New Perspectives about Drug Candidates Targeting HTLV-1 and Related Diseases
Abstract
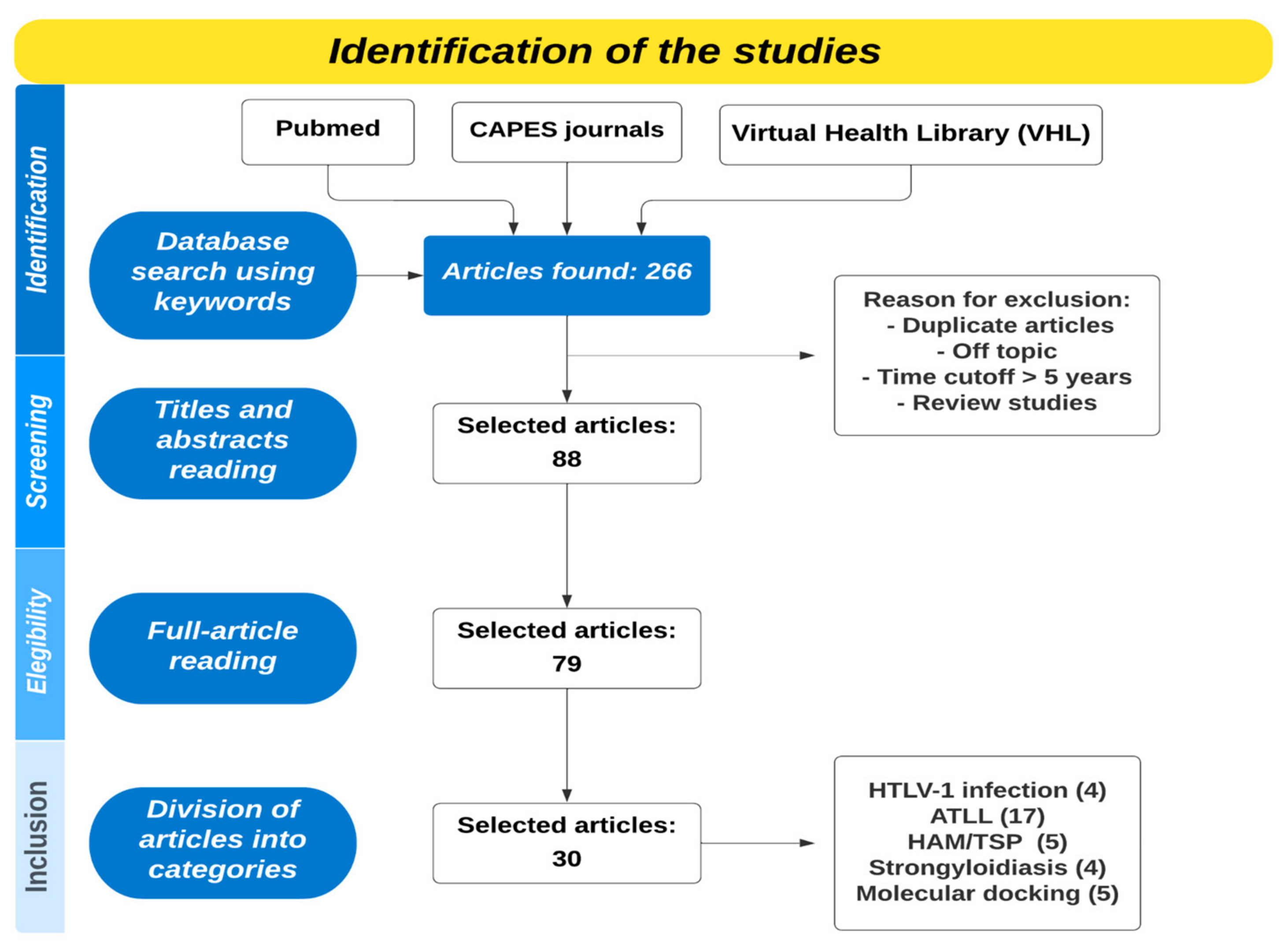
:1. Introduction
2. Materials and Methods
2.1. New Studies about Drugs to Treat HTLV-1 Infection
| HTLV-1 Infection | ||||
|---|---|---|---|---|
| Drug/Molecule/ Natural Product | Activity | Study Methodology | Year | Author |
| 1,2,3-Triazole tethered fused heterocyclic ring derivatives | Compounds induced S-phase cell cycle arrest; promoted apoptosis; and reduced GFP expression in an inducible-Tax reporter cell, which suggests an effect on Tax. | Cell-based assay using resazurin reduction method, and evaluation towards cell cycle, apoptosis and Tax/GFP expression analyzes through flow cytometry. | 2020 | [22] |
| Alcoholic extract from Eucalyptus camaldulensis | Inhibited Tax induced activation of NF-κB, SRF-dependent promoters, and HTLV-1 LTR. | Evaluated the activity of the extract by testing its influence on Tax-induced activity of NF-κB and HTLV-1 LTR in Jurkat cells. | 2020 | [28] |
| (E)-3-Phenyl-5-(phenylamino)-2-styryl-1,3,4-thiadiazol-3-ium chloride derivatives | Caused necrosis of Jurkat and MT-2 cells infected with HTLV-1 after 24 h, maybe due to its capacity to intercalate into DNA | Biological evaluation against MT-2 and C92 cell lines infected with human T-cell lymphotropic virus type-1 (HTLV-1) | 2020 | [23] |
| Pomalidomide (pom) | Can enhance the immune response to HTLV-1 infection, but this response is not maintained. | Rhesus macaque model. The pom (0.2 mg/kg) was administered orally to four HTLV-1-infected macaques over a 24-day period and collected blood, urine, and bone marrow samples. | 2022 | [24] |
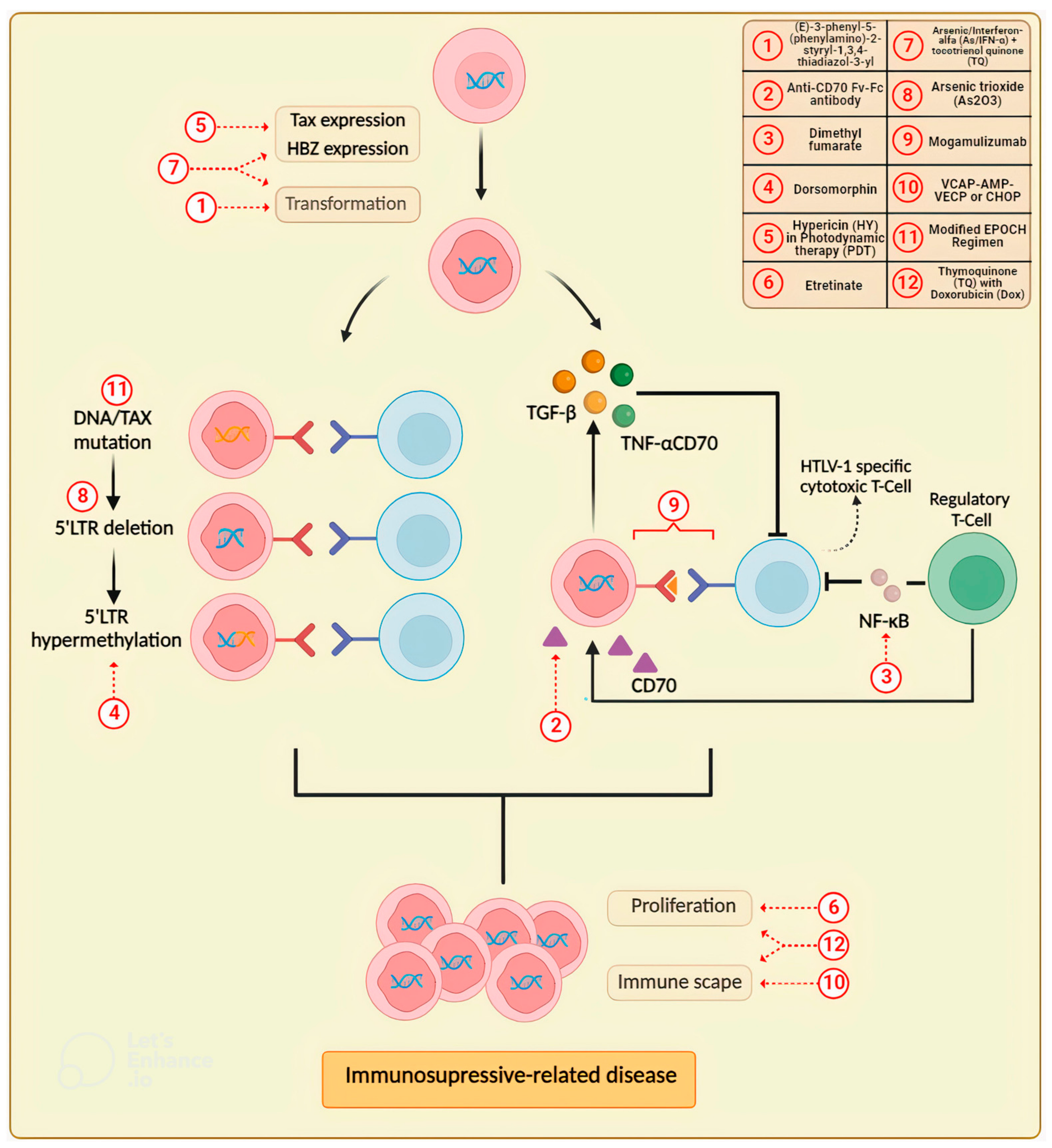
2.2. Updates in Drug Research for the Treatment of Adult T-Cell Leukemia/Lymphoma (ATLL)
| Adult T-Cell Leukemia/Lymphoma | ||||
|---|---|---|---|---|
| Drug/Molecule/Natural Product | Activity | Study Methodology | Year | Author |
| (E)-3-Phenyl-5-(phenylamino)-2-styryl-1,3,4-thiadiazol-3-ium chloride derivatives | The compounds showed cell death by necrosis and DNA interaction, especially those containing electron donor substituents. | Biological evaluation against MT-2 and C92 cell lines infected with human HTLV-1, and non-infected cell lines (Jurkat). Pharmacokinetic profile of the compounds was obtained through human serum albumin (HSA) binding affinity using multiple spectroscopic techniques (circular dichroism, steady-state and time-resolved fluorescence), zeta potential, and molecular docking calculations. | 2020 | [23] |
| Anti-CD70 single-chain Fv-Fc antibody conjugated with emtansine | Showed selective killing of peripheral blood mononuclear cells (PBMCs) from an ATLL patient. | Novel antibody drug conjugate (ADC) constructed using a novel antibody modification method. Its cell cytotoxicity and target specificity were assessed using a cell proliferation assay. | 2020 | [45] |
| Arsenic trioxide (As2O3) | As2O3 consolidation in combination with low-dose AZT/IFN maintenance may enhance long-term disease control in ATLL lymphoma with moderate side effects. | Retrospective study included nine newly diagnosed, previously untreated ATLL patients. | 2020 | [46] |
| Arsenic/interferon-alpha (As/IFN-α) with thymoquinone (TQ) | Led to a more pronounced and synergistic time-dependent inhibitory effect on HTLV-I-positive cells in comparison to As/IFN-α, as well as a significant decrease in tumor volume in a HuT-102 xenograft mouse model. | Trypan blue and flow cytometry were used to investigate viability and cell cycle effects. Annexin V staining, rhodamine assay, and Western blotting were used to determine apoptosis induction and changes in protein expression. Efficacy of single drugs and combinations were tested in an adult T-cell leukemia (HuT-102) mouse xenograft model. | 2020 | [47] |
