Dapagliflozin/Hesperidin Combination Mitigates Lipopolysaccharide-Induced Alzheimer’s Disease in Rats
Abstract
:1. Introduction
2. Results
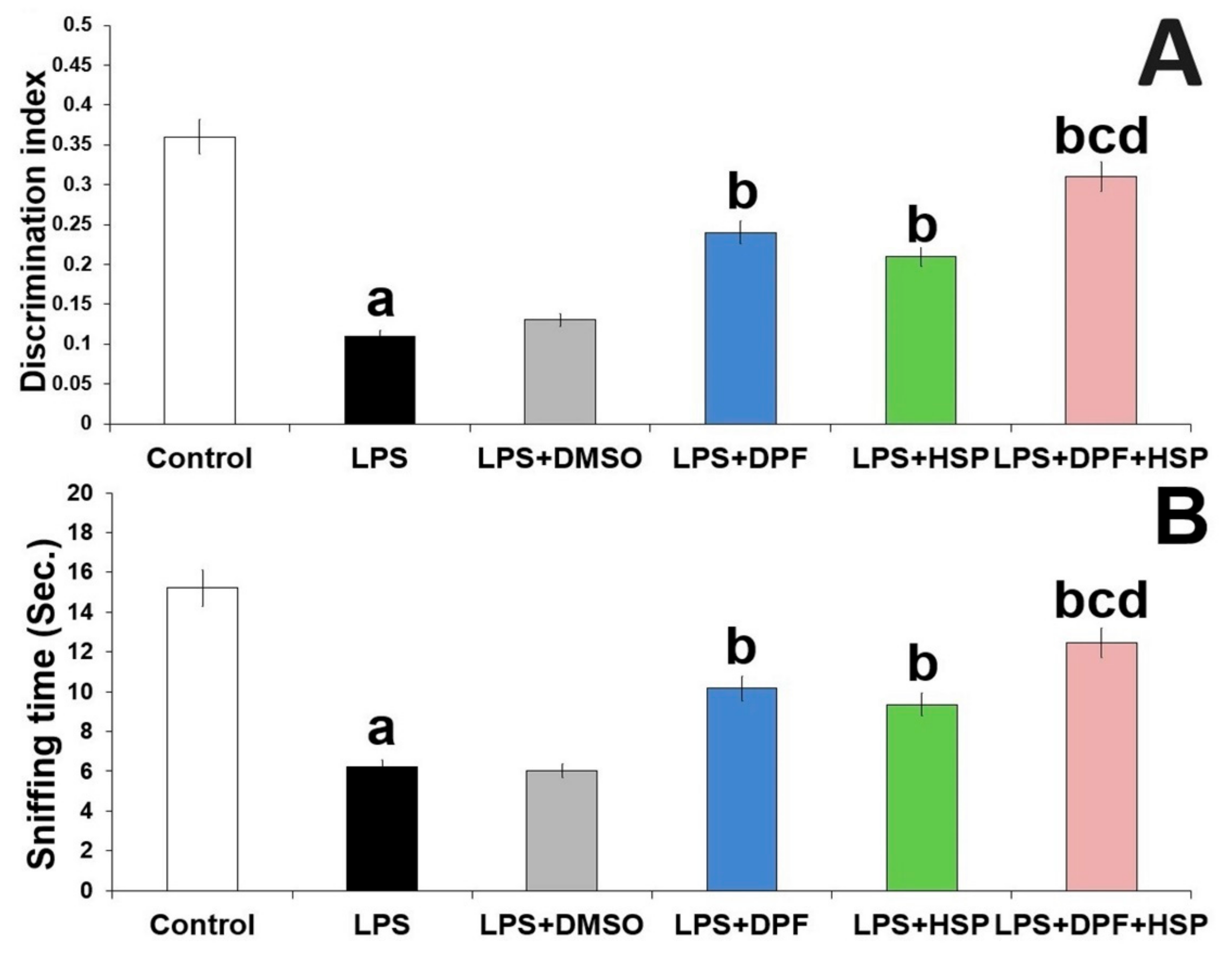
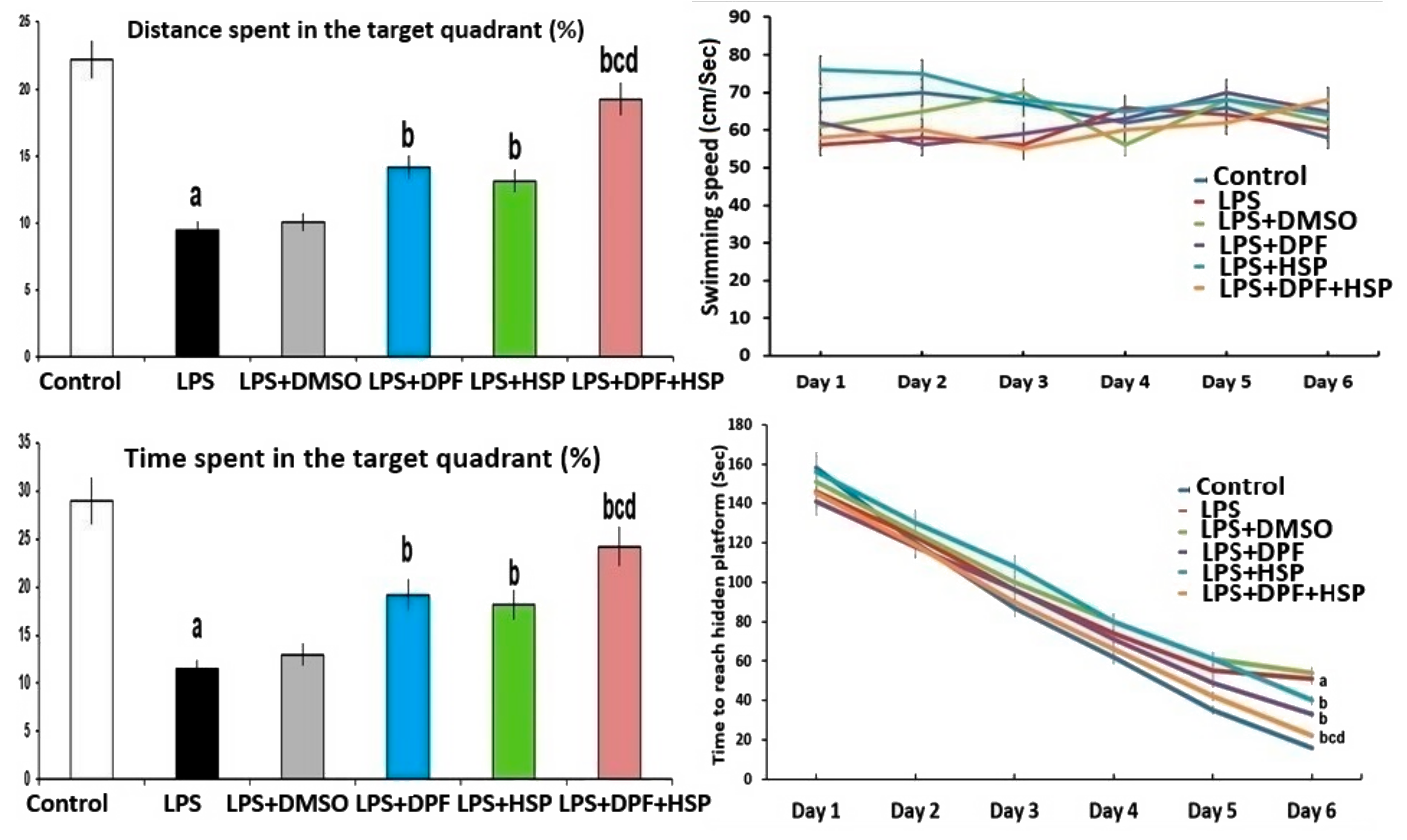
2.1. Dapagliflozin with or without Hesperidin Ameliorated the Changes in the Behavioral Tests Elicited by LPS Injection
2.2. Dapagliflozin with or without Hesperidin Mitigated the Effect of LPS Administration on the Antioxidant Status and Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) Content of the Hippocampal Tissues
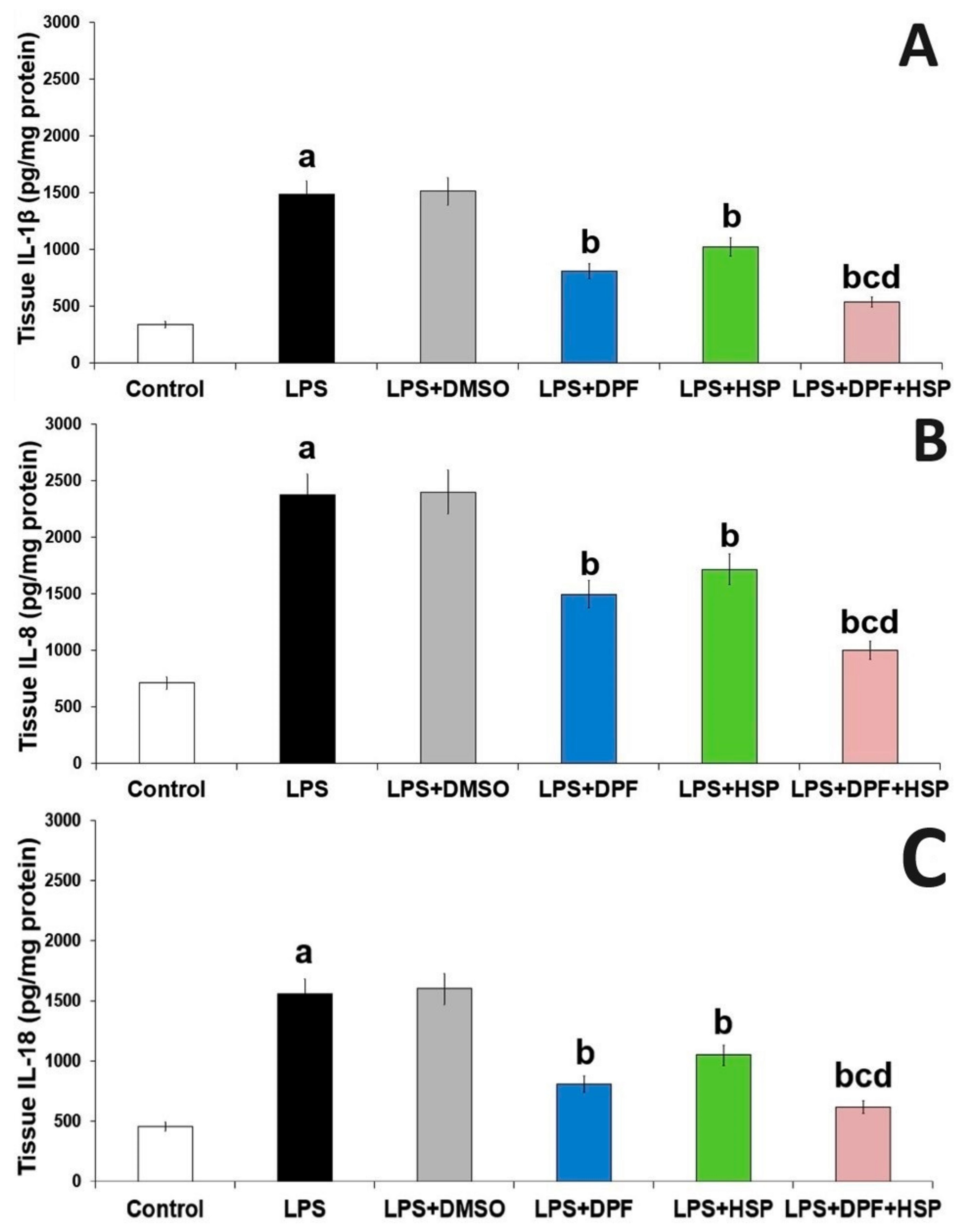
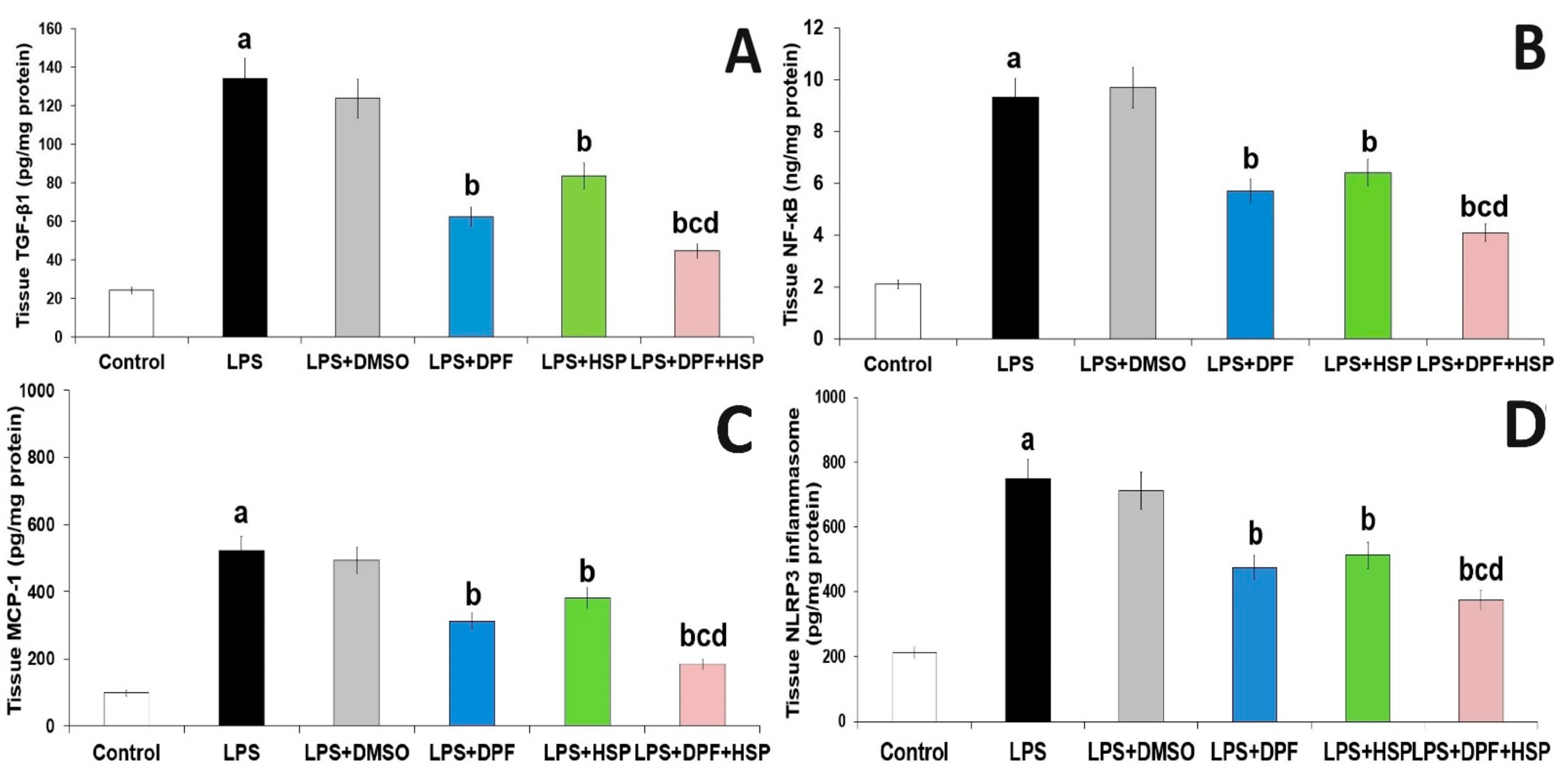
2.3. Dapagliflozin with or without Hesperidin Mitigated the Effect of LPS Injection on the Inflammatory Consequences in the Hippocampal Tissues
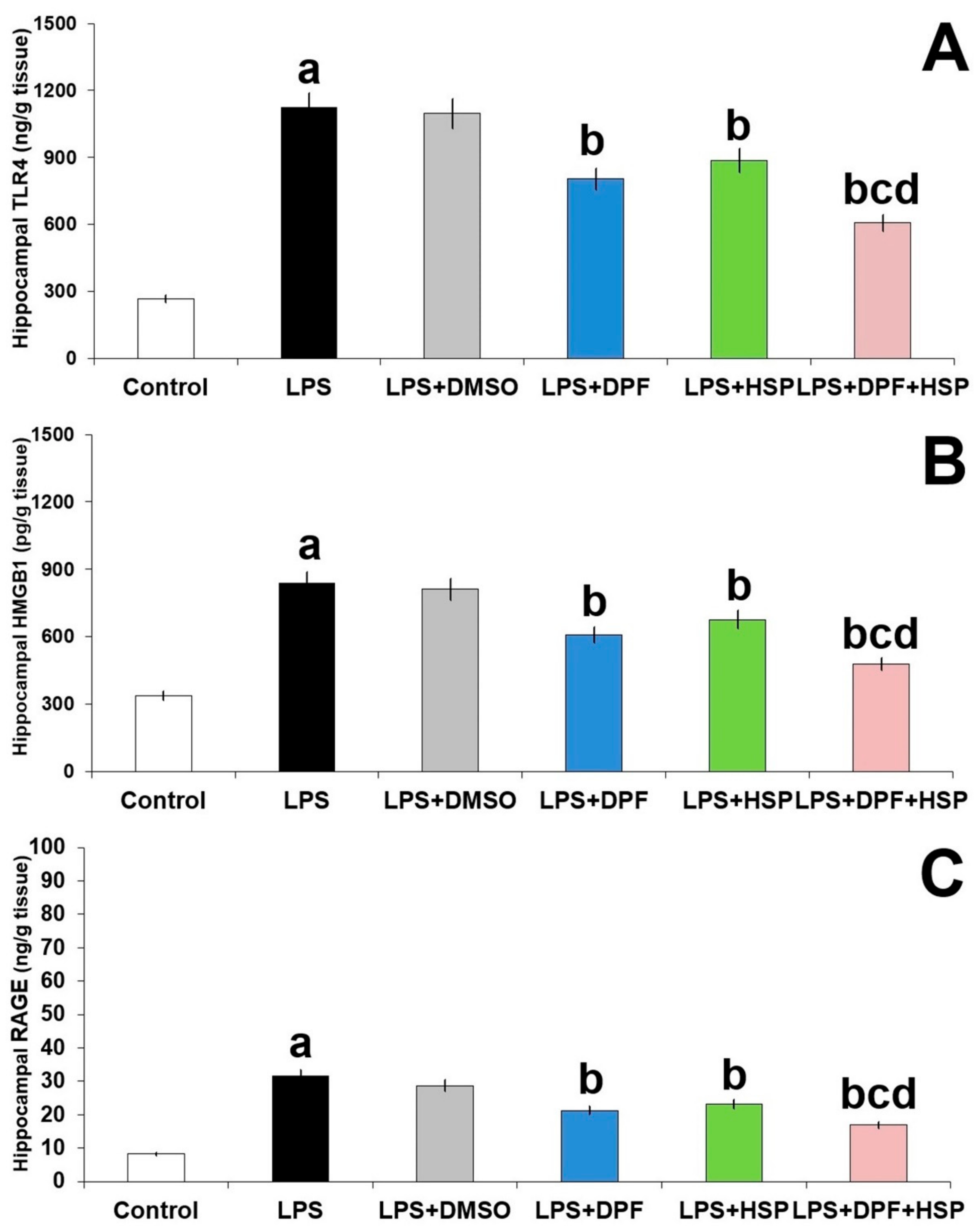
2.4. Dapagliflozin with or without Hesperidin Abrogated the Effect of LPS Injection on TLR4/RAGE/HMGB1 Signaling in the Hippocampal Tissues
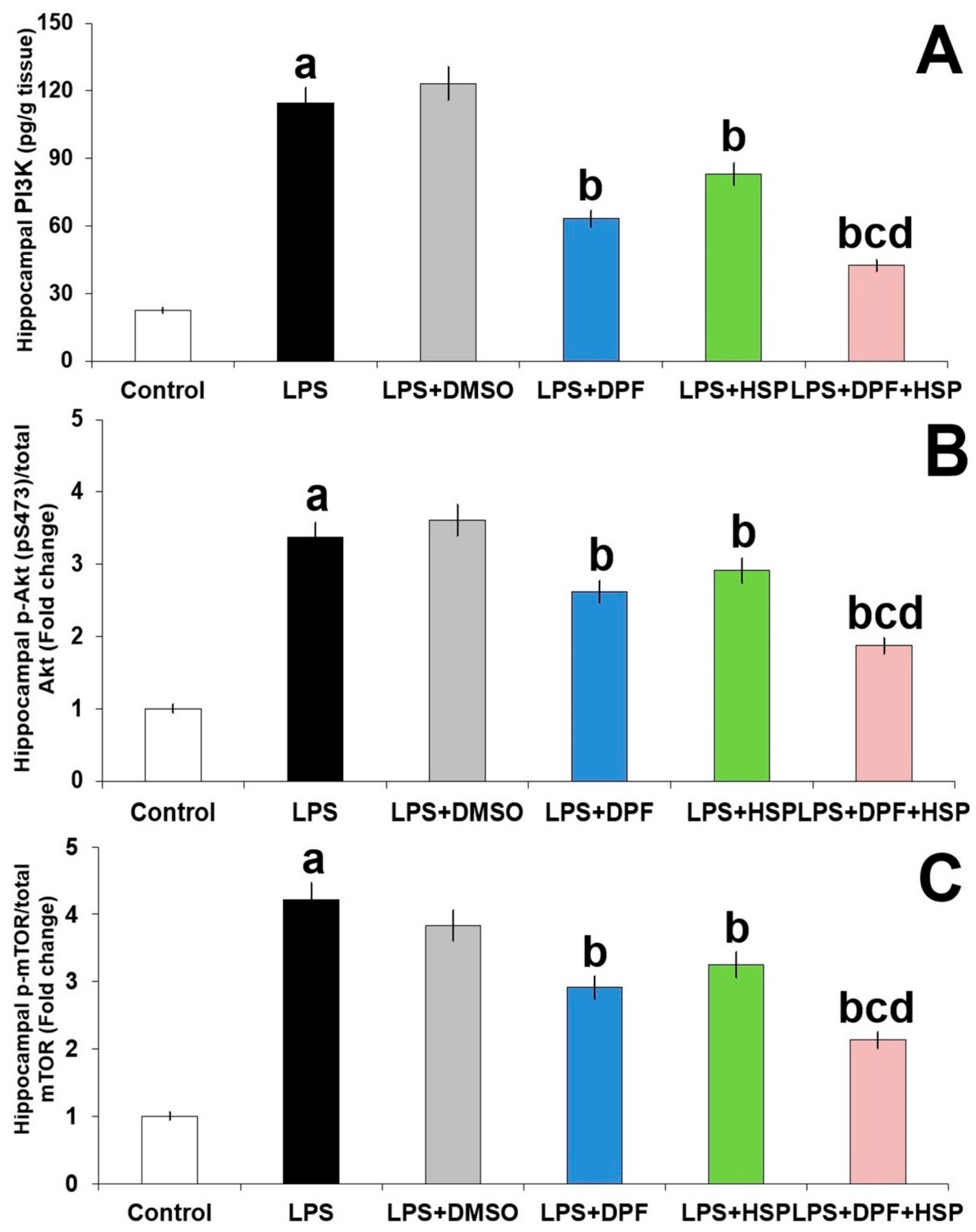
2.5. Dapagliflozin with or without Hesperidin Ameliorated the Effect of LPS Injection on PI3K/Akt/mTOR Axis in the Hippocampal Tissues
2.6. Dapagliflozin with or without Hesperidin Reversed the Perturbations in Autophagy Induced by LPS Injection in the Hippocampal Tissues
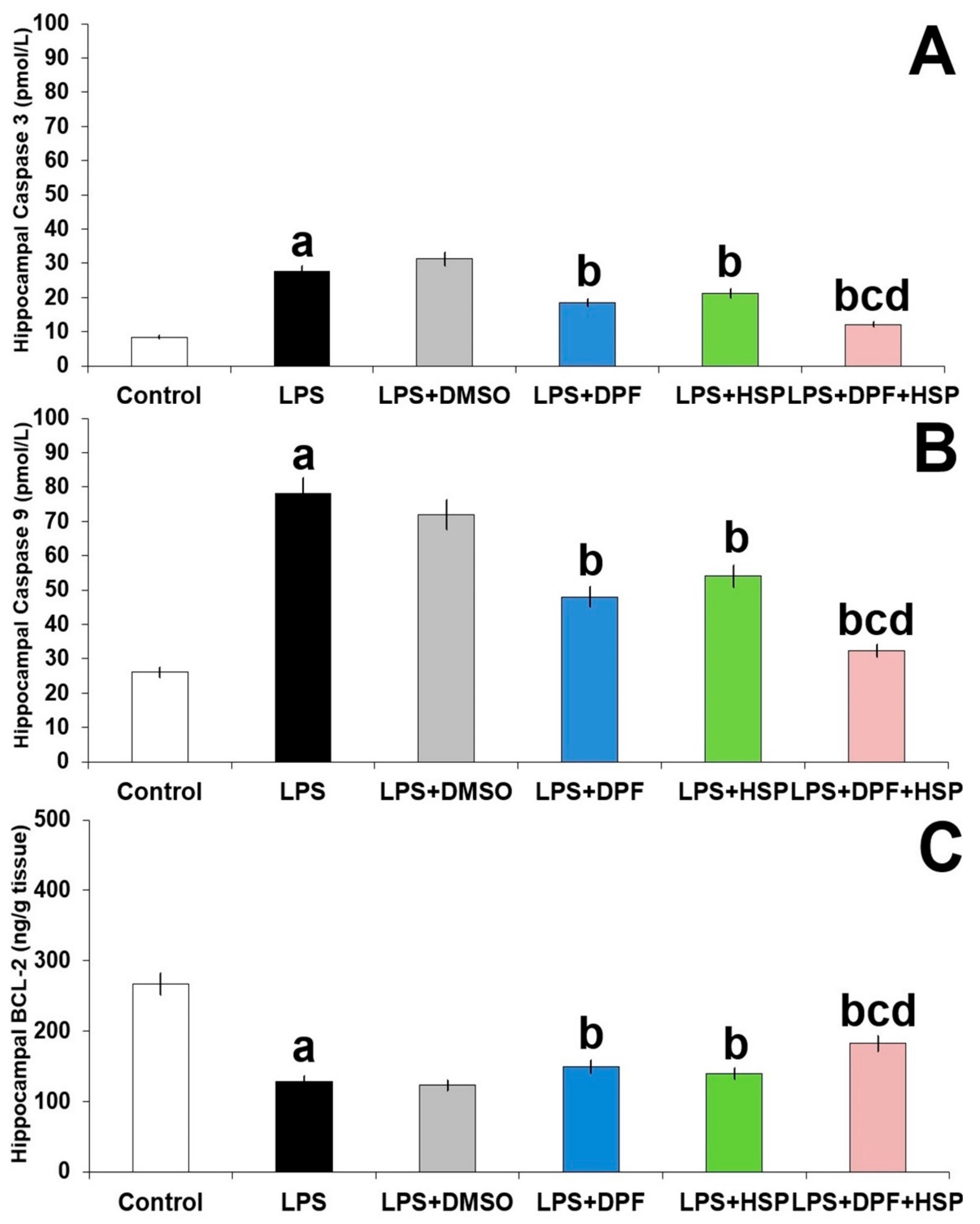
2.7. Dapagliflozin with or without Hesperidin Combatted the Apoptotic Changes Elicited by Lipopolysaccharide Injection in the Hippocampal Tissues
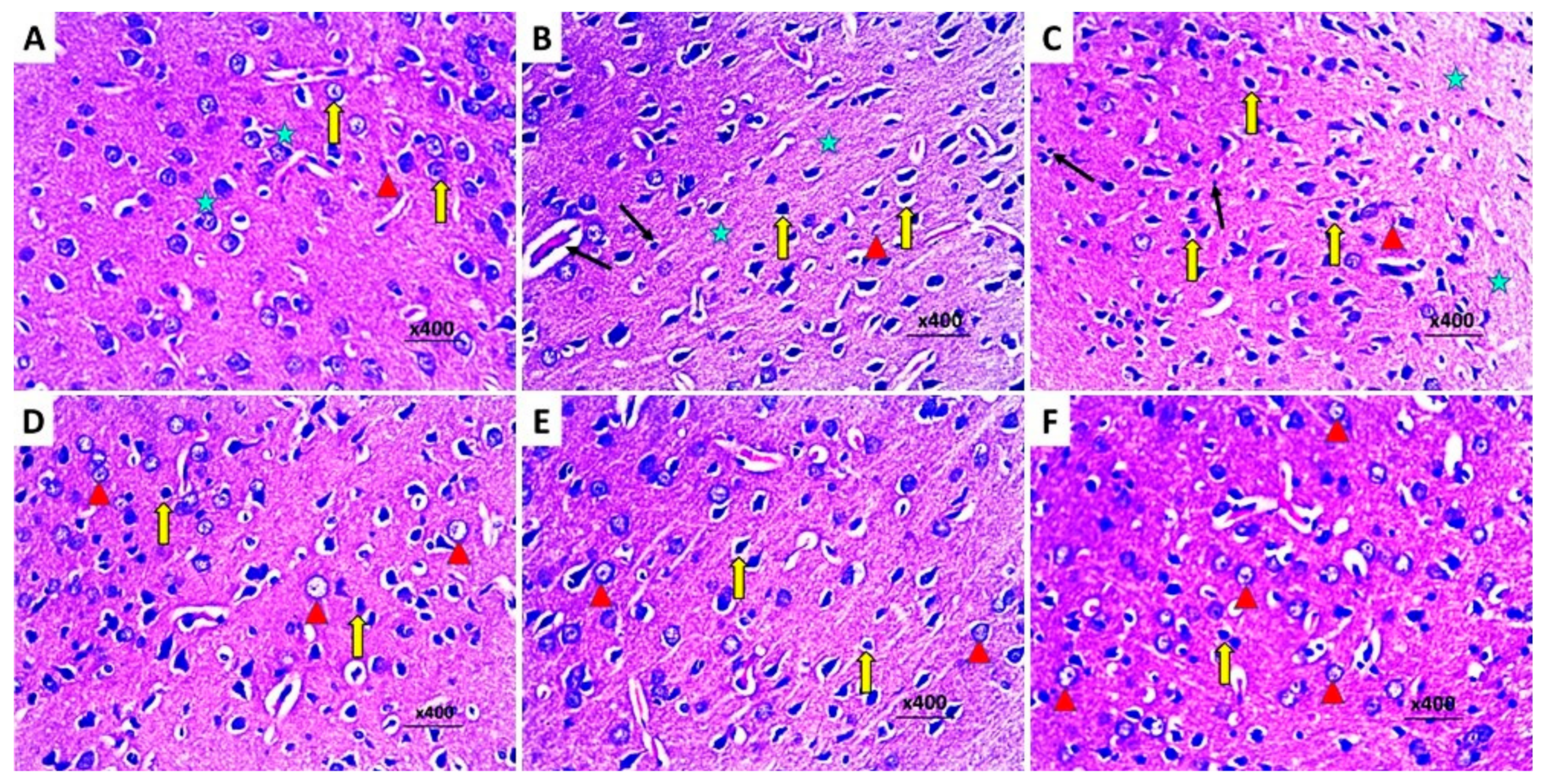
2.8. Dapagliflozin with or without Hesperidin Reversed the Changes in the Histopathological Morphology of the Hippocampus and the Frontal Lobe Induced by LPS Injection
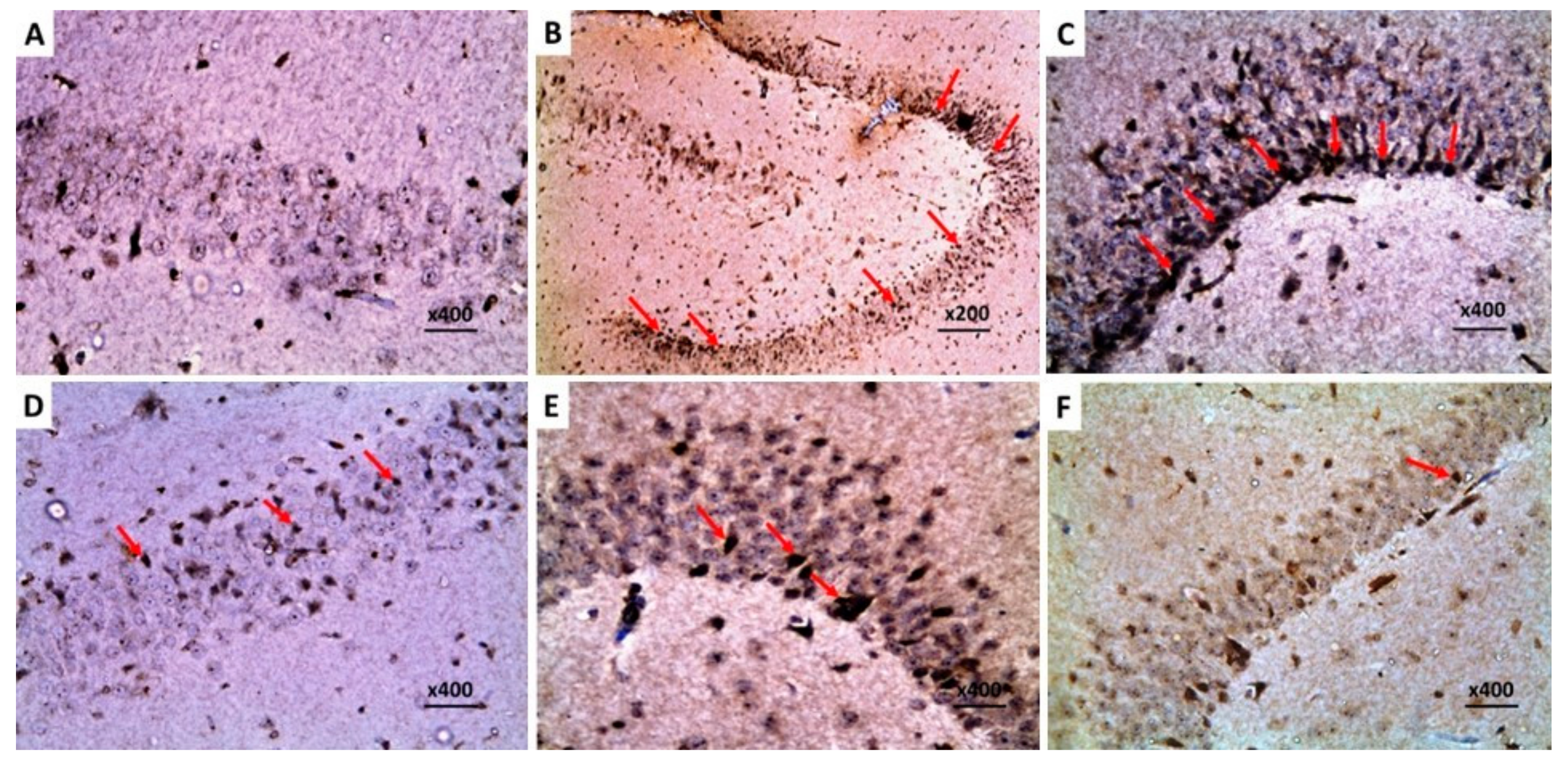
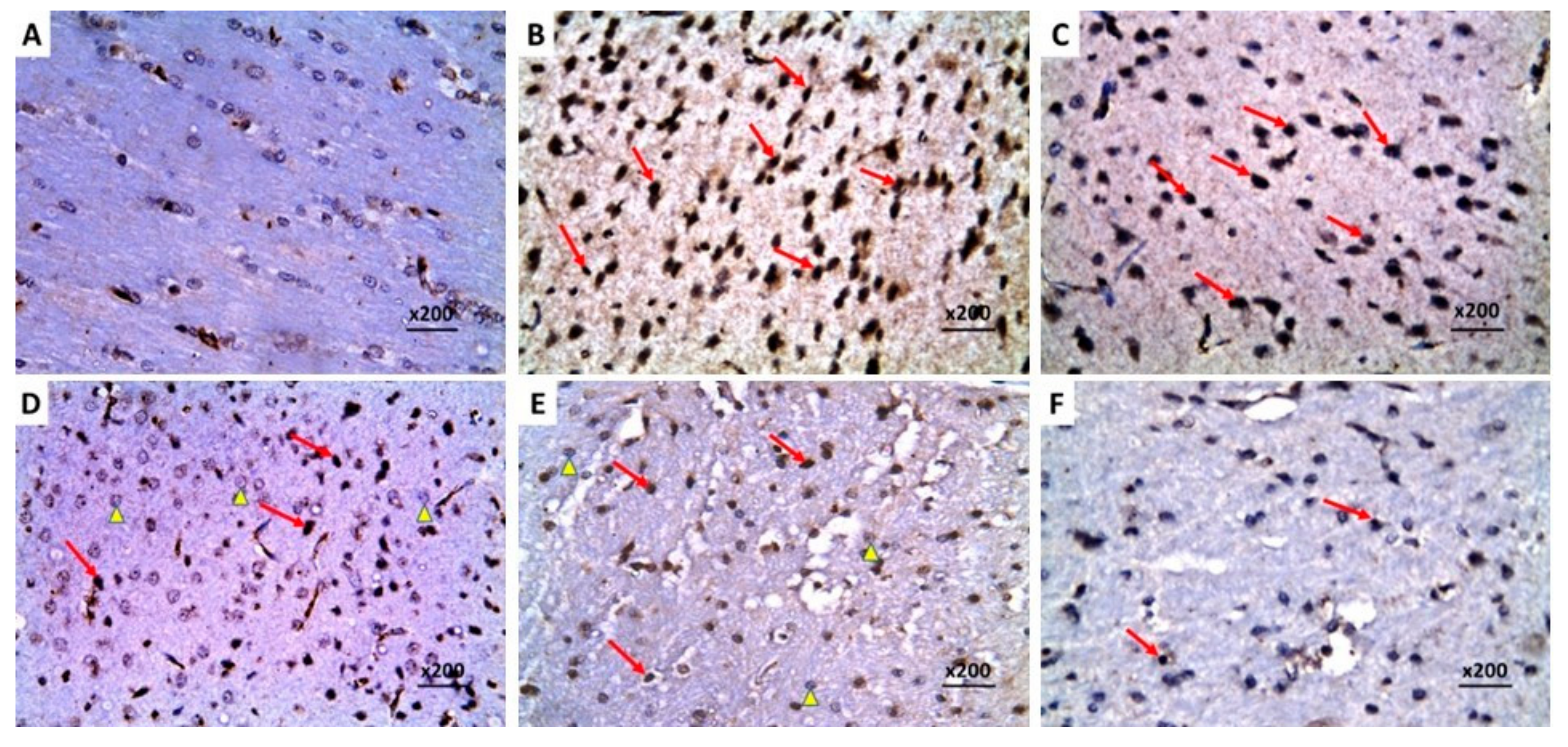
2.9. Dapagliflozin with or without Hesperidin Downregulated the Expression of Ki-67 in the Hippocampus and the Frontal Lobe Induced by LPS Injection
2.10. Dapagliflozin with or without Hesperidin Mitigated the Electron Microscopic Morphologic Changes of the Frontal Lobe Elicited by LPS Injection
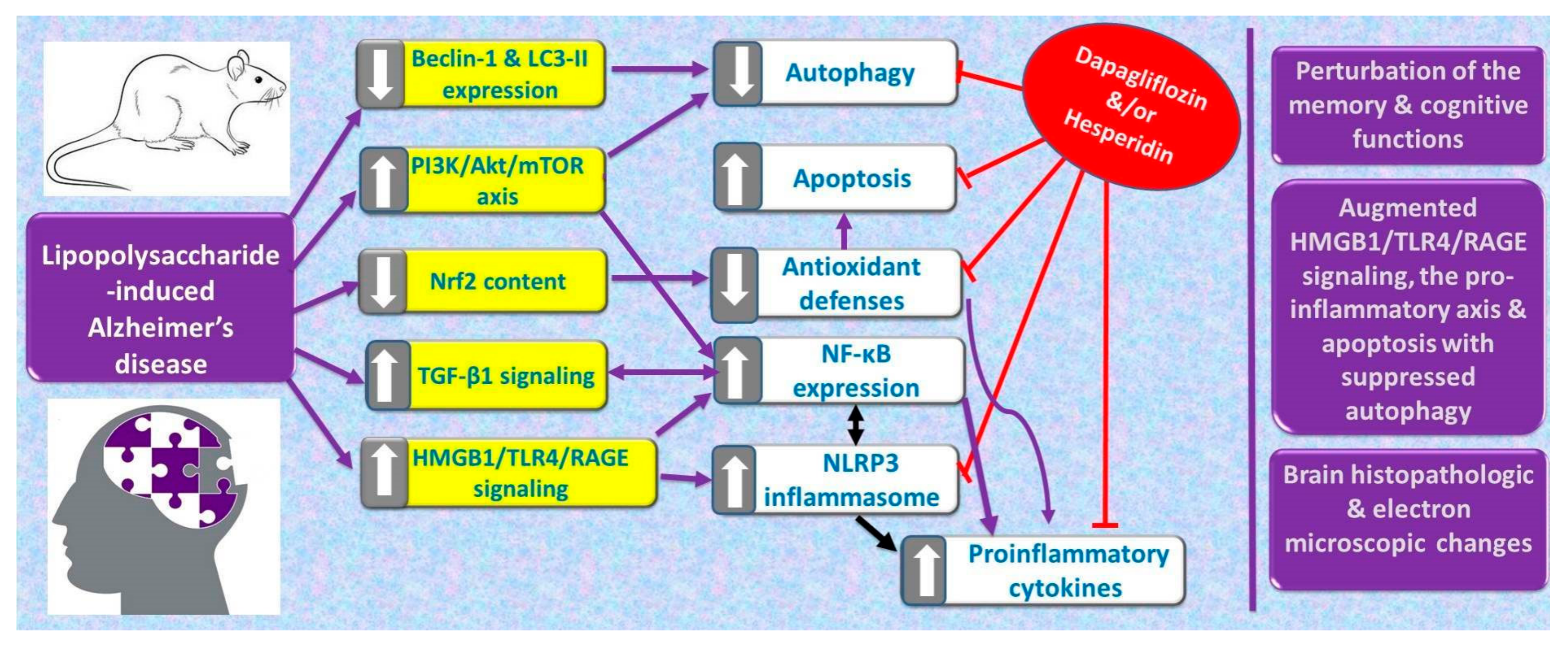
3. Discussion
4. Materials and Methods
4.1. Chemicals and Drugs
4.2. The Experimental Design
4.3. Determination of the Effect of Different Treatments on the Behavioral Tests
4.3.1. Open Field Locomotion Test (OFT)
4.3.2. Object Recognition Task
4.3.3. Morris Water Maze Test
4.4. Assessment of the Indicators of Oxidative Stress and Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) Content of the Hippocampal Tissue Specimens
4.5. Assessment of Interleukin 1 Beta (IL-1β), IL-8, IL-18, and Monocyte Chemoattractant Protein-1 (MCP-1) in the Hippocampal Tissues
