Current Overview of Metal Nanoparticles’ Synthesis, Characterization, and Biomedical Applications, with a Focus on Silver and Gold Nanoparticles
Abstract
:1. Introduction
2. Classification of NPs
2.1. Organic Nanoparticles
2.2. Inorganic Nanoparticles
2.3. Carbon-Based Nanoparticles
3. Synthesis of Metal Nanoparticles
3.1. Synthesis of Silver Nanoparticles (AgNPs)
| NPs Type | Plants | ||||
|---|---|---|---|---|---|
| AgNPs | Species | Biological material, conditions | Salt concentration (AgNO3) | Mixing conditions | Reference |
| Adenia trilobata | leaves, 5 g % (70 °C for 25 min) | 1–5 mM | 60 °C, 30 min and kept at room temperature for 1 h | [95] | |
| Buchanania lanzan | leaves, 5 g % (boiled for 5 min) | 0.5 mM | 70 °C, 1 h | [96] | |
| Cannabis sativa | leaves, 1 g % (60 °C) | 3 mM | 37 °C, 24 h | [97] | |
| Embelia ribes | fruits, extraction of embelin | 1 mM | 30 min in the dark and incubation for 24 h | [98] | |
| Lythrum salicaria | aerial part, 10 g % (40 °C) | 3 mM | 60 °C, pH = 8, 180 min | [99] | |
| Moringa oleifera | leaves, 10 g % (100 °C for 20–30 min) | 1 mM | 60–80 °C, 60 min | [100] | |
| Picea abies, Pinus nigra | bark, 10 g % (60 °C) | 1 mM | room temperature, 60 min | [101] | |
| Sanvitalia procumbens | plant, 10 g % (70 °C) | 0.01 M | 70 °C, pH = 8, 8 h | [102] | |
| Tagetes erecta | flowers, 10 g % | 3 mM | 40 °C, pH = 8, 75 min | [103] | |
| Theobroma cacao | cocoa pods and leaves, 15 g % (boiled for 10 min) | 1 mM | room temperature, 4–24 h | [104] | |
| Microorganisms | |||||
| Bacillus cereus | 37 °C, 24 h, 120 rpm | 1 mM | 48.5 °C, pH = 9, 69 h | [105] | |
| Bacillus subtilis, B. cereus, B. megaterium, B. pumilus, B. circulans | isolates grown aerobically, 37 °C, 24 h | 1 mM | 30 °C, 24 h | [106] | |
| Klebsiella pneumonia | 37 °C, 24 h | 4 mM | 37 °C, pH = 10, 24 h | [107] | |
| Lactobacillus bulgaricus | isolated colonies from raw milk, 37 °C | 1 mM | room temperature | [108] | |
| Aspergillus terreus | strain isolated from soil, 25 °C, 5 days, 200 rpm | 1 mM | 25 °C, 200 rpm | [109] | |
| Penicillium verrucosum | strain isolated from vegetable-cultivated greenhouse soil, 25 °C, 7 days, 150 rpm | 10 mM | 25 °C, in the dark | [110] | |
| Algae | |||||
| Chlorella vulgaris | algal suspension | 10 mM | 25 °C, 48 h | [111] | |
| Dunaliella salina | culture grown for 2 weeks, 12 h in light and 12 h in dark | 1 mM | in the dark, 48 h | [112] | |
| Ulva rigida (green alga) Cystoseira myrica (brown alga) Gracilaria foliifera (red alga) | ethanolic and aqueous extracts obtained from dried algal powder, room temperature, 24 h | 1 mM | 70 °C, 15 min | [113] | |
| AuNPs | Species | Biological material, conditions | Salt concentration (HAuCl4) | Mixing conditions | Reference |
| Plants | |||||
| Allium ampeloprasum | leaves, 10 g % (boiled) | 1 mM | room temperature, 30 min | [114] | |
| Annona muricata | leaves, ~ 13 g % (20 g–150 mL) (boiled 20 min) | 1 mM | room temperature, 22 h | [115] | |
| Citrus maxima | peel, 1 g % (boiled for 10 min) | 100 mM | room temperature, 1 h | [116] | |
| Dillenia indica | leaves, 1 g % (100 °C for 30 min) | 1 mM | dark, 30 min | [117] | |
| Dracocephalum kotschyi | leaves, ~ 7 g % (5 g–75 mL) boiled | 1 mM | 10 min | [118] | |
| Lonicera japonica | flowers, 10 g % (boiled 20 min) | 1 mM | 70 °C, 30 min | [119] | |
| Mentha and Pelargonium | plant (boiled 20 min) | - | boiling | [120] | |
| Pergularia daemia | leaves, 10 g % (boiling at 60 °C for 30 min) | 1 mM | 15 min | [121] | |
| Platycodon grandiflorum | leaves, 10 g % (boiled for 20 min) | 1 mM | 50 °C, 15 min | [122] | |
| Tecoma capensis | leaves, 5 g % (heated till boiling ~20 min) | 0.011 M | heating, 10 min | [123] | |
| Microorganisms | |||||
| Bacillus cereus Fusarium oxysporum | bacterial culture incubated at 37 °C, 150 rpm, 24 h fungal culture incubated at 30 °C, 150 rpm, 72 h | 1 mM | 37 °C, 200 rpm, 24 h | [124] | |
| Pseudomonas | culture, 24 h | 1 mM | 80–85 °C, 30 min | [125] | |
| Candida albicans | cytosolic extract | 1 mM | 24 h | [126] | |
| Algae | |||||
| Chlorella sorokiniana | aqueous extract, 80 °C, 20 min | 1 mM | 80 °C, pH = 8, 60 min | [127] | |
| Galaxaura elongata | powder form and ethanolic extract | 1 mM | room temperature, 120 rpm, 12 h | [128] | |
| Oscillatoria sp. Spirulina platensis | microalgal cultures | 5 mM | room temperature, 24 h | [129] | |
3.2. Synthesis of Gold Nanoparticles (AuNPs)
4. Characterization of Metal NPs
4.1. Visual Inspection and UV-Vis Spectroscopy
4.2. FTIR Spectroscopy
4.3. Dynamic Light Scattering (DLS) and Zeta Potential Analysis
4.4. SEM, TEM, EDX
4.5. XRD Analysis
4.6. Other Characterization Techniques
5. Biomedical Applications of Metal NPs
5.1. Diagnostics
5.2. Anticancer Activity
5.3. Angiogenesis Inhibition
5.4. Antimicrobial Properties
5.5. Antiviral Action
5.6. Anti-Inflammatory Activity
5.7. AgNPs and AuNPs in Clinical Trials
6. Cytotoxicity of Metal NPs
7. Key Challenges
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rana, A.; Yadav, K.; Jagadevan, S. A Comprehensive Review on Green Synthesis of Nature-Inspired Metal Nanoparticles: Mechanism, Application and Toxicity. J. Clean. Prod. 2020, 272, 122880. [Google Scholar] [CrossRef]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Pal, T. Interparticle Coupling Effect on the Surface Plasmon Resonance of Gold Nanoparticles: From Theory to Applications. Chem. Rev. 2007, 107, 4797–4862. [Google Scholar] [CrossRef]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and Nanoparticles: Sources and Toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef]
- Jacob, S.J.P.; Prasad, V.L.S.; Sivasankar, S.; Muralidharan, P. Biosynthesis of Silver Nanoparticles Using Dried Fruit Extract of Ficus Carica—Screening for Its Anticancer Activity and Toxicity in Animal Models. Food Chem. Toxicol. 2017, 109, 951–956. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Machado, S.; Pacheco, J.G.; Nouws, H.P.A.; Albergaria, J.T.; Delerue-Matos, C. Characterization of Green Zero-Valent Iron Nanoparticles Produced with Tree Leaf Extracts. Sci. Total Environ. 2015, 533, 76–81. [Google Scholar] [CrossRef]
- Talarska, P.; Boruczkowski, M.; Żurawski, J. Current Knowledge of Silver and Gold Nanoparticles in Laboratory Research—Application, Toxicity, Cellular Uptake. Nanomaterials 2021, 11, 2454. [Google Scholar] [CrossRef]
- Modena, M.M.; Rühle, B.; Burg, T.P.; Wuttke, S. Nanoparticle Characterization: What to Measure? Adv. Mater. 2019, 31, 1901556. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Yaqoob, S.B.; Adnan, R.; Rameez Khan, R.M.; Rashid, M. Gold, Silver, and Palladium Nanoparticles: A Chemical Tool for Biomedical Applications. Front. Chem. 2020, 8, 376. [Google Scholar] [CrossRef]
- Loiseau, A.; Asila, V.; Boitel-Aullen, G.; Lam, M.; Salmain, M.; Boujday, S. Silver-Based Plasmonic Nanoparticles for and Their Use in Biosensing. Biosensors 2019, 9, 78. [Google Scholar] [CrossRef]
- Khan, A.; Rashid, R.; Murtaza, G.; Zahra, A. Gold Nanoparticles: Synthesis and Applications in Drug Delivery. Trop. J. Pharm. Res. 2014, 13, 1169. [Google Scholar] [CrossRef]
- Păduraru, D.N.; Ion, D.; Niculescu, A.-G.; Mușat, F.; Andronic, O.; Grumezescu, A.M.; Bolocan, A. Recent Developments in Metallic Nanomaterials for Cancer Therapy, Diagnosing and Imaging Applications. Pharmaceutics 2022, 14, 435. [Google Scholar] [CrossRef]
- Heinemann, M.G.; Rosa, C.H.; Rosa, G.R.; Dias, D. Biogenic Synthesis of Gold and Silver Nanoparticles Used in Environmental Applications: A Review. Trends Environ. Anal. Chem. 2021, 30, e00129. [Google Scholar] [CrossRef]
- Ealia, S.A.M.; Saravanakumar, M.P. A Review on the Classification, Characterisation, Synthesis of Nanoparticles and Their Application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032019. [Google Scholar] [CrossRef]
- Khan, Y.; Sadia, H.; Shah, S.Z.A.; Khan, M.N.; Shah, A.A.; Ullah, N.; Ullah, M.F.; Bibi, H.; Bafakeeh, O.T.; Khedher, N.B.; et al. Classification, Synthetic, and Characterization Approaches to Nanoparticles, and Their Applications in Various Fields of Nanotechnology: A Review. Catalysts 2022, 12, 1386. [Google Scholar] [CrossRef]
- Esakkimuthu, T.; Sivakumar, D.; Akila, S. Application of Nanoparticles in Wastewater Treatment. Pollut. Res. 2014, 33, 567–571. [Google Scholar]
- Ahmad, Z.; Shah, A.; Siddiq, M.; Kraatz, H.-B. Polymeric Micelles as Drug Delivery Vehicles. RSC Adv. 2014, 4, 17028–17038. [Google Scholar] [CrossRef]
- Buse, J.; El-Aneed, A. Properties, Engineering and Applications of Lipid-Based Nanoparticle Drug-Delivery Systems: Current Research and Advances. Nanomedicine 2010, 5, 1237–1260. [Google Scholar] [CrossRef]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Oberholzer, T.; Luisi, P.L. The Use of Liposomes for Constructing Cell Models. J. Biol. Phys. 2002, 28, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.; Bugno, J.; Lee, S.; Hong, S. Dendrimer-based nanocarriers: A versatile platform for drug delivery. WIREs Nanomed. Nanobiotechnology 2017, 9, e1409. [Google Scholar] [CrossRef] [PubMed]
- Palmerston Mendes, L.; Pan, J.; Torchilin, V.P. Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy. Molecules 2017, 22, 1401. [Google Scholar] [CrossRef]
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Andronescu, E. Polymeric Nanoparticles for Antimicrobial Therapies: An up-to-date Overview. Polymers 2021, 13, 724. [Google Scholar] [CrossRef] [PubMed]
- Vardaxi, A.; Kafetzi, M.; Pispas, S. Polymeric Nanostructures Containing Proteins and Peptides for Pharmaceutical Applications. Polymers 2022, 14, 777. [Google Scholar] [CrossRef]
- Nemtsev, I.V.; Shabanova, O.V.; Shestakov, N.P.; Cherepakhin, A.V.; Zyryanov, V.Y. Morphology Stability of Polymethylmethacrylate Nanospheres Formed in Water–Acetone Dispersion Medium. Appl. Phys. A 2019, 125, 738. [Google Scholar] [CrossRef]
- Shabanova, O.V.; Korshunov, M.A.; Nemtsev, I.V.; Shabanov, A.V. Features of Self-Assembly of Opal-like Structures Based on Poly(Methyl Methacrylate) Submicron Dispersions. Nanotechnologies Russ. 2016, 11, 633–639. [Google Scholar] [CrossRef]
- Batista, F.A.; Fontele, S.B.C.; Santos, L.K.B.; Filgueiras, L.A.; Nascimento, S.Q.; Sousa, J.M.d.C.e.; Gonçalves, J.C.R.; Mendes, A.N. Synthesis, characterization of α-terpineol-loaded PMMA nanoparticles as proposed of therapy for melanoma. Mater. Today Commun. 2020, 22, 100762. [Google Scholar] [CrossRef]
- Aljabali, A.A.; Rezigue, M.; Alsharedeh, R.H.; Obeid, M.A.; Mishra, V.; Serrano-Aroca, Á.; El-Tanani, M.; Tambuwala, M.M. Protein-Based Nanomaterials: A New Tool for Targeted Drug Delivery. Ther. Deliv. 2022, 13, 321–338. [Google Scholar] [CrossRef]
- Kianfar, E. Protein Nanoparticles in Drug Delivery: Animal Protein, Plant Proteins and Protein Cages, Albumin Nanoparticles. J. Nanobiotechnol. 2021, 19, 159. [Google Scholar] [CrossRef] [PubMed]
- Estrada, L.P.H.; Champion, J.A. Protein nanoparticles for therapeutic protein delivery. Biomater. Sci. 2015, 3, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G.; Lee, W.; Park, H.H. Protein-Based Nanoparticles as Drug Delivery Systems. Pharmaceutics 2020, 12, 604. [Google Scholar] [CrossRef] [PubMed]
- Alemán, J.V.; Chadwick, A.V.; He, J.; Hess, M.; Horie, K.; Jones, R.G.; Kratochvíl, P.; Meisel, I.; Mita, I.; Moad, G.; et al. Definitions of Terms Relating to the Structure and Processing of Sols, Gels, Networks, and Inorganic-Organic Hybrid Materials (IUPAC Recommendations 2007). Pure Appl. Chem. 2007, 79, 1801–1829. [Google Scholar] [CrossRef]
- Neamtu, I.; Rusu, A.G.; Diaconu, A.; Nita, L.E.; Chiriac, A.P. Basic Concepts and Recent Advances in Nanogels as Carriers for Medical Applications. Drug Deliv. 2017, 24, 539–557. [Google Scholar] [CrossRef]
- Khan, M.U.; Ullah, H.; Honey, S.; Talib, Z.; Abbas, M.; Umar, A.; Ahmad, T.; Sohail, J.; Sohail, A.; Makgopa, K.; et al. Metal Nanoparticles: Synthesis Approach, Types and Applications—A Mini Review. Nano-Horizons 2023, 2, 1–21. [Google Scholar] [CrossRef]
- Salavati-Niasari, M.; Davar, F.; Mir, N. Synthesis and Characterization of Metallic Copper Nanoparticles via Thermal Decomposition. Polyhedron 2008, 27, 3514–3518. [Google Scholar] [CrossRef]
- Tai, C.Y.; Tai, C.-T.; Chang, M.-H.; Liu, H.-S. Synthesis of Magnesium Hydroxide and Oxide Nanoparticles Using a Spinning Disk Reactor. Ind. Eng. Chem. Res. 2007, 46, 5536–5541. [Google Scholar] [CrossRef]
- Vargas-Ortiz, J.R.; Gonzalez, C.; Esquivel, K. Magnetic Iron Nanoparticles: Synthesis, Surface Enhancements, and Biological Challenges. Processes 2022, 10, 2282. [Google Scholar] [CrossRef]
- Le, N.; Zhang, M.; Kim, K. Quantum Dots and Their Interaction with Biological Systems. Int. J. Mol. Sci. 2022, 23, 10763. [Google Scholar] [CrossRef]
- Bruckmann, F.d.S.; Nunes, F.B.; Salles, T.d.R.; Franco, C.; Cadoná, F.C.; Rhoden, C.R.B. Biological Applications of Silica-Based Nanoparticles. Magnetochemistry 2022, 8, 131. [Google Scholar] [CrossRef]
- Shahmoradi, S.; Shariati, A.; Amini, S.M.; Zargar, N.; Yadegari, Z.; Darban-Sarokhalil, D. The Application of Selenium Nanoparticles for Enhancing the Efficacy of Photodynamic Inactivation of Planktonic Communities and the Biofilm of Streptococcus Mutans. BMC Res. Notes 2022, 15, 84. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, Z.; Zheng, K.; Ren, Y.; Wang, M.; Wu, Q.; Zhou, F.; Liu, C.; Liu, L.; Song, J.; et al. Degradable Mesoporous Semimetal Antimony Nanospheres for Near-Infrared II Multimodal Theranostics. Nat. Commun. 2022, 13, 539. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, M.-A.; Faghfouri, L.; Ferreira, M.P.A.; Figueiredo, P.; Maleki, H.; Sefat, F.; Hirvonen, J.; Santos, H.A. The Versatile Biomedical Applications of Bismuth-Based Nanoparticles and Composites: Therapeutic, Diagnostic, Biosensing, and Regenerative Properties. Chem. Soc. Rev. 2020, 49, 1253–1321. [Google Scholar] [CrossRef]
- Vines, J.B.; Yoon, J.-H.; Ryu, N.-E.; Lim, D.-J.; Park, H. Gold Nanoparticles for Photothermal Cancer Therapy. Front. Chem. 2019, 7, 167. [Google Scholar] [CrossRef]
- Koga, K.; Tagami, T.; Ozeki, T. Gold Nanoparticle-Coated Thermosensitive Liposomes for the Triggered Release of Doxorubicin, and Photothermal Therapy Using a near-Infrared Laser. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 626, 127038. [Google Scholar] [CrossRef]
