Chitosan Nanoparticles for Enhanced Delivery of Sida cordifolia Extract: Formulation, Optimization and Bioactivity Assessment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Sida cordifolia-Loaded Chitosan NP
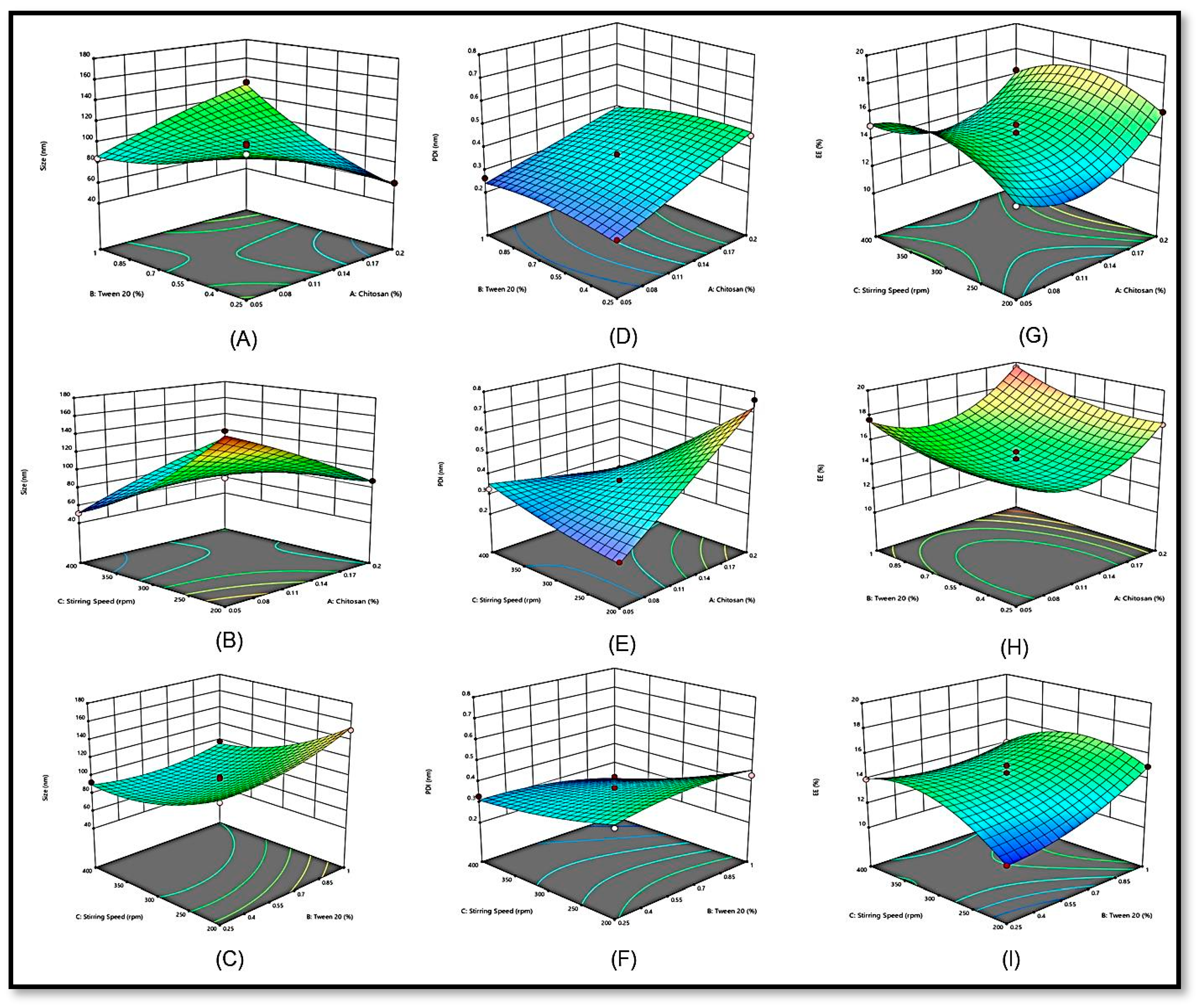
2.1.1. Effects of Independent Variables on Particle Size (Response Y1)
2.1.2. Effects of Independent Variables on the Polydispersity Index (Y2)
2.1.3. Effects of Independent Variables on the EE (Y3)
2.2. Point Prediction
2.3. FTIR