4.6. Quantification of Transforming Growth Factor Beta 1 (TGF-β1), TLR4, Nuclear Factor Kappa B (NF-κB), and NLRP3 Inflammasome Levels in the Hippocampal Tissues
4.7. Assessment of the Levels of HMGB1 and RAGE in the Hippocampal Tissues
4.8. Measurement of Phospho-Akt, Phosphotylinosital-3-Kinase (PI3K), and Phospho-Mammalian Target of Rapamycin (p-mTOR) Levels in the Hippocampal Tissues
4.9. Assay of the Levels of Beclin-1 and LC3-II in the Hippocampal Tissues
4.10. Quantification of Caspase 3, Caspase 9, and B-Cell Lymphoma (BCL-2) Protein Levels in the Hippocampal Tissues
4.11. Microscopic Detection of the Histopathological Changes of the Brain Tissues
4.12. Determination of the Immunohistochemical Expression of Protein Expression of Ki-67 in the Brain Tissues
4.13. Examination of the Electron Microscopic Morphologic Changes in the Brain Tissues
4.14. Statistical Comparisons between the Different Groups
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Drummond, E.; Kavanagh, T.; Pires, G.; Marta-Ariza, M.; Kanshin, E.; Nayak, S.; Faustin, A.; Berdah, V.; Ueberheide, B.; Wisniewski, T. The amyloid plaque proteome in early onset Alzheimer’s disease and Down syndrome. Acta Neuropathol. Commun. 2022, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer disease. Nat. Rev. Dis. Prim. 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Hoogmartens, J.; Cacace, R.; Van Broeckhoven, C. Insight into the genetic etiology of Alzheimer’s disease: A comprehensive review of the role of rare variants. Alzheimer’s Dement. 2021, 13, e12155. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Van Hoecke, L.; Vandenbroucke, R.E. The Impact of Systemic Inflammation on Alzheimer’s Disease Pathology. Front. Immunol. 2022, 12, 796867. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, E.C.; Bhatt, L.K.; Prabhavalkar, K.S. High Mobility Group Box-1 (HMGB1): A Potential Target in Therapeutics. Curr. Drug Targets 2019, 20, 1474–1485. [Google Scholar] [CrossRef]
- Fan, J.; Qiu, Y.; Zheng, Z.; Yu, L.; Shi, S.; Wu, X. NOD-like receptor protein 3 and high mobility group box-1 are associated with prognosis of patients with congenital heart disease. J. Int. Med. Res. 2020, 48, 300060519884500. [Google Scholar] [CrossRef]
- Tan, S.-W.; Zhao, Y.; Li, P.; Ning, Y.-L.; Huang, Z.-Z.; Yang, N.; Liu, D.; Zhou, Y.-G. HMGB1 mediates cognitive impairment caused by the NLRP3 inflammasome in the late stage of traumatic brain injury. J. Neuroinflammation 2021, 18, 241. [Google Scholar] [CrossRef]
