Functionalizing Dendrimers for Targeted Delivery of Bioactive Molecules to Macrophages: A Potential Treatment for Mycobacterium tuberculosis Infection—A Review
Abstract
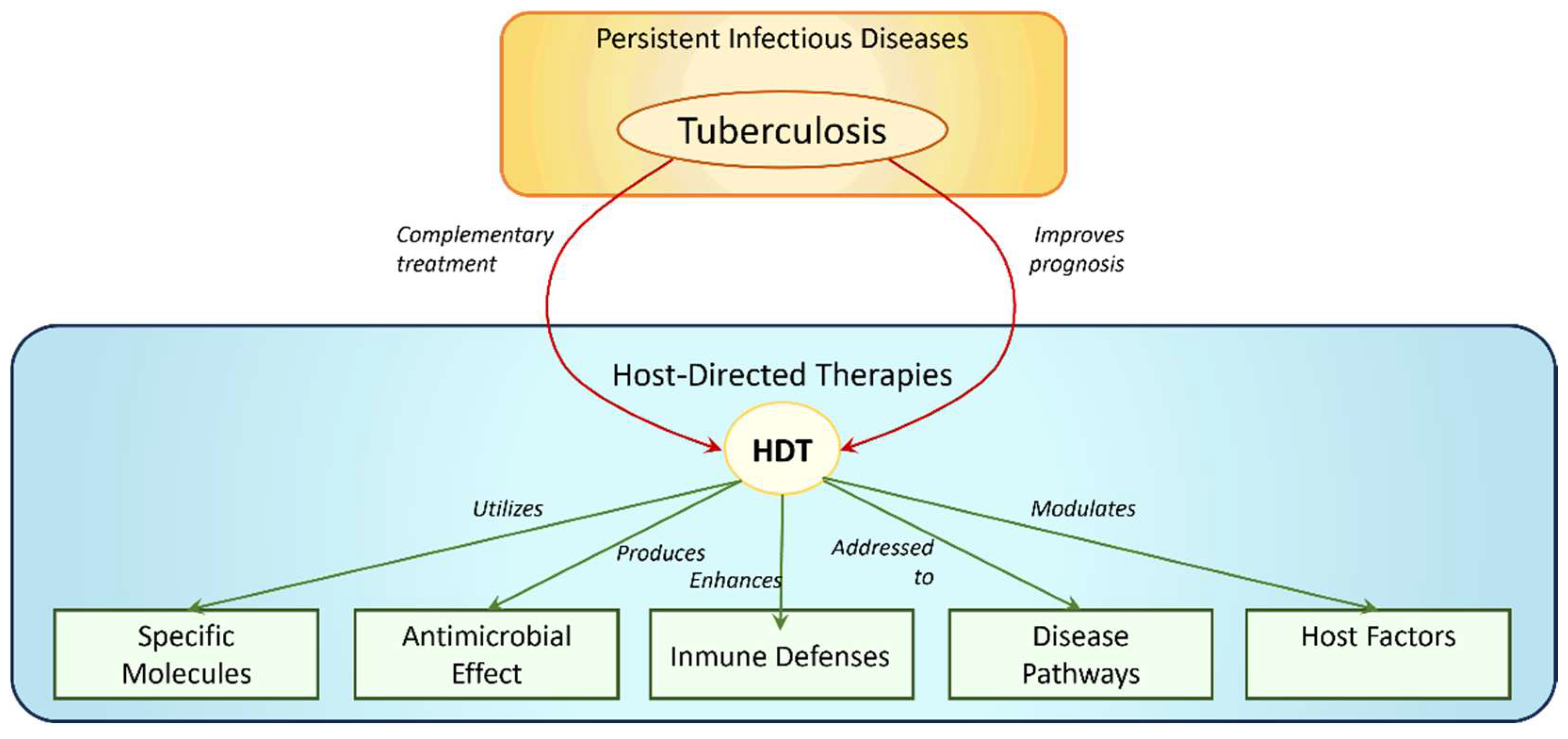
:1. Introduction
1.1. TB Current Therapies
1.2. miRNA Therapies

2. Dendrimers as Carriers
2.1. Current Studies Using Dendrimers in TB
2.2. Dendrimers for Gene Therapy
2.3. Targeting Dendrimers to Alveolar Macrophages
2.3.1. Mannose Functionalization
2.3.2. Tuftsin Functionalization
3. General Outlooks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chia, S.Z.G.; How, K.B.M.; Chlebicki, M.P.; Ling, M.L.; Gan, W.H. A retrospective review of tuberculosis exposure among health care workers in a tertiary hospital. Am. J. Infect. Control 2020, 48, 650–655. [Google Scholar] [CrossRef]
- World Health Organization Global Tuberculosis Report 2022. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2022 (accessed on 1 July 2023).
