A Bibliometric Analysis of 3D Printing in Personalized Medicine Research from 2012 to 2022
Abstract
:1. Introduction
2. Result
2.1. Trend and Annual Counts
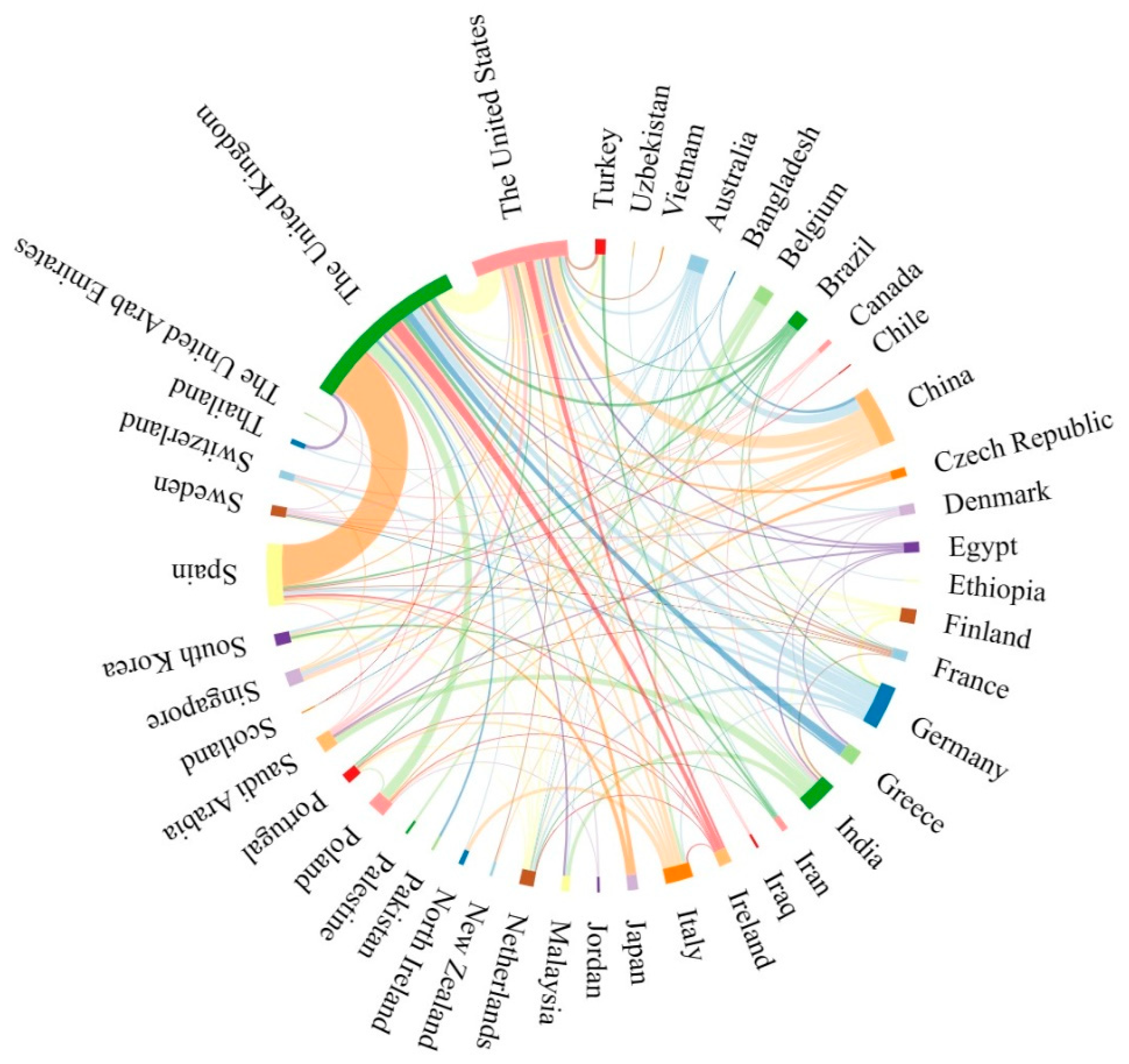
2.2. Contributions of Countries
2.3. Contributions of Journals
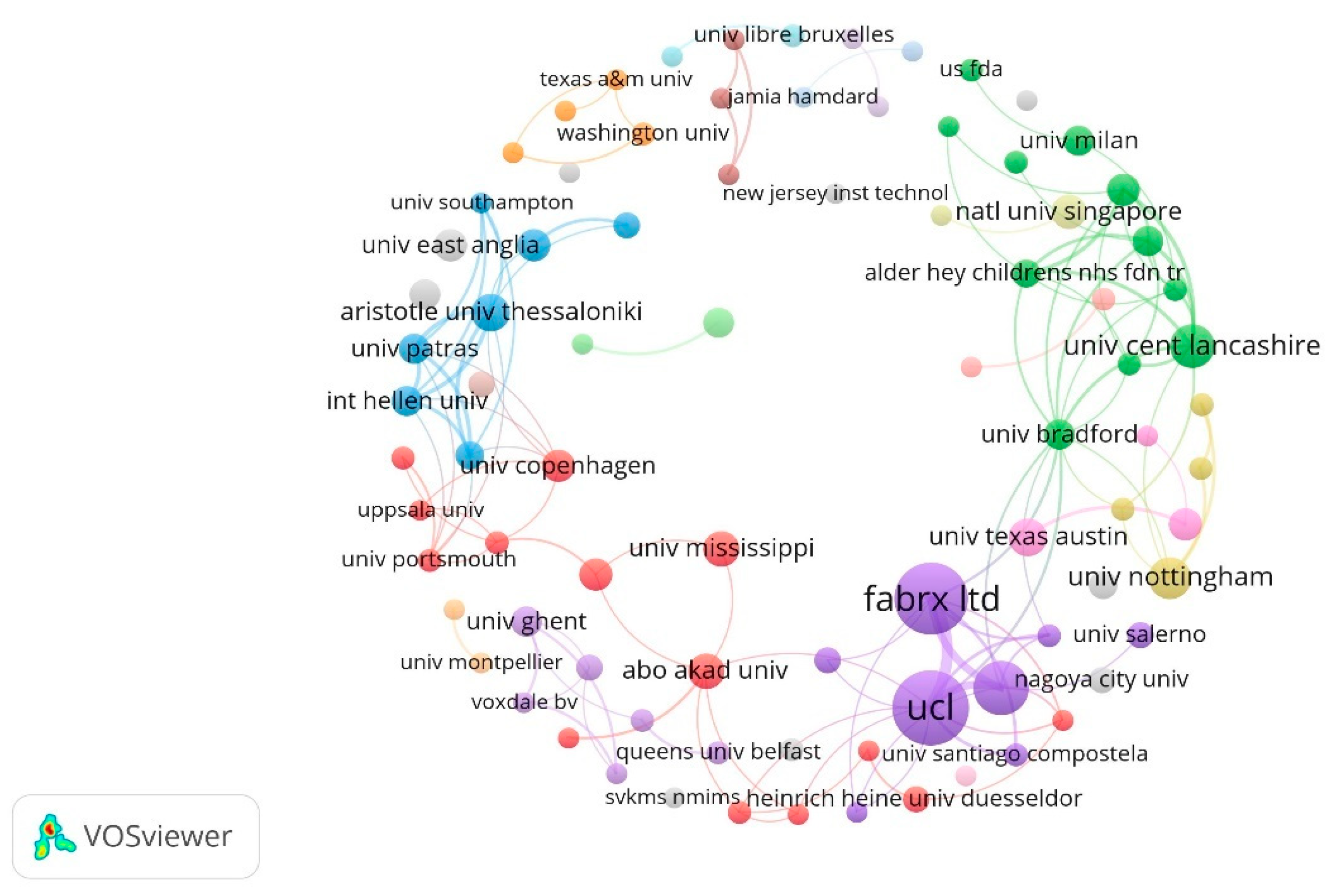
2.4. Contributions of Institutions
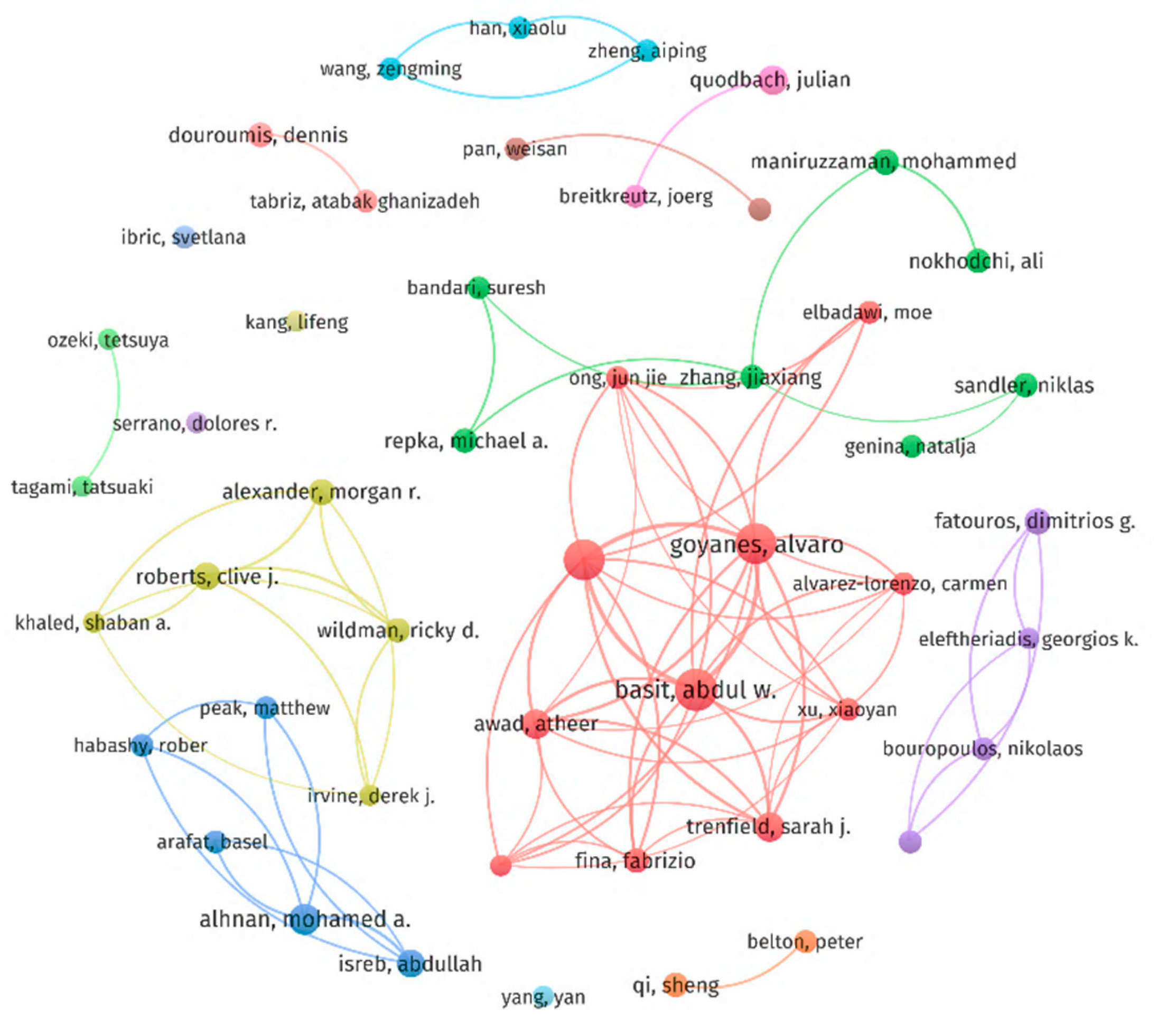
2.5. Contributions of Authors
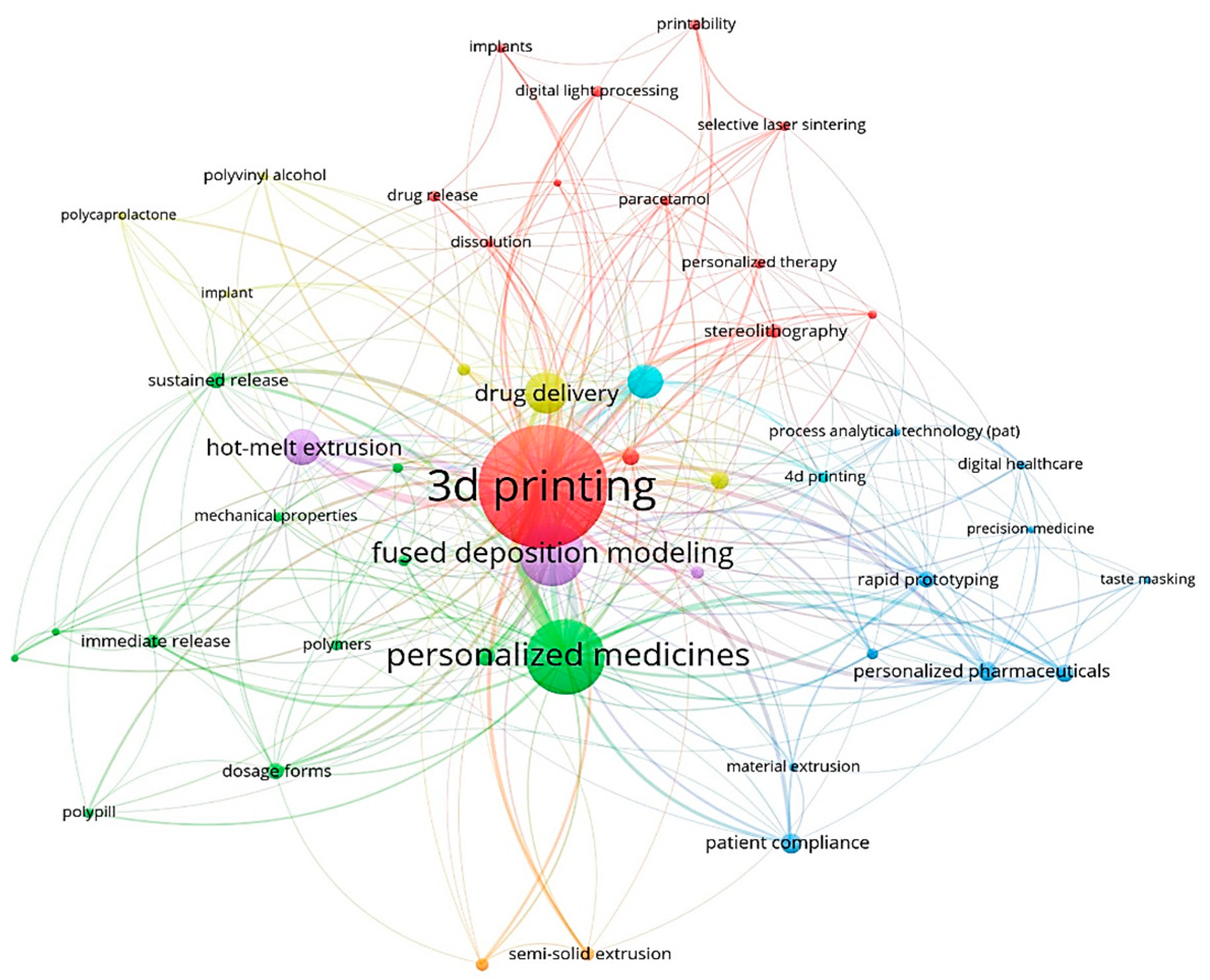
2.6. Keyword Analysis
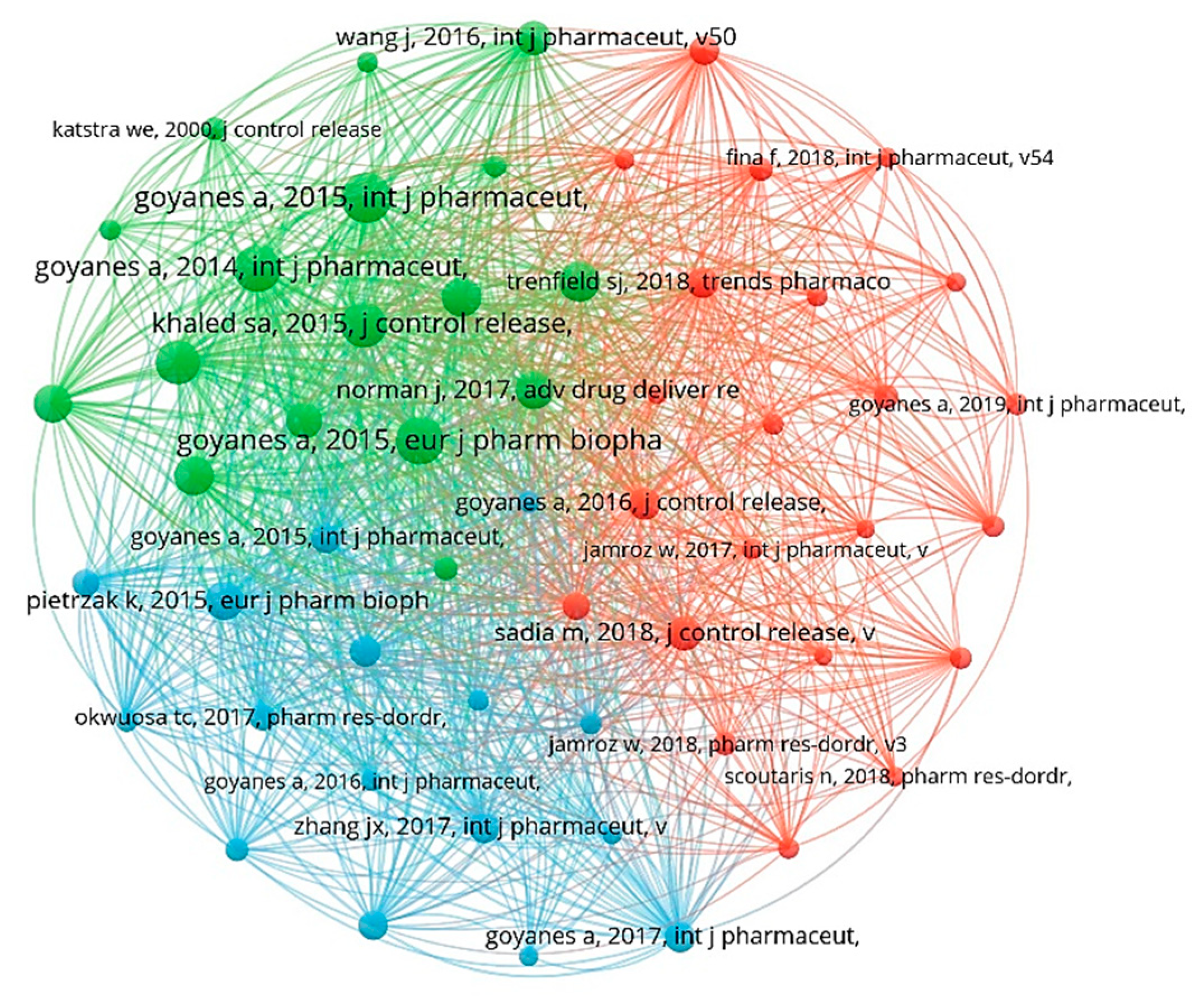
2.7. Co-Citation of Cited References
3. Discussion
3.1. Basic Information
3.2. Research Hotspots
4. Materials and Methods
4.1. Data Collection
4.2. Data Analysis
5. The Technology of 3D Printing
5.1. Fused Deposition Modeling (FDM)
5.2. Stereo Lithography Appearance (SLA)
5.3. Inject Printing (IP)
5.4. Semi-Solid Extrusion (SSE)
5.5. Selective Laser Sintering (SLS)
5.6. Binder Jet (BJ)
5.7. Direct Powder Extrusion (DPE)
| Technologies | Advantages | Disadvantages | Print Temperature | References |
|---|---|---|---|---|
| FDM | Small pieces of equipment, low cost, high mechanical strength, high efficiency, fast printing speeds (15~90 mm/s); generates amorphous solid dispersed filaments as well as amorphous forms of insoluble drugs. | High printing temperatures lead to degradation of thermal drugs; lack of suitable polymer materials. | High temperature (135~230 °C) | [36,37,38] |
| SLA | Low printing temperature reduces degradation of thermal components; high resolution and high printing accuracy. | UV-initiated polymerization may lead to drug polymerization; limited choice of biocompatible polymers and photo-initiators and high system costs; slow printing speeds. | Room temperature | [30,36,55,56,57] |
| IP | Simple production steps, high precision, and low cost; can be combined with FDM technology to print drugs. | Printing requires high physical and chemical properties of the drug ink; curing required. | Room temperature | [36,58] |
| SSE | Room-temperature printing can be carried out without heating, easy to operate, and fast printing speeds. | Low print accuracy; viscosity leads to clogging of easy nozzles; high drug loading capacity. | Room temperature | [5,13,59,60,62] |
| SLS | No additional drying steps are required; speeds up printing; improves the stability of easily hydrolysable drugs; the high resolution of the laser beam allows for the design of complex and fine dosage forms. | Drugs that are unstable toward light and heat are susceptible to drug degradation during this process; costs may be high. | High energy | [23,24,64,65] |
| BJ | Accuracy and flexibility are high; low cost. | Composed of organic solvents with safety risks; requires post-processing. | Room temperature | [66,67,68] |
| DPE | Allows a drug to exist in amorphous state; enhances the absorption and dissolution of the drug; there is no need to prepare a filament. | Limited number of drugs suitable for printing. | Room temperature | [15,16,34] |
| Polymers | Characteristics | Functions | Technology Applied | References |
|---|---|---|---|---|
| Poly (lactic acid) (PLA) | Good biodegradability, biocompatibility, thermoplastic processability, and eco-friendliness. | Filler component; controlled release. | FDM SLS | [72,73] |
| Polyvinylpyrrolidone (PVP) | Hygroscopic polymer; thermal resistance; biocompatibility. | Filler component; binder; immediate release. | FDM BJ SSE | [74,75,76,77] |
| Eudragit® | Thermoplastic properties; low glass transition temperatures (between 9 °C and >150 °C); high thermostability. | Filler component; various release modifiers; taste-masker agent. | SLS FDM BJ | [20,24,78,79] |
| Hydroxypropyl Cellulose (HPC) | Good thermoplasticity; solubility is determined by temperature. | Filler component; controlled release; binder. | FDM SLS SSE DPE | [47,48,80,81,82] |
| Ethylcellulose (EC) | Hydrophobic; thermal characteristics, thermoplasticity, and miscibility with incorporated plasticizers; degradation temperature (Td) is 280 °C. | Release retardant. | FDM | [20,81] |
| Hydroxypropyl Methylcellulose (HPMC) | Hydrophilic polymer; high melt viscosity; low degradation temperature. | Filler component; various release modifiers; controlled release. | FDM SLS SSE BJ | [68,81,83,84] |
| Polyvinyl alcohol (PVA) | Water-soluble polymer; melting point of PVA ranges from 180 °C to 220 °C; biocompatibility, non-toxicity, and good mechanical and swelling properties. | Filler component; immediate release. | FDM | [72,81] |
| Poly (Ethylene Glycol) (PEG) | Water-soluble, biocompatible, and amphiphilic polymer. | Plasticizer; controlled release; PEG derivatives are generally utilized as photopolymerizable (photocurable) polymers. | SLA FDM IP DPE | [21,41,58,82] |
| Polyethylene glycol diacrylate (PEGDA) | Good biocompatibility; low cost; and water solubility. | Photo-initiator. | SLA | [6,57,85] |
| 2,4,6-Trimethylbenzoyl-diphenylphosphine oxide (TPO) | High biocompatibility and excellent transparency. | Photo-initiator. | SLA | [6,86] |