- Gaikwad, S.; Puangmalai, N.; Bittar, A.; Montalbano, M.; Garcia, S.; McAllen, S.; Bhatt, N.; Sonawane, M.; Sengupta, U.; Kayed, R. Tau oligomer induced HMGB1 release contributes to cellular senescence and neuropathology linked to Alzheimer’s disease and frontotemporal dementia. Cell Rep. 2021, 36, 109419. [Google Scholar] [CrossRef]
- Han, Y.; Chen, R.; Lin, Q.; Liu, Y.; Ge, W.; Cao, H.; Li, J. Curcumin improves memory deficits by inhibiting HMGB1-RAGE/TLR4-NF-κB signalling pathway in APPswe/PS1dE9 transgenic mice hippocampus. J. Cell. Mol. Med. 2021, 25, 8947–8956. [Google Scholar] [CrossRef]
- Paudel, Y.N.; Angelopoulou, E.; Piperi, C.; Othman, I.; Aamir, K.; Shaikh, M.F. Impact of HMGB1, RAGE, and TLR4 in Alzheimer’s Disease (AD): From Risk Factors to Therapeutic Targeting. Cells 2020, 9, 383. [Google Scholar] [CrossRef] [PubMed]
- Abuelezz, S.A.; Hendawy, N. HMGB1/RAGE/TLR4 axis and glutamate as novel targets for PCSK9 inhibitor in high fat cholesterol diet induced cognitive impairment and amyloidosis. Life Sci. 2021, 273, 119310. [Google Scholar] [CrossRef] [PubMed]
- Francois, A.; Terro, F.; Quellard, N.; Fernandez, B.; Chassaing, D.; Janet, T.; Bilan, A.R.; Paccalin, M.; Page, G. Impairment of autophagy in the central nervous system during lipopolysaccharide-induced inflammatory stress in mice. Mol. Brain 2014, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Zhu, M.; Che, X.; Wang, H.; Liang, X.-J.; Wu, C.; Xue, X.; Yang, J. Lipopolysaccharide induces neuroinflammation in microglia by activating the MTOR pathway and downregulating Vps34 to inhibit autophagosome formation. J. Neuroinflammation 2020, 17, 18. [Google Scholar] [CrossRef]
- Lee, J.-W.; Nam, H.; Kim, L.E.; Jeon, Y.; Min, H.; Ha, S.; Lee, Y.; Kim, S.-Y.; Lee, S.J.; Kim, E.-K.; et al. TLR4 (toll-like receptor 4) activation suppresses autophagy through inhibition of FOXO3 and impairs phagocytic capacity of microglia. Autophagy 2019, 15, 753–770. [Google Scholar] [CrossRef]
- Sun, X.; Kaufman, P.D. Ki-67: More than a proliferation marker. Chromosoma 2018, 127, 175–186. [Google Scholar] [CrossRef]
- Essa, H.; Peyton, L.; Hasan, W.; León, B.E.; Choi, D.-S. Implication of Adult Hippocampal Neurogenesis in Alzheimer’s Disease and Potential Therapeutic Approaches. Cells 2022, 11, 286. [Google Scholar] [CrossRef]
- Mirza, F.J.; Zahid, S. Ursolic acid and rosmarinic acid ameliorate alterations in hippocampal neurogenesis and social memory induced by amyloid beta in mouse model of Alzheimer’s disease. Front. Pharmacol. 2022, 13, 1058358. [Google Scholar] [CrossRef]
- Batista, C.R.A.; Gomes, G.F.; Candelario-Jalil, E.; Fiebich, B.L.; De Oliveira, A.C.P. Lipopolysaccharide-Induced Neuroinflammation as a Bridge to Understand Neurodegeneration. Int. J. Mol. Sci. 2019, 20, 2293. [Google Scholar] [CrossRef]
