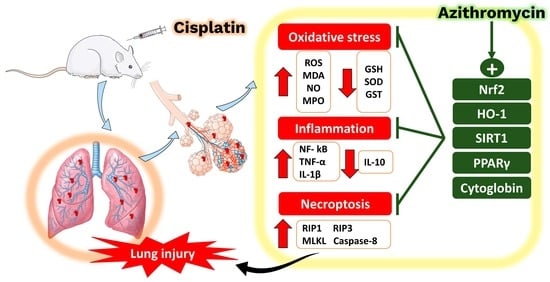
Azithromycin Mitigates Cisplatin-Induced Lung Oxidative Stress, Inflammation and Necroptosis by Upregulating SIRT1, PPARγ, and Nrf2/HO-1 Signaling
Abstract
1. Introduction
2. Results
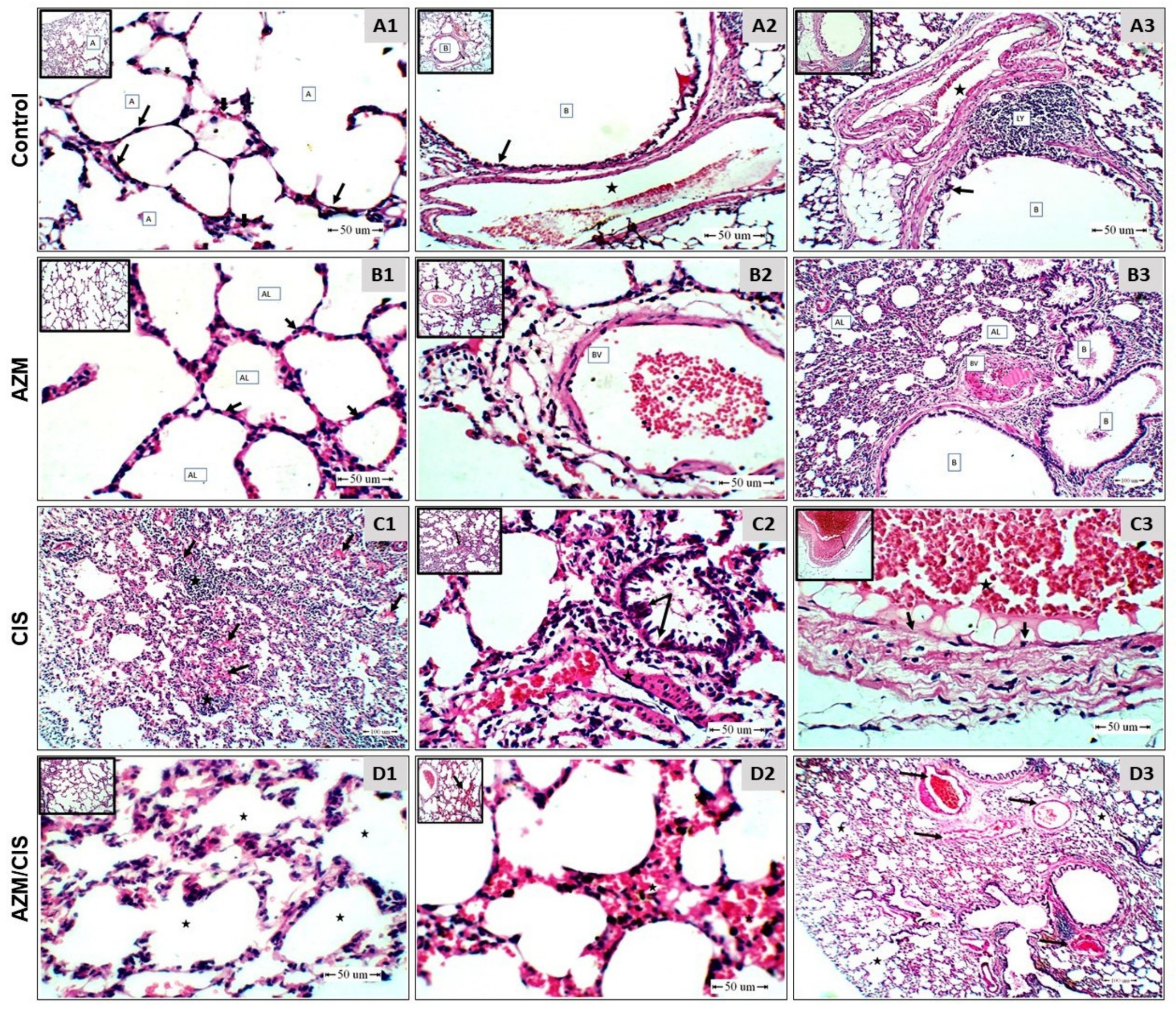
2.1. AZM Mitigates CIS-Induced Lung Injury
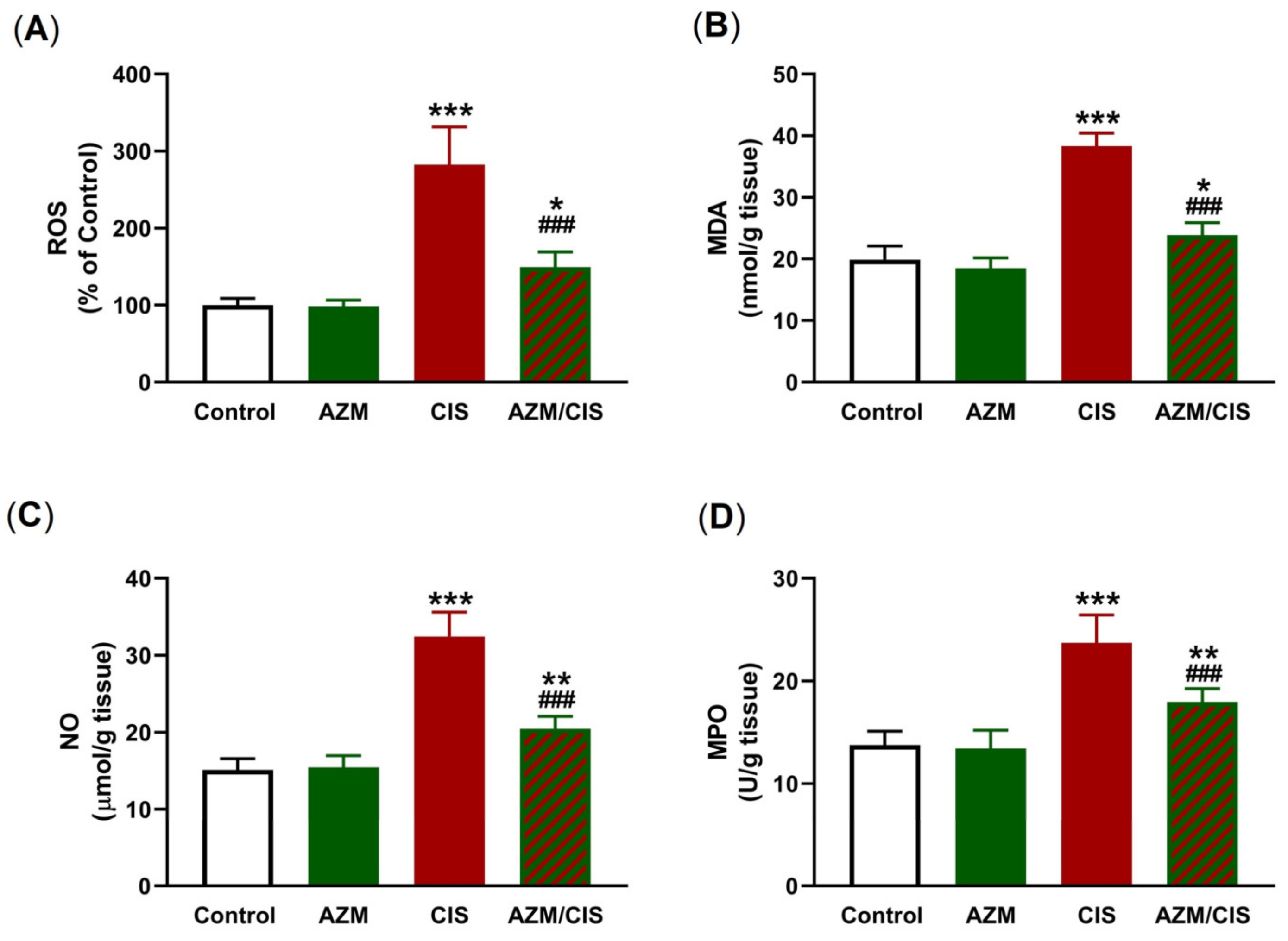
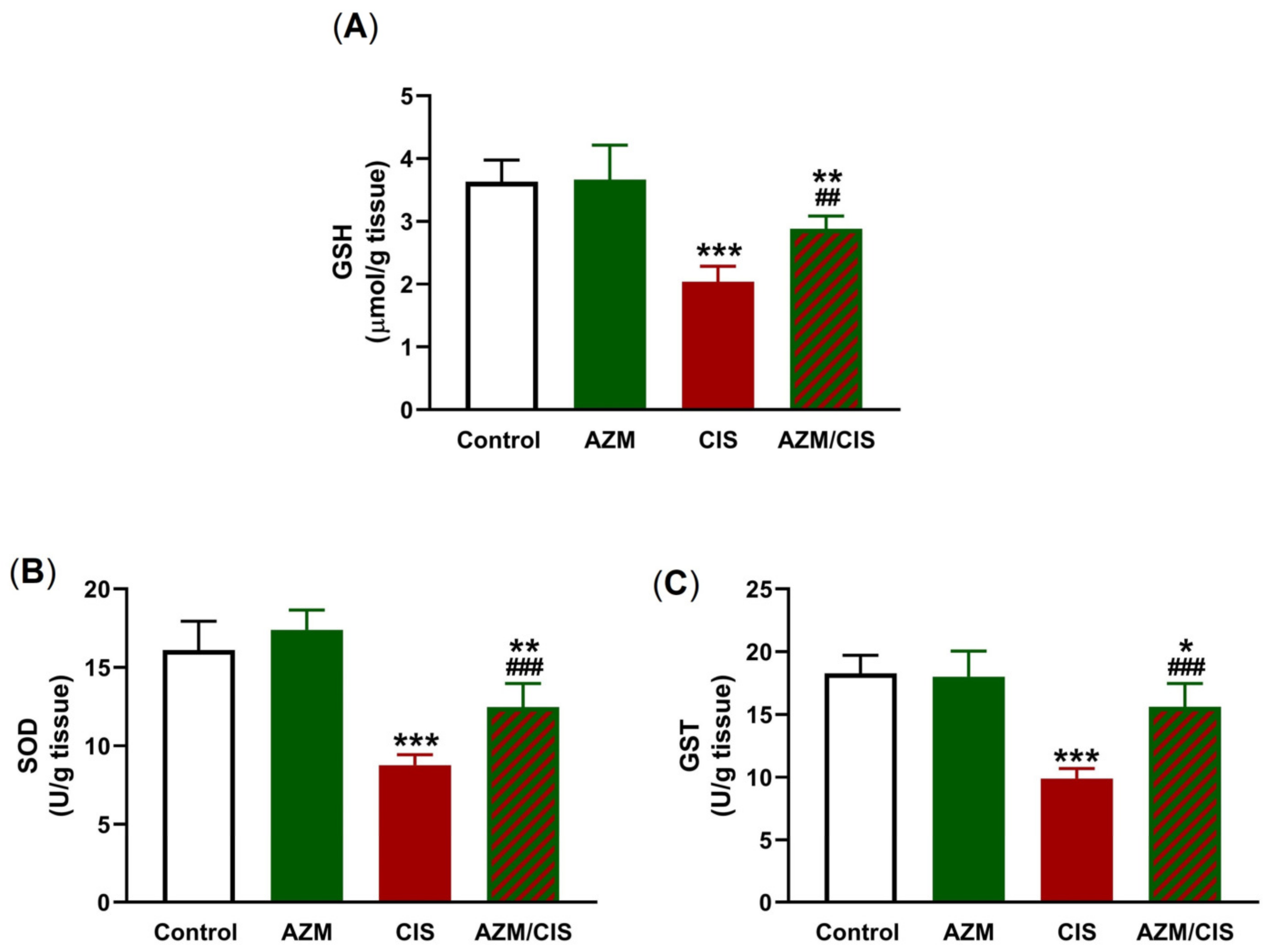
2.2. AZM Attenuates CIS-Induced Oxidative Stress
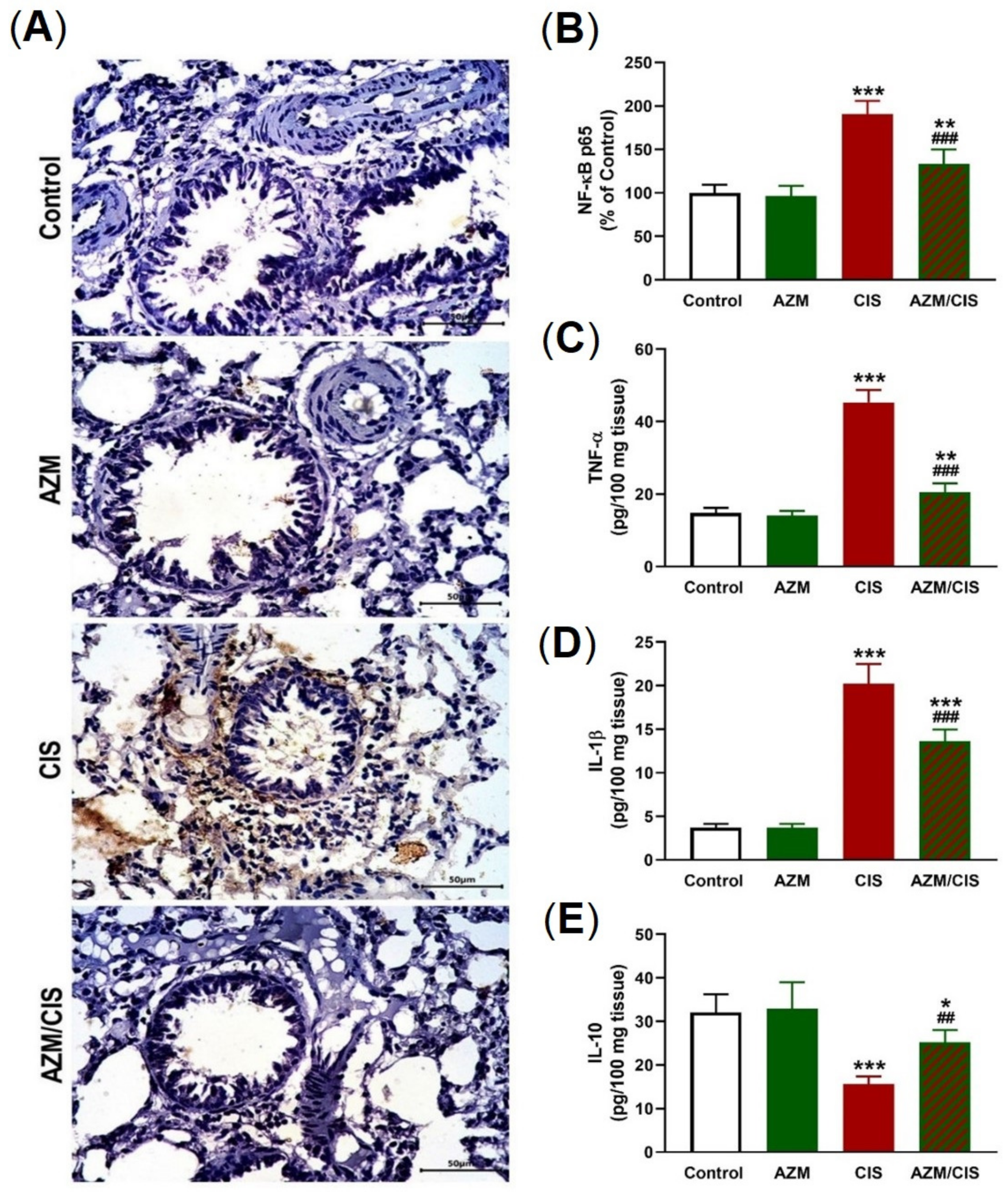
2.3. AZM Prevents CIS-Induced Inflammation
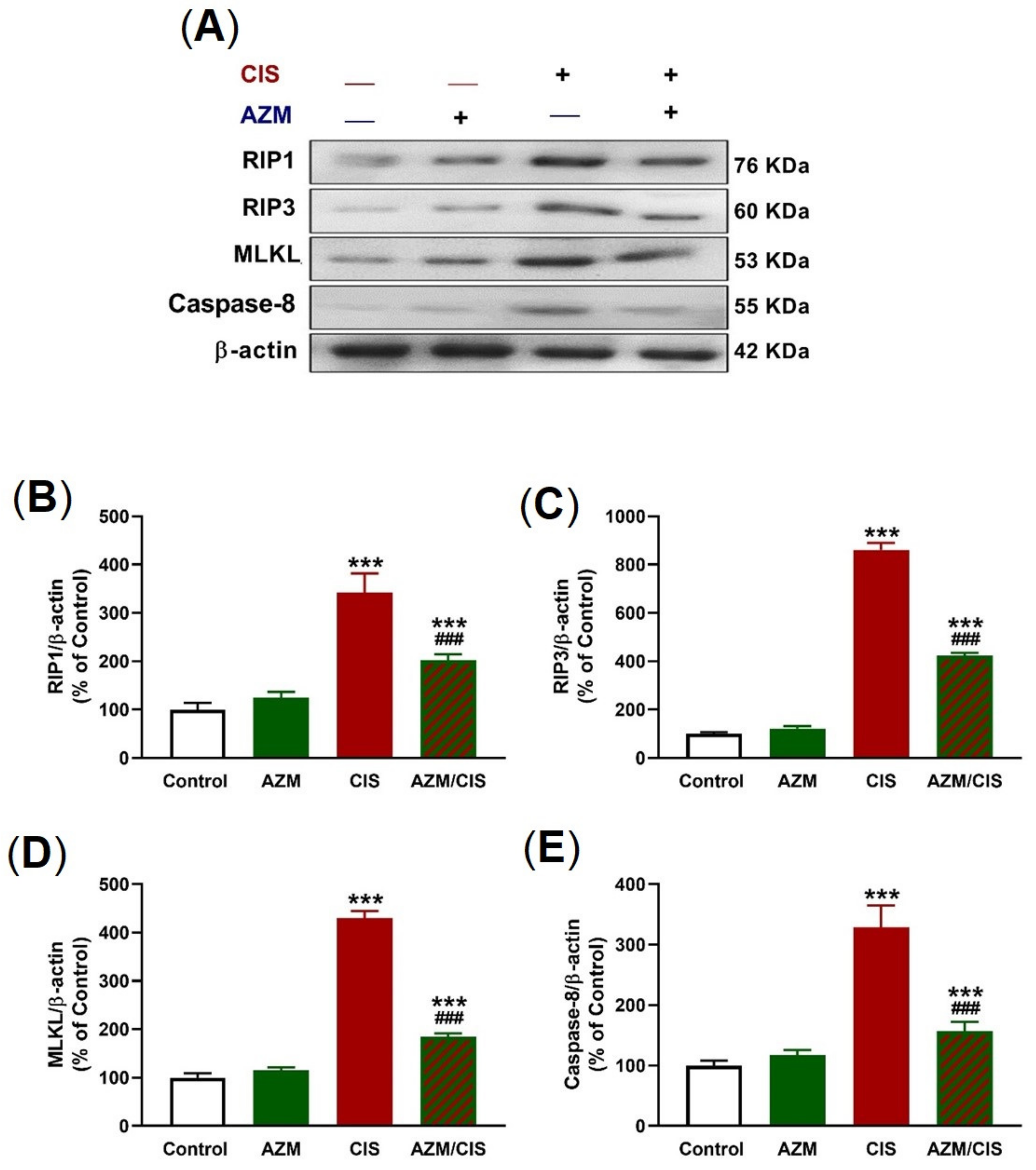
2.4. AZM Prevents Necroptosis in the Lungs of CIS-Treated Rats
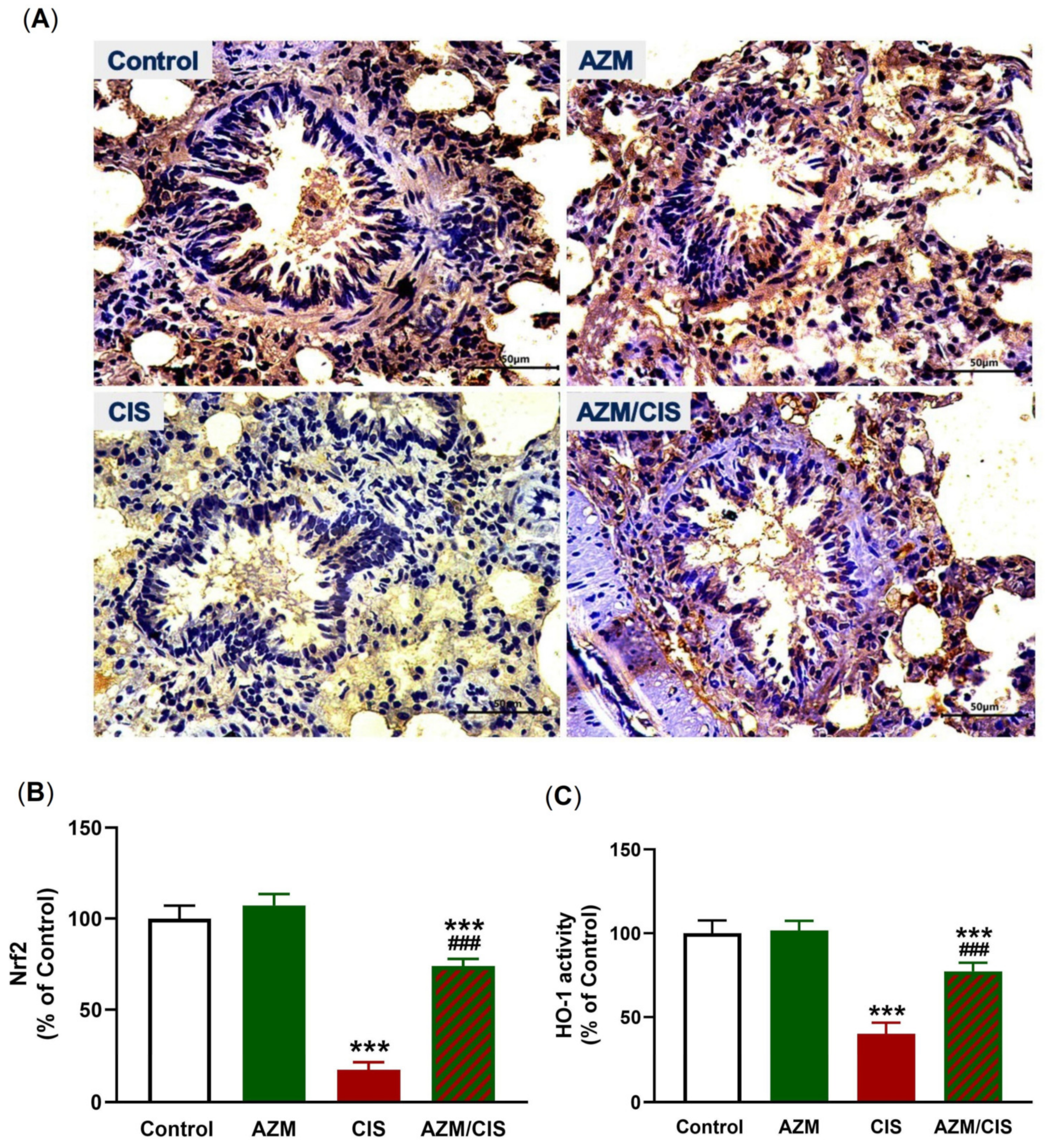
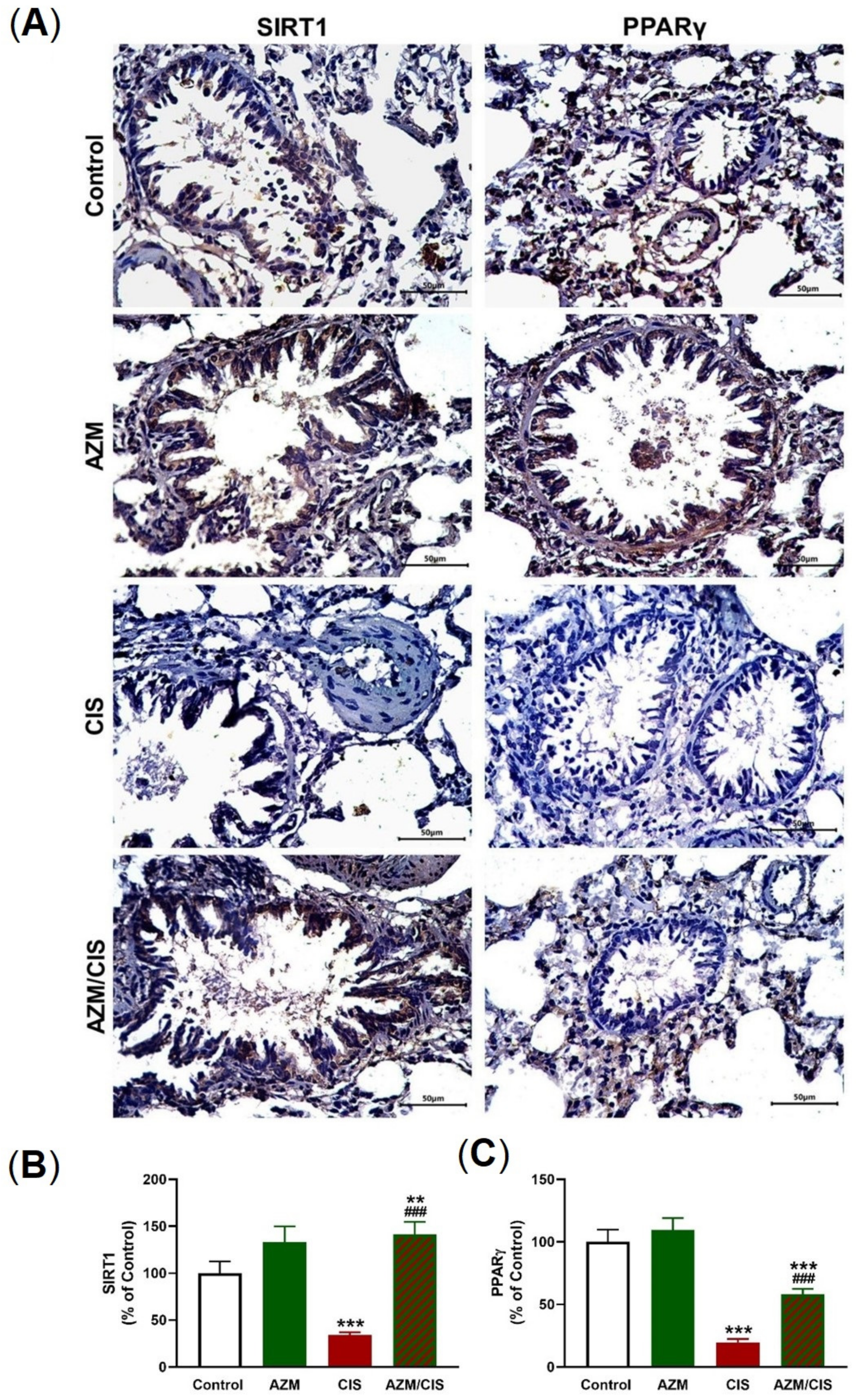
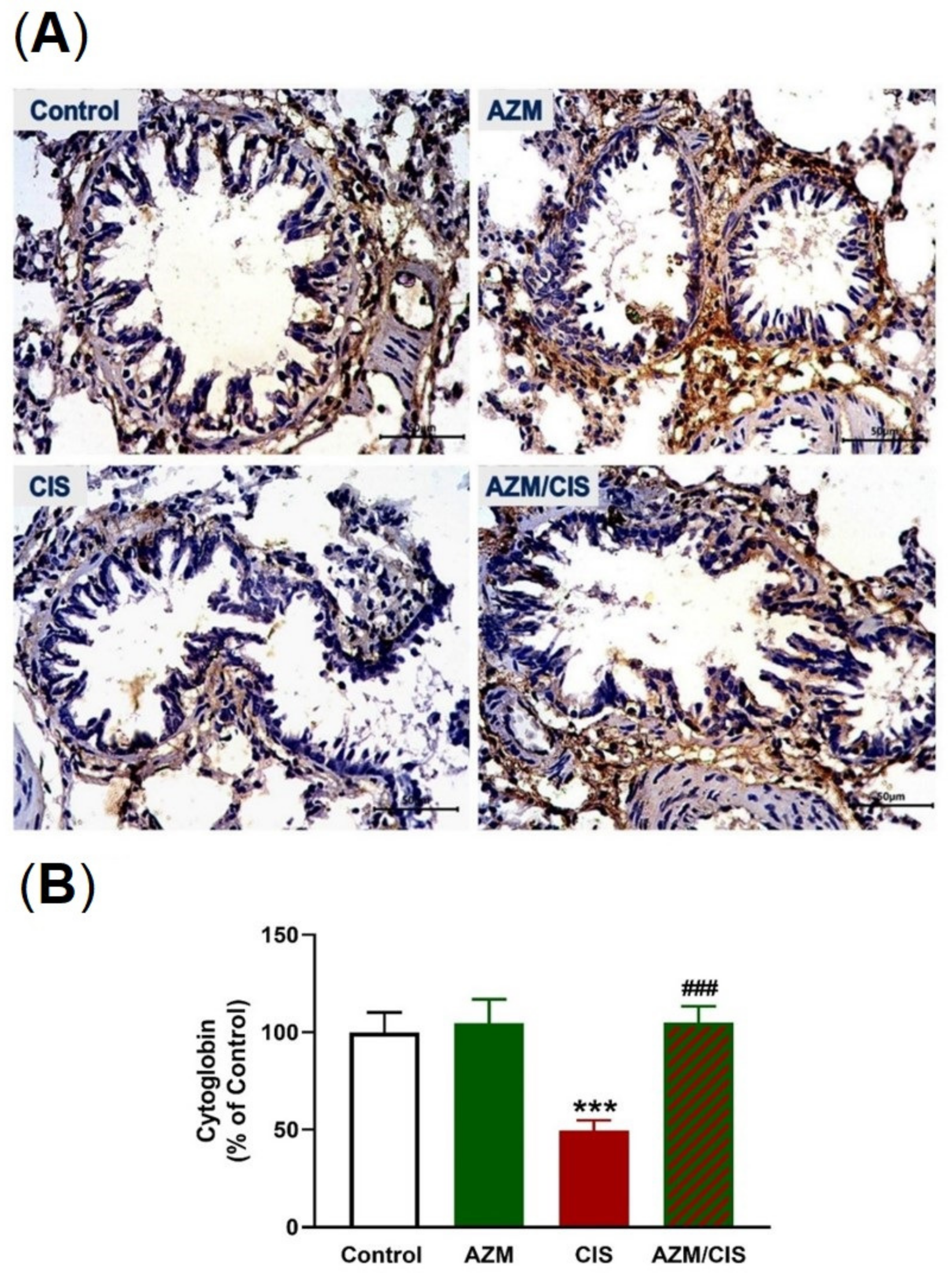
2.5. AZM Upregulates Nrf2/HO-1 Signaling, SIRT1, PPARγ, and Cytoglobin (Cygb) in CIS-Treated Rats
2.6. AZM Decreases Ang II and Increases Ang (1-7) in CIS-Administered Rats
3. Discussion
4. Materials and Methods
4.1. Animals and Treatments
4.2. Histopathology and IHC Examination
4.3. Biochemical Assays
4.4. Western Blotting
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorganic Chem. 2019, 88, 102925. [Google Scholar] [CrossRef] [PubMed]
- Tsang, R.Y.; Al-Fayea, T.; Au, H.J. Cisplatin overdose: Toxicities and management. Drug Saf. 2009, 32, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Ideguchi, H.; Kojima, K.; Hirosako, S.; Ichiyasu, H.; Fujii, K.; Kohrogi, H. Cisplatin-induced eosinophilic pneumonia. Case Rep. Pulmonol. 2014, 2014, 209732. [Google Scholar] [CrossRef] [PubMed]
- Kovach, M.A.; Standiford, T.J. Toll like receptors in diseases of the lung. Int. Immunopharmacol. 2011, 11, 1399–1406. [Google Scholar] [CrossRef]
- Aladaileh, S.H.; Al-Swailmi, F.K.; Abukhalil, M.H.; Ahmeda, A.F.; Mahmoud, A.M. Punicalagin prevents cisplatin-induced nephrotoxicity by attenuating oxidative stress, inflammatory response, and apoptosis in rats. Life Sci. 2021, 286, 120071. [Google Scholar] [CrossRef]
- Brozovic, A.; Ambriović-Ristov, A.; Osmak, M. The relationship between cisplatin-induced reactive oxygen species, glutathione, and BCL-2 and resistance to cisplatin. Crit. Rev. Toxicol. 2010, 40, 347–359. [Google Scholar] [CrossRef]
- Unver, E.; Tosun, M.; Olmez, H.; Kuzucu, M.; Cimen, F.K.; Suleyman, Z. The Effect of Taxifolin on Cisplatin-Induced Pulmonary Damage in Rats: A Biochemical and Histopathological Evaluation. Mediat. Inflamm. 2019, 2019, 3740867. [Google Scholar] [CrossRef]
- Geyikoglu, F.; Isikgoz, H.; Onalan, H.; Colak, S.; Cerig, S.; Bakir, M.; Hosseinigouzdagani, M.; Koc, K.; Erol, H.S.; Saglam, Y.S.; et al. Impact of high-dose oleuropein on cisplatin-induced oxidative stress, genotoxicity and pathological changes in rat stomach and lung. J. Asian Nat. Prod. Res. 2017, 19, 1214–1231. [Google Scholar] [CrossRef]
- Han, Y.K.; Kim, J.S.; Jang, G.; Park, K.M. Cisplatin induces lung cell cilia disruption and lung damage via oxidative stress. Free Radic. Biol. Med. 2021, 177, 270–277. [Google Scholar] [CrossRef]
- Zhang, Q.; Lenardo, M.J.; Baltimore, D. 30 Years of NF-κB: A Blossoming of Relevance to Human Pathobiology. Cell 2017, 168, 37–57. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Gaynor, R.B. Role of the NF-kB pathway in the pathogenesis of human disease states. Curr. Mol. Med. 2001, 1, 287–296. [Google Scholar] [CrossRef]
- Berghe, T.V.; Vanlangenakker, N.; Parthoens, E.; Deckers, W.; Devos, M.; Festjens, N.; Guerin, C.J.; Brunk, U.T.; Declercq, W.; Vandenabeele, P. Necroptosis, necrosis and secondary necrosis converge on similar cellular disintegration features. Cell Death Differ. 2010, 17, 922–930. [Google Scholar] [CrossRef]
- Faust, H.; Mangalmurti, N.S. Collateral damage: Necroptosis in the development of lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L215–L225. [Google Scholar] [CrossRef]
- Lu, Z.; Van Eeckhoutte, H.P.; Liu, G.; Nair, P.M.; Jones, B.; Gillis, C.M.; Nalkurthi, B.C.; Verhamme, F.; Buyle-Huybrecht, T.; Vandenabeele, P.; et al. Necroptosis Signaling Promotes Inflammation, Airway Remodeling, and Emphysema in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2021, 204, 667–681. [Google Scholar] [CrossRef]
- Declercq, W.; Vanden Berghe, T.; Vandenabeele, P. RIP kinases at the crossroads of cell death and survival. Cell 2009, 138, 229–232. [Google Scholar] [CrossRef]
- Chen, K.Y.; Chang, C.Y.; Hsu, H.J.; Shih, H.J.; Huang, I.T.; Patel, H.H.; Huang, C.J. Tumor Necrosis Factor-α Mediates Lung Injury in the Early Phase of Endotoxemia. Pharmaceuticals 2022, 15, 287. [Google Scholar] [CrossRef]
- Wang, H.; Sun, L.; Su, L.; Rizo, J.; Liu, L.; Wang, L.F.; Wang, F.S.; Wang, X. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol. Cell 2014, 54, 133–146. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, R.; Zhong, J.; Ying, Y.; Wang, W.; Cao, Y.; Cai, H.; Li, X.; Shuai, J.; Han, J. Mosaic composition of RIP1-RIP3 signalling hub and its role in regulating cell death. Nat. Cell Biol. 2022, 24, 471–482. [Google Scholar] [CrossRef]
- Li, W.; Yuan, X.; Nordgren, G.; Dalen, H.; Dubowchik, G.M.; Firestone, R.A.; Brunk, U.T. Induction of cell death by the lysosomotropic detergent MSDH. FEBS Lett. 2000, 470, 35–39. [Google Scholar] [CrossRef]