- Nemes, E.; Geldenhuys, H.; Rozot, V.; Rutkowski, K.T.; Ratangee, F.; Bilek, N.; Mabwe, S.; Makhethe, L.; Erasmus, M.; Toefy, A.; et al. Prevention of M. tuberculosis Infection with H4:IC31 Vaccine or BCG Revaccination. N. Engl. J. Med. 2018, 379, 138–149. [Google Scholar] [CrossRef]
- Young, C.; Walzl, G.; Du Plessis, N. Therapeutic host-directed strategies to improve outcome in tuberculosis. Mucosal Immunol. 2020, 13, 190–204. [Google Scholar] [CrossRef]
- Iannaccone, M.; Dorhoi, A.; Kaufmann, S.H.E. Host-directed therapy of tuberculosis: What is in it for microRNA? Expert Opin. Ther. Targets 2014, 18, 491–494. [Google Scholar] [CrossRef]
- Mortier, E.; Ma, A.; Malynn, B.A.; Neurath, M.F. Editorial: Modulating Cytokines as Treatment for Autoimmune Diseases and Cancer. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Sharma, D.; Sarkar, D. Pathophysiology of Tuberculosis: An Update Review. Pharmatutor 2018, 6, 15–21. [Google Scholar] [CrossRef]
- CDC. Core Curriculum on Tuberculosis: What the Clinician Should Know; Centers for Disease Control and Prevention National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention Division of Tuberculosis Elimination: Atlanta, GA, USA, 2021; pp. 9–12. [Google Scholar]
- Lee, W.H.; Loo, C.Y.; Traini, D.; Young, P.M. Nano- and micro-based inhaled drug delivery systems for targeting alveolar macrophages. Expert Opin. Drug Deliv. 2015, 12, 1009–1026. [Google Scholar] [CrossRef]
- Bah, A.; Vergne, I. Macrophage Autophagy and Bacterial Infections. Front. Immunol. 2017, 8, 1483. [Google Scholar] [CrossRef]
- Duan, L.; Yi, M.; Chen, J.; Li, S.; Chen, W. Mycobacterium tuberculosis EIS gene inhibits macrophage autophagy through up-regulation of IL-10 by increasing the acetylation of histone H3. Biochem. Biophys. Res. Commun. 2016, 473, 1229–1234. [Google Scholar] [CrossRef]
- Guirado, E.; Mbawuike, U.; Keiser, T.L.; Arcos, J.; Azad, A.K.; Wang, S.H.; Schlesinger, L.S. Characterization of host and microbial determinants in individuals with latent tuberculosis infection using a human granuloma model. mBio 2015, 6, e02537-14. [Google Scholar] [CrossRef]
- Silwal, P.; Kim, Y.S.; Basu, J.; Jo, E.K. The roles of microRNAs in regulation of autophagy during bacterial infection. Semin. Cell Dev. Biol. 2020, 101, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Shou, Z.; Jin, X.; Chen, Y. Emerging strategies in nanotechnology to treat respiratory tract infections: Realizing current trends for future clinical perspectives. Drug Deliv. 2022, 29, 2442–2458. [Google Scholar] [CrossRef] [PubMed]
- Kurz, S.G.; Furin, J.J.; Bark, C.M. Drug-Resistant Tuberculosis: Challenges and Progress. Infect. Dis. Clin. N. Am. 2016, 30, 509–522. [Google Scholar] [CrossRef]
- World Health Organization. Global Tuberculosis Report; WHO: Geneva, Switzerland, 2020; ISBN 9789241565714. [Google Scholar]
- Gong, W.; Liang, Y.; Wu, X. The current status, challenges, and future developments of new tuberculosis vaccines. Hum. Vaccin. Immunother. 2018, 14, 1697–1716. [Google Scholar] [CrossRef] [PubMed]
- Soleiman-Meignooni, S. Gene therapy and immunotherapy. ED Microbiol. 2018, 2, 851–852. [Google Scholar]
- Li, H.; Yang, Y.; Hong, W.; Huang, M.; Wu, M.; Zhao, X. Applications of genome editing technology in the targeted therapy of human diseases: Mechanisms, advances and prospects. Signal Transduct. Target. Ther. 2020, 5, 1. [Google Scholar] [CrossRef]
