Novel Siprulina platensis Bilosomes for Combating UVB Induced Skin Damage
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Characterization of Blank BS and SRP-BS
2.2. Percentage Entrapment Efficiency
2.3. Transmission Electron Microscopy
2.4. In Vitro Release Studies
2.5. Effect of Storage
2.6. In Vivo Studies
2.6.1. Skin Irritancy Test
2.6.2. UVB Exposure Test
2.6.3. Biochemical Analysis
Anti-Inflammatory Markers
Antioxidant Markers
Antiwrinkling Markers
2.7. Western Blotting
2.8. Histopathological Analysis and Immunohistochemical Staining
Limitations
3. Materials and Methods
3.1. Formulation of Blank and SPR-Loaded BS
3.2. In Vitro Characterization of Blank and SPR-Loaded BS
3.2.1. Particle Size and Zeta Potential Measurements
3.2.2. Entrapment Efficiency
3.2.3. In Vitro Drug Release
3.2.4. Morphological Examination by Transmission Electron Microscopy (TEM)
3.2.5. Stability during Storage
3.3. In Vivo Studies
3.3.1. Animals
3.3.2. Skin Irritation Test
- Group 1: served as a negative control group.
- Group 3: received 1 mL of blank BS that was applied once daily for 72 h.
- Group 4: received 1 mL of SPR suspension that was applied once daily for 72 h.
- Group 5: received 1 mL of SPR-BS that was applied once daily for 72 h.
3.3.3. UVB Exposure Test
- Group 1: Negative control group in which the dorsal skin was shaved while the UV lamp was turned off (non-UV-irradiated control).
- Group 2: Positive control group in which rats were subjected daily for 10 days to UVB irradiation (blank) [89].
- Group 3: Rats received blank BS topically 1 h prior to the UVB exposure for 10 days (UV + blank BS) [63].
- Group 4: Rats received SPR suspension topically 1 h prior to the UVB exposure for 10 days (UV + SPR suspension).
- Group 5: Rats received SPR-BS topically 1 h prior to the UVB exposure for 10 days (UV + SPR-BS).
3.3.4. Biochemical Analysis by Enzyme Linked Immunosorbent Assay
3.3.5. Western Blotting
3.3.6. Histopathological Study
3.3.7. Immunohistochemical Staining and Analysis
3.3.8. Quantitative Immunohistochemical Analysis
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.; Duan, E. Fighting against skin aging: The way from bench to bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Seleem, M.; Abulfadl, Y.; Hoffy, N.; Lotfy, N.M.; Ewida, H.A. Promising role of topical caffeine mesoporous gel in collagen resynthesis and UV protection through proline assessment. Future J. Pharm. Sci. 2022, 8, 1–11. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef]
- Dranseikienė, D.; Balčiūnaitė-Murzienė, G.; Karosienė, J.; Morudov, D.; Juodžiukynienė, N.; Hudz, N.; Gerbutavičienė, R.J.; Savickienė, N. Cyano-Phycocyanin: Mechanisms of Action on Human Skin and Future Perspectives in Medicine. Plants 2022, 11, 1249. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Ishida, K. Biological mechanisms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging I: Reduced skin elasticity, highly associated with enhanced dermal elastase activity, triggers wrinkling and sagging. Int. J. Mol. Sci. 2015, 16, 7753–7775. [Google Scholar] [CrossRef]
- Jo, K.; Bae, G.Y.; Cho, K.; Park, S.S.; Suh, H.J.; Hong, K.-B. An anthocyanin-enriched extract from vaccinium uliginosum improves signs of skin aging in UVB-induced photodamage. Antioxidants 2020, 9, 844. [Google Scholar] [CrossRef]
- Resende, D.I.; Ferreira, M.; Magalhães, C.; Lobo, J.S.; Sousa, E.; Almeida, I.F. Trends in the use of marine ingredients in anti-aging cosmetics. Algal Res. 2021, 55, 102273. [Google Scholar] [CrossRef]
- Guillerme, J.-B.; Couteau, C.; Coiffard, L. Applications for marine resources in cosmetics. Cosmetics 2017, 4, 35. [Google Scholar] [CrossRef]
- Uzair, B.; Liaqat, A.; Iqbal, H.; Menaa, B.; