The Use of Micro-Ribbons and Micro-Fibres in the Formulation of 3D Printed Fast Dissolving Oral Films
Abstract
1. Introduction
2. Results
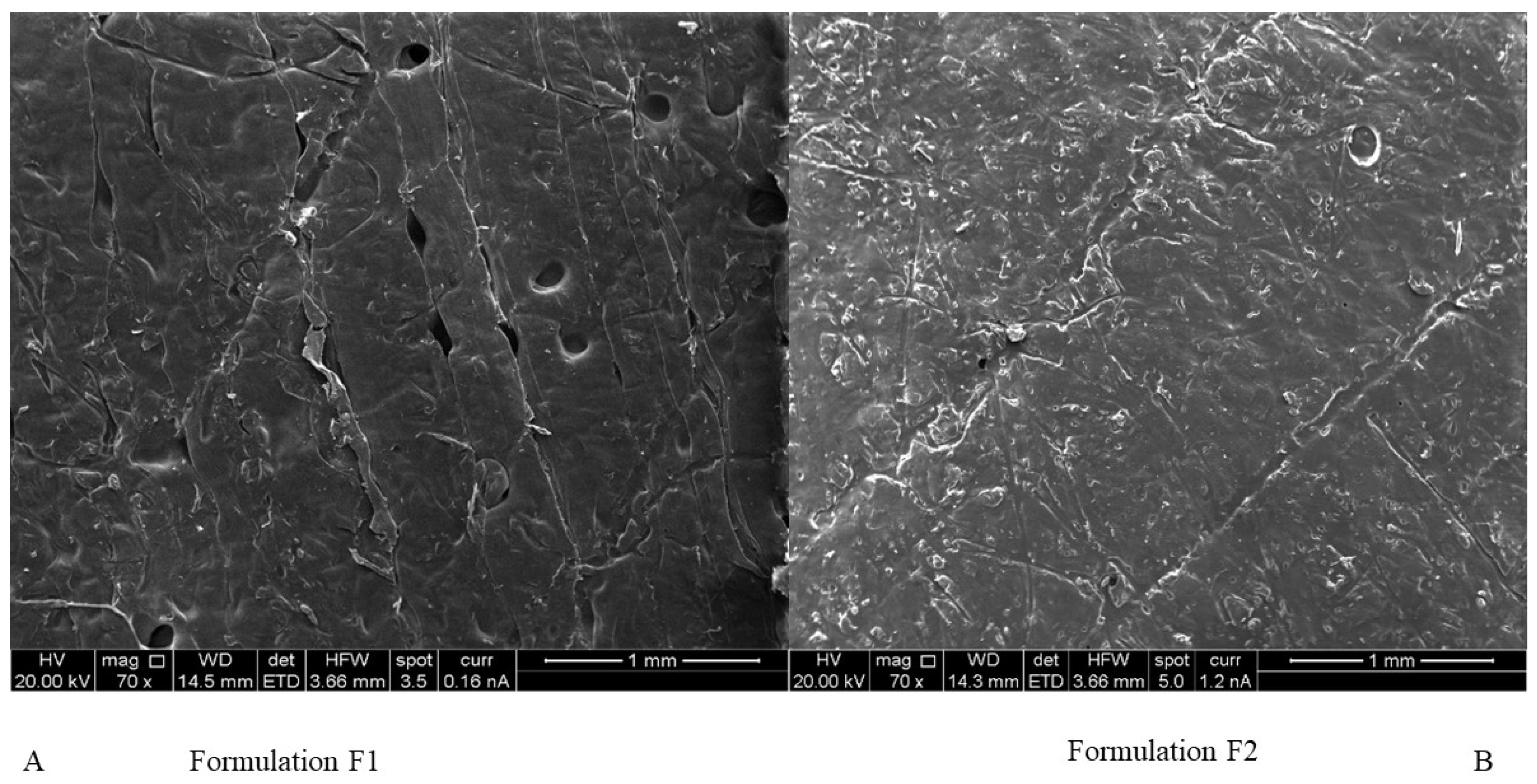
2.1. Morphology of Chitosan Micro-Ribbons and Cellulose Microfibres
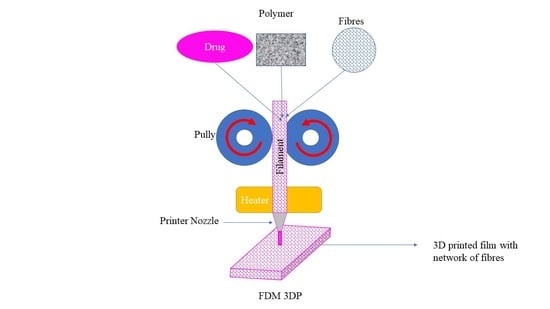
2.2. Formation of Filaments and Films
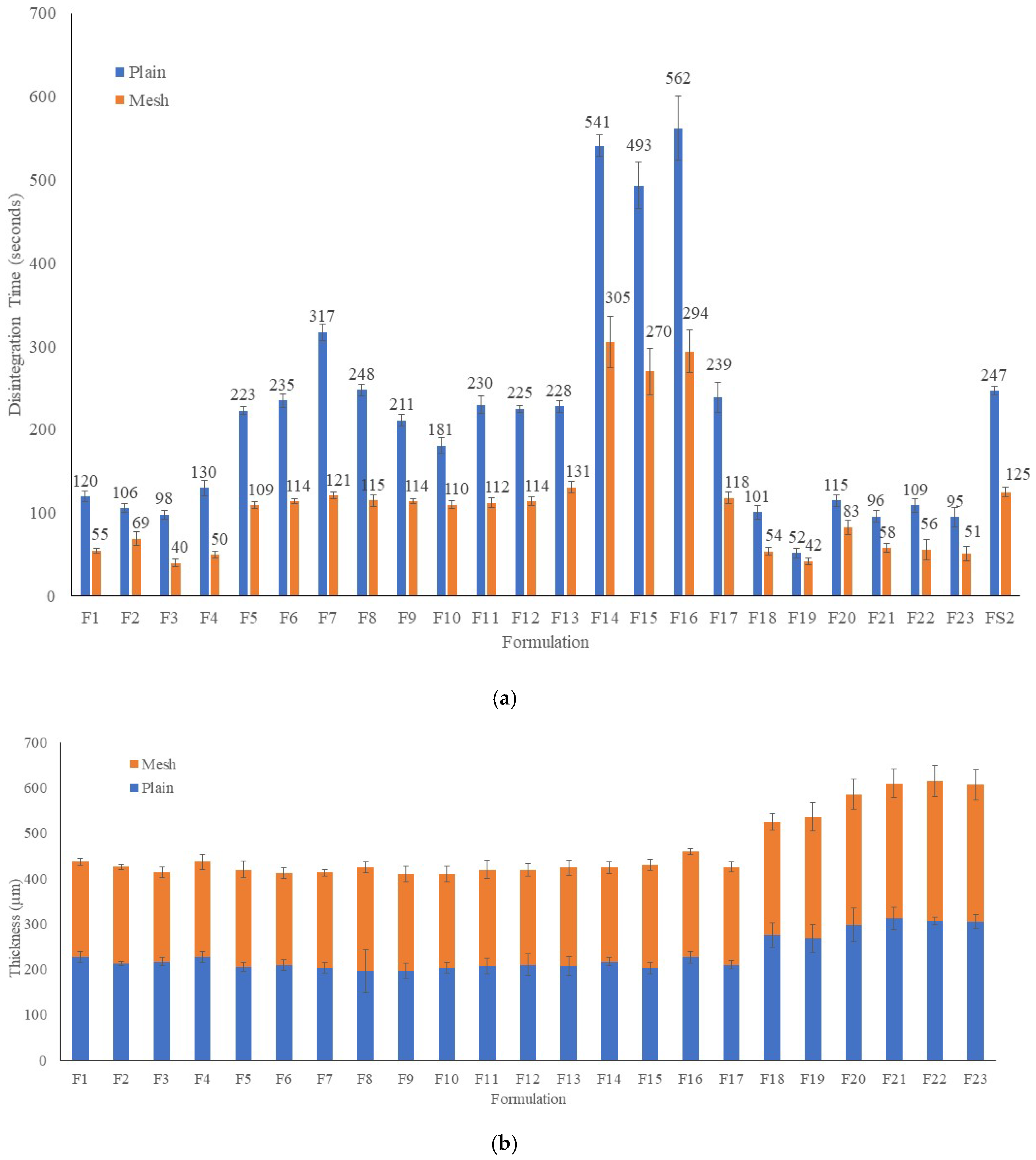
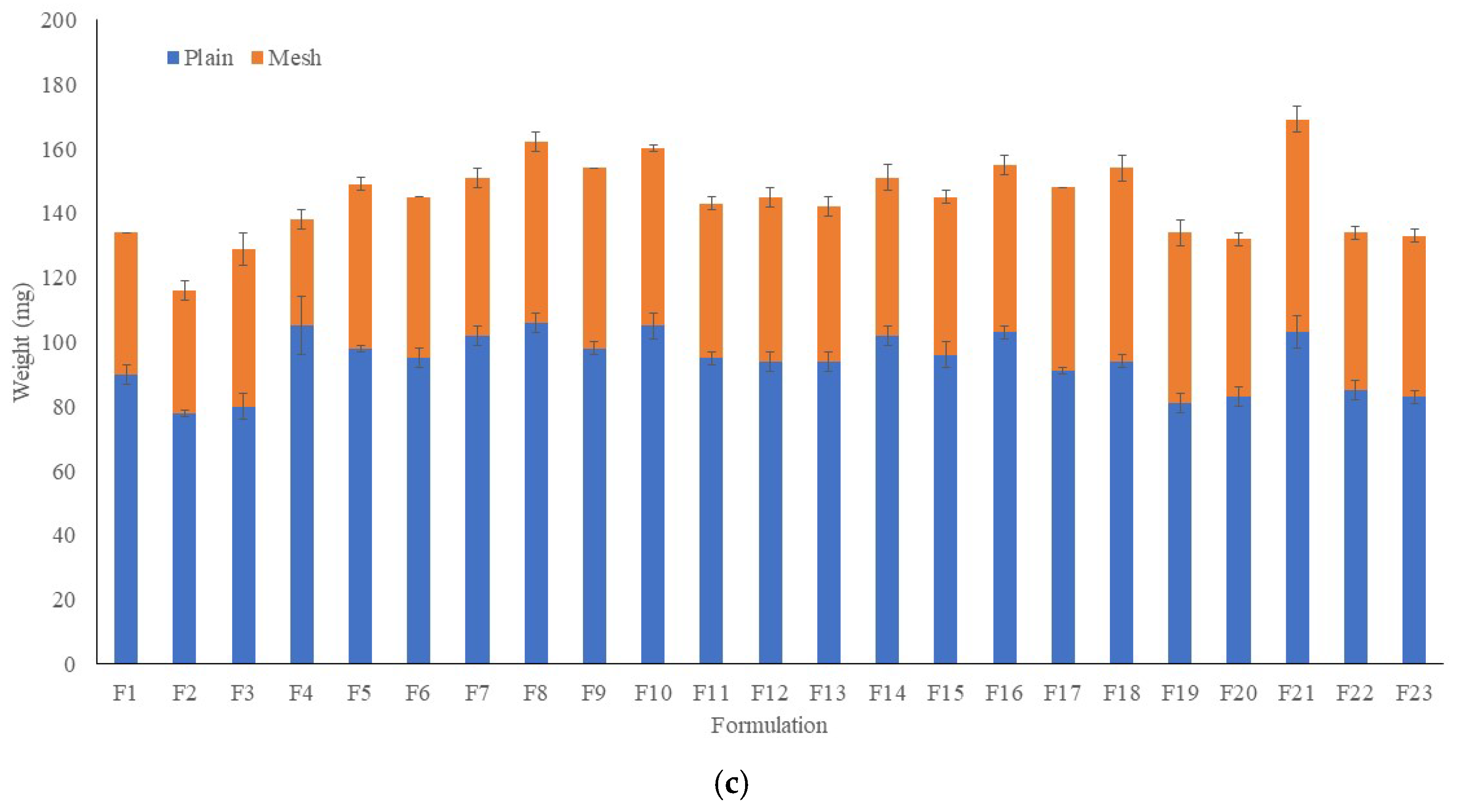
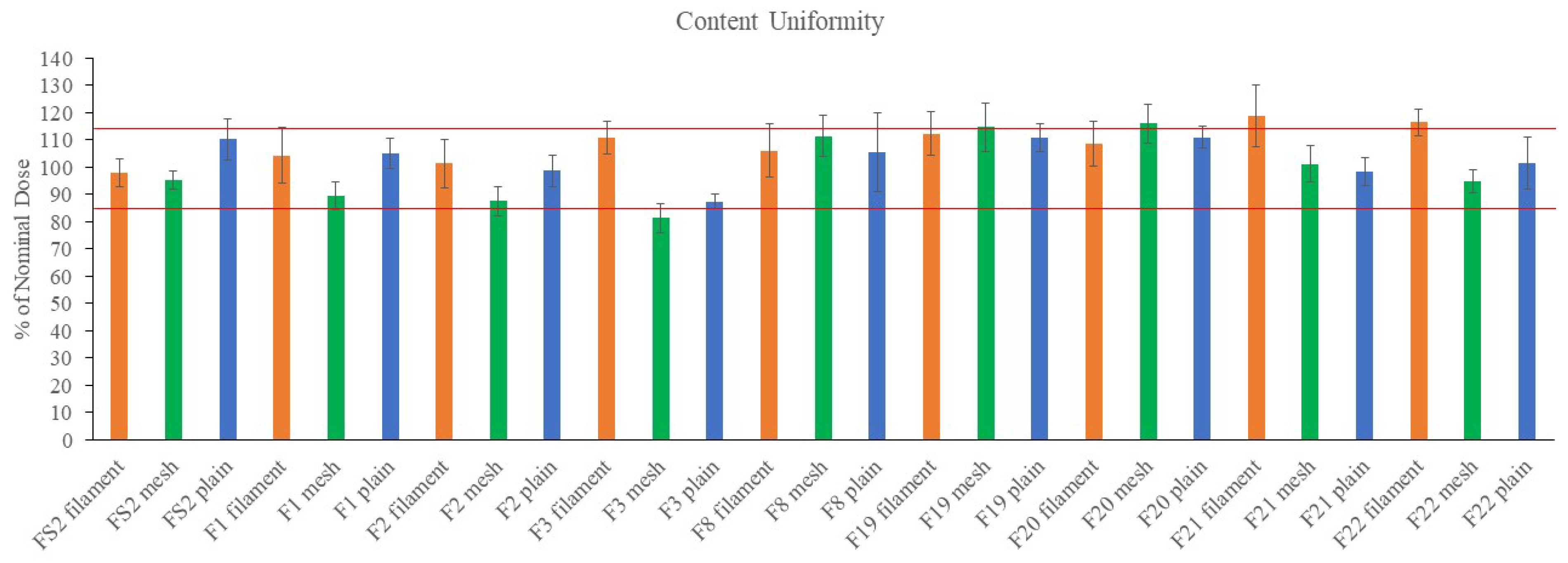
2.3. Weight, Thickness Disintegration Time and Content Uniformity of FDFs
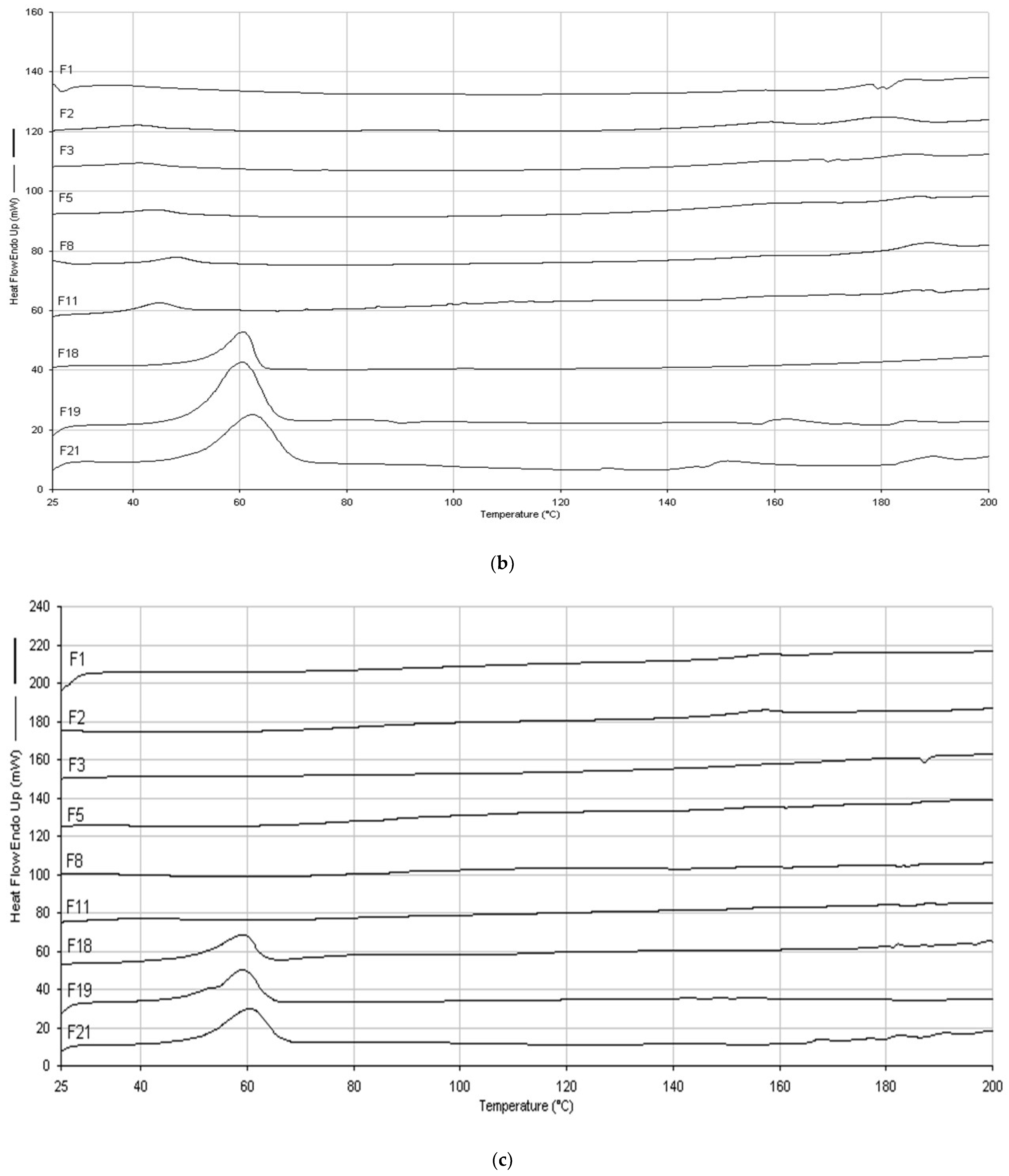
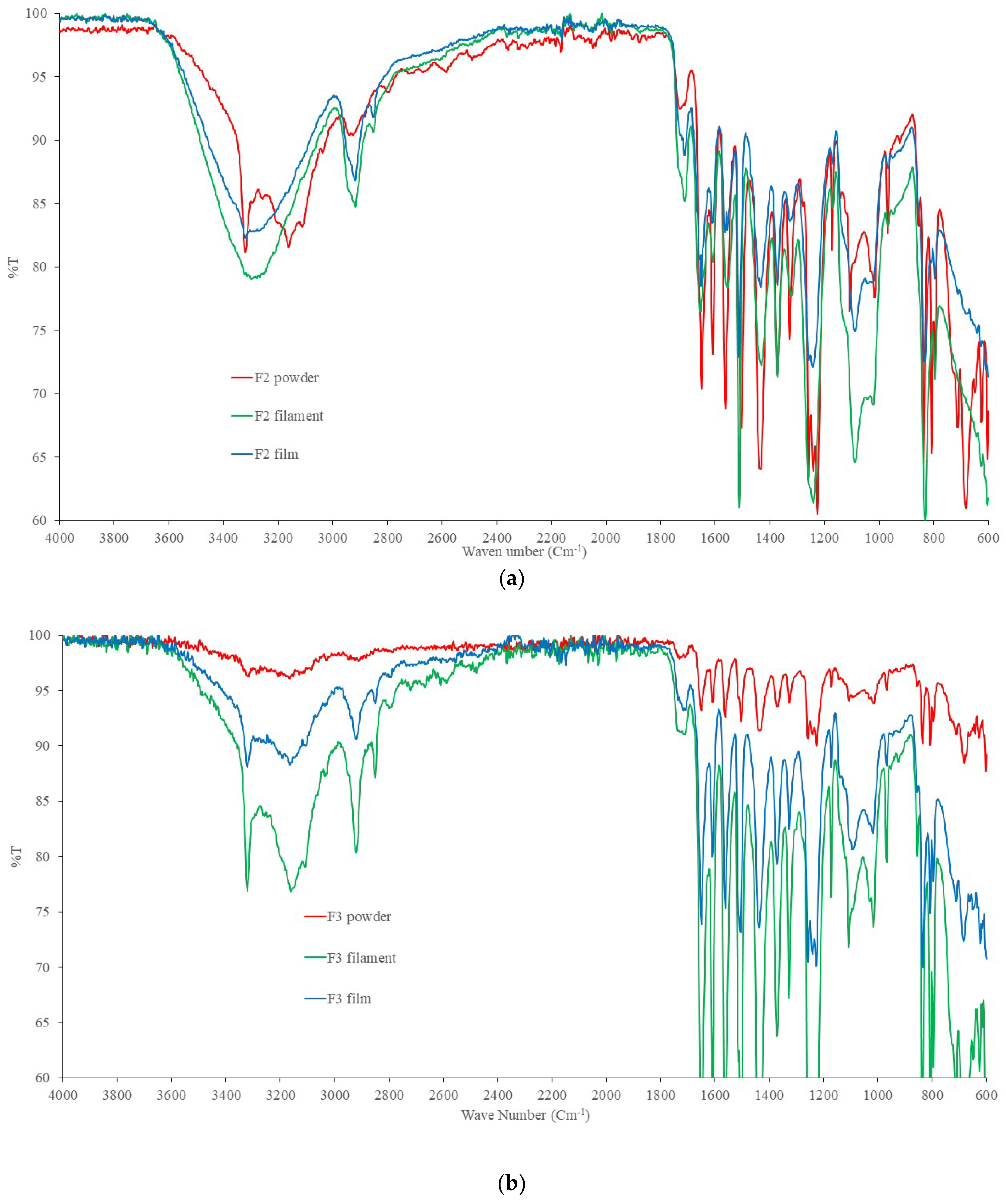
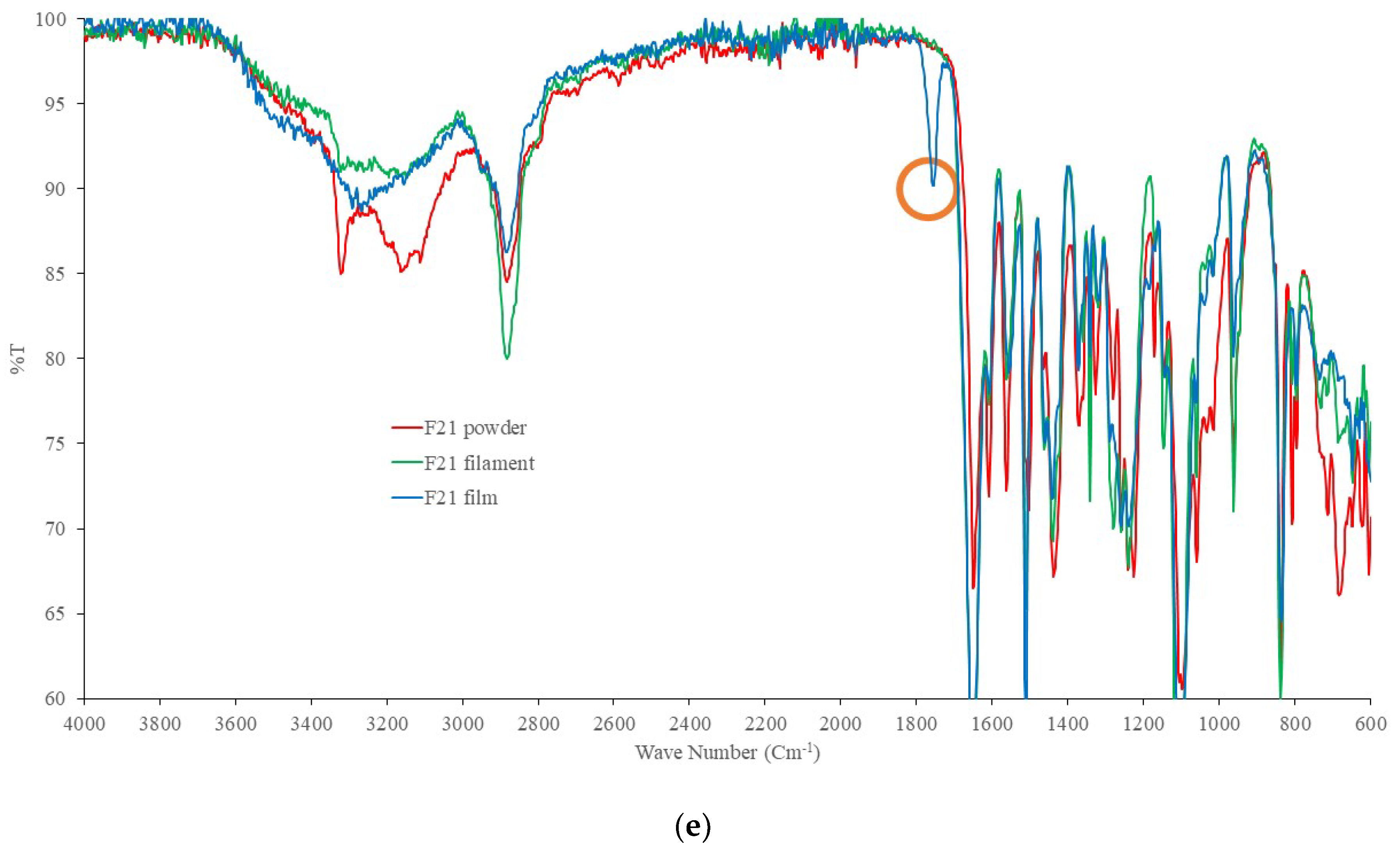
2.4. DSC and FTIR
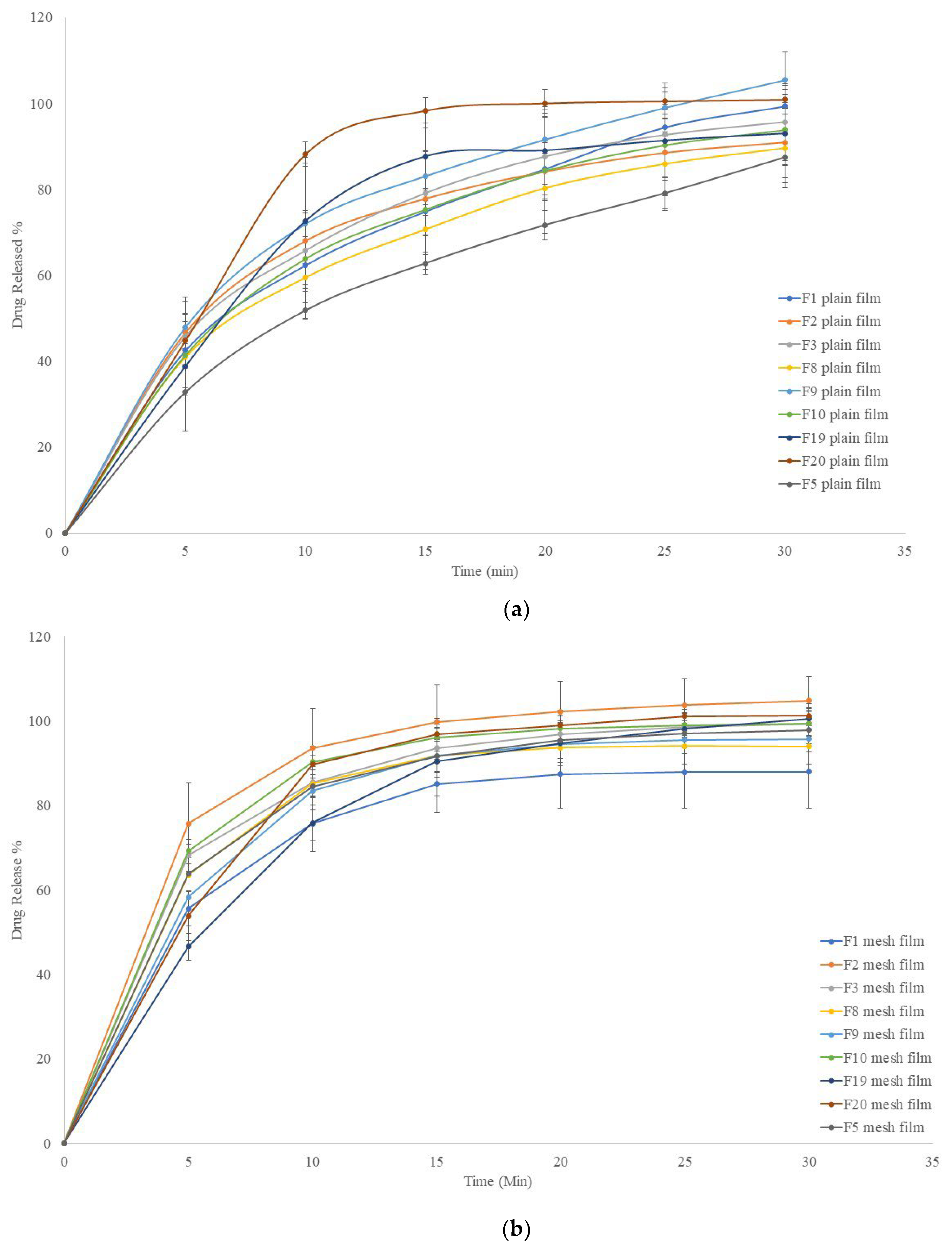
2.5. In Vitro Release Profiles
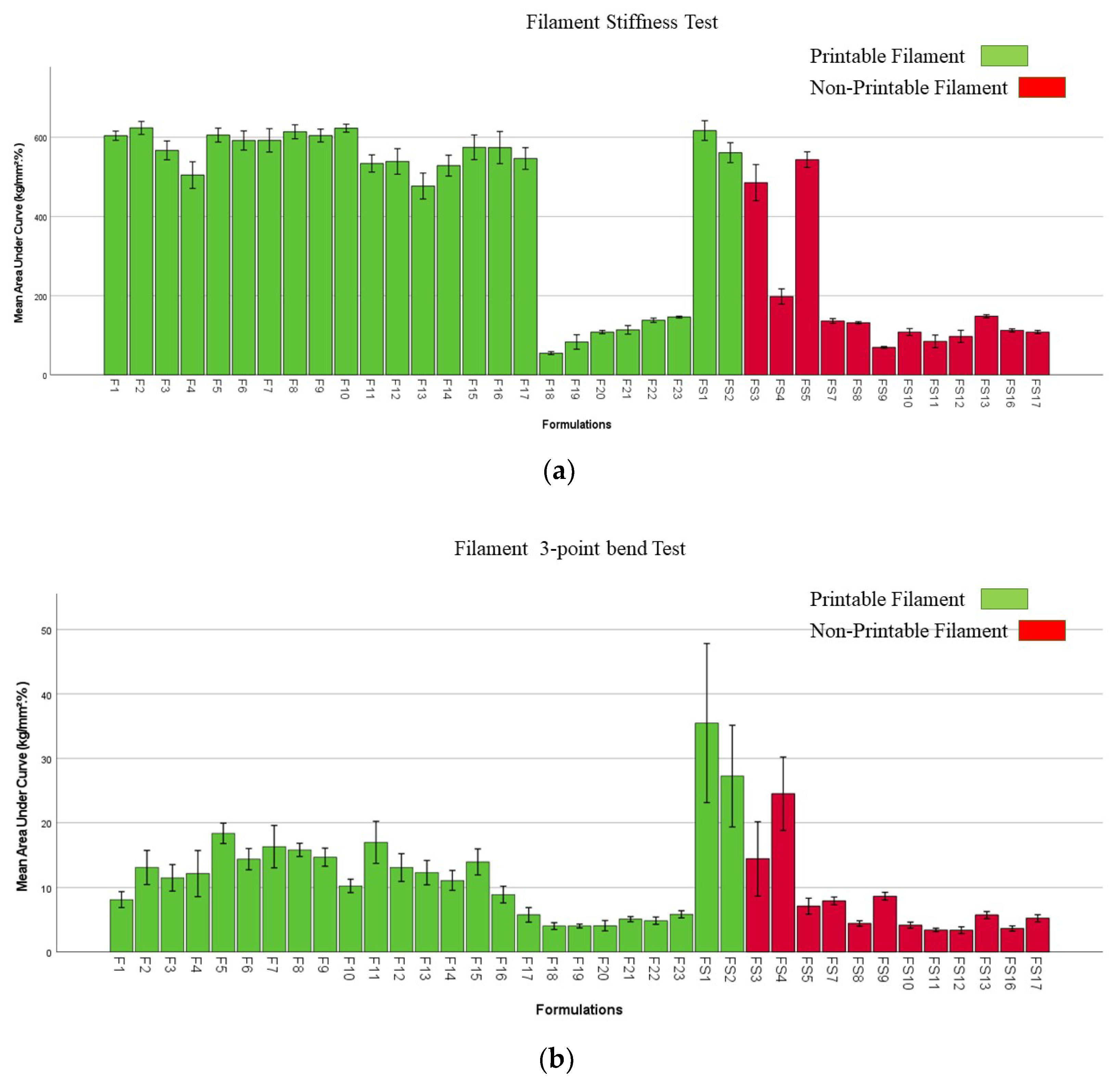
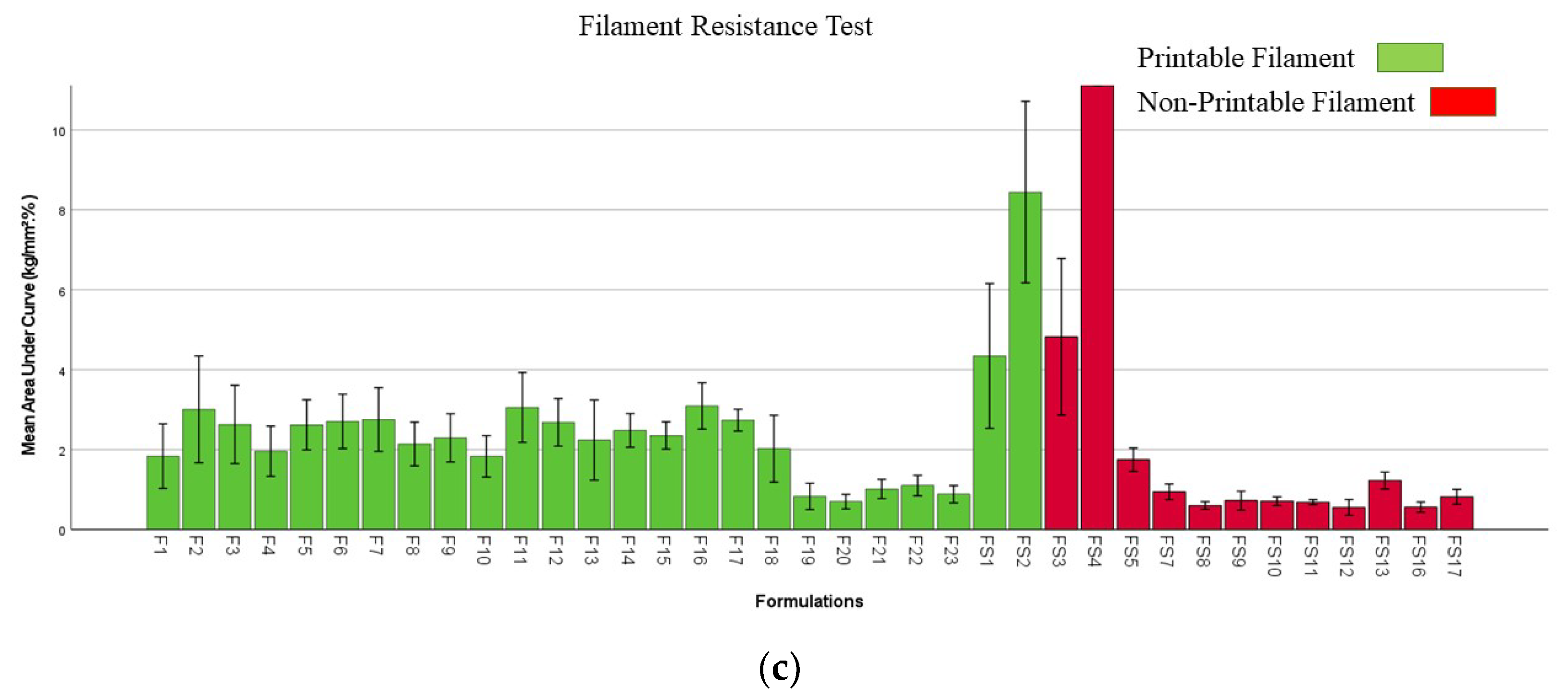
2.6. Mechanical Properties of Filaments and Films
2.7. Cell Toxicity Studies
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Chitosan Micro-Ribbons
4.3. Preparation of Filaments
| Formulation | PCM | PVP 40K | PEO 100K | PVA | CCS | Chi-MR | C500 | C1000 | C2000 | Chi |
|---|---|---|---|---|---|---|---|---|---|---|
| F1 | 30 | - | - | 63 | 7 | - | - | - | - | - |
| F2 | 30 | - | - | 62 | 7 | 1 | - | - | - | - |
| F3 | 30 | - | - | 69 | - | 1 | - | - | - | - |
| F4 | 30 | - | - | 68 | - | 2 | - | - | - | - |
| F5 | 30 | - | - | 69 | - | - | 1 | - | - | - |
| F6 | 30 | - | - | 68 | - | - | 2 | - | - | - |
| F7 | 30 | - | - | 67 | 3 | - | ||||
| F8 | 30 | - | - | 69 | - | - | - | 1 | - | - |
| F9 | 30 | - | - | 68 | - | - | - | 2 | - | - |
| F10 | 30 | - | - | 67 | 3 | - | ||||
| F11 | 30 | - | - | 69 | - | - | - | - | 1 | - |
| F12 | 30 | - | - | 68 | - | - | - | - | 2 | - |
| F13 | 30 | 67 | 3 | |||||||
| F14 | 30 | - | - | 62 | 7 | 1 | ||||
| F15 | 30 | - | - | 61 | 7 | 2 | ||||
| F16 | 30 | - | - | 60 | 7 | 3 | ||||