| Polycaprolactone (PCL) | Semi-crystalline, biocompatible polyester; melting point of 55–60 °C and Tg of −54 °C; low in vivo degradation; low tensile strength | Filler component. | FDM | [74,77] |
6. Application of 3D Printing
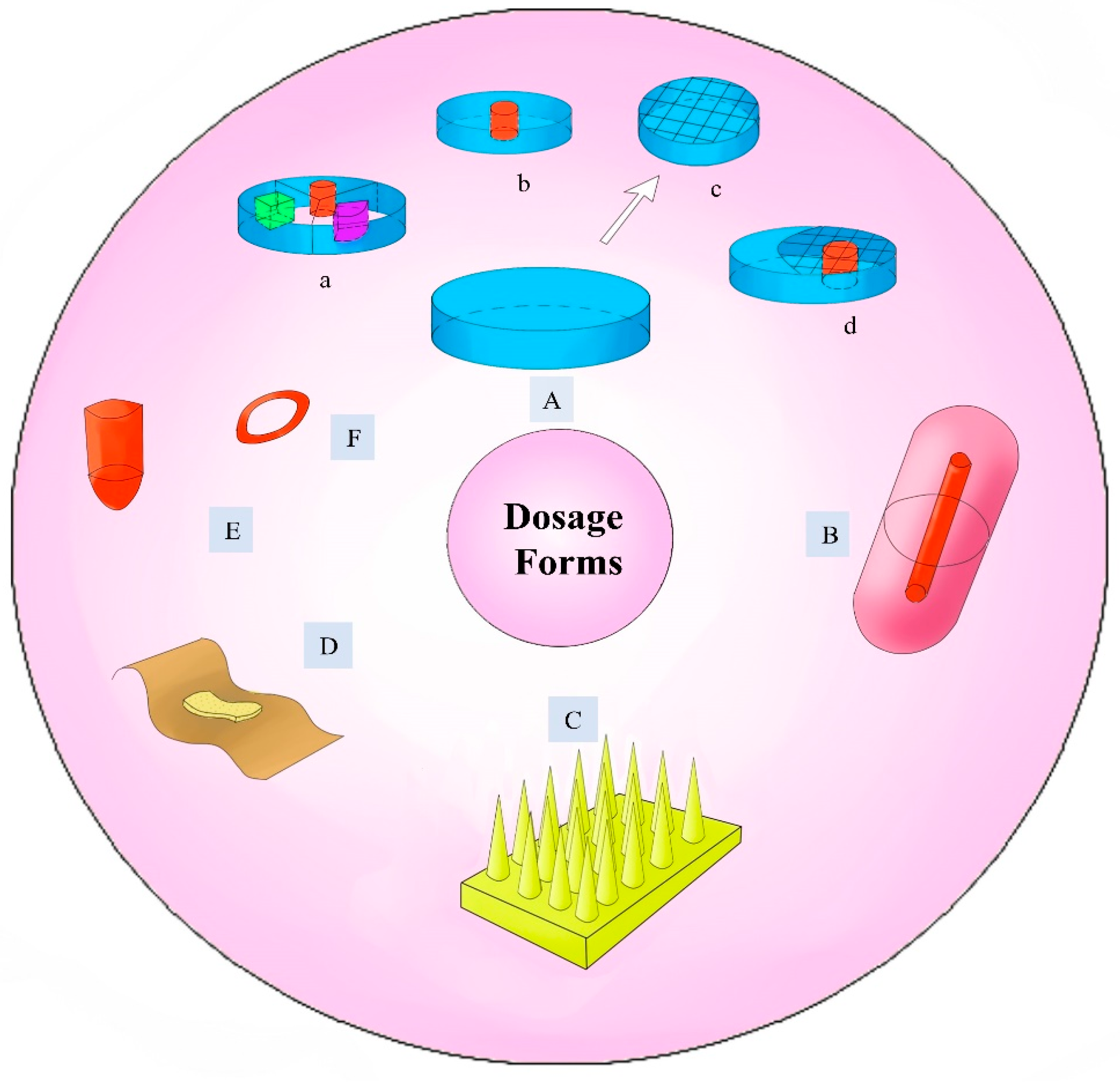
6.1. The Development of Different Dosage Forms
6.1.1. Tablets
6.1.2. Suppository
6.1.3. Orodispersible Films
6.1.4. Microneedles
6.1.5. Implants
6.1.6. Other Dosage Forms
6.2. Personalization of Drug Release
6.2.1. Rapid Release of the Drug
6.2.2. Extended Release of a Drug
7. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elkasabgy, N.A.; Mahmoud, A.A.; Maged, A. 3D printing: An appealing route for customized drug delivery systems. Int. J. Pharm. 2020, 588, 119732. [Google Scholar] [CrossRef]
- Zhu, X.; Li, H.J.; Huang, L.F.; Zhang, M.; Fan, W.G.; Cui, L. 3D printing promotes the development of drugs. Biomed. Pharmacother. 2020, 131, 110644. [Google Scholar] [CrossRef] [PubMed]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mulhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed]
- Mathew, E.; Pitzanti, G.; Larraneta, E.; Lamprou, D.A. 3D Printing of Pharmaceuticals and Drug Delivery Devices. Pharmaceutics 2020, 12, 266. [Google Scholar] [CrossRef] [PubMed]
- Abdella, S.; Youssef, S.H.; Afinjuomo, F.; Song, Y.M.; Fouladian, P.; Upton, R.; Garg, S. 3D Printing of Thermo-Sensitive Drugs. Pharmaceutics 2021, 13, 1524. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Stereolithographic (SLA) 3D printing of oral modified-release dosage forms. Int. J. Pharm. 2016, 503, 207–212. [Google Scholar] [CrossRef]
- Goyanes, A.; Buanz, A.B.M.; Hatton, G.B.; Gaisford, S.; Basit, A.W. 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets. Eur. J. Pharm. Biopharm. 2015, 89, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Kollamaram, G.; Croker, D.M.; Walker, G.M.; Goyanes, A.; Basit, A.W.; Gaisford, S. Low temperature fused deposition modeling (FDM) 3D printing of thermolabile drugs. Int. J. Pharm. 2018, 545, 144–152. [Google Scholar] [CrossRef]
- Okwuosa, T.C.; Stefaniak, D.; Arafat, B.; Isreb, A.; Wan, K.W.; Alhnan, M.A. A Lower Temperature FDM 3D Printing for the Manufacture of Patient-Specific Immediate Release Tablets. Pharm. Res. 2016, 33, 2704–2712. [Google Scholar] [CrossRef]
- Sadia, M.; Arafat, B.; Ahmed, W.; Forbes, R.T.; Alhnan, M.A. Channelled tablets: An innovative approach to accelerating drug release from 3D printed tablets. J. Control. Release 2018, 269, 355–363. [Google Scholar] [CrossRef]
- Skowyra, J.; Pietrzak, K.; Alhnan, M.A. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing. Eur. J. Pharm. Sci. 2015, 68, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Panraksa, P.; Zhang, B.; Rachtanapun, P.; Jantanasakulwong, K.; Qi, S.; Jantrawut, P. ‘Tablet-in-Syringe’: A Novel Dosing Mechanism for Dysphagic Patients Containing Fast-Disintegrating Tablets Fabricated Using Semisolid Extrusion 3D Printing. Pharmaceutics 2022, 14, 443. [Google Scholar] [CrossRef] [PubMed]
- Seoane-Viano, I.; Januskaite, P.; Alvarez-Lorenzo, C.; Basit, A.W.; Goyanes, A. Semi-solid extrusion 3D printing in drug delivery and biomedicine: Personalised solutions for healthcare challenges. J. Control. Release 2021, 332, 367–389. [Google Scholar] [CrossRef]
- Yan, T.T.; Lv, Z.F.; Tian, P.; Lin, M.M.; Lin, W.; Huang, S.Y.; Chen, Y.Z. Semi-solid extrusion 3D printing ODFs: An individual drug delivery system for small scale pharmacy. Drug Dev. Ind. Pharm. 2020, 46, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Boniatti, J.; Januskaite, P.; da Fonseca, L.B.; Vicosa, A.L.; Amendoeira, F.C.; Tuleu, C.; Basit, A.W.; Goyanes, A.; Re, M.I. Direct Powder Extrusion 3D Printing of Praziquantel to Overcome Neglected Disease Formulation Challenges in Paediatric Populations. Pharmaceutics 2021, 13, 1114. [Google Scholar] [CrossRef] [PubMed]
- Pistone, M.; Racaniello, G.F.; Arduino, I.; Laquintana, V.; Lopalco, A.; Cutrignelli, A.; Rizzi, R.; Franco, M.; Lopedota, A.; Denora, N. Direct cyclodextrin-based powder extrusion 3D printing for one-step production of the BCS class II model drug niclosamide. Drug Deliv. Transl. Res. 2022, 12, 1895–1910. [Google Scholar] [CrossRef]
- Acosta-Velez, G.F.; Zhu, T.Z.; Linsley, C.S.; Wu, B.M. Photocurable poly(ethylene glycol) as a bioink for the inkjet 3D pharming of hydrophobic drugs. Int. J. Pharm. 2018, 546, 145–153. [Google Scholar] [CrossRef]
- Chang, S.Y.; Li, S.W.; Kowsari, K.; Shetty, A.; Sorrells, L.; Sen, K.; Nagapudi, K.; Chaudhuri, B.; Ma, A.W.K. Binder-Jet 3D Printing of Indomethacin-laden Pharmaceutical Dosage Forms. J. Pharm. Sci. 2020, 109, 3054–3063. [Google Scholar] [CrossRef]
- Eleftheriadis, G.K.; Katsiotis, C.S.; Andreadis, D.A.; Tzetzis, D.; Ritzoulis, C.; Bouropoulos, N.; Kanellopoulou, D.; Andriotis, E.G.; Tsibouklis, J.; Fatouros, D.G. Inkjet printing of a thermolabile model drug onto FDM-printed substrates: Formulation and evaluation. Drug Dev. Ind. Pharm. 2020, 46, 1253–1264. [Google Scholar] [CrossRef]