| Dimethyl fumarate (DMF) | Inhibited proliferation and induced apoptosis in HTLV-1-infected and transformed T-cells by suppressing NF-ĸB and STAT3 signaling pathways. | Examined the proliferation and apoptosis by the trypan blue exclusion assay and annexin V/propidium iodide staining in HTLV-1-infected and transformed T-cell lines (MT-1 and MT-2 cells) and evaluated the NF-ĸB and STAT3 signaling pathways and anti-apoptotic proteins by immunoblotting. | 2022 | [43] |
| Dimethyl fumarate | Suppresses the proliferation of HTLV-1-infected T cells by inhibiting CBM-complex-triggered NF-ĸB signaling. | Assessed whether the BCL2 apoptosis regulator (BCL2)/BCL2-like 1 (BCL-xL) inhibitor navitoclax promoted the inhibitory effect of DMF on cell proliferation and apoptosis-associated proteins by trypan blue exclusion test and immunoblotting, respectively. | 2023 | [44] |
| Dorsomorphin | Induced apoptosis in PBMC from ATLL patients and dose- and time-dependent apoptosis in HTLV-1infected T-cell lines. | PBMCs were treated with dorsomorphin, stained with annexin V-fluorescein isothiocyanate (FITC) and 7-aminoactinomycin D (7-AAD), and analyzed by flow cytometry. | 2020 | [41] |
| Etretinate | It improves quality of life (QoL) by relieving the skin symptoms in cutaneous-type adult T-cell leukemia-lymphoma (cATLL). | Retrospective assessment of the efficacy and safety of etretinate in 9 patients with cATLL. | 2019 | [40] |
| Hypericin (HY) in photodynamic therapy (PDT) | It was highly effective against ATLL cells by induction of apoptosis and suppression of viral transcription. | Tested against ATLL cell lines and analyzed by colony formation assay, light and fluorescence microscopy, flow cytometry using an annexin V-FITC apoptosis detection kit, immunoblotting, luciferase assay, quantitative real-time PCR, chromatin immunoprecipitation assay, and HTLV-1 transmission assay. | 2019 | [48] |
| Modified EPOCH regimen | It was effective with tolerable adverse effects and prolonged overall survival mainly in patients that underwent allogeneic hematopoietic stem cell transplantation. | Retrospective analysis of untreated aggressive adult T-cell leukemia/lymphoma who received the modified EPOCH (mEPOCH) regimen. | 2020 | [33] |
| Mogamulizumab | The trial demonstrated the efficacy of mogamulizumab in comparison to other frequently used agents. | International, multicenter, open-label, randomized study conducted at 22 centers in Belgium, Brazil, France, Martinique, Peru, the UK, and the US; 18 centers screened and 17 randomized patients to determine the ORR of mogamulizumab that persisted and was confirmed at a subsequent response evaluation to compare cORR, PFS, OS, and DoR. | 2019 | [36] |
| Mogamulizumab | In clinical practice, mogamulizumab therapy was confirmed to be a feasible option for the treatment of patients with r/r ATLL, including the elderly, and the overall safety profile of mogamulizumab was manageable in most patients. | Prospective, observational, postmarketing surveillance conducted in patients with chemokine receptor 4 (CCR4)-positive, relapsed, or refractory adult T-cell leukemia-lymphoma (ATLL). | 2019 | [37] |
| Mogamulizumab | Exerts clinically meaningful antitumor activity in ATLL | Multicenter prospective observational study to establish the most effective and safe treatment strategy using mogamulizumab for ATLL patients. | 2020 | [39] |
| Mogamulizumab | Improved overall survival in patients with relapsed/refractory ATL, especially those with acute-type ATLL and skin rash. | Retrospective analysis of patients with acute- and lymphoma-type ATLL who received salvage therapy, and who received mogamulizumab | 2020 | [38] |