- Sabir, N.; Hussain, T.; Shah, S.Z.A.; Peramo, A.; Zhao, D.; Zhou, X. miRNAs in tuberculosis: New avenues for diagnosis and host-directed therapy. Front. Microbiol. 2018, 9, 602. [Google Scholar] [CrossRef]
- Singh, A.K.; Ghosh, M.; Kumar, V.; Aggarwal, S.; Patil, S.A. Interplay between miRNAs and Mycobacterium tuberculosis: Diagnostic and therapeutic implications. Drug Discov. Today 2021, 26, 1245–1255. [Google Scholar] [CrossRef]
- Ryan, B.; Joilin, G.; Williams, J.M. Plasticity-related microRNA and their potential contribution to the maintenance of long-term potentiation. Front. Mol. Neurosci. 2015, 8, 4. [Google Scholar] [CrossRef]
- Barbu, M.G.; Condrat, C.E.; Thompson, D.C.; Bugnar, O.L.; Cretoiu, D.; Toader, O.D.; Suciu, N.; Voinea, S.C. MicroRNA Involvement in Signaling Pathways During Viral Infection. Front. Cell Dev. Biol. 2020, 8, 143. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Song, Y. MicroRNA-26b inhibits the immune response to Mycobacterium tuberculosis (M.tb) infection in THP-1 cells via targeting TGFβ-activated kinase-1 (TAK1), a promoter of the NF-κB pathway. Int. J. Clin. Exp. Pathol. 2018, 11, 1218–1227. [Google Scholar] [PubMed]
- Sinigaglia, A.; Peta, E.; Riccetti, S.; Venkateswaran, S.; Manganelli, R.; Barzon, L. Tuberculosis-Associated MicroRNAs: From Pathogenesis to Disease Biomarkers. Cells 2020, 9, 2160. [Google Scholar] [CrossRef] [PubMed]
- Saunders, B.M.; Frank, A.A.; Orme, I.M.; Cooper, A.M. Interleukin-6 induces early gamma interferon production in the infected lung but is not required for generation of specific immunity to Mycobacterium tuberculosis infection. Infect. Immun. 2000, 68, 3322–3326. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.A.; Mazhar, H.; Saleha, S.; Tipu, H.N.; Muhammad, N.; Abbas, M.N. Interferon-Gamma Improves Macrophages Function against M. tuberculosis in Multidrug-Resistant Tuberculosis Patients. Chemother. Res. Pract. 2016, 2016, 7295390. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, K.; Zhou, L.; Wu, M.; Wu, Y.; Zhu, M.; Lai, X.M.; Chen, T.; Feng, L.; Li, M.; et al. MicroRNA-155 Promotes Autophagy to Eliminate Intracellular Mycobacteria by Targeting Rheb. PLoS Pathog. 2013, 9, e1003697. [Google Scholar] [CrossRef]
- Lee, S.W.L.; Paoletti, C.; Campisi, M.; Osaki, T.; Adriani, G.; Kamm, R.D.; Mattu, C.; Chiono, V. MicroRNA delivery through nanoparticles. J. Control. Release 2019, 313, 80–95. [Google Scholar] [CrossRef]
- Jain, N.K.; Mishra, V.; Mehra, N.K. Targeted drug delivery to macrophages. Expert Opin. Drug Deliv. 2013, 10, 353–367. [Google Scholar] [CrossRef]
- Yan, S.; Ren, B.Y.; Shen, J. Nanoparticle-mediated double-stranded RNA delivery system: A promising approach for sustainable pest management. Insect Sci. 2021, 28, 21–34. [Google Scholar] [CrossRef]
- Mehta, P.; Kadam, S.; Pawar, A.; Bothiraja, C. Dendrimers for pulmonary delivery: Current perspectives and future challenges. New J. Chem. 2019, 43, 8396–8409. [Google Scholar] [CrossRef]
- Mignani, S.; El Kazzouli, S.; Bousmina, M.; Majoral, J.P. Expand classical drug administration ways by emerging routes using dendrimer drug delivery systems: A concise overview. Adv. Drug Deliv. Rev. 2013, 65, 1316–1330. [Google Scholar] [CrossRef]