- Fina, F.; Goyanes, A.; Madla, C.M.; Awad, A.; Trenfield, S.J.; Kuek, J.M.; Patel, P.; Gaisford, S.; Basit, A.W. 3D printing of drug-loaded gyroid lattices using selective laser sintering. Int. J. Pharm. 2018, 547, 44–52. [Google Scholar] [CrossRef]
- Martinez, P.R.; Goyanes, A.; Basit, A.W.; Gaisford, S. Influence of Geometry on the Drug Release Profiles of Stereolithographic (SLA) 3D-Printed Tablets. AAPS PharmSciTech 2018, 19, 3355–3361. [Google Scholar] [CrossRef] [PubMed]
- Allahham, N.; Fina, F.; Marcuta, C.; Kraschew, L.; Mohr, W.; Gaisford, S.; Basit, A.W.; Goyanes, A. Selective Laser Sintering 3D Printing of Orally Disintegrating Printlets Containing Ondansetron. Pharmaceutics 2020, 12, 110. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.; Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D printing: Principles and pharmaceutical applications of selective laser sintering. Int. J. Pharm. 2020, 586, 14. [Google Scholar] [CrossRef] [PubMed]
- Fina, F.; Goyanes, A.; Gaisford, S.; Basit, A.W. Selective laser sintering (SLS) 3D printing of medicines. Int. J. Pharm. 2017, 529, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Vaz, V.M.; Kumar, L. 3D Printing as a Promising Tool in Personalized Medicine. AAPS PharmSciTech 2021, 22, 20. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, J.T. Twenty Years with Personalized Medicine: Past, Present, and Future of Individualized Pharmacotherapy. Oncologist 2019, 24, E432–E440. [Google Scholar] [CrossRef] [PubMed]
- Langreth, R.; Waldholz, M. New era of personalized medicine: Targeting drugs for each unique genetic profile. Oncologist 1999, 4, 426–427. [Google Scholar] [CrossRef] [PubMed]
- Jonathan, G.; Karim, A. 3D printing in pharmaceutics: A new tool for designing customized drug delivery systems. Int. J. Pharm. 2016, 499, 376–394. [Google Scholar]
- Bacskay, I.; Ujhelyi, Z.; Feher, P.; Arany, P. The Evolution of the 3D-Printed Drug Delivery Systems: A Review. Pharmaceutics 2022, 14, 1312. [Google Scholar] [CrossRef]
- Robles-Martinez, P.; Xu, X.Y.; Trenfield, S.J.; Awad, A.; Goyanes, A.; Telford, R.; Basit, A.W.; Gaisford, S. 3D Printing of a Multi-Layered Polypill Containing Six Drugs Using a Novel Stereolithographic Method. Pharmaceutics 2019, 11, 274. [Google Scholar] [CrossRef]
- Goyanes, A.; Martinez, P.R.; Buanz, A.; Basit, A.W.; Gaisford, S. Effect of geometry on drug release from 3D printed tablets. Int. J. Pharm. 2015, 494, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, K.; Larraza, I.; Berra, G.; Eceiza, A.; Gabilondo, N. 3D printing of customized all-starch tablets with combined release kinetics. Int. J. Pharm. 2022, 622, 11. [Google Scholar] [CrossRef] [PubMed]
- Thanawuth, K.; Limmatvapirat, S.; Rojviriya, C.; Sriamornsak, P. Controlled Release of Felodipine from 3D-Printed Tablets with Constant Surface Area: Influence of Surface Geometry. Pharmaceutics 2023, 15, 467. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Allahham, N.; Trenfield, S.J.; Stoyanov, E.; Gaisford, S.; Basit, A.W. Direct powder extrusion 3D printing: Fabrication of drug products using a novel single-step process. Int. J. Pharm. 2019, 567, 7. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.H.; Yu, X.; Jin, Y.G. 3D printing of vaginal rings with personalized shapes for controlled release of progesterone. Int. J. Pharm. 2018, 539, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, C.R.M.; Okafor-Muo, O.L.; Hassanin, H.; ElShaer, A. 3DP Printing of Oral Solid Formulations: A Systematic Review. Pharmaceutics 2021, 13, 358. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.; Shin, I.; Ham, G.; Abuzar, S.M.; Hyun, S.M.; Hwang, S.J. The advent of a novel manufacturing technology in pharmaceutics: Superiority of fused deposition modeling 3D printer. J. Pharm. Investig. 2020, 50, 131–145. [Google Scholar] [CrossRef]
- Sadia, M.; Sosnicka, A.; Arafat, B.; Isreb, A.; Ahmed, W.; Kelarakis, A.; Alhnan, M.A. Adaptation of pharmaceutical excipients to FDM 3D printing for the fabrication of patient-tailored immediate release tablets. Int. J. Pharm. 2016, 513, 659–668. [Google Scholar] [CrossRef]
- Saviano, M.; Aquino, R.P.; Del Gaudio, P.; Sansone, F.; Russo, P. Poly(vinyl alcohol) 3D printed tablets: The effect of polymer particle size on drug loading and process efficiency. Int. J. Pharm. 2019, 561, 1–8. [Google Scholar] [CrossRef]
- Raje, V.; Palekar, S.; Banella, S.; Patel, K. Tunable Drug Release from Fused Deposition Modelling (FDM) 3D-Printed Tablets Fabricated Using a Novel Extrudable Polymer. Pharmaceutics 2022, 14, 2192. [Google Scholar] [CrossRef]
- Li, R.; Pan, Y.; Chen, D.; Xu, X.; Yan, G.; Fan, T. Design, Preparation and In Vitro Evaluation of Core-Shell Fused Deposition Modelling 3D-Printed Verapamil Hydrochloride Pulsatile Tablets. Pharmaceutics 2022, 14, 437. [Google Scholar] [CrossRef]