| Thymoquinone (TQ) with low concentrations of doxorubicin (dox) | Caused greater inhibition of cell viability and increased sub-G1 cells in both cell lines compared to dox or TQ alone. The combination induced apoptosis by increasing ROS and caused a disruption of mitochondrial membrane potential. TQ and dox combination also reduced tumor volume in mice more significantly than single treatments through enhanced apoptosis without affecting the survival of mice. | HTLV-1-positive (HuT-102) and HTLV-1-negative (Jurkat) CD4+ malignant T-cell lines were treated with TQ, dox, and combinations. Viability and cell cycle effects were determined by MTT assay and flow cytometry analysis, respectively. Combination effects on mitochondrial membrane potential and generation of ROS were assessed. Expression levels of key cell death proteins were investigated by Western blotting. A mouse xenograft model of ATLL in NOD/SCID was used for testing drug effects, and tumor tissues were stained for Ki67 and TUNEL. | 2019 | [49] |
| VCAP-AMP-VECP or CHOP | Significantly higher response rates and overall survival after treatment with VCAP-AMP-VECP than CHOP in transplant-eligible patients with aggressive ATLL. | Retrospective analysis of transplant-eligible patients with ATLL who received only VCAP-AMP-VECP or CHOP, incorporating inverse probability of treatment weighting (IPTW) using propensity scoring. | 2019 | [34] |
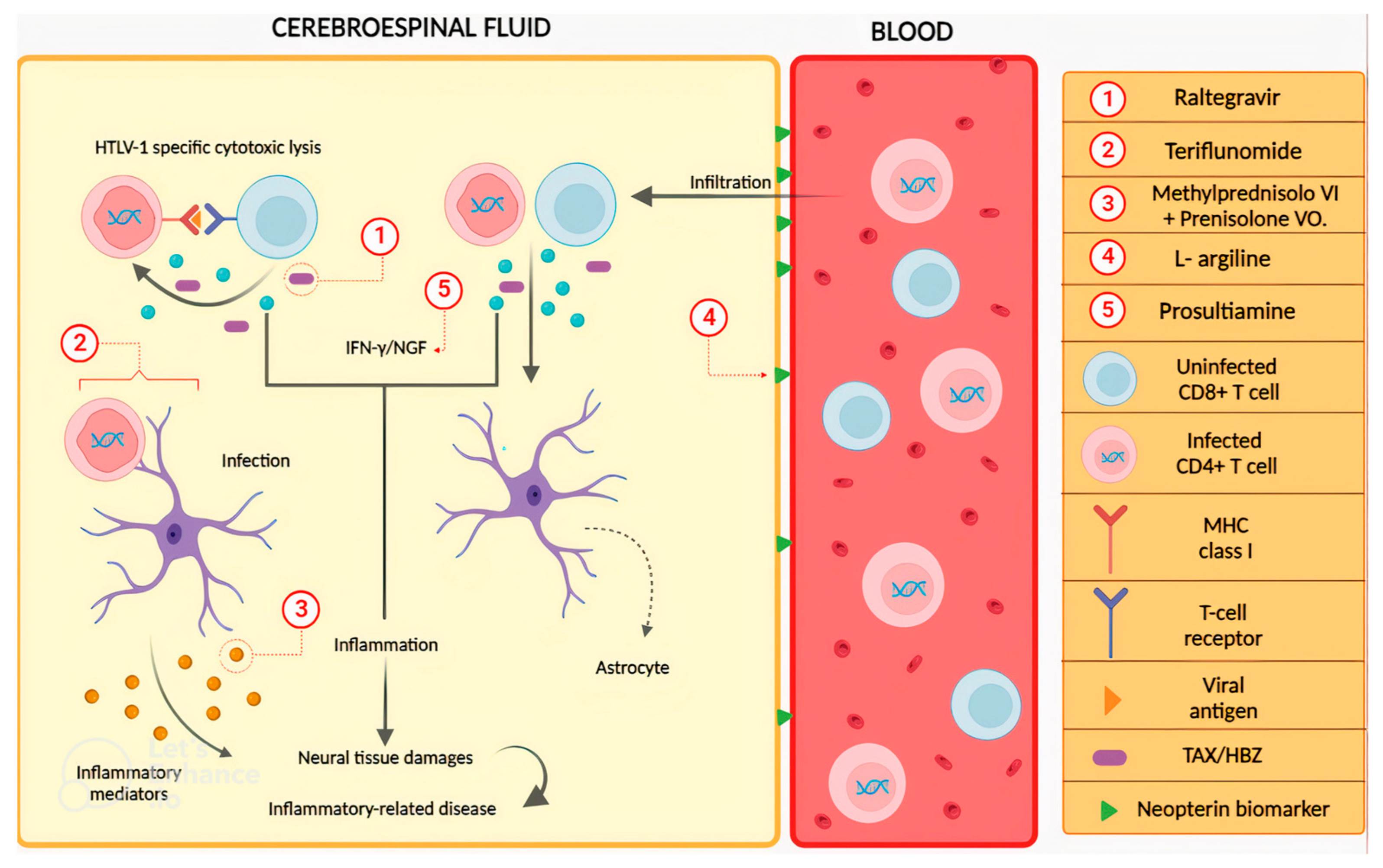
2.3. Drugs to Treat HTLV-1-Associated Myelopathy (HAM)/Tropical Spastic Paraparesis (TSP)
| HAM/TSP | ||||
|---|---|---|---|---|
| Drug | Activity | Study Methodology | Year | Author |
| Intravenous methylprednisolone plus oral prednisolone | Increase of one or more grades in Osame motor disability score (OMDS) and improvement of 15% or more in the 10 m walking test. | Randomized, controlled phase 2 study. Patients were divided into rapid and slow progression of HAM/TSP. Patients with rapid progression were allocated into groups (1:1) to receive or not combined corticosteroid therapy. Patients with slow progression were allocated into groups (1:1) to receive oral mono corticosteroid therapy or not. | 2022 | [55] |
| Raltegravir | Unequal reduction in proviral load and expression of Tax and HBZ, with no changes in the evaluated scores. | A pilot, single-center, single-arm, open-label study treated 18 patients with 400 mg of raltegravir twice daily for 6 months. Patients were evaluated using the expanded disability status scale, scripps neurological rating scale, time 25 foot walk, Instituto Evandro Chagas scale, ambulatory index, and nine-hole peg test. Viral load and immunological markers were evaluated using blood and cerebrospinal fluid (CSF) samples. | 2021 | [56] |
| Teriflunomide | Dose-dependent regulatory action on the spontaneous proliferation of CD4+ and CD8+ T lymphocytes; however, CD25 continued to be expressed, as well as the expression of Tax and HBZ mRNA and Tax protein. | PBMCs from 12 patients with HAM/TSP were collected and cultured in the presence and absence of teriflunomide to test cell viability, lymphocyte proliferation, activation markers, and the expression of Tax and HBZ mRNA and Tax protein. | 2021 | [58] |
| L-arginine | Increase in walking speed in the 10 m walk test over 14 days and improvement in gait function in the timed Up and Go test on the 14th and 28th days, along with a reduction in neopterin levels. | Phase 2, open-label, single-arm study: 20 HAM/TSP patients received oral L-arginine (20 g) for 1 week, followed by 3 weeks of observation without treatment. Assessments included walking tests, evaluation of inflammatory markers, and safety/tolerability. | 2023 | [59] |
| Prosultiamine | There was a decrease in night-time frequency, urgency, and levels of biomarkers for overactive bladder, such as nerve growth factor/creatinine and adenosine triphosphate/creatinine. | Prospective, single-center, open-label study. The patients received a once-daily treatment of 300 mg of prosultiamine, and their symptoms of overactive bladder and biomarkers were assessed at baseline and after 12 weeks. | 2019 | [60] |
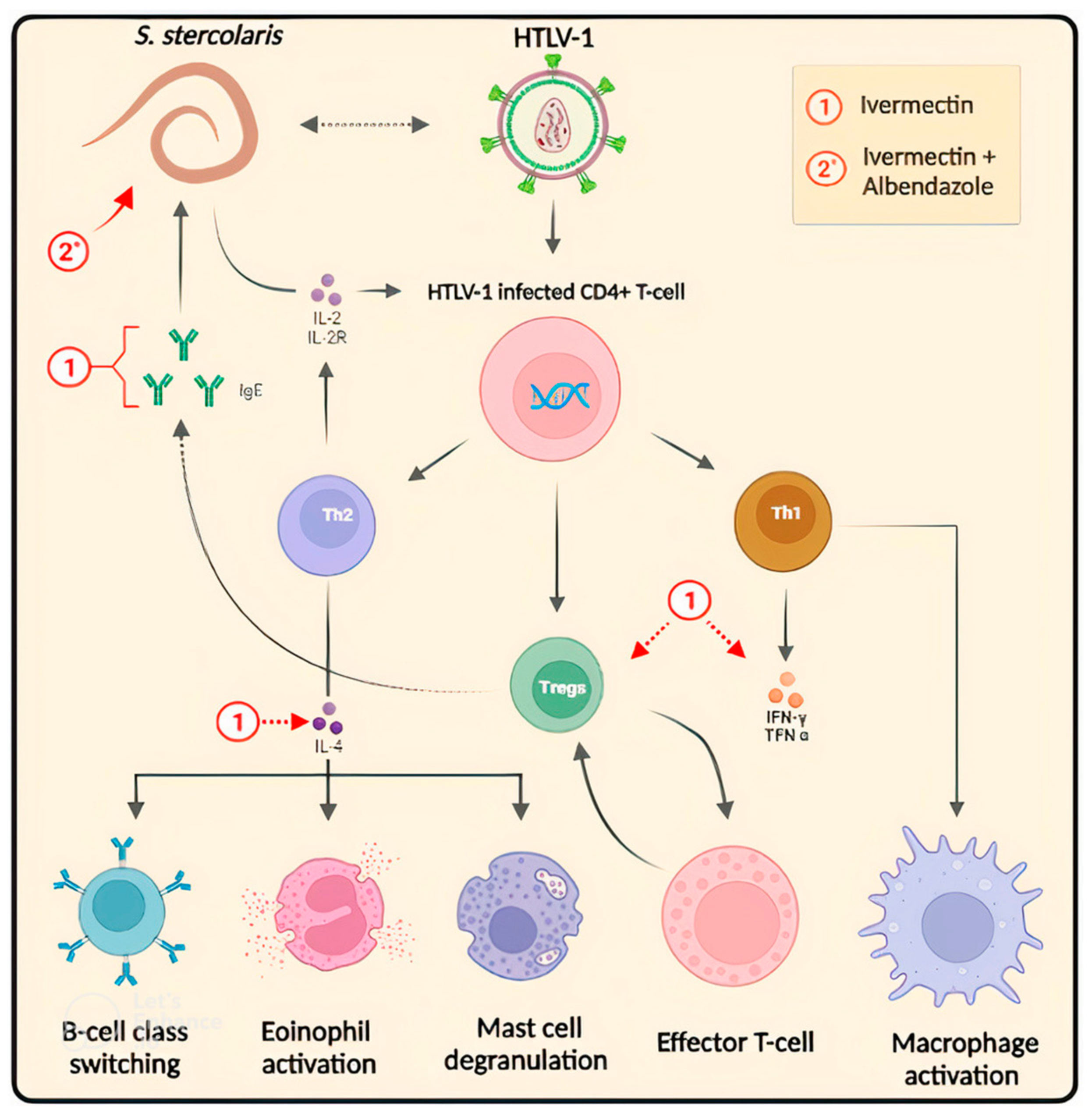
2.4. Challenges and Prospects in the Drug Treatment of HTLV-1-Associated Strongyloidiasis
| Strongyloidiasis | ||||
|---|---|---|---|---|
