Design, Synthesis and Biological Evaluation of Novel N-Arylpiperazines Containing a 4,5-Dihydrothiazole Ring
Abstract
:1. Introduction
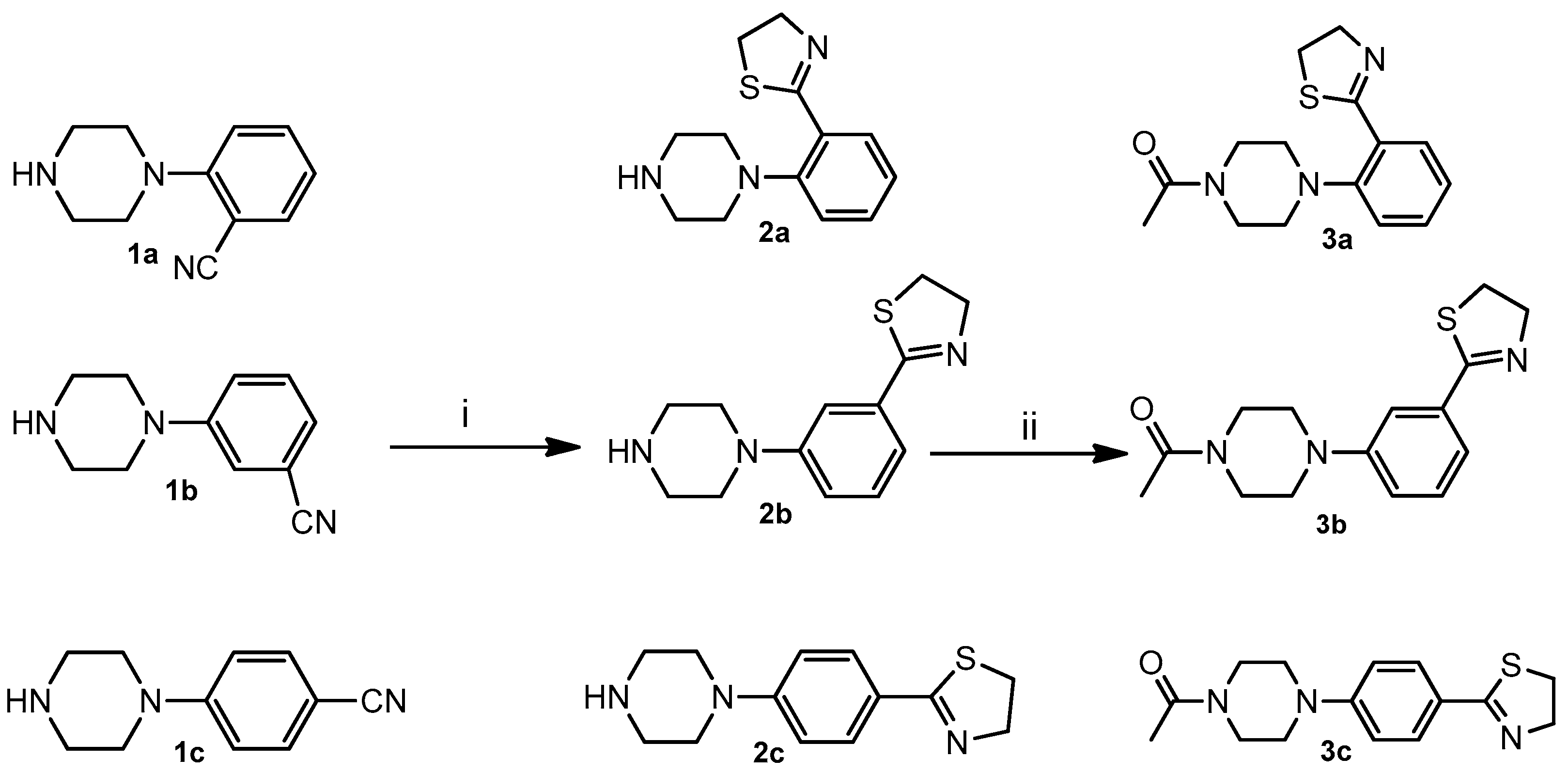
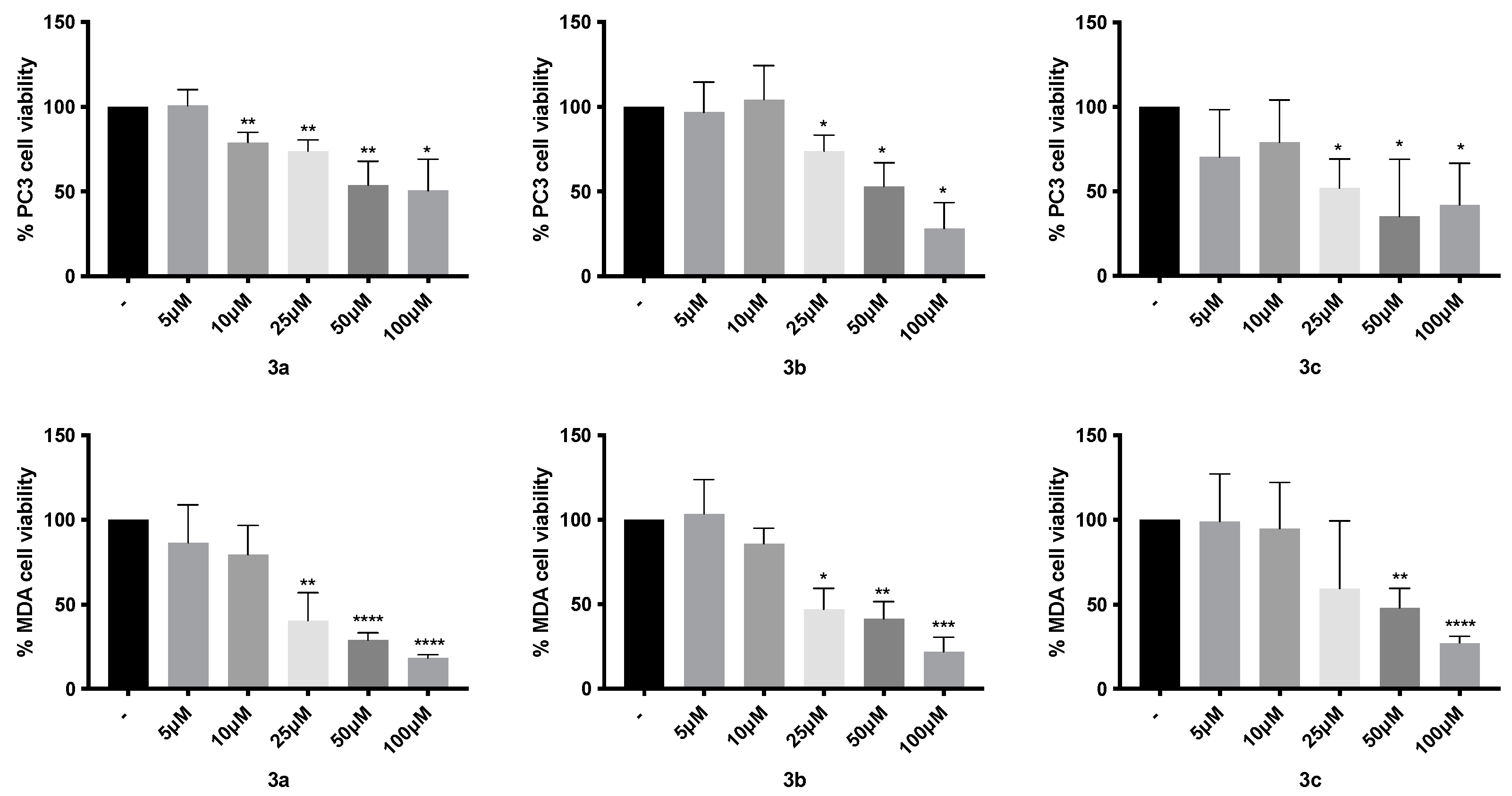
2. Results
3. Discussion
4. Materials and Methods
4.1. Synthesis
4.1.1. General Procedures
4.1.2. General Procedure for the Synthesis of 2-(x-(Piperazin-1-yl) phenyl)-4,5-dihydrothiazole (2a–c)
- 2-(2-(Piperazin-1-yl) phenyl)-4,5-dihydrothiazole (2a): Yield: 58%; mp: 78–80 °C; 1H-NMR (400 MHz, CDCl3) δ: 1.83 (s, 1H), 2.95 (bs, 4H, 2CH2 pip.), 3.13 (bs, 4H, 2CH2 pip.), 3.24(t, 2H, -CH2-, J = 8.3 Hz), 4.30 (t, 2H, -CH2-, J = 8.3 Hz), 7.10 (t, 1H, J = 7.7 Hz), 7.14 (d, 1H, J = 7.7 Hz), 7.40 (t, 1H, J = 7.7 Hz), 7.79 (d, 1H, J = 7.7 Hz); 13C-NMR (101 MHz, CDCl3) δ: 33.28, 45.80, 54.35, 62.83, 119.77, 123.34, 129.39, 130.04, 131.24, 152.07, 168.17 ESI-MS m/z [M + H]+ calculated for C13H17N3S 247.36, Found = 248.1 Anal. Calcd for C13H17N3S: C, 63.12; H, 6.93; N, 16.99. Found C, 63.30; H, 6.95; N, 17.04.