- Skrzypczak-Wiercioch, A.; Sałat, K. Lipopolysaccharide-Induced Model of Neuroinflammation: Mechanisms of Action, Research Application and Future Directions for Its Use. Molecules 2022, 27, 5481. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, X.; Chu, Y.; Chen, X.; Du, H.; Zhang, H.; Xu, C.; Xie, H.; Ruan, Q.; Lin, J.; et al. Dapagliflozin: A sodium–glucose cotransporter 2 inhibitor, attenuates angiotensin II-induced cardiac fibrotic remodeling by regulating TGFβ1/Smad signaling. Cardiovasc. Diabetol. 2021, 20, 121. [Google Scholar] [CrossRef] [PubMed]
- Tharmaraja, T.; Ho, J.S.; Sia, C.-H.; Lim, N.-A.; Chong, Y.F.; Lim, A.Y.; Rathakrishnan, R.R.; Yeo, L.L.; Sharma, V.K.; Tan, B.Y. Sodium-glucose cotransporter 2 inhibitors and neurological disorders: A scoping review. Ther. Adv. Chronic Dis. 2022, 13, 20406223221086996. [Google Scholar] [CrossRef] [PubMed]
- Arab, H.H.; Safar, M.M.; Shahin, N.N. Targeting ROS-Dependent AKT/GSK-3β/NF-κB and DJ-1/Nrf2 Pathways by Dapagliflozin Attenuates Neuronal Injury and Motor Dysfunction in Rotenone-Induced Parkinson’s Disease Rat Model. ACS Chem. Neurosci. 2021, 12, 689–703. [Google Scholar] [CrossRef]
- Faridvand, Y.; Kazemzadeh, H.; Vahedian, V.; Mirzajanzadeh, P.; Nejabati, H.R.; Safaie, N.; Maroufi, N.F.; Pezeshkian, M.; Nouri, M.; Jodati, A. Dapagliflozin attenuates high glucose-induced endothelial cell apoptosis and inflammation through AMPK/SIRT1 activation. Clin. Exp. Pharmacol. Physiol. 2022, 49, 643–651. [Google Scholar] [CrossRef]
- Pyrzynska, K. Hesperidin: A Review on Extraction Methods, Stability and Biological Activities. Nutrients 2022, 14, 2387. [Google Scholar] [CrossRef]
- Hajialyani, M.; Farzaei, M.H.; Echeverría, J.; Nabavi, S.M.; Uriarte, E.; Sobarzo-Sánchez, E. Hesperidin as a Neuroprotective Agent: A Review of Animal and Clinical Evidence. Molecules 2019, 24, 648. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Dhingra, A.K.; Chopra, B.; Guarve, K.; Bhateja, D. Therapeutic Potential and Clinical Evidence of Hesperidin as Neuroprotective Agent. Central Nerv. Syst. Agents Med. Chem. 2022, 22, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Zhang, Y.; Cheng, Q.; Huang, H.; Huang, C.; Tang, L. Research progress on the mechanism of action of hesperetin in cerebral ischemia: A narrative review. Ann. Transl. Med. 2022, 10, 806. [Google Scholar] [CrossRef]
- Teipel, S.; Gustafson, D.; Ossenkoppele, R.; Hansson, O.; Babiloni, C.; Wagner, M.; Riedel-Heller, S.G.; Kilimann, I.; Tang, Y. Alzheimer Disease: Standard of Diagnosis, Treatment, Care, and Prevention. J. Nucl. Med. 2022, 63, 981–985. [Google Scholar] [CrossRef]
