The p38-MITOGEN-ACTIVATED PROTEIN KINASE Signaling Pathway Is Involved in Leonurus artemisia Extract-Induced Inhibition of the Proliferation of Human Bladder Cancer BFTC-905 Cells via G1/G0 Arrest and Causes Apoptosis In Vitro
Abstract
:1. Introduction
2. Results
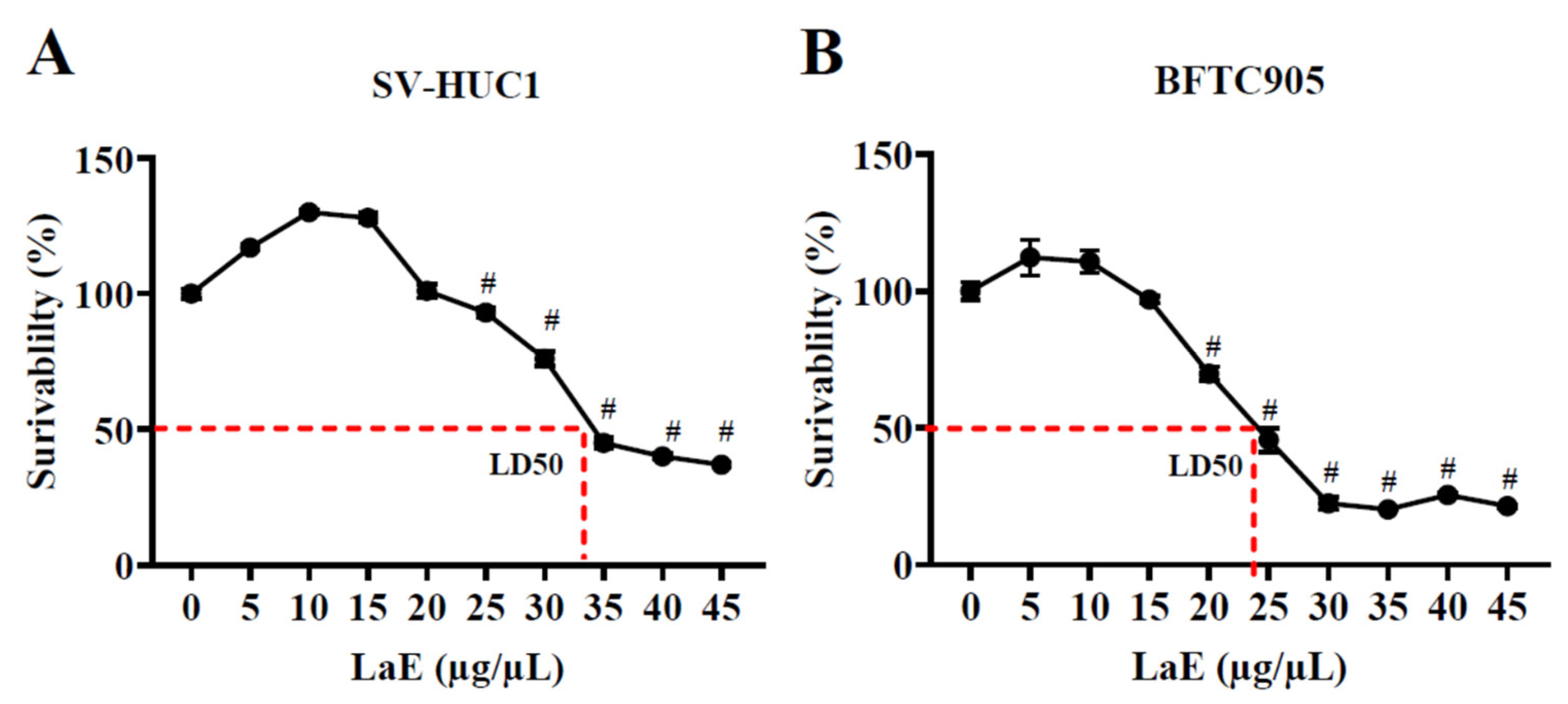
2.1. Leonurus artemisia Extract Inhibited the Proliferation of Human Bladder Cancer Cells
2.2. LaE Inhibited Migration Ability of Human Bladder Cancer Cells
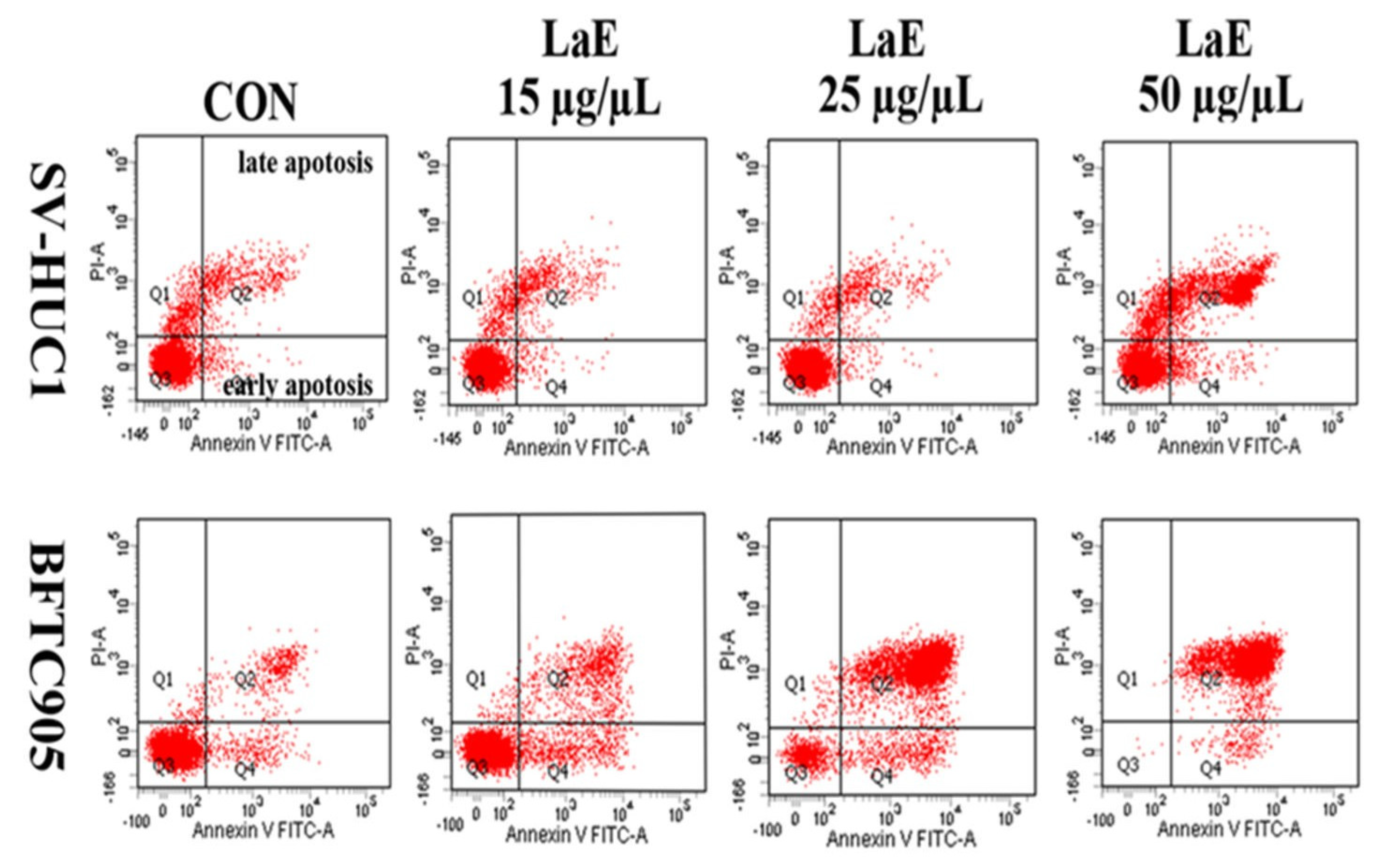
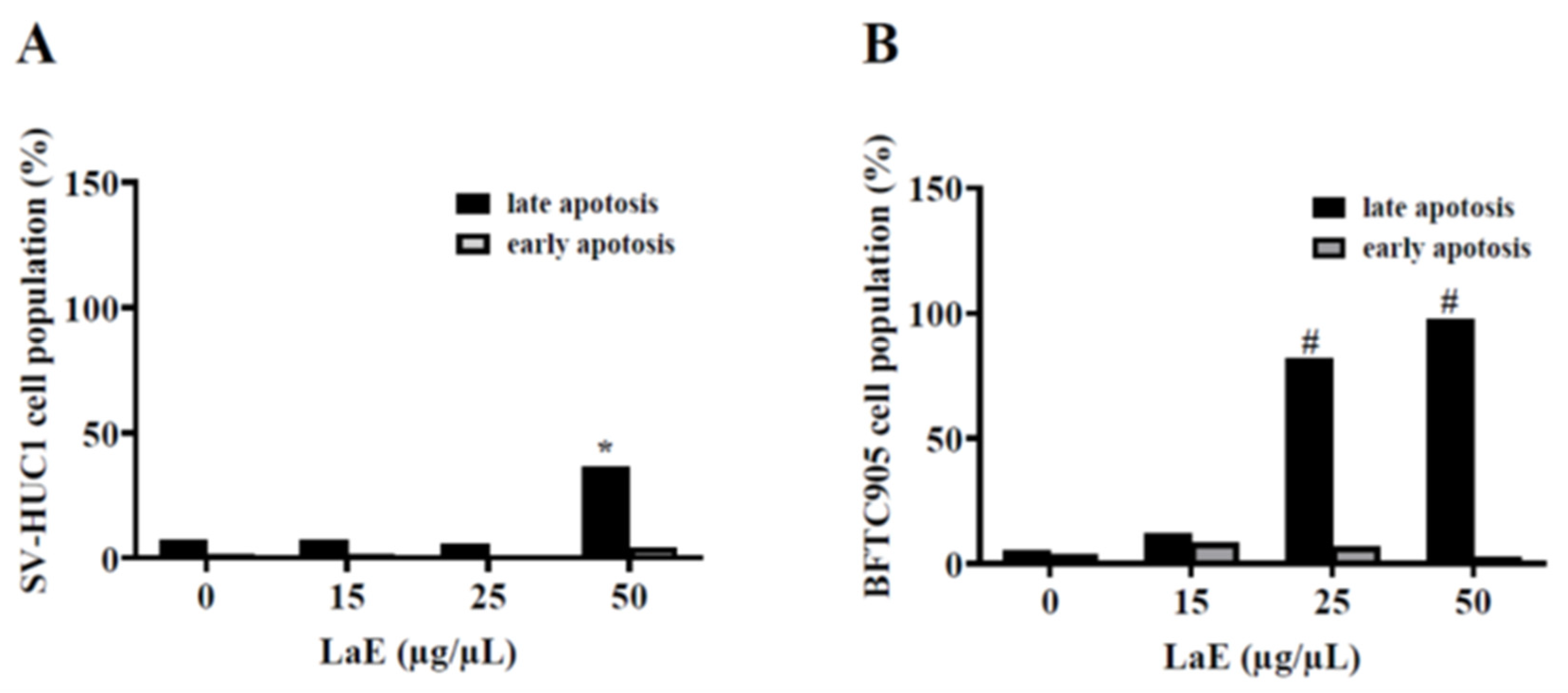
2.3. LaE Induced Apoptosis in Human Bladder Cancer Cells
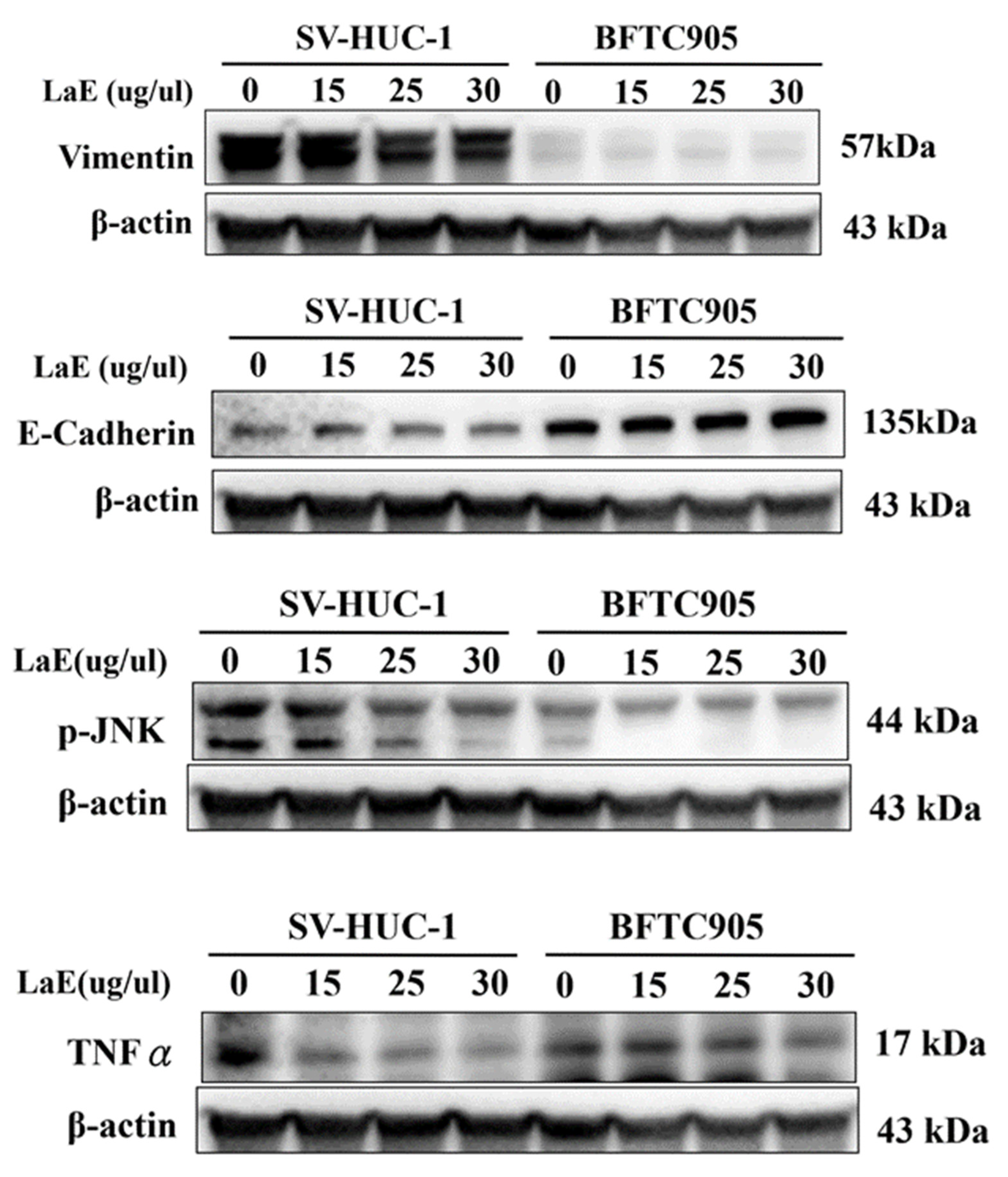
2.4. LaE Induced Apoptosis via Mitogen-Activated Protein Kinase Cascade Signaling
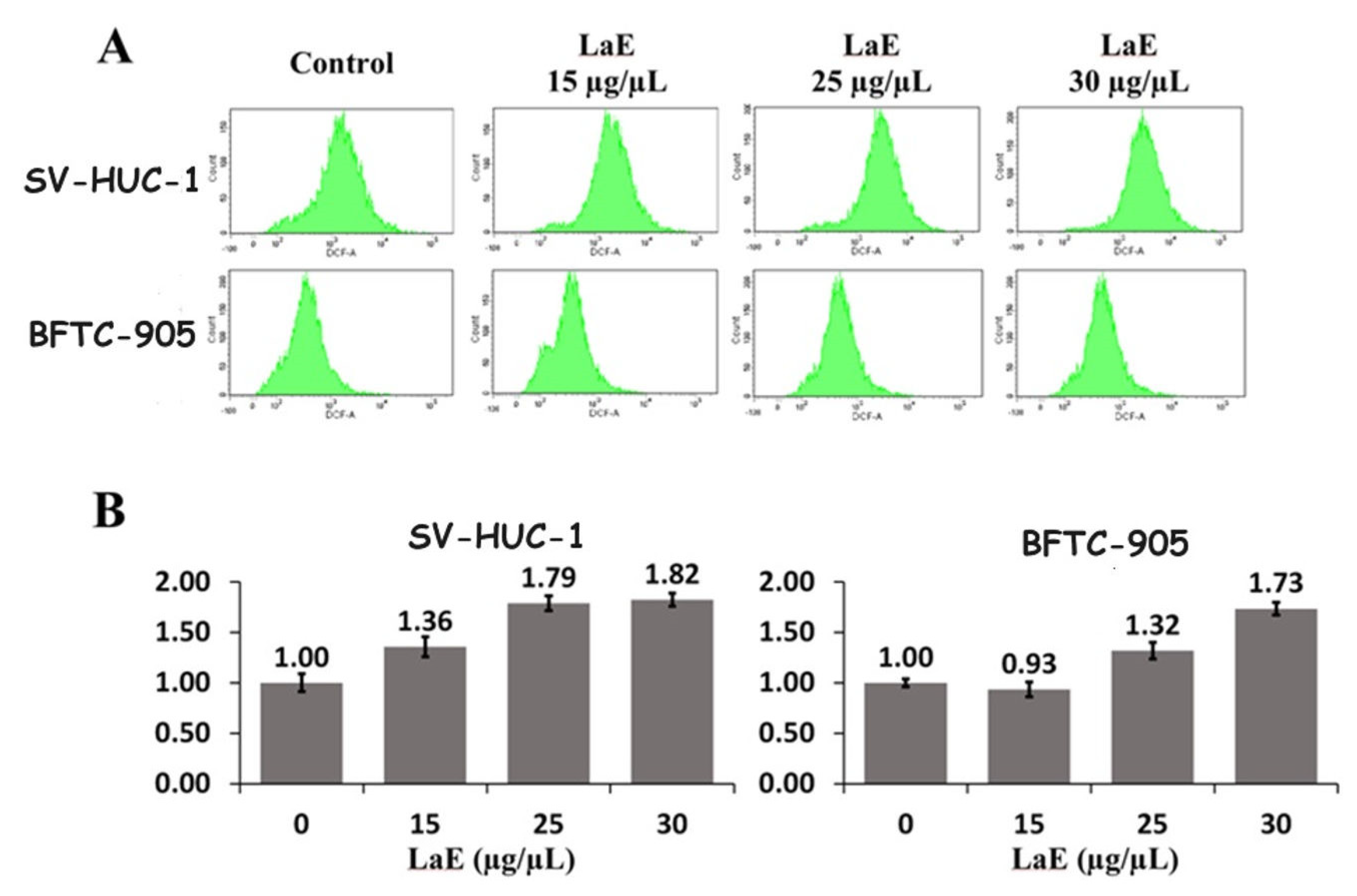
2.5. LaE Increased Reactive Oxygen Species Production
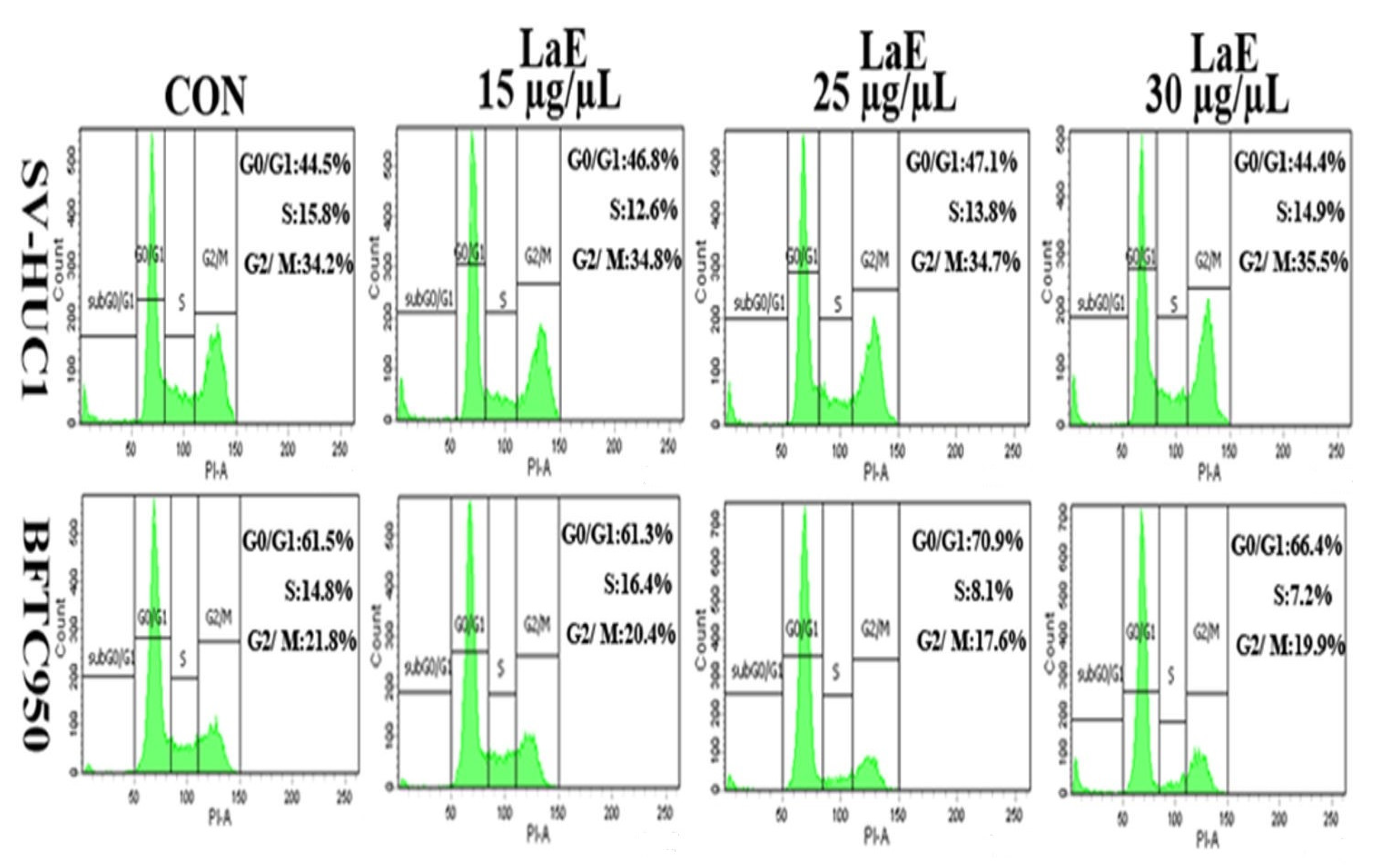
2.6. LaE Induced Cell Cycle Arrest in G1 and G0 Phases
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Extraction of Chinese Motherwort (L. artemisia)
4.4. Cell Proliferation and Cytotoxicity Assay
4.5. Wound Healing Assay
4.6. Cell Apoptosis Assay
4.7. Western Blot Analysis