- Dufès, C.; Uchegbu, I.F.; Schätzlein, A.G. Dendrimers in gene delivery. Adv. Drug Deliv. Rev. 2005, 57, 2177–2202. [Google Scholar] [CrossRef]
- Shcharbin, D.; Dzmitruk, V.; Shakhbazau, A.; Goncharova, N.; Seviaryn, I.; Kosmacheva, S.; Potapnev, M.; Pedziwiatr-Werbicka, E.; Bryszewska, M.; Talabaev, M.; et al. Fourth Generation Phosphorus-Containing Dendrimers: Prospective Drug and Gene Delivery Carrier. Pharmaceutics 2011, 3, 458–473. [Google Scholar] [CrossRef]
- Sun, M.; Müllen, K.; Yin, M. Water-soluble perylenediimides: Design concepts and biological applications. Chem. Soc. Rev. 2016, 45, 1513–1528. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Shum, K.-T.; Burnett, J.; Rossi, J. Nanoparticle-Based Delivery of RNAi Therapeutics: Progress and Challenges. Pharmaceuticals 2013, 6, 85–107. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.; Elmowafy, E.; Soliman, M.E. The Evolution of Dendrimers to Composite Dendrimers: A Review of the State of the Art; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128165041. [Google Scholar]
- Janaszewska, A.; Lazniewska, J.; Trzepiński, P.; Marcinkowska, M.; Klajnert-Maculewicz, B. Cytotoxicity of dendrimers. Biomolecules 2019, 9, 330. [Google Scholar] [CrossRef]
- Yan, S.; Hu, Q.; Li, J.; Chao, Z.; Cai, C.; Yin, M.; Du, X.; Shen, J. A Star Polycation Acts as a Drug Nanocarrier to Improve the Toxicity and Persistence of Botanical Pesticides. ACS Sustain. Chem. Eng. 2019, 7, 17406–17413. [Google Scholar] [CrossRef]
- Mendes, L.P.; Pan, J.; Torchilin, V.P. Dendrimers as nanocarriers for nucleic acid and drug delivery in cancer therapy. Molecules 2017, 22, 1401. [Google Scholar] [CrossRef]
- Bhadra, D.; Bhadra, S.; Jain, S.; Jain, N.K. A PEGylated dendritic nanoparticulate carrier of fluorouracil. Int. J. Pharm. 2003, 257, 111–124. [Google Scholar] [CrossRef]
- Rupp, R.; Rosenthal, S.L.; Stanberry, L.R. VivaGel (SPL7013 Gel): A candidate dendrimer—Microbicide for the prevention of HIV and HSV infection. Int. J. Nanomed. 2007, 2, 561–566. [Google Scholar]
- Price, C.F.; Tyssen, D.; Sonza, S.; Davie, A.; Evans, S.; Lewis, G.R.; Xia, S.; Spelman, T.; Hodsman, P.; Moench, T.R.; et al. SPL7013 Gel (VivaGel®) Retains Potent HIV-1 and HSV-2 Inhibitory Activity following Vaginal Administration in Humans. PLoS ONE 2011, 6, e24095. [Google Scholar] [CrossRef]
- McGowan, I.; Gomez, K.; Bruder, K.; Febo, I.; Chen, B.A.; Richardson, B.A.; Husnik, M.; Livant, E.; Price, C.; Jacobson, C. Phase 1 randomized trial of the vaginal safety and acceptability of SPL7013 gel (VivaGel) in sexually active young women (MTN-004). AIDS 2011, 25, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.V.; Asthana, A.; Dutta, T.; Jain, N.K. Intracellular macrophage uptake of rifampicin loaded mannosylated dendrimers. J. Drug Target. 2006, 14, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Rajabnezhad, S.; Casettari, L.; Lam, J.K.W.; Nomani, A.; Torkamani, M.R.; Palmieri, G.F.; Rajabnejad, M.R.; Darbandi, M.A. Pulmonary delivery of rifampicin microspheres using lower generation polyamidoamine dendrimers as a carrier. Powder Technol. 2016, 291, 366–374. [Google Scholar] [CrossRef]
- Ahmed, R.; Aucamp, M.; Ebrahim, N.; Samsodien, H. Supramolecular assembly of rifampicin and PEGylated PAMAM dendrimer as a novel conjugate for tuberculosis. J. Drug Deliv. Sci. Technol. 2021, 66, 102773. [Google Scholar] [CrossRef]