- Kearney, C.J.; Martin, S.J. An Inflammatory Perspective on Necroptosis. Mol. Cell 2017, 65, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Persson, H.L.; Vainikka, L.K. Increased Lysosomal Membrane Permeabilization in Oxidant-exposed Macrophages of Human Fibrotic Lungs. J. Cell Death 2013, 6, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Satta, S.; Mahmoud, A.M.; Wilkinson, F.L.; Yvonne Alexander, M.; White, S.J. The Role of Nrf2 in Cardiovascular Function and Disease. Oxidative Med. Cell. Longev. 2017, 2017, 9237263. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Gao, Y.; Ci, X. Role of Nrf2 and Its Activators in Respiratory Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 7090534. [Google Scholar] [CrossRef]
- Haigis, M.C.; Guarente, L.P. Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006, 20, 2913–2921. [Google Scholar] [CrossRef]
- Raynes, R.; Brunquell, J.; Westerheide, S.D. Stress Inducibility of SIRT1 and Its Role in Cytoprotection and Cancer. Genes Cancer 2013, 4, 172–182. [Google Scholar] [CrossRef]
- Chen, Q.; Ma, J.; Yang, X.; Li, Q.; Lin, Z.; Gong, F. SIRT1 Mediates Effects of FGF21 to Ameliorate Cisplatin-Induced Acute Kidney Injury. Front. Pharmacol. 2020, 11, 241. [Google Scholar] [CrossRef]
- Fu, C.; Hao, S.; Xu, X.; Zhou, J.; Liu, Z.; Lu, H.; Wang, L.; Jin, W.; Li, S. Activation of SIRT1 ameliorates LPS-induced lung injury in mice via decreasing endothelial tight junction permeability. Acta Pharmacol. Sin. 2019, 40, 630–641. [Google Scholar] [CrossRef]
- Carvalho, M.V.; Gonçalves-de-Albuquerque, C.F.; Silva, A.R. PPAR Gamma: From Definition to Molecular Targets and Therapy of Lung Diseases. Int. J. Mol. Sci. 2021, 22, 805. [Google Scholar] [CrossRef]
- Mateu, A.; Ramudo, L.; Manso, M.A.; De Dios, I. Cross-talk between TLR4 and PPARgamma pathways in the arachidonic acid-induced inflammatory response in pancreatic acini. Int. J. Biochem. Cell Biol. 2015, 69, 132–141. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Al Dera, H.S. 18β-Glycyrrhetinic acid exerts protective effects against cyclophosphamide-induced hepatotoxicity: Potential role of PPARγ and Nrf2 upregulation. Genes Nutr. 2015, 10, 1–13. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Abd El-Ghafar, O.A.M.; Alzoghaibi, M.A.; Hassanein, E.H.M. Agomelatine prevents gentamicin nephrotoxicity by attenuating oxidative stress and TLR-4 signaling, and upregulating PPARγ and SIRT1. Life Sci. 2021, 278, 119600. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Germoush, M.O.; Alotaibi, M.F.; Hussein, O.E. Possible involvement of Nrf2 and PPARgamma up-regulation in the protective effect of umbelliferone against cyclophosphamide-induced hepatotoxicity. Biomed Pharm. 2017, 86, 297–306. [Google Scholar] [CrossRef]
- Bakheit, A.H.; Al-Hadiya, B.M.; Abd-Elgalil, A.A. Azithromycin. In Profiles of Drug Substances, Excipients, and Related Methodology; Brittain, H.G., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 39, pp. 1–40. [Google Scholar] [CrossRef]
