Abstract
In the realm of gene therapy, a pivotal moment arrived with Paul Berg’s groundbreaking identification of the first recombinant DNA in 1972. This achievement set the stage for future breakthroughs. Conditions once considered undefeatable, like melanoma, pancreatic cancer, and a host of other ailments, are now being addressed at their root cause—the genetic level. Presently, the gene therapy landscape stands adorned with 22 approved in vivo and ex vivo products, including IMLYGIC, LUXTURNA, Zolgensma, Spinraza, Patisiran, and many more. In this comprehensive exploration, we delve into a rich assortment of 16 drugs, from siRNA, miRNA, and CRISPR/Cas9 to DNA aptamers and TRAIL/APO2L, as well as 46 carriers, from AAV, AdV, LNPs, and exosomes to naked mRNA, sonoporation, and magnetofection. The article also discusses the advantages and disadvantages of each product and vector type, as well as the current challenges faced in the practical use of gene therapy and its future potential.
1. Introduction
Gene therapy has become a rapidly growing field with significant advancements in recent years. This innovative therapeutic approach is revolutionizing the treatment of various diseases, including melanoma, pancreatic cancer, retinal dystrophy, spinal muscular atrophy, polyneuropathy, hereditary transthyretin-mediated amyloidosis, head and neck squamous cell carcinoma, atherosclerotic peripheral arterial disease, critical limb ischemia, nasopharyngeal cancer, SOS/VOD with multiorgan dysfunction, bacillus Calmette–Guérin (BCG)-unresponsive non-muscle invasive bladder cancer, hemophilia B, aromatic L-amino acid decarboxylase deficiency, multiple myeloma, cerebral adrenoleukodystrophy, lymphoma, ADA-SCID, large B cell lymphoma, acute lymphoblastic leukemia, beta-thalassemia, and metachromatic leukodystrophy, by addressing the root cause of the condition—the genetic level. By altering, repairing, or replacing defective genes in a patient’s body, gene therapy aims to restore the normal functioning of cells and tissues. Since the first gene therapy clinical trial in 1989, significant progress has been made in developing and refining the technology, leading to the approval of several gene therapy products for commercial use [1,2].
Delving into the annals of gene therapy’s history unveils a narrative shaped by pioneering discoveries and watershed moments. Frederick Griffith’s 1928 experiment resonates as a seminal exploration, elucidating the transference of genetic information among bacteria through the transformative process. A monumental breakthrough arrived in 1972 with Paul Berg’s revelation of the first recombinant DNA, a milestone that would reverberate across the scientific community. Berg’s paradigm-shifting achievement garnered him the 1980 Nobel Prize in Chemistry, underscoring the transformative power of recombining DNA molecules. In a pivotal moment on 19 January 1989, Dr. James A. Wyngaarden, director of the National Institutes of Health (NIH), granted approval for the inaugural clinical protocol, entailing the integration of a foreign gene into immune cells for cancer patients. On 14 September 1990, another indelible moment occurred, as W. French Anderson and his NIH colleagues conducted the first sanctioned gene therapy procedure, a life-altering intervention for a four year old afflicted with severe combined immunodeficiency (SCID). From 1990 to 2000, the landscape burgeoned with promise as approximately 300 clinical gene therapy trials embraced around 3000 individuals, marking a dynamic era of exploration. The march of progress ventured eastward as China’s State FDA granted approval for Gendicine in 2003 to address carcinoma and Oncorine in 2005 for nasopharyngeal cancer. Russia followed suit, gaining approval for Neovasculgen in 2011 to combat atherosclerotic peripheral arterial disease (PAD). The historical trajectory surged ahead, with the European FDA approving Defibrotide in 2013 for addressing SOS/VOD with multiorgan dysfunction. In 2015, the USA FDA’s imprimatur ushered in a new era with IMLYGIC, ratified to treat melanoma and pancreatic cancer. The progression was resounding, as 2023 bore witness to the culmination of these endeavors, witnessing the approval of 22 gene therapy products by entities such as the USA’s FDA, the EU’s FDA, the Chinese State’s FDA, and the Russian Ministry of Healthcare, emblematic of the multifaceted strides taken to harness the potential of gene therapy on a global scale [3,4,5,6].
Examining gene therapy from a broad perspective involves two critical components: genetic drugs (also referred to as passengers, which are technicians capable of repairing a malfunction) and carriers of the drugs (or vehicles capable of delivering the technicians to the point on the navigational map where the repair job can be performed).
Each drug and carrier have their advantages and drawbacks, and there is no one-size-fits-all solution. Therefore, the more effectively the pros and cons of the combinations of these two elements are used, the more successful the drugs are in reaching their destination and producing beneficial results.
Unfortunately, some combinations of these two elements are unable to reach their intended destination, while others get there, but the cost of repairing becomes prohibitively high, resulting in the rejection of the offer by the market. Only about 11.5% of these combinations succeed after years of experimentation and remedying failures; these are the gene therapy products that have been authorized by government authorities and embraced by the market demand. In this article, we provide an overview of the current state of gene therapy, focusing on approved products (Figure 1) [1,7,8].
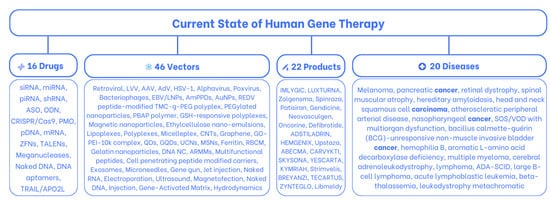
Figure 1.
The current state of human gene therapy: 16 drugs, 46 carriers, and 22 approved products that treat 20 diseases.
2. Sixteen Gene Therapy Drugs
Gene therapy drugs have revolutionized the field of medicine by providing a targeted approach to treating genetic disorders. In this section, and in Table 1, we will provide an overview of various gene therapy drugs that have been developed, their advantages and disadvantages, as well as the challenges faced in their practical use.

Table 1.
Gene therapy drugs.
2.1. Small Interfering RNA (siRNA)
siRNA is a synthetic molecule used to knock down the expression of any gene with a complementary sequence. The molecule works by targeting specific mRNA and cleaving it, preventing it from being translated into protein. Additionally, siRNA can also be used to regulate protein-coding genes and transposons, as well as functioning as an antiviral defense mechanism.
The size of siRNA ranges from 20 to 25 base pairs. One of the key advantages of siRNA is its high specificity due to its 100% complementarity to the target mRNA. This makes it an attractive drug candidate for diseases caused by specific gene mutations. Additionally, siRNA has been shown to be effective in delivering drugs to the brain, a feat that is notoriously difficult to achieve.
However, siRNA therapy also has some limitations. One major concern is off-target effects, which means that the siRNA may unintentionally target genes with similar sequences to the intended target. Another potential issue is innate immunity, which can cause an immune response and limit the effectiveness of the therapy [9,10,11,12,13,14,15].
2.2. MicroRNAs (miRNAs)
miRNAs are short non-coding RNA molecules that regulate gene expression at the post-transcriptional level. They play a significant role in a wide range of cellular processes, including differentiation, apoptosis, and development. In plants, miRNAs and their target mRNA are almost perfectly complementary, making them highly effective. They are involved in developmental timing, tissue growth, and left–right asymmetry in the nervous system. In animals, miRNAs comprise only approximately 1% of all genes, but they play an essential role in regulation, including mRNA degradation, translational repression, and the regulation of protein-coding genes.
MicroRNAs (miRNAs) offer a key advantage in gene therapy due to their small size and manipulability. Moreover, around 12 miRNAs have been identified for suppressing endogenous CFTR mRNA expression in the Caco-2 cell line. CFTR, responsible for the monogenic autosomal recessive cystic fibrosis (CF), impacts 1 in 3500 global live births. Maria V. Esposito et al. [76] examined 706 CF carriers, revealing undiagnosed CFTR-RD among a subset. Genetic testing scanning analysis aids in CFTR-RD identification, offering potential for tailored follow-up and therapies to enhance outcomes.
However, functional duplexes in animals can be more variable in structure than in plants, with only short complementary sequence stretches that may contain gaps and mismatches. Specific rules for functional miRNA–target pairing that capture all known functional targets have not been developed to date [11,16,76,77,78].
2.3. PIWI-Interacting RNAs (piRNAs)
piRNAs are small non-coding RNA molecules that interact with PIWI proteins to repress transposable elements in the genome.
piRNAs are known to have diverse functions such as transcriptional or post-transcriptional repression of transposons and multigenerational epigenetic phenomena in worms. In addition to transposon silencing, pre-pachytene piRNAs also have roles in the formation of the nuage, a perinuclear structure in germ cells.
These RNAs are larger than other small RNAs, typically ranging from 26 to 32 nucleotides in length.
While the exact mechanisms of piRNA biogenesis remain unclear, current models suggest that they are processed from long, single-stranded RNA precursors in a Dicer-independent manner. Studies on piRNAs are still ongoing to understand the full range of their functions and mechanisms of action [15,17].
2.4. Short Hairpin RNA (shRNA)
shRNA is an artificial RNA molecule used for gene silencing via RNA interference. This type of drug contains a hairpin turn that tightly binds to its target gene, leading to suppression of its expression.
shRNAs range in size from 19 to 29 base pairs and have the advantage of being relatively resistant to degradation and turnover, providing long-lasting gene silencing effects.
However, to use shRNA, an expression vector is required, which may cause side effects when used as a medicine.
Despite these limitations, shRNA is considered an effective tool for gene therapy due to its specific targeting ability and long-term effects [18,19,20,21,22].
2.5. Antisense Oligonucleotides (ASOs)
ASOs are a type of drug that have gained significant attention in recent years due to their potential in gene therapy. ASOs are single strands of DNA or RNA that are complementary to a specific sequence of mRNA. They work by binding to the targeted RNA and blocking the translation of certain proteins, thereby modulating gene expression. ASOs are relatively small in size, ranging from 18 to 30 base pairs, which enables them to easily penetrate cell membranes and target both nuclear- and cytoplasmic-located long non-coding RNAs (lncRNAs).
Despite their potential therapeutic benefits, ASOs have several limitations that need to be addressed. One major concern is off-target effects, where ASOs bind to unintended RNA sequences and cause unwanted biological effects. Additionally, ASOs may have insufficient biological activity, limiting their efficacy in gene therapy.
ASOs have shown promising results in clinical trials for treating genetic disorders, such as spinal muscular atrophy and Huntington’s disease, and several ASOs have been approved by the FDA [23,24,25,26,27,28,29].
2.6. Oligodeoxynucleotides (ODNs)
ODNs are synthetic DNA molecules that have shown potential as a gene therapy tool. ODNs work through two main mechanisms: the antisense strategy and the antigene strategy (also known as the decoy strategy). In the antisense strategy, ODNs bind to the targeted mRNA and block protein synthesis, while the antigene strategy involves the use of ODNs to bind to specific transcription factors and inhibit their activity, thus preventing the expression of downstream genes.
One of the benefits of using ODNs is the simplicity of their synthesis and manipulation, as well as the tissue specificity of their target transcription factors. This specificity enables precise targeting of specific genes or cell types, reducing the risk of off-target effects.
However, ODNs also have several limitations that need to be addressed. One major concern is their high rate of degradation by endocytosis or nucleases, which limits their stability and effectiveness. Additionally, their short lifetime may reduce the duration of therapeutic effects.
Despite these limitations, ODNs have shown potential in preclinical and clinical trials for treating various diseases, such as cancer and autoimmune disorders [30].
2.7. Clustered Regularly Interspersed Short Palindromic Repeats (CRISPR)/CRISPR-Associated Protein 9 (Cas9)
In recent years, CRISPR/Cas9 has been making waves in the field of gene therapy. This protein, formerly known as Cas5, Csn1, or Csx12, plays a critical role in the defense of certain bacteria against DNA viruses and plasmids. Its primary function is to cut DNA, which allows for the alteration of a cell’s genome.
One of the biggest advantages of CRISPR/Cas9 is its ease of design. It can be delivered via plasmid or viral vectors, which makes it accessible to many researchers. However, there are some downsides to consider. For example, off-target editing is common without an additional homologous sequence, which can be a challenge.
Another drawback is the requirement for a PAM or Protospacer Adjacent Motif, a short sequence downstream of the target DNA sequence. While this motif is necessary for CRISPR/Cas9 to work, it can also limit the range of targets available for editing [31,32,33,34,35].
2.8. Plasmid DNA (pDNA)
One of the most promising vectors for gene therapy is pDNA. These small, extrachromosomal DNA molecules replicate independently and are physically separated from chromosomal DNA within a cell. pDNA gene therapy has been shown to be particularly effective in the treatment of cardiovascular diseases because it allows for targeted transfer to cardiac or skeletal muscle.
One of the major advantages of using pDNA as a vector is its versatility in size. This allows for a wide range of genes to be delivered using this method. Additionally, plasmids can be engineered to include a variety of promoters and enhancers to increase gene expression in the target tissue.
However, like all gene therapy vectors, pDNA has its drawbacks. One of the biggest concerns is its potential for immunogenicity, which can cause an immune response and limit the effectiveness of the therapy. Therefore, careful consideration must be given to the design and delivery of pDNA to minimize this risk [36,37,38,39,40,41,42,43,44,45].
2.9. Messenger Ribonucleic Acid (mRNA)
mRNA is a promising drug for gene therapy. It is a single-stranded molecule of RNA that corresponds to the genetic sequence of a gene and is read by a ribosome in the process of protein synthesis. By delivering corrected mRNA into cells, they can receive the right blueprint for creating healthy proteins, which can help treat a variety of genetic disorders.
One of the major advantages of mRNA as a drug is its ease of manipulation. It can be rapidly produced and modified to fit specific needs. Additionally, it offers transient expression, meaning protein production is not permanent and can be turned off if necessary. Moreover, mRNA is adaptive and can be converted without mutagenesis.
However, there are some downsides to using mRNA for gene therapy. One of the major drawbacks is its immunogenicity, meaning the body’s immune system may recognize it as foreign and attack it, leading to negative side effects. Additionally, it can be challenging to control the concentration of reporter mRNA, which can lead to unintended effects [46,47,48].
2.10. Meganucleases
Meganucleases are a promising tool for targeted gene editing, characterized by a large recognition site and high specificity. They can be thought of as molecular DNA scissors, capable of replacing, eliminating, or modifying specific sequences in a highly precise manner.
The small size of meganucleases allows for their use with many viral vectors, and they can tolerate some mismatches, resulting in low off-target editing.
However, the design and reengineering of meganucleases for new specificities can be extremely challenging.
Despite these obstacles, recent research has demonstrated the potential of meganucleases to induce homologous recombination in yeast and mammalian cells, highlighting their potential as a tool for precise gene editing in various applications [49,50,51,52,53].
2.11. Zinc Finger Nucleases (ZFNs)
ZFNs are artificial endonucleases that have been developed for targeted gene editing. They are composed of a designed ZFP and the cleavage domain of the FokI restriction enzyme. The ZFP is engineered to recognize and bind to specific DNA sequences, while the FokI domain cleaves the DNA at the target site.
One advantage of ZFNs is that they can tolerate some mismatches, which reduces off-target editing. However, G-rich regions of DNA can be challenging for ZFNs, and their design can be difficult. Multiplexing, or targeting multiple genes at once, is also a challenge with ZFNs [54,55].
2.12. Transcription Activator-like Effector Nucleases (TALENs)
TALENs are a promising gene therapy tool that can be used to cut specific sequences of DNA. TALENs are restriction enzymes that have been engineered to bind and cut DNA in a highly specific manner.
Their size ranges from 32 to 40 base pairs, and they are capable of tolerating some mismatches, resulting in low off-target editing. Additionally, TALENs have moderate design requirements.
However, TALENs do have some limitations. Specifically, they require a 5’ T for each TALEN and are challenging to multiplex. Furthermore, their large size makes it difficult to utilize viral vectors, and repetitive sequences can lead to unwanted recombination [56,57,58,59,60].
2.13. DNA Aptamers
DNA aptamers are short sequences of artificial DNA that can bind specific target molecules with extremely high affinity based on their structural conformations. These aptamers are becoming increasingly popular in various biosensing and therapeutic applications due to their stability and the ease of their generation and synthesis. They are also known for having almost no immunogenicity and for their efficient penetration, lower batch variation, easy modification, cost-effectiveness, and short production times.
DNA aptamers have been compared with other biorecognition elements, such as antibody scFv and antibody Fab’ fragments. They have also been used to select molecules that bind to specific targets, such as gluten, cocaine, and malachite green. In addition, their secondary structural requirements have been investigated through thermodynamic and mutation studies. The 2.8 A crystal structure of the malachite green aptamer and the structural investigations of RNA and DNA aptamers in solution have been described in detail in previous studies [61,62,63,64,65,66,67,68,69,70].
2.14. Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand (TRAIL/APO2L)
TRAIL is a member of the tumor necrosis factor family. Its main function is to induce apoptosis, or programmed cell death, in cancerous cells by binding to the death receptor 4 (DR4) or DR5. Unlike other treatments, TRAIL has the advantage of selectively targeting tumor cells while avoiding side effects in normal tissues. This makes TRAIL a promising therapy for cancer treatment.