| F17 | 30 | - | - | 69 | 1 | |||||
| F18 | 30 | 40 | 30 | - | - | - | - | - | - | - |
| F19 | 30 | 33 | 30 | 7 | ||||||
| F20 | 30 | 33 | 29.8 | 7 | 0.2 | |||||
| F21 | 30 | 33 | 25 | 7 | 5 | |||||
| F22 | 30 | 33 | 25 | 7 | 5 | |||||
| F23 | 30 | 33 | 25 | 7 | 5 |
4.4. 3D Printing of FDFs
4.5. Scanning Electron Microscopy
4.6. Differential Scanning Calorimetry
4.7. Fourier Transform Spectroscopy
4.8. Disintegration Tests
4.9. In Vitro Dissolution Studies
4.10. Evaluating Mechanical Properties of Filaments and Films
4.11. Content Uniformity
4.12. Cell Toxicity Evaluations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhattacharyya, N. The Prevalence of Dysphagia among Adults in the United States. Otolaryngol. Neck Surg. 2014, 151, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhong, W.; Xu, L.; Li, H.; Yan, Q.; She, Y.; Yang, G. Recent progress of 3D-printed microneedles for transdermal drug delivery. Int. J. Pharm. 2021, 593, 120106. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Zhu, L.; Yang, N.; Li, H.; Yang, Q. Recent advances of oral film as platform for drug delivery. Int. J. Pharm. 2021, 604, 120759. [Google Scholar] [CrossRef] [PubMed]
- Ehtezazi, T.; Algellay, M.; Islam, Y.; Roberts, M.; Dempster, N.M.; Sarker, S.D. The Application of 3D Printing in the Formulation of Multilayered Fast Dissolving Oral Films. J. Pharm. Sci. 2018, 107, 1076–1085. [Google Scholar] [CrossRef] [PubMed]
- Elbl, J.; Gajdziok, J.; Kolarczyk, J. 3D printing of multilayered orodispersible films with in-process drying. Int. J. Pharm. 2020, 575, 118883. [Google Scholar] [CrossRef]
- Jamróz, W.; Kurek, M.; Łyszczarz, E.; Szafraniec, J.; Knapik-Kowalczuk, J.; Syrek, K.; Paluch, M.; Jachowicz, R. 3D printed orodispersible films with Aripiprazole. Int. J. Pharm. 2017, 533, 413–420. [Google Scholar] [CrossRef]