- Venditto, V.J.; Haydar, D.; Abdel-Latif, A.; Gensel, J.C.; Anstead, M.I.; Pitts, M.G.; Creameans, J.; Kopper, T.J.; Peng, C.; Feola, D.J. Immunomodulatory Effects of Azithromycin Revisited: Potential Applications to COVID-19. Front. Immunol. 2021, 12, 574425. [Google Scholar] [CrossRef]
- World Health Organization. World Health Organization Model List of Essential Medicines, 22nd ed.; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Li, H.; Liu, D.H.; Chen, L.L.; Zhao, Q.; Yu, Y.Z.; Ding, J.J.; Miao, L.Y.; Xiao, Y.L.; Cai, H.R.; Zhang, D.P.; et al. Meta-analysis of the adverse effects of long-term azithromycin use in patients with chronic lung diseases. Antimicrob. Agents Chemother. 2014, 58, 511–517. [Google Scholar] [CrossRef]
- Gautret, P.; Lagier, J.C.; Parola, P.; Hoang, V.T.; Meddeb, L.; Mailhe, M.; Doudier, B.; Courjon, J.; Giordanengo, V.; Vieira, V.E.; et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents 2020, 56, 105949. [Google Scholar] [CrossRef]
- Ray, W.A.; Murray, K.T.; Hall, K.; Arbogast, P.G.; Stein, C.M. Azithromycin and the risk of cardiovascular death. N. Engl. J. Med. 2012, 366, 1881–1890. [Google Scholar] [CrossRef]
- Firth, A.; Prathapan, P. Azithromycin: The First Broad-spectrum Therapeutic. Eur. J. Med. Chem. 2020, 207, 112739. [Google Scholar] [CrossRef]
- Thomsen, M.B.; Beekman, J.D.; Attevelt, N.J.; Takahara, A.; Sugiyama, A.; Chiba, K.; Vos, M.A. No proarrhythmic properties of the antibiotics Moxifloxacin or Azithromycin in anaesthetized dogs with chronic-AV block. Br. J. Pharmacol. 2006, 149, 1039–1048. [Google Scholar] [CrossRef]
- Takeda, A.; Takano, N.; Kokuba, H.; Hino, H.; Moriya, S.; Abe, A.; Hiramoto, M.; Tsukahara, K.; Miyazawa, K. Macrolide antibiotics enhance the antitumor effect of lansoprazole resulting in lysosomal membrane permeabilization-associated cell death. Int. J. Oncol. 2020, 57, 1280–1292. [Google Scholar] [CrossRef]
- Toriyama, K.; Takano, N.; Kokuba, H.; Kazama, H.; Moriya, S.; Hiramoto, M.; Abe, S.; Miyazawa, K. Azithromycin enhances the cytotoxicity of DNA-damaging drugs via lysosomal membrane permeabilization in lung cancer cells. Cancer Sci. 2021, 112, 3324–3337. [Google Scholar] [CrossRef]
- Atwa, A.M.; Abd El-Ghafar, O.A.M.; Hassanein, E.H.M.; Mahdi, S.E.; Sayed, G.A.; Alruhaimi, R.S.; Alqhtani, H.A.; Alotaibi, M.F.; Mahmoud, A.M. Candesartan Attenuates Cisplatin-Induced Lung Injury by Modulating Oxidative Stress, Inflammation, and TLR-4/NF-κB, JAK1/STAT3, and Nrf2/HO-1 Signaling. Pharmaceuticals 2022, 15, 1222. [Google Scholar]