| Drug | Activity | Study Methodology | Year | Author |
| Ivermectin 200 mcg/kg | In addition to the risk of developing multiorgan failure syndrome, a fact observed in the patient after starting ivermectin, following the neurological evolution, was unfavorable. | A case report illustrating the risk of occurrence of bacterial infections such as Gram-negative meningitis in the case of disseminated infection in a patient co-infected by Strongyloides-HTLV-1. | 2021 | [65] |
| Ivermectin 200 mcg/kg, albendazole 400 mg | In the patient, the medical procedure was initiated with broad-spectrum antibiotic therapy for bacterial co-infection, but despite this, there was no clinical response of improvement, leading to a fatal outcome. | A case report describing the clinical case of a patient with a history of HTLV-1 infection and ulcerative colitis that developed into Strongyloides stercoralis hyperinfection, including its diagnosis and treatment. | 2021 | [66] |
| Ivermectin 200 μg/kg | Treating strongyloidiasis infection decreases circulating Tregs, but antigen-specific cytokine remains altered. This may reflect the blunting of sensitization by Tregs. | Diagnosis of strongyloidiasis was made by stool examination using Baermann’s sedimentation method. All patients positive for Strongyoides larvae were tested for HTLV-1 infection by ELISA. Positive results were confirmed by Western blot. Then, patients received antihelminthic treatment with ivermectin 200 μg/kg dose on two consecutive days. A second course of two doses was given 15 days later. Clinical follow-up included the assessment of treatment efficacy by Baermann’s exam on stool. Then, flow cytometry and antigen-specific cytokine response tests were performed. | 2020 | [59] |
| Subcutaneous ivermectin, albendazole | Subcutaneous ivermectin was used as an anthelmintic treatment with an adequate therapeutic response. | A case report described a case of a man coinfected with Strongyloides stercoralis and HTLV-1. | 2019 | [67] |
2.5. Molecular Docking as a Tool to Discover New Drugs against HTLV-1 Infection and Related Diseases
| Molecular Doking | ||||
|---|---|---|---|---|
| Molecule | Target | Docking Methodology | New Findings | Author |
| Simeprevir, atazanavir, and saquinavir | Key residues in the HTLV-1 protease binding site. | Molecular simulation between drug, target protein, and the interaction between them. | It suggests the possibility of repurposing these drugs for the treatment of HTLV-1 infection. | [73] |
| Multiepitope immunization | Multiepitope domain targeting viral antigenic regions. | Molecular dynamics between the immunizer and the immune system. | The engineered vaccine has shown promise in structural stability and ability to induce an immune response. | [77] |
| Chloride derivatives (5a–d) | Sites of HTLV-1-infected MT-2 and C92 cell lines. | Simulations to predict how these compounds bind and interact with the replication target protein. | HSA-5 compounds can be active in HTLV-infected MT-2 and C92 cells, with potential bioavailability. | [23] |
| Indinavir | HTLV-1 protease reactive sites. | Conformational interaction between indinavir and HTLV-1 protease at an atomic level. | Simulation of indinavir binding to protease, revealing its molecular interactions. | [76] |
| Modified naphthyridines | Deltaretroviral intasome domains. | Analysis of structures of deltaretroviral intasomes obtained by cryo-EM. | Existence of specific interactions between intasome components and proviral DNA. | [78] |
3. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poiesz, B.J.; Ruscetti, F.W.; Gazdar, A.F.; Bunn, P.A.; Minna, J.D.; Gallo, R.C. Detection and isolation of type-c retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous t-cell lymphoma. Proc. Natl. Acad. Sci. USA 1980, 77, 7415–7419. [Google Scholar] [CrossRef] [PubMed]
- Ministério da Saúde. Guia de Manejo Clínico do Paciente com HTLV; Ministério da Saúde: Rio de Janeiro, Brazil, 2003.