- Amini, S.M.; Rezayat, S.M.; Dinarvand, R.; Kharrazi, S.; Jaafari, M.R. Gold Cluster Encapsulated Liposomes: Theranostic Agent with Stimulus Triggered Release Capability. Med. Oncol. 2023, 40, 126. [Google Scholar] [CrossRef]
- Koosha, F.; Farsangi, Z.J.; Samadian, H.; Amini, S.M. Mesoporous Silica Coated Gold Nanorods: A Multifunctional Theranostic Platform for Radiotherapy and X-Ray Imaging. J. Porous Mater. 2021, 28, 1961–1968. [Google Scholar] [CrossRef]
- Pangli, H.; Vatanpour, S.; Hortamani, S.; Jalili, R.; Ghahary, A. Incorporation of Silver Nanoparticles in Hydrogel Matrices for Controlling Wound Infection. J. Burn Care Res. 2021, 42, 785–793. [Google Scholar] [CrossRef]
- Bhaviripudi, S.; Mile, E.; Steiner, S.A.; Zare, A.T.; Dresselhaus, M.S.; Belcher, A.M.; Kong, J. CVD Synthesis of Single-Walled Carbon Nanotubes from Gold Nanoparticle Catalysts. J. Am. Chem. Soc. 2007, 129, 1516–1517. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Issaabadi, Z.; Sajjadi, M.; Sajadi, S.M.; Atarod, M. Types of Nanostructures. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 29–80. [Google Scholar]
- Lu, Z.; Dai, T.; Huang, L.; Kurup, D.B.; Tegos, G.P.; Jahnke, A.; Wharton, T.; Hamblin, M.R. Photodynamic Therapy with a Cationic Functionalized Fullerene Rescues Mice from Fatal Wound Infections. Nanomedicine 2010, 5, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Gaur, M.; Misra, C.; Yadav, A.B.; Swaroop, S.; Maolmhuaidh, F.Ó.; Bechelany, M.; Barhoum, A. Biomedical Applications of Carbon Nanomaterials: Fullerenes, Quantum Dots, Nanotubes, Nanofibers, and Graphene. Materials 2021, 14, 5978. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular Uptake of Nanoparticles: Journey inside the Cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Amini, F.; Ehrmann, A. Recent Advances in Carbon Nanofibers and Their Applications—A Review. Eur. Polym. J. 2020, 138, 109963. [Google Scholar] [CrossRef]
- Fan, Y.; Fowler, G.D.; Zhao, M. The Past, Present and Future of Carbon Black as a Rubber Reinforcing Filler—A Review. J. Clean. Prod. 2020, 247, 119115. [Google Scholar] [CrossRef]
- Silva, L.P.; Reis, I.G.; Bonatto, C.C. Green Synthesis of Metal Nanoparticles by Plants: Current Trends and Challenges. In Green Processes for Nanotechnology; Springer International Publishing: Cham, Switzerland, 2015; pp. 259–275. [Google Scholar]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green Synthesis of Nanoparticles: Current Developments and Limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Ramanathan, S.; Gopinath, S.C.B.; Arshad, M.K.M.; Poopalan, P.; Perumal, V. Nanoparticle Synthetic Methods: Strength and Limitations. In Nanoparticles in Analytical and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2021; pp. 31–43. [Google Scholar] [CrossRef]
- Krishnia, L.; Thakur, P.; Thakur, A. Synthesis of Nanoparticles by Physical Route. In Synthesis and Applications of Nanoparticles; Springer Nature: Singapore, 2022; pp. 45–59. [Google Scholar]
- Biswas, A.; Bayer, I.S.; Biris, A.S.; Wang, T.; Dervishi, E.; Faupel, F. Advances in Top–down and Bottom–up Surface Nanofabrication: Techniques, Applications & Future Prospects. Adv. Colloid Interface Sci. 2012, 170, 2–27. [Google Scholar] [CrossRef]
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of Nanomaterials Using Various Top-down and Bottom-up Approaches, Influencing Factors, Advantages, and Disadvantages: A Review. Adv. Colloid Interface Sci. 2022, 300, 102597. [Google Scholar] [CrossRef]
- Ali, A.; Shah, T.; Ullah, R.; Zhou, P.; Guo, M.; Ovais, M.; Tan, Z.; Rui, Y. Review on Recent Progress in Magnetic Nanoparticles: Synthesis, Characterization, and Diverse Applications. Front. Chem. 2021, 9, 629054. [Google Scholar] [CrossRef]
- Stolyar, S.V.; Komogortsev, S.V.; Gorbenko, A.S.; Knyazev, Y.V.; Yaroslavtsev, R.N.; Olkhovskiy, I.A.; Neznakhin, D.S.; Tyumentseva, A.V.; Bayukov, O.A.; Iskhakov, R.S. Maghemite Nanoparticles for DNA Extraction: Performance and Blocking Temperature. J. Supercond. Nov. Magn. 2022, 35, 1929–1936. [Google Scholar] [CrossRef]
- Ivănescu, B.; Burlec, A.F.; Crivoi, F.; Roșu, C.; Corciovă, A. Secondary Metabolites from Artemisia Genus as Biopesticides and Innovative Nano-Based Application Strategies. Molecules 2021, 26, 3061. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Park, J.H.; Han, J.W.; Kim, J.-H. Comparative Assessment of the Apoptotic Potential of Silver Nanoparticles Synthesized by Bacillus tequilensis and Calocybe indica in MDA-MB-231 Human Breast Cancer Cells: Targeting P53 for Anticancer Therapy. Int. J. Nanomed. 2015, 10, 4203–4223. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-R.; Xie, X.-B.; Shi, Q.-S.; Zeng, H.-Y.; OU-Yang, Y.-S.; Chen, Y.-B. Antibacterial Activity and Mechanism of Silver Nanoparticles on Escherichia Coli. Appl. Microbiol. Biotechnol. 2010, 85, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Parishcha, R.; Ajaykumar, P.V.; Alam, M.; Kumar, R.; et al. Fungus-Mediated Synthesis of Silver Nanoparticles and Their Immobilization in the Mycelial Matrix: A Novel Biological Approach to Nanoparticle Synthesis. Nano Lett. 2001, 1, 515–519. [Google Scholar] [CrossRef]
- Lee, S.; Jun, B.-H. Silver Nanoparticles: Synthesis and Application for Nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef]
- Kruis, F.E.; Fissan, H.; Rellinghaus, B. Sintering and Evaporation Characteristics of Gas-Phase Synthesis of Size-Selected PbS Nanoparticles. Mater. Sci. Eng. B 2000, 69–70, 329–334. [Google Scholar] [CrossRef]
- Jung, J.H.; Oh, C.H.; Noh, H.S.; Ji, J.H.; Kim, S.S. Metal nanoparticle generation using a small ceramic heater with a local heating area. J. Aerosol Sci. 2006, 37, 1662–1670. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Yeh, C.-S. Laser Ablation Method: Use of Surfactants to Form the Dispersed Ag Nanoparticles. Colloids Surfaces A Physicochem. Eng. Asp. 2002, 197, 133–139. [Google Scholar] [CrossRef]
- Chugh, H.; Sood, D.; Chandra, I.; Tomar, V.; Dhawan, G.; Chandra, R. Role of Gold and Silver Nanoparticles in Cancer Nano-Medicine. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1210–1220. [Google Scholar] [CrossRef]
- Elsupikhe, R.F.; Shameli, K.; Ahmad, M.B.; Ibrahim, N.A.; Zainudin, N. Green Sonochemical Synthesis of Silver Nanoparticles at Varying Concentrations of κ-Carrageenan. Nanoscale Res. Lett. 2015, 10, 302. [Google Scholar] [CrossRef] [PubMed]
- Shameli, K.; Ahmad, M.B.; Yunis, W.Z.; Ibrahim, N.A.; Darroudi, M.; Gharayebi, Y.; Sedaghat, S. Synthesis of Silver/Montmorillonite Nanocomposites Using γ-Irradiation. Int. J. Nanomed. 2010, 5, 1067–1077. [Google Scholar] [CrossRef]
- El-Nour, K.M.M.A.; Eftaiha, A.; Al-Warthan, A.; Ammar, R.A.A. Synthesis and Applications of Silver Nanoparticles. Arab. J. Chem. 2010, 3, 135–140. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of Silver Nanoparticles: Chemical, Physical and Biological Methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Kinnear, C.; Moore, T.L.; Rodriguez-Lorenzo, L.; Rothen-Rutishauser, B.; Petri-Fink, A. Form Follows Function: Nanoparticle Shape and Its Implications for Nanomedicine. Chem. Rev. 2017, 117, 11476–11521. [Google Scholar] [CrossRef] [PubMed]
- Pillai, Z.S.; Kamat, P.V. What Factors Control the Size and Shape of Silver Nanoparticles in the Citrate Ion Reduction Method? J. Phys. Chem. B 2004, 108, 945–951. [Google Scholar] [CrossRef]
- Turkevich, J. Colloidal Gold. Part I. Gold Bull. 1985, 18, 86–91. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of Thiol-Derivatised Gold Nanoparticles in a Two-Phase Liquid–Liquid System. J. Chem. Soc. Chem. Commun. 1994, 7, 801–802. [Google Scholar] [CrossRef]
- Evanoff, D.D.; Chumanov, G. Synthesis and Optical Properties of Silver Nanoparticles and Arrays. ChemPhysChem 2005, 6, 1221–1231. [Google Scholar] [CrossRef]
- Goulet, P.J.G.; Lennox, R.B. New Insights into Brust−Schiffrin Metal Nanoparticle Synthesis. J. Am. Chem. Soc. 2010, 132, 9582–9584. [Google Scholar] [CrossRef]
- Mallick, K.; Witcomb, M.J.; Scurrell, M.S. Polymer Stabilized Silver Nanoparticles: A Photochemical Synthesis Route. J. Mater. Sci. 2004, 39, 4459–4463. [Google Scholar] [CrossRef]
- Malik, M.A.; O’Brien, P.; Revaprasadu, N. A Simple Route to the Synthesis of Core/Shell Nanoparticles of Chalcogenides. Chem. Mater. 2002, 14, 2004–2010. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Kwon, D.-N.; Kim, J.-H. Enhanced Antibacterial and Anti-Biofilm Activities of Silver Nanoparticles against Gram-Negative and Gram-Positive Bacteria. Nanoscale Res. Lett. 2014, 9, 373. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Han, J.W.; Kim, J.-H. Green chemistry approach for the synthesis of biocompatible graphene. Int. J. Nanomed. 2013, 8, 2719–2732. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Park, J.-H.; Kim, E.S.; Choi, Y.-J.; Kwon, D.-N.; Kim, J.-H. Reduced graphene oxide–silver nanoparticle nanocomposite: A potential anticancer nanotherapy. Int. J. Nanomed. 2015, 10, 6257–6276. [Google Scholar] [CrossRef]
- Batir-Marin, D.; Mircea, C.; Boev, M.; Burlec, A.F.; Corciova, A.; Fifere, A.; Iacobescu, A.; Cioanca, O.; Verestiuc, L.; Hancianu, M. In Vitro Antioxidant, Antitumor and Photocatalytic Activities of Silver Nanoparticles Synthesized Using Equisetum Species: A Green Approach. Molecules 2021, 26, 7325. [Google Scholar] [CrossRef] [PubMed]
- Noah, N.M.; Ndangili, P.M. Green Synthesis of Nanomaterials from Sustainable Materials for Biosensors and Drug Delivery. Sensors Int. 2022, 3, 100166. [Google Scholar] [CrossRef]
- Amini, S.M.; Pour, M.S.S.; Vahidi, R.; Kouhbananinejad, S.M.; Bardsiri, M.S.; Farsinejad, A.; Mirzaei-Parsa, M.J. Green Synthesis of Stable Silver Nanoparticles Using Teucrium Polium Extract: In-Vitro Anticancer Activity on NALM-6. Nanomed. Res. J. 2021, 6, 170–178. [Google Scholar] [CrossRef]
- Sysak, S.; Czarczynska-Goslinska, B.; Szyk, P.; Koczorowski, T.; Mlynarczyk, D.T.; Szczolko, W.; Lesyk, R.; Goslinski, T. Metal Nanoparticle-Flavonoid Connections: Synthesis, Physicochemical and Biological Properties, as Well as Potential Applications in Medicine. Nanomaterials 2023, 13, 1531. [Google Scholar] [CrossRef]
- Amini, S.M.; Mohammadi, E.; Askarian-Amiri, S.; Azizi, Y.; Shakeri-Zadeh, A.; Neshastehriz, A. Investigating the in Vitro Photothermal Effect of Green Synthesized Apigenin-coated Gold Nanoparticle on Colorectal Carcinoma. IET Nanobiotechnology 2021, 15, 329–337. [Google Scholar] [CrossRef]
- Islam, M.J.; Kamaruzzaman; Hossain, M.F.; Awal, M.A.; Rahman, M.R.; Alam, M.M. Green Synthesis of Silver Nanoparticles (AgNPs) Incorporated with Adenia Trilobata Leaf Extracts and Its Anti-Bacterial Application. AIP Adv. 2022, 12, 115116. [Google Scholar] [CrossRef]
- Purohit, A.; Sharma, R.; Ramakrishnan, R.S.; Sharma, S.; Kumar, A.; Jain, D.; Kushwaha, H.S.; Maharjan, E. Biogenic Synthesis of Silver Nanoparticles (AgNPs) Using Aqueous Leaf Extract of Buchanania lanzan Spreng and Evaluation of Their Antifungal Activity against Phytopathogenic Fungi. Bioinorg. Chem. Appl. 2022, 2022, 6825150. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, S.; Guleria, S. Green Synthesis of AgNPs Using Cannabis sativa Leaf Extract: Characterization, Antibacterial, Anti-Yeast and α-Amylase Inhibitory Activity. Mater. Sci. Energy Technol. 2020, 3, 536–544. [Google Scholar] [CrossRef]
- Jagtap, R.R.; Garud, A.; Puranik, S.S.; Rudrapal, M.; Ansari, M.A.; Alomary, M.N.; Alshamrani, M.; Salawi, A.; Almoshari, Y.; Khan, J.; et al. Biofabrication of Silver Nanoparticles (AgNPs) Using Embelin for Effective Therapeutic Management of Lung Cancer. Front. Nutr. 2022, 9, 960674. [Google Scholar] [CrossRef] [PubMed]
- Corciovă, A.; Mircea, C.; Burlec, A.F.; Fifere, A.; Moleavin, I.T.; Sarghi, A.; Tuchiluș, C.; Ivănescu, B.; Macovei, I. Green Synthesis and Characterization of Silver Nanoparticles Using a Lythrum salicaria Extract and in Vitro Exploration of Their Biological Activities. Life 2022, 12, 1643. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Yasmin, R.; Asif, R.; Ambreen, A.; Mustafa, M.; Umbreen, S. Green Synthesis of Silver Nanoparticles (AgNPs), Structural Characterization, and Their Antibacterial Potential. Dose-Response 2022, 20, 155932582210887. [Google Scholar] [CrossRef]
- Macovei, I.; Luca, S.V.; Skalicka-Woźniak, K.; Sacarescu, L.; Pascariu, P.; Ghilan, A.; Doroftei, F.; Ursu, E.-L.; Rimbu, C.M.; Horhogea, C.E.; et al. Phyto-Functionalized Silver Nanoparticles Derived from Conifer Bark Extracts and Evaluation of Their Antimicrobial and Cytogenotoxic Effects. Molecules 2022, 27, 217. [Google Scholar] [CrossRef]
- Aslam, M.; Fozia, F.; Gul, A.; Ahmad, I.; Ullah, R.; Bari, A.; Mothana, R.A.; Hussain, H. Phyto-Extract-Mediated Synthesis of Silver Nanoparticles Using Aqueous Extract of Sanvitalia procumbens, and Characterization, Optimization and Photocatalytic Degradation of Azo Dyes Orange g and Direct Blue-15. Molecules 2021, 26, 6144. [Google Scholar] [CrossRef]
- Burlec, A.F.; Hăncianu, M.; Macovei, I.; Mircea, C.; Fifere, A.; Turin-Moleavin, I.-A.; Tuchiluș, C.; Robu, S.; Corciovă, A. Eco-Friendly Synthesis and Comparative in Vitro Biological Evaluation of Silver Nanoparticles Using Tagetes erecta Flower Extracts. Appl. Sci. 2022, 12, 887. [Google Scholar] [CrossRef]
- Efavi, J.K.; Nyankson, E.; Kyeremeh, K.; Manu, G.P.; Asare, K.; Yeboah, N. Monodispersed AgNPs Synthesized from the Nanofactories of Theobroma Cacao (Cocoa) Leaves and Pod Husk and Their Antimicrobial Activity. Int. J. Biomater. 2022, 2022, 4106558. [Google Scholar] [CrossRef]
- Ibrahim, S.; Ahmad, Z.; Manzoor, M.Z.; Mujahid, M.; Faheem, Z.; Adnan, A. Optimization for Biogenic Microbial Synthesis of Silver Nanoparticles through Response Surface Methodology, Characterization, Their Antimicrobial, Antioxidant, and Catalytic Potential. Sci. Rep. 2021, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Alsamhary, K.I. Eco-Friendly Synthesis of Silver Nanoparticles by Bacillus subtilis and Their Antibacterial Activity. Saudi J. Biol. Sci. 2020, 27, 2185–2191. [Google Scholar] [CrossRef]
- Saleh, M.N.; Alwan, S.K. Bio-Synthesis of Silver Nanoparticles from Bacteria Klebsiella Pneumonia: Their Characterization and Antibacterial Studies. J. Phys. Conf. Ser. 2020, 1664, 012115. [Google Scholar] [CrossRef]