One of the most significant benefits of TRAIL is its ability to efficiently kill tumor cells. By activating the apoptotic pathway in cancer cells, TRAIL induces their death, halting their growth and spread. However, some tumor cells are resistant to TRAIL, which limits its effectiveness. The existence of TRAIL-resistant tumor cells remains a challenge that needs to be overcome to maximize the benefits of TRAIL therapy.
Despite the limitations, TRAIL is a promising therapy for cancer treatment due to its selective toxicity towards cancer cells. Researchers are working to overcome the problem of TRAIL resistance by combining it with other treatments such as chemotherapy or radiation therapy. These combinations have shown promising results in clinical trials and may improve the effectiveness of TRAIL therapy in the future.
In conclusion, TRAIL has emerged as a promising therapy for cancer treatment due to its ability to selectively target tumor cells. While the existence of TRAIL-resistant tumor cells limits its effectiveness, ongoing research aims to overcome this challenge and improve the therapeutic potential of TRAIL [71,72].
2.15. Phosphorodiamidate Morpholino Oligomers (PMOs)
PMOs are short, single-stranded DNA analogs built upon a backbone of morpholine rings connected by phosphorodiamidate linkages. These uncharged nucleic acid analogs are designed to bind to complementary sequences of target mRNA through Watson–Crick base pairing, which results in the blocking of protein translation through steric blockade.
PMO oligomers range in size from 6 to 22 base pairs and have been shown to be resistant to a variety of enzymes present in biologic fluids, making them ideal for in vivo applications.
The resistance of PMO to nucleases and other enzymes is a major advantage for their use in gene therapy. By preventing degradation, they can efficiently target and inhibit translation of specific mRNA molecules. PMOs have been used in clinical trials to treat various diseases such as Duchenne muscular dystrophy and spinal muscular atrophy, demonstrating their therapeutic potential [73,74].
2.16. Naked DNA
Naked DNA, which is simply DNA without any associated proteins, has been widely investigated as a gene transfer tool for several tissues including skin, thymus, cardiac muscle, skeletal muscle, and liver cells. This method involves direct injection of DNA into the target tissue, allowing for the transfer of a gene with a size range of 2–19 kb.
Naked DNA-based gene transfer is a safe and straightforward approach, but its application is limited to certain areas such as DNA vaccination. In skeletal muscle, long-term expression has been observed after injection for more than 19 months.
Despite these advantages, the efficiency of naked DNA for gene delivery is low, with less than 1% of total myofibers showing transgenic expression following a single injection. However, repeated injections can improve the overall transfection efficiency, making naked DNA a viable option for certain gene therapy applications [75].
3. Forty-Six Gene Therapy Carriers
Within this section, and subsequently in Table 2, our focal point shall rest upon the second facet of gene therapy, namely carriers, which serve as conduits for the delivery of gene therapy drugs to their designated destinations. We aim to proffer an exhaustive compendium of carriers presently employed within gene therapy practices, accompanied by a thorough discourse on their respective constraints, benefits, drawbacks, and authorized utility within gene therapy products, as well as illustrative instances highlighting their applications.

Table 2.
Gene therapy carriers.
3.1. Viral Vectors
As nature’s original “gene therapists”, viruses possess the necessary tools for efficient cellular entry and dissemination of their genetic payloads. Scientists have taken advantage of this natural capability and repurposed viruses for human gene therapy by replacing their original genetic material with therapeutic gene therapy drugs. This process has led to the development of numerous viral vectors that are now used in gene therapy. In the following sections, we will explore some of the most widely used viral vectors for gene therapy.
3.1.1. Retroviral
Ex vivo gene therapy is a process of genetically modifying cells outside of the body and then returning them back to the patient. Retroviral vectors are one of the commonly used viral vectors for ex vivo gene therapy, and they have been used in various clinical trials. Retroviruses are small RNA viruses that replicate through a DNA intermediate. These vectors are suitable for ex vivo gene therapy, such as transducing CD34+ bone marrow hematopoietic stem cells or peripheral blood lymphocytes. The retroviral vector has a size limit of 10 kb, which means that it can deliver genetic material of a limited size.
However, retroviral vectors have some limitations. They have a low vector titer, a low in vitro transfection efficiency, particle instability, and difficulty in concentrating. Moreover, they cannot transduce non-dividing post-mitotic cells, and their particles can only infect proliferating cells. This can limit the therapeutic application of these vectors.
Despite these limitations, retroviral vectors have been used in several approved gene therapies. Invossa (TissueGene-C or Tonogenchoncel-L), YESCARTA (axicabtagene ciloleucel), Zalmoxis (Allogenic T cells encoding LNGFR and HSV-TK), Strimvelis (GSK-2696273), and TECARTUS (brexucabtagene autoleucel) are examples of retroviral vector-based approved products. The targets of these products include growth factor β1 (TGF β1), ΔLNGFR and HSV-TK Mut2, ADA cDNA, Anti-CD19 CAR gene, and Cyclin G1.
In summary, retroviral vectors have shown their potential in ex vivo gene therapy, especially for transducing hematopoietic stem cells or peripheral blood lymphocytes. However, a low efficiency and limitations of retroviral vectors have also been reported [75,79,80].
3.1.2. Lentiviral Vector (LVV)
LVVs are a type of viral vector that is spherical and composed of single-stranded RNA. These vectors are commonly used in ex vivo gene therapy applications for T cells. The LVVs have a higher limit of 8 kb, making them suitable for larger genes. One of the key advantages of LVVs is their ability to persistently transfer genes in dividing cells. However, they may also pose a safety risk as they can lead to unnecessary non-target insertion mutations.
LVVs have the ability to transduce both dividing and non-dividing cells, which can be beneficial in some applications but may lead to oncogenesis in others. Despite the potential risks, there are several approved gene therapy products that utilize LVVs. These include ABECMA (idecabtagene vicleucel), CARVYKTI (ciltacabtagene autoleucel), SKYSONA (elivaldogene autotemcel), Kymriah (tisagenlecleucel CTL 019), BREYANZI, and ZYNTEGLO (betibeglogene autotemcel). The approved products use LVVs to deliver genes such as a chimeric antigen receptor (CAR), anti-B cell maturation antigen (BCMA), ABCD1 gene, and Beta-A-T87Q-globin [75,80].
3.1.3. Adeno-Associated Virus (AAV)
Adeno-associated virus (AAV) is a non-enveloped, small, double-stranded DNA virus that has gained popularity as a vector for gene therapy. AAV is a good choice for in vivo gene therapy as it has good biological characteristics and is non-immunogenic and non-pathogenic. AAV has a high gene transduction efficiency and large-scale ease of use, which has led to its use in a range of somatic gene therapy applications.
One of the limitations of AAV is its packaging capacity, which is limited to 4.5 kb. However, AAV is known for its genetic stability, making it an attractive vector for gene therapy. The production process for AAV is complicated and expensive, which may be a barrier to its wider use.
Despite these challenges, there have been several approved products that use AAV as the vector for somatic gene therapy, including LUXTURNA and Zolgensma. LUXTURNA targets the RPE65 gene to treat inherited retinal dystrophy, while Zolgensma targets the SMN1 gene in motor neurons to treat spinal muscular atrophy [75,80,81].
3.1.4. Adenovirus (AdV)
Adenovirus (AdV) is an in vivo somatic viral vector with an icosahedral nucleocapsid structure.
It is easy to purify and has genetic stability and a large foreign gene loading capacity of up to 48 kb, making it efficient for transduction in most tissues.
Gendicine and Oncorine are two approved AdV-based gene therapies targeting the Tp53 tumor-suppressor gene.
However, AdV may initiate a strong inflammatory response, which is a major limitation of its use [7].
3.1.5. Herpes Simplex Virus Type 1 (HSV-1)
Herpes simplex virus type 1 (HSV-1) is a viral vector that has shown great promise for somatic gene therapy in vivo. With a packaging capacity of up to 40 kb (replication defective) and 150 kb (amplicon), HSV-1 can accommodate large transgene cassettes, making it an attractive option for gene therapy. In addition, the strong tropism of HSV-1 for neurons makes it an ideal candidate for therapy of central nervous system diseases.
However, the use of HSV-1 as a vector has some limitations. It may initiate a strong inflammatory response, which can be a concern for safety in some cases. Transient gene expression in cells other than neurons is also a limitation. Despite these limitations, the use of HSV-1 as a vector has been approved for certain products, including IMLYGIC (Talimogenelaherparepvec).
IMLYGIC is a modified HSV-1 vector encoding the human granulocyte-macrophage colony-stimulating factor (GM-CSF) gene, which has been approved for the treatment of advanced melanoma [80,82].
3.1.6. Alphavirus
Alphaviruses are single-stranded RNA viruses that have been used in gene therapy as viral vectors for in vivo somatic cell delivery. These enveloped alphavirus particles are made up of a protein capsid structure surrounded by spike membrane proteins. They recognize surface proteins such as laminin and heparin receptors on mammalian and insect cells, delivering the RNA genome to the cell cytoplasm for immediate RNA replication.
One of the advantages of using alphavirus vectors is their broad host range and high level of transient heterologous gene expression. However, the production of expensive virus stocks and the highly transient nature of heterologous gene expression are some of the disadvantages. The limit of alphavirus vectors is less than 12 kb [83,84].
3.1.7. Poxvirus
Poxviruses are a class of double-stranded DNA viruses that belong to the family Poxviridae.
They have been used as viral vectors for gene therapy due to their ability to induce long-lasting immunological responses. In nature, vertebrates and arthropods serve as natural hosts for this virus. With 83 species currently identified, divided among 22 genera and two subfamilies, poxviruses have shown great potential as oncolytic therapeutics for various cancer types, with some successes observed in phase I/II clinical trials. The maximum cargo capacity of poxviral vectors is limited to 500–1000 bp.
However, the therapeutic potential of most poxviruses has yet to be fully characterized and developed [85,86,87,88].
3.1.8. Bacteriophage
Bacteriophages, also known as phages, are a type of viral vector that have become increasingly popular in gene therapy. These viruses infect and replicate only in bacterial cells and are the most abundant biological agent on Earth. They consist of a nucleic acid genome encased in a shell of phage-encoded capsid proteins.
Bacteriophages are highly specific, making them ideal for targeted therapy. They are also easy and inexpensive to propagate, highly stable, and can be used to package non-phage nucleic acid, proteins, or other types of materials. Additionally, they are easy to modify and are GRAS agents.
There are various types of bacteriophages with different genome sizes, including 5 Kbp, 49 Kbp, 40 Kbp, and 160 Kbp–250 Kbp. Their small size makes them useful for gene therapy applications that require small vectors [89,90,91,92,93,94,95].
3.1.9. Epstein–Barr Virus (EBV), Human Gammaherpesvirus 4
The Epstein–Barr virus (EBV), also known as human gammaherpesvirus 4, is a double-stranded DNA virus with a limit of 172 Kbp.
One of the advantages of using the EBV vector is that it provides both long-term and timely controlled exogenous gene expression in neurons. Additionally, the vector remains in the nucleus as an episome and does not integrate into the host genome. This feature helps reduce the risk of insertional mutagenesis, a common concern in gene therapy [96,97,98,99,100].
3.2. Non-Viral Vectors
The development of non-viral vectors for gene therapy has been a major focus in recent years in order to address the limitations of viral vectors, such as their high cost of production, complex manufacturing processes, potential for inducing inflammatory responses, etc. Non-viral vectors offer a more affordable, simpler, and effective alternative. Numerous non-viral vectors have been created, each with unique advantages and disadvantages. In the following section, we will explore some of the most notable non-viral vectors used in gene therapy.
3.2.1. Lipid Nanoparticles (LNPs)
Lipid nanoparticles (LNPs) are a type of non-viral vector that are formed by the orientation of phospholipid biomolecules. These biomolecules can encapsulate both fat-soluble and water-soluble drugs, and deliver genetic drugs to the body through fusion with cell membranes. LNPs have a limit of approximately 22 base pairs and have several advantages such as good biocompatibility, reduced drug toxicity, drug resistance, and endosomal escape facilitation. These characteristics make LNPs an attractive option for gene therapy [75,101].
3.2.2. Amphiphilic Phospholipid Peptide Dendrimers (AmPPDs)
Non-viral vectors, such as amphiphilic phospholipid peptide dendrimers (AmPPDs), have been extensively studied in recent years due to their potential as effective siRNA delivery systems for anticancer therapy. AmPPDs are capable of compacting siRNA into nanoparticles to protect it from enzymatic degradation. This vector bears the natural lipid derivative DSPE as the hydrophobic tail and different dendritic l-lysine as the hydrophilic head, with a limit of about 13 kDa.
One of the advantages of AmPPDs is their simplicity of synthesis and versatility of functionalization. However, they also have some limitations.
One drawback is their relatively low loading capacity for siRNA, which may limit their efficacy in some applications. Another potential issue is the premature leakage of payloads, which can lead to non-specific effects and potential toxicity [102].
3.2.3. Gold Nanoparticles (AuNPs)
Gold nanoparticles (AuNPs) have become a promising non-viral vector in gene therapy, with advantages including facile surface modification and unique optical properties. These inorganic biomaterials can be synthesized and modified with chemical and biological molecules, making them a versatile tool for drug delivery and molecular diagnostics.
The surface of AuNPs supports the efficient attachment of various biomacromolecules via chemisorption, chemical conjugation, and electrostatic interactions. In addition, their small size of 1–100 nm and non-toxicity make them an attractive option for use in gene therapy.
AuNPs have been extensively used as drug carriers for the intracellular delivery of therapeutics and as molecular nanoprobes for the detection and monitoring of target molecules of interest [103,104,105,106,107,108].
3.2.4. REDV Peptide-Modified TMC-g-PEG Polyplex
The REDV peptide-modified TMC-g-PEG polyplex is designed to target vascular endothelial cells (VECs) and deliver miRNA-126 to these cells. The vector is composed of a short peptide, Arg-Glu-Asp-Val (REDV), linked to trimethyl chitosan (TMC) via a bifunctional poly(ethylene glycol) (PEG) linker.
This complex has potential as a miRNA carrier for rapid endothelialization in artificial blood vessels [109].
3.2.5. PEGylated Nanoparticles
PEGylated nanoparticles are a non-viral vector, formed by coating the surface of nanoparticles with polyethylene glycol (PEG), a process known as “PEGylation”. PEGylation has several advantages over traditional viral vectors, including an improved systemic circulation time and decreased immunogenicity.
By shielding the surface of the nanoparticles from aggregation, opsonization, and phagocytosis, PEGylation can prolong the systemic circulation time, allowing for more efficient delivery of therapeutic genes. This technology has shown great promise in preclinical studies and is currently being tested in clinical trials for a variety of diseases, including cancer, cystic fibrosis, and cardiovascular disease [110].
3.2.6. PBAT (Polybutylene Adipate Terephthalate) Polymer
PBAT (polybutylene adipate terephthalate) polymer is a biodegradable random copolymer consisting of adipic acid, 1,4-butanediol, and terephthalic acid, and has shown great potential for both in vitro and in vivo gene delivery.
One of the major benefits of PBAT is its biodegradability. Unlike other non-viral vectors, PBAT is fully biodegradable, making it an attractive option for gene therapy applications. In addition, PBAT has demonstrated a high transfection efficiency and low cytotoxicity when delivering a variety of genetic material, including DNA, mRNA, and Cas9 RNP.
PBAT’s ability to deliver genetic material with high transfection efficiency and low cytotoxicity is attributed to its unique structure. The random copolymer has a hydrophobic core and a hydrophilic shell, which allows it to encapsulate the genetic material and protect it from degradation while also promoting cell uptake [111,112,113,114,115,116].
3.2.7. GSH-Responsive Polyplexes
GSH-responsive polyplexes are an exciting non-viral vector for gene therapy that show great promise in delivering genetic biomacromolecules. These polyplexes are capable of forming electrostatic interactions with negatively charged DNA, mRNA, and Cas9/sgRNA ribonucleoprotein (RNP), allowing for efficient cellular uptake, endosomal escape, and cytosol unpacking of payloads. Furthermore, this delivery system is universal and glutathione-responsive, making it highly adaptable for a wide range of therapeutic applications.
In vitro studies have shown a relatively high transfection efficiency with low cytotoxicity, making GSH-responsive polyplexes an excellent alternative to viral vectors. Additionally, the convenient surface functionalization of these polyplexes makes them easy to modify for specific applications [117].
3.2.8. Magnetic Nanoparticles
Magnetic nanoparticles, a type of non-viral vector, have garnered considerable interest in recent years for their potential in gene delivery. These nanoparticles consist of a magnetic material, typically iron, nickel, or cobalt, and a functional chemical component. Magnetic nanoparticles are intrinsically biocompatible, and their magnetic moments can be controlled using externally applied magnetic fields to leverage their nanoscale behavior. Additionally, magnetic nanoparticles are relatively easy to synthesize and modify, making them an attractive option for gene therapy applications.
One potential application of magnetic nanoparticles in gene therapy is their use in targeted delivery to specific cells or tissues. By functionalizing the surface of magnetic nanoparticles with targeting moieties, such as antibodies or peptides, these particles can be directed to specific cell types or even individual cells within a tissue. This can increase the specificity and efficacy of gene therapy while minimizing off-target effects.
Another potential advantage of magnetic nanoparticles is their ability to penetrate barriers such as the blood–brain barrier, allowing for non-invasive gene delivery to the central nervous system. However, further research is needed to fully understand the potential of magnetic nanoparticles in gene therapy and to optimize their delivery efficiency and safety [118,119,120,121,122,123,124].