4.8. ROS Assay
4.9. Cell Cycle Assay
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Dominguez Escrig, J.L.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur. Urol. 2022, 81, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Youssef, R.F.; Lotan, Y. Predictors of outcome of non-muscle-invasive and muscle-invasive bladder cancer. Sci. World J. 2011, 11, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Kassouf, W.; Traboulsi, S.L.; Kulkarni, G.S.; Breau, R.H.; Zlotta, A.; Fairey, A.; So, A.; Lacombe, L.; Rendon, R.; Aprikian, A.G.; et al. CUA guidelines on the management of non-muscle invasive bladder cancer. Can. Urol. Assoc. J. 2015, 9, E690–E704. [Google Scholar] [CrossRef]
- Sylvester, R.J.; van der Meijden, A.P.; Oosterlinck, W.; Witjes, J.A.; Bouffioux, C.; Denis, L.; Newling, D.W.; Kurth, K. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: A combined analysis of 2596 patients from seven EORTC trials. Eur. Urol. 2006, 49, 466–467. [Google Scholar] [CrossRef]
- Koch, G.E.; Smelser, W.W.; Chang, S.S. Side Effects of Intravesical BCG and Chemotherapy for Bladder Cancer: What They Are and How to Manage Them. Urology 2021, 149, 11–20. [Google Scholar] [CrossRef]