- Naseer, Q.A.; Xue, X.; Wang, X.; Dang, S.; Din, S.U.; Kalsoom; Jamil, J. Synthesis of Silver Nanoparticles Using Lactobacillus bulgaricus and Assessment of Their Antibacterial Potential. Braz. J. Biol. 2022, 82, e232434. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, W.A.; Alkersh, B.M.; Sabry, S.A.; Ghozlan, H.A. Biosynthesis of Silver Nanoparticles by Aspergillus Terreus: Characterization, Optimization, and Biological Activities. Front. Bioeng. Biotechnol. 2021, 9, 633468. [Google Scholar] [CrossRef]
- Yassin, M.A.; Elgorban, A.M.; El-Samawaty, A.E.-R.M.A.; Almunqedhi, B.M.A. Biosynthesis of Silver Nanoparticles Using Penicillium verrucosum and Analysis of Their Antifungal Activity. Saudi J. Biol. Sci. 2021, 28, 2123–2127. [Google Scholar] [CrossRef]
- Soleimani, M.; Habibi-Pirkoohi, M. Biosynthesis of Silver Nanoparticles Using Chlorella vulgaris and Evaluation of the Antibacterial Efficacy against Staphylococcus aureus. Avicenna J. Med. Biotechnol. 2017, 9, 120–125. [Google Scholar]
- Shantkriti, S.; Pradeep, M.; Unish, K.; Viji Das, M.; Nidhin, S.; Gugan, K.; Murugan, A. Bioynthesis of Silver Nanoparticles Using Dunaliella Salina and Its Antibacterial Applications. Appl. Surf. Sci. Adv. 2023, 13, 100377. [Google Scholar] [CrossRef]
- Algotiml, R.; Gab-Alla, A.; Seoudi, R.; Abulreesh, H.H.; El-Readi, M.Z.; Elbanna, K. Anticancer and Antimicrobial Activity of Biosynthesized Red Sea Marine Algal Silver Nanoparticles. Sci. Rep. 2022, 12, 2421. [Google Scholar] [CrossRef]
- Hatipoğlu, A. Rapid Green Synthesis of Gold Nanoparticles: Synthesis, Characterization, and Antimicrobial Activities. Prog. Nutr. 2021, 23, e2021242. [Google Scholar] [CrossRef]
- Folorunso, A.; Akintelu, S.; Oyebamiji, A.K.; Ajayi, S.; Abiola, B.; Abdusalam, I.; Morakinyo, A. Biosynthesis, Characterization and Antimicrobial Activity of Gold Nanoparticles from Leaf Extracts of Annona muricata. J. Nanostruct. Chem. 2019, 9, 111–117. [Google Scholar] [CrossRef]
- Yuan, C.; Huo, C.; Gui, B.; Cao, W. Green Synthesis of Gold Nanoparticles Using Citrus Maxima Peel Extract and Their Catalytic/Antibacterial Activities. IET Nanobiotechnol. 2017, 11, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Luo, A.; Jiang, L.; Zhou, Y.; Yang, Y.; Liu, Q.; Zhang, C. Disinfection Efficacy of Green Synthesized Gold Nanoparticles for Medical Disinfection Applications. Afr. Health Sci. 2019, 19, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Dorosti, N.; Jamshidi, F. Plant-Mediated Gold Nanoparticles by Dracocephalum kotschyi as Anticholinesterase Agent: Synthesis, Characterization, and Evaluation of Anticancer and Antibacterial Activity. J. Appl. Biomed. 2016, 14, 235–245. [Google Scholar] [CrossRef]
- Nagajyothi, P.C.; Lee, S.-E.; An, M.; Lee, K.-D. Green Synthesis of Silver and Gold Nanoparticles Using Lonicera Japonica Flower Extract. Bull. Korean Chem. Soc. 2012, 33, 2609–2612. [Google Scholar] [CrossRef]
- Jafarizad, A.; Safaee, K.; Gharibian, S.; Omidi, Y.; Ekinci, D. Biosynthesis and In-Vitro Study of Gold Nanoparticles Using Mentha and Pelargonium Extracts. Procedia Mater. Sci. 2015, 11, 224–230. [Google Scholar] [CrossRef]
- Rajendran, A. Antibacterial Properties and Mechanism of Gold Nanoparticles Obtained from Pergularia daemia Leaf Extract. J. Nanomed. Res. 2017, 6, 00146. [Google Scholar] [CrossRef]
- Anbu, P.; Gopinath, S.C.; Jayanthi, S. Synthesis of Gold Nanoparticles Using Platycodon Grandiflorum Extract and Its Antipathogenic Activity under Optimal Conditions. Nanomater. Nanotechnol. 2020, 10, 184798042096169. [Google Scholar] [CrossRef]
- Hosny, M.; Fawzy, M.; El-Badry, Y.A.; Hussein, E.E.; Eltaweil, A.S. Plant-Assisted Synthesis of Gold Nanoparticles for Photocatalytic, Anticancer, and Antioxidant Applications. J. Saudi Chem. Soc. 2022, 26, 101419. [Google Scholar] [CrossRef]
- Pourali, P.; Badiee, S.H.; Manafi, S.; Noorani, T.; Rezaei, A.; Yahyaei, B. Biosynthesis of Gold Nanoparticles by Two Bacterial and Fungal Strains, Bacillus cereus and Fusarium oxysporum, and Assessment and Comparison of Their Nanotoxicity in Vitro by Direct and Indirect Assays. Electron. J. Biotechnol. 2017, 29, 86–93. [Google Scholar] [CrossRef]
- Nadhim, K.; Mahmood, N.N.; Mustafa, A. Synthesized Gold Nanoparticles Using Pseudomonas Supernatant and Study the Physical Characterization–Antproliferative Activity of Breast Cancer Cells (MCF-7). Medico-Legal Updat. 2021, 21, 1281–1286. [Google Scholar] [CrossRef]
- Owais, M.; Chauhan, A.; Tufail; Sherwani; Sajid, M.; Suri, C.R.; Owais, M. Fungus-Mediated Biological Synthesis of Gold Nanoparticles: Potential in Detection of Liver Cancer. Int. J. Nanomed. 2011, 6, 2305–2319. [Google Scholar] [CrossRef] [PubMed]
- Gürsoy, N.; Yilmaz Öztürk, B.; Dağ, İ. Synthesis of Intracellular and Extracellular Gold Nanoparticles with a Green Machine and Its Antifungal Activity. Turk. J. Biol. 2021, 45, 196–213. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Enazi, N.M.; Ibraheem, I.B.M. Green Biosynthesis of Gold Nanoparticles Using Galaxaura Elongata and Characterization of Their Antibacterial Activity. Arab. J. Chem. 2017, 10, S3029–S3039. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Shabaan, M.T.; Hassan, L.; Morsi, H.H. Antiviral Activity of Algae Biosynthesized Silver and Gold Nanoparticles against Herps Simplex (HSV-1) Virus in Vitro Using Cell-Line Culture Technique. Int. J. Environ. Health Res. 2022, 32, 616–627. [Google Scholar] [CrossRef]
- Pal, K.; Chakroborty, S.; Nath, N. Limitations of Nanomaterials Insights in Green Chemistry Sustainable Route: Review on Novel Applications. Green Process. Synth. 2022, 11, 951–964. [Google Scholar] [CrossRef]
- Khan, M.; Khan, M.S.A.; Borah, K.K.; Goswami, Y.; Hakeem, K.R.; Chakrabartty, I. The Potential Exposure and Hazards of Metal-Based Nanoparticles on Plants and Environment, with Special Emphasis on ZnO NPs, TiO2 NPs, and AgNPs: A Review. Environ. Adv. 2021, 6, 100128. [Google Scholar] [CrossRef]
- Qamar, S.U.R.; Ahmad, J.N. Nanoparticles: Mechanism of Biosynthesis Using Plant Extracts, Bacteria, Fungi, and Their Applications. J. Mol. Liq. 2021, 334, 116040. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Sheikh, H.I.; Sarkar, T.; Edinur, H.A.; Pati, S.; Ray, R.R. Microbiologically-Synthesized Nanoparticles and Their Role in Silencing the Biofilm Signaling Cascade. Front. Microbiol. 2021, 12, 636588. [Google Scholar] [CrossRef]
- Iravani, S. Bacteria in Nanoparticle Synthesis: Current Status and Future Prospects. Int. Sch. Res. Not. 2014, 2014, 359316. [Google Scholar] [CrossRef]
- Khan, F.; Shahid, A.; Zhu, H.; Wang, N.; Javed, M.R.; Ahmad, N.; Xu, J.; Alam, M.A.; Mehmood, M.A. Prospects of Algae-Based Green Synthesis of Nanoparticles for Environmental Applications. Chemosphere 2022, 293, 133571. [Google Scholar] [CrossRef] [PubMed]
- Michael, A.; Singh, A.; Roy, A.; Islam, M.R. Fungal- and Algal-Derived Synthesis of Various Nanoparticles and Their Applications. Bioinorg. Chem. Appl. 2022, 2022, 3142674. [Google Scholar] [CrossRef] [PubMed]
- Nahvi, I.; Belkahla, S.; Asiri, S.M.; Rehman, S. Overview and Prospectus of Algal Biogenesis of Nanoparticles. In Microbial Nanotechnology: Green Synthesis and Applications; Springer: Singapore, 2021; pp. 121–134. [Google Scholar]
- Velgosová, O.; Mražíková, A. Limitations and Possibilities of Green Synthesis and Long-Term Stability of Colloidal Ag Nanoparticles. AIP Conf. Proc. 2017, 1918, 020004. [Google Scholar]
- Košević, M.G.; Zarić, M.M.; Stopić, S.R.; Stevanović, J.S.; Weirich, T.E.; Friedrich, B.G.; Panić, V.V. Structural and Electrochemical Properties of Nesting and Core/Shell Pt/TiO2 Spherical Particles Synthesized by Ultrasonic Spray Pyrolysis. Metals 2019, 10, 11. [Google Scholar] [CrossRef]
- Yu, X.; Pham, J.T.; Subramani, C.; Creran, B.; Yeh, Y.-C.; Du, K.; Patra, D.; Miranda, O.R.; Crosby, A.J.; Rotello, V.M. Direct Patterning of Engineered Ionic Gold Nanoparticles via Nanoimprint Lithography. Adv. Mater. 2012, 24, 6330–6334. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.-L.; O’Brien, J.; Gun’ko, Y.K. Rare Earth Doped Silica Nanoparticles via Thermolysis of a Single Source Metallasilsesquioxane Precursor. Sci. Rep. 2017, 7, 45862. [Google Scholar] [CrossRef]
- Ielo, I.; Rando, G.; Giacobello, F.; Sfameni, S.; Castellano, A.; Galletta, M.; Drommi, D.; Rosace, G.; Plutino, M.R. Synthesis, Chemical–Physical Characterization, and Biomedical Applications of Functional Gold Nanoparticles: A Review. Molecules 2021, 26, 5823. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A Study of the Nucleation and Growth Processes in the Synthesis of Colloidal Gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Noruzi, M.; Zare, D.; Khoshnevisan, K.; Davoodi, D. Rapid Green Synthesis of Gold Nanoparticles Using Rosa Hybrida Petal Extract at Room Temperature. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 1461–1465. [Google Scholar] [CrossRef]
- Shankar, S.S.; Rai, A.; Ahmad, A.; Sastry, M. Rapid Synthesis of Au, Ag, and Bimetallic Au Core–Ag Shell Nanoparticles Using Neem (Azadirachta indica) Leaf Broth. J. Colloid Interface Sci. 2004, 275, 496–502. [Google Scholar] [CrossRef]
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Biosynthesis of Nanoparticles: Technological Concepts and Future Applications. J. Nanoparticle Res. 2008, 10, 507–517. [Google Scholar] [CrossRef]
- Singh, M.; Kalaivani, R.; Manikandan, S.; Sangeetha, N.; Kumaraguru, A.K. Facile Green Synthesis of Variable Metallic Gold Nanoparticle Using Padina gymnospora, a Brown Marine Macroalga. Appl. Nanosci. 2013, 3, 145–151. [Google Scholar] [CrossRef]
- Saleh, T.A. Nanomaterials: Classification, Properties, and Environmental Toxicities. Environ. Technol. Innov. 2020, 20, 101067. [Google Scholar] [CrossRef]
- Shnoudeh, A.J.; Hamad, I.; Abdo, R.W.; Qadumii, L.; Jaber, A.Y.; Surchi, H.S.; Alkelany, S.Z. Synthesis, Characterization, and Applications of Metal Nanoparticles. In Biomaterials and Bionanotechnology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 527–612. [Google Scholar]
- Liaqat, N.; Jahan, N.; Rahman, K.U.; Anwar, T.; Qureshi, H. Green synthesized silver nanoparticles: Optimization, characterization, antimicrobial activity, and cytotoxicity study by hemolysis assay. Front. Chem. 2022, 10, 952006. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Ghoshal, G.; Jain, A.; Goyal, M. Rapid Green Synthesis of Silver Nanoparticles (AgNPs) Using (Prunus persica) Plants Extract: Exploring Its Antimicrobial and Catalytic Activities. J. Nanomed. Nanotechnol. 2017, 8, 1000452. [Google Scholar] [CrossRef]
- Correa, S.N.; Naranjo, A.M.; Herrera, A.P. Biosynthesis and Characterization of Gold Nanoparticles Using Extracts of Tamarindus indica L Leaves. J. Phys. Conf. Ser. 2016, 687, 012082. [Google Scholar] [CrossRef]
- Dzimitrowicz, A.; Jamróz, P.; DiCenzo, G.C.; Sergiel, I.; Kozlecki, T.; Pohl, P. Preparation and Characterization of Gold Nanoparticles Prepared with Aqueous Extracts of Lamiaceae Plants and the Effect of Follow-up Treatment with Atmospheric Pressure Glow Microdischarge. Arab. J. Chem. 2019, 12, 4118–4130. [Google Scholar] [CrossRef]
- Femi-Adepoju, A.G.; Dada, A.O.; Otun, K.O.; Adepoju, A.O.; Fatoba, O.P. Green Synthesis of Silver Nanoparticles Using Terrestrial Fern (Gleichenia pectinata (Willd.) C. Presl.): Characterization and Antimicrobial Studies. Heliyon 2019, 5, e01543. [Google Scholar] [CrossRef]
- Lima, A.K.O.; Vasconcelos, A.A.; Kobayashi, R.K.T.; Nakazato, G.; Braga, H.d.C.; Taube, P.S. Green Synthesis: Characterization and Biological Activity of Silver Nanoparticles Using Aqueous Extracts of Plants from the Arecaceae Family. Acta Sci. Technol. 2021, 43, e52011. [Google Scholar] [CrossRef]
- TYAl-Abdullah, Z.; Al-Shawi, A.A.A.; Aboud, M.N.; Abdulaziz, B.A.A.; Al-Furaiji, H.Q.M.; Luaibi, I.N. Synthesis and Analytical Characterization of Gold Nanoparticles Using Microwave-Assisted Extraction System and Study Their Application in Degradation. J. Nanostruct. 2020, 10, 682–690. [Google Scholar] [CrossRef]
- Geraldes, A.N.; da Silva, A.A.; Leal, J.; Estrada-Villegas, G.M.; Lincopan, N.; Katti, K.V.; Lugão, A.B. Green Nanotechnology from Plant Extracts: Synthesis and Characterization of Gold Nanoparticles. Adv. Nanoparticles 2016, 05, 176–185. [Google Scholar] [CrossRef]
- Reda, M.; Ashames, A.; Edis, Z.; Bloukh, S.; Bhandare, R.; Abu Sara, H. Green Synthesis of Potent Antimicrobial Silver Nanoparticles Using Different Plant Extracts and Their Mixtures. Processes 2019, 7, 510. [Google Scholar] [CrossRef]
- Miškovská, A.; Rabochová, M.; Michailidu, J.; Masák, J.; Čejková, A.; Lorinčík, J.; Maťátková, O. Antibiofilm Activity of Silver Nanoparticles Biosynthesized Using Viticultural Waste. PLoS ONE 2022, 17, e0272844. [Google Scholar] [CrossRef]
- Lee, K.-C.; Lin, S.-J.; Lin, C.-H.; Tsai, C.-S.; Lu, Y.-J. Size Effect of Ag Nanoparticles on Surface Plasmon Resonance. Surf. Coatings Technol. 2008, 202, 5339–5342. [Google Scholar] [CrossRef]
- Sau, T.K.; Rogach, A.L.; Jäckel, F.; Klar, T.A.; Feldmann, J. Properties and Applications of Colloidal Nonspherical Noble Metal Nanoparticles. Adv. Mater. 2010, 22, 1805–1825. [Google Scholar] [CrossRef] [PubMed]
- Saputra, I.; Suhartati, S.; Yulizar, Y.; Sudirman, S. Synthesis and Characterization of Gold Nanoparticles (AuNPs) by Utilizing Bioactive Compound of Imperata cylndrica L. Indones. J. Appl. Chem. 2020, 22, 1–7. [Google Scholar]
- Jamil, S.; Dastagir, G.; Foudah, A.I.; Alqarni, M.H.; Yusufoglu, H.S.; Alkreathy, H.M.; Ertürk, Ö.; Shah, M.A.R.; Khan, R.A. Carduus edelbergii Rech. f. Mediated Fabrication of Gold Nanoparticles; Characterization and Evaluation of Antimicrobial, Antioxidant and Antidiabetic Potency of the Synthesized AuNPs. Molecules 2022, 27, 6669. [Google Scholar] [CrossRef]
- Akkus, Z.B.; Imran Nazir, A.J.; Tribus, M.; Bernkop-Schnürch, A. Zeta Potential Changing Polyphosphate Nanoparticles: A Promising Approach to Overcome the Mucus and Epithelial Barrier. Mol. Pharm. 2019, 16, 2817–2825. [Google Scholar] [CrossRef]
- Erdogan, O.; Abbak, M.; Demirbolat, G.M.; Birtekocak, F.; Aksel, M.; Pasa, S.; Cevik, O. Green Synthesis of Silver Nanoparticles via Cynara scolymus Leaf Extracts: The Characterization, Anticancer Potential with Photodynamic Therapy in MCF7 Cells. PLoS ONE 2019, 14, e0216496. [Google Scholar] [CrossRef]
- Elamawi, R.M.; Al-Harbi, R.E.; Hendi, A.A. Biosynthesis and Characterization of Silver Nanoparticles Using Trichoderma longibrachiatum and Their Effect on Phytopathogenic Fungi. Egypt. J. Biol. Pest Control 2018, 28, 28. [Google Scholar] [CrossRef]
- Mukherjee, S.; Sushma, V.; Patra, S.; Barui, A.K.; Bhadra, M.P.; Sreedhar, B.; Patra, C.R. Green Chemistry Approach for the Synthesis and Stabilization of Biocompatible Gold Nanoparticles and Their Potential Applications in Cancer Therapy. Nanotechnology 2012, 23, 455103. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Ismail, M.; Ahmad, B.; Zahid, N.; Jabbour, G.; Khan, M.S.; Mutreja, V.; Sareen, S.; Rafiq, A.; Faheem, M.; et al. Characterizations and Analysis of the Antioxidant, Antimicrobial, and Dye Reduction Ability of Green Synthesized Silver Nanoparticles. Green Process. Synth. 2020, 9, 693–705. [Google Scholar] [CrossRef]
- Hodoroaba, V.; Rades, S.; Unger, W. Inspection of Morphology and Elemental Imaging of Single Nanoparticles by High-resolution SEM/EDX in Transmission Mode. Surf. Interface Anal. 2014, 46, 945–948. [Google Scholar] [CrossRef]
- Mubeen, B.; Rasool, M.G.; Ullah, I.; Rasool, R.; Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Alzarea, S.I.; Nadeem, M.S.; Kazmi, I. Phytochemicals Mediated Synthesis of AuNPs from Citrullus colocynthis and Their Characterization. Molecules 2022, 27, 1300. [Google Scholar] [CrossRef]
- Ojemaye, M.O.; Okoh, S.O.; Okoh, A.I. Silver Nanoparticles (AgNPs) Facilitated by Plant Parts of Crataegus ambigua Becker AK Extracts and Their Antibacterial, Antioxidant and Antimalarial Activities. Green Chem. Lett. Rev. 2021, 14, 51–61. [Google Scholar] [CrossRef]
- Botteon, C.E.A.; Silva, L.B.; Ccana-Ccapatinta, G.V.; Silva, T.S.; Ambrosio, S.R.; Veneziani, R.C.S.; Bastos, J.K.; Marcato, P.D. Biosynthesis and Characterization of Gold Nanoparticles Using Brazilian Red Propolis and Evaluation of Its Antimicrobial and Anticancer Activities. Sci. Rep. 2021, 11, 1974. [Google Scholar] [CrossRef]
- Philip, D. Rapid Green Synthesis of Spherical Gold Nanoparticles Using Mangifera indica Leaf. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 77, 807–810. [Google Scholar] [CrossRef]
- Corciovă, A.; Mircea, C.; Burlec, A.-F.; Cioancă, O.; Tuchiluş, C.; Fifere, A.; Lungoci, A.-L.; Marangoci, N.; Hăncianu, M. Antioxidant, Antimicrobial Activities and Photocatalytic Degradation Efficacy of Silver Nanoparticles Obtained by Bee Propolis Extract Assisted Biosynthesis. Farmacia 2019, 67, 482–489. [Google Scholar] [CrossRef]
- Verma, A.; Mehata, M.S. Controllable Synthesis of Silver Nanoparticles Using Neem Leaves and Their Antimicrobial Activity. J. Radiat. Res. Appl. Sci. 2016, 9, 109–115. [Google Scholar] [CrossRef]
- Bhaskaran, S.; Sharma, N.; Tiwari, P.; Singh, S.R.; Sahi, S.V. Fabrication of Innocuous Gold Nanoparticles Using Plant Cells in Culture. Sci. Rep. 2019, 9, 12040. [Google Scholar] [CrossRef]
- Siakavella, I.K.; Lamari, F.; Papoulis, D.; Orkoula, M.; Gkolfi, P.; Lykouras, M.; Avgoustakis, K.; Hatziantoniou, S. Effect of Plant Extracts on the Characteristics of Silver Nanoparticles for Topical Application. Pharmaceutics 2020, 12, 1244. [Google Scholar] [CrossRef] [PubMed]
- Meva, F.E.; Ntoumba, A.A.; Kedi, P.B.E.; Tchoumbi, E.; Schmitz, A.; Schmolke, L.; Klopotowski, M.; Moll, B.; Kökcam-Demir, Ü.; Mpondo, E.A.M.; et al. Silver and palladium nanoparticles produced using a plant extract as reducing agent, stabilized with an ionic liquid: Sizing by X-ray powder diffraction and dynamic light scattering. J. Mater. Res. Technol. 2019, 8, 1991–2000. [Google Scholar] [CrossRef]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer Equation to Estimate More Accurately Nano-Crystallite Size Using XRD. World J. Nano Sci. Eng. 2012, 2, 154–160. [Google Scholar] [CrossRef]
- Sajid, M.; Płotka-Wasylka, J. Nanoparticles: Synthesis, Characteristics, and Applications in Analytical and Other Sciences. Microchem. J. 2020, 154, 104623. [Google Scholar] [CrossRef]
- Mansor, M.; Drabesch, S.; Bayer, T.; Van Le, A.; Chauhan, A.; Schmidtmann, J.; Peiffer, S.; Kappler, A. Application of Single-Particle ICP-MS to Determine the Mass Distribution and Number Concentrations of Environmental Nanoparticles and Colloids. Environ. Sci. Technol. Lett. 2021, 8, 589–595. [Google Scholar] [CrossRef]
- Teulon, J.-M.; Godon, C.; Chantalat, L.; Moriscot, C.; Cambedouzou, J.; Odorico, M.; Ravaux, J.; Podor, R.; Gerdil, A.; Habert, A.; et al. On the Operational Aspects of Measuring Nanoparticle Sizes. Nanomaterials 2018, 9, 18. [Google Scholar] [CrossRef]
- Sharma, J.N.; Pattadar, D.K.; Mainali, B.P.; Zamborini, F.P. Size Determination of Metal Nanoparticles Based on Electrochemically Measured Surface-Area-to-Volume Ratios. Anal. Chem. 2018, 90, 9308–9314. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, A. Green Synthesis of Metallic Nanoparticles and Their Prospective Biotechnological Applications: An Overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef]
- Li, Y.; Jin, R. Seeing Ligands on Nanoclusters and in Their Assemblies by X-ray Crystallography: Atomically Precise Nanochemistry and Beyond. J. Am. Chem. Soc. 2020, 142, 13627–13644. [Google Scholar] [CrossRef]
- Badirzadeh, A.; Alipour, M.; Najm, M.; Vosoogh, A.; Vosoogh, M.; Samadian, H.; Hashemi, A.S.; Farsangi, Z.J.; Amini, S.M. Potential Therapeutic Effects of Curcumin Coated Silver Nanoparticle in the Treatment of Cutaneous Leishmaniasis Due to Leishmania Major In-Vitro and in a Murine Model. J. Drug Deliv. Sci. Technol. 2022, 74, 103576. [Google Scholar] [CrossRef]
- Blaškovičová, J.; Vyskočil, V.; Augustín, M.; Purdešová, A. Ethanol and NaCl-Induced Gold Nanoparticle Aggregation Toxicity toward DNA Investigated with a DNA/GCE Biosensor. Sensors 2023, 23, 3425. [Google Scholar] [CrossRef]
- Cuenca, A.G.; Jiang, H.; Hochwald, S.N.; Delano, M.; Cance, W.G.; Grobmyer, S.R. Emerging Implications of Nanotechnology on Cancer Diagnostics and Therapeutics. Cancer 2006, 107, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, F.; Mertens, M.E.; Grimm, J.; Lammers, T. Nanoparticles for Imaging: Top or Flop? Radiology 2014, 273, 10–28. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, J.T.; Stark, D.D. Iron Oxide-Enhanced MR Imaging of the Liver and Spleen: Review of the First 5 Years. Am. J. Roentgenol. 1990, 155, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Marchal, G.; Van Hecke, P.; Demaerel, P.; Decrop, E.; Kennis, C.; Baert, A.; van der Schueren, E. Detection of Liver Metastases with Superparamagnetic Iron Oxide in 15 Patients: Results of MR Imaging at 1.5 T. Am. J. Roentgenol. 1989, 152, 771–775. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Shang, W.; Wang, K.; Guo, K.; Liu, Y.; Tian, J.; Fang, C. Targeted-Detection and Sequential-Treatment of Small Hepatocellular Carcinoma in the Complex Liver Environment by GPC-3-Targeted Nanoparticles. J. Nanobiotechnology 2022, 20, 156. [Google Scholar] [CrossRef] [PubMed]
- Santoro, L.; Grazioli, L.; Filippone, A.; Grassedonio, E.; Belli, G.; Colagrande, S. Resovist Enhanced MR Imaging of the Liver: Does Quantitative Assessment Help in Focal Lesion Classification and Characterization? J. Magn. Reson. Imaging 2009, 30, 1012–1020. [Google Scholar] [CrossRef]
- Neuwelt, E.A.; Hamilton, B.E.; Varallyay, C.G.; Rooney, W.R.; Edelman, R.D.; Jacobs, P.M.; Watnick, S.G. Ultrasmall Superparamagnetic Iron Oxides (USPIOs): A Future Alternative Magnetic Resonance (MR) Contrast Agent for Patients at Risk for Nephrogenic Systemic Fibrosis (NSF)? Kidney Int. 2009, 75, 465–474. [Google Scholar] [CrossRef]
- Johnson, J.M.; Mohamed, A.S.R.; Ding, Y.; Wang, J.; Lai, S.Y.; Fuller, C.D.; Shah, R.; Butler, R.T.; Weber, R.S. Ultra-small Superparamagnetic Iron Oxide (USPIO) Magnetic Resonance Imaging in Benign Mixed Tumor of the Parotid Gland. Clin. Case Rep. 2021, 9, 123–127. [Google Scholar] [CrossRef]
- Weissleder, R.; Nahrendorf, M.; Pittet, M.J. Imaging Macrophages with Nanoparticles. Nat. Mater. 2014, 13, 125–138. [Google Scholar] [CrossRef]
- Lobatto, M.E.; Fuster, V.; Fayad, Z.A.; Mulder, W.J.M. Perspectives and Opportunities for Nanomedicine in the Management of Atherosclerosis. Nat. Rev. Drug Discov. 2011, 10, 835–852. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, C.; Nitta, N.; Tsuchiya, K.; Watanabe, S.; Nitta-Seko, A.; Ohta, S.; Otani, H.; Sonoda, A.; Murata, K.; Shiomi, M. MRI Study of Atherosclerotic Plaque Progression Using Ultrasmall Superparamagnetic Iron Oxide in Watanabe Heritable Hyperlipidemic Rabbits. Br. J. Radiol. 2015, 88, 20150167. [Google Scholar] [CrossRef] [PubMed]
- Kooi, M.E.; Cappendijk, V.C.; Cleutjens, K.B.J.M.; Kessels, A.G.H.; Kitslaar, P.J.E.H.M.; Borgers, M.; Frederik, P.M.; Daemen, M.J.A.P.; van Engelshoven, J.M.A. Accumulation of Ultrasmall Superparamagnetic Particles of Iron Oxide in Human Atherosclerotic Plaques Can Be Detected by in Vivo Magnetic Resonance Imaging. Circulation 2003, 107, 2453–2458. [Google Scholar] [CrossRef] [PubMed]
- Tourdias, T.; Roggerone, S.; Filippi, M.; Kanagaki, M.; Rovaris, M.; Miller, D.H.; Petry, K.G.; Brochet, B.; Pruvo, J.-P.; Radüe, E.-W.; et al. Assessment of Disease Activity in Multiple Sclerosis Phenotypes with Combined Gadolinium- and Superparamagnetic Iron Oxide–Enhanced MR Imaging. Radiology 2012, 264, 225–233. [Google Scholar] [CrossRef]
- Fatima, A.; Ahmad, M.W.; Al Saidi, A.K.A.; Choudhury, A.; Chang, Y.; Lee, G.H. Recent Advances in Gadolinium Based Contrast Agents for Bioimaging Applications. Nanomaterials 2021, 11, 2449. [Google Scholar] [CrossRef]
- Boisselier, E.; Astruc, D. Gold Nanoparticles in Nanomedicine: Preparations, Imaging, Diagnostics, Therapies and Toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef]
- Şologan, M.; Padelli, F.; Giachetti, I.; Aquino, D.; Boccalon, M.; Adami, G.; Pengo, P.; Pasquato, L. Functionalized Gold Nanoparticles as Contrast Agents for Proton and Dual Proton/Fluorine MRI. Nanomaterials 2019, 9, 879. [Google Scholar] [CrossRef]
- Eskandarinezhad, S.; Wani, I.A.; Nourollahileilan, M.; Khosla, A.; Ahmad, T. Review—Metal and Metal Oxide Nanoparticles/Nanocomposites as Electrochemical Biosensors for Cancer Detection. J. Electrochem. Soc. 2022, 169, 047504. [Google Scholar] [CrossRef]
- Babu, A.; Templeton, A.K.; Munshi, A.; Ramesh, R. Nanoparticle-Based Drug Delivery for Therapy of Lung Cancer: Progress and Challenges. J. Nanomater. 2013, 2013, 863951. [Google Scholar] [CrossRef]
- Nejati, K.; Rastegar, M.; Fathi, F.; Dadashpour, M.; Arabzadeh, A. Nanoparticle-Based Drug Delivery Systems to Overcome Gastric Cancer Drug Resistance. J. Drug Deliv. Sci. Technol. 2022, 70, 103231. [Google Scholar] [CrossRef]
- Bowman, M.-C.; Ballard, T.E.; Ackerson, C.J.; Feldheim, D.L.; Margolis, D.M.; Melander, C. Inhibition of HIV Fusion with Multivalent Gold Nanoparticles. J. Am. Chem. Soc. 2008, 130, 6896–6897. [Google Scholar] [CrossRef] [PubMed]
- Xi, D.; Luo, X.; Ning, Q.; Lu, Q.; Yao, K.; Liu, Z. The Detection of HBV DNA with Gold Nanoparticle Gene Probes. J. Nanjing Med. Univ. 2007, 21, 207–212. [Google Scholar] [CrossRef]
- Yen, C.-W.; de Puig, H.; Tam, J.O.; Gómez-Márquez, J.; Bosch, I.; Hamad-Schifferli, K.; Gehrke, L. Multicolored Silver Nanoparticles for Multiplexed Disease Diagnostics: Distinguishing Dengue, Yellow Fever, and Ebola Viruses. Lab Chip 2015, 15, 1638–1641. [Google Scholar] [CrossRef] [PubMed]
- Balakumar, V.; Prakash, P.; Muthupandi, K.; Rajan, A. Nanosilver for Selective and Sensitive Sensing of Saturnism. Sensors Actuators B Chem. 2017, 241, 814–820. [Google Scholar] [CrossRef]
- Baptista, P.V.; Koziol-Montewka, M.; Paluch-Oles, J.; Doria, G.; Franco, R. Gold-Nanoparticle-Probe–Based Assay for Rapid and Direct Detection of Mycobacterium tuberculosis DNA in Clinical Samples. Clin. Chem. 2006, 52, 1433–1434. [Google Scholar] [CrossRef]
- Georganopoulou, D.G.; Chang, L.; Nam, J.-M.; Thaxton, C.S.; Mufson, E.J.; Klein, W.L.; Mirkin, C.A. Nanoparticle-Based Detection in Cerebral Spinal Fluid of a Soluble Pathogenic Biomarker for Alzheimer’s Disease. Proc. Natl. Acad. Sci. USA 2005, 102, 2273–2276. [Google Scholar] [CrossRef]
- Haes, A.J.; Chang, L.; Klein, W.L.; Van Duyne, R.P. Detection of a Biomarker for Alzheimer’s Disease from Synthetic and Clinical Samples Using a Nanoscale Optical Biosensor. J. Am. Chem. Soc. 2005, 127, 2264–2271. [Google Scholar] [CrossRef]
- dos Santos Tramontin, N.; da Silva, S.; Arruda, R.; Ugioni, K.S.; Canteiro, P.B.; de Bem Silveira, G.; Mendes, C.; Silveira, P.C.L.; Muller, A.P. Gold Nanoparticles Treatment Reverses Brain Damage in Alzheimer’s Disease Model. Mol. Neurobiol. 2020, 57, 926–936. [Google Scholar] [CrossRef]
- Esmaeili-bandboni, A.; Amini, S.M.; Faridi-majidi, R.; Bagheri, J.; Mohammadnejad, J.; Sadroddiny, E. Cross-linking Gold Nanoparticles Aggregation Method Based on Localised Surface Plasmon Resonance for Quantitative Detection of MiR-155. IET Nanobiotechnol. 2018, 12, 453–458. [Google Scholar] [CrossRef]
- Fortunati, S.; Pedrini, F.; Del Grosso, E.; Baranda Pellejero, L.; Bertucci, A. Design of Specific Nucleic Acid-Based Biosensors for Protein Binding Activity. Anal. Sens. 2022, 2, e202200037. [Google Scholar] [CrossRef]
- Fatemi, F.; Amini, S.M.; Kharrazi, S.; Rasaee, M.J.; Mazlomi, M.A.; Asadi-Ghalehni, M.; Rajabibazl, M.; Sadroddiny, E. Construction of Genetically Engineered M13K07 Helper Phage for Simultaneous Phage Display of Gold Binding Peptide 1 and Nuclear Matrix Protein 22 ScFv Antibody. Colloids Surf. B Biointerfaces 2017, 159, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Andleeb, A.; Andleeb, A.; Asghar, S.; Zaman, G.; Tariq, M.; Mehmood, A.; Nadeem, M.; Hano, C.; Lorenzo, J.M.; Abbasi, B.H. A Systematic Review of Biosynthesized Metallic Nanoparticles as a Promising Anti-Cancer-Strategy. Cancers 2021, 13, 2818. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L.; Fan, Y.; Feng, Q.; Cui, F. Biocompatibility and Toxicity of Nanoparticles and Nanotubes. J. Nanomater. 2012, 2012, 548389. [Google Scholar] [CrossRef]
- Singh, A. Comparative Therapeutic Effects of Plant-Extract Synthesized and Traditionally Synthesized Gold Nanoparticles on Alcohol-Induced Inflammatory Activity in SH-SY5Y Cells In Vitro. Biomedicines 2017, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Thiesen, B.; Jordan, A. Clinical Applications of Magnetic Nanoparticles for Hyperthermia. Int. J. Hyperth. 2008, 24, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Tassa, C.; Shaw, S.Y.; Weissleder, R. Dextran-Coated Iron Oxide Nanoparticles: A Versatile Platform for Targeted Molecular Imaging, Molecular Diagnostics, and Therapy. Acc. Chem. Res. 2011, 44, 842–852. [Google Scholar] [CrossRef]
- Amatya, R.; Hwang, S.; Park, T.; Min, K.A.; Shin, M.C. In Vitro and in Vivo Evaluation of PEGylated Starch-Coated Iron Oxide Nanoparticles for Enhanced Photothermal Cancer Therapy. Pharmaceutics 2021, 13, 871. [Google Scholar] [CrossRef]
- Ge, R.; Liu, C.; Zhang, X.; Wang, W.; Li, B.; Liu, J.; Liu, Y.; Sun, H.; Zhang, D.; Hou, Y.; et al. Photothermal-Activatable Fe3O4 Superparticle Nanodrug Carriers with PD-L1 Immune Checkpoint Blockade for Anti-Metastatic Cancer Immunotherapy. ACS Appl. Mater. Interfaces 2018, 10, 20342–20355. [Google Scholar] [CrossRef]
- Mohamadkazem, M.; Neshastehriz, A.; Amini, S.M.; Moshiri, A.; Janzadeh, A. Radiosensitising Effect of Iron Oxide-gold Nanocomplex for Electron Beam Therapy of Melanoma in Vivo by Magnetic Targeting. IET Nanobiotechnol. 2023, 17, 212–223. [Google Scholar] [CrossRef]
- Zhou, R.; Zhao, D.; Beeraka, N.M.; Wang, X.; Lu, P.; Song, R.; Chen, K.; Liu, J. Novel Implications of Nanoparticle-Enhanced Radiotherapy and Brachytherapy: Z-Effect and Tumor Hypoxia. Metabolites 2022, 12, 943. [Google Scholar] [CrossRef]
- Rezaeian, A.; Amini, S.M.; Najafabadi, M.R.H.; Farsangi, Z.J.; Samadian, H. Plasmonic Hyperthermia or Radiofrequency Electric Field Hyperthermia of Cancerous Cells through Green-Synthesized Curcumin-Coated Gold Nanoparticles. Lasers Med. Sci. 2022, 37, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, W.G. Electroporation for Nanomedicine: A Review. J. Mater. Chem. B 2017, 5, 2726–2738. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi Kamalabadi, M.; Neshastehriz, A.; Ghaznavi, H.; Amini, S.M. Folate Functionalized Gold-Coated Magnetic Nanoparticles Effect in Combined Electroporation and Radiation Treatment of HPV-Positive Oropharyngeal Cancer. Med. Oncol. 2022, 39, 196. [Google Scholar] [CrossRef]
- Tian, L.; Lu, L.; Qiao, Y.; Ravi, S.; Salatan, F.; Melancon, M. Stimuli-Responsive Gold Nanoparticles for Cancer Diagnosis and Therapy. J. Funct. Biomater. 2016, 7, 19. [Google Scholar] [CrossRef]
- Puvanakrishnan, P. In Vivo Tumor Targeting of Gold Nanoparticles: Effect of Particle Type and Dosing Strategy. Int. J. Nanomed. 2012, 7, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.; Shah, N.; Visaria, R.; Paciotti, G.F.; Bischof, J.C. Biodistribution of TNF-α-Coated Gold Nanoparticles in an in Vivo Model System. Nanomedicine 2009, 4, 401–410. [Google Scholar] [CrossRef]
- Coelho, S.C.; Almeida, G.M.; Pereira, M.C.; Santos-Silva, F.; Coelho, M.A. Functionalized Gold Nanoparticles Improve Afatinib Delivery into Cancer Cells. Expert Opin. Drug Deliv. 2016, 13, 133–141. [Google Scholar] [CrossRef]
- Cryer, A.M.; Chan, C.; Eftychidou, A.; Maksoudian, C.; Mahesh, M.; Tetley, T.D.; Spivey, A.C.; Thorley, A.J. Tyrosine Kinase Inhibitor Gold Nanoconjugates for the Treatment of Non-Small Cell Lung Cancer. ACS Appl. Mater. Interfaces 2019, 11, 16336–16346. [Google Scholar] [CrossRef]
- Ramalingam, V.; Varunkumar, K.; Ravikumar, V.; Rajaram, R. Target Delivery of Doxorubicin Tethered with PVP Stabilized Gold Nanoparticles for Effective Treatment of Lung Cancer. Sci. Rep. 2018, 8, 3815. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Bhat, F.A.; Raja Singh, P.; Mukherjee, S.; Elumalai, P.; Das, S.; Patra, C.R.; Arunakaran, J. Gold Nanoparticle-Conjugated Quercetin Inhibits Epithelial-Mesenchymal Transition, Angiogenesis and Invasiveness via EGFR/VEGFR-2-Mediated Pathway in Breast Cancer. Cell Prolif. 2016, 49, 678–697. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Lee, Y.-C.; Huang, C.-H.; Chang, L.-S. Gallic Acid-Capped Gold Nanoparticles Inhibit EGF-Induced MMP-9 Expression through Suppression of P300 Stabilization and NFκB/c-Jun Activation in Breast Cancer MDA-MB-231 Cells. Toxicol. Appl. Pharmacol. 2016, 310, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Jun, B.-H.; Noh, M.S.; Kim, J.; Kim, G.; Kang, H.; Kim, M.-S.; Seo, Y.-T.; Baek, J.; Kim, J.-H.; Park, J.; et al. Multifunctional Silver-Embedded Magnetic Nanoparticles as SERS Nanoprobes and Their Applications. Small 2010, 6, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Newell, B.B.; Irudayaraj, J. Folic Acid Protected Silver Nanocarriers for Targeted Drug Delivery. J. Biomed. Nanotechnol. 2012, 8, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Kievit, F.M.; Cho, Y.-C.; Mok, H.; Press, O.W.; Zhang, M. Effect of Cationic Side-Chains on Intracellular Delivery and Cytotoxicity of PH Sensitive Polymer–Doxorubicin Nanocarriers. Nanoscale 2012, 4, 7012–7020. [Google Scholar] [CrossRef] [PubMed]
- AshaRani, P.; Sethu, S.; Lim, H.; Balaji, G.; Valiyaveettil, S.; Hande, M.P. Differential Regulation of Intracellular Factors Mediating Cell Cycle, DNA Repair and Inflammation Following Exposure to Silver Nanoparticles in Human Cells. Genome Integr. 2012, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Foldbjerg, R.; Irving, E.S.; Hayashi, Y.; Sutherland, D.S.; Thorsen, K.; Autrup, H.; Beer, C. Global Gene Expression Profiling of Human Lung Epithelial Cells after Exposure to Nanosilver. Toxicol. Sci. 2012, 130, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Mundekkad, D.; Cho, W.C. Mitophagy Induced by Metal Nanoparticles for Cancer Treatment. Pharmaceutics 2022, 14, 2275. [Google Scholar] [CrossRef]
- Gurunathan, S.; Jeong, J.-K.; Han, J.W.; Zhang, X.-F.; Park, J.H.; Kim, J.-H. Multidimensional Effects of Biologically Synthesized Silver Nanoparticles in Helicobacter pylori, Helicobacter felis, and Human Lung (L132) and Lung Carcinoma A549 Cells. Nanoscale Res. Lett. 2015, 10, 35. [Google Scholar] [CrossRef]
- Yeh, Y.-C.; Creran, B.; Rotello, V.M. Gold Nanoparticles: Preparation, Properties, and Applications in Bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef]
- Hashemi Goradel, N.; Ghiyami-Hour, F.; Jahangiri, S.; Negahdari, B.; Sahebkar, A.; Masoudifar, A.; Mirzaei, H. Nanoparticles as New Tools for Inhibition of Cancer Angiogenesis. J. Cell. Physiol. 2018, 233, 2902–2910. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Mukherjee, P. Biological Properties of “Naked” Metal Nanoparticles. Adv. Drug Deliv. Rev. 2008, 60, 1289–1306. [Google Scholar] [CrossRef] [PubMed]
- Ngernyuang, N.; Wongwattanakul, M.; Charusirisawad, W.; Shao, R.; Limpaiboon, T. Green Synthesized Apigenin Conjugated Gold Nanoparticles Inhibit Cholangiocarcinoma Cell Activity and Endothelial Cell Angiogenesis in Vitro. Heliyon 2022, 8, e12028. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Xing, Y.; Zhu, L.; Zhuo, J.; Cai, M. Sorafenib Derivatives-Functionalized Gold Nanoparticles Confer Protection against Tumor Angiogenesis and Proliferation via Suppression of EGFR and VEGFR-2. Exp. Cell Res. 2021, 406, 112633. [Google Scholar] [CrossRef]
- Kalishwaralal, K.; Banumathi, E.; Pandian, S.R.K.; Deepak, V.; Muniyandi, J.; Eom, S.H.; Gurunathan, S. Silver Nanoparticles Inhibit VEGF Induced Cell Proliferation and Migration in Bovine Retinal Endothelial Cells. Colloids Surf. B Biointerfaces 2009, 73, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Lee, K.-J.; Kalishwaralal, K.; Sheikpranbabu, S.; Vaidyanathan, R.; Eom, S.H. Antiangiogenic Properties of Silver Nanoparticles. Biomaterials 2009, 30, 6341–6350. [Google Scholar] [CrossRef]
- Pavan, S.R.; Venkatesan, J.; Prabhu, A. Anticancer Activity of Silver Nanoparticles from the Aqueous Extract of Dictyota Ciliolata on Non-Small Cell Lung Cancer Cells. J. Drug Deliv. Sci. Technol. 2022, 74, 103525. [Google Scholar] [CrossRef]
- Baharara, J.; Namvar, F.; Ramezani, T.; Hosseini, N.; Mohamad, R. Green Synthesis of Silver Nanoparticles Using Achillea Biebersteinii Flower Extract and Its Anti-Angiogenic Properties in the Rat Aortic Ring Model. Molecules 2014, 19, 4624–4634. [Google Scholar] [CrossRef]
- Baharara, J.; Namvar, F.; Mousavi, M.; Ramezani, T.; Mohamad, R. Anti-Angiogenesis Effect of Biogenic Silver Nanoparticles Synthesized Using Saliva officinalis on Chick Chorioalantoic Membrane (CAM). Molecules 2014, 19, 13498–13508. [Google Scholar] [CrossRef]
- Baharara, J.; Namvar, F.; Ramezani, T.; Mousavi, M.; Mohamad, R. Silver Nanoparticles Biosynthesized Using Achillea biebersteinii Flower Extract: Apoptosis Induction in MCF-7 Cells via Caspase Activation and Regulation of Bax and Bcl-2 Gene Expression. Molecules 2015, 20, 2693–2706. [Google Scholar] [CrossRef]
- Ozdal, M.; Gurkok, S. A Recent advances in nanoparticles as antibacterial agent. ADMET DMPK 2022, 10, 115–129. [Google Scholar] [CrossRef]
- Lei, C.; Sun, N.; Wu, H.; Zhao, Y.; Yu, C.; Janani, B.J.; Fakhri, A. Bio-Photoelectrochemical Degradation, and Photocatalysis Process by the Fabrication of Copper Oxide/Zinc Cadmium Sulfide Heterojunction Nanocomposites: Mechanism, Microbial Community and Antifungal Analysis. Chemosphere 2022, 308, 136375. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hadi, M.A.; Aljuboory, D.S.; Ali, F.A.; Jawad, M.A.; AL-Alwany, A.; Hadrawi, S.K.; Mundher, T.; Riadi, Y.; Amer, R.F.; et al. High Efficiency of Ag0 Decorated Cu2MoO4 Nanoparticles for Heterogeneous Photocatalytic Activation, Bactericidal System, and Detection of Glucose from Blood Sample. J. Photochem. Photobiol. B Biol. 2022, 236, 112571. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jihad, A.; Hussam, F.; Al-Abdeen, S.H.Z.; Hussein, J.M.; Adhab, Z.H.; Alzahraa, Z.H.A.; Ahmad, I.; Fatolahi, L.; Janani, B.J. A Facile Preparation Method for Efficiency a Novel LaNiO3/SrCeO3 (p-n Type) Heterojunction Catalyst in Photocatalytic Activities, Bactericidal Assessment and Dopamine Detection. Surf. Interfaces 2023, 38, 102830. [Google Scholar] [CrossRef]
- Mahdi, A.A.; Obeid, R.A.; Abdullah, K.; Mohammed, S.; Kadhim, A.J.; Ramadan, M.F.; Hussien, B.M.; Alkahtani, A.; Ali, F.A.; Alkhathami, A.G.; et al. A Facile Construction of NiV2O6/CeO2 Nano-Heterojunction for Photo-Operated Process in Water Remediation Reaction, Antibacterial Studies, and Detection of D-Amino Acid in Peroxidase System. Surf. Interfaces 2023, 40, 102970. [Google Scholar] [CrossRef]
- Aldhalmi, A.K.; Alkhayyat, S.; Younis Albahadly, W.K.; Jawad, M.A.; Alsaraf, K.M.; Riyad Muedii, Z.A.-H.; Ali, F.A.; Ahmed, M.; Asiri, M.; Al-Fatolahi, L.; et al. A Novel Fabricate of Iron and Nickel-Introduced Bimetallic MOFs for Quickly Catalytic Degradation via the Peroxymonosulfate, Antibacterial Efficiency, and Cytotoxicity Assay. Inorg. Chem. Commun. 2023, 153, 110823. [Google Scholar] [CrossRef]
- Lai, Y.; Fakhri, A.; Janani, B.J. Synergistic Activities of Silver Indium Sulfide/Nickel Molybdenum Sulfide Nanostructures Anchored on Clay Mineral for Light-Driven Bactericidal Performance, and Detection of Uric Acid from Gout Patient Serum. J. Photochem. Photobiol. B Biol. 2022, 234, 112526. [Google Scholar] [CrossRef]
- Syed, A.; Elgorban, A.M.; Bahkali, A.H.; Eswaramoorthy, R.; Verma, M.; Varma, R.S.; Janani, B.J. Highly-Impressive Performances of Novel NiCo2O4/Bi2O3/Ag2ZrO3 Nanocomposites in Photocatalysis System, Removal Pathway, Toxicity Estimation, and Antibacterial Activities. J. Taiwan Inst. Chem. Eng. 2023, 149, 105004. [Google Scholar] [CrossRef]
- Maťátková, O.; Michailidu, J.; Miškovská, A.; Kolouchová, I.; Masák, J.; Čejková, A. Antimicrobial Properties and Applications of Metal Nanoparticles Biosynthesized by Green Methods. Biotechnol. Adv. 2022, 58, 107905. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal Nanoparticles: Understanding the Mechanisms behind Antibacterial Activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef]
- Chatterjee, A.K.; Chakraborty, R.; Basu, T. Mechanism of Antibacterial Activity of Copper Nanoparticles. Nanotechnology 2014, 25, 135101. [Google Scholar] [CrossRef]
- Tao, C. Antimicrobial Activity and Toxicity of Gold Nanoparticles: Research Progress, Challenges and Prospects. Lett. Appl. Microbiol. 2018, 67, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Durán, N.; Durán, M.; de Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver Nanoparticles: A New View on Mechanistic Aspects on Antimicrobial Activity. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.A.; Das, S.S.; Khatoon, A.; Ansari, M.T.; Afzal, M.; Hasnain, S.; Nayak, A.K. Bactericidal activity of silver nanoparticles: A mechanistic review. Mater. Sci. Energy Technol. 2020, 3, 756–769. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver Nanoparticles as Antimicrobial Agent: A Case Study on E. Coli as a Model for Gram-Negative Bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Baker, C.; Pradhan, A.; Pakstis, L.; Pochan, D.; Shah, S.I. Synthesis and Antibacterial Properties of Silver Nanoparticles. J. Nanosci. Nanotechnol. 2005, 5, 244–249. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The Bactericidal Effect of Silver Nanoparticles. Nanotechnol. 2005, 16, 2346–2353. [Google Scholar] [CrossRef]
- Yuan, Y.-G.; Peng, Q.-L.; Gurunathan, S. Effects of Silver Nanoparticles on Multiple Drug-Resistant Strains of Staphylococcus aureus and Pseudomonas aeruginosa from Mastitis-Infected Goats: An Alternative Approach for Antimicrobial Therapy. Int. J. Mol. Sci. 2017, 18, 569. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.-H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.-Y.; et al. Antimicrobial Effects of Silver Nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Allahverdiyev, A.M.; Kon, K.V.; Abamor, E.S.; Bagirova, M.; Rafailovich, M. Coping with Antibiotic Resistance: Combining Nanoparticles with Antibiotics and Other Antimicrobial Agents. Expert Rev. Anti Infect. Ther. 2011, 9, 1035–1052. [Google Scholar] [CrossRef]
- Deng, H.; McShan, D.; Zhang, Y.; Sinha, S.S.; Arslan, Z.; Ray, P.C.; Yu, H. Mechanistic Study of the Synergistic Antibacterial Activity of Combined Silver Nanoparticles and Common Antibiotics. Environ. Sci. Technol. 2016, 50, 8840–8848. [Google Scholar] [CrossRef]