3.2.9. Ethylcellulose Nano-Emulsions
Ethylcellulose nano-emulsions can be prepared using a low-energy approach with aqueous components, resulting in folate–ethylcellulose nanoparticle complexes.
Ethylcellulose is an abundant and biocompatible material, listed by the Food and Drug Administration as “generally recognized as safe” (GRAS). The surface charge of these nanoparticles can be adjusted to be either positive or negative, allowing for optimal interaction with the desired genetic biomacromolecule. This versatility makes ethylcellulose nano-emulsions an attractive candidate for gene delivery in various applications [125].
3.2.10. Lipoplexes
Lipoplexes are a class of non-viral vectors used in gene therapy that have attracted attention due to their ability to effectively deliver genetic material into target cells. These vectors consist of cationic lipids that are amphiphilic in nature, meaning they have both hydrophilic and hydrophobic regions. Typically, a charged cationic headgroup is attached via a linker, such as glycerol, to a double hydrocarbon chain or cholesterol derivative. Lipoplexes can be used both in vitro and in vivo for gene delivery applications.
One of the main advantages of lipoplexes is that they are relatively easy to produce compared to viral vectors. In addition, they do not induce a host inflammatory or immune response, which can be a significant advantage for gene therapy applications.
Despite these benefits, there are still challenges associated with the use of lipoplexes, including concerns about their stability and efficiency in delivering genetic material to target cells [126,127].
3.2.11. Polyplexes
Non-viral vectors such as polyplexes can overcome some of the limitations of viral vectors, such as safety concerns and host immune responses. Polyplexes consist of cationic polymers and genes, which form nanoscale complexes through electrostatic interactions.
These complexes have shown high versatility, low toxicity, low immunogenicity, and biodegradability. Furthermore, polyplexes are able to form complexes with small RNAs, leading to RNA protection, cellular delivery, and intracellular release [128,129].
3.2.12. Micelleplexes
Micelleplexes, denoting nanostructured NA/micelle-like complexes, are characterized by their distinctive attributes forged by the chemical composition of amphiphilic copolymers. These copolymers showcase regions that are both cationic and hydrophilic/hydrophobic, thereby facilitating the compaction of nucleic acids through interaction with one or more cationic constituents.
The virtues of micelleplexes extend to proficient binding, conveyance, and precision delivery of nucleic acids to neoplastic cells. These micelleplexes notably outperform their polyplex counterparts in the domains of gene silencing, cellular internalization, toxicity mitigation, colloidal stability enhancement, and payload trafficking efficiency.
Nevertheless, the progressive landscape of innovation is attended by a dualistic complexity, where nanosystems characterized by an excessive positive charge density beckon forth discerning considerations concerning their in vivo toxicity potential, thereby warranting scrupulous evaluation [130,131].
3.2.13. Carbon Nanotubes (CNTs)
Carbon nanotubes (CNTs) are non-viral vectors with unique physical and chemical properties. These allotropic forms of carbon are cylinder-shaped and can be produced through chemical vapor deposition. CNTs have a high surface area, a needle-like structure, considerable strength, flexible interaction with cargo, high drug loading capacity, and outstanding optical and electrical features, making them promising for targeted gene delivery. Additionally, CNTs have high stability, biocompatibility, and the ability to release therapeutic agents at targeted sites.
However, one important drawback is the concern over nanotoxicology. Further studies are necessary to fully understand the potential risks and benefits of using CNTs as a gene delivery system [132].
3.2.14. Graphene
Graphene is a non-viral vector that has been widely studied for its potential use in gene therapy. As a single layer of carbon atoms arranged in a hexagonal lattice, graphene is incredibly thin, lightweight, and strong. However, its use as a gene therapy vector is not without challenges.
One major concern is its potential toxicity, as well as the risk of inappropriate release of therapeutic agents.
Despite these challenges, the unique properties of graphene have led researchers to explore its use as a gene delivery vehicle. Its high surface area, flexibility, and biocompatibility make it an attractive option for delivering therapeutic agents to target cells. Additionally, its ability to penetrate cell membranes and interact with DNA makes it a promising tool for gene therapy [133,134].
3.2.15. GO-PEI-10k Complex
The GO-PEI-10k complex has gained attention for its low cytotoxicity and high transfection efficiency. This complex is formed by binding GO with a cationic polymer, polyethyleneimine (PEI), with two different molecular weights of 1.2 kDa and 10 kDa. The resulting complex is stable in physiological solutions and has been shown to exhibit a significantly reduced toxicity to treated cells compared to the bare PEI-10k polymer.
Further studies have demonstrated the potential of GO–PEI complexes to bind with pDNA for intracellular transfection of genes. In particular, the GO-PEI-10k complex has been shown to efficiently transfect the enhanced green fluorescence protein (EGFP) gene in HeLa cells [134].
3.2.16. Quantum Dots (QDs)
Quantum dots (QDs) have been proposed as an innovative type of non-viral vector in gene therapy. These semiconductor nanocrystals have unique electronic and optical properties that are different to the bulk material thanks to quantum mechanics. QDs have a narrow emission peak, a size-dependent emission wavelength, and a broad excitation range that could be exploited for several biomedical applications such as molecular imaging, biosensing, and diagnostic systems.
One significant advantage of QDs is that they are biocompatible, stable, and soluble in the biomatrix. However, like many other nanomaterials, QDs also have some potential toxicity concerns that should be taken into account [135,136].
3.2.17. Graphene Quantum Dots (GQDs)
Graphene quantum dots (GQDs) are nanoparticles of graphene with sizes smaller than 100 nm that exhibit low toxicity, stable photoluminescence, and excellent chemical and thermal stabilities. They also have a pronounced quantum confinement effect, which makes them suitable for biological, opto-electronic, energy, and environmental applications.
As a non-viral vector, GQDs have been found to have dual functions for gene delivery and nuclear targeting. They are nontoxic carriers that can effectively deliver therapeutic genes to the target cells, making them an attractive option for gene therapy. Additionally, GQDs can be modified to enhance their stability and biocompatibility, further increasing their potential as a gene delivery vector [137,138,139,140,141,142].
3.2.18. Up-Conversion Nanoparticles (UCNs)
These optical nanocrystals are doped with lanthanide ions and can convert low-energy near-infrared (NIR) light into high-energy visible or ultraviolet light, which makes them a photoactive delivery platform for gene carriers. UCNs have unique properties such as a high quantum yield, high stability, and low photobleaching, which make them attractive for biomedical applications. Additionally, surface modifications can be made to improve UCNP-based gene carriers, allowing for an improved biological efficiency [143].
3.2.19. Mesoporous Silica Nanoparticles (MSNs)
Mesoporous silica nanoparticles (MSNs) are an attractive vector for gene therapy due to their tunable size, biocompatibility, and high surface area. The porous structure of MSNs provides a large surface area, allowing functional groups to be attached to the particle surface. MSNs offer easily tunable particle and pore sizes, greater surface areas, and a simple mesoporous or hollow structure, making them ideal for drug delivery applications.
Additionally, MSNs are stimuli-responsive, with pH-sensitive and redox-sensitive drug release profiles. Although MSNs have been mainly tested in vitro, recent studies have also demonstrated their efficacy in vivo [144,145,146].
3.2.20. Ferritin
Ferritin, a spherical nanocage formed by the self-assembly of heavy and light polypeptide chains, is a promising non-viral vector for gene therapy due to its natural transport function for metals and versatility in cargo loading. Ferritin has a small and uniform size, limited to 8 nm in diameter, which allows for efficient cellular uptake and intracellular trafficking. It is also highly stable under various conditions, including high pHs and temperatures.
Additionally, ferritin can be visualized using magnetic resonance imaging (MRI) for in vivo tracking purposes.
However, it is important to note that ferritin can elicit robust humoral immunogenicity, which may limit its application in certain gene therapy contexts [147,148,149,150].
3.2.21. Red Blood Cell Membrane (RBCM)
The Red Blood Cell Membrane (RBCM) is a non-viral vector that has gained attention for its biocompatibility, biodegradability, and long circulating half-life. Erythrocytes, or RBCs, are the most abundant circulating cells in the blood, and have been widely used in drug delivery systems (DDSs). By using a “camouflage” consisting of erythrocyte membranes, nanoparticles can imitate RBCs and achieve long-term circulation in the blood of animal models. This approach combines the advantages of native erythrocyte membranes with those of nanomaterials.
Coating nanoparticles with RBC membranes has several advantages. Firstly, they are able to escape the immune system and achieve long-term circulation in the body. Secondly, they have inherent biocompatibility and biodegradability and can avoid some of the toxicities associated with other nano-preparations. Additionally, RBCs have a lifespan of up to 120 days, allowing for sustained release of cargo. Furthermore, the large quantities of cell membranes make it easy to achieve a high load capacity, while also improving nanoparticle stability, enhancing in vitro storage time, and discouraging aggregation [151].
3.2.22. Gelatin Nanoparticles
Gelatin nanoparticles have shown great potential due to their excellent immune evasion capabilities. One of the advantages of these nanoparticles is their susceptibility to hydrolysis by broad-spectrum bacterial gelatinases, which can convert them into small biomolecules. This property can be advantageous in cases where the nanoparticles need to be rapidly degraded and cleared from the body. Furthermore, the biodegradable and biocompatible nature of gelatin makes it an attractive choice for therapeutic applications [151].
3.2.23. DNA Nanoclews (NCs)
DNA nanoclews (NCs) are made using rolling circle amplification (RCA) and have a uniform size, high biodegradability, and spatial addressability. They are particularly effective at escaping endosomes due to the use of PEI, which coats the Cas9/sgRNA/NC complex.
While DNA NCs are still in the early stages of development, studies have shown that they can be used in both in vitro and in vivo settings. In fact, these vectors have been used to treat diseases such as cystic fibrosis and HIV. In addition, they are easy to synthesize and can be modified to target specific cells or tissues.
One of the key advantages of DNA NCs is their uniform size, which allows for better control over their delivery and release. This is important because it can reduce toxicity and increase the efficiency of the treatment. Additionally, DNA NCs have been shown to be highly biodegradable, meaning they are easily broken down by the body and do not accumulate in tissues.
Another important advantage of DNA NCs is their spatial addressability, which refers to their ability to be modified with various functional groups. This allows them to be targeted to specific cells or tissues, which can improve the efficiency and effectiveness of the therapy. Finally, DNA NCs have been shown to be effective at escaping endosomes, which is a critical step in delivering the therapeutic payload to the target cell [152,153,154].
3.2.24. Arrestin Domain-Containing Protein 1 (ARRDC1)-Mediated Microvesicles (ARMMs)
Arrestin domain-containing protein 1 (ARRDC1)-mediated microvesicles (ARMMs) are capable of delivering various macromolecules, including the tumor suppressor p53 protein, RNAs, and the genome-editing CRISPR-Cas9/guide RNA complex, both in vitro and in vivo. ARMMs are particularly attractive for gene therapy due to their lipid bilayer membrane which protects the enclosed cargo from degradation by proteases or nucleases and also shields them from being recognized as foreign antigens by the immune system. ARMMs have demonstrated high efficiency in targeted gene delivery with minimal off-target effects.
ARMMs are produced through the ARRD1 protein, which packages the desired cargo into small vesicles that are transported out of the cell. These vesicles can then be taken up by other cells where the contents can be released to perform their therapeutic function.
One of the major advantages of using ARMMs for gene therapy is their ability to target specific tissues or organs. This feature could potentially reduce side effects associated with current gene therapy approaches [155,156].
3.2.25. Multifunctional Peptides
Multifunctional peptides are able to serve as building blocks for self-assembling nanoscale structures. Peptides can be rationally designed to target molecular recognition sites, and their biocompatibility and biodegradability make them an attractive option. Moreover, peptides offer the possibility of endless combinations and modifications of amino acid residues, allowing for the assembly of modular, multiplexed delivery systems [157].
3.2.26. Cell-Penetrating Peptide (CPP), Cell-Permeable Peptides, Protein Transduction Domains (PTDs)
Cell-penetrating peptide (CPP)-modified carriers are short peptides that facilitate cellular uptake of various molecular cargo, including DNA, by crossing cell membranes. These carriers typically consist of small peptide domains of less than 40 amino acids, which have the ability to penetrate cells easily. CPPs recognize specific types of cells and have been shown to translocate across their cellular membranes.
One of the major advantages of CPP-modified carriers is their specificity in targeting certain types of cells. This specificity is derived from the amino acid sequence of the CPP, which determines its binding and internalization properties. In addition, CPPs can translocate across cellular membranes without the need for any additional endosomal escape mechanism, which can further enhance gene delivery efficiency.
CPP-modified carriers have been used for both in vitro and in vivo applications in gene therapy. They have been successfully employed to deliver various types of genetic material, such as DNA, RNA, and proteins, to a wide range of cell types. These carriers can also protect the genetic material from degradation and clearance, leading to sustained gene expression [158,159].
3.2.27. Exosomes (Endogenous Nanocarriers)
Exosomes are secreted nanoparticles that can be defined by their size, surface protein, and lipid composition. They are produced by many types of cells and can be harvested from various bodily fluids. One of the advantages of exosomes is that they can be tailored for specific therapeutic purposes. Exosomes can be derived from different cell types, such as immune cells, stem cells, and cancer cells, to achieve specific effects.
Additionally, exosomes can be loaded with various therapeutic cargoes, including small molecules, RNA, and proteins. Targeting peptides can also be added to exosomes to enhance their specificity for particular cells or tissues. Moreover, the method of loading and the administration route can be optimized to improve the efficiency and specificity of exosome-mediated delivery [160].
3.3. Physical Delivery
In the world of gene therapy, in addition to the therapeutic genes and their carriers, there are also physical delivery methods that can enhance the efficiency and effectiveness of gene delivery. We can think of these physical vectors as accelerators that improve the performance of the gene therapy vehicles. Some of these physical vectors can even act as gene delivery vehicles themselves. Let us explore the various physical vectors used in gene therapy to learn more about their unique characteristics and advantages.
3.3.1. Microneedles (MNs)
Microneedles (MNs) are a promising tool in gene therapy that can penetrate the stratum corneum, which is the main barrier for drug delivery through the skin. There are different types of MNs, such as metal MNs, coated MNs, and dissolving MNs. MNs have been shown to be effective in delivering both low-molecular-weight and high-molecular-weight agents, including nucleic acids, with ease of administration and without significant pain. This pain-free and patient-friendly feature of MNs lends it the potential for self-administration. Moreover, the low production cost of MNs makes it an attractive option for marketing. MNs have been used for the transdermal delivery of siRNA, low-molecular-weight drugs, oligonucleotides, DNA, peptides, proteins, and inactivated viruses.
However, MNs do have some limitations. The application site is limited to areas such as the arms, hands, and abdomen. Additionally, the duration time for application is critical since the microneedles dissolve completely in 20 min. Although MNs may not be better than intradermal injection alone, the combination of MNs and electroporation has been found to be more effective [161].
3.3.2. Gene Gun (Biolistic Particle Delivery System)
The gene gun, also known as a biolistic particle delivery system, is a technique used to incorporate DNA or RNA into cells that are typically difficult to transfect using traditional methods. This approach involves using microparticles, tiny projectiles with a diameter of about 1 μm, which are propelled into tissues by pressurized gas. This method is gaining popularity due to its high efficiency in delivering DNA into cells.
However, there is a possibility of significant tissue damage during the process. Despite these limitations, biolistic transfection has shown promising results in inducing immune responses against infectious diseases and cancer [162].
3.3.3. Jet Injection
Jet injection is a physical delivery method that uses high pressure to force microdroplets of liquid to penetrate the skin to deliver a drug or vaccine into intradermal, subcutaneous, or intramuscular tissues. Unlike needle-based injections, jet injection minimizes patient discomfort and eliminates the risk of needlestick injury. A high-pressure liquid stream is used to pierce the skin or target tissue, with the pressure reaching up to 3–4 bar and the velocity of the droplets ranging from 100 ms−1 to 200 ms−1.
Jet injection has been used in DNA vaccination to induce the host’s immune response to the gene product encoded and expressed by the plasmid used. However, it has been noted that the method may cause tissue damage due to the high-pressure stream and it may not be suitable for delivering molecules with certain physical properties, such as a high viscosity or a high molecular weight.
Overall, jet injection remains a promising method of gene delivery that offers improved patient comfort and reduced risk of needlestick injury. Further research is needed to optimize the technique for specific molecules and tissue types [163].
3.3.4. Electroporation
Electroporation has emerged as a widely used method for efficient gene delivery in gene therapy. It involves the use of short high-voltage pulses to temporarily break down the cell membrane, allowing for the transfer of genetic material into cells.
The advantages of electroporation include a significant increase in gene delivery and expression, as well as a decrease in development costs and time. It is also safe and highly effective in delivering larger and more complex payloads, such as CRISPR/Cas9 systems, DNA vectors, and even small molecules, to various tissues, including the muscles, skin, heart, liver, lungs, and vasculature.
However, optimization is required for each new setting, as there can be variable transfection efficiency, limited cell viability, and potential tissue damage [164,165,166,167,168].