- George, M.; Tharakan, M.; Culberson, J.; Reddy, A.P.; Reddy, P.H. Role of Nrf2 in aging, Alzheimer’s and other neurodegenerative diseases. Ageing Res. Rev. 2022, 82, 101756. [Google Scholar] [CrossRef]
- Kabel, A.M.; Arab, H.H.; Atef, A.; Estfanous, R.S. Omarigliptin/galangin combination mitigates lipopolysaccharide-induced neuroinflammation in rats: Involvement of glucagon-like peptide-1, toll-like receptor-4, apoptosis and Akt/GSK-3β signaling. Life Sci. 2022, 295, 120396. [Google Scholar] [CrossRef] [PubMed]
- Tejo, F.V.; Quintanilla, R. Contribution of the Nrf2 Pathway on Oxidative Damage and Mitochondrial Failure in Parkinson and Alzheimer’s Disease. Antioxidants 2021, 10, 1069. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-J.; Wang, T.-J.; Chen, S.-D.; Lin, K.-L.; Liou, C.-W.; Lan, M.-Y.; Chuang, Y.-C.; Chuang, J.-H.; Wang, P.-W.; Lee, J.-J.; et al. Two Birds One Stone: The Neuroprotective Effect of Antidiabetic Agents on Parkinson Disease—Focus on Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors. Antioxidants 2021, 10, 1935. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Muhammad, T.; Rehman, S.U.; Khan, A.; Jo, M.G.; Ali, T.; Kim, M.O. Hesperetin Confers Neuroprotection by Regulating Nrf2/TLR4/NF-κB Signaling in an Aβ Mouse Model. Mol. Neurobiol. 2019, 56, 6293–6309. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Wie, M.-B.; Ahn, M.; Tanaka, A.; Matsuda, H.; Shin, T. Benefits of hesperidin in central nervous system disorders: A review. Anat. Cell Biol. 2019, 52, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Dallas, M.L.; Widera, D. TLR2 and TLR4-mediated inflammation in Alzheimer’s disease: Self-defense or sabotage? Neural Regen. Res. 2021, 16, 1552–1553. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Zhang, Y.; Wu, S.; Chen, Q.; Wang, L. The Role of NLRP3 Inflammasome in Alzheimer’s Disease and Potential Therapeutic Targets. Front. Pharmacol. 2022, 13, 845185. [Google Scholar] [CrossRef]
- Zhao, X.; Rouhiainen, A.; Li, Z.; Guo, S.; Rauvala, H. Regulation of Neurogenesis in Mouse Brain by HMGB1. Cells 2020, 9, 1714. [Google Scholar] [CrossRef]
- Fan, H.; Tang, H.-B.; Chen, Z.; Wang, H.-Q.; Zhang, L.; Jiang, Y.; Li, T.; Yang, C.-F.; Wang, X.-Y.; Li, X.; et al. Inhibiting HMGB1-RAGE axis prevents pro-inflammatory macrophages/microglia polarization and affords neuroprotection after spinal cord injury. J. Neuroinflammation 2020, 17, 295. [Google Scholar] [CrossRef]
- Rizzo, M.R.; Di Meo, I.; Polito, R.; Auriemma, M.C.; Gambardella, A.; di Mauro, G.; Capuano, A.; Paolisso, G. Cognitive impairment and type 2 diabetes mellitus: Focus of SGLT2 inhibitors treatment. Pharmacol. Res. 2022, 176, 106062. [Google Scholar] [CrossRef]