- Martinez, M.P.; Al-Saleem, J.; Green, P.L. Comparative virology of HTLV-1 and HTLV-2. Retrovirology 2019, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Proietti, F.A.; Carneiro-Proietti, A.B.; Catalan-Soares, B.C.; Murphy, E.L. Global epidemiology of HTLV-I infection and associated diseases. Oncogene 2005, 24, 6058–6068. [Google Scholar] [CrossRef] [PubMed]
- Gessain, A.; Cassar, O. Epidemiological aspects and world distribution of HTLV-1 infection. Front. Microbiol. 2012, 3, 388. [Google Scholar] [CrossRef] [PubMed]
- Calattini, S.; Chevalier, S.A.; Duprez, R.; Bassot, S.; Froment, A.; Mahieux, R.; Gessain, A. Discovery of a new human T-cell lymphotropic virus (HTLV-3) in Central Africa. Retrovirology 2005, 2, 30. [Google Scholar] [CrossRef]
- Wolfe, N.D.; Heneine, W.; Carr, J.K.; Garcia, A.D.; Shanmugam, V.; Tamoufe, U.; Torimiro, J.N.; Prosser, A.T.; Lebreton, M.; Mpoudi-Ngole, E.; et al. Emergence of unique primate T-lymphotropic viruses among central African bushmeat hunters. Proc. Nat. Acad. Sci. USA 2005, 102, 7994–7999. [Google Scholar] [CrossRef]
- Calattini, S.; Betsem, E.; Bassot, S.; Chevalier, S.A.; Mahieux, R.; Froment, A.; Gessain, A. New strain of human T lymphotropic virus (HTLV) type 3 in a Pygmy from Cameroon with peculiar HTLV serologic results. J. Infect. Dis. 2009, 199, 561–564. [Google Scholar] [CrossRef]
- Zheng, H.; Wolfe, N.D.; Sintasath, D.M.; Tamoufe, U.; Lebreton, M.; Djoko, C.F.; Diffo Jle, D.; Pike, B.L.; Heneine, W.; Switzer, W.M. Emergence of a novel and highly divergent HTLV-3 in a primate hunter in Cameroon. Virology 2010, 401, 137–145. [Google Scholar] [CrossRef]
- Hoshino, H. Cellular Factors Involved in HTLV-1 Entry and Pathogenicit. Front Microbiol. 2012, 21, 222. [Google Scholar] [CrossRef]
- Tezuka, K.; Fuchi, N.; Okuma, K.; Tsukiyama, T.; Miura, S.; Hasegawa, Y.; Nagata, A.; Komatsu, N.; Hasegawa, H.; Sasaki, D. HTLV-1 targets human placental trophoblasts in seropositive pregnant women. J. Clin. Investig. 2020, 130, 6171–6186. [Google Scholar] [CrossRef]
- Eusebio-Ponce, E.; Anguita, E.; Paulino-Ramirez, R.; Candel, F.J. HTLV-1 infection: An emerging risk. Pathogenesis, epidemiology, diagnosis and associated diseases. Rev. Esp. Quimioter. 2019, 32, 485–496. [Google Scholar] [PubMed]
- Koyanagi, Y.; Itoyama, Y.; Nakamura, M.; Takamatsu, K.; Kira, J.; Iwamasa, T.; Goto, I.; Yamamoto, N. In vivo infection of human T-cell leukemia virus type I in non-T cells. Virology 1993, 196, 25–33. [Google Scholar] [CrossRef]
- World Health Organization. Human T-Lymphotropic Virus Type 1; Technical Report; World Health Organization: Geneva, Italy, 2021. [Google Scholar]
- Van-Leeuwen, R.; Katlam, C.; Kitchen, V.; Boucher, C.A.; Tubiana, R.; McBride, M.; Ingrand, D.; Weber, J.; Hill, A.; McDade, H.; et al. Evaluation of safety and efficacy of 3TC (lamivudine) in patients with asymptomatic or mildly symptomatic human immunodeficiency virus infection: A phase I/II study. J. Infect. Dis. 1995, 171, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.P.; Hall, S.E.; Navarrete, S.; Michie, C.A.; Davis, R.; Witkover, A.D.; Rossor, M.; Nowak, M.A.; Rudge, P.; Matutes, E.; et al. Effect of Lamivudine on Human T-Cell Leukemia Virus Type 1 (HTLV-1) DNA Copy Number, T-Cell Phenotype, and Anti-Tax Cytotoxic T-Cell Frequency in Patients with HTLV-1-Associated Myelopathy. J. Virol. 1999, 73, 10289–10295. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, M. Epidemiology of HTLV-1 Infection and ATL in Japan: An Update. Front. Microbiol. 2020, 11, 1124. [Google Scholar] [CrossRef]
- Cook, L.B.; Fuji, S.; Hermine, O.; Bazarbachi, A.; Ramos, J.C.; Ratner, L.; Horwitz, S.; Fields, P.; Tanase, A.; Bumbea, H.; et al. Revised Adult T-Cell Leukemia-Lymphoma International Consensus Meeting Report. J. Clin. Oncol. 2019, 37, 677–687. [Google Scholar] [CrossRef]
- Araujo, A.; Bangham, C.R.M.; Casseb, J.; Gotuzzo, E.; Jacobson, S.; Martin, F.; Penalva de Oliveira, A.; Puccioni-Sohler, M.; Taylor, G.P.; Yamano, Y. Management of HAM/TSP: Systematic Review and Consensus-based Recommendations 2019. Neurol. Clin. Pract. 2021, 11, 49–56. [Google Scholar] [CrossRef]
- Soltani, A.; Hashemy, S.I.; Zahedi, A.F.; Soleimani, A.; Rafatpanah, H.; Rezaee, S.A.; Griffith, R.; Mashkani, B. Molecular targeting for treatment of human T-lymphotropic virus type 1 infection. Biomed. Pharmacother. 2019, 109, 770–778. [Google Scholar] [CrossRef]
- Boxus, M.; Willems, L. Mechanisms of HTLV-1 persistence and transformation. Br. J. Cancer 2009, 101, 1497–1501. [Google Scholar] [CrossRef]
- Santos, D.F.; De Pilger, D.R.B.; Vandermeulen, C.; Khouri, R.; Mantoani, S.P.; Nunes, P.S.G.; De andrade, P.; Carvalho, I.; Casseb, J.; Twizere, J.C.; et al. Non-cytotoxic 1,2,3-triazole tethered fused heterocyclic ring derivatives display Tax protein inhibition and impair HTLV-1 infected cells. Bioorg. Med. Chem. 2020, 28, 115746. [Google Scholar] [CrossRef]
- Sousa-Pereira, D.; Oliveira, T.S.; Paiva, R.O.; Chaves, O.A.; Netto-Ferreira, J.C.; Echavarria-lima, J.; Echevarria, A. Synthetic (E)-3-Phenyl-5-(phenylamino)-2-styryl-1,3,4-thiadiazol-3-ium Chloride Derivatives as Promising Chemotherapy Agents on Cell Lines Infected with HTLV-1. Molecules 2020, 25, 2537. [Google Scholar] [CrossRef]