- Alqahtani, A.A.; Mohammed, A.A.; Fatima, F.; Ahmed, M.M. Fused Deposition Modelling 3D-Printed Gastro-Retentive Floating Device for Propranolol Hcl Tablets. Polymers 2023, 15, 3554. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Salvage, J.P.; Maniruzzaman, M.; Nokhodchi, A. Role of release modifiers to modulate drug release from fused deposition modelling (FDM) 3D printed tablets. Int. J. Pharm. 2021, 597, 120315. [Google Scholar] [CrossRef] [PubMed]
- Kempin, W.; Domsta, V.; Grathoff, G.; Brecht, I.; Semmling, B.; Tillmann, S.; Weitschies, W.; Seidlitz, A. Immediate Release 3D-Printed Tablets Produced via Fused Deposition Modeling of a Thermo-Sensitive Drug. Pharm. Res. 2018, 35, 124. [Google Scholar] [CrossRef] [PubMed]
- Alhijjaj, M.; Belton, P.; Qi, S. An investigation into the use of polymer blends to improve the printability of and regulate drug release from pharmaceutical solid dispersions prepared via fused deposition modeling (FDM) 3D printing. Eur. J. Pharm. Biopharm. 2016, 108, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Beck, R.C.R.; Chaves, P.S.; Goyanes, A.; Vukosavljevic, B.; Buanz, A.; Windbergs, M.; Basit, A.W.; Gaisford, S. 3D printed tablets loaded with polymeric nanocapsules: An innovative approach to produce customized drug delivery systems. Int. J. Pharm. 2017, 528, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Buyukgoz, G.G.; Soffer, D.; Defendre, J.; Pizzano, G.M.; Dave, R.N. Exploring tablet design options for tailoring drug release and dose via fused deposition modeling (FDM) 3D printing. Int. J. Pharm. 2020, 591, 13. [Google Scholar]
- Dumpa, N.R.; Bandari, S.; Repka, M.A. Novel Gastroretentive Floating Pulsatile Drug Delivery System Produced via Hot-Melt Extrusion and Fused Deposition Modeling 3D Printing. Pharmaceutics 2020, 12, 52. [Google Scholar] [CrossRef] [PubMed]
- Bandari, S.; Nyavanandi, D.; Dumpa, N.; Repka, M.A. Coupling hot melt extrusion and fused deposition modeling: Critical properties for successful performance q. Adv. Drug Deliv. Rev. 2021, 172, 52–63. [Google Scholar] [CrossRef]
- Dumpa, N.; Butreddy, A.; Wang, H.H.; Komanduri, N.; Bandari, S.; Repka, M.A. 3D printing in personalized drug delivery: An overview of hot-melt extrusion-based fused deposition modeling. Int. J. Pharm. 2021, 600, 15. [Google Scholar] [CrossRef]
- Figueiredo, S.; Fernandes, A.I.; Carvalho, F.G.; Pinto, J.F. Performance and paroxetine stability in tablets manufactured by fused deposition modelling-based 3D printing. J. Pharm. Pharmacol. 2022, 74, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Nashed, N.; Lam, M.; Nokhodchi, A. A comprehensive overview of extended release oral dosage forms manufactured through hot melt extrusion and its combination with 3D printing. Int. J. Pharm. 2021, 596, 17. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. Advanced Pharmaceutical Applications of Hot-Melt Extrusion Coupled with Fused Deposition Modelling (FDM) 3D Printing for Personalised Drug Delivery. Pharmaceutics 2018, 10, 203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.X.; Thakkar, R.; Zhang, Y.; Maniruzzaman, M. Structure-function correlation and personalized 3D printed tablets using a quality by design (QbD) approach. Int. J. Pharm. 2020, 590, 14. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, Q.L.; Qiang, W.; Li, H.J.; Zhong, W.Z.; Pan, S.Y.; Yang, G.S. Hydrophilic Excipient-Independent Drug Release from SLA-Printed Pellets. Pharmaceutics 2021, 13, 1717. [Google Scholar] [CrossRef] [PubMed]
- Curti, C.; Kirby, D.J.; Russell, C.A. Stereolithography Apparatus Evolution: Enhancing Throughput and Efficiency of Pharmaceutical Formulation Development. Pharmaceutics 2021, 13, 616. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Det-Amornrat, U.; Wang, J.; Basit, A.W.; Gaisford, S. 3D scanning and 3D printing as innovative technologies for fabricating personalized topical drug delivery systems. J. Control. Release 2016, 234, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Cader, H.K.; Rance, G.A.; Alexander, M.R.; Goncalves, A.D.; Roberts, C.J.; Tuck, C.J.; Wildman, R.D. Water-based 3D inkjet printing of an oral pharmaceutical dosage form. Int. J. Pharm. 2019, 564, 359–368. [Google Scholar] [CrossRef]
- van Kampen, E.E.M.; Ayyoubi, S.; Willemsteijn, L.; van Bommel, K.J.C.; Ruijgrok, E.J. The Quest for Child-Friendly Carrier Materials Used in the 3D Semi-Solid Extrusion Printing of Medicines. Pharmaceutics 2023, 15, 28. [Google Scholar] [CrossRef]
- Dores, F.; Kuzminska, M.; Soares, C.; Bohus, M.; Shervington, L.A.; Habashy, R.; Pereira, B.C.; Peak, M.; Isreb, A.; Alhnan, M.A. Temperature and solvent facilitated extrusion based 3D printing for pharmaceuticals. Eur. J. Pharm. Sci. 2020, 152, 9. [Google Scholar] [CrossRef]