- Feijóo-Bandín, S.; Aragón-Herrera, A.; Otero-Santiago, M.; Anido-Varela, L.; Moraña-Fernández, S.; Tarazón, E.; Roselló-Lletí, E.; Portolés, M.; Gualillo, O.; González-Juanatey, J.R.; et al. Role of Sodium-Glucose Co-Transporter 2 Inhibitors in the Regulation of Inflammatory Processes in Animal Models. Int. J. Mol. Sci. 2022, 23, 5634. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.-F.; Chen, Y.-L.; Chiou, T.T.-Y.; Chu, T.-H.; Li, L.-C.; Ng, H.-Y.; Lee, W.-C.; Lee, C.-T. Emergence of SGLT2 Inhibitors as Powerful Antioxidants in Human Diseases. Antioxidants 2021, 10, 1166. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, T.; Ikram, M.; Ullah, R.; Rehman, S.; Kim, M. Hesperetin, a Citrus Flavonoid, Attenuates LPS-Induced Neuroinflammation, Apoptosis and Memory Impairments by Modulating TLR4/NF-κB Signaling. Nutrients 2019, 11, 648. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Gu, Z.; Liu, H.; Jia, B.; Wang, Y.; Cao, M.; Song, R.; Zhang, Z.; Bian, Y. The Anti-Depressive Effects of Hesperidin and the Relative Mechanisms Based on the NLRP3 Inflammatory Signaling Pathway. Front. Pharmacol. 2020, 11, 1251. [Google Scholar] [CrossRef] [PubMed]
- Long, H.-Z.; Cheng, Y.; Zhou, Z.-W.; Luo, H.-Y.; Wen, D.-D.; Gao, L.-C. PI3K/AKT Signal Pathway: A Target of Natural Products in the Prevention and Treatment of Alzheimer’s Disease and Parkinson’s Disease. Front. Pharmacol. 2021, 12, 648636. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Zhou, Y.; Xiao, M.; Yan, L.-J.; He, W. Activation of mTOR: A culprit of Alzheimer’s disease? Neuropsychiatr. Dis. Treat. 2015, 11, 1015–1030. [Google Scholar] [CrossRef]
- Razani, E.; Pourbagheri-Sigaroodi, A.; Safaroghli-Azar, A.; Zoghi, A.; Shanaki-Bavarsad, M.; Bashash, D. The PI3K/Akt signaling axis in Alzheimer’s disease: A valuable target to stimulate or suppress? Cell Stress Chaperones 2021, 26, 871–887. [Google Scholar] [CrossRef]
- Evans, J.A.; Mendonca, P.; Soliman, K.F.A. Neuroprotective Effects and Therapeutic Potential of the Citrus Flavonoid Hesperetin in Neurodegenerative Diseases. Nutrients 2022, 14, 2228. [Google Scholar] [CrossRef]
- Liu, J.; Li, L. Targeting Autophagy for the Treatment of Alzheimer’s Disease: Challenges and Opportunities. Front. Mol. Neurosci. 2019, 12, 203. [Google Scholar] [CrossRef]
- Condello, M.; Pellegrini, E.; Caraglia, M.; Meschini, S. Targeting Autophagy to Overcome Human Diseases. Int. J. Mol. Sci. 2019, 20, 725. [Google Scholar] [CrossRef]