- Panraksa, P.; Qi, S.; Udomsom, S.; Tipduangta, P.; Rachtanapun, P.; Jantanasakulwong, K.; Jantrawut, P. Characterization of Hydrophilic Polymers as a Syringe Extrusion 3D Printing Material for Orodispersible Film. Polymers 2021, 13, 3454. [Google Scholar] [CrossRef]
- Elbadawi, M.; Nikjoo, D.; Gustafsson, T.; Gaisford, S.; Basit, A.W. Pressure-assisted microsyringe 3D printing of oral films based on pullulan and hydroxypropyl methylcellulose. Int. J. Pharm. 2021, 595, 120197. [Google Scholar] [CrossRef]
- Cailleaux, S.; Sanchez-Ballester, N.M.; Gueche, Y.A.; Bataille, B.; Soulairol, I. Fused Deposition Modeling (FDM), the new asset for the production of tailored medicines. J. Control. Release 2021, 330, 821–841. [Google Scholar] [CrossRef]
- Eleftheriadis, G.K.; Ritzoulis, C.; Bouropoulos, N.; Tzetzis, D.; Andreadis, D.A.; Boetker, J.; Rantanen, J.; Fatouros, D.G. Unidirectional drug release from 3D printed mucoadhesive buccal films using FDM technology: In vitro and ex vivo evaluation. Eur. J. Pharm. Biopharm. 2019, 144, 180–192. [Google Scholar] [CrossRef]
- Ehtezazi, T.; Algellay, M.; Hardy, A. Next Steps in 3D Printing of Fast Dissolving Oral Films for Commercial Production. Recent Patents Drug Deliv. Formul. 2020, 14, 5–20. [Google Scholar] [CrossRef]
- Chennakesava, P.; Narayan, Y.S. Fused deposition modeling-insights. In Proceedings of the International Conference on Advances in Design and Manufacturing (ICAD&M’14), Tiruchirappalli, India, 5–7 December 2014; p. 1345. [Google Scholar]
- Rajpurohit, S.R.; Dave, H.K. Analysis of tensile strength of a fused filament fabricated PLA part using an open-source 3D printer. Int. J. Adv. Manuf. Technol. 2019, 101, 1525–1536. [Google Scholar] [CrossRef]