- Gutowska, A.; Mckinnon, K.; Sarkis, S.; Doster, M.N.; Bissa, M.; Moles, R.; Stamos, J.D.; Rahman, M.A.; Washington-Parks, R.; Davis, D.; et al. Transient Viral Activation in Human T Cell Leukemia Virus Type 1-Infected Macaques Treated with Pomalidomide. Front. Med. 2022, 9, 897264. [Google Scholar] [CrossRef] [PubMed]
- Karbalaei, M.; Keikha, M. Curcumin as an Herbal Inhibitor Candidate Against HTLV-1 Protease. Jentashapir J. Cell. Mol. Biol. 2019, 10, e92813. [Google Scholar] [CrossRef]
- Nakama, S.; Ishikawa, C.; Nakachi, S.; Mori, N. Anti-adult T-cell leukemia effects of Bidens pilosa. Int. J. Oncol. 2011, 38, 1163–1173. [Google Scholar]
- Xu, J.; Xu, Z.; Zheng, W. A Review of the Antiviral Role of Green Tea Catechins. Molecules 2017, 22, 1337. [Google Scholar] [CrossRef]
- Abu-Jafar, A.; Suleiman, M.; Nesim, N.; Huleihel, M. The effect of alcoholic extract from Eucalyptus camaldulensis leaves on HTLV-1 Tax activities. Cell Cycle 2020, 19, 1768–1776. [Google Scholar] [CrossRef]
- Mulherkar, R.; Karabudak, A.; Ginwala, R.; Huang, X.; Rowan, A.; Philip, R.; Murphy, E.L.; Clements, D.; Ndhlovu, L.C.; Khan, Z.K.; et al. In vivo and in vitro immunogenicity of novel MHC class I presented epitopes to confer protective immunity against chronic HTLV-1 infection. Vaccine 2019, 36, 5046–5057. [Google Scholar] [CrossRef]
- Imaizumi, Y.; Iwanaga, M.; Nosaka, K.; Ishitsuka, K.; Ishizawa, K.; Ito, S.; Amano, M.; Ishida, T.; Uike, N.; Utsunomiya, A.; et al. Prognosis of patients with adult T-cell leukemia/lymphoma in Japan: A nationwide hospital-based study. Cancer Sci. 2020, 111, 4567–4580. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, A.; Choi, I.; Chihara, D.; Seto, M. Recent advances in the treatment of adult T-cell leukemia-lymphomas. Cancer Sci. 2015, 106, 344–351. [Google Scholar] [CrossRef]
- Kinpara, S.; Kijiyama, M.; Takamori, A.; Hasegawa, A.; Sasada, A.; Masuda, T.; Tanaka, Y.; Utsunomiya, A.; Kannagi, M. Interferon-a (IFN-a) suppresses HTLV-1 gene expression and cell cycling, while IFN-acombined with zido-vudine induces p53 signaling and apoptosis in HTLV-1-infected cells. Retrovirology 2013, 10, 52. [Google Scholar] [CrossRef]
- Tsukamoto, Y.; Kiyasu, J.; Choi, I.; Kozuru, M.; Uike, N.; Utsunomiya, H.; Hirata, A.; Fujioka, E.; Ohno, H.; Nakashima, E.; et al. Efficacy and Safety of the Modified EPOCH Regimen (Etoposide, Vincristine, Doxorubicin, Carboplatin, and Prednisolone) for Adult T-cell Leukemia/Lymphoma: A Multicenter Retrospective Study. Clin. Lymphoma Myeloma Leuk. 2020, 20, e445–e453. [Google Scholar] [CrossRef]
- Fuji, S.; Yamaguchi, T.; Inoue, Y.; Utsunomiya, A.; Moriuchi, Y.; Owatari, S.; Miyagi, T.; Sawayama, Y.; Otsuka, E.; Yoshida, S.I.; et al. VCAP-AMP-VECP as a preferable induction chemotherapy in transplant-eligible patients with aggressive adult T-cell leukemia-lymphoma: A propensity score analysis. Bone Marrow Transpl. 2019, 54, 1399–1405. [Google Scholar] [CrossRef]
- Ishida, T.; Utsunomiya, A.; Iida, S.; Inagaki, H.; Takatsuka, Y.; Kusumoto, S.; Takeuchi, G.; Shimizu, S.; Ito, M.; Komatsu, H.; et al. Clinical significance of CCR4 expression in adult T-cell leukemia/lymphoma: Its close association with skin involvement and unfavorable outcome. Clin. Cancer Res. 2003, 9, 3625–3634. [Google Scholar]
- Phillips, A.A.; Fields, P.A.; Hermine, O.; Ramos, J.C.; Beltran, B.E.; Pereira, J.; Wandroo, F.; Feldman, T.; Taylor, G.P.; Sawas, A.; et al. Mogamulizumab versus investigator’s choice of chemotherapy regimen in relapsed/refractory adult T-cell leukemia/lymphoma. Haematologica 2019, 104, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Joh, T.; Uike, N.; Yamamoto, K.; Utsunomiya, A.; Yoshida, S.; Saburi, Y.; Miyamoto, T.; Takemoto, S.; Suzushima, H.; et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: A multicenter phase II study. J. Clin. Oncol. 2012, 30, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Satake, A.; Konishi, A.; Azuma, Y.; Tsubokura, Y.; Yoshimura, H.; Hotta, M.; Nakanishi, T.; Fujita, S.; Nakaya, A.; Ito, T.; et al. Clinical efficacy of mogamulizumab for relapsed/refractory aggressive adult T-cell leukemia/lymphoma: A retrospective analysis. Eur. J. Haematol. 2020, 105, 704–711. [Google Scholar] [CrossRef]
- Ishitsuka, K.; Yurimoto, S.; Tsuji, Y.; Iwabuchi, M.; Takahashi, T.; Tobinai, K. Safety and effectiveness of mogamulizumab in relapsed or refractory adult T-cell leukemia-lymphoma. Eur. J. Haematol. 2019, 102, 407–415. [Google Scholar] [CrossRef]
- Yonekura, K.; Takeda, K.; Kawakami, N.; Kanzaki, T.; Kanekura, T.; Utsunomiya, A. Therapeutic Efficacy of Etretinate on Cutaneous-type Adult T-cell Leukemia-Lymphoma. Acta Derm Venereol. 2019, 99, 774–776. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, A.; Kozako, T.; Uchida, Y.; Yoshimitsu, M.; Ishitsuka, K.; Ohsugi, T.; Honda, S.I. Cell death induced by dorsomorphin in adult T-cell leukemia/lymphoma is AMPK-independent. FEBS J. 2020, 287, 4005–4015. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.; Xu, W.; Bucher, P.; Grimm, M.; Konantz, M.; Horn, H.; Zapukhlyak, M.; Berning, P.; Brändle, M.; Jarboui, M.A.; et al. Dimethyl fumarate induces ferroptosis and impairs NF-κB/STAT3 signaling in DLBCL. Blood 2021, 138, 871–884. [Google Scholar] [CrossRef]