- Bellini, R.G.; Guimarães, A.P.; Pacheco, M.A.C.; Dias, D.M.; Furtado, V.R.; de Alencastro, R.B.; Horta, B.A.C. Association of the anti-tuberculosis drug rifampicin with a PAMAM dendrimer. J. Mol. Graph. Model. 2015, 60, 34–42. [Google Scholar] [CrossRef]
- Rodrigues, B.; Shende, P. Monodispersed metal-based dendrimeric nanoclusters for potentiation of anti-tuberculosis action. J. Mol. Liq. 2020, 304, 112731. [Google Scholar] [CrossRef]
- Mignani, S.; Tripathi, V.D.; Soam, D.; Tripathi, R.P.; Das, S.; Singh, S.; Gandikota, R.; Laurent, R.; Karpus, A.; Caminade, A.M.; et al. Safe Polycationic Dendrimers as Potent Oral in Vivo Inhibitors of Mycobacterium tuberculosis: A New Therapy to Take down Tuberculosis. Biomacromolecules 2021, 22, 2659–2675. [Google Scholar] [CrossRef]
- Sarkar, B.; Mahapa, A.; Dey, K.; Manhas, R.; Chatterji, D.; Jayaraman, N. Aza-Michael promoted glycoconjugation of PETIM dendrimers and selectivity in mycobacterial growth inhibitions. RSC Adv. 2023, 13, 4669–4677. [Google Scholar] [CrossRef]
- Sharma, R.; Zhang, I.; Shiao, T.C.; Pavan, G.M.; Maysinger, D.; Roy, R. Low generation polyamine dendrimers bearing flexible tetraethylene glycol as nanocarriers for plasmids and siRNA. Nanoscale 2016, 8, 5106–5119. [Google Scholar] [CrossRef]
- Márquez-Miranda, V.; Peñaloza, J.P.; Araya-Durán, I.; Reyes, R.; Vidaurre, S.; Romero, V.; Fuentes, J.; Céric, F.; Velásquez, L.; González-Nilo, F.D.; et al. Effect of Terminal Groups of Dendrimers in the Complexation with Antisense Oligonucleotides and Cell Uptake. Nanoscale Res. Lett. 2016, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Tarach, P.; Janaszewska, A. Recent Advances in Preclinical Research Using PAMAM Dendrimers for Cancer Gene Therapy. Int. J. Mol. Sci. 2021, 22, 2912. [Google Scholar] [CrossRef] [PubMed]
- Luong, D.; Kesharwani, P.; Deshmukh, R.; Mohd Amin, M.C.I.; Gupta, U.; Greish, K.; Iyer, A.K. PEGylated PAMAM dendrimers: Enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery. Acta Biomater. 2016, 43, 14–29. [Google Scholar] [CrossRef]
- Lee, J.M.; Yoon, T.J.; Cho, Y.S. Recent developments in nanoparticle-based siRNA delivery for cancer therapy. Biomed Res. Int. 2013, 2013, 782041. [Google Scholar] [CrossRef]
- Maghsoudnia, N.; Baradaran Eftekhari, R.; Naderi Sohi, A.; Norouzi, P.; Akbari, H.; Ghahremani, M.H.; Soleimani, M.; Amini, M.; Samadi, H.; Dorkoosh, F.A. Mitochondrial delivery of microRNA mimic let-7b to NSCLC cells by PAMAM-based nanoparticles. J. Drug Target. 2020, 28, 818–830. [Google Scholar] [CrossRef] [PubMed]
- Michlewska, S.; Ionov, M.; Maroto-Díaz, M.; Szwed, A.; Ihnatsyeu-Kachan, A.; Loznikova, S.; Shcharbin, D.; Maly, M.; Ramirez, R.G.; de la Mata, F.J.; et al. Ruthenium dendrimers as carriers for anticancer siRNA. J. Inorg. Biochem. 2018, 181, 18–27. [Google Scholar] [CrossRef]
- Inapagolla, R.; Guru, B.R.; Kurtoglu, Y.E.; Gao, X.; Lieh-Lai, M.; Bassett, D.J.P.; Kannan, R.M. In vivo efficacy of dendrimer-methylprednisolone conjugate formulation for the treatment of lung inflammation. Int. J. Pharm. 2010, 399, 140–147. [Google Scholar] [CrossRef]
- Dong, Z.; Hamid, K.A.; Gao, Y.; Lin, Y.; Katsumi, H.; Sakane, T.; Yamamoto, A. Polyamidoamine Dendrimers Can Improve the Pulmonary Absorption of Insulin and Calcitonin in Rats. J. Pharm. Sci. 2011, 100, 1866–1878. [Google Scholar] [CrossRef]