- Goyanes, A.; Allahham, N.; Trenfield, S.J.; Stoyanov, E.; Gaisford, S.; Basit, A.W. Direct powder extrusion 3D printing: Fabrication of drug products using a novel single-step process. Int. J. Pharm. 2019, 567, 118471. [Google Scholar] [CrossRef]
- Zhang, L.G.; Leong, K.; Fisher, J.P. 3D Bioprinting and Nanotechnology in Tissue Engineering and Regenerative Medicine; Zhang, L.G., Leong, K., Fisher, J.P., Eds.; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Francis, V.; Jain, P.K. Experimental investigations on fused deposition modelling of polymer-layered silicate nanocomposite. Virtual Phys. Prototyp. 2016, 11, 109–121. [Google Scholar] [CrossRef]
- Cataldi, A.; Rigotti, D.; Nguyen, V.; Pegoretti, A. Polyvinyl alcohol reinforced with crystalline nanocellulose for 3D printing application. Mater. Today Commun. 2018, 15, 236–244. [Google Scholar] [CrossRef]
- Bilkar, D.; Keshavamurthy, R.; Tambrallimath, V. Influence of carbon nanofiber reinforcement on mechanical properties of polymer composites developed by FDM. Mater. Today Proc. 2020, 46, 4559–4562. [Google Scholar] [CrossRef]
- Shao, D.; Qin, L.; Sawyer, S. Optical properties of polyvinyl alcohol (PVA) coated In2O3 nanoparticles. Opt. Mater. 2013, 35, 563–566. [Google Scholar] [CrossRef]
- Patanwala, H.S.; Hong, D.; Vora, S.R.; Bognet, B.; Ma, A.W.K. The microstructure and mechanical properties of 3D printed carbon nanotube-polylactic acid composites. Polym. Compos. 2017, 39, 1060–1071. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, S.; Liu, K.; Wen, W.; Lu, L.; Ding, S.; Zhou, C.; Luo, B. 3D poly (L-lactide)/chitosan micro/nano fibrous scaffolds functionalized with quercetin-polydopamine for enhanced osteogenic and anti-inflammatory activities. Chem. Eng. J. 2020, 391, 123524. [Google Scholar] [CrossRef]
- Zhao, J.; Han, W.; Chen, H.; Tu, M.; Zeng, R.; Shi, Y.; Cha, Z.; Zhou, C. Preparation, structure and crystallinity of chitosan nano-fibers by a solid–liquid phase separation technique. Carbohydr. Polym. 2011, 83, 1541–1546. [Google Scholar] [CrossRef]