3.3.5. Ultrasound
Ultrasound, a noninvasive technique, has been explored as a means of delivering genes into cells in a process known as sonoporation. The ultrasound waves cause cavitation, resulting in the formation of pores in the cell membrane that allow the entry of therapeutic DNA into cells. This method of gene transfer is highly efficient and has a more favorable safety profile than other non-viral delivery methods. In addition, ultrasound-mediated gene transfer is considered to be less invasive than electroporation and is more readily accepted in clinical settings.
However, careful control of ultrasound parameters is required to avoid potential side effects. Despite this limitation, ultrasound-mediated gene transfer has proven to be an efficient and effective technique, with systemic injection of DNA possible. Researchers have found that sonoporation can significantly increase the uptake and expression of DNA in cells across many organ systems [168,169,170,171].
3.3.6. Magnetofection
Magnetofection is a nanoparticle-based gene delivery method that uses SPIONs to transport therapeutic genes to cells, tissues, and tumors. This approach has shown great potential in enhancing the efficacy of gene delivery up to several hundred-fold and in reducing the duration of gene delivery to minutes. To achieve this, SPIONs are complexed with DNA and exposed to an external magnetic field that guides them towards target cells.
Magnetofection is effective in vitro and in vivo, and it has been successfully used to achieve local transfection in the gastrointestinal tract and blood vessels. However, in vivo localization of SPIONs can be challenging, and the size of the particles can affect their ability to enter cells. Moreover, there are concerns over the cytotoxicity associated with the use of SPIONs [168,172,173].
3.3.7. Naked RNA Injection
Naked mRNA offers an innovative approach to deliver genetic material without the need for traditional carrier molecules. This strategy entails the direct administration of mRNA through a process known as naked mRNA injection.
The allure of this approach lies in its simplicity, efficient preparation, cost-effectiveness, and facile storage. Notably, the technique has demonstrated its versatility through intramuscular injection in murine models, yielding diverse protein expression profiles. Similarly, human studies employing intradermal injection have showcased the successful translation of exogenous mRNA, underscoring its potential for human applications. Beyond these achievements, the intratumoral delivery of saline-formulated mRNA encoding four cytokines has emerged as a potent tool to effectively impede tumor growth.
However, despite these strides, the realm of naked mRNA is not devoid of challenges. Swift degradation within extracellular and intracellular environments by exo- and endonucleases poses a notable hurdle, significantly truncating the duration of protein translation stemming from the mRNA. Addressing this issue, innovative strategies such as packaging mRNA within the core of polyplex micelles and employing PEG-conjugated cationic polymers, have arisen. Remarkably, this approach confers substantial protection, shielding intact mRNA from degradation by over 10,000-fold compared to naked mRNA, even in conditions rich with ribonucleases [174,175,176,177,178].
3.3.8. Naked DNA Injection
Naked DNA injection is the simplest and least expensive delivery method for gene therapy. This approach involves the direct injection of DNA at the targeted site. Although it offers the advantage of localized DNA uptake, the technique has several drawbacks. Poor and variable expression levels of the desired gene are often observed, and the method can cause damage to the tissue surrounding the injection site [168].
3.3.9. Gene-Activated Matrix (GAM)
Gene therapy and tissue engineering can be combined to create a novel solution for repairing damaged tissues, known as the Gene-Activated Matrix (GAM). A GAM offers the potential to restore the structure and function of damaged or dysfunctional tissues. A GAM is a scaffold made of biomaterials that can be seeded with therapeutic genes to direct and sustain gene expression. It can be used for both in vivo and ex vivo approaches, providing a three-dimensional template for tissue regeneration.
While GAMs hold great potential, they may require other viral or non-viral vectors to increase expression, and DNA damage is possible during scaffold formation. Nonetheless, the ability to direct and sustain gene expression in a 3D template makes GAMs a promising candidate for tissue engineering and regenerative medicine [168,179].
3.3.10. Hydrodynamic High-Pressure Injection
A hydrodynamic high-pressure injection is a gene delivery method that involves the rapid injection of a large volume of pDNA. This technique is simple, convenient, and highly efficient, making it a versatile tool for a variety of applications in gene therapy. Hydrodynamic injection uses a high-pressure flow of fluid to deliver DNA directly into the liver or other targeted tissues. The process is thought to work by briefly disrupting the plasma membrane, allowing the DNA to enter the cells.
One of the major advantages of hydrodynamic high-pressure injections is its simplicity. This method does not require specialized equipment or extensive training and can be easily performed by researchers with minimal experience in gene delivery. Additionally, hydrodynamic injections are highly efficient, with transfection rates as high as 60% in some studies. This makes it a useful tool for a variety of applications, including gene therapy, vaccine development, and gene function studies.
However, hydrodynamic injections do have some limitations. The high pressure used in this method can cause tissue damage, and the transient nature of the transgene expression limits its usefulness for long-term applications. Additionally, hydrodynamic injection is primarily useful for liver-specific gene expression, and its application to other tissues is still under investigation [180,181].
4. Twenty-Two Approved Human Gene Therapy Products
In this section, and in Table 3, our attention will be directed towards the culmination of gene therapy endeavors, namely the assortment of approved human gene therapy products that have successfully navigated the rigors of the market. These products have ushered gene therapy from a realm of promising potentiality to the realm of practical application. We shall endeavor to present an exhaustive inventory of these products, accompanied by an in-depth analysis of their underlying mechanisms, as well as an elucidation of their respective merits and limitations.

Table 3.
Approved human gene therapy products.
4.1. Approved Human Gene Therapy Products and Their Applications for In Vivo Treatment
4.1.1. IMLYGIC/Melanoma, Pancreatic Cancer
IMLYGIC (Talimogenelaherparepvec) is an FDA-approved gene therapy product used to treat melanoma and pancreatic cancer in adults.
It utilizes the HSV-1 oncolytic virus vector, which has deletions in the y34.5 and a47 regions, and the GM-CSF gene is inserted into the deleted y34.5 loci. The therapy works by inducing tumor lysis and antitumor immune responses.
Priced at USD 65,000 per treatment, IMLYGIC has shown promise in clinical trials and has been approved by the USA FDA for use in patients [7,182,183].
4.1.2. LUXTURNA/Retinal Dystrophy
Retinal dystrophy, caused by biallelic RPE65 mutation, leads to progressive vision loss and eventually blindness. However, LUXTURNA, a gene therapy product, has been approved by the USA FDA and EU FDA for the treatment of this condition.
LUXTURNA utilizes a recombinant adeno-associated virus (AAV2) vector to deliver a normal copy of the RPE65 gene to the retinal pigment epithelium (RPE) cells. This compensates for the biallelic mutation, allowing the RPE65 protein to convert trans-retinyl esters to 11-cis-retinal, the natural ligand and chromophore of the photoreceptors in the eye.
LUXTURNA is administered through a subretinal injection following a vitrectomy, and its application has shown significant improvement in visual function. The therapy is priced at USD 850,000 for both eyes or USD 425,000 per eye [7,184,185].
4.1.3. Zolgensma/Spinal Muscular Atrophy
Spinal muscular atrophy (SMA) is a rare genetic disorder caused by mutations in the SMN1 gene, leading to the progressive degeneration of motor neurons and ultimately to muscle weakness and atrophy. Among SMA types, type I is the most severe and is often fatal. However, the approval of Zolgensma (Onasemnogene Abeparvovec) by the USA FDA offers new hope to pediatric patients less than 2 years of age with SMA type I.
Zolgensma is a gene therapy product that utilizes a non-replicating recombinant adeno-associated virus 9 (AAV9) vector to deliver a functional copy of the SMN1 gene under the control of the CMV enhancer/chicken-β-actin-hybrid promoter (CB) to express SMN1 in motor neurons of SMA patients. The AAV9 capsid is unique and has the ability to cross the blood–brain barrier, allowing efficient CNS delivery by intravenous administration. Furthermore, the AAV ITR modification in Zolgensma produces a self-complementary DNA molecule that forms a double-stranded transgene, enhancing active transcription.
Zolgensma offers a promising cure for SMA type I. However, it comes with a high price tag of USD 4.2–USD 6.6 million per patient. Nevertheless, the approval of Zolgensma is a significant milestone in the field of gene therapy [7,186].
4.1.4. Spinraza/Spinal Muscular Atrophy
Another approved treatment available for spinal muscular atrophy (SMA) is Spinraza (Nusinersen), an ASO approved by the USA FDA and EU FDA. Spinraza targets intron 7 on the SMN2 hnRNA, modulating alternative splicing to increase the inclusion of exon 7 in the final processed RNA, resulting in higher levels of functional SMN protein in the central nervous system (CNS). Spinraza is indicated for both pediatric and adult patients with SMA. The price of Spinraza is USD 125,000 per injection [7,187].
4.1.5. Patisiran/Polyneuropathy
Polyneuropathy, also known as familial amyloidotic polyneuropathy, is a progressive and fatal disease caused by the accumulation of abnormal protein in the nerves and organs. Hereditary transthyretin-mediated amyloidosis, also called ATTRv amyloidosis, is one type of polyneuropathy. Patisiran (Onpattro) is a novel treatment for ATTRv amyloidosis that was approved by the FDA in 2018 and the EU FDA in 2019.
Patisiran is an LNP containing an RNAi that targets the transthyretin (TTR) gene. When Patisiran is administered, it enters the cell and cleaves the transthyretin mRNA, leading to a reduction in the circulating transthyretin protein. This decrease in protein levels reduces the amyloid accumulation associated with transthyretin-mediated amyloidosis, ultimately slowing the progression of the disease.
Patisiran is administered through intravenous infusion at a recommended dose of 0.3 mg/kg once every three weeks. In clinical trials, Patisiran demonstrated a significant reduction in the progression of neuropathy and an improvement in quality of life. As of 2023, Patisiran is the only approved RNAi-based therapy for the treatment of a genetic disease. Patisiran is priced at USD 345,000 per 2 mg/mL [7,188].
4.1.6. Gendicine/Carcinoma
Gendicine (rAd-p53) has been approved by the Chinese State Food and Drug Administration. Gendicine is a recombinant adenovirus vector that contains the wild-type p53 gene. The target of this therapy is the Tp53 tumor-suppressor gene, which is often mutated or lost in cancer cells. The adenovirus vector delivers the wild-type p53 gene into the cancer cells, where it inhibits cell proliferation and induces apoptosis. The mechanism of action of Gendicine involves the restoration of p53’s function in cancer cells.
Gendicine has been shown to be effective in preclinical and clinical trials, with a good safety profile. Its use in combination with other therapies has also been investigated. In terms of cost, Gendicine is relatively affordable, with a price of USD 387 per dose. However, its approval is currently limited to China [7,189,190].
4.1.7. Neovasculgen/Atherosclerotic Peripheral Arterial Disease
Atherosclerotic peripheral arterial disease (PAD), including critical limb ischemia (CLI), is a debilitating disease that can lead to amputation of the affected limb. Neovasculgen, a pDNA therapy approved by the Russian Ministry of Healthcare, aims to promote angiogenesis and improve the blood flow to the affected area.
This therapy targets the 165-amino-acid isoform of human vascular endothelial growth factor (VEGF165) using a recombinant DNA construct that contains the necessary genetic information to produce the protein. Upon administration, the plasmid enters the cells and VEGF165 is produced, stimulating angiogenesis, endothelial migration, and cellular proliferation. The DNA construct contains a transcription start site, splicing signal, and transcription terminator, as well as a polyadenylation signal to ensure proper mRNA processing. The therapy is administered via transmuscular transfer to the calf muscles. The price of the treatment course is USD 6600 [7,191].
4.1.8. Oncorine/Nasopharyngeal Cancer
Nasopharyngeal cancer, a type of head and neck cancer, along with lung, liver, and pancreatic cancers, has been targeted by an oncolytic adenovirus type 5 product called Oncorine, which has been approved by the Chinese State Food and Drug Administration.
The vector of Oncorine is a replication-selective adenovirus with the E1B-55 kDa gene entirely deleted. This gene is responsible for the inactivation of the p53 tumor-suppressor gene. The mechanism of action is based on the selective replication of Oncorine in p53-deficient cancer cells, leading to their lysis. Oncorine propagates selectively in cancer cells, while normal cells do not get infected because of the lack of E1b-55KD in the adenovirus, thereby ensuring the safety of the product. Subsequently, Oncorine-mediated cell cytotoxicity is initiated, and the adenoviruses released from lysed cancer cells can infect neighboring cancer cells, triggering a cascade of cancer cell death [7,192,193].
4.1.9. Defitelio/SOS/VOD
Defibrotide, also known as Defitelio or defibrotide sodium, is a gene therapy product that has been approved by the USA FDA and EU FDA for the treatment of a rare and life-threatening disease known as sinusoidal obstruction syndrome (SOS) or veno-occlusive disease (VOD) with multiorgan dysfunction. Defibrotide is a combination of single-stranded oligo DNAs with aptameric functions that are obtained from porcine mucosa tissue through controlled depolymerization.
The mechanism of action of Defibrotide involves its ability to act on vascular endothelial cells. It binds to the endothelial cell membrane and prevents thrombosis and inflammation, thereby promoting fibrinolysis and reducing endothelial cell damage. The aptameric function of Defibrotide allows it to act as an anticoagulant and also exhibit anti-inflammatory properties. Defibrotide costs USD 7425 per day [7,192,193,194].
4.1.10. ADSTILADRIN/Cancer (BCG-NMIBC)
One of the most recent breakthroughs in this field has been the development of ADSTILADRIN (nadofaragene firadenovec-vncg), a gene therapy product approved by the USA FDA in December 2022. ADSTILADRIN is specifically designed for the treatment of bacillus Calmette–Guérin (BCG)-unresponsive non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS) with or without papillary tumors in adults.
ADSTILADRIN is a non-replicating adenoviral vector-based gene therapy that utilizes a recombinant adenovirus serotype 5 vector. The vector is designed to deliver a copy of a gene encoding human interferon-alfa 2b (IFNα2b) to the bladder urothelium. Upon intravesical instillation, ADSTILADRIN results in cell transduction and transient local expression of the IFNα2b protein, which is anticipated to have anti-tumor effects.
One of the advantages of ADSTILADRIN is its targeted delivery system, which allows for precise treatment of the affected area while minimizing systemic side effects. The therapy’s mechanism of action is centered around the localized expression of IFNα2b, a protein known to have anti-tumor effects. While the treatment is only approved for BCG-unresponsive non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS) with or without papillary tumors, it is expected that the technology underlying ADSTILADRIN may be applicable to a wider range of cancers [195,196].
4.1.11. HEMGENIX/Hemophilia B
Hemophilia B is caused by a deficiency in clotting factor IX and is characterized by bleeding episodes that can lead to joint damage and other serious health issues. In November 2022, the US FDA approved HEMGENIX, an adeno-associated virus serotype 5 (AAV5)-based gene therapy developed by CSL Behring.
HEMGENIX delivers a copy of a gene encoding the Padua variant of human coagulation factor IX (hFIX598 Padua) to the liver cells. A single intravenous infusion of HEMGENIX leads to cell transduction and an increase in circulating factor IX activity in patients with hemophilia B. This therapy has shown significant potential in providing long-term benefits for adult patients with hemophilia B [197,198,199].
4.1.12. Upstaza/AADC Deficiency
Aromatic L-amino acid decarboxylase (AADC) deficiency is a rare genetic disorder caused by mutations in the gene responsible for producing the AADC enzyme, which is essential for the production of dopamine. Dopamine is a neurotransmitter that plays a critical role in movement control, and patients with AADC deficiency experience very little or no dopamine production in the brain.
Fortunately, a new gene therapy product called Upstaza (eladocagene exuparvovec) has been approved by the EU FDA as of July 2022 for the treatment of AADC deficiency in adults and children aged 18 months and older.
The product uses adeno-associated virus (AAV) as a vector to carry the functional AADC gene into nerve cells. This enables the cells to produce the missing enzyme, which in turn leads to the production of the dopamine necessary for proper brain function and an improvement in the symptoms of the condition [200,201].
4.2. Approved Human Gene Therapy Products and Their Applications for Ex Vivo Treatment
4.2.1. ABECMA/Multiple Myeloma
Multiple myeloma is a type of blood cancer that affects plasma cells. It occurs when these cells become abnormal and grow uncontrollably, leading to the production of excessive amounts of abnormal protein. The USA FDA and EU FDA have approved ABECMA (idecabtagene vicleucel), a gene therapy for multiple myeloma.
ABECMA is an autologous human T cell gene therapy that uses a lentiviral vector (LVV) to introduce a chimeric antigen receptor (CAR) that recognizes B cell maturation antigen (BCMA) in the patient’s own T cells. Once modified, these T cells are infused back into the patient, where they recognize and kill the cancerous plasma cells. ABECMA is approved for use in adults who have not responded to other types of treatment or who have relapsed after previous treatments [202,203,204,205].
4.2.2. CARVYKTI/Multiple Myeloma
CARVYKTI (ciltacabtagene autoleucel) is a gene therapy product approved by both the USA FDA and EU FDA for the treatment of adults with multiple myeloma. The product uses a lentiviral vector (LVV) to introduce an anti-B cell maturation antigen (BCMA) chimeric antigen receptor (CAR) into the patient’s own T cells. After being genetically modified ex vivo, the T cells can target and destroy the cancer cells expressing BCMA.
The therapy involves extracting the patient’s T cells and genetically modifying them with the lentiviral vector encoding the anti-BCMA CAR, which is then infused back into the patient. The CAR T cells recognize the cancer cells expressing BCMA and destroy them [206,207,208,209].