- Ipe, D.S.; Kumar, P.T.S.; Love, R.M.; Hamlet, S.M. Silver Nanoparticles at Biocompatible Dosage Synergistically Increases Bacterial Susceptibility to Antibiotics. Front. Microbiol. 2020, 11, 1074. [Google Scholar] [CrossRef] [PubMed]
- León-Buitimea, A.; Garza-Cárdenas, C.R.; Román-García, M.F.; Ramírez-Díaz, C.A.; Ulloa-Ramírez, M.; Morones-Ramírez, J.R. Nanomaterials-Based Combinatorial Therapy as a Strategy to Combat Antibiotic Resistance. Antibiotics 2022, 11, 794. [Google Scholar] [CrossRef] [PubMed]
- Nirmala Grace, A.; Pandian, K. Antibacterial Efficacy of Aminoglycosidic Antibiotics Protected Gold Nanoparticles—A Brief Study. Colloids Surfaces A Physicochem. Eng. Asp. 2007, 297, 63–70. [Google Scholar] [CrossRef]
- Ribeiro, A.I.; Dias, A.M.; Zille, A. Synergistic Effects between Metal Nanoparticles and Commercial Antimicrobial Agents: A Review. ACS Appl. Nano Mater. 2022, 5, 3030–3064. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef]
- Shahverdi, A.R.; Fakhimi, A.; Shahverdi, H.R.; Minaian, S. Synthesis and Effect of Silver Nanoparticles on the Antibacterial Activity of Different Antibiotics against Staphylococcus aureus and Escherichia coli. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 168–171. [Google Scholar] [CrossRef]
- Gajbhiye, M.; Kesharwani, J.; Ingle, A.; Gade, A.; Rai, M. Fungus-Mediated Synthesis of Silver Nanoparticles and Their Activity against Pathogenic Fungi in Combination with Fluconazole. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 382–386. [Google Scholar] [CrossRef]
- Jain, J.; Arora, S.; Rajwade, J.M.; Omray, P.; Khandelwal, S.; Paknikar, K.M. Silver Nanoparticles in Therapeutics: Development of an Antimicrobial Gel Formulation for Topical Use. Mol. Pharm. 2009, 6, 1388–1401. [Google Scholar] [CrossRef]
- Mallmann, E.J.J.; Cunha, F.A.; Castro, B.N.M.F.; Maciel, A.M.; Menzes, E.A.; Fechine, P.B.A. Antifungal Activity of Silver Nanoparticles Obtained by Green Synthesis. Rev. Inst. Med. Trop. Sao Paulo 2015, 57, 165–167. [Google Scholar] [CrossRef]
- Peralta, L.C.F.; Almeida, N.L.M.; Pontes, F.M.L.; Rinaldo, D.; Carneiro, C.A.; Neppelenbroek, K.H.; Lara, V.S.; Porto, V.C. Silver Nanoparticles in Denture Adhesive: An Antimicrobial Approach against Candida albicans. J. Dent. 2023, 131, 104445. [Google Scholar] [CrossRef] [PubMed]
- Golli, R.; Thummaneni, C.; Pabbathi, D.D.; Srungarapu, T.; Jayasri, G.; Vangalapati, M. Silver Nanoparticles Synthesized by Brassica oleracea (Broccoli) Acting as Antifungal Agent against Candida albicans. Mater. Today Proc. 2023, 80, 1495–1500. [Google Scholar] [CrossRef]
- Robles-Martínez, M.; Patiño-Herrera, R.; Pérez-Vázquez, F.J.; Montejano-Carrizales, J.M.; González, J.F.C.; Pérez, E. Mentha Piperita as a Natural Support for Silver Nanoparticles: A New Anti-Candida albicans Treatment. Colloid Interface Sci. Commun. 2020, 35, 100253. [Google Scholar] [CrossRef]
- Ahmad, T.; Wani, I.A.; Lone, I.H.; Ganguly, A.; Manzoor, N.; Ahmad, A.; Ahmed, J.; Al-Shihri, A.S. Antifungal Activity of Gold Nanoparticles Prepared by Solvothermal Method. Mater. Res. Bull. 2013, 48, 12–20. [Google Scholar] [CrossRef]
- Umamaheswari, K.; Abirami, M. Assessment of Antifungal Action Mechanism of Green Synthesized Gold Nanoparticles (AuNPs) Using Allium sativum on Candida Species. Mater. Lett. 2023, 333, 133616. [Google Scholar] [CrossRef]
- Kareem, H.A.; Samaka, H.M.; Abdulridha, W.M. Evaluation of the gold nanoparticles prepared by green chemistry in the treatment of cutaneous candidiasis. Curr. Med. Mycol. 2021, 7, 1–5. [Google Scholar] [CrossRef]
- Rai, M.; Deshmukh, S.D.; Ingle, A.P.; Gupta, I.R.; Galdiero, M.; Galdiero, S. Metal Nanoparticles: The Protective Nanoshield against Virus Infection. Crit. Rev. Microbiol. 2016, 42, 46–56. [Google Scholar] [CrossRef]
- Ratan, Z.A.; Mashrur, F.R.; Chhoan, A.P.; Shahriar, S.M.; Haidere, M.F.; Runa, N.J.; Kim, S.; Kweon, D.-H.; Hosseinzadeh, H.; Cho, J.Y. Silver Nanoparticles as Potential Antiviral Agents. Pharmaceutics 2021, 13, 2034. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, G.; Ran, D.; Krishnan, N.; Fang, R.H.; Gao, W.; Spector, S.A.; Zhang, L. T-cell-mimicking Nanoparticles Can Neutralize HIV Infectivity. Adv. Mater. 2018, 30, 1802233. [Google Scholar] [CrossRef]
- Orłowski, P.; Kowalczyk, A.; Tomaszewska, E.; Ranoszek-Soliwoda, K.; Węgrzyn, A.; Grzesiak, J.; Celichowski, G.; Grobelny, J.; Eriksson, K.; Krzyzowska, M. Antiviral Activity of Tannic Acid Modified Silver Nanoparticles: Potential to Activate Immune Response in Herpes Genitalis. Viruses 2018, 10, 524. [Google Scholar] [CrossRef]
- Gaikwad, S.; Ingle, A.; Gade, A.; Rai, M.; Falanga, A.; Incoronato, N.; Russo, L.; Galdiero, S.; Galdiero, M. Antiviral Activity of Mycosynthesized Silver Nanoparticles against Herpes Simplex Virus and Human Parainfluenza Virus Type 3. Int. J. Nanomed. 2013, 8, 4303–4314. [Google Scholar] [CrossRef]
- Jeremiah, S.S.; Miyakawa, K.; Morita, T.; Yamaoka, Y.; Ryo, A. Potent Antiviral Effect of Silver Nanoparticles on SARS-CoV-2. Biochem. Biophys. Res. Commun. 2020, 533, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.H.; Ayala-Nuñez, N.V.; Ixtepan-Turrent, L.; Rodriguez-Padilla, C. Mode of Antiviral Action of Silver Nanoparticles against HIV-1. J. Nanobiotechnol. 2010, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, S.; Ghirlando, R.; Grisshammer, R. Biophysical Characterization of Membrane Proteins in Nanodiscs. Methods 2013, 59, 287–300. [Google Scholar] [CrossRef]
- Dos Santos, C.A.; Seckler, M.M.; Ingle, A.P.; Gupta, I.; Galdiero, S.; Galdiero, M.; Gade, A.; Rai, M. Silver Nanoparticles: Therapeutical Uses, Toxicity, and Safety Issues. J. Pharm. Sci. 2014, 103, 1931–1944. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Zheng, C.; Zheng, Y.; Li, X.; Yin, J.; O’Conner, M.; Marappan, M.; Miao, Y.; Xiang, B.; Duan, W.; et al. Inhibition of A/Human/Hubei/3/2005 (H3N2) Influenza Virus Infection by Silver Nanoparticles In Vitro and In Vivo. Int. J. Nanomed. 2013, 8, 4103–4114. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, N.; Kaur, G.; Chaubey, K.K.; Singh, P.; Sharma, S.; Tiwari, A.; Singh, S.V.; Kumar, N. Silver Nanoparticles Impair Peste Des Petits Ruminants Virus Replication. Virus Res. 2014, 190, 1–7. [Google Scholar] [CrossRef]
- Mehranfar, A.; Izadyar, M. Theoretical Design of Functionalized Gold Nanoparticles as Antiviral Agents against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). J. Phys. Chem. Lett. 2020, 11, 10284–10289. [Google Scholar] [CrossRef]
- Chen, L.; Liang, J. An Overview of Functional Nanoparticles as Novel Emerging Antiviral Therapeutic Agents. Mater. Sci. Eng. C 2020, 112, 110924. [Google Scholar] [CrossRef]
- Babaei, A.; Mousavi, S.M.; Ghasemi, M.; Pirbonyeh, N.; Soleimani, M.; Moattari, A. Gold Nanoparticles Show Potential in Vitro Antiviral and Anticancer Activity. Life Sci. 2021, 284, 119652. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Gold Nanoparticles: Biosynthesis and Potential of Biomedical Application. J. Funct. Biomater. 2021, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Draz, M.S.; Shafiee, H. Applications of Gold Nanoparticles in Virus Detection. Theranostics 2018, 8, 1985–2017. [Google Scholar] [CrossRef] [PubMed]
- Maduray, K.; Parboosing, R. Metal Nanoparticles: Apromising Treatment for Viral and Arboviral Infections. Biol. Trace Elem. Res. 2021, 199, 3159–3176. [Google Scholar] [CrossRef] [PubMed]
- Murugan, K.; Wei, J.; Alsalhi, M.S.; Nicoletti, M.; Paulpandi, M.; Samidoss, C.M.; Dinesh, D.; Chandramohan, B.; Paneerselvam, C.; Subramaniam, J.; et al. Magnetic Nanoparticles Are Highly Toxic to Chloroquine-Resistant Plasmodium Falciparum, Dengue Virus (DEN-2), and Their Mosquito Vectors. Parasitol. Res. 2017, 116, 495–502. [Google Scholar] [CrossRef]
- Gutierrez, L.; Li, X.; Wang, J.; Nangmenyi, G.; Economy, J.; Kuhlenschmidt, T.B.; Kuhlenschmidt, M.S.; Nguyen, T.H. Adsorption of Rotavirus and Bacteriophage MS2 Using Glass Fiber Coated with Hematite Nanoparticles. Water Res. 2009, 43, 5198–5208. [Google Scholar] [CrossRef]
- Kumar, R.; Nayak, M.; Sahoo, G.C.; Pandey, K.; Sarkar, M.C.; Ansari, Y.; Das, V.N.R.; Topno, R.K.; Bhawna; Madhukar, M.; et al. Iron Oxide Nanoparticles Based Antiviral Activity of H1N1 Influenza A Virus. J. Infect. Chemother. 2019, 25, 325–329. [Google Scholar] [CrossRef]
- Abo-zeid, Y.; Ismail, N.S.M.; McLean, G.R.; Hamdy, N.M. A Molecular Docking Study Repurposes FDA Approved Iron Oxide Nanoparticles to Treat and Control COVID-19 Infection. Eur. J. Pharm. Sci. 2020, 153, 105465. [Google Scholar] [CrossRef]
- Wong, C.K.; Cheung, P.F.Y.; Ip, W.K.; Lam, C.W.K. Intracellular Signaling Mechanisms Regulating Toll-like Receptor–Mediated Activation of Eosinophils. Am. J. Respir. Cell Mol. Biol. 2007, 37, 85–96. [Google Scholar] [CrossRef]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in Wound Repair: Molecular and Cellular Mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef]
- Aththanayaka, S.; Thiripuranathar, G.; Ekanayake, S. Green Synthesized Nanomaterials as Antioxidant and Antiinflammatory Substances. In Synthesis of Bionanomaterials for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2023; pp. 299–317. [Google Scholar]
- Lan Chi, N.T.; Narayanan, M.; Chinnathambi, A.; Govindasamy, C.; Subramani, B.; Brindhadevi, K.; Pimpimon, T.; Pikulkaew, S. Fabrication, Characterization, Anti-Inflammatory, and Anti-Diabetic Activity of Silver Nanoparticles Synthesized from Azadirachta Indica Kernel Aqueous Extract. Environ. Res. 2022, 208, 112684. [Google Scholar] [CrossRef]
- Londhe, S.; Haque, S.; Patra, C.R. Silver and Gold Nanoparticles: Potential Cancer Theranostic Applications, Recent Development, Challenges, and Future Perspectives. In Gold and Silver Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2023; pp. 247–290. [Google Scholar]
- Pauna, A.-M.R. The Prolongation of the Antinociceptive Effects of Novel Nanosystems Entrapping Diclofenac Sodium in Somatic Pain in Rats. Rev. Med. Chir. 2022, 126, 557–564. [Google Scholar] [CrossRef]
- Afshari, A.R.; Sanati, M.; Mollazadeh, H.; Kesharwani, P.; Johnston, T.P.; Sahebkar, A. Nanoparticle-Based Drug Delivery Systems in Cancer: A Focus on Inflammatory Pathways. Semin. Cancer Biol. 2022, 86, 860–872. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, R.F.; de Araújo, A.A.; Pessoa, J.B.; Freire Neto, F.P.; da Silva, G.R.; Leitão Oliveira, A.L.C.S.; de Carvalho, T.G.; Silva, H.F.O.; Eugênio, M.; Sant’Anna, C.; et al. Anti-Inflammatory, Analgesic and Anti-Tumor Properties of Gold Nanoparticles. Pharmacol. Rep. 2017, 69, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Somasuntharam, I.; Yehl, K.; Carroll, S.L.; Maxwell, J.T.; Martinez, M.D.; Che, P.-L.; Brown, M.E.; Salaita, K.; Davis, M.E. Knockdown of TNF-α by DNAzyme Gold Nanoparticles as an Anti-Inflammatory Therapy for Myocardial Infarction. Biomaterials 2016, 83, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, G.M.; Waheeb, H.M.; Jabir, M.S.; Khazaal, S.H.; Dewir, Y.H.; Naidoo, Y. Hesperidin Loaded on Gold Nanoparticles as a Drug Delivery System for a Successful Biocompatible, Anti-Cancer, Anti-Inflammatory and Phagocytosis Inducer Model. Sci. Rep. 2020, 10, 9362. [Google Scholar] [CrossRef]
- Gao, W.; Wang, Y.; Xiong, Y.; Sun, L.; Wang, L.; Wang, K.; Lu, H.Y.; Bao, A.; Turvey, S.E.; Li, Q.; et al. Size-Dependent Anti-Inflammatory Activity of a Peptide-Gold Nanoparticle Hybrid in Vitro and in a Mouse Model of Acute Lung Injury. Acta Biomater. 2019, 85, 203–217. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Chung, I.-M.; Gomathi, T.; Ansari, M.A.; Khanna, V.G.; Babu, V.; Rajakumar, G. Synthesis, characterization and pharmacological potential of green synthesized copper nanoparticles. Bioprocess Biosyst. Eng. 2019, 42, 1769–1777. [Google Scholar] [CrossRef]
- Agarwal, H.; Shanmugam, V. A Review on Anti-Inflammatory Activity of Green Synthesized Zinc Oxide Nanoparticle: Mechanism-Based Approach. Bioorg. Chem. 2020, 94, 103423. [Google Scholar] [CrossRef]
- Aafreen, M.M.; Anitha, R.; Preethi, R.C.; Rajeshkumar, S.; Lakshmi, T. Anti-Inflammatory Activity of Silver Nanoparticles Prepared from Ginger Oil—An Invitro Approach. Indian J. Public Health Res. Dev. 2019, 10, 145. [Google Scholar] [CrossRef]
- Singh, P.; Ahn, S.; Kang, J.-P.; Veronika, S.; Huo, Y.; Singh, H.; Chokkaligam, M.; El-Agamy Farh, M.; Aceituno, V.C.; Kim, Y.J.; et al. In Vitro Anti-Inflammatory Activity of Spherical Silver Nanoparticles and Monodisperse Hexagonal Gold Nanoparticles by Fruit Extract of Prunus serrulata: A Green Synthetic Approach. Artif. Cells Nanomed. Biotechnol. 2017, 46, 2022–2032. [Google Scholar] [CrossRef]
- Bhol, K.C.; Schechter, P.J. Effects of Nanocrystalline Silver (NPI 32101) in a Rat Model of Ulcerative Colitis. Dig. Dis. Sci. 2007, 52, 2732–2742. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Wong, K.K.Y.; Ho, C.-M.; Lok, C.-N.; Yu, W.-Y.; Che, C.-M.; Chiu, J.-F.; Tam, P.K.H. Topical Delivery of Silver Nanoparticles Promotes Wound Healing. ChemMedChem 2007, 2, 129–136. [Google Scholar] [CrossRef] [PubMed]
- David, L.; Moldovan, B.; Vulcu, A.; Olenic, L.; Perde-Schrepler, M.; Fischer-Fodor, E.; Florea, A.; Crisan, M.; Chiorean, I.; Clichici, S.; et al. Green Synthesis, Characterization and Anti-Inflammatory Activity of Silver Nanoparticles Using European Black Elderberry Fruits Extract. Colloids Surf. B Biointerfaces 2014, 122, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Nadworny, P.L.; Wang, J.; Tredget, E.E.; Burrell, R.E. Anti-Inflammatory Activity of Nanocrystalline Silver in a Porcine Contact Dermatitis Model. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Pandit, S.; Mokkapati, V.R.S.S.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef]
- Boroumand, Z.; Golmakani, N.; Boroumand, S. Clinical Trials on Silver Nanoparticles for Wound Healing. Nanomed. J 2018, 5, 186–191. [Google Scholar] [CrossRef]
- Carrasco-Esteban, E.; Domínguez-Rullán, J.A.; Barrionuevo-Castillo, P.; Pelari-Mici, L.; Leaman, O.; Sastre-Gallego, S.; López-Campos, F. Current Role of Nanoparticles in the Treatment of Lung Cancer. J. Clin. Transl. Res. 2021, 7, 140–155. [Google Scholar]
- National Library of Medicine; National Institutes of Health; U.S. Department of Health and Human Services. ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=&term=gold+nanoparticles&cntry=&state=&city=&dist= (accessed on 20 June 2023).