- Berglund, L.A.; Peijs, T. Cellulose Biocomposites—From Bulk Moldings to Nanostructured Systems. MRS Bull. 2010, 35, 201–207. [Google Scholar] [CrossRef]
- Al-Turaif, H.A. Relationship between tensile properties and film formation kinetics of epoxy resin reinforced with nanofibrillated cellulose. Prog. Org. Coat. 2013, 76, 477–481. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Saurabh, C.K.; Asniza, M.; Tye, Y.Y.; Nurul Fazita, M.R.; Syakir, M.I.; Fizree, H.M.; Yusra, A.F.I.; Haafiz, M.K.M.; Kassim, M.A.; et al. Nanofibrillated cellulose reinforcement in thermoset polymer composites. In Cellulose-Reinforced Nanofibre Composites; Jawaid, M., Boufi, S., Abdul Khalil, H.P.S., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 1–24. [Google Scholar] [CrossRef]
- Shaghaleh, H.; Xu, X.; Wang, S. Current progress in production of biopolymeric materials based on cellulose, cellulose nanofibers, and cellulose derivatives. RSC Adv. 2018, 8, 825–842. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-U. Degradation Classification of 3D Printing Thermoplastics Using Fourier Transform Infrared Spectroscopy and Artificial Neural Networks. Appl. Sci. 2018, 8, 1224. [Google Scholar] [CrossRef]
- Elbadawi, M.; Gustaffson, T.; Gaisford, S.; Basit, A.W. 3D printing tablets: Predicting printability and drug dissolution from rheological data. Int. J. Pharm. 2020, 590, 119868. [Google Scholar] [CrossRef]
- Wang, Q.; Backman, O.; Nuopponen, M.; Xu, C.; Wang, X. Rheological and Printability Assessments on Biomaterial Inks of Nanocellulose/Photo-Crosslinkable Biopolymer in Light-Aided 3D Printing. Front. Chem. Eng. 2021, 3, 723429. [Google Scholar] [CrossRef]
- Cho, H.-W.; Baek, S.-H.; Lee, B.-J.; Jin, H.-E. Orodispersible Polymer Films with the Poorly Water-Soluble Drug, Olanzapine: Hot-Melt Pneumatic Extrusion for Single-Process 3D Printing. Pharmaceutics 2020, 12, 692. [Google Scholar] [CrossRef]
- Janigová, N.; Elbl, J.; Pavloková, S.; Gajdziok, J. Effects of Various Drying Times on the Properties of 3D Printed Orodispersible Films. Pharmaceutics 2022, 14, 250. [Google Scholar] [CrossRef]
- Musazzi, U.M.; Selmin, F.; Ortenzi, M.A.; Mohammed, K.G.; Franzé, S.; Minghetti, P.; Cilurzo, F. Personalized orodispersible films by hot melt ram extrusion 3D printing. Int. J. Pharm. 2018, 551, 52–59. [Google Scholar] [CrossRef]
- Okwuosa, T.C.; Stefaniak, D.; Arafat, B.; Isreb, A.; Wan, K.-W.; Alhnan, M.A. A Lower Temperature FDM 3D Printing for the Manufacture of Patient-Specific Immediate Release Tablets. Pharm. Res. 2016, 33, 2704–2712. [Google Scholar] [CrossRef]
- Xu, P.; Li, J.; Meda, A.; Osei-Yeboah, F.; Peterson, M.L.; Repka, M.; Zhan, X. Development of a quantitative method to evaluate the printability of filaments for fused deposition modeling 3D printing. Int. J. Pharm. 2020, 588, 119760. [Google Scholar] [CrossRef]
- Gupta, S.; Webster, T.J.; Sinha, A. Evolution of PVA gels prepared without crosslinking agents as a cell adhesive surface. J. Mater. Sci. Mater. Med. 2011, 22, 1763–1772. [Google Scholar] [CrossRef]
- Satyanarayana, D.A.; Keshavarao, K.P. Fast disintegrating films containing anastrozole as a dosage form for dysphagia patients. Arch. Pharmacal Res. 2012, 35, 2171–2182. [Google Scholar] [CrossRef]
- Wang, H.; Yu, X.; Su, C.; Shi, Y.; Zhao, L. Chitosan nanoparticles triggered the induction of ROS-mediated cytoprotective autophagy in cancer cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, 293–301. [Google Scholar] [CrossRef]