- Royo-Rubio, E.; Rodríguez-Izquierdo, I.; Moreno-Domene, M.; Lozano-Cruz, T.; de la Mata, F.J.; Gómez, R.; Muñoz-Fernández, M.A.; Jiménez, J.L. Promising PEGylated cationic dendrimers for delivery of miRNAs as a possible therapy against HIV-1 infection. J. Nanobiotechnol. 2021, 19, 158. [Google Scholar] [CrossRef]
- Dzmitruk, V.; Apartsin, E.; Ihnatsyeu-Kachan, A.; Abashkin, V.; Shcharbin, D.; Bryszewska, M. Dendrimers Show Promise for siRNA and microRNA Therapeutics. Pharmaceutics 2018, 10, 126. [Google Scholar] [CrossRef]
- Biswas, S.; Torchilin, V. Dendrimers for siRNA Delivery. Pharmaceuticals 2013, 6, 161–183. [Google Scholar] [CrossRef] [PubMed]
- Bohr, A.; Tsapis, N.; Foged, C.; Andreana, I.; Yang, M.; Fattal, E. Treatment of acute lung inflammation by pulmonary delivery of anti-TNF-α siRNA with PAMAM dendrimers in a murine model. Eur. J. Pharm. Biopharm. 2020, 156, 114–120. [Google Scholar] [CrossRef]
- Liu, J.; Gray, W.D.; Davis, M.E.; Luo, Y. Peptide- and saccharide-conjugated dendrimers for targeted drug delivery: A concise review. Interface Focus 2012, 2, 307–324. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Sarmento, B.; Seabra, V. Mannose-functionalized solid lipid nanoparticles are effective in targeting alveolar macrophages. Eur. J. Pharm. Sci. 2018, 114, 103–113. [Google Scholar] [CrossRef]
- Jaynes, J.M.; Sable, R.; Ronzetti, M.; Bautista, W.; Knotts, Z.; Abisoye-Ogunniyan, A.; Li, D.; Calvo, R.; Dashnyam, M.; Singh, A.; et al. Mannose receptor (CD206) activation in tumor-associated macrophages enhances adaptive and innate antitumor immune responses. Sci. Transl. Med. 2020, 12, eaax6337. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Liaw, K.; Sharma, A.; Jimenez, A.; Chang, M.; Salazar, S.; Amlani, I.; Kannan, S.; Kannan, R.M. Glycosylation of PAMAM Dendrimers Significantly Improves Tumor Macrophage Targeting and Specificity in Glioblastoma. J. Control. Release 2021, 337, 179–192. [Google Scholar] [CrossRef]
- Serwer, L.P.; Noble, C.O.; Michaud, K.; Drummond, D.C.; Kirpotin, D.B.; Ozawa, T.; Prados, M.D.; Park, J.W.; James, C.D. Investigation of intravenous delivery of nanoliposomal topotecan for activity against orthotopic glioblastoma xenografts. Neuro. Oncol. 2011, 13, 1288–1295. [Google Scholar] [CrossRef]
- Khan, M.A. Targeted Drug Delivery Using Tuftsin-bearing Liposomes: Implications in the Treatment of Infectious Diseases and Tumors. Curr. Drug Targets 2021, 22, 770–778. [Google Scholar] [CrossRef]
- Siebert, A.; Gensicka-Kowalewska, M.; Cholewinski, G.; Dzierzbicka, K. Tuftsin—Properties and Analogs. Curr. Med. Chem. 2017, 24, 3711–3727. [Google Scholar] [CrossRef]
- Nissen, J.C.; Selwood, D.L.; Tsirka, S.E. Tuftsin signals through its receptor neuropilin-1 via the transforming growth factor beta pathway. J. Neurochem. 2013, 127, 394–402. [Google Scholar] [CrossRef]
- Dutta, T.; Garg, M.; Jain, N.K. Targeting of efavirenz loaded tuftsin conjugated poly(propyleneimine) dendrimers to HIV infected macrophages in vitro. Eur. J. Pharm. Sci. 2008, 34, 181–189. [Google Scholar] [CrossRef]
- Fridkin, M.; Tsubery, H.; Tzehoval, E.; Vonsover, A.; Biondi, L.; Filira, F.; Rocchi, R. Tuftsin-AZT conjugate: Potential macrophage targeting for AIDS therapy. J. Pept. Sci. 2005, 11, 37–44. [Google Scholar] [CrossRef]