- Miao, L.L.; Zhou, Q.M.; Peng, C.; Liu, Z.H.; Xiong, L. Leonurus japonicus (Chinese motherwort), an excellent traditional medicine for obstetrical and gynecological diseases: A comprehensive overview. Biomed. Pharmacother. 2019, 117, 109060. [Google Scholar] [CrossRef]
- Wojtyniak, K.; Szymański, M.; Matławska, I. Leonurus cardiaca L. (motherwort): A review of its phytochemistry and pharmacology. Phytother. Res. 2013, 27, 1115–1120. [Google Scholar] [CrossRef]
- Chinwala, M.G.; Gao, M.; Dai, J.; Shao, J. In vitro anticancer activities of Leonurus heterophyllus sweet (Chinese motherwort herb). J. Altern. Complement. Med. 2003, 9, 511–518. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, P.; Liu, G.; Yan, H.; Bu, X.; Ma, Z.; Wang, N.; Wang, G.; Jia, W. Cytotoxicity of Chinese motherwort (YiMuCao) aqueous ethanol extract is non-apoptotic and estrogen receptor independent on human breast cancer cells. J. Ethnopharmacol. 2009, 122, 234–239. [Google Scholar] [CrossRef]
- Mao, F.; Zhang, L.; Cai, M.H.; Guo, H.; Yuan, H.H. Leonurine hydrochloride induces apoptosis of H292 lung cancer cell by a mitochondria-dependent pathway. Pharm. Biol. 2015, 53, 1684–1690. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Subramaniam, S.; Chia-Chuan, C.; Venkatachalam, B.; Thomas Cheeran, A.; Chi-Ying, F.H. Anticancer Activity of Leonurus sibiricus L.: Possible Involvement of Intrinsic Apoptotic Pathway. Nutr. Cancer 2022, 74, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Pan, H.; Wang, X.; He, H.; Li, M. Leonurus japonicus Houtt.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2014, 152, 14–32. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xu, D.Q.; Chen, Y.Y.; Yue, S.J.; Tang, Y.P. Leonurine, a potential drug for the treatment of cardiovascular system and central nervous system diseases. Brain Behav. 2021, 11, e01995. [Google Scholar] [CrossRef]
- Yue, J.; López, J.M. Understanding MAPK Signaling Pathways in Apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Fierascu, I.; Ortan, A.; Fierascu, I.C.; Anuta, V.; Velescu, B.S.; Pituru, S.M.; Dinu-Pirvu, C.E. Leonurus cardiaca L. as a Source of Bioactive Compounds: An Update of the European Medicines Agency Assessment Report (2010). BioMed Res. Int. 2019, 2019, 4303215. [Google Scholar] [CrossRef]