- Bieri, G.; Lucin, K.M.; O’brien, C.E.; Zhang, H.; Villeda, S.A.; Wyss-Coray, T. Proteolytic cleavage of Beclin 1 exacerbates neurodegeneration. Mol. Neurodegener. 2018, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Shrestha, Y.; Jayatunga, D.P.W.; Rea, S.; Martins, R.; Bharadwaj, P. Activate or Inhibit? Implications of Autophagy Modulation as a Therapeutic Strategy for Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 6739. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Kitada, M.; Ogura, Y.; Liu, H.; Koya, D. Dapagliflozin Restores Impaired Autophagy and Suppresses Inflammation in High Glucose-Treated HK-2 Cells. Cells 2021, 10, 1457. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xu, Q.; Li, H.; Meng, Z.; Hao, M.; Ma, X.; Lin, W.; Kuang, H. Dapagliflozin Reduces Apoptosis of Diabetic Retina and Human Retinal Microvascular Endothelial Cells Through ERK1/2/cPLA2/AA/ROS Pathway Independent of Hypoglycemic. Front. Pharmacol. 2022, 13, 827896. [Google Scholar] [CrossRef]
- Wdowiak, K.; Walkowiak, J.; Pietrzak, R.; Bazan-Woźniak, A.; Cielecka-Piontek, J. Bioavailability of Hesperidin and Its Aglycone Hesperetin—Compounds Found in Citrus Fruits as a Parameter Conditioning the Pro-Health Potential (Neuroprotective and Antidiabetic Activity)—Mini-Review. Nutrients 2022, 14, 2647. [Google Scholar] [CrossRef]
- Kong, W.; Ling, X.; Chen, Y.; Wu, X.; Zhao, Z.; Wang, W.; Wang, S.; Lai, G.; Yu, Z. Hesperetin reverses P-glycoprotein-mediated cisplatin resistance in DDP-resistant human lung cancer cells via modulation of the nuclear factor-κB signaling pathway. Int. J. Mol. Med. 2020, 45, 1213–1224. [Google Scholar] [CrossRef]
- Filippatos, T.D.; Liberopoulos, E.N.; Elisaf, M.S. Dapagliflozin in patients with type 2 diabetes mellitus. Ther. Adv. Endocrinol. Metab. 2015, 6, 29–41. [Google Scholar] [CrossRef]
- Obermeier, M.; Yao, M.; Khanna, A.; Koplowitz, B.; Zhu, M.; Li, W.; Komoroski, B.; Kasichayanula, S.; Discenza, L.; Washburn, W.; et al. In Vitro Characterization and Pharmacokinetics of Dapagliflozin (BMS-512148), a Potent Sodium-Glucose Cotransporter Type II Inhibitor, in Animals and Humans. Drug Metab. Dispos. 2010, 38, 405–414. [Google Scholar] [CrossRef]
- Khan, M.S.; Muhammad, T.; Ikram, M.; Kim, M.O. Dietary Supplementation of the Antioxidant Curcumin Halts Systemic LPS-Induced Neuroinflammation-Associated Neurodegeneration and Memory/Synaptic Impairment via the JNK/NF-κB/Akt Signaling Pathway in Adult Rats. Oxidative Med. Cell. Longev. 2019, 2019, 7860650. [Google Scholar] [CrossRef]
- Tanajak, P.; Sa-Nguanmoo, P.; Sivasinprasasn, S.; Thummasorn, S.; Siri-Angkul, N.; Chattipakorn, S.C.; Chattipakorn, N. Cardioprotection of dapagliflozin and vildagliptin in rats with cardiac ischemia-reperfusion injury. J. Endocrinol. 2018, 236, 69–84. [Google Scholar] [CrossRef]