- 2-(3-(Piperazin-1-yl) phenyl)-4,5-dihydrothiazole (2b): Yield: 65%; mp: 109–110 °C; 1H-NMR (400 MHz, CDCl3) δ: 1.80 (s, 1H), 3.06 (m, 4H, 2CH2 pip.), 3.22 (m, 4H, 2CH2 pip.), 3.41 (t, 2H, -CH2-, J = 8.4 Hz), 4.46 (t, 2H, -CH2-, J = 8.4 Hz), 7.15 (bs, 1H), 7.29 (bs, 2H), 7.45 (bs, 1H); 13C-NMR (101 MHz, CDCl3) δ: 33.60, 46.07, 50.18, 65.18, 115.15, 118.86, 120.07, 129.20, 134.03, 151.79, 169.00 ESI-MS m/z [M + H]+ calculated for C13H17N3S 247.36, Found = 248.14 Anal. Calcd. for C13H17N3S: C, 63.12; H, 6.93; N, 16.99. Found C, 63.24; H, 6.73; N, 17.05.
- 2-(4-(Piperazin-1-yl) phenyl)-4,5-dihydrothiazole (2c): Yield: 60%; mp: 152–153 °C; 1H-NMR (400 MHz, CDCl3) δ: 1.80 (s, 1H), 3.02 (m, 4H, 2CH2 pip.), 3.26 (m, 4H, 2CH2 pip.), 3.36 (t, 2H, -CH2-, J = 8.2 Hz), 4.42 (t, 2H, -CH2-, J = 8.2 Hz), 6.88 (d, 2H, J = 8.4 Hz), 7.24 (d, 2H, J = 8.4 Hz); 13C-NMR (101 MHz, CDCl3) δ: 33.53, 45.88, 49.04, 64.97, 114.39, 123.86, 129.70, 153.44, 167.32 ESI-MS m/z [M + H]+ calculated for C13H17N3S: 247.36, Found = 248.13 Anal. Calcd. for C13H17N3S: C, 63.12; H, 6.93; N, 16.99. Found C, 62.99; H, 6.95; N, 16.97.
4.1.3. General Procedure for the Synthesis of 1-(4-(x-(4,5-Dihydrothiazol-2-yl) phenyl)piperazin-1-yl)ethan-1-one (3a–c):
- 1-(4-(2-(4,5-Dihydrothiazol-2-yl) phenyl)piperazin-1-yl)ethan-1-one (3a): Yield: 62%; mp: 90–92 °C; 1H-NMR (400 MHz, CDCl3) δ: 2.15 (s, 3H), 2.98 (bs, 4H, 2CH2 pip.), 3.26 (t, 2H, -CH2-, J = 8.0 Hz) 3.72 (bs, 2H, -CH2 pip.), 3.88 (bs, 2H, -CH2 pip.), 4.32 (t, 2H, -CH2-, J = 8.0 Hz), 7.10 (d, 1H, J = 7.8 Hz), 7.15 (t, 1H, J = 7.8 Hz), 7.41 (t, 1H, J = 7.8 Hz), 7.82 (d, 1H, J = 7.8 Hz); 13C-NMR (101 MHz, CDCl3) δ: 21.40, 33.30, 41.35, 46.26, 52.58, 53.39, 63.01, 119.89, 124.01, 129.55, 130.26, 131.34, 150.94, 167.64, 169.21 ESI-MS m/z [M + H]+ calculated for C15H19N3OS 289,40 Found = 290.2 Anal. Calcd. for C15H19N3OS: C, 62.26; H, 6.62; N, 14.52. Found C, 62.44; H, 6.59; N, 14.56.
- 1-(4-(3-(4,5-Dihydrothiazol-2-yl)phenyl)piperazin-1-yl)ethan-1-one (3b): Yield: 65%; mp: 91–93 °C; 1H-NMR (400 MHz, CDCl3) δ: 2.16 (s, 3H), 3.22 (t, 2H, CH2 pip., J = 5.2 Hz), 3.25 (t, 2H, CH2 pip., J = 5.2 Hz), 3.42(t, 2H, -CH2-, J = 8.4 Hz) 3.64 (t, 2H, -CH2 pip., J = 5.2 Hz), 3.79 (t, 2H, -CH2 pip. J = 5.2 Hz), 4.46 (t, 2H, -CH2-, J = 8.4 Hz), 7.04 (m, 2H), 7.32 (bs, 1H,), 7.46 (bs, 1H); 13C-NMR (101 MHz, CDCl3) δ: 21.37, 33.66, 41.29, 46.14, 49.20, 49.41, 65.17, 115.65, 119.35, 120.90, 129.38, 134.19, 150.95, 168.92, 169.19 ESI-MS m/z [M + H]+ calculated for C15H19N3OS 289,40 Found = 290.2 Anal. Calcd. for C15H19N3OS: C, 62.26; H, 6.62; N, 14.52. Found C, 62.07; H, 6.63; N, 14.50.