4.2.3. SKYSONA/Cerebral Adrenoleukodystrophy (CALD)
Cerebral adrenoleukodystrophy (CALD) is a rare neurodegenerative disease that affects boys aged 4–17. SKYSONA (elivaldogene autotemcel) is an autologous hematopoietic stem cell (HSC) gene therapy that was approved by the USA FDA in September 2022 for the treatment of CALD through an accelerated approval pathway.
SKYSONA is prepared from the patient’s own HSCs, which are collected via apheresis procedures. The cells are then transduced ex vivo with Lenti-D LVV, which is a replication-incompetent, self-inactivating lentiviral vector (LVV) that carries a functional ABCD1 gene that encodes normal adrenoleukodystrophy protein (ALDP). The ABCD1 gene is under the control of an internal MNDU3 promoter, which is a modified viral promoter that has been shown to effectively control the expression of the transgene in HSCs and their progeny in all lineages.
SKYSONA has been shown to slow the progression of neurologic dysfunction in patients with early, active CALD. The therapy works by replacing the faulty gene with a functional one, which can produce the missing protein that is needed for proper brain function. SKYSONA is administered via a single infusion and has shown promising results in clinical trials, with patients demonstrating significant improvements in neurological function [210,211,212].
4.2.4. YESCARTA/Large B Cell Lymphoma
The advent of cancer immunotherapy has transformed the treatment landscape for certain types of cancer. One such example is YESCARTA (axicabtagene ciloleucel), a gene therapy product that was approved by the FDA in October 2017 for the treatment of adults with certain types of cancer, specifically, relapsed or refractory large B cell lymphoma after two or more lines of systemic therapy, including diffuse large B cell lymphoma (DLBCL) not otherwise specified, primary mediastinal large B cell lymphoma, high-grade B cell lymphoma, and DLBCL arising from follicular lymphoma. YESCARTA is an autologous CAR T cell therapy that uses a gamma-retroviral vector to transfect anti-CD19 CAR genes into the patient’s T cells.
The ex vivo modulated autologous T cells are transfected with the gamma-retroviral vector, which expresses a chimeric antigen receptor (CAR) consisting of a murine anti-CD19 single-chain variable fragment and co-stimulatory domains of CD28 and CD3-zeta. After collecting autologous T cells from patients through leukapheresis, the cells are then enriched in a closed system in the YESCARTA manufacturing center. The transfected T cells are activated with anti-CD3 and IL-2, transduced with a retroviral vector expressing the anti-CD19 CAR gene, and then infused back into the patient after less than 10 days of manufacturing processes. The CAR T cell infusion targets and eliminates CD19-expressing cancer cells in the patient.
YESCARTA is priced at USD 373,000 per dose. While YESCARTA was initially approved for the treatment of large B cell lymphoma, ongoing clinical trials are also investigating the potential of YESCARTA in the treatment of other types of cancer, such as melanoma and pancreatic cancer [7,213,214].
4.2.5. KYMRIAH/Lymphoma
KYMRIAH (tisagenlecleucel CTL019) is a genetically engineered product composed of autologous T cells that have been genetically modified with a lentiviral vector to produce a CAR consisting of a murine single-chain antibody fragment (scFv) that is specific for CD19, which is linked to an intracellular cytoplasmic domain for 4-1BB (CD137) and CD3 zeta with a CD8 transmembrane hinge. The FDA and the EU have approved KYMRIAH for the treatment of pediatric and young adult patients up to 25 years of age with relapsed or refractory follicular lymphoma.
The lentiviral vector used in the manufacturing process of KYMRIAH is pseudotyped with a VSV g envelope derived from the HIV-1 genome in ex vivo conditions. This vector integrates into the genome of the transduced cells, resulting in the production of the tisagenlecleucel CAR under the regulation of a constitutively active promoter. When the tisagenlecleucel CAR binds to target cells, which are CD19-expressing cells in this case, it initiates antitumor activity through the CD3 domain. The intracellular 4-1BB costimulatory domain enhances the antitumor response and also ensures durable persistence of the CAR T cells. The price of KYMRIAH is approximately USD 475,000 per dose [6,7,166,215,216,217].
4.2.6. Strimvelis/ADA-SCID
Strimvelis (GSK-2696273) is a gene therapy product approved by the EU FDA in May 2016 to treat patients with severe combined immunodeficiency due to adenosine deaminase deficiency (ADA-SCID), a rare inherited condition in which patients lack the ADA enzyme required to maintain healthy lymphocytes, leading to a dysfunctional immune system. This disease is life-threatening, and without effective treatment, patients rarely survive more than two years. Strimvelis is used for patients who cannot undergo a bone marrow transplant due to the unavailability of a suitable, matched, related donor.
The product contains cells derived from the patient’s own bone marrow, including CD34+ cells genetically modified to contain a working ADA cDNA gene. This retroviral-based vector therapy functions by delivering genes into the patient’s body to produce the necessary protein. The medicine has been approved for adult use, and the cost of treatment is EUR 163,900 [7,218].
4.2.7. BREYANZI/Large B Cell Lymphoma (LBCL)
Large B cell lymphoma (LBCL) is a type of blood cancer that affects B cells, a type of white blood cell that produce antibodies. B cell lymphomas are the most common type of non-Hodgkin’s lymphoma (NHL) and can be aggressive or indolent. BREYANZI (lisocabtagene maraleucel) is a type of gene therapy that is used to treat adult patients with LBCL who have not responded to or have relapsed after at least two other types of therapy. It is a type of CAR T cell therapy that uses the patient’s own T cells to fight the cancer.
BREYANZI works by using a lentiviral vector to genetically modify the patient’s T cells to express a chimeric antigen receptor (CAR) that targets CD19, a protein found on the surface of B cells. The CAR is made up of CD8+ and CD4+ cell components, which work together to recognize and attack the cancer cells. The modified T cells are then infused back into the patient, where they multiply and attack the cancer cells.
BREYANZI was approved by the US FDA in 2021 and the EU FDA in 2022. It is the first CAR T cell therapy that can be given as a single infusion [219,220,221].
4.2.8. TECARTUS/Acute Lymphoblastic Leukemia (ALL)
Acute lymphoblastic leukemia (ALL) is a devastating disease that affects adults. Fortunately, TECARTUS (brexucabtagene autoleucel), a gene therapy product, has been approved by both the USA FDA and the EU FDA to combat this disease.
TECARTUS uses a retroviral vector to genetically modify autologous T cells ex vivo with an anti-CD19 chimeric antigen receptor (CAR) consisting of a murine anti-CD19 single-chain variable fragment (scFv) linked to a CD28 co-stimulatory domain and a CD3-zeta signaling domain. The mechanism of action of TECARTUS is the targeting of CD19-positive cells in the patient, which is achieved through the expression of the anti-CD19 CAR on the modified T cells. The modified T cells are then infused back into the patient where they multiply and attack the CD19-positive cells. The price of TECARTUS is USD 373,000 [217,222,223,224].
4.2.9. ZYNTEGLO/Beta-Thalassemia
Beta-thalassemia is a genetic disorder that affects the production of hemoglobin, causing anemia and other complications. ZYNTEGLO (betibeglogene autotemcel) is a promising gene therapy product for this disease, which has been approved by the USA FDA and EU FDA. This product uses a Lentiglobin BB305 lentiviral vector to deliver the beta-A-T87Q-globin gene to autologous CD34+ cells enriched with hematopoietic stem cells. This process enhances the production of hemoglobin and improves the quality of life for patients with beta-thalassemia. It is a one-time treatment and has demonstrated significant efficacy in clinical trials.
The approval of ZYNTEGLO marks a significant milestone in the field of gene therapy, as it is the first approved gene therapy product for beta-thalassemia. This product provides hope to patients suffering from this debilitating disease, offering a potentially curative treatment option. However, the high cost of ZYNTEGLO, currently priced at USD 1.8 million, has sparked discussions regarding the affordability and accessibility of gene therapies. Despite this, the approval of ZYNTEGLO represents a major step forward for gene therapy and offers renewed optimism for the development of future gene therapies for genetic diseases [225].
4.2.10. Libmeldy/Metachromatic Leukodystrophy
Leukodystrophy is a rare and debilitating disease that affects the nervous system in children. A new gene therapy called Libmeldy has been developed and was approved by the EU FDA in December 2020 to help treat the condition. Libmeldy is an autologous ex vivo lentiviral gene therapy that uses a type of virus called a lentivirus to introduce a healthy copy of the ARSA gene into CD34+ cells extracted from the patient’s blood or bone marrow. These CD34+ cells are then infused back into the patient’s bloodstream where they migrate to the bone marrow and begin producing functional ARSA.
The ARSA protein helps to break down sulfatides, which build up in the nervous system of patients with metachromatic leukodystrophy. By introducing healthy ARSA-producing cells into the patient, Libmeldy helps to control the symptoms of the disease, with long-lasting effects expected [226].
5. Future Directions
As we cast our gaze towards the horizon, the landscape of gene therapy reveals a realm brimming with potentialities yet to be harnessed. With a deepening comprehension of the intricacies of the human genome and the maturation of our genetic manipulation tools, the vista of gene therapy seems poised for continued expansion. The path ahead is one of unwavering research and innovative strides, a journey that holds the promise of ushering forth more potent treatments and transformative remedies for a diverse spectrum of ailments. The transformative impact on the lives of patients globally is on the precipice of realization. For instance, a paramount obstacle on the path to clinical translation resides in the nonspecific sequestration of gene-therapeutic-loaded delivery carriers by reticuloendothelial system (RES) organs, notably liver sinusoidal scavenger wall cells. This not only compromises targeted delivery but also accentuates concerns of toxicity and immunogenicity. Stealth coating with PEG-oligopeptides has emerged as a strategic intervention, transiently and selectively obstructing the scavenging functions of liver sinusoidal wall cells. This breakthrough approach circumvents sinusoidal capture, thus elevating gene transfer efficacy within target tissues. Moreover, the lingering challenge of undesired hepatic off-target accumulation of FDA-approved mRNA-encapsulated LNPs persists, even following local intramuscular administration. This inadvertently triggers adverse hepatic side effects and inflammation, constraining their broad application. A paradigm shift beckons, characterized by minimizing hepatic off-targeting while optimizing injection site delivery. Such precision targeting not only augments vaccine applications but also holds the promise of aiding patients beset by systemic inflammation. The confluence of innovative solutions with the pursuit of precision heralds the dawn of an era where gene therapy’s potential can reach unprecedented heights [226,227,228,229,230,231].
6. Conclusions
Our in-depth analysis of the current state of human gene therapy elucidates the significant progress and the transformative potential of this field. The range of approved in vivo and ex vivo products, such as IMLYGIC, LUXTURNA, Zolgensma, Spinraza, Patisiran, Gendicine, Neovasculgen, Oncorine, Defibrotide, ADSTILADRIN, HEMGENIX, Upstaza, ABECMA, CARVYKTI, SKYSONA, YESCARTA, KYMRIAH, Strimvelis, BREYANZI, TECARTUS, ZYNTEGLO, and Libmeldy, demonstrates the growing applicability of gene therapy across various diseases, including melanoma, pancreatic cancer, retinal dystrophy, spinal muscular atrophy, polyneuropathy, hereditary transthyretin-mediated amyloidosis, head and neck squamous cell carcinoma, atherosclerotic peripheral arterial disease, critical limb ischemia, nasopharyngeal cancer, SOS/VOD with multiorgan dysfunction, bacillus Calmette–Guérin (BCG)-unresponsive non-muscle invasive bladder cancer, hemophilia B, aromatic L-amino acid decarboxylase deficiency, multiple myeloma, cerebral adrenoleukodystrophy, lymphoma, ADA-SCID, large B cell lymphoma, acute lymphoblastic leukemia, beta-thalassemia, and metachromatic leukodystrophy.
Gene therapy has emerged as a robust and versatile tool poised to catalyze the advancement of personalized medicine, revolutionizing therapy efficacy and ameliorating the collateral effects of widely used pharmaceutical agents. The most rapid evolution of personalized medicine is witnessed in the realms of cancer treatment and hereditary disorders, where gene therapy strategies and products play a pivotal role. However, the potential of gene therapy extends far beyond these domains, encompassing a diverse array of pathologies, such as neurodegenerative ailments, immune disorders, inflammatory processes, and more, calling for further investigation.
Despite remarkable progress, challenges remain in the practical use of gene therapy. These include, but are not limited to, issues related to safety, specificity, delivery efficiency, and immune responses. For instance, while viral vectors are highly efficient, they can provoke immune responses. Non-viral vectors, while less immunogenic, are less efficient. Balancing these contrasting attributes is a key concern.
Moreover, certain ethical, regulatory, and commercialization challenges must be addressed for gene therapy to become more widely adopted. Researchers embarking on the nascent phases of product development are tasked with a multifaceted problem encompassing current technological frontiers, product commercialization, and the potential for costly solutions. While some of these innovative solutions hold transformative promise, their accessibility for patients could be constrained by elevated costs. It is imperative that these pioneering commercial decisions are underpinned by judicious forethought, as ill-conceived approaches could jeopardize not only production but also the integration of gene therapy products into medical practice. Given the potentially transformative impact of gene therapy, it is critical that these challenges are met with thoughtful and comprehensive solutions.
Looking forward, the field of gene therapy holds immense potential. As our understanding of the human genome deepens and our tools for genetic manipulation become more sophisticated, the scope of gene therapy will likely continue to broaden. Continued research and innovation in this field will undoubtedly lead to more effective treatments and cures for a range of diseases, transforming the lives of patients worldwide.
It is our hope that this review provides researchers, clinicians, and policymakers with a comprehensive understanding of the current state of human gene therapy, serving as a launchpad for future advancements in this promising field.
Author Contributions
Conceptualization, supervision, G.D.T.; writing—original draft preparation, A.Y.S.; writing—review and editing, S.V.A.; writing—review and editing, M.G.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Edelstein, M.L.; Abedi, M.R.; Wixon, J.; Edelstein, R.M. Gene therapy clinical trials worldwide 1989–2004—An overview. J. Gene Med. 2004, 6, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Arabi, F.; Mansouri, V.; Ahmadbeigi, N. Gene therapy clinical trials, where do we go? An overview. Biomed. Pharmacother. 2022, 153, 113324. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, C. Discovery of DNA as the hereditary material using Streptococcus pneumoniae. Nat. Educ. 2008, 1, 104. [Google Scholar]
- Scheller, E.L.; Krebsbach, P.H. Gene therapy: Design and prospects for craniofacial regeneration. J. Dent. Res. 2009, 88, 585–596. [Google Scholar] [CrossRef]
- Baltimore, D. Paul Berg (1926–2023). Science 2023, 379, 1095. [Google Scholar] [CrossRef]
- Approval Letter—KYMRIAH. Available online: https://www.fda.gov/media/106989/download (accessed on 4 July 2023).