2.4. DSC
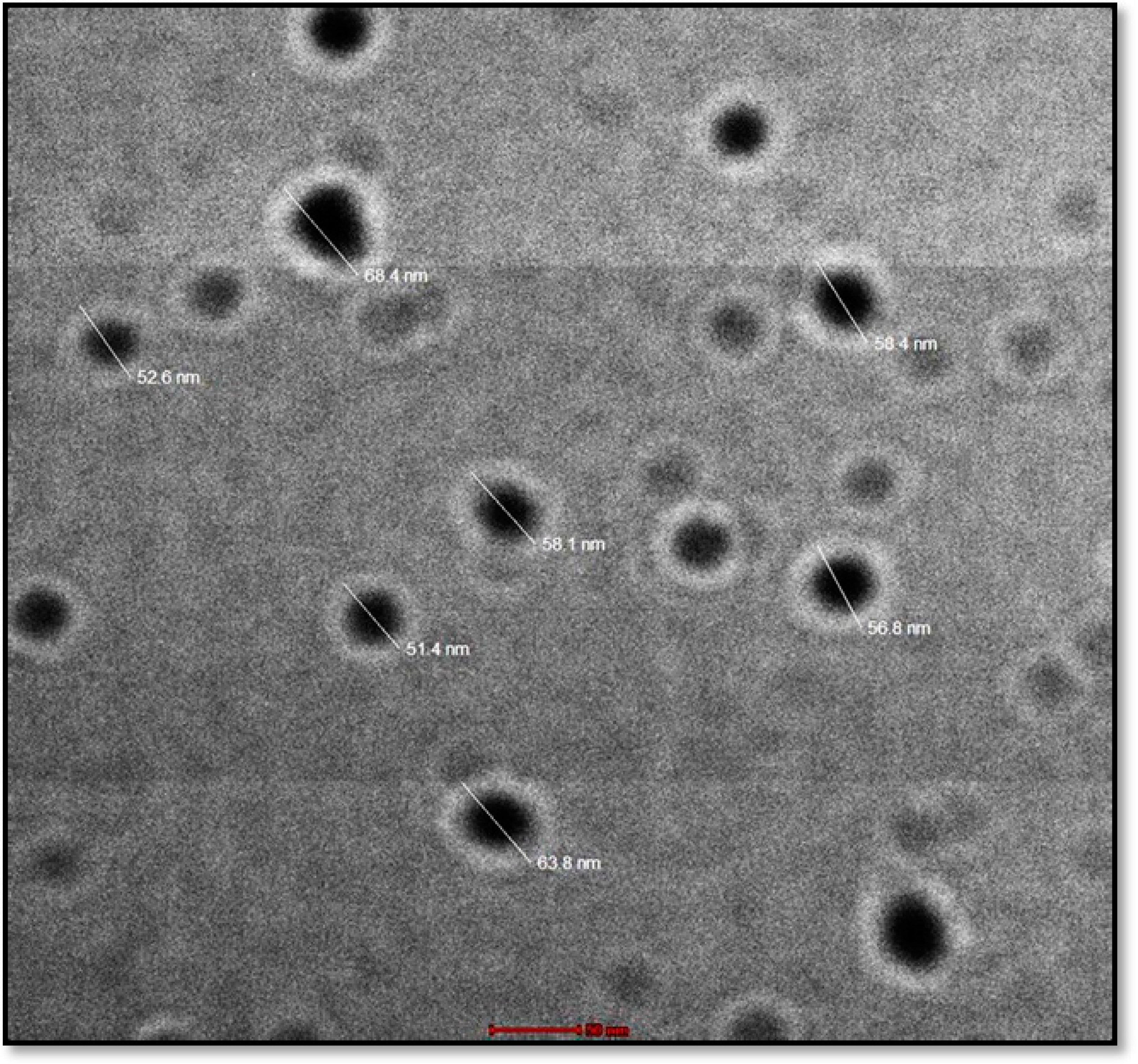
2.5. TEM
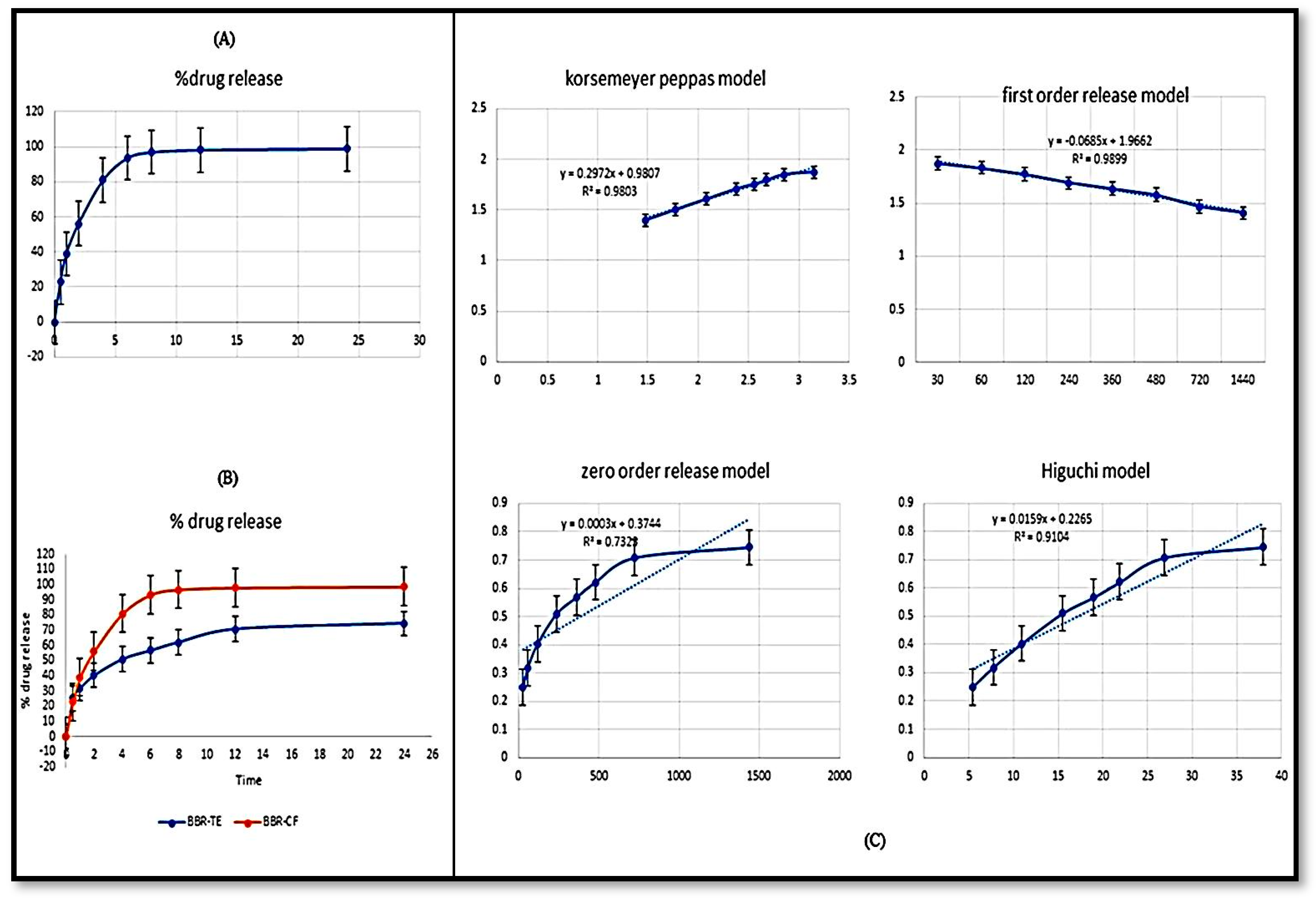
2.6. In Vitro Release Study
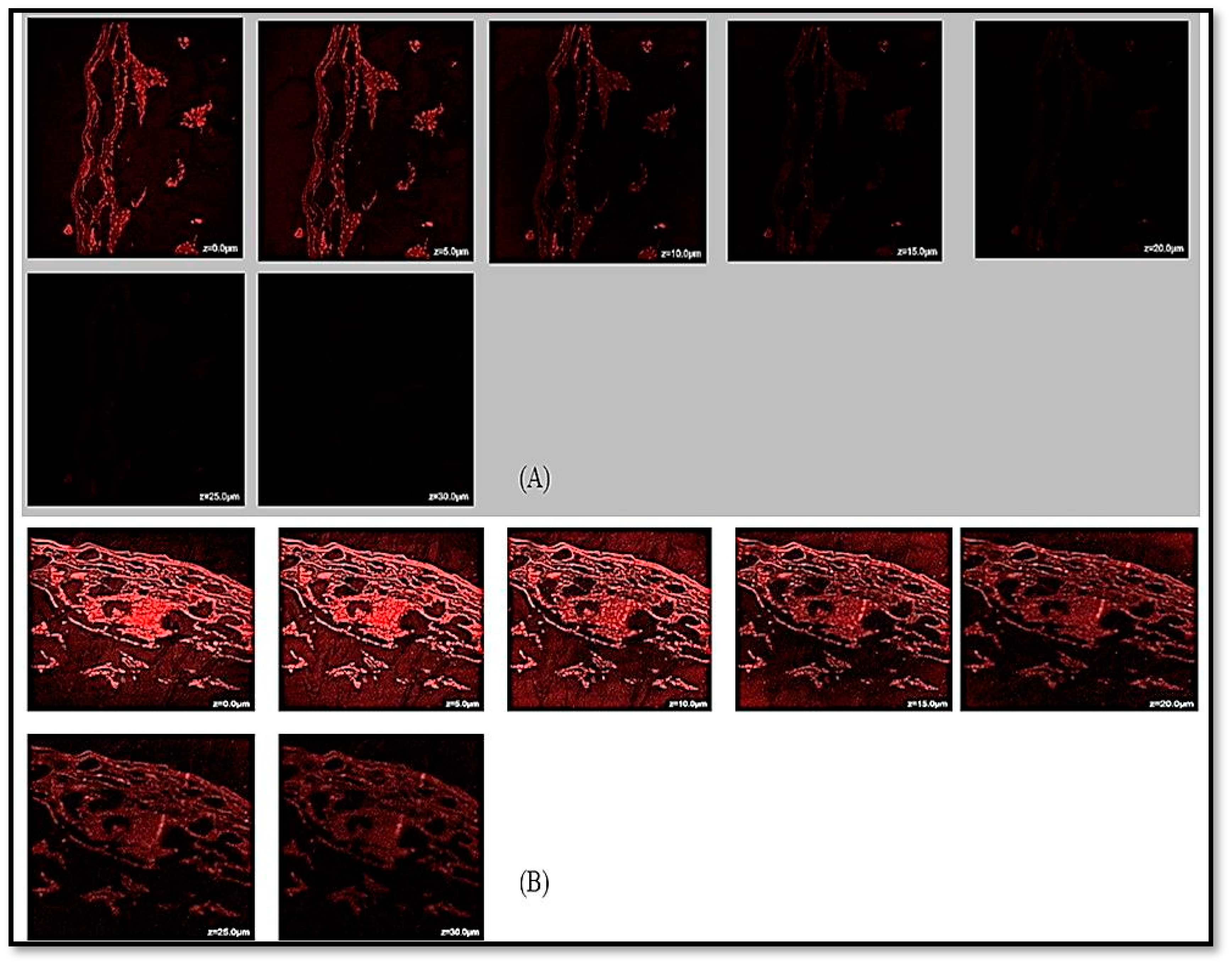
2.7. CLSM Visualization
2.8. Antioxidant and α-Amylase Assay
3. Materials and Methods
3.1. Materials
3.2. Extraction
3.3. Optimization and Development of NPs
3.4. Preparation of SCHA-CS-NPs
3.5. Characterization of SCHA-CS-NPs
3.5.1. FTIR Analysis
3.5.2. DSC Analysis
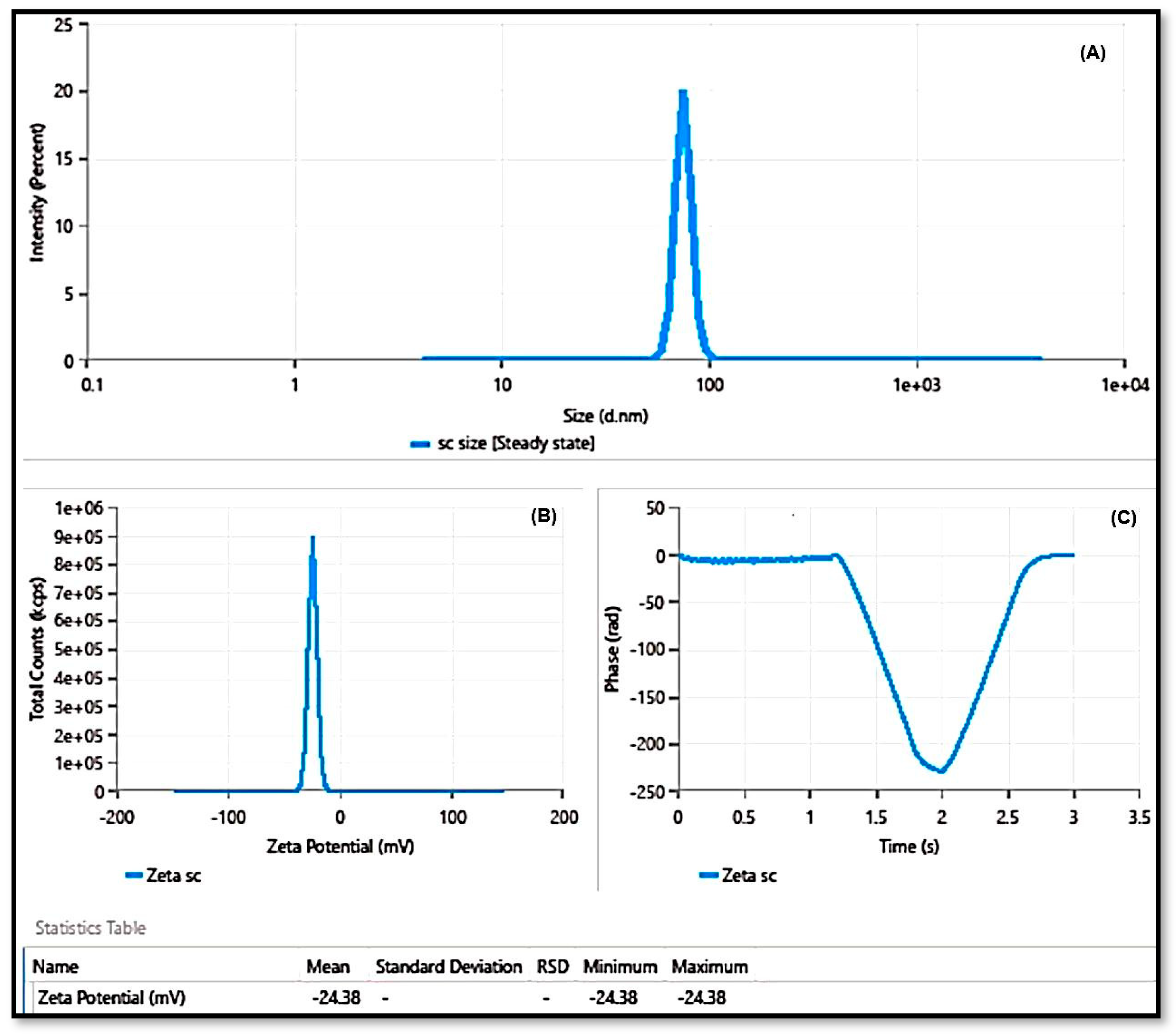
3.5.3. Particle Size and PDI Determination
3.5.4. EE (%)
3.5.5. TEM
3.5.6. Drug Release Study
3.5.7. CLSM Analysis
3.6. Antioxidant Assay
3.7. α-Amylase Assay
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berbudi, A.; Rahmadika, N.; Tjahjadi, A.I.; Ruslami, R. Type 2 Diabetes and Its Impact on the Immune System. Curr. Diabetes Rev. 2020, 16, 442–449. Available online: https://www.ingentaconnect.com/content/ben/cdr/2020/00000016/00000005/art00004 (accessed on 1 November 2023). [PubMed]
- Barathikannan, K.; Venkatadri, B.; Khusro, A.; Al-Dhabi, N.A.; Agastian, P.; Arasu, M.V.; Choi, H.S.; Kim, Y.O. Chemical analysis of Punica granatum fruit peel and its in vitro and in vivo biological properties. BMC Complement. Altern. Med. 2016, 16, 264. [Google Scholar]
- Kanth, V.R.; Diwan, P.V. Analgesic, Antiinflammatory and Hypoglycaemic Activities of Sida cordifolia. Available online: https://onlinelibrary.wiley.com/doi/10.1002/ (accessed on 1 November 2023).