- 1-(4-(4-(4,5-Dihydrothiazol-2-yl)phenyl)piperazin-1-yl)ethan-1-one (3c): Yield: 61%; mp: 199–200 °C; 1H-NMR (400 MHz, CDCl3) δ: 2.16 (s, 3H), 3.29 (bs, 2H, CH2 pip.), 3.33 (bs, 2H, CH2 pip.), 3.39 (t, 2H, -CH2-, J = 8.2 Hz) 3.65 (bs, 2H, -CH2 pip.), 3.80 (bs, 2H, -CH2 pip.), 4.42 (t, 2H, -CH2-, J = 8.2 Hz), 6.90 (d, 2H, J = 8.2 Hz), 7.77 (d, 2H, J = 8.2 Hz); 13C-NMR (101 MHz, CDCl3) δ: 21.33, 33.60, 41.06, 45.92, 48.03, 48.32, 65.03, 114.86, 124.69, 129.78, 152.58, 167.67, 169.26 ESI-MS m/z [M + H]+ calculated for C15H19N3OS 289,40 Found = 290.2 Anal. Calcd. for C15H19N3OS: C, 62.26; H, 6.62; N, 14.52. Found C, 62.50; H, 6.60; N, 14.55.
4.2. In Vitro Receptor Binding
4.2.1. Membrane Preparation
4.2.2. Competitive 5-HT1A Assay
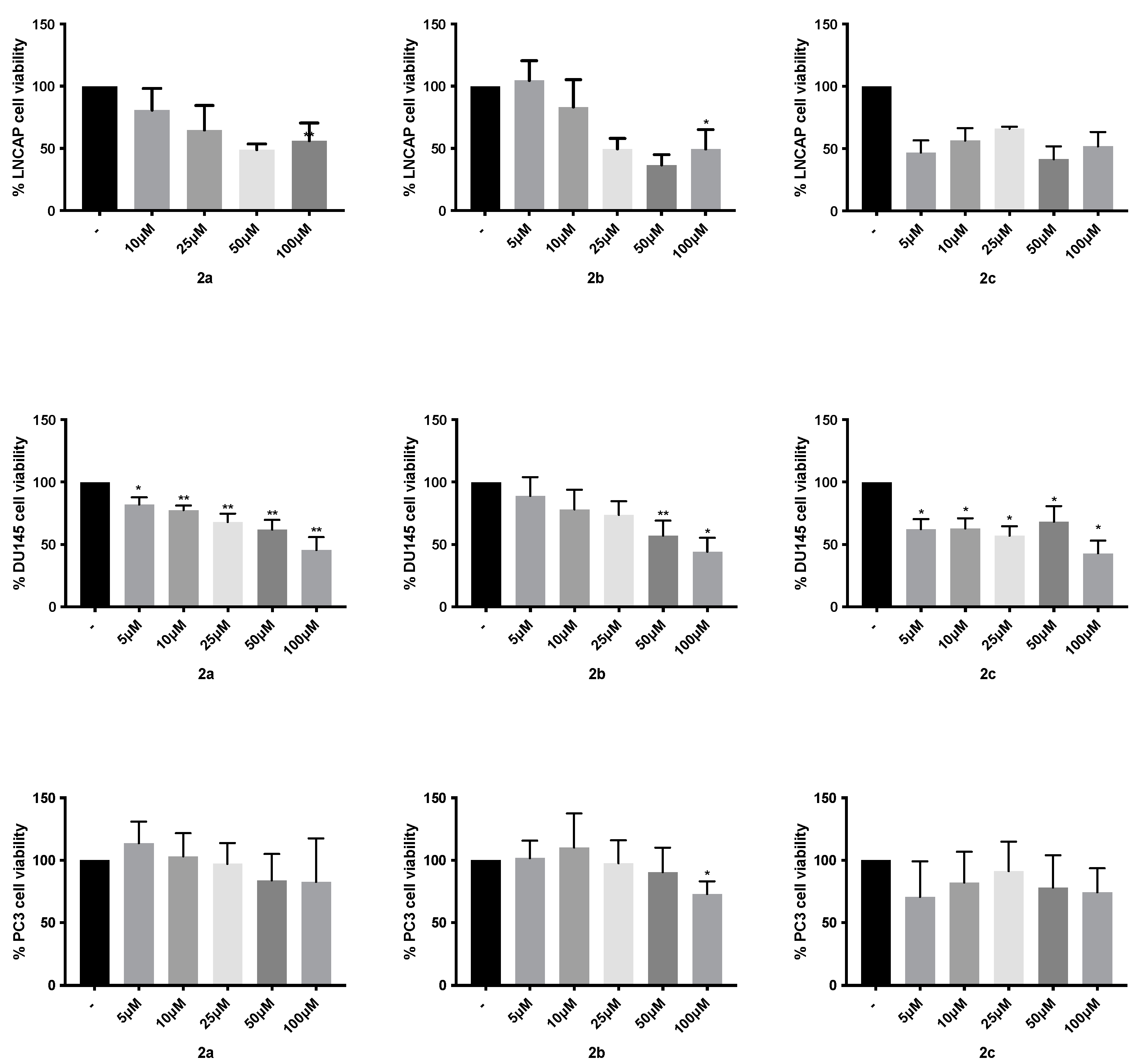
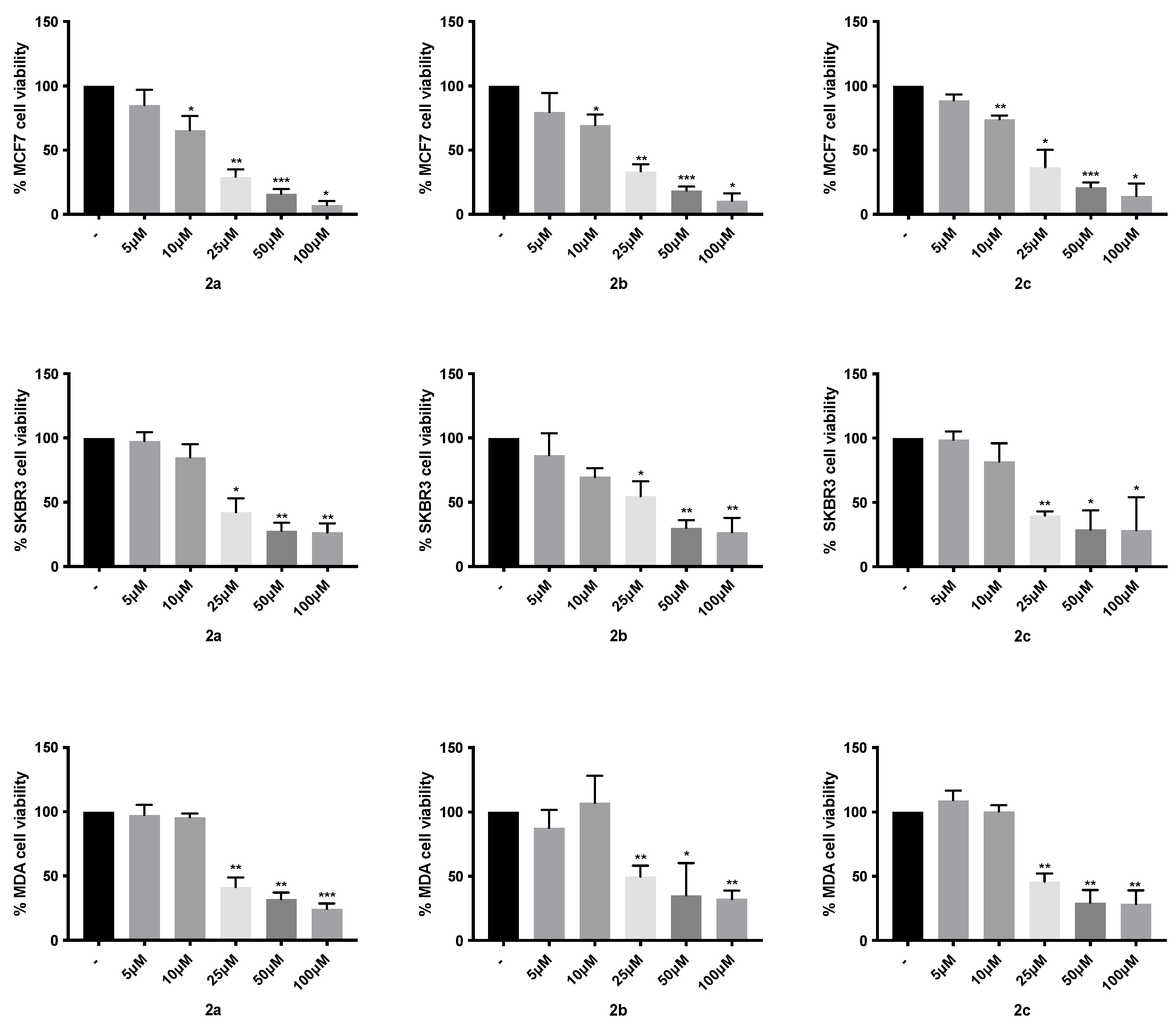
4.3. Cytotoxic Activity
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ravilla, L.; Naidu Subba Venkata, N.; Nagarajan, K. An efficient scale up process for synthesis of N-arylpiperazines. Tetrahedron Lett. 2015, 56, 4541–4544. [Google Scholar] [CrossRef]
- Mao, Z.W.; Zheng, X.; Lin, Y.P.; Hu, C.Y.; Wang, X.L.; Wan, C.P.; Rao, G.X. Design, synthesis, and anticancer activity of novel hybrid compounds between benzofuran and N-aryl piperazine. Bioorg. Med. Chem. Lett. 2016, 26, 3421–3424. [Google Scholar] [CrossRef] [PubMed]
- Corvino, A.; Fiorino, F.; Severino, B.; Saccone, I.; Frecentese, F.; Perissutti, E.; Di Vaio, P.; Caliendo, G.; Magli, E. The Role of 5-HT1A Receptor in the Cancer as a New Opportunity in Medicinal Chemistry. Curr. Med. Chem. 2018, 25, 3214–3227. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, M.R.; Magli, E.; Caliendo, G.; Sparaco, R.; Massarelli, P.; D’Esposito, V.; Migliaccio, T.; Mosca, G.; Fiorino, F.; Formisano, P. Serotoninergic receptor ligands improve Tamoxifen effectiveness on breast cancer cells. BMC Cancer 2022, 22, 171. [Google Scholar] [CrossRef] [PubMed]
- Fiorino, F.; Magli, E.; Perissutti, E.; Severino, B.; Frecentese, F.; Esposito, A.; De Angelis, F.; Incisivo, G.M.; Massarelli, P.; Nencini, C.; et al. Synthesis of 1-naphtylpiperazine derivatives as serotoninergic ligands and their evaluation as antiproliferative agents. Eur. J. Med. Chem. 2011, 46, 2206–2216. [Google Scholar] [CrossRef]