- Shahryari, A.; Saghaeian Jazi, M.; Mohammadi, S.; Razavi Nikoo, H.; Nazari, Z.; Hosseini, E.S.; Burtscher, I.; Mowla, S.J.; Lickert, H. Development and Clinical Translation of Approved Gene Therapy Products for Genetic Disorders. Front. Genet. 2019, 10, 868. [Google Scholar] [CrossRef]
- Mullard, A. Parsing clinical success rates. Nat. Rev. Drug Discov. 2016, 15, 447. [Google Scholar] [CrossRef]
- Dana, H.; Chalbatani, G.M.; Mahmoodzadeh, H.; Karimloo, R.; Rezaiean, O.; Moradzadeh, A.; Mehmandoost, N.; Moazzen, F.; Mazraeh, A.; Marmari, V.; et al. Molecular mechanisms and biological functions of siRNA. Int. J. Biomed. Sci. 2017, 13, 48–57. [Google Scholar] [CrossRef]
- Xu, W.; Jiang, X.; Huang, L. RNA Interference Technology. Compr. Biotechnol. 2019, 5, 560–575. [Google Scholar] [CrossRef]
- Lam, J.K.W.; Chow, M.Y.T.; Zhang, Y.; Leung, S.W.S. siRNA Versus miRNA as Therapeutics for Gene Silencing. Mol. Ther. Nucleic Acids 2015, 4, e252. [Google Scholar] [CrossRef]
- Flemming, A. siRNA: Brain delivery breakthrough. Nat. Rev. Neurosci. 2007, 8, 570. [Google Scholar] [CrossRef]
- Jackson, A.L.; Linsley, P.S. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat. Rev. Drug Discov. 2010, 9, 57–67. [Google Scholar] [CrossRef]
- Robbins, M.; Judge, A.; MacLachlan, I. siRNA and innate immunity. Oligonucleotides 2009, 19, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Brennecke, J.; Stark, A.; Russell, R.B.; Cohen, S.M. Principles of MicroRNA–Target Recognition. PLoS Biol. 2005, 3, e85. [Google Scholar] [CrossRef] [PubMed]
- Eslava-Avilés, E.; Arenas-Huertero, F. piRNAs: Nature, biogenesis, regulation, and their potential clinical utility. Bol. Med. Hosp. Infant. Mex. 2021, 78, 432–442. [Google Scholar] [CrossRef]
- Moore, C.B.; Guthrie, E.H.; Huang, M.T.-H.; Taxman, D.J. Short Hairpin RNA (shRNA): Design, Delivery, and Assessment of Gene Knockdown. Methods Mol. Biol. 2010, 629, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Ilves, H.; Dallas, A.; Kumar, P.; Shorenstein, J.; Kazakov, S.A.; Johnston, B.H. Minimal-length short hairpin RNAs: The relationship of structure and RNAi activity. RNA 2010, 16, 106–117. [Google Scholar] [CrossRef]
- Larsson, E.; Sander, C.; Marks, D. mRNA turnover rate limits siRNA and microRNA efficacy. Mol. Syst. Biol. 2010, 6, 433. [Google Scholar] [CrossRef]
- Fellmann, C.; Lowe, S.W. Stable RNA interference rules for silencing. Nat. Cell Biol. 2014, 16, 10–18. [Google Scholar] [CrossRef]
- Mcintyre, G.J.; Yu, Y.-H.; Lomas, M.; Fanning, G.C. The effects of stem length and core placement on shRNA activity. BMC Mol. Biol. 2011, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Dhuri, K.; Bechtold, C.; Quijano, E.; Pham, H.; Gupta, A.; Vikram, A.; Bahal, R. Antisense Oligonucleotides: An Emerging Area in Drug Discovery and Development. J. Clin. Med. 2020, 9, 2004. [Google Scholar] [CrossRef] [PubMed]
- Scoles, D.R.; Minikel, E.V.; Pulst, S.M. Antisense oligonucleotides. Neurol. Genet. 2019, 5, e323. [Google Scholar] [CrossRef] [PubMed]
- McClorey, G.; Banerjee, S. Cell-Penetrating Peptides to Enhance Delivery of Oligonucleotide-Based Therapeutics. Biomedicines 2018, 6, 51. [Google Scholar] [CrossRef]
- Liang, X.-H.; Sun, H.; Nichols, J.G.; Crooke, S.T. RNase H1-Dependent Antisense Oligonucleotides Are Robustly Active in Directing RNA Cleavage in Both the Cytoplasm and the Nucleus. Mol. Ther. 2017, 25, 2075–2092. [Google Scholar] [CrossRef]
- Yoshida, T.; Naito, Y.; Yasuhara, H.; Sasaki, K.; Kawaji, H.; Kawai, J.; Naito, M.; Okuda, H.; Obika, S.; Inoue, T. Evaluation of off-target effects of gapmer antisense oligonucleotides using human cells. Genes Cells 2019, 24, 827–835. [Google Scholar] [CrossRef]
- Rinaldi, C.; Wood, M.J.A. Antisense oligonucleotides: The next frontier for treatment of neurological disorders. Nat. Rev. Neurol. 2018, 14, 9–21. [Google Scholar] [CrossRef]
- Rook, M.E.; Southwell, A.L. Antisense Oligonucleotide Therapy: From Design to the Huntington Disease Clinic. BioDrugs 2022, 36, 105–119. [Google Scholar] [CrossRef]
- Gardlík, R.; Pálffy, R.; Hodosy, J.; Lukács, J.; Turna, J.; Celec, P. Vectors and delivery systems in gene therapy. Med. Sci. Monit. 2005, 11, RA110–RA121. [Google Scholar]
- Asmamaw, M.; Zawdie, B. Mechanism and Applications of CRISPR/Cas-9-Mediated Genome Editing. Biologics 2021, 15, 353–361. [Google Scholar] [CrossRef]
- Xu, C.L.; Ruan, M.Z.C.; Mahajan, V.B.; Tsang, S.H. Viral Delivery Systems for CRISPR. Viruses 2019, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Majeed, S.; Hoque, M.Z.; Ahmad, I. Latest Developed Strategies to Minimize the Off-Target Effects in CRISPR-Cas-Mediated Genome Editing. Cells 2020, 9, 1608. [Google Scholar] [CrossRef] [PubMed]
- Rawashdeh, O.; Rawashdeh, R.Y.; Kebede, T.; Kapp, D.; Ralescu, A. Bio-informatic analysis of CRISPR protospacer adjacent motifs (PAMs) in T4 genome. BMC Genom. Data 2022, 23, 40. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, J.; Ge, S.; Lai, L. CRISPR/Cas: Advances, Limitations, and Applications for Precision Cancer Research. Front. Med. 2021, 8, 649896. [Google Scholar] [CrossRef]
- Taniyama, Y.; Azuma, J.; Kunugiza, Y.; Iekushi, K.; Rakugi, H.; Morishita, R. Therapeutic option of plasmid-DNA based gene transfer. Curr. Top. Med. Chem. 2012, 12, 1630–1637. [Google Scholar] [CrossRef]
- Sousa, F.; Passarinha, L.; Queiroz, J.A. Biomedical application of plasmid DNA in gene therapy: A new challenge for chromatography. Biotechnol. Genet. Eng. Rev. 2009, 26, 83–116. [Google Scholar] [CrossRef]
- Dishart, K.L.; Work, L.M.; Denby, L.; Baker, A.H. Gene Therapy for Cardiovascular Disease. J. Biomed. Biotechnol. 2003, 2, 138–148. [Google Scholar] [CrossRef]
- Enghiad, B.; Xue, P.; Singh, N.; Boob, A.G.; Shi, C.; Petrov, V.A.; Liu, R.; Peri, S.S.; Lane, S.T.; Gaither, E.D.; et al. PlasmidMaker is a versatile, automated, and high throughput end-to-end platform for plasmid construction. Nat. Commun. 2022, 13, 2697. [Google Scholar] [CrossRef]
- Shintani, M.; Sanchez, Z.K.; Kimbara, K. Genomics of microbial plasmids: Classification and identification based on replication and transfer systems and host taxonomy. Front. Microbiol. 2015, 6, 242. [Google Scholar] [CrossRef]
- Barnhart, K.M.; Hartikka, J.; Manthorpe, M.; Norman, J.; Hobart, P. Enhancer and promoter chimeras in plasmids designed for intramuscular injection: A comparative in vivo and in vitro study. Hum. Gene Ther. 1998, 9, 2545–2553. [Google Scholar] [CrossRef]
- Eusébio, D.; Neves, A.R.; Costa, D.; Biswas, S.; Alves, G.; Cui, Z.; Sousa, Â. Methods to improve the immunogenicity of plasmid DNA vaccines. Drug Discov. Today 2021, 26, 2575–2592. [Google Scholar] [CrossRef] [PubMed]
- Antonenko, S.; Gurianov, D.; Telegeev, G. Colocalization of USP1 and РН domain of Bcr-Abl oncoprotein in terms of chronic myeloid leukemia cell rearrangements. Tsitol. Genet. 2016, 50, 352–356. [Google Scholar]
- Antonenko, S.; Telegeev, G. Inhibition of USP1, a new partner of bcr-abl, results in decrease of Bcr-Abl level in k562 cells. Exp. Oncol. 2020, 42, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Antonenko, S.; Kravchuk, I.; Telegeev, G. GLG1 in K562 cells: Role in pathogenesis of chronic myeloid leukemia. Cytol. Genet. 2020, 54, 62–70. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, S.; Fu, R.; Zhang, L.; Huang, K.; Peng, H.; Dai, L.; Chen, Q. Therapeutic Prospects of mRNA-Based Gene Therapy for Glioblastoma. Front. Oncol. 2019, 9, 2019. [Google Scholar] [CrossRef]
- Andreev, D.E.; Terenin, I.M.; Dmitriev, S.E.; Shatsky, I.N. Pros and cons of pDNA and mRNA transfection to study mRNA translation in mammalian cells. Gene 2016, 578, 1–6. [Google Scholar] [CrossRef]
- Damase, T.R.; Sukhovershin, R.; Boada, C.; Taraballi, F.; Pettigrew, R.I.; Cooke, J.P. The Limitless Future of RNA Therapeutics. Front. Bioeng. Biotechnol. 2021, 9, 628137. [Google Scholar] [CrossRef]
- Iqbal, Z.; Iqbal, M.S.; Ahmad, A.; Memon, A.G.; Ansari, M.I. New prospects on the horizon: Genome editing to engineer plants for desirable traits. Curr. Plant Biol. 2020, 24, 100171. [Google Scholar] [CrossRef]
- Flisikowska, T.; Kind, A.; Schnieke, A. 10—Production of Transgenic Rabbits. In Transgenic Animal Technology, 3rd ed.; Pinkert, C.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 275–304. [Google Scholar] [CrossRef]
- Silva, G.; Poirot, L.; Galetto, R.; Smith, J.; Montoya, G.; Duchateau, P.; Pâques, F. Meganucleases and Other Tools for Targeted Genome Engineering: Perspectives and Challenges for Gene Therapy. Curr. Gene Ther. 2011, 11, 11–27. [Google Scholar] [CrossRef]
- Daboussi, F.; Stoddard, T.J.; Zhang, F. Engineering Meganuclease for Precise Plant Genome Modification. In Advances in New Technology for Targeted Modification of Plant Genomes; Zhang, F., Puchta, H., Thomson, J., Eds.; Springer: New York, NY, USA, 2015; pp. 21–38. [Google Scholar] [CrossRef]
- Epinat, J.C.; Arnould, S.; Chames, P.; Rochaix, P.; Desfontaines, D.; Puzin, C.; Patin, A.; Zanghellini, A.; Pâques, F.; Lacroix, E. A novel engineered meganuclease induces homologous recombination in yeast and mammalian cells. Nucleic Acids Res. 2003, 31, 2952–2962. [Google Scholar] [CrossRef]
- Urnov, F.D.; Rebar, E.J.; Holmes, M.C.; Zhang, H.S.; Gregory, P.D. Genome editing with engineered zinc finger nucleases. Nat. Rev. Genet. 2010, 11, 636–646. [Google Scholar] [CrossRef] [PubMed]
- Durai, S.; Mani, M.; Kandavelou, K.; Wu, J.; Porteus, M.H.; Chandrasegaran, S. Zinc finger nucleases: Custom-designed molecular scissors for genome engineering of plant and mammalian cells. Nucleic Acids Res. 2005, 33, 5978–5990. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kweon, J.; Kim, A.; Chon, J.K.; Yoo, J.Y.; Kim, H.J.; Kim, S.; Lee, C.; Jeong, E.; Chung, E.; et al. A genome-wide TALEN resource. Nat. Methods 2013, 10, 286. [Google Scholar] [CrossRef]
- Heigwer, F.; Kerr, G.; Walther, N.; Glaeser, K.; Pelz, O.; Breinig, M.; Boutros, M. E-TALEN: A web tool to design TALENs for genome engineering. Nucleic Acids Res. 2013, 41, e190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Lin, Y.; Qi, X.; Li, L.; Wang, Q.; Ma, Y. TALENs-Assisted Multiplex Editing for Accelerated Genome Evolution To Improve Yeast Phenotypes. ACS Synth. Biol. 2015, 4, 1101–1111. [Google Scholar] [CrossRef]
- Wang, L.; Li, F.; Dang, L.; Liang, C.; Wang, C.; He, B.; Liu, J.; Li, D.; Wu, X.; Xu, X.; et al. In Vivo Delivery Systems for Therapeutic Genome Editing. Int. J. Mol. Sci. 2016, 17, 626. [Google Scholar] [CrossRef]
- Lau, C.-H.; Zhu, H.; Tay, J.C.-K.; Li, Z.; Tay, F.C.; Chen, C.; Tan, W.-K.; Du, S.; Sia, V.-K.; Phang, R.-Z.; et al. Genetic rearrangements of variable di-residue (RVD)-containing repeat arrays in a baculoviral TALEN system. Mol. Ther. Methods Clin. Dev. 2014, 1, 14050. [Google Scholar] [CrossRef]
- Adachi, T.; Nakamura, Y. Aptamers: A Review of Their Chemical Properties and Modifications for Therapeutic Application. Molecules 2019, 24, 4229. [Google Scholar] [CrossRef]
- Mou, Q.; Xue, X.; Ma, Y.; Banik, M.; Garcia, V.; Guo, W.; Wang, J.; Song, T.; Chen, L.-Q.; Lu, Y. Efficient delivery of a DNA aptamer-based biosensor into plant cells for glucose sensing through thiol-mediated uptake. Sci. Adv. 2022, 8, eabo0902. [Google Scholar] [CrossRef]
- Kong, H.Y.; Byun, J. Nucleic Acid Aptamers: New Methods for Selection, Stabilization, and Application in Biomedical Science. Biomol. Ther. 2013, 21, 423–434. [Google Scholar] [CrossRef]
- Zhu, Q.; Liu, G.; Kai, M. DNA Aptamers in the Diagnosis and Treatment of Human Diseases. Molecules 2015, 20, 20979–20997. [Google Scholar] [CrossRef] [PubMed]
- Crivianu-Gaita, V.; Thompson, M. Aptamers, antibody scFv, and antibody Fab’ fragments: An overview and comparison of three of the most versatile biosensor biorecognition elements. Biosens. Bioelectron. 2016, 85, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Castro, R.; de-los-Santos-Álvarez, N.; Miranda-Ordieres, A.J.; Lobo-Castañón, M.J. Harnessing Aptamers to Overcome Challenges in Gluten Detection. Biosensors 2016, 6, 16. [Google Scholar] [CrossRef]
- Kawano, R.; Osaki, T.; Sasaki, H.; Takinoue, M.; Yoshizawa, S.; Takeuchi, S. Rapid detection of a cocaine-binding aptamer using biological nanopores on a chip. J. Am. Chem. Soc. 2011, 133, 8474–8477. [Google Scholar] [CrossRef]
- Xie, M.; Chen, Z.; Zhao, F.; Lin, Y.; Zheng, S.; Han, S. Selection and Application of ssDNA Aptamers for Fluorescence Biosensing Detection of Malachite Green. Foods 2022, 11, 801. [Google Scholar] [CrossRef]
- Neves, M.A.D.; Reinstein, O.; Saad, M.; Johnson, P.E. Defining the secondary structural requirements of a cocaine-binding aptamer by a thermodynamic and mutation study. Biophys. Chem. 2010, 153, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Baugh, C.; Grate, D.; Wilson, C. 2.8 Å crystal structure of the malachite green aptamer. J. Mol. Biol. 2000, 301, 117–128. [Google Scholar] [CrossRef]
- Zhong, H.; Wang, H.; Li, J.; Huang, Y. TRAIL-based gene delivery and therapeutic strategies. Acta Pharmacol. Sin. 2019, 40, 1373–1385. [Google Scholar] [CrossRef]
- Griffith, T.S.; Stokes, B.; Kucaba, T.A.; Earel, J.K., Jr.; VanOosten, R.L.; Brincks, E.L.; Norian, L.A. TRAIL gene therapy: From preclinical development to clinical application. Curr. Gene Ther. 2009, 9, 9–19. [Google Scholar] [CrossRef]
- Deere, J.; Iversen, P.; Geller, B.L. Antisense phosphorodiamidate morpholino oligomer length and target position effects on gene-specific inhibition in Escherichia coli. Antimicrob. Agents Chemother. 2005, 49, 249–255. [Google Scholar] [CrossRef]
- Nan, Y.; Zhang, Y.-J. Antisense phosphorodiamidate morpholino oligomers as novel antiviral compounds. Front. Microbiol. 2018, 9, 750. [Google Scholar] [CrossRef] [PubMed]
- Nayerossadat, N.; Maedeh, T.; Ali, P.A. Viral and nonviral delivery systems for gene delivery. Adv. Biomed. Res. 2012, 1, 27. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.V.; Aveta, A.; Comegna, M.; Cernera, G.; Iacotucci, P.; Carnovale, V.; Taccetti, G.; Terlizzi, V.; Castaldo, G. Extensive CFTR Gene Analysis Revealed a Higher Occurrence of Cystic Fibrosis Transmembrane Regulator-Related Disorders (CFTR-RD) among CF Carriers. J. Clin. Med. 2020, 9, 3853. [Google Scholar] [CrossRef] [PubMed]
- Gillen, A.E.; Gosalia, N.; Leir, S.H.; Harris, A. MicroRNA regulation of expression of the cystic fibrosis transmembrane conductance regulator gene. Biochem. J. 2011, 438, 25–32. [Google Scholar] [CrossRef]
- De Palma, F.D.E.; Raia, V.; Kroemer, G.; Maiuri, M.C. The Multifaceted Roles of MicroRNAs in Cystic Fibrosis. Diagnostics 2020, 10, 1102. [Google Scholar] [CrossRef]
- Vargas, J.E.; Chicaybam, L.; Stein, R.T.; Tanuri, A.; Delgado-Cañedo, A.; Bonamino, M.H. Retroviral vectors and transposons for stable gene therapy: Advances, current challenges and perspectives. J. Transl. Med. 2016, 14, 288. [Google Scholar] [CrossRef]
- Ghosh, S.; Brown, A.M.; Jenkins, C.; Campbell, K. Viral Vector Systems for Gene Therapy: A Comprehensive Literature Review of Progress and Biosafety Challenges. Appl. Biosaf. 2020, 25, 7–18. [Google Scholar] [CrossRef]
- Naso, M.F.; Tomkowicz, B.; Perry, W.L., III; Strohl, W.R. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs 2017, 31, 317–334. [Google Scholar] [CrossRef]
- Hukkanen, V. Herpesvirus Vectors in Gene Therapy. Open Virol. J. 2010, 4, 94–95. [Google Scholar] [CrossRef]
- Lundstrom, K. Alphaviruses in Gene Therapy. Viruses 2015, 7, 2321–2333. [Google Scholar] [CrossRef]
- Kim, A.S.; Diamond, M.S. A molecular understanding of alphavirus entry and antibody protection. Nat. Rev. Microbiol. 2023, 21, 396–407. [Google Scholar] [CrossRef]
