induced Bcl-2/Bax-Mediated Apoptosis of Schwann Cells via MTORC1/S6K1 Inhibition in Diabetic Peripheral Neuropathy. Exp. Cell Res. 2018, 367, 186–195. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bana, S.; Kumar, N.; Sartaj, A.; Alhalmi, A.; Qurtam, A.A.; Nasr, F.A.; Al-Zharani, M.; Singh, N.; Gaur, P.; Mishra, R.; et al. Rubia cordifolia L. Attenuates Diabetic Neuropathy by Inhibiting Apoptosis and Oxidative Stress in Rats. Pharmaceuticals 2023, 16, 1586. https://doi.org/10.3390/ph16111586
Bana S, Kumar N, Sartaj A, Alhalmi A, Qurtam AA, Nasr FA, Al-Zharani M, Singh N, Gaur P, Mishra R, et al. Rubia cordifolia L. Attenuates Diabetic Neuropathy by Inhibiting Apoptosis and Oxidative Stress in Rats. Pharmaceuticals. 2023; 16(11):1586. https://doi.org/10.3390/ph16111586
Chicago/Turabian StyleBana, Sweeti, Nitin Kumar, Ali Sartaj, Abdulsalam Alhalmi, Ashraf Ahmed Qurtam, Fahd A. Nasr, Mohammed Al-Zharani, Neelam Singh, Praveen Gaur, Rosaline Mishra, and et al. 2023. "Rubia cordifolia L. Attenuates Diabetic Neuropathy by Inhibiting Apoptosis and Oxidative Stress in Rats" Pharmaceuticals 16, no. 11: 1586. https://doi.org/10.3390/ph16111586
APA StyleBana, S., Kumar, N., Sartaj, A., Alhalmi, A., Qurtam, A. A., Nasr, F. A., Al-Zharani, M., Singh, N., Gaur, P., Mishra, R., Bhardwaj, S., Ali, H., & Goel, R. (2023). Rubia cordifolia L. Attenuates Diabetic Neuropathy by Inhibiting Apoptosis and Oxidative Stress in Rats. Pharmaceuticals, 16(11), 1586. https://doi.org/10.3390/ph16111586