- Maeta, T.; Sato, T.; Asano, K.; Ito, S. Dimethyl Fumarate Induces Apoptosis via Inhibiting NF-κB and STAT3 Signaling in Adult T-cell Leukemia/Lymphoma Cells. Anticancer Res. 2022, 42, 2301–2309. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Maeta, T.; Ito, S. Dimethyl Fumarate Suppresses the Proliferation of HTLV-1-infected T Cells by Inhibiting CBM Complex-triggered NF-B Signaling. Anticancer Res. 2023, 43, 1901–1908. [Google Scholar] [CrossRef] [PubMed]
- Yokota, R.; Hashimoto, S.; Watanabe, I.; Kishimoto, S.; Toyama, M.; Okamoto, M.; Yoshimtsu, M.; Ishitsuka, K.; Ito, I.; Baba, M. Novel Anti-CD70 Antibody Drug Conjugate for the Treatment of Adult T-Cell Leukemia (ATL). Anticancer Res. 2020, 40, 4471–4479. [Google Scholar] [CrossRef] [PubMed]
- Marçais, A.; Cook, L.; Witkover, A.; Asnafi, V.; Avettand-Fenoel, V.; Delarue, R.; Cheminant, M.; Sibon, D.; Frenzel, L.; De Thé, H.; et al. Arsenic trioxide (As2O3) as a maintenance therapy for adult T cell leukemia/lymphoma. Retrovirology 2020, 17, 5. [Google Scholar] [CrossRef]
- Houssein, M.; Fatfat, M.; Habli, Z.; Ghazal, N.; Moodad, S.; Khalife, H.; Khalil, M.; Gali-Muhtasib, H. Thymoquinone synergizes with arsenic and interferon alpha to target human T-cell leukemia/lymphoma. Life Sci. 2020, 251, 117639. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, X.; Cheng, W.; Wang, Y.; Yi, K.; Wang, Z.; Zhang, Y.; Shao, L.; Zhao, T. Hypericin-photodynamic therapy inhibits the growth of adult T-cell leukemia cells through induction of apoptosis and suppression of viral transcription. Retrovirology 2019, 16, 5. [Google Scholar] [CrossRef]
- Fatfat, M.; Fakhoury, I.; Habli, Z.; Mismar, R.; Gali-Muhtasib, H. Thymoquinone enhances the anticancer activity of doxorubicin against adult T-cell leukemia in vitro and in vivo through ROS-dependent mechanisms. Life Sci. 2019, 232, 116628. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Shafer III, A.L.; Ceribelli, M.; Zhang, M.; Wright, G.; Huang, D.W.; Xiao, W.; Powell, J.; Petrus, M.N.; Yang, Y.; et al. Targeting the HTLV-I-Regulated BATF3/IRF4 Transcriptional Network in Adult T Cell Leukemia/Lymphoma. Cancer Cell 2018, 34, 286–297. [Google Scholar] [CrossRef]
- Futsch, N.; Mahieux, R.; Dutartre, H. HTLV-1, the other pathogenic yet neglected human retrovirus: From transmission to therapeutic treatment. Viruses 2017, 10, 1. [Google Scholar] [CrossRef]
- Rajaei, T.; Farajifard, H.; Rezaee, S.A.; Azarpazhooh, M.R.; Mahmoudi, M.; Valizadeh, N.; Rafatpanah, H. Different roles of CXCR1 and CXCR2 in HTLV-1 carriers and HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) patients. Med. Microbiol. Immunol. 2019, 208, 641–650. [Google Scholar] [CrossRef]
- Nozuma, S.; Jacobson, S. Neuroimmunology of Human T-Lymphotropic Virus Type 1-Associated Myelopathy/Tropical Spastic Paraparesis. Front. Microbiol. 2019, 10, 885. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T. HAM/TSP Pathogenesis: The Transmigration Activity of HTLV-1-Infected T Cells into Tissues. Pathogens 2023, 12, 492. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, J.; Tanabe, K.; Sato, T.; Nakagawa, M.; Matsuura, E.; Tsuboi, Y.; Tamaki, K.; Sakima, H.; Ishihara, S.; Ohta, Y.; et al. Efficacy of Costicosteroid Therapy for HTLV-1-Associated Myelopathy: A Randomized Controlled Trial (HAMLET-P). Viruses 2022, 14, 136. [Google Scholar] [CrossRef] [PubMed]
- Enose-Akahata, Y.; Billioux, B.J.; Azodi, S.; Dwyer, J.; Velluci, A.; Ngouth, N.; Nozuma, S.; Massoud, R.; Cortese, I.; Ohayon, N.; et al. Clinical trial of raltegravir, an integrase inhibitor, in HAM/TSP. Ann. Clin. Transl. Neurol. 2021, 8, 1970–1985. [Google Scholar] [CrossRef]
- Schneiderman†, B.S.; Barski†, M.S.; Maertens, G.N. Cabotegravir, the Long-Acting Integrase Strand Transfer Inhibitor, Potently Inhibits Human T-Cell Lymphotropic Virus Type 1 Transmission in vitro. Front. Med. 2022, 9, 889621. [Google Scholar] [CrossRef]
- Enose-Akahata, Y.; Ngouth, N.; Ohayon, J.; Mandel, M.; Chavin, J.; Turner, T.J.; Jacobson, S. Effect of Teriflunomide on cells from patients with Human T-cell Lymphotropic Virus type 1-associated neurological disease. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e986. [Google Scholar] [CrossRef]
- Hoces, D.; Barros, N.; Woll, F.; Bauer, A.; White, A.C., Jr.; Montes, M. Regulatory T cell expansion resolves after effective strongyloidiasis treatment in subjects with HTLV-1 co- infection. Parasitol. Int. 2020, 76, 102092. [Google Scholar] [CrossRef]
- Gordon, C.A.; Shield, J.M.; Bradbury, R.S.; Muhi, S.; Page, W.; Judd, J.A.; Lee, R.; Biggs, B.A.; Ross, K.; Kurscheid, J.; et al. HTLV-I and Strongyloides in Australia: The worm lurking beneath. Adv. Parasitol. 2021, 111, 119–201. [Google Scholar]
- Nozuma, S.; Matsuura, E.; Tashiro, Y.; Nagata, R.; Ando, M.; Hiramatsu, Y.; Higuchi, Y.; Sakiyama, Y.; Hashiguchi, A.; Michizono, K.; et al. Efficacy of l-Arginine treatment in patients with HTLV-1-associated neurological disease. Ann. Clin. Transl. Neurol. 2023, 10, 237–245. [Google Scholar] [CrossRef]
- Matsuo, T.; Miyata, Y.; Nakamura, T.; Satoh, K.; Sakai, H. Prosultiamine for treatment of lower urinary tract dysfunction accompanied by human T-lymphotropic virus type 1-associated myelopathy/tropical spastic paraparesis. Int. J. Urol. 2018, 25, 54–60. [Google Scholar] [CrossRef]