- Johannesson, J.; Khan, J.; Hubert, M.; Teleki, A.; Bergstrom, C.A.S. 3D-printing of solid lipid tablets from emulsion gels. Int. J. Pharm. 2021, 597, 8. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.A.; Alexander, M.R.; Irvine, D.J.; Wildman, R.D.; Wallace, M.J.; Sharpe, S.; Yoo, J.; Roberts, C.J. Extrusion 3D Printing of Paracetamol Tablets from a Single Formulation with Tunable Release Profiles Through Control of Tablet Geometry. AAPS PharmSciTech 2018, 19, 3403–3413. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.A.; Alexander, M.R.; Wildman, R.D.; Wallace, M.J.; Sharpe, S.; Yoo, J.; Roberts, C.J. 3D extrusion printing of high drug loading immediate release paracetamol tablets. Int. J. Pharm. 2018, 538, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Charoo, N.A.; Ali, S.F.B.; Mohamed, E.M.; Kuttolamadom, M.A.; Ozkan, T.; Khan, M.A.; Rahman, Z. Selective laser sintering 3D printing—An overview of the technology and pharmaceutical applications. Drug Dev. Ind. Pharm. 2020, 46, 869–877. [Google Scholar] [CrossRef]
- Gueche, Y.A.; Sanchez-Ballester, N.M.; Cailleaux, S.; Bataille, B.; Soulairol, I. Selective Laser Sintering (SLS), a New Chapter in the Production of Solid Oral Forms (SOFs) by 3D Printing. Pharmaceutics 2021, 13, 1212. [Google Scholar] [CrossRef]
- Chen, X.J.; Wang, S.S.; Wu, J.; Duan, S.W.; Wang, X.L.; Hong, X.X.; Han, X.L.; Li, C.H.; Kang, D.Z.; Wang, Z.M.; et al. The Application and Challenge of Binder Jet 3D Printing Technology in Pharmaceutical Manufacturing. Pharmaceutics 2022, 14, 2589. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Dharani, D.; Dong, X.; Maiorana, C.; Chaudhuri, B.; Nagapudi, K.; Chang, S.-Y.; Ma, A.W.K. Pilot-scale binder jet 3D printing of sustained release solid dosage forms. Int. J. Pharm. 2023, 631, 122540. [Google Scholar] [CrossRef] [PubMed]
- Sen, K.; Mehta, T.; Sansare, S.; Sharifi, L.; Ma, A.W.K.; Chaudhuri, B. Pharmaceutical applications of powder-based binder jet 3D printing process—A review. Adv. Drug Deliv. Rev. 2021, 177, 12. [Google Scholar] [CrossRef]
- Yu, X.H.; Xing, R.B.; Peng, Z.X.; Lin, Y.M.; Du, Z.H.; Ding, J.Q.; Wang, L.X.; Han, Y.C. To inhibit coffee ring effect in inkjet printing of light-emitting polymer films by decreasing capillary force. Chin. Chem. Lett. 2019, 30, 135–138. [Google Scholar] [CrossRef]
- Hong, X.X.; Han, X.L.; Li, X.F.; Li, J.L.; Wang, Z.M.; Zheng, A.P. Binder Jet 3D Printing of Compound LEV-PN Dispersible Tablets: An Innovative Approach for Fabricating Drug Systems with Multicompartmental Structures. Pharmaceutics 2021, 13, 1780. [Google Scholar] [CrossRef]
- Kuzminska, M.; Pereira, B.C.; Habashy, R.; Peak, M.; Isreb, M.; Gough, T.D.; Isreb, A.; Alhnan, M.A. Solvent-free temperature-facilitated direct extrusion 3D printing for pharmaceuticals. Int. J. Pharm. 2021, 598, 9. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T.; Nagata, N.; Hayashi, N.; Ogawa, E.; Fukushige, K.; Sakai, N.; Ozeki, T. Defined drug release from 3D-printed composite tablets consisting of drugloaded polyvinylalcohol and a water-soluble or water-insoluble polymer filler. Int. J. Pharm. 2018, 543, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Abaci, A.; Gedeon, C.; Kuna, A.; Guvendiren, M. Additive Manufacturing of Oral Tablets: Technologies, Materials and Printed Tablets. Pharmaceutics 2021, 13, 156. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.; Pegoretti, A.; Fredi, G. An overview of poly(vinyl alcohol) and poly(vinyl pyrrolidone) in pharmaceutical additive manufacturing. J. Vinyl Addit. Technol. 2023, 29, 223–239. [Google Scholar] [CrossRef]
- Jovanovic, M.; Petrovic, M.; Cvijic, S.; Tomic, N.; Stojanovic, D.; Ibric, S.; Uskokovic, P. 3D Printed Buccal Films for Prolonged-Release of Propranolol Hydrochloride: Development, Characterization and Bioavailability Prediction. Pharmaceutics 2021, 13, 2143. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.A.; Olawuni, D.; Kimbell, G.; Badruddoza, A.Z.M.; Hossain, M.S.; Sultana, T. Polymers for Extrusion-Based 3D Printing of Pharmaceuticals: A Holistic Materials-Process Perspective. Pharmaceutics 2020, 12, 124. [Google Scholar] [CrossRef]
- Dos Santos, J.; da Silva, G.S.; Velho, M.C.; Beck, R.C.R. Eudragit(R): A Versatile Family of Polymers for Hot Melt Extrusion and 3D Printing Processes in Pharmaceutics. Pharmaceutics 2021, 13, 1424. [Google Scholar] [CrossRef]
- Rowe, C.W.; Katstra, W.E.; Palazzolo, R.D.; Giritlioglu, B.; Teung, P.; Cima, M.J. Multimechanism oral dosage forms fabricated by three dimensional printing™. J. Control. Release 2000, 66, 11–17. [Google Scholar] [CrossRef]
- Mathiyalagan, R.; Sjo, E.; Manandhar, S.; Lakio, S.; Rosenholm, J.M.; Kaasalainen, M.; Wang, X.; Sandler, N. Personalizing oral delivery of nanoformed piroxicam by semi-solid extrusion 3D printing. Eur. J. Pharm. Sci. 2023, 188, 106497. [Google Scholar] [CrossRef]
- Giri, B.R.; Poudel, S.; Kim, D.W. Cellulose and its derivatives for application in 3D printing of pharmaceuticals. J. Pharm. Investig. 2021, 51, 1–22. [Google Scholar] [CrossRef]