- Kumar, R.R.; Sahu, B.; Pathania, S.; Kumar Singh, P.; Jawaid Akhtar, M.; Kumar, B. Piperazine, a Key Substructure for Antidepressants: Its Role in Developments and Structure-Activity Relationships. ChemMedChem 2021, 16, 1878–1901. [Google Scholar] [CrossRef]
- Quaglia, W.; Cifani, C.; Del Bello, F.; Giannella, M.; Giorgioni, G.; Micioni Di Bonaventura, M.V.; Piergentili, A. Serotonin—A Chemical Messenger Between All Types of Living Cells; InTechOpen: London, UK, 2017; Chapter 4; pp. 67–108. [Google Scholar]
- Chen, H.; Xu, F.; Liang, X.; Xu, B.B.; Yang, Z.L.; He, X.L.; Huang, B.Y.; Yuan, M. Design, synthesis and biological evaluation of novel arylpiperazine derivatives on human prostate cancer cell lines. Bioorg. Med. Chem. Lett. 2015, 25, 285. [Google Scholar] [CrossRef]
- Del Bello, F.; Bonifazi, A.; Giorgioni, G.; Quaglia, W.; Amantini, C.; Morelli, M.B.; Santoni, G.; Battiti, F.O.; Vistoli, G.; Cilia, A.; et al. Chemical manipulations on the 1,4-dioxane ring of 5-HT1A receptor agonists lead to antagonists endowed with antitumor activity in prostate cancer cells. Eur. J. Med. Chem. 2019, 168, 461–473. [Google Scholar] [CrossRef]
- Caliendo, G.; Fiorino, F.; Grieco, P.; Perissutti, E.; Santagada, V.; Severino, B.; Bruni, G.; Romeo, M.R. Synthesis of new 1,2,3-benzotriazin-4-one-arylpiperazine derivatives as 5-HT1A serotonin receptor ligands. Bioorg. Med. Chem. 2000, 8, 533–538. [Google Scholar] [CrossRef]
- Fiorino, F.; Perissutti, E.; Severino, B.; Santagada, V.; Cirillo, D.; Terracciano, S.; Massarelli, P.; Bruni, G.; Collavoli, E.; Renner, C.; et al. New 5-hydroxytryptamine(1A) receptor ligands containing a norbornene nucleus: Synthesis and in vitro pharmacological evaluation. J. Med. Chem. 2005, 48, 5495–5503. [Google Scholar] [CrossRef]
- Fiorino, F.; Severino, B.; De Angelis, F.; Perissutti, E.; Frecentese, F.; Massarelli, P.; Bruni, G.; Collavoli, E.; Santagada, V.; Caliendo, G. Synthesis and in-vitro pharmacological evaluation of new 5-HT1A receptor ligands containing a benzotriazinone nucleus. Arch. Pharm. 2008, 341, 20–27. [Google Scholar] [CrossRef]
- Fiorino, F.; Severino, B.; De Angelis, F.; Perissutti, E.; Magli, E.; Frecentese, F.; Esposito, A.; Massarelli, P.; Nencini, C.; Viti, B.; et al. Synthesis and in vitro pharmacological evaluation of a new series of 5-HT1A 5-HT2A and 5-HT2C receptor ligands containing a norbornene nucleus. Pharmazie 2009, 64, 555–564. [Google Scholar]