- Ahmad, M.; Prawez, S.; Sultana, M.; Raina, R.; Pankaj, N.K.; Verma, P.K.; Rahman, S. Anti-hyperglycemic, anti-hyperlipidemic and antioxidant potential of alcoholic-extract of Sida cordifolia (Areal Part) in streptozotocin-induced-diabetes in wistar-rats. Proc. Natl. Acad. Sci. India Sect. B—Biol. Sci. 2014, 84, 397–405. [Google Scholar]
- Dinda, B.; Das, N.; Dinda, S.; Dinda, M.; SilSharma, I. The Genus Sida L.–A Traditional Medicine: Its Ethnopharmacological, Phytochemical and Pharmacological Data for Commercial Exploitation in Herbal Drugs Industry. J. Ethnopharmacol. 2015, 176, 135–176. Available online: https://www.sciencedirect.com/science/article/pii/S0378874115301860 (accessed on 1 November 2023). [PubMed]
- Ahmad, M.; Prawez, S.; Sultana, M.; Raina, R.; Kumar, P.; Verma, A.A.A.; Pankaj, N.K. Antidiabetic effect of sida cordifolia (aqueous extract) on diabetes induced in wistar rats using streptozotocin and its phytochemistry. Int. J. Pharm. Res. Innov. 2015, 8, 11–22. [Google Scholar]
- Galal, A.; Raman, V.A.; Khan, I. Sida cordifolia, a Traditional Herb in Modern Perspective—A review. Curr. Tradit. Med. 2015, 1, 5–17. [Google Scholar] [CrossRef]
- Nagpal, K.; Singh, S.; Mishra, D.N. Chitosan Nanoparticles: A Promising System in Novel Drug Delivery. Chem. Pharm. Bull. 2010, 58, 1424. Available online: https://www.jstage.jst.go.jp/article/cpb/58/11/58_11_1423/_article/-char/ja/ (accessed on 1 November 2023).
- Sharifi-Rad, J.; Quispe, C.; Butnariu, M.; Rotariu, L.S.; Sytar, O.; Sestito, S.; Rapposelli, S.; Akram, M.; Iqbal, M.; Krishna, A.; et al. Chitosan nanoparticles as a Promising Tool in Nanomedicine with Particular Emphasis on Oncological Treatment. Cancer Cell Int. 2021, 21, 318. Available online: http://repositoriodigital.ucsc.cl/handle/25022009/2591 (accessed on 1 November 2023).
- Yoshioka, T.; Sternberg, B.; Florence, A.T. Preparation and Properties of Vesicles (niosomes) of Sorbitan Monoesters (Span 20, 40, 60 and 80) and a Sorbitan Triester (Span 85). Int. J. Pharm. 1994, 105, 1–6. Available online: https://www.sciencedirect.com/science/article/pii/0378517394902283 (accessed on 1 November 2023). [CrossRef]
- Bouarab, L.; Maherani, B.; Kheirolomoom, A.; Hasan, M.; Aliakbarian, B.; Linder, M.; Arab-Tehrany, E. Influence of Lecithin–lipid Composition on Physico-Chemical Properties of Nanoliposomes Loaded with a Hydrophobic Molecule. Colloids Surf. B Biointerfaces 2014, 115, 197–204. Available online: https://www.sciencedirect.com/science/article/pii/S0927776513007327 (accessed on 1 November 2023).