- Auten, R.L.; Davis, J.M. Oxygen Toxicity and Reactive Oxygen Species: The Devil Is in the Details. Pediatr. Res. 2009, 66, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Afsar, T.; Razak, S.; Almajwal, A.; Khan, M.R. Acacia hydaspica R. Parker ameliorates cisplatin induced oxidative stress, DNA damage and morphological alterations in rat pulmonary tissue. BMC Complement. Altern. Med. 2018, 18, 49. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Hu, H.; Keat, K. Myeloperoxidase and associated lung disease: Review of the latest developments. Int. J. Rheum. Dis. 2021, 24, 1460–1466. [Google Scholar] [CrossRef]
- Zhang, R.; Brennan, M.L.; Shen, Z.; MacPherson, J.C.; Schmitt, D.; Molenda, C.E.; Hazen, S.L. Myeloperoxidase functions as a major enzymatic catalyst for initiation of lipid peroxidation at sites of inflammation. J. Biol. Chem. 2002, 277, 46116–46122. [Google Scholar] [CrossRef]
- Jiang, D.; Liang, J.; Li, Y.; Noble, P.W. The role of Toll-like receptors in non-infectious lung injury. Cell Res. 2006, 16, 693–701. [Google Scholar] [CrossRef]
- Popovic, M.; Janicijevic-Hudomal, S.; Kaurinovic, B.; Rasic, J.; Trivic, S. Antioxidant effects of some drugs on ethanol-induced ulcers. Molecules 2009, 14, 816–826. [Google Scholar] [CrossRef]
- Chen, M.; Yang, T.; Meng, X.; Sun, T. Azithromycin attenuates cigarette smoke extract-induced oxidative stress injury in human alveolar epithelial cells. Mol. Med. Rep. 2015, 11, 3414–3422. [Google Scholar] [CrossRef]
- Cuevas, S.; Yang, Y.; Armando, I.; Jose, P.A. Mechanisms involved in the antioxidant properties of azithromycin in lung epithelial cells stimulated with cigarette smoke extract. FASEB J. 2016, 30, 982.2. [Google Scholar]
- Bosnar, M.; Čužić, S.; Bošnjak, B.; Nujić, K.; Ergović, G.; Marjanović, N.; Pašalić, I.; Hrvačić, B.; Polančec, D.; Glojnarić, I.; et al. Azithromycin inhibits macrophage interleukin-1β production through inhibition of activator protein-1 in lipopolysaccharide-induced murine pulmonary neutrophilia. Int. Immunopharmacol. 2011, 11, 424–434. [Google Scholar] [CrossRef]
- Wang, J.; Xie, L.; Wang, S.; Lin, J.; Liang, J.; Xu, J. Azithromycin promotes alternatively activated macrophage phenotype in systematic lupus erythematosus via PI3K/Akt signaling pathway. Cell Death Dis. 2018, 9, 1080. [Google Scholar] [CrossRef]
- Vos, R.; Verleden, S.E.; Ruttens, D.; Vandermeulen, E.; Bellon, H.; Neyrinck, A.; Van Raemdonck, D.E.; Yserbyt, J.; Dupont, L.J.; Verbeken, E.K.; et al. Azithromycin and the treatment of lymphocytic airway inflammation after lung transplantation. Am. J. Transpl. 2014, 14, 2736–2748. [Google Scholar] [CrossRef]
- Khezri, M.R.; Zolbanin, N.M.; Ghasemnejad-Berenji, M.; Jafari, R. Azithromycin: Immunomodulatory and antiviral properties for SARS-CoV-2 infection. Eur. J. Pharmacol. 2021, 905, 174191. [Google Scholar] [CrossRef]
- Aghai, Z.H.; Kode, A.; Saslow, J.G.; Nakhla, T.; Farhath, S.; Stahl, G.E.; Eydelman, R.; Strande, L.; Leone, P.; Rahman, I. Azithromycin suppresses activation of nuclear factor-kappa B and synthesis of pro-inflammatory cytokines in tracheal aspirate cells from premature infants. Pediatr. Res. 2007, 62, 483–488. [Google Scholar] [CrossRef]
