Thermosensitive Polyurethane-Based Hydrogels as Potential Vehicles for Meloxicam Delivery
Abstract
:1. Introduction
2. Results and Discussion
2.1. FTIR Spectra
2.2. Rheological Behavior
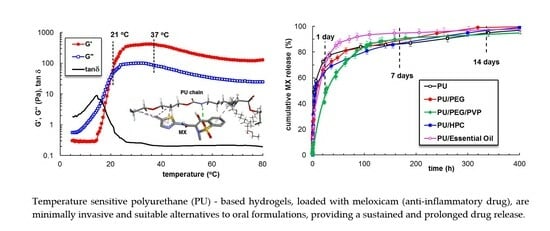
2.2.1. Temperature-Induced Gelation Monitored by Rheological Measurements
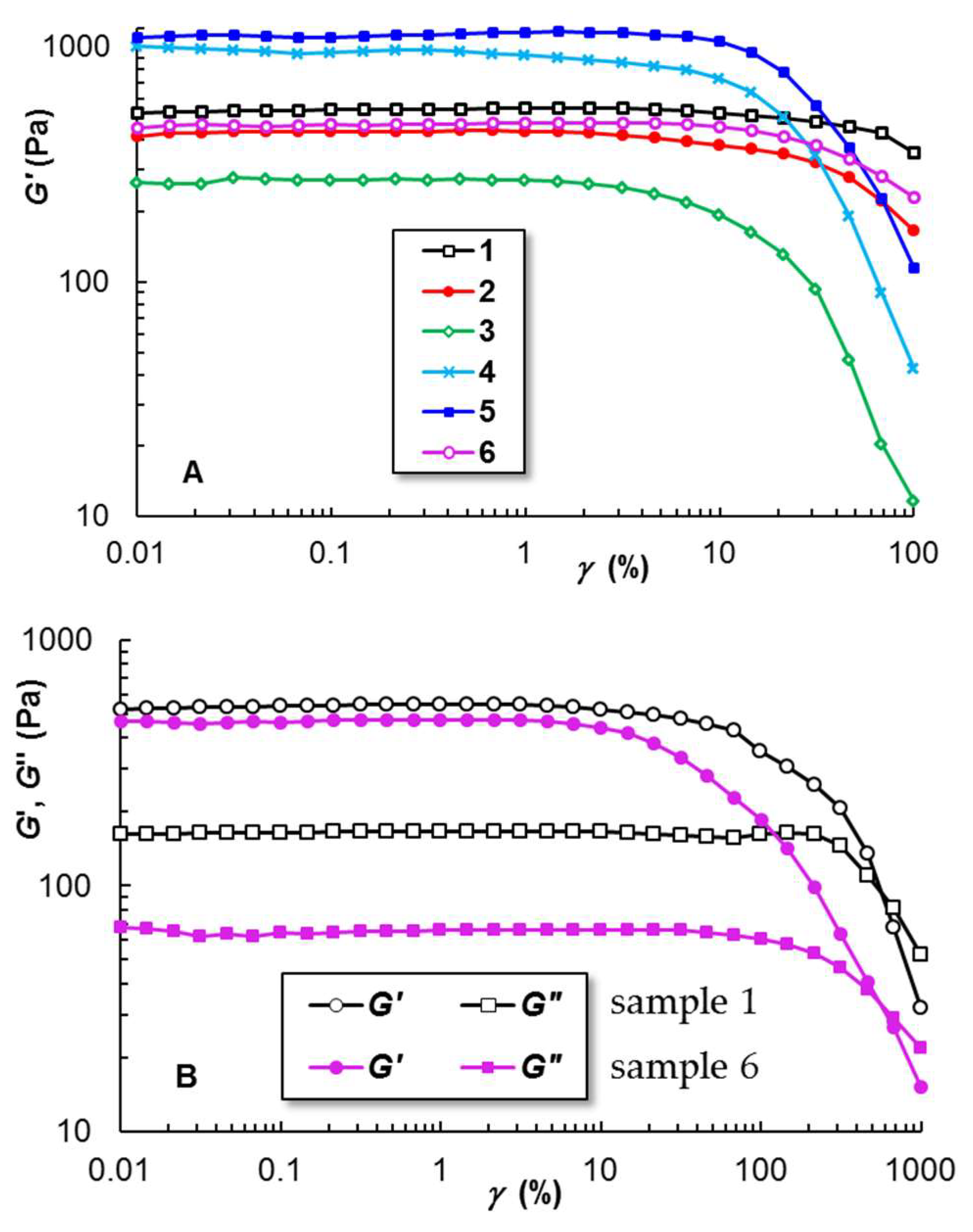
2.2.2. Linear and Non-Linear Viscoelasticity
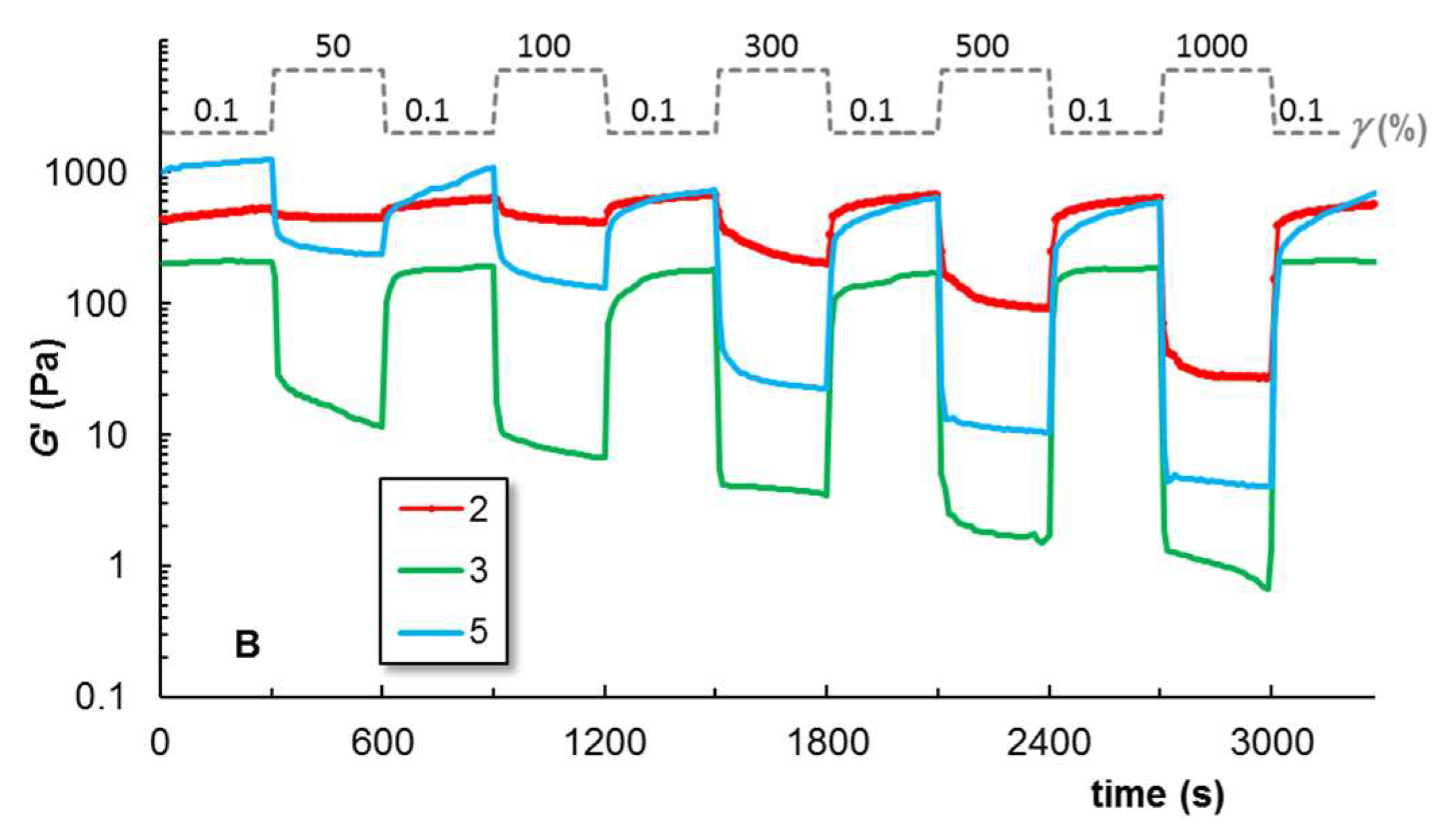
2.2.3. Self-Healing Behavior
2.2.4. Shear Flow Behavior at 37 °C
2.2.5. Creep and Recovery Behavior
2.3. Zeta Potential and Average Diameter of PU Micelles
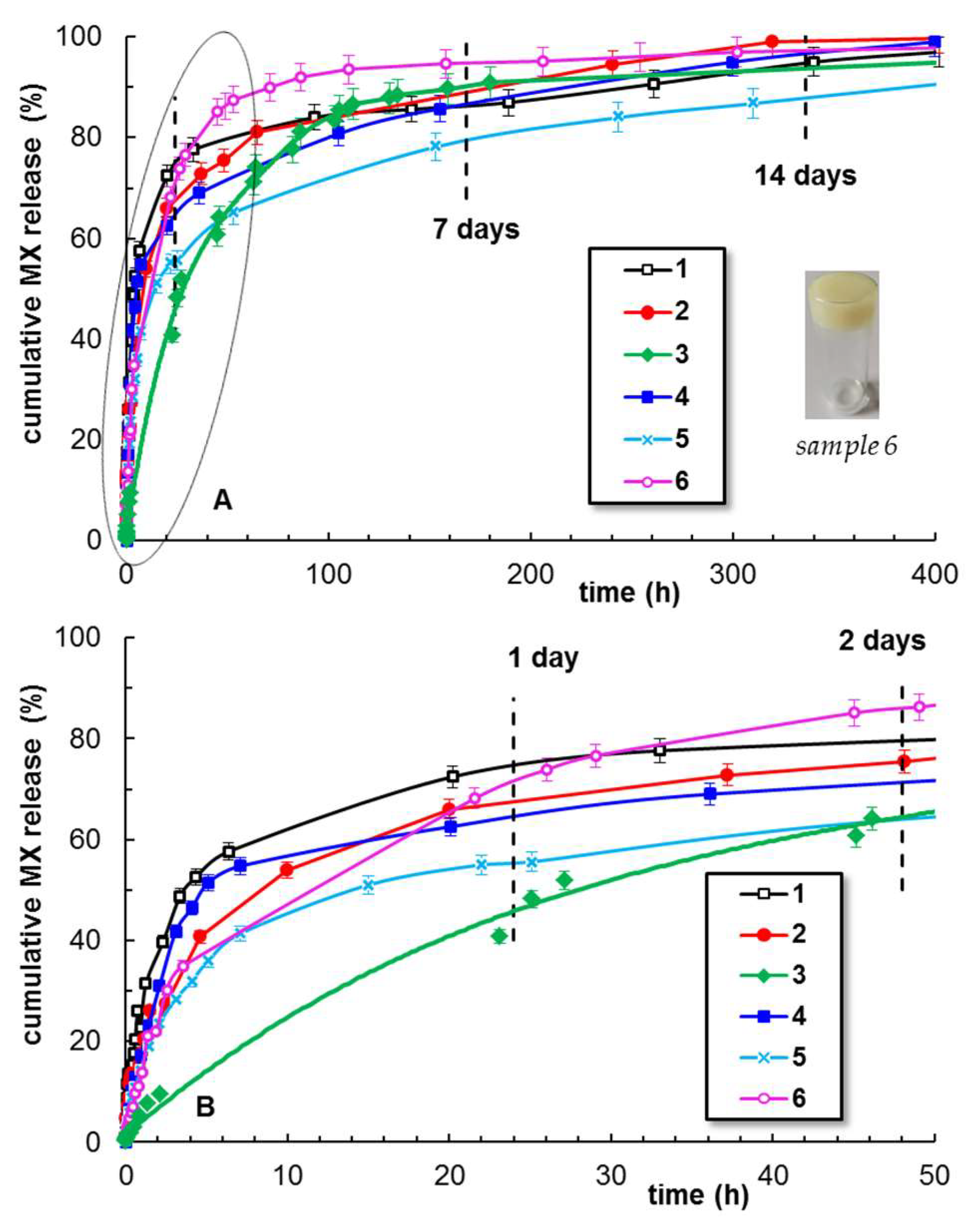
2.4. Meloxicam Delivery from PU-Based Hydrogels
3. Materials and Methods
3.1. Materials
3.1.1. Chemicals
3.1.2. Synthesis of Thermoresponsive Polyurethane
3.1.3. Preparation of Thermoresponsive PU-Based Hydrogels
3.2. Methods
3.2.1. Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy
3.2.2. In Silico Investigations of Polymer-Drug Binding
3.2.3. Rheology
3.2.4. Zeta Potential and Average Diameter
3.2.5. Meloxicam Delivery
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deshmukh, R. Rheumatoid arthritis: Pathophysiology, current therapeutic strategies and recent advances in targeted drug delivery system. Mater. Today Commun. 2023, 35, 105877. [Google Scholar] [CrossRef]
- Luger, P.; Daneck, K.; Engel, W.; Trummlitz, G.; Wagner, K. Structure and physicochemical properties of meloxicam, a new NSAID. Eur. J. Pharm. Sci. 1996, 4, 175–187. [Google Scholar] [CrossRef]
- Sostres, C.; Gargallo, C.J.; Lanas, A. Nonsteroidal anti-inflammatory drugs and upper and lower gastrointestinal mucosal damage. Arthritis Res. Ther. 2013, 15 (Suppl. 3), S3. [Google Scholar] [CrossRef]
- Gerwin, N.; Hops, C.; Lucke, A. Intraarticular drug delivery in osteoarthritis. Adv. Drug Deliv. Rev. 2006, 58, 226–242. [Google Scholar] [CrossRef] [PubMed]
- Noble, S.; Balfour, J.A. Meloxicam. Drugs 1996, 51, 424–432. [Google Scholar] [CrossRef]
- Fleischmann, R.; Iqbal, I.; Slobodin, G. Meloxicam. Expert Opin. Pharmacother. 2002, 3, 1501–1512. [Google Scholar] [CrossRef]
- Tavakoli, M. Modelling therapeutic strategies in the treatment of osteoarthritis: An economic evaluation of meloxicam versus diclofenac and piroxicam. Pharmacoeconomics 2003, 21, 443–454. [Google Scholar] [CrossRef]
- Beetge, E.; du Plessis, J.; Muller, D.G.; Goosen, C.; van Rensburg, F.J. The influence of the physicochemical characteristics and pharmacokinetic properties of selected NSAIDs on their transdermal absorption. Int. J. Pharm. 2000, 193, 261–264. [Google Scholar] [CrossRef]
- Fetih, G. Meloxicam formulations for transdermal delivery: Hydrogels versus organogels. J. Drug Deliv. Sci. Technol. 2010, 20, 451–456. [Google Scholar] [CrossRef]
- Fattahpour, S.; Shamanian, M.; Tavakoli, N.; Fathi, M.; Sheykhi, S.; Fattahpour, S. Design and optimization of alginate-chitosan-pluronic nanoparticles as a novel meloxicam drug delivery system. Appl. Polym. Sci. 2015, 132, 42241. [Google Scholar] [CrossRef]
- Sadeghi-Aliabadi, H.; Sheykhi, S.R.; Fesharaki, M.; Fattahpour, S. An injectable carboxymethyl chitosan-methylcellulose-pluronic hydrogel for the encapsulation of meloxicam loaded nanoparticles. Int. J. Biol. Macromol. 2020, 151, 220–229. [Google Scholar] [CrossRef]
- Kolawole, O.M.; Cook, M.T. In situ gelling drug delivery systems for topical drug delivery. Eur. J. Pharm. Biopharm. 2023, 184, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Inal, O.; Yapar, E.A. Effect of mechanical properties on the release of meloxicam from poloxamer gel bases. Indian J. Pharm. Sci. 2013, 75, 700–706. [Google Scholar] [PubMed]
- McKenzie, M.; Betts, D.; Suh, A.; Bui, K.; Kim, L.D.; Cho, H. Hydrogel-based drug delivery systems for poorly water-soluble drugs. Molecules 2015, 20, 20397–20408. [Google Scholar] [CrossRef]
- Lupu, A.; Gradinaru, L.M.; Rusu, D.; Bercea, M. Self-healing of Pluronic® F127 hydrogels in the presence of various polysaccharides. Gels 2023, 9, 719. [Google Scholar] [CrossRef]
- Lupu, A.; Rosca, I.; Gradinaru, R.; Bercea, M. Temperature induced gelation and antimicrobial properties of Pluronic F127 based systems. Polymers 2023, 15, 355. [Google Scholar] [CrossRef]
- Khan, B.; Arbab, A.; Khan, S.; Fatima, H.; Bibi, I.; Chowdhry, N.P.; Ansari, A.Q.; Ursani, A.A.; Kumar, S.; Hussain, J.; et al. Recent progress in thermosensitive hydrogels and their applications in drug delivery area. MedComm-Biomater. Appl. 2023, 2, e55. [Google Scholar] [CrossRef]
- Khurana, S.; Jain, N.K.; Bedi, P.M.S. Development and characterization of a novel controlled release drug delivery system based on nanostructured lipid carriers gel for meloxicam. Life Sci. 2013, 93, 763–772. [Google Scholar] [CrossRef]
- Wendels, S.; Aveous, L. Biobased polyurethanes for biomedical applications. Bioact. Mater. 2021, 6, 1083–1106. [Google Scholar] [CrossRef]
- Joseph, J.; Patel, R.M.; Wenham, A.; Smith, J.R. Biomedical applications of polyurethane materials and coatings. Trans. IMF 2018, 96, 121–129. [Google Scholar] [CrossRef]
- Das, A.; Mahanwar, P. A brief discussion on advances in polyurethane applications. Adv. Ind. Eng. Polym. Res. 2020, 3, 93–101. [Google Scholar] [CrossRef]
- Wang, J.; Dai, D.; Xie, H.; Li, D.; Xiong, G.; Zhang, C. Biological effects, applications and design strategies of medical polyurethanes modified by nanomaterials. Int. J. Nanomed. 2022, 17, 6791–6819. [Google Scholar] [CrossRef] [PubMed]
- Boffito, M.; Pontremoli, C.; Fiorilli, S.; Laurano, R.; Ciardelli, G.; Vitale-Brovarone, C. Injectable thermosensitive formulation based on polyurethane hydrogel/mesoporous glasses for sustained co-delivery of functional ions and drugs. Pharmaceutics 2019, 11, 501. [Google Scholar] [CrossRef] [PubMed]
- Laurano, R.; Boffito, M.; Cassino, C.; Liberti, F.; Ciardelli, G.; Chiono, V. Design of injectable bioartificial hydrogels by green chemistry for mini-invasive applications in the biomedical or aesthetic medicine fields. Gels 2023, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Huynh, C.T.; Nguyen, M.K.; Jeong, I.K.; Kim, S.W.; Lee, D.S. Synthesis, characteristics and potential application of poly(β-amino ester urethane)-based multiblock copolymers as an injectable, biodegradable and pH/temperature-sensitive hydrogel system. Biomater. Sci. Polym. Ed. 2012, 23, 1091–1106. [Google Scholar] [CrossRef]
- Gradinaru, L.M.; Ciobanu, C.; Vlad, S.; Bercea, M.; Popa, M. Thermoreversible poly(isopropyl lactate diol)-based polyurethane hydrogels: Effect of isocyanate on some physical properties. Ind. Eng. Chem. Res. 2012, 51, 12344–12354. [Google Scholar] [CrossRef]
- Yu, Y.; Cheng, Y.; Tong, J.; Zhang, L.; Wei, Y.; Tian, M. Recent advances in thermo-sensitive hydrogels for drug delivery. J. Mater. Chem. B 2021, 9, 2979–2992. [Google Scholar] [CrossRef]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Available online: https://www.ashland.com/file_source/Ashland/Product/Documents/Pharmaceutical/PC_11229_Klucel_HPC.pdf (accessed on 28 September 2023).
- Gao, J.; Haidar, G.; Lu, X.; Hu, Z. Self-association of hydroxypropylcellulose in water. Macromolecules 2001, 34, 2242–2247. [Google Scholar] [CrossRef]
- Jantharaprapap, R.; Stagni, G. Effects of penetration enhancers on in vitro permeability of meloxicam gels. Int. J. Pharm. 2007, 343, 26–33. [Google Scholar] [CrossRef]
- Chen, J.; Yunhua Gao, Y. Strategies for meloxicam delivery to and across the skin: A review. Drug Deliv. 2016, 23, 3146–3156. [Google Scholar] [CrossRef]
- El-Badry, M.; Fathy, M. Enhancement of the dissolution and permeation rates of meloxicam by formation of its freeze-dried solid dispersions in polyvinylpyrrolidone K-30. Drug Dev. Ind. Pharm. 2006, 32, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Mártha, C.; Kürti, L.; Farkas, G.; Jójárt-Laczkovich, O.; Szalontai, B.; Glässer, E.; Deli, M.A.; Szabó-Révész, P. Effects of polymers on the crystallinity of nanonized meloxicam during a co-grinding process. Eur. Polym. J. 2013, 49, 2426–2432. [Google Scholar] [CrossRef]
- Ochi, M.; Takaki Kawachi, T.; Toita, E.; Hashimoto, I.; Yuminoki, K.; Onoue, S.; Hashimoto, N. Development of nanocrystal formulation of meloxicam with improved dissolution and pharmacokinetic behaviors. Int. J. Pharm. 2014, 474, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Kurti, L.; Kukovecz, A.; Kozma, G.; Ambrus, R.; Deli, M.; Szabó-Révész, P. Study of the parameters influencing the co-grinding process for the production of meloxicam nanoparticles. Powder Technol. 2011, 212, 210–217. [Google Scholar] [CrossRef]
- Shi, X.; Huang, W.; Xu, T.; Fan, B.; Sheng, X. Investigation of drug–polymer miscibility and solubilization on meloxicam binary solid dispersion. J. Pharm. Innov. 2020, 15, 125–137. [Google Scholar] [CrossRef]
- Leuner, C.; Dressman, J. Improving drug solubility for oral delivery using solid dispersions. Eur. J. Pharm. Biopharm. 2000, 50, 47–60. [Google Scholar] [CrossRef]
- Sugimoto, M.; Okagi, T.; Narisawa, S.; Koida, Y.; Nakjima, K. Improvement of dissolution characteristics and bioavailability of poorly water soluble drugs by novel co-grinding method using water soluble polymer. Int. J. Pharm. 1998, 160, 11–19. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Essential oils and their constituents as skin penetration enhancer for transdermal drug delivery: A review. J. Pharm. Pharmacol. 2014, 67, 473–485. [Google Scholar] [CrossRef]
- Bouyahya, A.; Abrini, J.; Dakka, N.; Bakri, Y. Essential oils of Origanum Compactum increase membrane permeability, disturb cell membrane integrity, and suppress quorumsensing phenotype in bacteria. J. Pharm. Anal. 2019, 9, 301–311. [Google Scholar] [CrossRef]
- Kuo, S.H.; Shen, C.J.; Shen, C.F.; Cheng, C.M. Role of pH value in clinically relevant diagnosis. Diagnostics 2020, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Lukic, M.; Pantelic, I.; Savic, S.D. Towards optimal pH of the skin and topical formulations: From the current state of the art to tailored products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]
- Percival, S.L.; McCarty, S.; Hunt, J.A.; Woods, E.J. The effects of pH on wound healing, biofilms, and antimicrobial efficacy. Wound Rep. Reg. 2014, 22, 174–186. [Google Scholar] [CrossRef]
- Huang, S.; Venables, D.S.; Lawrence, S.E. Pharmaceutical salts of piroxicam and meloxicam with organic counterions. Cryst. Growth Des. 2022, 22, 6504–6520. [Google Scholar] [CrossRef] [PubMed]
- Gradinaru, L.M.; Bercea, M.; Lupu, A.; Gradinaru, V.R. Development of polyurethane/peptide-based carriers with self-healing properties. Polymers 2023, 15, 1697. [Google Scholar] [CrossRef] [PubMed]
- Mandru, M.; Bercea, M.; Gradinaru, L.M.; Ciobanu, C.; Drobota, M.; Vlad, S.; Albulescu, R. Polyurethane/poly(vinyl alcohol) hydrogels: Preparation, characterization and drug delivery. Eur. Polym. J. 2019, 118, 137–145. [Google Scholar] [CrossRef]
- McFarlane, N.L.; Wagner, N.J.; Kaler, E.W.; Lynch, M.L. Poly(ethylene oxide) (PEO) and poly(vinyl pyrolidone) (PVP) induce different changes in the colloid stability of nanoparticles. Langmuir 2010, 26, 13823–13830. [Google Scholar] [CrossRef]
- Kiran Kumar, K.K.; Ravi, M.; Pavani, Y.; Bhavani, S.; Sharma, A.K.; Narasimha Rao, V.V.R. Electrical conduction mechanism in NaCl complexed PEO/PVP polymer blend electrolytes. J. Non-Cryst. Solids 2012, 358, 3205–3211. [Google Scholar] [CrossRef]
- Coussot, P. Yield stress fluid flows: A review of experimental data. J. Non-Newton. Fluid Mech. 2014, 211, 31–49. [Google Scholar] [CrossRef]
- Maya, S.; Sarmento, B.; Nair, A.; Rejinold, N.S.; Nair, S.V.; Jayakumar, R. Smart stimuli sensitive nanogels in cancer drug delivery and imaging: A review. Curr. Pharm. Des. 2013, 19, 7203–7218. [Google Scholar] [CrossRef]
- Peppas, N.A.; Narasimhan, B. Mathematical models in drug delivery: How modeling has shaped the way we design new drug delivery systems. J. Control. Release 2014, 190, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Peppas, N.A. Higuchi equation: Derivation, applications, use and misuse. Int. J. Pharm. 2011, 418, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Korsmeyer, R.W.; Gumy, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Peppas, N.A. Analysis of Fickian and non-Fickian drug release from polymers. Pharm. Acta Helv. 1985, 60, 110–111. [Google Scholar] [PubMed]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable device. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Gupta, S.K.; Bansal, P.; Bhardwaj, R.K.; Jaiswal, J.; Velpandian, T. Comparison of analgesic and anti-inflammatory activity of meloxicam gel with diclofenac and piroxicam gels in animal models: Pharmacokinetic parameters after topical application. Skin Pharmacol. Physiol. 2002, 15, 105–111. [Google Scholar] [CrossRef]
- Chiriac, A.P.; Rusu, A.G.; Nita, L.E.; Chiriac, V.M.; Neamtu, I.; Sandu, A. Polymeric carriers designed for encapsulation of essential oils with biological activity. Pharmaceutics 2021, 13, 631. [Google Scholar] [CrossRef]
- Sindi, A.M.; Hosny, K.M. Lyophilized composite loaded with meloxicam-peppermint oil nanoemulsion for periodontal pain. Polymers 2021, 13, 2317. [Google Scholar] [CrossRef]
- Panja, S.; Dietrich, B.; Adams, D.J. Controlling syneresis of hydrogels using organic salts. Angew. Chem. Int. Ed. 2022, 61, e202115021. [Google Scholar] [CrossRef]
- Available online: https://www.rcsb.org/chemical-sketch (accessed on 10 October 2023).
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.3ds.com/products-services/biovia (accessed on 10 October 2023).