- Shaikh, M.V.; Kala, M.; Nivsarkar, M. Formulation and Optimization of Doxorubicin Loaded Polymeric Nanoparticles Using Box-Behnken Design: Ex-Vivo Stability and In-Vitro Activity. Eur. J. Pharm. Sci. 2017, 100, 262–272. Available online: https://www.sciencedirect.com/science/article/pii/S0928098717300507 (accessed on 1 November 2023). [PubMed]
- Gupta, M.; Mondal, A.; Ahmed, K. International Journal of Modern Pharmaceutical Research. Available online: https://ijmpronline.com/download/article/228/1591178545.pdf (accessed on 1 November 2023).
- Pallela, P.N.V.K.; Ummey, S.; Ruddaraju, L.K.; Pammi, S.V.N.; Yoon, S.-G. Ultra Small, Mono Dispersed Green Synthesized Silver Nanoparticles Using Aqueous Extract of Sida Cordifolia Plant and Investigation of Antibacterial Activity. Microb. Pathog. 2018, 124, 63–69. Available online: https://www.sciencedirect.com/science/article/pii/S088240101731762X (accessed on 1 November 2023). [PubMed]
- Pallela, P.N.V.K.; Ummey, S.; Ruddaraju, L.K.; Gadi, S.; Cherukuri, C.S.; Barla, S.; Pammi, S.V.N. Antibacterial efficacy of green synthesized α-Fe2O3 nanoparticles using Sida cordifolia plant extract. Heliyon 2019, 5, e02765. [Google Scholar] [CrossRef] [PubMed]
- Srinithya, B.; Kumar, V.; Vadivel, V.; Pemaiah, B.; Anthony, S.P.; Muthuraman, M.S. Synthesis of Biofunctionalized AgNPs Using Medicinally Important Sida Cordifolia Leaf Extract for Enhanced Antioxidant and Anticancer Activities. Mater. Lett. 2016, 170, 101–104. Available online: https://www.sciencedirect.com/science/article/pii/S0167577X1630180X (accessed on 1 November 2023).
- Jain, A.; Jain, A.; Parajuli, P.; Mishra, V.; Ghoshal, G.; Singh, B.; Shivhare, U.S.; Katare, O.P.; Kesharwani, P. Recent Advances in Galactose-Engineered Nanocarriers for the Site-Specific Delivery of siRNA and Anticancer Drugs. Drug Discov. Today 2018, 23, 960–973. Available online: https://www.sciencedirect.com/science/article/pii/S1359644617303409 (accessed on 1 November 2023).
- Misof, B.; Roschger, P.; Fratzl, P. Imaging mineralized tissues in vertebrates. Compr. Biomater. II 2017, 549–578. [Google Scholar] [CrossRef]
- Desai, K.G. Chitosan NANOPARTICLES prepared by Ionotropic Gelation: An Overview of Recent Advances. Crit. Rev. ™ Ther. Drug Carr. Syst. 2016, 33, 107–158. Available online: https://www.dl.begellhouse.com/journals/3667c4ae6e8fd136,3e868ea50400cfbb,4e5b2d994c2873af.html (accessed on 1 November 2023). [CrossRef]
- Ebert, D.D.; Nobis, S.; Lehr, D.; Baumeister, H.; Riper, H.; Auerbach, R.P.; Snoek, F.; Cuijpers, P.; Berking, M. The 6-Month Effectiveness of Internet-Based Guided Self-Help for Depression in Adults with Type 1 and 2 Diabetes Mellitus. Diabet. Med. 2017, 34, 99–107. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1111/dme.13173 (accessed on 1 November 2023).
- Praveen, A.; Aqil, M.; Imam, S.S.; Ahad, A.; Moolakkadath, T.; Ahmad, F.J. Lamotrigine Encapsulated Intra-Nasal Nanoliposome Formulation for Epilepsy Treatment: Formulation Design, Characterization and Nasal Toxicity Study. Colloids Surf. B Biointerfaces 2019, 174, 553–562. Available online: https://www.sciencedirect.com/science/article/pii/S0927776518308014 (accessed on 1 November 2023).
- Wallenwein, C.M.; Nova, M.V.; Janas, C.; Jablonka, L.; Gao, G.F.; Thurn, M.; Albrecht, V.; Wiehe, A.; Wacker, M.G. A Dialysis-Based In Vitro Drug Release Assay to Study Dynamics of the Drug-Protein Transfer of Temoporfin Liposomes. Eur. J. Pharm. Biopharm. 2019, 143, 44–50. Available online: https://www.sciencedirect.com/science/article/pii/S0939641119305466 (accessed on 1 November 2023). [CrossRef]
- Carter, J.D.; Dula, S.B.; Corbin, K.L.; Wu, R.; Nunemaker, C.S. A Practical Guide to Rodent Islet Isolation and Assessment. Biol. Proced Online 2009, 11, 3–31. Available online: https://biologicalproceduresonline.biomedcentral.com/articles/10.1007/s12575-009-9021-0 (accessed on 1 November 2023). [CrossRef] [PubMed]
- Alam, M.S.; Ahad, A.; Abidin, L.; Aqil, M.; Mir, S.R.; Mujeeb, M. Embelin-Loaded Oral Niosomes Ameliorate Streptozotocin-induced Diabetes in Wistar Rats. Biomed. Pharmacother. 2018, 97, 1514–1520. Available online: https://www.sciencedirect.com/science/article/pii/S0753332217337162 (accessed on 1 November 2023). [CrossRef] [PubMed]
- Nair, S.; Kavrekar, V.; Mishra, A. In Vitro Studies on Alpha Amylase and Alpha Glucosidase Inhibitory Activities of Selected Plant Extracts. Eur. J. Exp. Biol. 2013, 3, 128–132. Available online: https://www.researchgate.net/profile/Mabrur-Mabrur/post/alpha_amylase_assay/attachment/5ca4822fcfe4a7df4ae533de/AS%3A743539768045568%401554285103167/download/in-vitro-studies-on-alpha-amylase-and-alpha-glucosidase-inhibitory-activities-of-selected-plant-extracts.pdf (accessed on 1 November 2023).