- Fiorino, F.; Severino, B.; De Angelis, F.; Perissutti, E.; Magli, E.; Frecentese, F.; Esposito, A.; Massarelli, P.; Nencini, C.; Santagada, V.; et al. New 5-HT1A receptor ligands containing a N′-cyanoisonicotinamidine nucleus: Synthesis and in vitro pharmacological evaluation. Bioorganic Med. Chem. Lett. 2010, 20, 2978–2982. [Google Scholar] [CrossRef]
- Fiorino, F.; Severino, B.; Magli, E.; Perissutti, E.; Frecentese, F.; Esposito, A.; Incisivo, G.M.; Ciano, A.; Massarelli, P.; Nencini, C.; et al. New Potent 5-HT2A Receptor Ligands Containing a N′-Cyanopicolinamidine Nucleus: Synthesis and In Vitro Pharmacolog-ical Evaluation. Eur. J. Med.Chem. 2012, 47, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Fiorino, F.; Magli, E.; Severino, B.; Corvino, A.; Ciano, A.; Perissutti, E.; Frecentese, F.; Massarelli, P.; Nencini, C.; Santagada, V.; et al. Synthesis and In Vitro Pharmacological Evaluation of Novel 2-Hydroxypropyl-4-arylpiperazine Derivatives as Seroto-ninergic Ligands. Arch. Pharm. 2014, 347, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Fiorino, F.; Ciano, A.; Magli, E.; Severino, B.; Corvino, A.; Perissutti, E.; Frecentese, F.; Di Vaio, P.; Izzo, A.A.; Capasso, R.; et al. Synthesis, in vitro and in vivo pharmacological evaluation of serotoninergic ligands containing an isonicotinic nucleus. Eur. J. Med. Chem. 2016, 110, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Magli, E.; Kędzierska, E.; Kaczor, A.A.; Severino, B.; Corvino, A.; Perissutti, E.; Frecentese, F.; Saccone, I.; Massarelli, P.; Gibuła-Tarłowska, E.; et al. Synthesis, docking studies, and pharmacological evaluation of 5HT2C ligands containing the N′-cyanoisonicotinamidine or N′-cyanopicolinamidine nucleus. Arch. Pharm. 2019, 352, 1800373. [Google Scholar] [CrossRef]
- Magli, E.; Kędzierska, E.; Kaczor, A.A.; Bielenica, A.; Severino, B.; Gibuła-Tarłowska, E.; Kotlinska, J.H.; Corvino, A.; Sparaco, R.; Esposito, G.; et al. Synthesis, docking studies, and pharmacological evaluation of 2-hydroxypropyl-4-arylpiperazine derivatives as seroto-ninergic ligands. Arch. Pharm. 2021, 354, 2000414. [Google Scholar] [CrossRef]