- Liu, H.; Han, Y.; Wang, T.; Zhang, H.; Xu, Q.; Yuan, J.; Li, Z. Targeting Microglia for Therapy of Parkinson’s Disease by Using Biomimetic Ultrasmall Nanoparticles. J. Am. Chem. Soc. 2020, 142, 21730–21742. [Google Scholar] [CrossRef]
- Calvo, P.; Remuñán-López, C.; Vila-Jato, J.L.; Alonso, M.J. Chitosan and Chitosan/Ethylene Oxide-Propylene Oxide Block Copolymer Nanoparticles as Novel Carriers for Proteins and Vaccines. Pharm. Res. 1997, 14, 1431–1436. [Google Scholar] [CrossRef]
- Selmin, F.; Franceschini, I.; Cupone, I.E.; Minghetti, P.; Cilurzo, F. Aminoacids as non-traditional plasticizers of maltodextrins fast-dissolving films. Carbohydr. Polym. 2015, 115, 613–616. [Google Scholar] [CrossRef]
- Gaisford, S.; Verma, A.; Saunders, M.; Royall, P.G. Monitoring crystallisation of drugs from fast-dissolving oral films with isothermal calorimetry. Int. J. Pharm. 2009, 380, 105–111. [Google Scholar] [CrossRef]
- Azarmi, S.; Roa, W.; Löbenberg, R. Current perspectives in dissolution testing of conventional and novel dosage forms. Int. J. Pharm. 2007, 328, 12–21. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, P.; Vo, A.Q.; Bandari, S.; Yang, F.; Durig, T.; Repka, M.A. Development and evaluation of pharmaceutical 3D printability for hot melt extruded cellulose-based filaments. J. Drug Deliv. Sci. Technol. 2019, 52, 292–302. [Google Scholar] [CrossRef]
- Zhang, J.; Feng, X.; Patil, H.; Tiwari, R.V.; Repka, M.A. Coupling 3D printing with hot-melt extrusion to produce controlled-release tablets. Int. J. Pharm. 2017, 519, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Nasereddin, J.M.; Wellner, N.; Alhijjaj, M.; Belton, P.; Qi, S. Development of a Simple Mechanical Screening Method for Predicting the Feedability of a Pharmaceutical FDM 3D Printing Filament. Pharm. Res. 2018, 35, 151. [Google Scholar] [CrossRef] [PubMed]