- Fanous, M.; Gold, S.; Muller, S.; Hirsch, S.; Ogorka, J.; Imanidis, G. Simplification of fused deposition modeling 3D-printing paradigm: Feasibility of 1-step direct powder printing for immediate release dosage form production. Int. J. Pharm. 2020, 578, 119124. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Qin, H.; Acevedo, N.C.; Jiang, X.; Shi, X. 3D printing of extended-release tablets of theophylline using hydroxypropyl methylcellulose (HPMC) hydrogels. Int. J. Pharm. 2020, 591, 119983. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; Kim, D.W. Fabrication of Gastro-Floating Famotidine Tablets: Hydroxypropyl Methylcellulose-Based Semisolid Extrusion 3D Printing. Pharmaceutics 2023, 15, 316. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zou, B.; Lai, Q.; Zhu, K. SLA-3d printing and compressive strength of PEGDA/nHAP biomaterials. Ceram. Int. 2022, 48, 30917–30926. [Google Scholar] [CrossRef]
- Liu, M.; Chen, J.; Zheng, L.; Gao, Y.; Liu, Y. Synthesis and characterization of stereolithography 3D printing fluorescent resin. Polym. Eng. Sci. 2023, 63, 1961–1973. [Google Scholar] [CrossRef]
- McDonagh, T.; Belton, P.; Qi, S. Manipulating drug release from 3D printed dual-drug loaded polypills using challenging polymer compositions. Int. J. Pharm. 2023, 637, 11. [Google Scholar] [CrossRef] [PubMed]
- Tabriz, A.G.; Nandi, U.; Scoutaris, N.; Sanfo, K.; Alexander, B.; Gong, Y.C.; Hui, H.W.; Kumar, S.; Douroumis, D. Personalised paediatric chewable Ibuprofen tablets fabricated using 3D micro-extrusion printing technology. Int. J. Pharm. 2022, 626, 12. [Google Scholar] [CrossRef]
- Huanbutta, K.; Sangnim, T. Design and development of zero-order drug release gastroretentive floating tablets fabricated by 3D printing technology. J. Drug Deliv. Sci. Technol. 2019, 52, 831–837. [Google Scholar] [CrossRef]
- Oladeji, S.A.; Dadou, S.M.; Zhao, M.; Li, S.; Jones, D.S.; Andrews, G.P. The development and optimisation of gastro-retentive floating tablets using fused deposition modelling 3D printing. J. Pharm. Pharmacol. 2022, 74, 1450–1466. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Mohammed, A.A.; Ahmad, J.; Saleh, E. Development of a 3D Printed Coating Shell to Control the Drug Release of Encapsulated Immediate-Release Tablets. Polymers 2020, 12, 1395. [Google Scholar] [CrossRef] [PubMed]
- Solanki, N.G.; Tahsin, M.; Shah, A.V.; Serajuddin, A.M. Formulation of 3D Printed Tablet for Rapid Drug Release by Fused Deposition Modeling: Screening Polymers for Drug Release, Drug-Polymer Miscibility and Printability. J. Pharm. Sci. 2018, 107, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Tabriz, A.G.; Nandi, U.; Hurt, A.P.; Hui, H.W.; Karki, S.; Gong, Y.C.; Kumar, S.; Douroumis, D. 3D printed bilayer tablet with dual controlled drug release for tuberculosis treatment. Int. J. Pharm. 2021, 593, 10. [Google Scholar]
- Liu, B.S.; Han, X.L.; Wang, Z.M.; Zhang, H.; Liu, N.; Gao, X.; Gao, J.; Zheng, A.P. Three-dimensional printing personalized acetaminophen sustained-release tablets using hot melt extrusion. J. Drug Deliv. Sci. Technol. 2021, 66, 9. [Google Scholar] [CrossRef]
- Parhi, R.; Jena, G.K. An updated review on application of 3D printing in fabricating pharmaceutical dosage forms. Drug Deliv. Transl. Res. 2022, 12, 2428–2462. [Google Scholar] [CrossRef] [PubMed]
- Purohit, T.J.; Hanning, S.M.; Wu, Z. Advances in rectal drug delivery systems. Pharm. Dev. Technol. 2018, 23, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Chatzitaki, A.T.; Tsongas, K.; Tzimtzimis, E.K.; Tzetzis, D.; Bouropoulos, N.; Barmpalexis, P.; Eleftheriadis, G.K.; Fatouros, D.G. 3D printing of patient-tailored SNEDDS-based suppositories of lidocaine. J. Drug Deliv. Sci. Technol. 2021, 61, 8. [Google Scholar] [CrossRef]
- Seoane-Viano, I.; Ong, J.J.; Luzardo-Alvarez, A.; Gonzalez-Barcia, M.; Basit, A.W.; Otero-Espinar, F.J.; Goyanes, A. 3D printed tacrolimus suppositories for the treatment of ulcerative colitis. Asian J. Pharm. Sci. 2021, 16, 110–119. [Google Scholar] [CrossRef]
- Tagami, T.; Hayashi, N.; Sakai, N.; Ozeki, T. 3D printing of unique water-soluble polymer-based suppository shell for controlled drug release. Int. J. Pharm. 2019, 568, 8. [Google Scholar] [CrossRef]
- Morath, B.; Sauer, S.; Zaradzki, M.; Wagner, A.H. Orodispersible films-Recent developments and new applications in drug delivery and therapy. Biochem. Pharmacol. 2022, 200, 13. [Google Scholar] [CrossRef]
- Musazzi, U.M.; Khalid, G.M.; Selmin, F.; Minghetti, P.; Cilurzo, F. Trends in the production methods of orodispersible films. Int. J. Pharm. 2020, 576, 10. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, C.; Song, I.O.; Lee, B.J.; Kang, C.Y.; Park, J.B. Investigation of Patient-Centric 3D-Printed Orodispersible Films Containing Amorphous Aripiprazole. Pharmaceuticals 2022, 15, 895. [Google Scholar] [CrossRef] [PubMed]
- Panraksa, P.; Udomsom, S.; Rachtanapun, P.; Chittasupho, C.; Ruksiriwanich, W.; Jantrawut, P. Hydroxypropyl Methylcellulose E15: A Hydrophilic Polymer for Fabrication of Orodispersible Film Using Syringe Extrusion 3D Printer. Polymers 2020, 12, 2666. [Google Scholar] [CrossRef] [PubMed]