- Stellari, F.F.; Sala, A.; Donofrio, G.; Ruscitti, F.; Caruso, P.; Topini, T.M.; Francis, K.P.; Li, X.; Carnini, C.; Civelli, M.; et al. Azithromycin inhibits nuclear factor-κB activation during lung inflammation: An in vivo imaging study. Pharm. Res. Perspect. 2014, 2, e00058. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Sami, D.H.; Soliman, A.S.; Khowailed, A.A.; Hassanein, E.H.M.; Kamel, E.M.; Mahmoud, A.M. 7-hydroxycoumarin modulates Nrf2/HO-1 and microRNA-34a/SIRT1 signaling and prevents cisplatin-induced oxidative stress, inflammation, and kidney injury in rats. Life Sci. 2022, 310, 121104. [Google Scholar] [CrossRef]
- Alanezi, A.A.; Almuqati, A.F.; Alfwuaires, M.A.; Alasmari, F.; Namazi, N.I.; Althunibat, O.Y.; Mahmoud, A.M. Taxifolin Prevents Cisplatin Nephrotoxicity by Modulating Nrf2/HO-1 Pathway and Mitigating Oxidative Stress and Inflammation in Mice. Pharmaceuticals 2022, 15, 1310. [Google Scholar] [CrossRef]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, S.P.; Reddy, A.T.; Banno, A.; Reddy, R.C. Airway Epithelial Cell Peroxisome Proliferator-Activated Receptor γ Regulates Inflammation and Mucin Expression in Allergic Airway Disease. J. Immunol. 2018, 201, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, H.E.; Thatcher, T.H.; Olsen, K.C.; Garcia-Bates, T.M.; Baglole, C.J.; Kottmann, R.M.; Strong, E.R.; Phipps, R.P.; Sime, P.J. Peroxisome proliferator-activated receptor-gamma ligands induce heme oxygenase-1 in lung fibroblasts by a PPARgamma-independent, glutathione-dependent mechanism. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 297, L912–L919. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cho, R.L.; Lin, W.N.; Wang, C.Y.; Yang, C.C.; Hsiao, L.D.; Lin, C.C.; Yang, C.M. Heme oxygenase-1 induction by rosiglitazone via PKCα/AMPKα/p38 MAPKα/SIRT1/PPARγ pathway suppresses lipopolysaccharide-mediated pulmonary inflammation. Biochem. Pharmacol. 2018, 148, 222–237. [Google Scholar] [CrossRef]
- Jia, L.; Wang, J.; Cao, H.; Zhang, X.; Rong, W.; Xu, Z. Activation of PGC-1α and Mitochondrial Biogenesis Protects Against Prenatal Hypoxic-ischemic Brain Injury. Neuroscience 2020, 432, 63–72. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, F.; Lei, X.; Dong, W. Resveratrol alleviates alveolar epithelial cell injury induced by hyperoxia by reducing apoptosis and mitochondrial dysfunction. Exp. Biol. Med. 2021, 246, 596–606. [Google Scholar] [CrossRef]
- Wang, X.L.; Li, T.; Li, J.H.; Miao, S.Y.; Xiao, X.Z. The Effects of Resveratrol on Inflammation and Oxidative Stress in a Rat Model of Chronic Obstructive Pulmonary Disease. Molecules 2017, 22, 1529. [Google Scholar] [CrossRef]
- McRonald, F.E.; Risk, J.M.; Hodges, N.J. Protection from intracellular oxidative stress by cytoglobin in normal and cancerous oesophageal cells. PLoS ONE 2012, 7, e30587. [Google Scholar] [CrossRef]
- Hodges, N.J.; Innocent, N.; Dhanda, S.; Graham, M. Cellular protection from oxidative DNA damage by over-expression of the novel globin cytoglobin in vitro. Mutagenesis 2008, 23, 293–298. [Google Scholar] [CrossRef]
- Zweier, J.L.; Hemann, C.; Kundu, T.; Ewees, M.G.; Khaleel, S.A.; Samouilov, A.; Ilangovan, G.; El-Mahdy, M.A. Cytoglobin has potent superoxide dismutase function. Proc. Natl. Acad. Sci. USA 2021, 118, e2105053118. [Google Scholar] [CrossRef]
- Santos, R.A.S.; Sampaio, W.O.; Alzamora, A.C.; Motta-Santos, D.; Alenina, N.; Bader, M.; Campagnole-Santos, M.J. The ACE2/Angiotensin-(1-7)/MAS Axis of the Renin-Angiotensin System: Focus on Angiotensin-(1-7). Physiol. Rev. 2018, 98, 505–553. [Google Scholar] [CrossRef]
- Lelis, D.F.; Freitas, D.F.; Machado, A.S.; Crespo, T.S.; Santos, S.H.S. Angiotensin-(1-7), Adipokines and Inflammation. Metab. Clin. Exp. 2019, 95, 36–45. [Google Scholar] [CrossRef]
- Hitomi, H.; Kiyomoto, H.; Nishiyama, A. Angiotensin II and oxidative stress. Curr. Opin. Cardiol. 2007, 22, 311–315. [Google Scholar] [CrossRef]
- Mateo, T.; Abu Nabah, Y.N.; Abu Taha, M.; Mata, M.; Cerdá-Nicolás, M.; Proudfoot, A.E.; Stahl, R.A.; Issekutz, A.C.; Cortijo, J.; Morcillo, E.J.; et al. Angiotensin II-induced mononuclear leukocyte interactions with arteriolar and venular endothelium are mediated by the release of different CC chemokines. J. Immunol. 2006, 176, 5577–5586. [Google Scholar] [CrossRef]
- Funakoshi, Y.; Ichiki, T.; Shimokawa, H.; Egashira, K.; Takeda, K.; Kaibuchi, K.; Takeya, M.; Yoshimura, T.; Takeshita, A. Rho-kinase mediates angiotensin II-induced monocyte chemoattractant protein-1 expression in rat vascular smooth muscle cells. Hypertension 2001, 38, 100–104. [Google Scholar] [CrossRef]
- Nair, A.R.; Ebenezer, P.J.; Saini, Y.; Francis, J. Angiotensin II-induced hypertensive renal inflammation is mediated through HMGB1-TLR4 signaling in rat tubulo-epithelial cells. Exp. Cell Res. 2015, 335, 238–247. [Google Scholar] [CrossRef]
- Ferreira, A.J.; Bader, M.; Santos, R.A. Therapeutic targeting of the angiotensin-converting enzyme 2/Angiotensin-(1-7)/Mas cascade in the renin-angiotensin system: A patent review. Expert Opin. Ther. Pat. 2012, 22, 567–574. [Google Scholar] [CrossRef]