- Hoetelmans, R.; van Slooten, H.J.; Keijzer, R.; Erkeland, S.; van de Velde, C.J.; Dierendonck, J.H. Bcl-2 and Bax proteins are present in interphase nuclei of mammalian cells. Cell Death Differ. 2000, 7, 384–392. [Google Scholar] [CrossRef]
- Ajili, F.; Kaabi, B.; Darouiche, A.; Tounsi, H.; Kourda, N.; Chebil, M.; Manai, M.; Boubaker, S. Prognostic value of Bcl-2 and Bax tumor cell expression in patients with non muscle-invasive bladder cancer receiving bacillus Calmette-Guerin immunotherapy. Ultrastruct. Pathol. 2012, 36, 31–39. [Google Scholar] [CrossRef]
- Chai, X.; Zhang, J.W.; Li, S.H.; Cheng, Q.S.; Qin, M.M.; Yang, C.Y.; Gao, J.L.; Huang, H.B. Xanthoceraside induces cell apoptosis through downregulation of the PI3K/Akt/Bcl-2/Bax signaling pathway in cell lines of human bladder cancer. Indian. J. Pathol. Microbiol. 2021, 64, 294–301. [Google Scholar]
- Liu, D.; Qiu, X.; Xiong, X.; Chen, X.; Pan, F. Current updates on the role of reactive oxygen species in bladder cancer pathogenesis and therapeutics. Clin. Transl. Oncol. 2020, 22, 1687–1697. [Google Scholar] [CrossRef]