- Meltzer, H.Y.; Massey, B.W. The role of serotonin receptors in the action of atypical antipsychotic drugs. Curr. Opin. Pharmacol. 2011, 1, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, J.A.; Mandos, L.A.; Rickels, K.; Lohoff, F.W. Pharmacological treatment of generalized anxiety disorder. Expert Opin. Pharmacother. 2011, 12, 2457–2467. [Google Scholar] [CrossRef]
- Perez-Lloret, S.; Rascol, O. Piribedil for the Treatment of Motor and Non-motor Symptoms of Parkinson Disease. CNS Drugs 2016, 30, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.C.; Bansal, K.K.; Sharma, A.; Sharma, D.; Deep, A. Thiazole-containing compounds as therapeutic targets for cancer therapy. Eur. J. Med. Chem. 2020, 188, 112016. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Devgun, M.; Narang, R.; Lal, S.; Rana, A.C. Thiazoles: A Retrospective Study on Synthesis, Structure-activity Relationship and Therapeutic Significanc. Indian J. Pharm. Edu. Res. 2022, 56, 646–666. [Google Scholar] [CrossRef]
- Szczepańska, K.; Karcz, T.; Kotańska, M.; Siwek, A.; Kuder, K.J.; Latacz, G.; Mogilski, S.; Hagenow, S.; Lubelska, A.; Sobolewski, M.; et al. Optimization and preclinical evaluation of novel histamine H3 receptor ligands: Acetyl and propionyl phenoxyalkyl piperazine derivatives. Bioorganic Med. Chem. 2018, 26, 6056–6066. [Google Scholar] [CrossRef]
- León-González, A.J.; Sáez-Martínez, P.; Jiménez-Vacas, J.M.; Herrero-Aguayo, V.; Montero-Hidalgo, A.J.; Gómez-Gómez, E.; Madrona, A.; Castaño, J.P.; Espartero, J.L.; Gahete, M.D.; et al. Comparative Cytotoxic Activity of Hydroxytyrosol and Its Semisynthetic Lipophilic Derivatives in Prostate Cancer Cells. Antioxidants 2021, 10, 1348. [Google Scholar] [CrossRef]
- Kumar, B.; Kumar, N.; Thakur, A.; Kumar, V.; Kumar, R.; Kumar, V. A Review on the Arylpiperazine Derivatives as Potential Therapeutics for the Treatment of Various Neurological Disorders. Curr. Drug Targets 2022, 23, 729–751. [Google Scholar] [CrossRef]
- Ambrosio, M.R.; D’Esposito, V.; Costa, V.; Liguoro, D.; Collina, F.; Cantile, M.; Prevete, N.; Passaro, C.; Mosca, G.; De Laurentiis, M.; et al. Glucose impairs tamoxifen responsiveness modulating connective tissue growth factor in breast cancer cells. Oncotarget 2017, 8, 109000. [Google Scholar] [CrossRef]