- Unagolla, J.M.; Ambalangodage, C.; Jayasuriya, A.C. Drug transport mechanisms and in vitro release kinetics of vancomycin encapsulated chitosan-alginate polyelectrolyte microparticles as a controlled drug delivery system. Eur. J. Pharm. Sci. 2018, 114, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, K.; Nakagawa, T.; Uno, T. Application of Akaike’s information criterion (AIC) in the evaluation of linear pharmacokinetic equations. J. Pharmacokinet. Biophacodyn. 1978, 6, 165–175. [Google Scholar] [CrossRef]











| Sample Code | PU (% wt.) | Excipient Type | Excipient Content (% wt.) | Solvent (% wt.) | MX (%) | τo (a) (Pa) | (%) | G′ (b) (Pa) |
|---|---|---|---|---|---|---|---|---|
| 1 | 25 | - | - | 75 | 1 | 79.2 | 31.6 | 525.5 |
| 2 | 25 | PEG | 3 | 72 | 1 | 72.8 | 14.7 | 456.1 |
| 3 | 25 | PEG PVP | 3 3 | 69 | 1 | 9.4 | 3.2 | 208.6 |
| 4 | 18.75 | HPC | 6.25 | 75 | 1 | 87.3 | 10.9 | 1330 |
| 5 | 12.5 | HPC | 12.5 | 75 | 1 | 85.6 | 6.8 | 1203 |
| 6 | 25 | EO | 6 | 69 | 1 | 46.1 | 21.5 | 474.3 |
| Sample | Temperature (°C) | DH ± SD * (nm) | PDI |
|---|---|---|---|
| PU | 25 | 32.8 ± 0.98 | 0.120 |
| PU loaded with MX | 25 | 31.6 ± 0.94 | 0.132 |
| PU | 37 | 35.8 ± 1.06 | 0.245 |
| PU loaded with MX | 37 | 37.0 ± 1.10 | 0.246 |
| Sample Code | First Order Equation (2) | Higuchi Equation (3) | Korsmeyer-Peppas Equation (4) | Peppas-Sahlin Equation (5) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 102·k11 (min−1) | AIC | 102·kh (min−1/2) | AIC | n | AIC | 10·k1 (min−m) | 102·k2 (min−2m) | m | AIC | |
| 1 | 2.250 | −78.600 | 2.588 | −73.785 | 0.831 | −34.492 | 1.446 | 0.778 | 0.465 | −108.299 |
| 2 | 0.984 | −71.619 | 1.294 | −111.686 | 0.863 | −31.354 | 1.896 | 3.274 | 0.744 | −51.282 |
| 3 | 3.274 | −84.467 | 1.422 | −150.85 | 0.672 | −37.223 | 1.659 | 2.642 | 0.186 | −109.711 |
| 4 | 0.431 | −46.850 | 7.428 | −44.058 | 0.806 | −41.852 | 2.337 | 1.678 | 0.713 | −90.105 |
| 5 | 2.590 | −42.543 | 7.328 | −46.382 | 0.835 | −43.257 | 2.352 | 2.190 | 0.843 | −116.873 |
| 6 | 1.375 | −89.8648 | 2.040 | −96.243 | 0.770 | −74.9818 | 5.061 | 6.918 | 0.120 | −105.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plugariu, I.-A.; Gradinaru, L.M.; Avadanei, M.; Rosca, I.; Nita, L.E.; Maxim, C.; Bercea, M. Thermosensitive Polyurethane-Based Hydrogels as Potential Vehicles for Meloxicam Delivery. Pharmaceuticals 2023, 16, 1510. https://doi.org/10.3390/ph16111510
Plugariu I-A, Gradinaru LM, Avadanei M, Rosca I, Nita LE, Maxim C, Bercea M. Thermosensitive Polyurethane-Based Hydrogels as Potential Vehicles for Meloxicam Delivery. Pharmaceuticals. 2023; 16(11):1510. https://doi.org/10.3390/ph16111510
Chicago/Turabian StylePlugariu, Ioana-Alexandra, Luiza Madalina Gradinaru, Mihaela Avadanei, Irina Rosca, Loredana Elena Nita, Claudia Maxim, and Maria Bercea. 2023. "Thermosensitive Polyurethane-Based Hydrogels as Potential Vehicles for Meloxicam Delivery" Pharmaceuticals 16, no. 11: 1510. https://doi.org/10.3390/ph16111510
APA StylePlugariu, I.-A., Gradinaru, L. M., Avadanei, M., Rosca, I., Nita, L. E., Maxim, C., & Bercea, M. (2023). Thermosensitive Polyurethane-Based Hydrogels as Potential Vehicles for Meloxicam Delivery. Pharmaceuticals, 16(11), 1510. https://doi.org/10.3390/ph16111510