- Liu, J.; Li, A.P.; Li, C.P.; Zhang, Z.D.; Zhou, J.W. The role of reactive oxygen species in N-[4-hydroxyphenyl] retinamide induced apoptosis in bladder cancer cell lineT24. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2005, 23, 191–194. [Google Scholar] [PubMed]
- Zarubin, T.; Han, J. Activation and signaling of the p38 MAP kinase pathway. Cell Res. 2005, 15, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Perdiguero, E.; Ruiz-Bonilla, V.; Gresh, L.; Hui, L.; Ballestar, E.; Sousa-Victor, P.; Baeza-Raja, B.; Jardí, M.; Bosch-Comas, A.; Esteller, M.; et al. Genetic analysis of p38 MAP kinases in myogenesis: Fundamental role of p38alpha in abrogating myoblast proliferation. EMBO J. 2007, 26, 1245–1256. [Google Scholar] [CrossRef]
- Farley, N.; Pedraza-Alva, G.; Serrano-Gomez, D.; Nagaleekar, V.; Aronshtam, A.; Krahl, T.; Thornton, T.; Rincón, M. p38 mitogen-activated protein kinase mediates the Fas-induced mitochondrial death pathway in CD8+ T cells. Mol. Cell. Biol. 2006, 26, 2118–2129. [Google Scholar] [CrossRef] [PubMed]
- Thornton, T.M.; Rincon, M. Non-classical p38 map kinase functions: Cell cycle checkpoints and survival. Int. J. Biol. Sci. 2009, 5, 44–51. [Google Scholar] [CrossRef]