| 5-HT1A Receptor Binding Affinity | ||
|---|---|---|
| Compd. | pKi ± SEM | Ki (µM, 95% CI) |
| 2a | 5.6 ± 0.14 | 2.295 (1.155–4.559) |
| 2b | 6.4 ± 0.11 | 0.412 (0.246–0.688) |
| 2c | 4.3 ± 0.084 | 49.460 (33.100–73.900) |
| 8-OH-DPAT | 9.16 ± 0.09 | 0.00068 (0.00045–0.00102) |
| Compd. | MCF-7 | SKBR-3 | MDA-MB231 | LNCAP | DU-145 | PC-3 |
|---|---|---|---|---|---|---|
| 2a | 14.7 ± 1.9 | 28.17 ± 4.1 | 31.37 ± 5.1 | 67.24 ± 13.1 | 66.63 ± 7.3 | - |
| 2b | 15.93 ± 1.8 | 27.58 ± 5.6 | 39.96 ± 9.8 | 48.48 ± 7.3 | 66.58 ± 9.4 | - |
| 2c | 19.47 ± 2.3 | 27.65 ± 4.8 | 36.32 ± 7.7 | 31.93 ± 9.2 | 47.74 ± 11.7 | - |
| 3a | - | - | 23.27 ± 3.4 | - | - | 73.3 ± 10.3 |
| 3b | - | - | 34.6 ± 5.4 | - | - | 64.96 ± 7.4 |
| 3c | - | - | 47.15 ± 6.7 | - | - | 32.09 ± 10.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreozzi, G.; Ambrosio, M.R.; Magli, E.; Maneli, G.; Severino, B.; Corvino, A.; Sparaco, R.; Perissutti, E.; Frecentese, F.; Santagada, V.; et al. Design, Synthesis and Biological Evaluation of Novel N-Arylpiperazines Containing a 4,5-Dihydrothiazole Ring. Pharmaceuticals 2023, 16, 1483. https://doi.org/10.3390/ph16101483
Andreozzi G, Ambrosio MR, Magli E, Maneli G, Severino B, Corvino A, Sparaco R, Perissutti E, Frecentese F, Santagada V, et al. Design, Synthesis and Biological Evaluation of Novel N-Arylpiperazines Containing a 4,5-Dihydrothiazole Ring. Pharmaceuticals. 2023; 16(10):1483. https://doi.org/10.3390/ph16101483
Chicago/Turabian StyleAndreozzi, Giorgia, Maria Rosaria Ambrosio, Elisa Magli, Giovanni Maneli, Beatrice Severino, Angela Corvino, Rosa Sparaco, Elisa Perissutti, Francesco Frecentese, Vincenzo Santagada, and et al. 2023. "Design, Synthesis and Biological Evaluation of Novel N-Arylpiperazines Containing a 4,5-Dihydrothiazole Ring" Pharmaceuticals 16, no. 10: 1483. https://doi.org/10.3390/ph16101483
APA StyleAndreozzi, G., Ambrosio, M. R., Magli, E., Maneli, G., Severino, B., Corvino, A., Sparaco, R., Perissutti, E., Frecentese, F., Santagada, V., Leśniak, A., Bujalska-Zadrożny, M., Caliendo, G., Formisano, P., & Fiorino, F. (2023). Design, Synthesis and Biological Evaluation of Novel N-Arylpiperazines Containing a 4,5-Dihydrothiazole Ring. Pharmaceuticals, 16(10), 1483. https://doi.org/10.3390/ph16101483