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron Oxide Nanoparticles: Diagnostic, Therapeutic and Theranostic Applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef]
- Singh, S.P.; Bhargava, C.S.; Dubey, V.; Mishra, A.; Singh, Y. Silver Nanoparticles: Biomedical Applications, Toxicity, and Safety Issues. Int. J. Res. Pharm. Pharm. Sci. 2017, 2, 2455–2698. [Google Scholar]
- Xiong, P.; Huang, X.; Ye, N.; Lu, Q.; Zhang, G.; Peng, S.; Wang, H.; Liu, Y. Cytotoxicity of Metal-based Nanoparticles: From Mechanisms and Methods of Evaluation to Pathological Manifestations. Adv. Sci. 2022, 9, 2106049. [Google Scholar] [CrossRef]
- Lansdown, A.B.G. Silver in Health Care: Antimicrobial Effects and Safety in Use. In Biofunctional Textiles and the Skin; KARGER: Basel, Switzerland, 2006; pp. 17–34. [Google Scholar]
- Xu, L.; Wang, Y.-Y.; Huang, J.; Chen, C.-Y.; Wang, Z.-X.; Xie, H. Silver Nanoparticles: Synthesis, Medical Applications and Biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, T.; Li, P.; Huang, W.; Tang, J.; Wang, P.; Liu, J.; Yuan, Q.; Bai, R.; Li, B.; et al. Use of Synchrotron Radiation-Analytical Techniques to Reveal Chemical Origin of Silver-Nanoparticle Cytotoxicity. ACS Nano 2015, 9, 6532–6547. [Google Scholar] [CrossRef]
- Kus-Liśkiewicz, M.; Fickers, P.; Ben Tahar, I. Biocompatibility and Cytotoxicity of Gold Nanoparticles: Recent Advances in Methodologies and Regulations. Int. J. Mol. Sci. 2021, 22, 10952. [Google Scholar] [CrossRef] [PubMed]
- Lewinski, N.; Colvin, V.; Drezek, R. Cytotoxicity of Nanoparticles. Small 2008, 4, 26–49. [Google Scholar] [CrossRef]
- Murphy, C.J.; Gole, A.M.; Stone, J.W.; Sisco, P.N.; Alkilany, A.M.; Goldsmith, E.C.; Baxter, S.C. Gold Nanoparticles in Biology: Beyond Toxicity to Cellular Imaging. Acc. Chem. Res. 2008, 41, 1721–1730. [Google Scholar] [CrossRef] [PubMed]
- Hawley, A.E.; Davis, S.S.; Illum, L. Targeting of Colloids to Lymph Nodes: Influence of Lymphatic Physiology and Colloidal Characteristics. Adv. Drug Deliv. Rev. 1995, 17, 129–148. [Google Scholar] [CrossRef]
- Sabourian, P.; Yazdani, G.; Ashraf, S.S.; Frounchi, M.; Mashayekhan, S.; Kiani, S.; Kakkar, A. Effect of Physico-Chemical Properties of Nanoparticles on Their Intracellular Uptake. Int. J. Mol. Sci. 2020, 21, 8019. [Google Scholar] [CrossRef] [PubMed]
- Sonavane, G.; Tomoda, K.; Sano, A.; Ohshima, H.; Terada, H.; Makino, K. In Vitro Permeation of Gold Nanoparticles through Rat Skin and Rat Intestine: Effect of Particle Size. Colloids Surf. B Biointerfaces 2008, 65, 1–10. [Google Scholar] [CrossRef]
- Sonavane, G.; Tomoda, K.; Makino, K. Biodistribution of Colloidal Gold Nanoparticles after Intravenous Administration: Effect of Particle Size. Colloids Surf. B Biointerfaces 2008, 66, 274–280. [Google Scholar] [CrossRef]
- Steckiewicz, K.P.; Barcinska, E.; Malankowska, A.; Zauszkiewicz–Pawlak, A.; Nowaczyk, G.; Zaleska-Medynska, A.; Inkielewicz-Stepniak, I. Impact of Gold Nanoparticles Shape on Their Cytotoxicity against Human Osteoblast and Osteosarcoma in in Vitro Model. Evaluation of the Safety of Use and Anti-Cancer Potential. J. Mater. Sci. Mater. Med. 2019, 30, 22. [Google Scholar] [CrossRef]
- Hussain, S.M.; Hess, K.L.; Gearhart, J.M.; Geiss, K.T.; Schlager, J.J. In Vitro Toxicity of Nanoparticles in BRL 3A Rat Liver Cells. Toxicol. Vitr. 2005, 19, 975–983. [Google Scholar] [CrossRef]
- Ling, D.; Hyeon, T. Chemical Design of Biocompatible Iron Oxide Nanoparticles for Medical Applications. Small 2013, 9, 1450–1466. [Google Scholar] [CrossRef]
- Jeng, H.A.; Swanson, J. Toxicity of Metal Oxide Nanoparticles in Mammalian Cells. J. Environ. Sci. Health Part A 2006, 41, 2699–2711. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.L.; Gustafsson, J.; Cronholm, P.; Möller, L. Size-Dependent Toxicity of Metal Oxide Particles—A Comparison between Nano- and Micrometer Size. Toxicol. Lett. 2009, 188, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Möller, L. Copper Oxide Nanoparticles Are Highly Toxic: A Comparison between Metal Oxide Nanoparticles and Carbon Nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Jenkins, G.J.S.; Asadi, R.; Doak, S.H. Potential Toxicity of Superparamagnetic Iron Oxide Nanoparticles (SPION). Nano Rev. 2010, 1, 5358. [Google Scholar] [CrossRef]
- Valdiglesias, V.; Kiliç, G.; Costa, C.; Fernández-Bertólez, N.; Pásaro, E.; Teixeira, J.P.; Laffon, B. Effects of Iron Oxide Nanoparticles: Cytotoxicity, Genotoxicity, Developmental Toxicity, and Neurotoxicity. Environ. Mol. Mutagen. 2015, 56, 125–148. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic Potential of Materials at the Nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Shubayev, V.I.; Pisanic, T.R.; Jin, S. Magnetic Nanoparticles for Theragnostics. Adv. Drug Deliv. Rev. 2009, 61, 467–477. [Google Scholar] [CrossRef]
- Unfried, K.; Albrecht, C.; Klotz, L.-O.; Von Mikecz, A.; Grether-Beck, S.; Schins, R.P.F. Cellular Responses to Nanoparticles: Target Structures and Mechanisms. Nanotoxicology 2007, 1, 52–71. [Google Scholar] [CrossRef]
- Malhotra, N.; Lee, J.-S.; Liman, R.A.D.; Ruallo, J.M.S.; Villaflores, O.B.; Ger, T.-R.; Hsiao, C.-D. Potential Toxicity of Iron Oxide Magnetic Nanoparticles: A Review. Molecules 2020, 25, 3159. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outliningtheir Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ding, T.; Sun, J. Neurotoxic Potential of Iron Oxide Nanoparticles in the Rat Brain Striatum and Hippocampus. Neurotoxicology 2013, 34, 243–253. [Google Scholar] [CrossRef]
- Soenen, S.J.H.; Himmelreich, U.; Nuytten, N.; De Cuyper, M. Cytotoxic Effects of Iron Oxide Nanoparticles and Implications for Safety in Cell Labelling. Biomaterials 2011, 32, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Pinzaru, I.; Coricovac, D.; Dehelean, C.; Moacă, E.-A.; Mioc, M.; Baderca, F.; Sizemore, I.; Brittle, S.; Marti, D.; Calina, C.D.; et al. Stable PEG-Coated Silver Nanoparticles—A Comprehensive Toxicological Profile. Food Chem. Toxicol. 2018, 111, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.S.; Ravikumar, M.; John, A.J.; Selvarajan, E.; Patel, H.; Chander, P.S.; Soundarya, J.; Vuppala, S.; Balaji, R.; Chandrasekar, N. A Review on Green Synthesis of Nanoparticles and Their Diverse Biomedical and Environmental Applications. Catalysts 2022, 12, 459. [Google Scholar] [CrossRef]
- Joseph, T.M.; Mahapatra, D.K.; Esmaeili, A.; Piszczyk, Ł.; Hasanin, M.S.; Kattali, M.; Haponiuk, J.; Thomas, S. Nanoparticles: Taking a Unique Position in Medicine. Nanomaterials 2023, 13, 574. [Google Scholar] [CrossRef]
- Anjum, S.; Ishaque, S.; Fatima, H.; Farooq, W.; Hano, C.; Abbasi, B.H.; Anjum, I. Emerging Applications of Nanotechnology in Healthcare Systems: Grand Challenges and Perspectives. Pharmaceuticals 2021, 14, 707. [Google Scholar] [CrossRef]
- Zhang, N.; Xiong, G.; Liu, Z. Toxicity of Metal-Based Nanoparticles: Challenges in the Nano Era. Front. Bioeng. Biotechnol. 2022, 10, 1001572. [Google Scholar] [CrossRef]
- Murali, M.; Gowtham, H.G.; Shilpa, N.; Singh, S.B.; Aiyaz, M.; Sayyed, R.Z.; Shivamallu, C.; Achar, R.R.; Silina, E.; Stupin, V.; et al. Zinc Oxide Nanoparticles Prepared through Microbial Mediated Synthesis for Therapeutic Applications: A Possible Alternative for Plants. Front. Microbiol. 2023, 14, 1227951. [Google Scholar] [CrossRef]
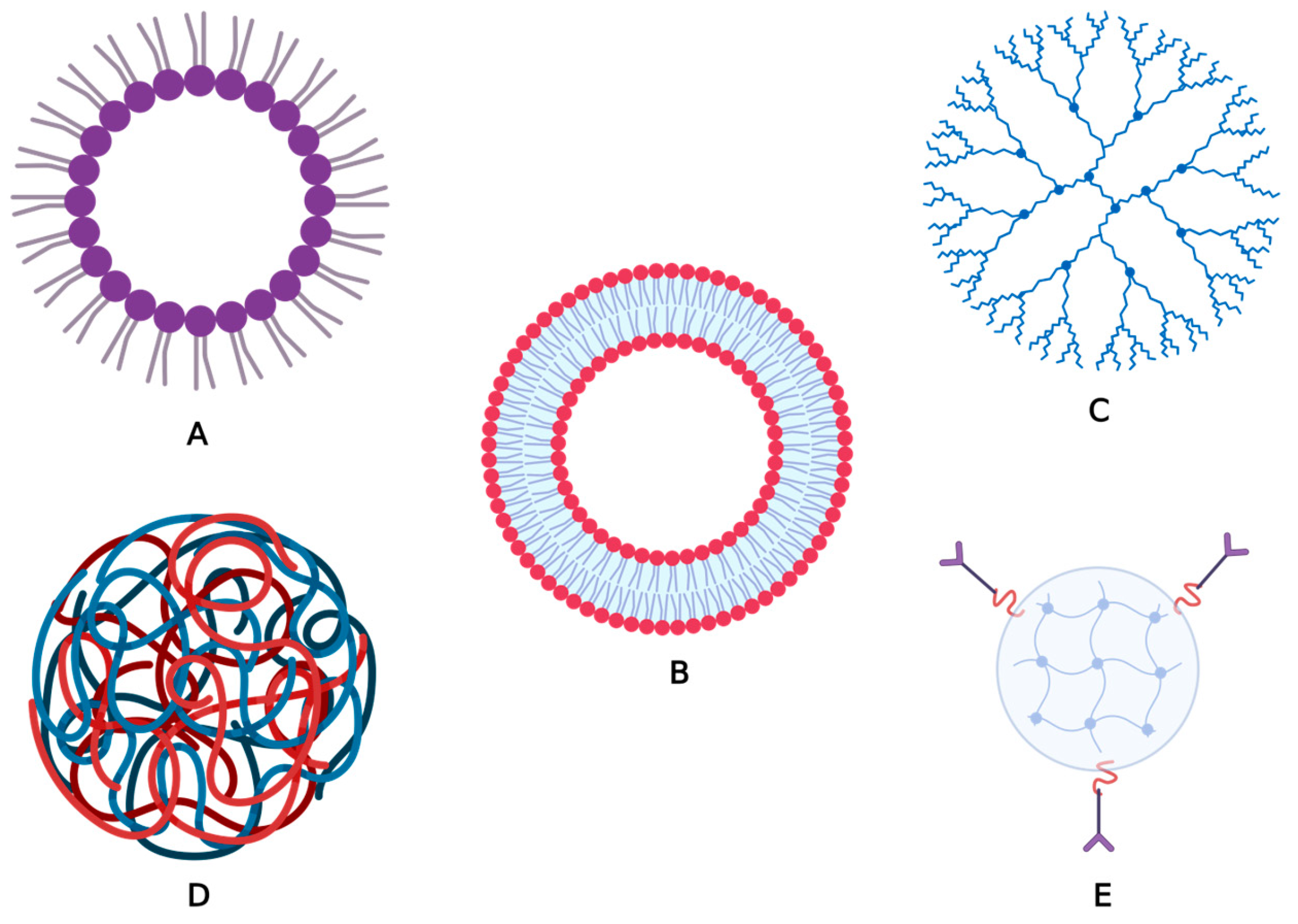
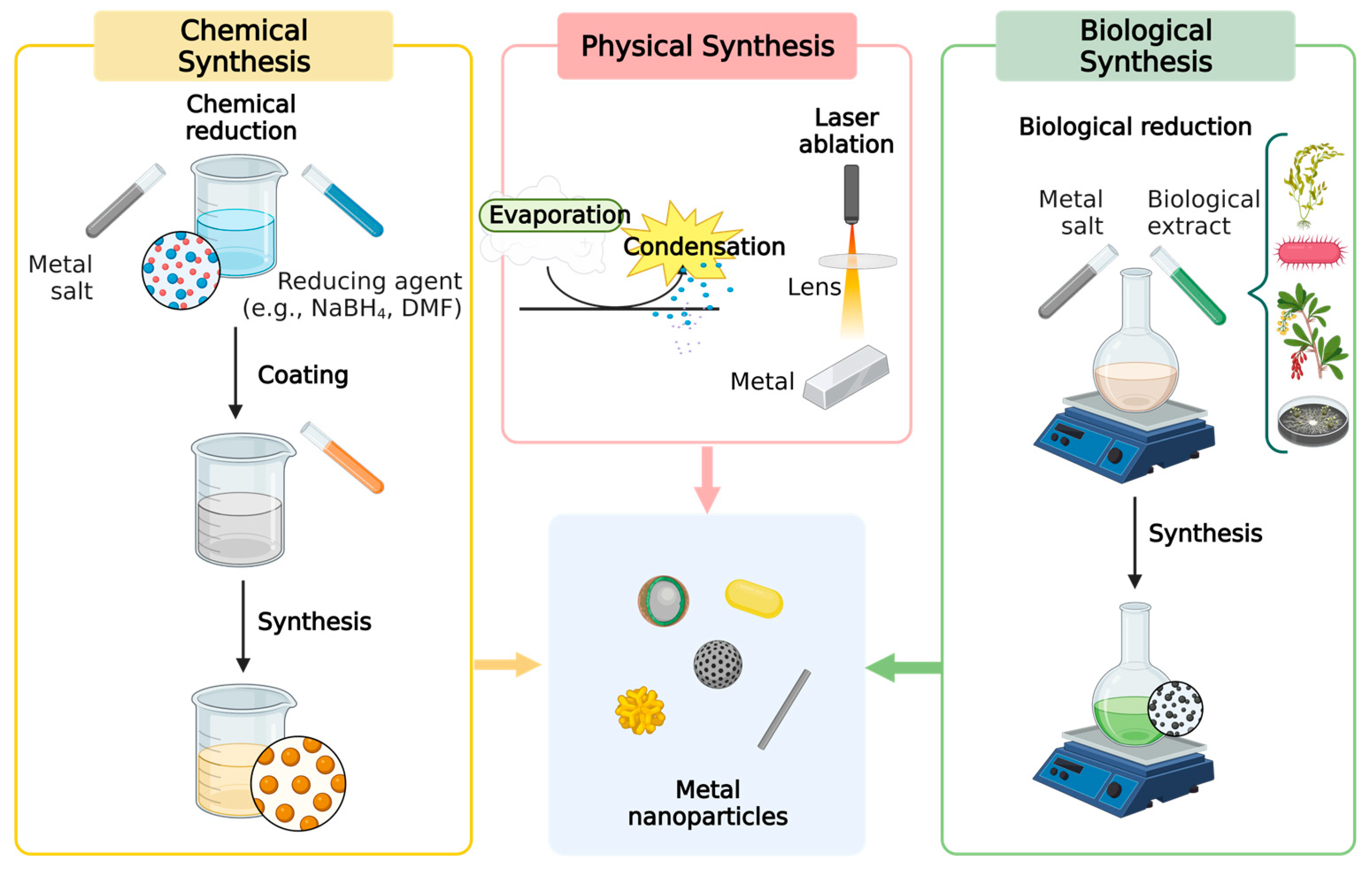

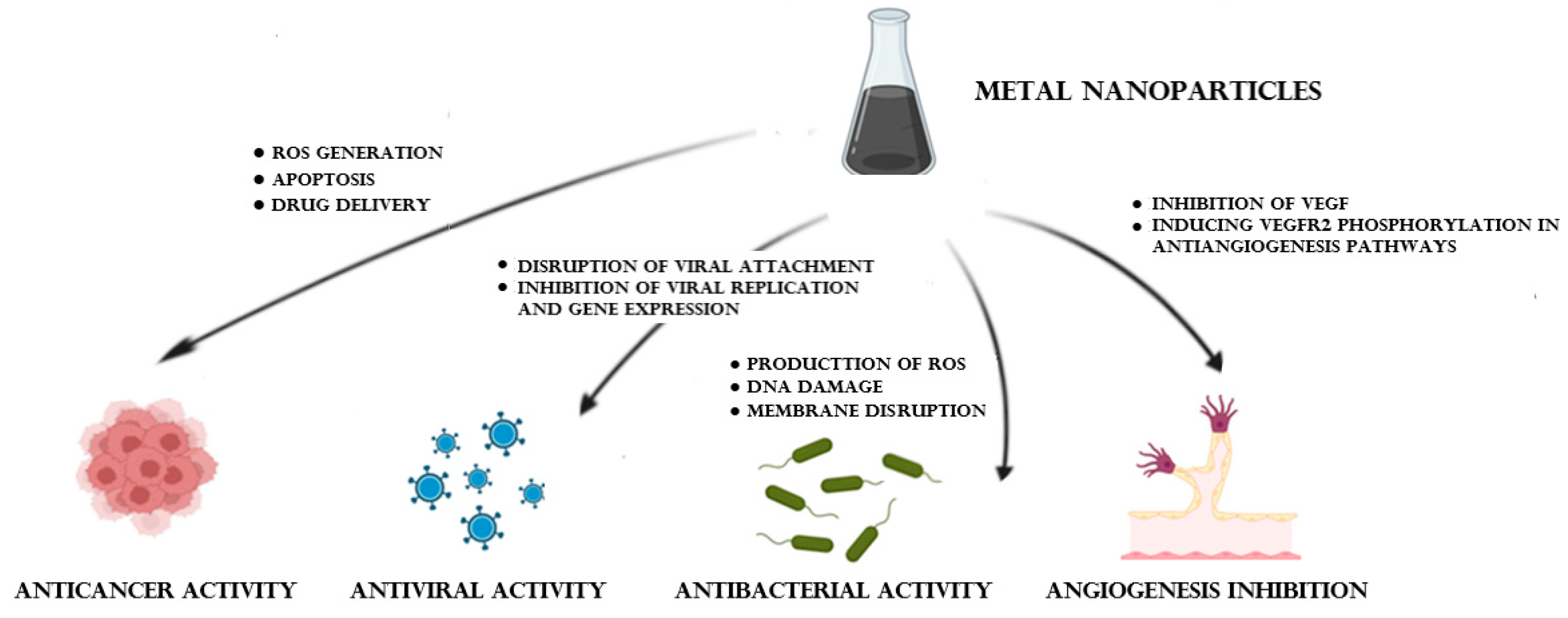
| Biological Material | Advantages | Ref. | Limitations | Ref. |
|---|---|---|---|---|
| Plants |
| [95] |
| [130] |
| [100] | |||
| [96] | |||
| [98] |
| [59] | |
| [130] | |||
| [97] | |||
| [100] |
| [131] | |
| [99] | |||
| [100] | |||
| Microorganisms |
| [132] |
| [133] |
| [134] | |||
| [134] | |||
| Algae |
| [135] |
| [136] |
| [137] | |||
| [138] |
| [59] |