- Conrad, S.J.; Liu, J. Poxviruses as Gene Therapy Vectors: Generating Poxviral Vectors Expressing Therapeutic Transgenes. Methods Mol. Biol. 2019, 1937, 189–209. [Google Scholar] [CrossRef]
- Jenne, L.; Hauser, C.; Arrighi, J.F.; Saurat, J.H.; Hügin, A.W. Poxvirus as a vector to transduce human dendritic cells for immunotherapy: Abortive infection but reduced APC function. Gene Ther. 2000, 7, 1575–1583. [Google Scholar] [CrossRef]
- Oliveira, G.P.; Rodrigues, R.A.L.; Lima, M.T.; Drumond, B.P.; Abrahão, J.S. Poxvirus Host Range Genes and Virus–Host Spectrum: A Critical Review. Viruses 2017, 9, 331. [Google Scholar] [CrossRef] [PubMed]
- Saghazadeh, A.; Rezaei, N. Poxviruses and the immune system: Implications for monkeypox virus. Int. Immunopharmacol. 2022, 113, 109364. [Google Scholar] [CrossRef] [PubMed]
- Gibb, B.; Hyman, P.; Schneider, C.L. The Many Applications of Engineered Bacteriophages—An Overview. Pharmaceuticals 2021, 14, 634. [Google Scholar] [CrossRef] [PubMed]
- Kasman, L.M.; Porter, L.D. Bacteriophages. In StatPearls [Internet]; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Sanger, F.; Coulson, A.R.; Hong, G.F.; Hill, D.F.; Petersen, G.B. Nucleotide sequence of bacteriophage λ DNA. J. Mol. Biol. 1982, 162, 729–773. [Google Scholar] [CrossRef]
- Dunn, J.J.; Studier, F.W.; Gottesman, M. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J. Mol. Biol. 1983, 166, 477–535. [Google Scholar] [CrossRef]
- Petrov, V.M.; Ratnayaka, S.; Nolan, J.M.; Miller, E.S.; Karam, J.D. Genomes of the T4-related bacteriophages as windows on microbial genome evolution. Virol. J. 2010, 7, 292. [Google Scholar] [CrossRef]
- Hatfull, G.F. Bacteriophage genomics. Curr. Opin. Microbiol. 2008, 11, 447–453. [Google Scholar] [CrossRef]
- Principi, N.; Silvestri, E.; Esposito, S. Advantages and Limitations of Bacteriophages for the Treatment of Bacterial Infections. Front. Pharmacol. 2019, 10, 513. [Google Scholar] [CrossRef] [PubMed]
- Young, L.S. Epstein-Barr Virus: General Features. In Encyclopedia of Virology, 3rd ed.; Mahy, B.W.J., van Regenmortel, M.H.V., Eds.; Academic Press: Oxford, UK, 2008; pp. 148–157. [Google Scholar]
- Tomkinson, B.; Robertson, E.; Yalamanchili, R.; Longnecker, R.; Kieff, E. Epstein-Barr virus recombinants from overlapping cosmid fragments. J. Virol. 1993, 67, 7298–7306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zuo, Y.; Jiang, L.; Peng, Y.; Huang, X.; Zuo, L. Epstein-Barr Virus and Neurological Diseases. Front. Mol. Biosci. 2021, 8, 816098. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Laing, J.; Yan, B.; Zhou, H.; Ke, L.; Wang, C.; Narita, Y.; Zhang, Z.; Olson, M.R.; Afzali, B.; et al. Epstein-Barr Virus Episome Physically Interacts with Active Regions of the Host Genome in Lymphoblastoid Cells. J. Virol. 2020, 94, e01390-20. [Google Scholar] [CrossRef]
- Wu, L.; Borza, C.M.; Hutt-Fletcher, L.M. Mutations of Epstein-Barr Virus gH That Are Differentially Able To Support Fusion with B Cells or Epithelial Cells. J. Virol. 2005, 79, 10923–10930. [Google Scholar] [CrossRef]
- Lujan, H.; Griffin, W.C.; Taube, J.H.; Sayes, C.M. Synthesis and characterization of nanometer-sized liposomes for encapsulation and microRNA transfer to breast cancer cells. Int. J. Nanomed. 2019, 14, 5159–5173. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, Y.; Zhu, D.; Shi, K.; Ma, C.; Zhang, W.; Rocchi, P.; Jiang, L.; Liu, X. Self-assembly of amphiphilic phospholipid peptide dendrimer-based nanovectors for effective delivery of siRNA therapeutics in prostate cancer therapy. J. Control. Release 2020, 322, 416–425. [Google Scholar] [CrossRef]
- Paz, M.M.; Peinador Veiga, A.; Regueira, T.; Vázquez Vázquez, C.; López Quintela, M.A. Facile generation of surface diversity in gold nanoparticles. J. Colloid Interface Sci. 2023, 641, 719–728. [Google Scholar] [CrossRef]
- Si, P.; Razmi, N.; Nur, O.; Solanki, S.; Pandey, C.M.; Gupta, R.K.; Malhotra, B.D.; Willander, M.; de la Zerda, A. Gold nanomaterials for optical biosensing and bioimaging. Nanoscale Adv. 2021, 3, 2679–2698. [Google Scholar] [CrossRef]
- Jeong, E.H.; Jung, G.; Hong, C.A.; Lee, H. Gold nanoparticle (AuNP)-based drug delivery and molecular imaging for biomedical applications. Arch. Pharm. Res. 2014, 37, 53–59. [Google Scholar] [CrossRef]
- Ackerson, C.J.; Jadzinsky, P.D.; Sexton, J.Z.; Bushnell, D.A.; Kornberg, R.D. Synthesis and Bioconjugation of 2 and 3 nm-diameter Gold Nanoparticles. Bioconjug. Chem. 2010, 21, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Hammami, I.; Alabdallah, N.M.; Al Jomaa, A.; Kamoun, M. Gold nanoparticles: Synthesis properties and applications. J. King Saud Univ.-Sci. 2021, 33, 101560. [Google Scholar] [CrossRef]
- Sani, A.; Cao, C.; Cui, D. Toxicity of gold nanoparticles (AuNPs): A review. Biochem. Biophys. Rep. 2021, 26, 100991. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Jia, X.; Yang, Q.; Yang, Y.; Zhao, Y.; Fan, Y.; Yuan, X. Targeted delivery of microRNA-126 to vascular endothelial cells via REDV peptide modified PEG-trimethyl chitosan. Biomater. Sci. 2016, 4, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99 Pt A, 28–51. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, M.; Xie, R.; Gong, S. Enhancing the In Vitro and In Vivo Stabilities of Polymeric Nucleic Acid Delivery Nanosystems. Bioconjug. Chem. 2019, 30, 325–337. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, B.; Abdeen, A.A.; Chen, G.; Xie, R.; Saha, K.; Gong, S. Versatile Redox-Responsive Polyplexes for the Delivery of Plasmid DNA, Messenger RNA, and CRISPR-Cas9 Genome-Editing Machinery. ACS Appl. Mater. Interfaces 2018, 10, 31915–31927. [Google Scholar] [CrossRef]
- Jian, J.; Xiangbin, Z.; Xianbo, H. An overview on synthesis, properties and applications of poly(butylene-adipate-co-terephthalate)–PBAT. Adv. Ind. Eng. Polym. Res. 2020, 3, 19–26. [Google Scholar] [CrossRef]
- Weng, Y.-X.; Jin, Y.-J.; Meng, Q.-Y.; Wang, L.; Zhang, M.; Wang, Y.-Z. Biodegradation behavior of poly(butylene adipate-co-terephthalate) (PBAT), poly(lactic acid) (PLA), and their blend under soil conditions. Polym. Test. 2013, 32, 918–926. [Google Scholar] [CrossRef]
- Phothisarattana, D.; Wongphan, P.; Promhuad, K.; Promsorn, J.; Harnkarnsujarit, N. Biodegradable Poly(Butylene Adipate-Co-Terephthalate) and Thermoplastic Starch-Blended TiO2 Nanocomposite Blown Films as Functional Active Packaging of Fresh Fruit. Polymers 2021, 13, 4192. [Google Scholar] [CrossRef]
- Fukushima, K.; Rasyida, A.; Yang, M.-C. Characterization, degradation and biocompatibility of PBAT based nanocomposites. Appl. Clay Sci. 2013, 80–81, 291–298. [Google Scholar] [CrossRef]
- Chen, G.; Ma, B.; Wang, Y.; Gong, S. A Universal GSH-Responsive Nanoplatform for the Delivery of DNA, mRNA, and Cas9/sgRNA Ribonucleoprotein. ACS Appl. Mater. Interfaces 2018, 10, 18515–18523. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Shah, T.; Ullah, R.; Zhou, P.; Guo, M.; Ovais, M.; Tan, Z.; Rui, Y. Review on Recent Progress in Magnetic Nanoparticles: Synthesis, Characterization, and Diverse Applications. Front. Chem. 2021, 9, 629054. [Google Scholar] [CrossRef]
- Ernst, C.; Bartel, A.; Elferink, J.W.; Huhn, J.; Eschbach, E.; Schönfeld, K.; Feßler, A.T.; Oberheitmann, B.; Schwarz, S. Improved DNA extraction and purification with magnetic nanoparticles for the detection of methicillin-resistant Staphylococcus aureus. Vet. Microbiol. 2019, 230, 45–48. [Google Scholar] [CrossRef]
- Savliwala, S.; Chiu-Lam, A.; Unni, M.; Rivera-Rodriguez, A.; Fuller, E.; Sen, K.; Threadcraft, M.; Rinaldi, C. Chapter 13—Magnetic nanoparticles. In Nanoparticles for Biomedical Applications; Chung, E.J., Leon, L., Rinaldi, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 195–221. [Google Scholar] [CrossRef]
- Kim, I.; Yang, H.-M.; Park, C.W.; Yoon, I.-H.; Sihn, Y. 20—Environmental applications of magnetic nanoparticles. In Magnetic Nanoparticle-Based Hybrid Materials; Ehrmann, A., Nguyen, T.A., Ahmadi, M., Farmani, A., Nguyen-Tri, P., Eds.; Woodhead Publishing: Cambridge, UK, 2021; pp. 529–545. [Google Scholar] [CrossRef]
- Alagiri, M.; Muthamizhchelvan, C.; Ponnusamy, S. Structural and magnetic properties of iron, cobalt and nickel nanoparticles. Synth. Met. 2011, 161, 1776–1780. [Google Scholar] [CrossRef]
- Markides, H.; Rotherham, M.; El Haj, A.J. Biocompatibility and Toxicity of Magnetic Nanoparticles in Regenerative Medicine. J. Nanomater. 2012, 2012, 614094. [Google Scholar] [CrossRef]
- Busquets, M.A.; Espargaró, A.; Sabaté, R.; Estelrich, J. Magnetic Nanoparticles Cross the Blood-Brain Barrier: When Physics Rises to a Challenge. Nanomaterials 2015, 5, 2231–2248. [Google Scholar] [CrossRef]
- Leitner, S.; Solans, C.; García-Celma, M.J.; Morral-Ruíz, G.; Melgar-Lesmes, P.; Calderó, G. Ethylcellulose nanoparticles prepared from nano-emulsion templates as new folate binding haemocompatible platforms. Mater. Sci. Eng. C 2021, 120, 111682. [Google Scholar] [CrossRef]
- Tros de Ilarduya, C.; Sun, Y.; Düzgüneş, N. Gene delivery by lipoplexes and polyplexes. Eur J Pharm Sci. 2010, 40, 159–170. [Google Scholar] [CrossRef]
- Wasungu, L.; Hoekstra, D. Cationic lipids, lipoplexes and intracellular delivery of genes. J. Control. Release 2006, 116, 255–264. [Google Scholar] [CrossRef]
- Kim, N.J.; Yoo, J.J.; Atala, A.; Lee, S.J. Chapter 7—Small RNA Delivery for In Situ Tissue Regeneration. In Situ Tissue Regeneration; Academic Press: London, UK, 2016; pp. 111–135. [Google Scholar]
- Vasile, C. Chapter 1—Polymeric Nanomaterials: Recent Developments, Properties and Medical Applications. In Polymeric Nanomaterials in Nanotherapeutics; Vasile, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–66. [Google Scholar] [CrossRef]
- Pereira-Silva, M.; Jarak, I.; Alvarez-Lorenzo, C.; Concheiro, A.; Santos, A.C.; Maleki, R.; Veiga, F.; Figueiras, A. Micelleplexes as nucleic acid delivery systems for cancer-targeted therapies. J. Control. Release 2020, 323, 442–462. [Google Scholar] [CrossRef] [PubMed]
- Grimme, C.J.; Hanson, M.G.; Corcoran, L.G.; Reineke, T.M. Polycation Architecture Affects Complexation and Delivery of Short Antisense Oligonucleotides: Micelleplexes Outperform Polyplexes. Biomacromolecules 2022, 23, 3257–3271. [Google Scholar] [CrossRef] [PubMed]
- Zare, H.; Ahmadi, S.; Ghasemi, A.; Ghanbari, M.; Rabiee, N.; Bagherzadeh, M.; Karimi, M.; Webster, T.J.; Hamblin, M.R.; Mostafavi, E. Carbon Nanotubes: Smart Drug/Gene Delivery Carriers. Int. J. Nanomed. 2021, 16, 1681–1706. [Google Scholar] [CrossRef]
- Ghuge, A.D.; Shirode, A.R.; Kadam, V.J. Graphene: A Comprehensive Review. Curr. Drug Targets 2017, 18, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Han, X.M.; Zheng, K.W.; Wang, R.L.; Yue, S.F.; Chen, J.; Zhao, Z.W.; Song, F.; Su, Y.; Ma, Q. Functionalization and optimization-strategy of graphene oxide-based nanomaterials for gene and drug delivery. Am. J. Transl. Res. 2020, 12, 1515–1534. [Google Scholar]
- Devi, S.; Kumar, M.; Tiwari, A.; Tiwari, V.; Kaushik, D.; Verma, R.; Bhatt, S.; Sahoo, B.M.; Bhattacharya, T.; Alshehri, S.; et al. Quantum Dots: An Emerging Approach for Cancer Therapy. Front. Mater. 2022, 8, 798440. [Google Scholar] [CrossRef]
- Singh, S.; Dhawan, A.; Karhana, S.; Bhat, M.; Dinda, A.K. Quantum Dots: An Emerging Tool for Point-of-Care Testing. Micromachines 2020, 11, 1058. [Google Scholar] [CrossRef]
- Biswas, M.C.; Islam, M.T.; Nandy, P.K.; Hossain, M.M. Graphene Quantum Dots (GQDs) for Bioimaging and Drug Delivery Applications: A Review. ACS Mater. Lett. 2021, 3, 889–911. [Google Scholar] [CrossRef]
- Kumar, Y.R.; Deshmukh, K.; Sadasivuni, K.K.; Pasha, S.K.K. Graphene quantum dot based materials for sensing, bio-imaging and energy storage applications: A review. RSC Adv. 2020, 10, 23861–23898. [Google Scholar] [CrossRef]
- Henna, T.K.; Pramod, K. Graphene quantum dots redefine nanobiomedicine. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110651. [Google Scholar] [CrossRef]
- Tian, P.; Tang, L.; Teng, K.S.; Lau, S.P. Graphene quantum dots from chemistry to applications. Mater. Today Chem. 2018, 10, 221–258. [Google Scholar] [CrossRef]
- Ghafary, S.M.; Nikkhah, M.; Hatamie, S.; Hosseinkhani, S. Simultaneous Gene Delivery and Tracking through Preparation of Photo-Luminescent Nanoparticles Based on Graphene Quantum Dots and Chimeric Peptides. Sci. Rep. 2017, 7, 9552. [Google Scholar] [CrossRef] [PubMed]
- Fasbender, S.; Zimmermann, L.; Cadeddu, R.-P.; Luysberg, M.; Moll, B.; Janiak, C.; Heinzel, T.; Haas, R. The Low Toxicity of Graphene Quantum Dots is Reflected by Marginal Gene Expression Changes of Primary Human Hematopoietic Stem Cells. Sci. Rep. 2019, 9, 12028. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Cao, T.; Dai, G.; Liu, B.; Duan, H.; Kong, C.; Tian, N.; Hou, D.; Sun, Z. Recent advances in functionalized upconversion nanoparticles for light-activated tumor therapy. RSC Adv. 2021, 11, 35472–35488. [Google Scholar] [CrossRef] [PubMed]
- Rajani, C.; Borisa, P.; Karanwad, T.; Borade, Y.; Patel, V.; Rajpoot, K.; Tekade, R.K. Cancer-targeted chemotherapy: Emerging role of the folate anchored dendrimer as drug delivery nanocarrier. In Pharmaceutical Applications of Dendrimers; Chauhan, A., Kulhari, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 151–198. [Google Scholar] [CrossRef]
- Pednekar, P.P.; Godiyal, S.C.; Jadhav, K.R.; Kadam, V.J. Mesoporous silica nanoparticles: A promising multifunctional drug delivery system. In Nanostructures for Cancer Therapy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 593–621. [Google Scholar] [CrossRef]
- Watermann, A.; Brieger, J. Mesoporous Silica Nanoparticles as Drug Delivery Vehicles in Cancer. Nanomaterials 2017, 7, 189. [Google Scholar] [CrossRef]
- Szulc, D.A.; Lee, X.A.; Cheng, H.-Y.M.; Cheng, H.-L.M. Bright Ferritin—A Reporter Gene Platform for On-Demand, Longitudinal Cell Tracking on MRI. iScience 2020, 23, 101350. [Google Scholar] [CrossRef]
- Tariq, H.; Batool, S.; Asif, S.; Ali, M.; Abbasi, B.H. Virus-Like Particles: Revolutionary Platforms for Developing Vaccines Against Emerging Infectious Diseases. Front. Microbiol. 2021, 12, 790121. [Google Scholar] [CrossRef]
- Kanekiyo, M.; Buck, C.B. Virus-Like Particle and Nanoparticle Vaccines. Hum. Vaccines 2017, 13, 87–98. [Google Scholar] [CrossRef]
- Khoshnejad, M.; Parhiz, H.; Shuvaev, V.V.; Dmochowski, I.J.; Muzykantov, V.R. Ferritin-based drug delivery systems: Hybrid nanocarriers for vascular immunotargeting. J. Control. Release 2018, 282, 13–24. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, Y.; Li, Z.; Hou, X.; Feng, N. Red blood cell membrane-camouflaged nanoparticles: A novel drug delivery system for antitumor application. Acta Pharm. Sin. B 2019, 9, 675–689. [Google Scholar] [CrossRef]
- Sun, W.; Ji, W.; Hall, J.M.; Hu, Q.; Wang, C.; Beisel, C.L.; Gu, Z. Self-assembled DNA nanoclews for the efficient delivery of CRISPR-Cas9 for genome editing. Angew Chem. Int. Ed. Engl. 2015, 54, 12029–12033. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wagner, E.; Lächelt, U. Non-viral delivery of the CRISPR/Cas system: DNA versus RNA versus RNP. Biomater. Sci. 2022, 10, 1166–1192. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, Z.; Liu, Y. Recent Progress on Nonviral Delivery Carriers for CRISPR/Cas9 Systems. Mater. Matters 2020, 15, 104–111. [Google Scholar]
- Wang, Q.; Yu, J.; Kadungure, T.; Beyene, J.; Zhang, H.; Lu, Q. ARMMs as a versatile platform for intracellular delivery of macromolecules. Nat. Commun. 2018, 9, 960. [Google Scholar] [CrossRef]
- ARRDC1-Mediated Microvesicles (ARMMs) and Uses Thereof. Available online: https://patents.google.com/patent/US9737480B2/en (accessed on 4 July 2023).