- Sjoholm, E.; Sandler, N. Additive manufacturing of personalized orodispersible warfarin films. Int. J. Pharm. 2019, 564, 117–123. [Google Scholar] [CrossRef]
- Cheung, K.; Das, D.B. Microneedles for drug delivery: Trends and progress. Drug Deliv. 2016, 23, 2338–2354. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.R.T.; Tekko, I.; McAvoy, K.; McMillan, H.; Jones, D.; Donnelly, R.F. Minimally invasive microneedles for ocular drug delivery. Expert Opin. Drug Deliv. 2017, 14, 525–537. [Google Scholar] [CrossRef]
- Sharma, S.; Hatware, K.; Bhadane, P.; Sindhikar, S.; Mishra, D.K. Recent advances in microneedle composites for biomedical applications: Advanced drug delivery technologies. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 103, 14. [Google Scholar] [CrossRef]
- Wang, R.; Jiang, G.H.; Aharodnikau, U.E.; Yunusov, K.; Sun, Y.F.; Liu, T.Q.; Solomevich, S.O. Recent Advances in Polymer Microneedles for Drug Transdermal Delivery: Design Strategies and Applications. Macromol. Rapid Commun. 2022, 43, 19. [Google Scholar] [CrossRef]
- Li, R.; Liu, X.; Yuan, X.; Wu, S.S.; Li, L.; Jiang, X.B.; Li, B.; Jiang, X.; Gou, M.L. Fast Customization of Hollow Microneedle Patches for Insulin Delivery. Int. J. Bioprinting 2022, 8, 124–135. [Google Scholar] [CrossRef]
- Economidou, S.N.; Pere, C.P.P.; Reid, A.; Uddin, M.J.; Windmill, J.F.C.; Lamprou, D.A.; Douroumis, D. 3D printed microneedle patches using stereolithography (SLA) for intradermal insulin delivery. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 102, 743–755. [Google Scholar] [CrossRef]
- Sirbubalo, M.; Tucak, A.; Muhamedagic, K.; Hindija, L.; Rahic, O.; Hadziabdic, J.; Cekic, A.; Begic-Hajdarevic, D.; Husic, M.C.; Dervisevic, A.; et al. 3D Printing-A “Touch-Button” Approach to Manufacture Microneedles for Transdermal Drug Delivery. Pharmaceutics 2021, 13, 924. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.L.; Zhong, W.Z.; Xu, L.; Li, H.J.; Yan, Q.Y.; She, Y.B.; Yang, G.S. Recent progress of 3D-printed microneedles for transdermal drug delivery. Int. J. Pharm. 2021, 593, 15. [Google Scholar] [CrossRef] [PubMed]
- Han, H.K.; Park, B.J.; Choi, H.J.; Moon, S.J.; Kim, S.J.; Bajracharya, R.; Min, J.Y. Pharmaceutical applications of 3D printing technology: Current understanding and future perspectives. J. Pharm. Investig. 2019, 49, 575–585. [Google Scholar]
- Yang, Y.T.; Wu, H.C.; Fu, Q.L.; Xie, X.F.; Song, Y.M.; Xu, M.; Li, J. 3D-printed polycaprolactone-chitosan based drug delivery implants for personalized administration. Mater. Des. 2022, 214, 10. [Google Scholar] [CrossRef]
- Beg, S.; Almalki, W.H.; Malik, A.; Farhan, M.; Aatif, M.; Rahman, Z.; Alruwaili, N.K.; Alrobaian, M.; Tarique, M.; Rahman, M. 3D printing for drug delivery and biomedical applications. Drug Discov. Today 2020, 25, 1668–1681. [Google Scholar] [CrossRef] [PubMed]
- El-Habashy, S.E.; El-Kamel, A.H.; Essawy, M.M.; Abdelfattah, E.Z.A.; Eltaher, H.M. 3D printed bioinspired scaffolds integrating doxycycline nanoparticles: Customizable implants for in vivo osteoregeneration. Int. J. Pharm. 2021, 607, 16. [Google Scholar] [CrossRef] [PubMed]
- Gaurav; Hasan, N.; Malik, A.K.; Singh, V.; Raza, K.; Ahmad, F.J.; Kesharwani, P.; Jain, G.K. Recent update of 3D printing technology in pharmaceutical formulation development. J. Biomater. Sci.-Polym. Ed. 2021, 32, 2306–2330. [Google Scholar] [CrossRef]
- Awad, A.; Fina, F.; Trenfield, S.J.; Patel, P.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printed Pellets (Miniprintlets): A Novel, Multi-Drug, Controlled Release Platform Technology. Pharmaceutics 2019, 11, 148. [Google Scholar] [CrossRef]
- Maroni, A.; Melocchi, A.; Parietti, F.; Foppoli, A.; Zema, L.; Gazzaniga, A. 3D printed multi-compartment capsular devices for two-pulse oral drug delivery. J. Control. Release 2017, 268, 10–18. [Google Scholar] [CrossRef]
- Long, J.J.J.; Etxeberria, A.E.; Nand, A.V.; Bunt, C.R.; Ray, S.; Seyfoddin, A. A 3D printed chitosan-pectin hydrogel wound dressing for lidocaine hydrochloride delivery. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 104, 9. [Google Scholar] [CrossRef]
- McDonagh, T.; Belton, P.; Qi, S. An investigation into the effects of geometric scaling and pore structure on drug dose and release of 3D printed solid dosage forms. Eur. J. Pharm. Biopharm. 2022, 177, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Shi, H.X.; Yang, S.D. 3D printed oral solid dosage form: Modified release and improved solubility. J. Control. Release 2022, 351, 407–431. [Google Scholar] [CrossRef] [PubMed]