- Hofmann, D.; Sayasone, S.; Sengngam, K.; Chongvilay, B.; Hattendorf, J.; Keiser, J. Efficacy and safety of ascending doses of moxidectin against Strongyloides stercoralis infections in adults: A randomised, parallel-group, single-blinded, placebo-controlled, dose-ranging, phase 2a trial. Lancet Infect. Dis. 2021, 21, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Taylor, G.P.; Rosadas, C. Human T-Cell Lymphotropic Virus Type 1 and Strongyloides stercoralis Co-infection: A Systematic Review and Meta-Analysis. Front. Med. 2022, 9, 832430. [Google Scholar] [CrossRef] [PubMed]
- Guerin, E.; Poirier, P.; Nervo, M.; Terrier, C.L. Fatal Multiorgan Failure Syndrome in a Strongyloides-HTLV-1 Coinfected Patient, after Treatment with Ivermectin. Case Rep. Crit. Care. 2021, 2021, 5554810. [Google Scholar] [CrossRef]
- Rivera, A.; Patiño, M.; Ocampo, J.M.; Suárez, J.; López, G.; Salazar, W. Strongyloides stercoralis hyperinfection in a Young Patient with HTLV-1 Infection and Ulcerative Colitis. Rev. Colomb. Gastroenterol. 2022, 36, 408–413. [Google Scholar] [CrossRef]
- Hunter, M.; Beltramino, S.; Ocampo, C.V.; Marull, R.S.; Badariotti, G.; De Diego, B. Strongyloides Hyperinfection in Patient from Patagonia with HTLV-1 Co-infection. Medicina 2019, 79, 147–149. [Google Scholar] [PubMed]
- Dykie, A.; Wijesinghe, T.; Rabson, A.B.; Madugula, K.; Farinas, C.; Wilson, S.; Abraham, D.; Jain, P. Human T-cell Leukemia Virus Type 1 and Strongyloides stercoralis: Partners in Pathogenesis. Pathogens 2020, 29, 904. [Google Scholar] [CrossRef]
- Park, K.B.; Dalton-Brown, E.; Hirst, C.; Williams, D.P. Selection of new chemical entities with decreased potential for adverse drug reactions. Eur. J. Pharmacol. 2006, 549, 1–8. [Google Scholar] [CrossRef]
- Jakhar, R.; Hooda, M.D.; Khici, A.; Chhillar, A. Relevance of molecular docking studies in drug designing. Curr. Bioinform. 2020, 15, 270–278. [Google Scholar] [CrossRef]
- Marino-Merlo, F.; Balestrieri, E.; Matteucci, C.; Mastino, A.; Grelli, S.; Macchi, B. Antiretroviral Therapy in HTLV-1 Infection: An Updated Overview. Pathogens 2020, 9, 342. [Google Scholar] [CrossRef]
- Kassay, N.; Motyan, J.A.; Matuz, K.; Golda, M.; Tozser, J. Biochemical characterization, specificity and inhibition studies of HTLV-1, HTLV-2, and HTLV-3 proteases. Life 2021, 11, 127. [Google Scholar] [CrossRef]
- Jahantigh, H.; Ahmadi, N.; Lovreglio, P.; Stufano, A.; Enayatkhani, M.; Shahbazi, B.; Ahmadi, K. Repurposing antiviral drugs against HTLV-1 protease by molecular docking and molecular dynamics simulation. J. Biomol. Struct. Dyn. 2023, 41, 5057–5066. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, C.; Singh, P.; Singh, S.K. Molecular modeling studies and comparative analysis on structurally similar HTLV and HIV protease using HIV-PR inhibitors. J. Recept. Signal Transduct. Res. 2014, 34, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.; Das, S.; Pearson, M.; Kozubek, J.; Strivens, M.; Gardner, S. Systematic drug repurposing to enable precision medicine: A case study in breast cancer. Digital Medicine. 2019, 5, 180–186. [Google Scholar] [CrossRef]
- Sohraby, F.; Aryapour, H. Reconstruction of the binding pathway of an anti-HIV drug, Indinavir, in complex with the HTLV-1 protease using unaggregated unbiased molecular dynamics simulation. Comput. Biol. Chem. 2022, 96, 107616. [Google Scholar] [CrossRef]
- Pandey, R.K.; Ojha, R.; Chatterjee, N.; Upadhyay, N.; Mishra, A.; Prajapati, V.K. Combinatorial screening algorithm to engineer multiepitope subunit vaccine targeting human T-lymphotropic virus-1 infection. J. Cell Physiol. 2019, 234, 8717–8726. [Google Scholar] [CrossRef]
- Barski, M.S.; Vanzo, T.; Zhao, X.Z.; Smith, S.J.; Ballandras-Colas, A.; Cronin, N.B.; Pye, V.E.; Hughes, S.H.; Burke, T.R., Jr.; Cherepanov, P.; et al. Structural basis for the inhibition of HTLV-1 integration inferred from cryo-EM deltaretroviral intasome structures. Nat. Commun. 2021, 12, 4996. [Google Scholar] [CrossRef]
- Alam, S.; Hasan, M.K.; Manjur, O.H.B.; Khan, A.M.; Sharmin, Z.; Pavel, M.A.; Hossain, M.F. Predicting and Designing Epitope Ensemble Vaccines against HTLV-1. J. Integr. Bioinform. 2020, 16, 20180051. [Google Scholar] [CrossRef]
- Jahantigh, H.R.; Stufano, A.; Lovreglio, P.; Rezaee, S.A.; Ahmadi, K. In silico identification of epitope-based vaccine candidates against HTLV-1. J. Biomol. Struct Dyn. 2022, 40, 6737–6754. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.C.M.d.; Pereira, R.S.B.; Araujo, A.C.A.; Filho, E.G.d.S.; Dias, A.d.L.; Cavalcante, K.S.; Sousa, M.S.d. New Perspectives about Drug Candidates Targeting HTLV-1 and Related Diseases. Pharmaceuticals 2023, 16, 1546. https://doi.org/10.3390/ph16111546
Silva MCMd, Pereira RSB, Araujo ACA, Filho EGdS, Dias AdL, Cavalcante KS, Sousa MSd. New Perspectives about Drug Candidates Targeting HTLV-1 and Related Diseases. Pharmaceuticals. 2023; 16(11):1546. https://doi.org/10.3390/ph16111546
Chicago/Turabian StyleSilva, Milena Cristina Martins da, Renan Stefferson Barradas Pereira, Antonia Cherlly Aparecida Araujo, Ednilson Gregorio da Silva Filho, Anderson de Lima Dias, Kassio Silva Cavalcante, and Maísa Silva de Sousa. 2023. "New Perspectives about Drug Candidates Targeting HTLV-1 and Related Diseases" Pharmaceuticals 16, no. 11: 1546. https://doi.org/10.3390/ph16111546
APA StyleSilva, M. C. M. d., Pereira, R. S. B., Araujo, A. C. A., Filho, E. G. d. S., Dias, A. d. L., Cavalcante, K. S., & Sousa, M. S. d. (2023). New Perspectives about Drug Candidates Targeting HTLV-1 and Related Diseases. Pharmaceuticals, 16(11), 1546. https://doi.org/10.3390/ph16111546