- Agarwal, A.; Kandpal, H.; Gupta, H.P.; Singh, N.B.; Gupta, C.M. Tuftsin-bearing liposomes as rifampin vehicles in treatment of tuberculosis in mice. Antimicrob. Agents Chemother. 1994, 38, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, S.P.; Carvalho, K.V.; de Oliveira Aguiar Soares, R.D.; Carneiro, C.M.; de Andrade, M.H.G.; Duarte, R.S.; dos Santos, O.D.H. Functionalized rifampicin-loaded nanostructured lipid carriers enhance macrophages uptake and antimycobacterial activity. Colloids Surf. B Biointerfaces 2019, 175, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Horváti, K.; Bacsa, B.; Kiss, É.; Gyulai, G.; Fodor, K.; Balka, G.; Rusvai, M.; Szabó, E.; Hudecz, F.; Bősze, S. Nanoparticle Encapsulated Lipopeptide Conjugate of Antitubercular Drug Isoniazid: In Vitro Intracellular Activity and in Vivo Efficacy in a Guinea Pig Model of Tuberculosis. Bioconjug. Chem. 2014, 25, 2260–2268. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Zhai, X.; Zhu, K.; Ji, J.; Liu, W. Tuftsin-tailored fusion protein inhibits the growth of circulating gastric tumor cells associated with macrophage phagocytosis. Biochem. Biophys. Reports 2023, 34, 101443. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Amiji, M. Tuftsin-modified alginate nanoparticles as a noncondensing macrophage-targeted DNA delivery system. Biomacromolecules 2012, 13, 1074–1085. [Google Scholar] [CrossRef]
- Khan, A.; Zhang, K.; Singh, V.K.; Mishra, A.; Kachroo, P.; Bing, T.; Won, J.H.; Mani, A.; Papanna, R.; Mann, L.K.; et al. Human M1 macrophages express unique innate immune response genes after mycobacterial infection to defend against tuberculosis. Commun. Biol. 2022, 5, 480. [Google Scholar] [CrossRef]
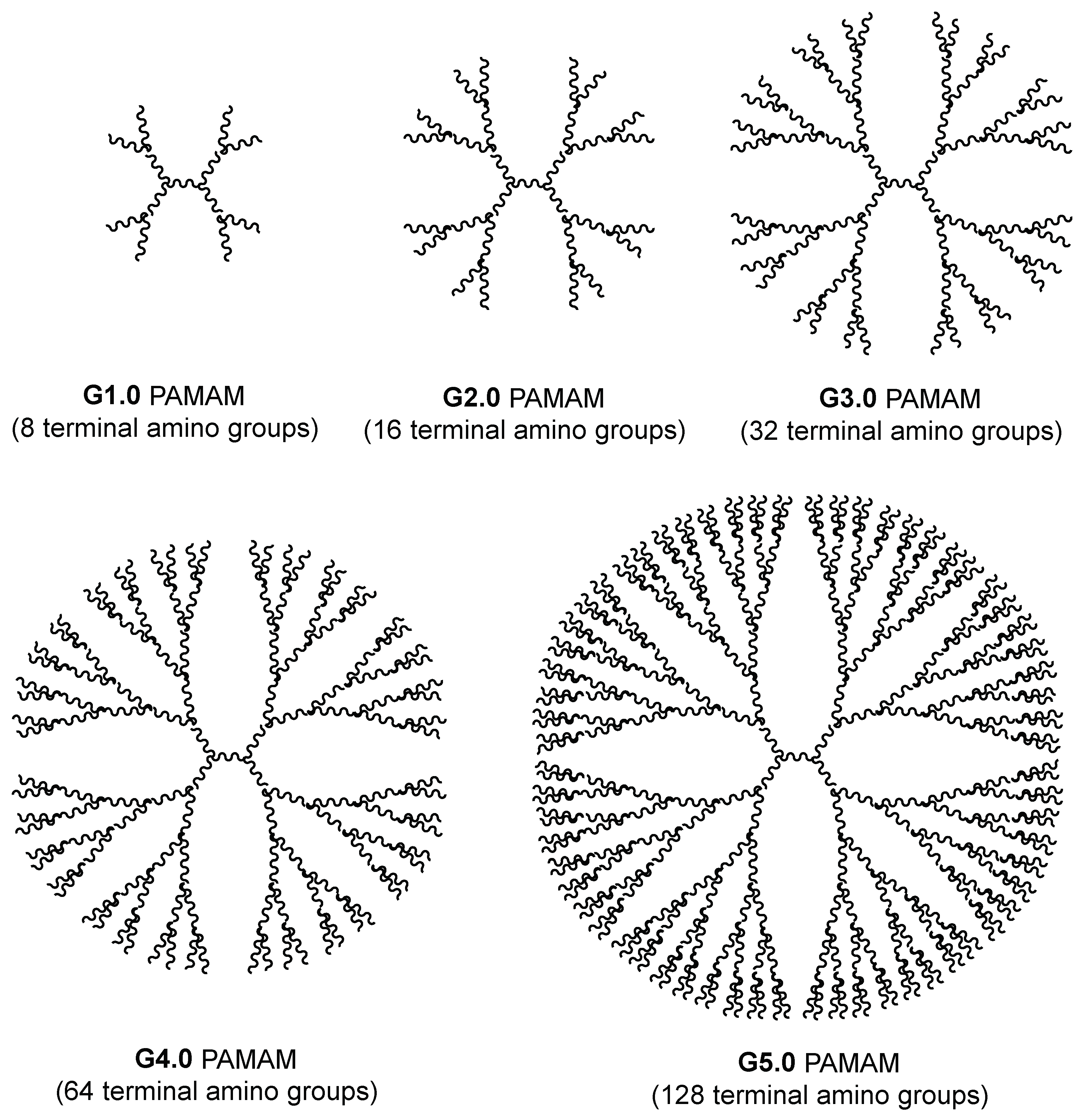
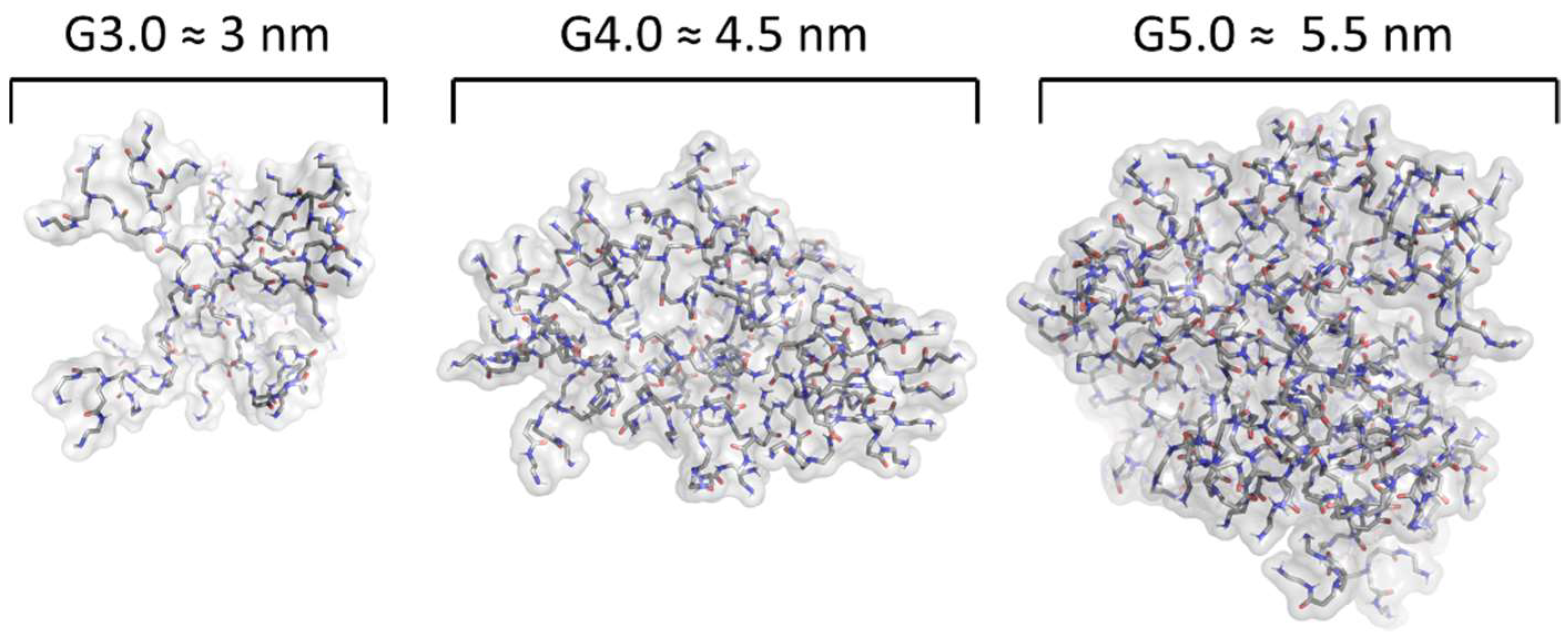
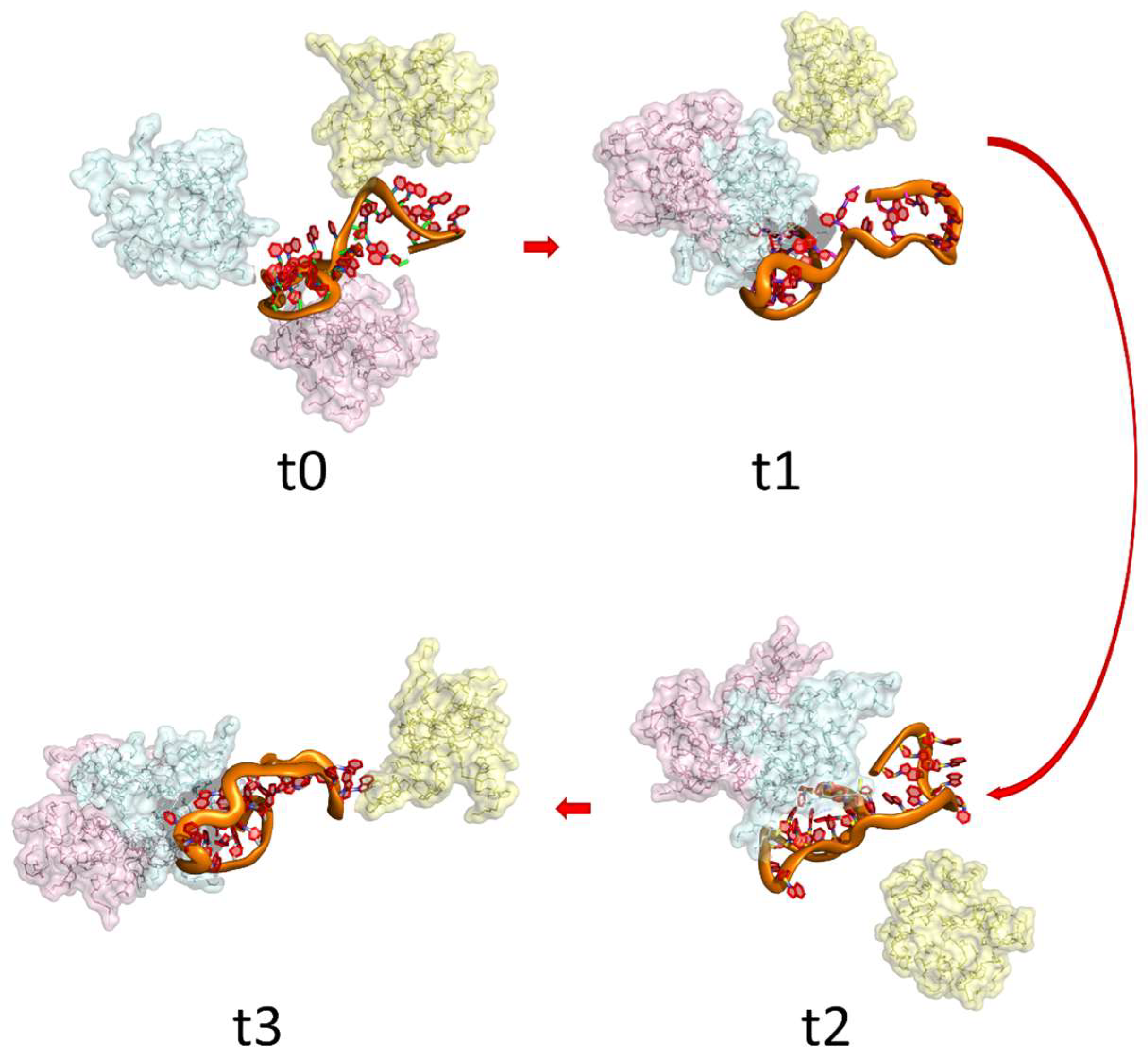

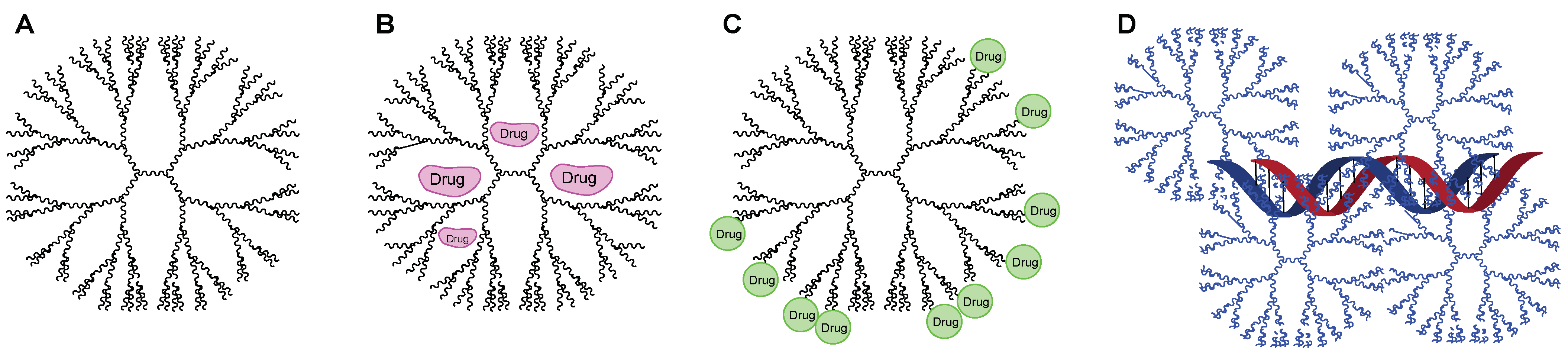
| Anti-TB | Target | Polymers | Generation | Key Findings | Ref. |
|---|---|---|---|---|---|
| Rifampicin | Vero cells (ATCC-CCL-81e) | ethylene diamine, acrylonitrile (mannosylated dendrimer) | G5.0 | The rifampicin-loaded mannosylated dendrimer reduced the drug release rate at pH 7.4, hemolytic toxicity, and cytotoxicity. Conversely, increased drug release at pH 5.0 and alveolar macrophages absorption were observed. | [47] |
| Rifampicin | - | Poly(amidoamine) PAMAM | G4.0 | The results demonstrated the remarkable stability of the rifampicin–PAMAM complex at physiological pH, as well as the rapid release of rifampicin molecules in an acidic environment. This release pattern closely resembles the acidic domains found within macrophages, which are the host cells where Mycobacterium tuberculosis resides. | [50] |
| Rifampicin | Wister rats (male)—in vivo pulmonary drug absorption | Poly(amidoamine) PAMAM | G1.0-G3.0 | The lower generation PAMAM microspheres were found to have a significant impact on the pharmacokinetic parameters of rifampicin, ultimately affecting the bioavailability of the drug. This study identified PAMAM G3 dendritic microspheres as suitable carriers for the pulmonary delivery of rifampicin to lung tissues. | [48] |
| Isoniazid and copper | M. tuberculosis H37Ra (ATCC25177) cells | Poly(amidoamine) PAMAM Methylmethacrylate | G4.0 | The combination of copper and isoniazid showed a synergistic effect against M. tuberculosis H37Ra, resulting in a high inhibition rate of 96% and a significant dose reduction of up to 85 μg/mL. Copper nanoclusters containing isoniazid, synthesized using G4 PAMAM dendrimers, exhibited a controlled release profile with a cumulative drug release of 75% over 24 h. | [51] |