- Yong, H.Y.; Koh, M.S.; Moon, A. The p38 MAPK inhibitors for the treatment of inflammatory diseases and cancer. Expert. Opin. Investig. Drugs 2009, 18, 1893–1905. [Google Scholar] [CrossRef]
- Martínez-Limón, A.; Joaquin, M.; Caballero, M.; Posas, F.; de Nadal, E. The p38 Pathway: From Biology to Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 1913. [Google Scholar] [CrossRef]
- Kurata, S. Selective activation of p38 MAPK cascade and mitotic arrest caused by low level oxidative stress. J. Biol. Chem. 2000, 275, 23413–23416. [Google Scholar] [CrossRef]
- Cuenda, A.; Rousseau, S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim. Biophys. Acta 2007, 1773, 1358–1375. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.-H.; Hung, C.-H.; Huang, Y.-C.; Chen, C.-S.; Ho, D.-R. The p38-MITOGEN-ACTIVATED PROTEIN KINASE Signaling Pathway Is Involved in Leonurus artemisia Extract-Induced Inhibition of the Proliferation of Human Bladder Cancer BFTC-905 Cells via G1/G0 Arrest and Causes Apoptosis In Vitro. Pharmaceuticals 2023, 16, 1338. https://doi.org/10.3390/ph16101338