- Adefegha, S.A.; Leal, D.B.R.; Olabiyi, A.A.; Oboh, G.; Castilhos, L.G. Hesperidin attenuates inflammation and oxidative damage in pleural exudates and liver of rat model of pleurisy. Redox Rep. 2017, 22, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Whyte, L.S.; Hemsley, K.M.; Lau, A.A.; Hassiotis, S.; Saito, T.; Saido, T.C.; Hopwood, J.J.; Sargeant, T.J. Reduction in open field activity in the absence of memory deficits in the AppNL−G−F knock-in mouse model of Alzheimer’s disease. Behav. Brain Res. 2018, 336, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.J.; Bengoetxea, X.; Rodriguez-Perdigon, M. Object recognition test for studying cognitive impairments in animal models of Alzheimer s disease. Front. Biosci. 2015, 7, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Ding, N.; Guo, M.; Wang, S.; Wang, Z.; Liu, H.; Yang, J.; Li, Y.; Ren, J.; Jiang, J.; et al. Analysis of Learning and Memory Ability in an Alzheimer’s Disease Mouse Model using the Morris Water Maze. J. Vis. Exp. 2019, 152, e60055. [Google Scholar] [CrossRef]
- Dzidziguri, D.; Modebadze, I.; Bakuradze, E.; Mosidze, G.; Berulava, M. Determination of The Properties of Rat Brain Thermostable Protein Complex which Inhibit Cell Proliferation. Cell J. 2018, 19, 552–558. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd Elmaaboud, M.A.; Estfanous, R.S.; Atef, A.; Kabel, A.M.; Alnemari, K.A.; Naguib, T.M.; Alsufyani, S.E.; Darwish, H.W.; Arab, H.H. Dapagliflozin/Hesperidin Combination Mitigates Lipopolysaccharide-Induced Alzheimer’s Disease in Rats. Pharmaceuticals 2023, 16, 1370. https://doi.org/10.3390/ph16101370
Abd Elmaaboud MA, Estfanous RS, Atef A, Kabel AM, Alnemari KA, Naguib TM, Alsufyani SE, Darwish HW, Arab HH. Dapagliflozin/Hesperidin Combination Mitigates Lipopolysaccharide-Induced Alzheimer’s Disease in Rats. Pharmaceuticals. 2023; 16(10):1370. https://doi.org/10.3390/ph16101370
Chicago/Turabian StyleAbd Elmaaboud, Maaly A., Remon S. Estfanous, Aliaa Atef, Ahmed M. Kabel, Khalid A. Alnemari, Tamer M. Naguib, Shuruq E. Alsufyani, Hany W. Darwish, and Hany H. Arab. 2023. "Dapagliflozin/Hesperidin Combination Mitigates Lipopolysaccharide-Induced Alzheimer’s Disease in Rats" Pharmaceuticals 16, no. 10: 1370. https://doi.org/10.3390/ph16101370
APA StyleAbd Elmaaboud, M. A., Estfanous, R. S., Atef, A., Kabel, A. M., Alnemari, K. A., Naguib, T. M., Alsufyani, S. E., Darwish, H. W., & Arab, H. H. (2023). Dapagliflozin/Hesperidin Combination Mitigates Lipopolysaccharide-Induced Alzheimer’s Disease in Rats. Pharmaceuticals, 16(10), 1370. https://doi.org/10.3390/ph16101370