- Festing, M.F.W. Design and Statistical Methods in Studies Using Animal Models of Development. ILAR J. 2006, 47, 5–14. Available online: https://academic.oup.com/ilarjournal/article-abstract/47/1/5/662269 (accessed on 1 November 2023). [CrossRef]

| Variables | Low | Medium | High |
|---|---|---|---|
| Independent variables | |||
| X1 Chitosan (%) | 0.05 | 0.125 | 0.2 |
| X2 Tween 20 (%) | 0.25 | 0.625 | 1 |
| X3 Stirring speed (Rpm) | 200 | 300 | 400 |
| Dependent variables | |||
| Y1 Particle size (Nm) | |||
| Y2 Polydispersity index (%) | |||
| Y3 Entrapment efficiency (%) | |||
| Runs | Independent Variables | Dependent Variables | |||||
|---|---|---|---|---|---|---|---|
| Formulation | Chitosan (%) (X1) | Tween 20 (%) (X2) | Stirring Speed (rpm) (X3) | Particle Size (nm) (Y1) | Polydispersity Index (Y2) | Entrapment Efficiency (%) (Y3) | |
| 1 | F1 | 0.125 | 0.625 | 300 | 91.09 | 0.342 | 78.21 |
| 2 | F2 | 0.125 | 1 | 200 | 151 | 0.432 | 74 |
| 3 | F3 | 0.2 | 0.625 | 400 | 106 | 0.231 | 81.23 |
| 4 | F4 | 0.125 | 0.625 | 300 | 94 | 0.359 | 73.9 |
| 5 | F5 | 0.05 | 0.25 | 300 | 121 | 0.243 | 80.21 |
| 6 | F6 | 0.2 | 1 | 300 | 134.47 | 0.391 | 84.54 |
| 7 | F7 | 0.2 | 0.625 | 2000 | 88.06 | 0.761 | 79.25 |
| 8 | F8 | 0.125 | 0.25 | 400 | 93 | 0.329 | 65 |
| 9 | F9 | 0.125 | 0.625 | 300 | 97.09 | 0.359 | 67.89 |
| 10 | F10 | 0.05 | 0.625 | 400 | 51 | 0.326 | 75.54 |
| 11 | F11 | 0.125 | 0.625 | 300 | 99 | 0.371 | 72 |
| 12 | F12 | 0.2 | 0.25 | 300 | 60.03 | 0.453 | 79.09 |
| 13 | F13 | 0.05 | 0.625 | 200 | 169 | 0.216 | 55.90 |
| 14 | F14 | 0.125 | 1 | 400 | 98 | 0.223 | 61.89 |
| 15 | F15 | 0.05 | 1 | 300 | 84.31 | 0.264 | 80.07 |
| 16 | F16 | 0.125 | 0.625 | 300 | 93 | 0.359 | 56 |
| 17 | F17 | 0.125 | 0.25 | 200 | 120.08 | 0.409 | 44.76 |