- Windolf, H.; Chamberlain, R.; Quodbach, J. Predicting Drug Release from 3D Printed Oral Medicines Based on the Surface Area to Volume Ratio of Tablet Geometry. Pharmaceutics 2021, 13, 1453. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Roberts, C.J. Desktop 3D printing of controlled release pharmaceutical bilayer tablets. Int. J. Pharm. 2014, 461, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.J.N.; Yong, W.P.; Low, H.R.; Kochhar, J.S.; Khanolkar, J.; Lim, T.S.E.; Sun, Y.J.; Wong, J.Z.E.; Soh, S.L. Customizable drug tablets with constant release profiles via 3D printing technology. Int. J. Pharm. 2021, 598, 11. [Google Scholar] [CrossRef] [PubMed]
- Omari, S.; Ashour, E.A.; Elkanayati, R.; Alyahya, M.; Almutairi, M.; Repka, M.A. Formulation development of loratadine immediate- release tablets using hot-melt extrusion and 3D printing technology. J. Drug Deliv. Sci. Technol. 2022, 74, 9. [Google Scholar]
- Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. Development and Optimisation of Novel Polymeric Compositions for Sustained Release Theophylline Caplets (PrintCap) via FDM 3D Printing. Polymers 2020, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Teoh, X.Y.; Yan, J.Y.; Gleadall, A.; Belton, P.; Bibb, R.; Qi, S. Development of combi-pills using the coupling of semi-solid syringe extrusion 3D printing with fused deposition modelling. Int. J. Pharm. 2022, 625, 14. [Google Scholar] [CrossRef]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J. Control. Release 2015, 217, 308–314. [Google Scholar] [CrossRef]


| Rank | Journals | Papers | IF (2022) | JCR | Publisher |
|---|---|---|---|---|---|
| 1 | International Journal of Pharmaceutics | 109 | 5.8 | Q1 | ELSEVIER |
| 2 | Pharmaceutics | 74 | 5.4 | Q1 | MDPI |
| 3 | Journal of Controlled Release | 18 | 10.8 | Q1 | ELSEVIER |
| 4 | AAPS Pharmscitech | 14 | 3.3 | Q2 | SPRINGER |
| 5 | European Journal of Pharmaceutics and Biopharmaceutics | 14 | 4.9 | Q1 | ELSEVIER |
| 6 | Advanced Drug Delivery Reviews | 12 | 16.1 | Q1 | ELSEVIER |
| 7 | Current Pharmaceutical Design | 12 | 3.1 | Q3 | BENTHAM SCIENCE PUBL LTD |
| 8 | Journal of Drug Delivery Science and Technology | 12 | 5.0 | Q2 | ELSEVIER |
| 9 | Journal of Pharmaceutical Sciences | 12 | 3.8 | Q2 | ELSEVIER |
| 10 | European Journal of Pharmaceutical Sciences | 11 | 4.6 | Q2 | ELSEVIER |
| Rank | Institutions | Country | Papers | Citation |
|---|---|---|---|---|
| 1 | University College London | The United Kingdom | 52 | 5898 |
| 2 | FabRx Ltd. | The United Kingdom | 47 | 5673 |
| 3 | University of Santiago De Compostela | Spanish | 25 | 1867 |
| 4 | University of Cent Lancashire | The United Kingdom | 16 | 1994 |
| 5 | University of Nottingham | The United Kingdom | 14 | 1578 |
| 6 | Aristotle University of Thessaloniki | Greece | 11 | 297 |
| 7 | University of Texas Austin | The United States | 11 | 479 |
| 8 | Abo Akademi University | Finland | 10 | 525 |
| 9 | University of Mississippi | The United States | 10 | 605 |
| 10 | National University of Singapore | Singapore | 9 | 392 |
| Rank | Cited References | Year | First Author | Citation Frequency |
|---|---|---|---|---|
| 1 | Effect of geometry on drug release from 3D-printed tablets | 2015 | Goyanes, A. | 154 |
| 2 | 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets | 2015 | Goyanes, A. | 146 |
| 3 | Fused-filament 3D printing (3DP) for fabrication of tablets | 2014 | Goyanes, A. | 142 |
| 4 | 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles | 2015 | Khaled, S.A. | 138 |
| 5 | Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modelling (FDM) 3D printing | 2015 | Skowyra, J. | 131 |
| 6 | Emergence of 3D Printed Dosage Forms: Opportunities and Challenges | 2016 | Alhnan, M.A. | 118 |
| 7 | 3D printing of Medicines: Engineering Novel Oral Devices with Unique Design and Drug Release Characteristics | 2015 | Goyanes, A. | 116 |
| 8 | 3D printing of tablets containing multiple drugs with defined release profiles | 2015 | Khaled, S.A. | 116 |
| 9 | Desktop 3D printing of controlled release pharmaceutical bilayer tablets | 2014 | Khaled, S.A. | 111 |
| 10 | A flexible-dose dispenser for immediate and extended release 3D-printed tablets | 2015 | Pietrzak, K. | 111 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, A.; Li, W.; Tian, W.; Zheng, M.; Shen, L.; Hong, Y. A Bibliometric Analysis of 3D Printing in Personalized Medicine Research from 2012 to 2022. Pharmaceuticals 2023, 16, 1521. https://doi.org/10.3390/ph16111521
Xue A, Li W, Tian W, Zheng M, Shen L, Hong Y. A Bibliometric Analysis of 3D Printing in Personalized Medicine Research from 2012 to 2022. Pharmaceuticals. 2023; 16(11):1521. https://doi.org/10.3390/ph16111521
Chicago/Turabian StyleXue, Aile, Wenjie Li, Wenxiu Tian, Minyue Zheng, Lan Shen, and Yanlong Hong. 2023. "A Bibliometric Analysis of 3D Printing in Personalized Medicine Research from 2012 to 2022" Pharmaceuticals 16, no. 11: 1521. https://doi.org/10.3390/ph16111521
APA StyleXue, A., Li, W., Tian, W., Zheng, M., Shen, L., & Hong, Y. (2023). A Bibliometric Analysis of 3D Printing in Personalized Medicine Research from 2012 to 2022. Pharmaceuticals, 16(11), 1521. https://doi.org/10.3390/ph16111521