Lin J-H, Hung C-H, Huang Y-C, Chen C-S, Ho D-R. The p38-MITOGEN-ACTIVATED PROTEIN KINASE Signaling Pathway Is Involved in Leonurus artemisia Extract-Induced Inhibition of the Proliferation of Human Bladder Cancer BFTC-905 Cells via G1/G0 Arrest and Causes Apoptosis In Vitro. Pharmaceuticals. 2023; 16(10):1338. https://doi.org/10.3390/ph16101338
Chicago/Turabian StyleLin, Jian-Hui, Chein-Hui Hung, Yun-Ching Huang, Chih-Shou Chen, and Dong-Ru Ho. 2023. "The p38-MITOGEN-ACTIVATED PROTEIN KINASE Signaling Pathway Is Involved in Leonurus artemisia Extract-Induced Inhibition of the Proliferation of Human Bladder Cancer BFTC-905 Cells via G1/G0 Arrest and Causes Apoptosis In Vitro" Pharmaceuticals 16, no. 10: 1338. https://doi.org/10.3390/ph16101338
APA StyleLin, J.-H., Hung, C.-H., Huang, Y.-C., Chen, C.-S., & Ho, D.-R. (2023). The p38-MITOGEN-ACTIVATED PROTEIN KINASE Signaling Pathway Is Involved in Leonurus artemisia Extract-Induced Inhibition of the Proliferation of Human Bladder Cancer BFTC-905 Cells via G1/G0 Arrest and Causes Apoptosis In Vitro. Pharmaceuticals, 16(10), 1338. https://doi.org/10.3390/ph16101338