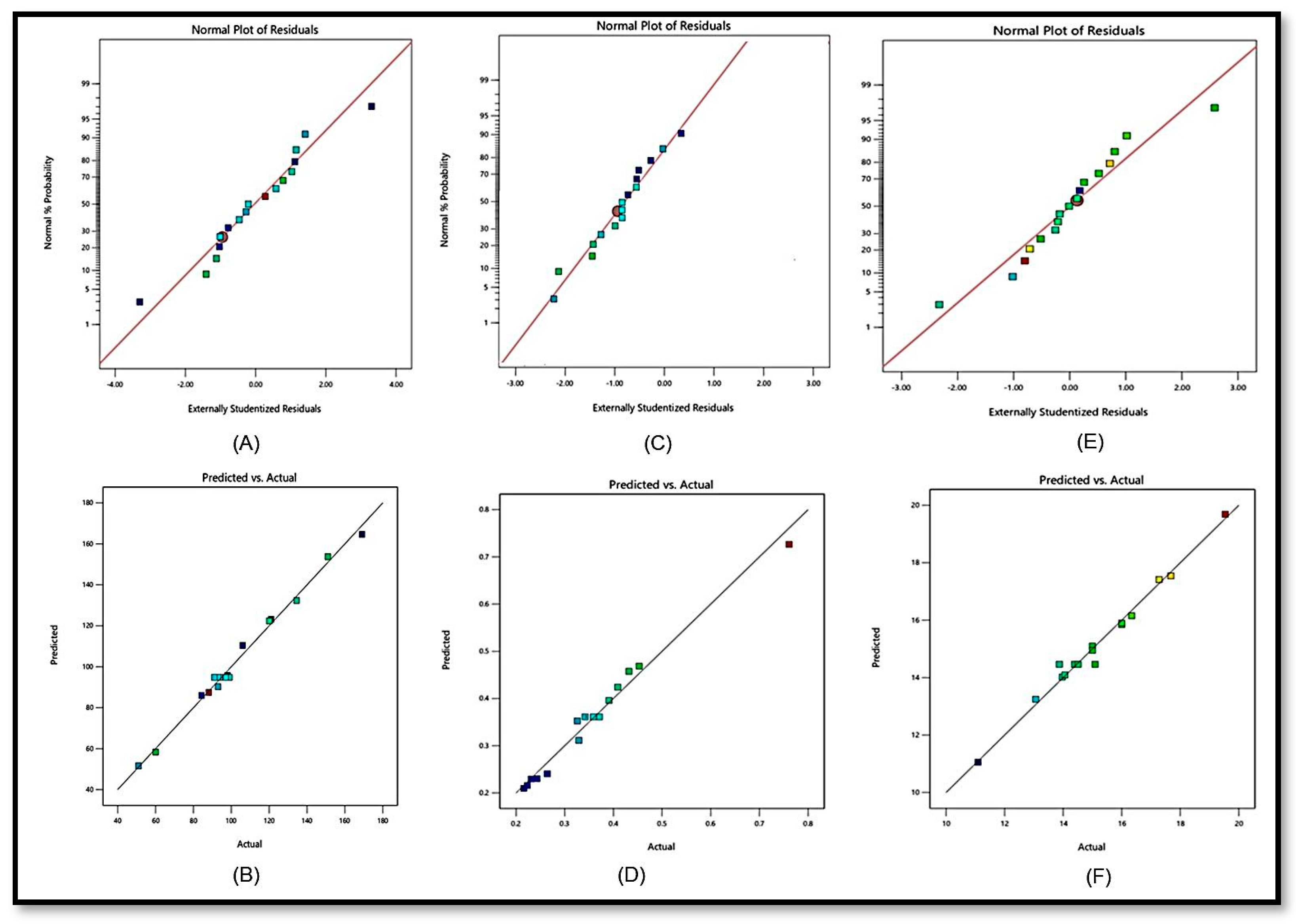
| Responses | R2 | Adjusted R2 | Predicted R2 | SD | % CV | Model |
|---|---|---|---|---|---|---|
| Y1 (nm) | 0.9914 | 0.9804 | 0.9046 | 4.13 | 4.01 | Quadratic |
| Y2 | 0.9822 | 0.9643 | 0.8371 | 0.0240 | 6.74 | Quadratic |
| Y3 (%) | 0.9844 | 0.9644 | 0.9327 | 0.3644 | 2.41 | Quadratic |
| S. No. | Name of the Drug | IC50 (µg/mL) |
|---|---|---|
| 1 | SCHA extract | 82.36 ± 1.31 |
| 2 | SCHA-CS-NPs | 86.45 ± 2.24 |
| 3 | Ascorbic acid (standard) | 88.23 ± 1.43 |
| S. No. | α-Amylase Activity | IC50 (µg/mL) |
|---|---|---|
| 1 | SCHA extract | 90.23 ± 1.64 |
| 2 | SCHA-CS-NPs | 93.71 ± 1.79 |
| 3 | Acarbose (standard) | 97.25 ± 1.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, P.; Imran, M.; Ahmed, S.; Majid, H.; Akhtar, A. Chitosan Nanoparticles for Enhanced Delivery of Sida cordifolia Extract: Formulation, Optimization and Bioactivity Assessment. Pharmaceuticals 2023, 16, 1561. https://doi.org/10.3390/ph16111561
Alam P, Imran M, Ahmed S, Majid H, Akhtar A. Chitosan Nanoparticles for Enhanced Delivery of Sida cordifolia Extract: Formulation, Optimization and Bioactivity Assessment. Pharmaceuticals. 2023; 16(11):1561. https://doi.org/10.3390/ph16111561
Chicago/Turabian StyleAlam, Perwez, Mohd Imran, Shahnawaz Ahmed, Haya Majid, and Ali Akhtar. 2023. "Chitosan Nanoparticles for Enhanced Delivery of Sida cordifolia Extract: Formulation, Optimization and Bioactivity Assessment" Pharmaceuticals 16, no. 11: 1561. https://doi.org/10.3390/ph16111561
APA StyleAlam, P., Imran, M., Ahmed, S., Majid, H., & Akhtar, A. (2023). Chitosan Nanoparticles for Enhanced Delivery of Sida cordifolia Extract: Formulation, Optimization and Bioactivity Assessment. Pharmaceuticals, 16(11), 1561. https://doi.org/10.3390/ph16111561