- Liu, Y.; Pu, Y.; Li, D.; Zhou, L.; Wan, L. Azithromycin ameliorates airway remodeling via inhibiting airway epithelium apoptosis. Life Sci. 2017, 170, 1–8. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Suvarna, K.S.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Hozayen, W.G.; Mahmoud, A.M.; Desouky, E.M.; El-Nahass, E.S.; Soliman, H.A.; Farghali, A.A. Cardiac and pulmonary toxicity of mesoporous silica nanoparticles is associated with excessive ROS production and redox imbalance in Wistar rats. Biomed Pharm. 2019, 109, 2527–2538. [Google Scholar] [CrossRef]
- Mihara, M.; Uchiyama, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, H.A.C.; Dymock, J.F. Determination of nitrite in water. Analyst 1961, 86, 414–416. [Google Scholar]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Keen, J.H.; Habig, W.H.; Jakoby, W.B. Mechanism for the several activities of the glutathione S-transferases. J. Biol. Chem. 1976, 251, 6183–6188. [Google Scholar] [CrossRef]
- Abraham, N.G.; Lutton, J.D.; Levere, R.D. Heme metabolism and erythropoiesis in abnormal iron states: Role of delta-aminolevulinic acid synthase and heme oxygenase. Exp. Hematol. 1985, 13, 838–843. [Google Scholar]
- Krawisz, J.; Sharon, P.; Stenson, W.F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity: Assessment of inflammation in rat and hamster models. Gastroenterology 1984, 87, 1344–1350. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassanein, E.H.M.; Sayed, G.A.; Alzoghaibi, A.M.; Alammar, A.S.; Abdel-Wahab, B.A.; Abd El-Ghafar, O.A.M.; Mahdi, S.E.; Atwa, A.M.; Alzoghaibi, M.A.; Mahmoud, A.M. Azithromycin Mitigates Cisplatin-Induced Lung Oxidative Stress, Inflammation and Necroptosis by Upregulating SIRT1, PPARγ, and Nrf2/HO-1 Signaling. Pharmaceuticals 2023, 16, 52. https://doi.org/10.3390/ph16010052
Hassanein EHM, Sayed GA, Alzoghaibi AM, Alammar AS, Abdel-Wahab BA, Abd El-Ghafar OAM, Mahdi SE, Atwa AM, Alzoghaibi MA, Mahmoud AM. Azithromycin Mitigates Cisplatin-Induced Lung Oxidative Stress, Inflammation and Necroptosis by Upregulating SIRT1, PPARγ, and Nrf2/HO-1 Signaling. Pharmaceuticals. 2023; 16(1):52. https://doi.org/10.3390/ph16010052
Chicago/Turabian StyleHassanein, Emad H. M., Ghadir A. Sayed, Abdullah M. Alzoghaibi, Abdalmohsen S. Alammar, Basel A. Abdel-Wahab, Omnia A. M. Abd El-Ghafar, Somya E. Mahdi, Ahmed M. Atwa, Mohammed A. Alzoghaibi, and Ayman M. Mahmoud. 2023. "Azithromycin Mitigates Cisplatin-Induced Lung Oxidative Stress, Inflammation and Necroptosis by Upregulating SIRT1, PPARγ, and Nrf2/HO-1 Signaling" Pharmaceuticals 16, no. 1: 52. https://doi.org/10.3390/ph16010052
APA StyleHassanein, E. H. M., Sayed, G. A., Alzoghaibi, A. M., Alammar, A. S., Abdel-Wahab, B. A., Abd El-Ghafar, O. A. M., Mahdi, S. E., Atwa, A. M., Alzoghaibi, M. A., & Mahmoud, A. M. (2023). Azithromycin Mitigates Cisplatin-Induced Lung Oxidative Stress, Inflammation and Necroptosis by Upregulating SIRT1, PPARγ, and Nrf2/HO-1 Signaling. Pharmaceuticals, 16(1), 52. https://doi.org/10.3390/ph16010052