| NPs Type | Study Title | Material | Targeted Condition | Clinical trials.gov Identifier |
|---|---|---|---|---|
| AgNPs | Topical Silver Nanoparticles for Microbial Activity | Topical cream containing silver NPs |
| NCT03752424 |
| Topical Application of Silver Nanoparticles and Oral Pathogens in Ill Patients | Innocuous gel compound with 12 ppm of AgNPs |
| NCT02761525 | |
| Silver Nanoparticles in Multidrug Resistant Bacteria | AgNPs |
| NCT04431440 | |
| Nanosilver Fluoride to Prevent Dental Biofilms Growth | Nanosilver fluoride |
| NCT01950546 | |
| Remineralization of Dentine Caries Using Two Remineralizing Agents Which Are Nanosilver Fluoride and Casein phosphopeptides amorphous Calcium Phosphate | Nanosilver fluoride vs. sodium fluoride with casein phosphopeptides amorphous calcium phosphate on carious lesion |
| NCT04930458 | |
| Radiographic Assessment of Glass Ionomer Restorations with and Without Prior Application of Nano Silver Fluoride in Occlusal Carious Molars Treated with Partial Caries Removal Technique | Nano silver fluoride solution |
| NCT03193606 | |
| Clinical Evaluation of Silver Nanoparticles in Comparison to Silver Diamine Fluoride in Management of Deep Carious Lesions | AgNPs vs. silver diamine fluoride |
| NCT05231330 | |
| Addition of Silver Nanoparticles to an Orthodontic Primer in Preventing Enamel Demineralization Adjacent Brackets | AgNPs incorporated into the primer orthodontic Transbond XT |
| NCT02400957 | |
| Evaluation of Antimicrobial Efficacy and Adaptability of Bioceramic Sealer Containing Nanoparticles | Bioceramic sealers with classic mix vs. AgNPs vs. chitosan |
| NCT04481945 | |
| Silver Nanoparticle Investigation for Treating Chronic Sinusitis | Topical colloidal silver |
| NCT03243201 | |
| The Antibacterial Effect of Nanosilver Fluoride on Primary Teeth | Topic solution of silver fluoride NPs |
| NCT05221749 | |
| Colloidal Silver, Treatment of COVID-19 | Inhalation colloidal silver solution |
| NCT04978025 | |
| Evaluation of Silver Nanoparticles for the Prevention of COVID-19 | AgNPs solution with 1 wt% concentration (0.6 mg/mL metallic silver) for mouthwash and nose rinse |
| NCT04894409 | |
| SARS-CoV-2-Spike-Ferritin-Nanoparticle (SpFN) Vaccine with ALFQ Adjuvant for Prevention of COVID-19 in Healthy Adults | SpFN COVID-19 vaccine with Army Liposomal Formulation QS21 (ALFQ) adjuvant |
| NCT04784767 | |
| Fluor Varnish with Silver Nanoparticles for Dental Remineralization in Patients with Trisomy 21 | Fluor dental varnish plus 25% 50 nm AgNPs vs. fluor dental varnish |
| NCT01975545 | |
| Comparison of Central Venous Catheters with Silver Nanoparticles Versus Conventional Catheters | Central venous catheter impregnated with AgNPs (AgTive®) |
| NCT00337714 | |
| Evaluation of Diabetic Foot Wound Healing Using Hydrogel/Nano Silver-based Dressing vs. Traditional Dressing | Hydrogel/nano silver-based dressing |
| NCT04834245 | |
| Efficacy of Silver Nanoparticle Gel Versus a Common Antibacterial Hand Gel | Nano-silver gel exposed vs. alcohol-based hand gel |
| NCT00659204 | |
| Post-operative Pain Reduction | Conventional calcium hydroxide paste vs. combined calcium hydroxide with AgNPs |
| NCT04338633 | |
| A Phase I/II Double-blind Safety and Efficacy Evaluation of Nowarta110 in Patients with Plantar Warts | Nowarta 110, a colloidal silver fig extract for topical administration |
| NCT02338336 | |
| Research on the Key Technology of Burn Wound Treatment | Nano-silver ion gel and dressings for wounds |
| NCT03279549 | |
| Altrazeal Range of Motion Study Comparing with Typical Carboxymethyl | Hydrogel nanoparticle wound dressing |
| NCT01062191 | |
| Assessment of Postoperative Pain After Using Various Intracanal Medication in Patients with Necrotic Pulp | AgNPs in gel form vs. calcium hydroxide intracanal medication |
| NCT03692286 | |
| Preadmission Skin Wipe Use for Surgical Site Infection Prophylaxis in Adult Orthopaedic Surgery Patients | Bath wipes impregnated with multiple ingredients, among which colloid silver |
| NCT03401749 | |
| Thyme and Carvacroll Nanoparticle Effect on Fungi | Thyme and carvacrol AgNPs |
| NCT04431804 | |
| Clinical and Radiographic Evaluation of the Synergistic Effect of Nano Silver Particles and Calcium Hydroxide Versus Triple Antibiotic Paste as Antibacterial Agents for Lesion Sterilization and Tissue Repair (LSTR) in Necrotic Primary Molars | Nano-silver particles and calcium hydroxide vs. triple antibiotic paste |
| NCT05681221 | |
| Effect of Metallic Nanoparticles on Nosocomial Bacteria | AgNPs and CuNPs |
| NCT04775238 | |
| Evaluation of the Antimicrobial Fiber Reinforced Composite Resin Space Maintainer Modified With Silver Nano Particles | Fiber reinforced composite resin space maintainer modified with silver nano particles |
| NCT05902975 | |
| AuNPs | Gold Factor on Knee Joint Health and Function | Dietary supplement: Gold factor (AuNPs) |
| NCT05347602 |
| Diagnostic and Prognostic Accuracy of Gold Nanoparticles in Salivary Gland Tumors | CD24-Gold nanocomposites |
| NCT04907422 | |
| Plasmonic Photothermal and Stem Cell Therapy of Atherosclerosis Versus Stenting | Stenting and micro-infusion of NP |
| NCT01436123 | |
| Enhanced Epidermal Antigen Specific Immunotherapy Trial -1 | C19-A3 GNP drug |
| NCT02837094 | |
| NU-0129 in Treating Patients with Recurrent Glioblastoma or Gliosarcoma Undergoing Surgery | NU-0129 drug |
| NCT03020017 | |
| Plasmonic Nanophotothermal Therapy of Atherosclerosis | Transplantation of NPs |
| NCT01270139 | |
| Effect of Nano Care Gold on Marginal Integrity of Resin Composite | Nano Care Gold (silver and gold NPs) suspended in 70% isopropyl alcohol, for cavity pre-treatment. |
| NCT03669224 | |
| A Phase-I Study of a Nanoparticle-based Peptide Vaccine Against Dengue Virus | Nanoparticle-based peptide vaccine |
| NCT04935801 | |
| A Phase-I Study of a Nanoparticle-based Peptide Vaccine Against SARS-CoV-2 | Nanoparticle-based peptide vaccine |
| NCT05113862 | |
| Diagnosis of Gastric Lesions from Exhaled Breath and Saliva | Chemical nanosensors based on organically functionalized AuNPs and carbon nanotubes |
| NCT01420588 | |
| Exploratory Study Using Nanotechnology to Detect Biomarkers of Parkinson’s Disease from Exhaled Breath | Combinations of nanomaterial-based sensors |
| NCT01246336 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burlec, A.F.; Corciova, A.; Boev, M.; Batir-Marin, D.; Mircea, C.; Cioanca, O.; Danila, G.; Danila, M.; Bucur, A.F.; Hancianu, M. Current Overview of Metal Nanoparticles’ Synthesis, Characterization, and Biomedical Applications, with a Focus on Silver and Gold Nanoparticles. Pharmaceuticals 2023, 16, 1410. https://doi.org/10.3390/ph16101410
Burlec AF, Corciova A, Boev M, Batir-Marin D, Mircea C, Cioanca O, Danila G, Danila M, Bucur AF, Hancianu M. Current Overview of Metal Nanoparticles’ Synthesis, Characterization, and Biomedical Applications, with a Focus on Silver and Gold Nanoparticles. Pharmaceuticals. 2023; 16(10):1410. https://doi.org/10.3390/ph16101410
Chicago/Turabian StyleBurlec, Ana Flavia, Andreia Corciova, Monica Boev, Denisa Batir-Marin, Cornelia Mircea, Oana Cioanca, Gabriela Danila, Marius Danila, Anca Florentina Bucur, and Monica Hancianu. 2023. "Current Overview of Metal Nanoparticles’ Synthesis, Characterization, and Biomedical Applications, with a Focus on Silver and Gold Nanoparticles" Pharmaceuticals 16, no. 10: 1410. https://doi.org/10.3390/ph16101410
APA StyleBurlec, A. F., Corciova, A., Boev, M., Batir-Marin, D., Mircea, C., Cioanca, O., Danila, G., Danila, M., Bucur, A. F., & Hancianu, M. (2023). Current Overview of Metal Nanoparticles’ Synthesis, Characterization, and Biomedical Applications, with a Focus on Silver and Gold Nanoparticles. Pharmaceuticals, 16(10), 1410. https://doi.org/10.3390/ph16101410