- Hafezi, F.; Scoutaris, N.; Douroumis, D.; Boateng, J. 3D printed chitosan dressing crosslinked with genipin for potential healing of chronic wounds. Int. J. Pharm. 2019, 560, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Arany, P.; Papp, I.; Zichar, M.; Regdon, G., Jr.; Béres, M.; Szalóki, M.; Kovács, R.; Fehér, P.; Ujhelyi, Z.; Vecsernyés, M.; et al. Manufacturing and Examination of Vaginal Drug Delivery System by FDM 3D Printing. Pharmaceutics 2021, 13, 1714. [Google Scholar] [CrossRef]
- Ajibade, P.A.; Fatokun, A.A.; Andrew, F.P. Synthesis, characterization and anti-cancer studies of Mn(II), Cu(II), Zn(II) and Pt(II) dithiocarbamate complexes-crystal structures of the Cu(II) and Pt(II) complexes. Inorg. Chim. Acta 2020, 504, 119431. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Algellay, M.; Roberts, M.; Bosworth, L.; Sarker, S.D.; Fatokun, A.A.; Ehtezazi, T. The Use of Micro-Ribbons and Micro-Fibres in the Formulation of 3D Printed Fast Dissolving Oral Films. Pharmaceuticals 2023, 16, 79. https://doi.org/10.3390/ph16010079
Algellay M, Roberts M, Bosworth L, Sarker SD, Fatokun AA, Ehtezazi T. The Use of Micro-Ribbons and Micro-Fibres in the Formulation of 3D Printed Fast Dissolving Oral Films. Pharmaceuticals. 2023; 16(1):79. https://doi.org/10.3390/ph16010079
Chicago/Turabian StyleAlgellay, Marwan, Matthew Roberts, Lucy Bosworth, Satyajit D. Sarker, Amos A. Fatokun, and Touraj Ehtezazi. 2023. "The Use of Micro-Ribbons and Micro-Fibres in the Formulation of 3D Printed Fast Dissolving Oral Films" Pharmaceuticals 16, no. 1: 79. https://doi.org/10.3390/ph16010079
APA StyleAlgellay, M., Roberts, M., Bosworth, L., Sarker, S. D., Fatokun, A. A., & Ehtezazi, T. (2023). The Use of Micro-Ribbons and Micro-Fibres in the Formulation of 3D Printed Fast Dissolving Oral Films. Pharmaceuticals, 16(1), 79. https://doi.org/10.3390/ph16010079