| Rifampicin | Raw 264.7 macrophage cells | Poly(amidoamine) PAMAM Poly(ethylene glycol) (PEG) | G4.0 | The PEGylated G4 PAMAM dendrimers developed in this study are proposed as an ideal drug carrier for rifampicin, offering minimal cytotoxicity, high loading capacity, and extendedrelease characteristics. | [49] |
| Active per se | M. tuberculosis H37Ra M. tuberculosis H37Rv M. bovis BCG Balb/C mice | Polycationic phosphorus | G0.0–G4.0 | The 2G0HCl polycationic phosphorus dendrimer, has shown promise for treating TB based on in vitro and in vitro studies. It is a safe and chemically stable compound that remains intact in aerated aqueous solutions for up to 9 months, which is crucial for its potential use in clinical development. Notably, 2G0HCl exhibits impressive efficacy against single drug-resistant strains of M. tuberculosis H37Rv that are resistant to rifampicin, isoniazid, ethambutol, or streptomycin. In vivo experiments using infected Balb/C mice have demonstrated significant effectiveness in reducing bacterial counts in the lungs when administered orally at a dose of 50 mg/kg once a day for 2 weeks, surpassing the efficacy of ethambutol and rifampicin. | [52] |
| Active per se | M. tuberculosis H37Rv | Glycoconjugated amine-terminated poly(ether imine) (PETIM) | G0.0 to G3.0 | The Glyco-conjugated dendrimers PETIM possess antibacterial activity against M. tuberculosis by inhibiting its growth. The selectivity of the dendrimers towards mycobacterial growth inhibition was attributed to their glycosil moieties. The MIC values for M. tuberculosis H37Rv were found to be 100 mg/mL and 200 mg/mL, for both glycoconjugated G1.0 and G2.0, respectively. | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanhueza, C.; Vergara, D.; Chávez-Aravena, C.; Gálvez-Jiron, F.; Chavez-Angel, E.; Castro-Alvarez, A. Functionalizing Dendrimers for Targeted Delivery of Bioactive Molecules to Macrophages: A Potential Treatment for Mycobacterium tuberculosis Infection—A Review. Pharmaceuticals 2023, 16, 1428. https://doi.org/10.3390/ph16101428
Sanhueza C, Vergara D, Chávez-Aravena C, Gálvez-Jiron F, Chavez-Angel E, Castro-Alvarez A. Functionalizing Dendrimers for Targeted Delivery of Bioactive Molecules to Macrophages: A Potential Treatment for Mycobacterium tuberculosis Infection—A Review. Pharmaceuticals. 2023; 16(10):1428. https://doi.org/10.3390/ph16101428
Chicago/Turabian StyleSanhueza, Claudia, Daniela Vergara, Catalina Chávez-Aravena, Felipe Gálvez-Jiron, Emigdio Chavez-Angel, and Alejandro Castro-Alvarez. 2023. "Functionalizing Dendrimers for Targeted Delivery of Bioactive Molecules to Macrophages: A Potential Treatment for Mycobacterium tuberculosis Infection—A Review" Pharmaceuticals 16, no. 10: 1428. https://doi.org/10.3390/ph16101428
APA StyleSanhueza, C., Vergara, D., Chávez-Aravena, C., Gálvez-Jiron, F., Chavez-Angel, E., & Castro-Alvarez, A. (2023). Functionalizing Dendrimers for Targeted Delivery of Bioactive Molecules to Macrophages: A Potential Treatment for Mycobacterium tuberculosis Infection—A Review. Pharmaceuticals, 16(10), 1428. https://doi.org/10.3390/ph16101428