- Tarvirdipour, S.; Huang, X.; Mihali, V.; Schoenenberger, C.-A.; Palivan, C.G. Peptide-Based Nanoassemblies in Gene Therapy and Diagnosis: Paving the Way for Clinical Application. Molecules 2020, 25, 3482. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Cell penetrating peptide-modified pharmaceutical nanocarriers for intracellular drug and gene delivery. Biopolymers 2008, 90, 604–610. [Google Scholar] [CrossRef]
- Taylor, R.E.; Zahid, M. Cell Penetrating Peptides, Novel Vectors for Gene Therapy. Pharmaceutics 2020, 12, 225. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Gudbergsson, J.M.; Skov, M.N.; Pilgaard, L.; Moos, T.; Duroux, M. A comprehensive overview of exosomes as drug delivery vehicles—Endogenous nanocarriers for targeted cancer therapy. Biochim. Biophys. Acta. 2014, 1846, 75–87. [Google Scholar] [CrossRef]
- Chen, W.; Li, H.; Shi, D.; Liu, Z.; Yuan, W. Microneedles as a delivery system for gene therapy. Front. Pharmacol. 2016, 7, 137. [Google Scholar] [CrossRef]
- O’Brien, J.A.; Lummis, S.C.R. Nano-biolistics: A method of biolistic transfection of cells and tissues using a gene gun with novel nanometer-sized projectiles. BMC Biotechnol. 2011, 11, 66. [Google Scholar] [CrossRef]
- Walther, W.; Stein, U.; Fichtner, I.; Malcherek, L.; Lemm, M.; Schlag, P.M. Nonviral in vivo gene delivery into tumors using a novel low volume jet-injection technology. Gene Ther. 2001, 8, 173–180. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gehl, J. Electroporation: Theory and methods, perspectives for drug delivery, gene therapy and research. Acta. Physiol. Scand. 2003, 177, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Young, J.L.; Dean, D.A. Electroporation-mediated gene delivery. Adv. Genet. 2015, 89, 49–88. [Google Scholar] [CrossRef] [PubMed]
- Package Insert—Kymriah. Available online: https://www.fda.gov/media/107296/download (accessed on 4 July 2023).
- Shirley, S.A.; Heller, R.; Heller, L.C. Chapter 7—Electroporation Gene Therapy. In Gene Therapy of Cancer, 3rd ed.; Lattime, E.C., Gerson, S.L., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 93–106. [Google Scholar]
- Krut, Z.; Gazit, D.; Gazit, Z.; Pelled, G. Applications of Ultrasound-Mediated Gene Delivery in Regenerative Medicine. Bioengineering 2022, 9, 190. [Google Scholar] [CrossRef]
- Bez, M.; Foiret, J.; Shapiro, G.; Pelled, G.; Ferrara, K.W.; Gazit, D. Nonviral ultrasound-mediated gene delivery in small and large animal models. Nat. Protoc. 2019, 14, 1015–1026. [Google Scholar] [CrossRef]
- Huang, S.-L.; McPherson, D.D. Chapter 16—Ultrasound for Drug/Gene Delivery. In Cancer Theranostics, 1st ed.; Chen, X., Wong, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 269–283. [Google Scholar] [CrossRef]
- Walsh, A.P.G.; Gordon, H.N.; Peter, K.; Wang, X. Ultrasonic particles: An approach for targeted gene delivery. Adv. Drug Deliv. Rev. 2021, 179, 113998. [Google Scholar] [CrossRef]
- Scherer, F.; Anton, M.; Schillinger, U.; Henke, J.; Bergemann, C.; Krüger, A.; Gänsbacher, B.; Plank, C. Magnetofection: Enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002, 9, 102–109. [Google Scholar] [CrossRef]
- Prosen, L.; Prijic, S.; Music, B.; Lavrencak, J.; Cemazar, M.; Sersa, G. Magnetofection: A Reproducible Method for Gene Delivery to Melanoma Cells. BioMed Res. Int. 2013, 2013, 209452. [Google Scholar] [CrossRef]
- Ramachandran, S.; Satapathy, S.R.; Dutta, T. Delivery Strategies for mRNA Vaccines. Pharm. Med. 2022, 36, 11–20. [Google Scholar] [CrossRef]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247 Pt 1, 1465–1468. [Google Scholar] [CrossRef]
- Probst, J.; Weide, B.; Scheel, B.; Pichler, B.J.; Hoerr, I.; Rammensee, H.G.; Pascolo, S. Spontaneous cellular uptake of exogenous messenger RNA in vivo is nucleic acid-specific, saturable and ion dependent. Gene Ther. 2007, 14, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Hotz, C.; Wagenaar, T.R.; Gieseke, F.; Bangari, D.S.; Callahan, M.; Cao, H.; Diekmann, J.; Diken, M.; Grunwitz, C.; Hebert, A.; et al. Local delivery of mRNA-encoded cytokines promotes antitumor immunity and tumor eradication across multiple preclinical tumor models. Sci. Transl. Med. 2021, 13, eabc7804. [Google Scholar] [CrossRef] [PubMed]
- Dirisala, A.; Uchida, S.; Tockary, T.A.; Yoshinaga, N.; Li, J.; Osawa, S.; Gorantla, L.; Fukushima, S.; Osada, K.; Kataoka, K. Precise tuning of disulphide crosslinking in mRNA polyplex micelles for optimising extracellular and intracellular nuclease tolerability. J. Drug Target. 2019, 27, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ma, L.; Gao, C. Design of gene-activated matrix for the repair of skin and cartilage. Polym. J. 2014, 46, 476–482. [Google Scholar] [CrossRef]
- Suda, T.; Liu, D. Hydrodynamic Gene Delivery: Its Principles and Applications. Mol. Ther. 2007, 15, 2063–2069. [Google Scholar] [CrossRef]
- Zhang, G.; Gao, X.; Song, Y.K.; Vollmer, R.; Stolz, D.B.; Gasiorowski, J.Z.; Dean, D.A.; Liu, D. Hydroporation as the mechanism of hydrodynamic delivery. Gene Ther. 2004, 11, 675–682. [Google Scholar] [CrossRef]
- IMLYGIC. Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/imlygic (accessed on 4 July 2023).
- Detela, G.; Lodge, A. EU Regulatory Pathways for ATMPs: Standard, Accelerated and Adaptive Pathways to Marketing Authorisation. Mol. Ther. Methods Clin. Dev. 2019, 13, 205–232. [Google Scholar] [CrossRef]
- LUXTURNA. Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/luxturna (accessed on 4 July 2023).
- Beheshtizadeh, N.; Gharibshahian, M.; Pazhouhnia, Z.; Rostami, M.; Rajabi Zangi, A.; Maleki, R.; Kolahi Azar, H.; Zalouli, V.; Rajavand, H.; Farzin, A.; et al. Commercialization and regulation of regenerative medicine products: Promises, advances and challenges. Biomed. Pharmacother. 2022, 153, 113431. [Google Scholar] [CrossRef]
- ZOLGENSMA. Available online: https://www.fda.gov/vaccines-blood-biologics/zolgensma (accessed on 4 July 2023).
- Spinraza. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/spinraza (accessed on 4 July 2023).
- Onpattro. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/onpattro (accessed on 4 July 2023).
- Zhang, W.W.; Li, L.; Li, D.; Liu, J.; Li, X.; Li, W.; Xu, X.; Zhang, M.J.; Chandler, L.A.; Lin, H.; et al. The First Approved Gene Therapy Product for Cancer Ad-p53 (Gendicine): 12 Years in the Clinic. Hum. Gene Ther. 2018, 29, 160–179. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Lu, Y.-Y.; Peng, Z.-H. 10—Recombinant Adenoviral-p53 Agent (Gendicine®): Quality Control, Mechanism of Action, and Its Use for Treatment of Malignant Tumors. In Recent Advances in Cancer Research and Therapy; Elsevier: Amsterdam, The Netherlands, 2012; pp. 215–243. [Google Scholar] [CrossRef]
- Vinokurov, M.M.; Yakovlev, A.A.; Ushnitsky, I.D.; Palshin, G.A.; Petrov, A.P.; Popov, V.V.; Yalinskaya, T.V. The Experience of Using “Neovasculgen” in the Complex Treatment of Patients with Critical Lower Limb Ischemia. Int. J. Biomed. 2020, 10, 287–290. [Google Scholar] [CrossRef]
- Cao, G.; He, X.; Sun, Q.; Chen, S.; Wan, K.; Xu, X.; Feng, X.; Li, P.; Chen, B.; Xiong, M. The Oncolytic Virus in Cancer Diagnosis and Treatment. Front. Oncol. 2020, 10, 1786. [Google Scholar] [CrossRef] [PubMed]
- Liang, M. Oncorine, the World First Oncolytic Virus Medicine and its Update in China. Curr. Cancer Drug Targets 2018, 18, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Defitelio. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/defitelio (accessed on 4 July 2023).
- ADSTILADRIN. Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/adstiladrin (accessed on 4 July 2023).
- Package Insert—ADSTILADRIN. Available online: https://www.fda.gov/media/164029/download (accessed on 4 July 2023).
- Hemgenix. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/hemgenix (accessed on 4 July 2023).
- HEMGENIX. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/hemgenix (accessed on 4 July 2023).
- Package Insert—HEMGENIX. Available online: https://www.fda.gov/media/163467/download (accessed on 4 July 2023).
- Upstaza. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/upstaza (accessed on 4 July 2023).
- Upstaza (Eladocagene Exuparvovec). Available online: https://www.ema.europa.eu/en/documents/overview/upstaza-epar-medicine-overview_en.pdf (accessed on 4 July 2023).
- Abecma—Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/abecma-epar-product-information_en.pdf (accessed on 4 July 2023).
- ABECMA (Idecabtagene Vicleucel). Available online: https://www.fda.gov/vaccines-blood-biologics/abecma-idecabtagene-vicleucel (accessed on 4 July 2023).
- Approval Letter—ABECMA. Available online: https://www.fda.gov/media/147062/download (accessed on 4 July 2023).
- Abecma. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/abecma (accessed on 4 July 2023).
- Carvykti. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/carvykti (accessed on 4 July 2023).
- Carvykti—Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/carvykti-epar-product-information_en.pdf (accessed on 4 July 2023).
- Approval Letter—CARVYKTI. Available online: https://www.fda.gov/vaccines-blood-biologics/carvykti (accessed on 4 July 2023).
- Package Insert—CARVYKTI. Available online: https://www.fda.gov/media/156560/download (accessed on 4 July 2023).
- Approval Letter—SKYSONA. Available online: https://www.fda.gov/media/161665/download (accessed on 4 July 2023).
- Evans, C.H. The vicissitudes of gene therapy. Bone Joint Res. 2019, 8, 469–471. [Google Scholar] [CrossRef]
- Skysona. Available online: https://www.fda.gov/vaccines-blood-biologics/skysona (accessed on 4 July 2023).
- YESCARTA (Axicabtagene Ciloleucel). Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/yescarta-axicabtagene-ciloleucel (accessed on 4 July 2023).
- Package Insert—YESCARTA (Axicabtagene Ciloleucel). Available online: https://www.fda.gov/media/108377/download (accessed on 4 July 2023).
- KYMRIAH (Tisagenlecleucel). Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/kymriah-tisagenlecleucel (accessed on 4 July 2023).
- Kymriah. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/kymriah (accessed on 4 July 2023).
- Labbé, R.P.; Vessillier, S.; Rafiq, Q.A. Lentiviral Vectors for T Cell Engineering: Clinical Applications, Bioprocessing and Future Perspectives. Viruses 2021, 13, 1528. [Google Scholar] [CrossRef] [PubMed]
- Strimvelis. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/strimvelis (accessed on 4 July 2023).
- Breyanzi. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/breyanzi (accessed on 4 July 2023).
- Breyanzi. Available online: https://www.ema.europa.eu/en/documents/product-information/breyanzi-epar-product-information_en.pdf (accessed on 4 July 2023).
- Breyanzi (Lisocabtagene Maraleucel). Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/breyanzi-lisocabtagene-maraleucel (accessed on 4 July 2023).
- Tecartus (Brexucabtagene Autoleucel). Available online: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/tecartus-brexucabtagene-autoleucel (accessed on 4 July 2023).
- Tecartus. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/tecartus (accessed on 4 July 2023).
- Zynteglo. Available online: https://www.fda.gov/vaccines-blood-biologics/zynteglo (accessed on 4 July 2023).
- Libmeldy. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/libmeldy (accessed on 4 July 2023).
- Ganesan, L.P.; Mohanty, S.; Kim, J.; Clark, K.R.; Robinson, J.M.; Anderson, C.L. Rapid and Efficient Clearance of Blood-borne Virus by Liver Sinusoidal Endothelium. PLoS Pathog. 2011, 7, e1002281. [Google Scholar] [CrossRef]
- Dirisala, A.; Uchida, S.; Toh, K.; Li, J.; Osawa, S.; Tockary, T.A.; Liu, X.; Abbasi, S.; Hayashi, K.; Mochida, Y.; et al. Transient stealth coating of liver sinusoidal wall by anchoring two-armed PEG for retargeting nanomedicines. Sci. Adv. 2020, 6, eabb8133. [Google Scholar] [CrossRef]
- Lindsay, K.E.; Bhosle, S.M.; Zurla, C.; Beyersdorf, J.; Rogers, K.A.; Vanover, D.; Xiao, P.; Araínga, M.; Shirreff, L.M.; Pitard, B.; et al. Visualization of early events in mRNA vaccine delivery in non-human primates via PET–CT and near-infrared imaging. Nat. Biomed. Eng. 2019, 3, 371–380. [Google Scholar] [CrossRef]
- Chen, J.; Ye, Z.; Huang, C.; Qiu, M.; Song, D.; Li, Y.; Xu, Q. Lipid nanoparticle-mediated lymph node-targeting delivery of mRNA cancer vaccine elicits robust CD8+ T cell response. Proc. Natl. Acad. Sci. USA 2022, 119, e2207841119. [Google Scholar] [CrossRef]
- Parhiz, H.; Brenner, J.S.; Patel, P.N.; Papp, T.E.; Shahnawaz, H.; Li, Q.; Shi, R.; Zamora, M.E.; Yadegari, A.; Marcos-Contreras, O.A.; et al. Added to pre-existing inflammation, mRNA-lipid nanoparticles induce inflammation exacerbation (IE). J. Control. Release 2022, 344, 50–61. [Google Scholar] [CrossRef]
- Dirisala, A.; Li, J.; Gonzalez-Carter, D.; Wang, Z. Editorial: Delivery systems in biologics-based therapeutics. Front. Bioeng. Biotechnol. 2023, 11, 1274210. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).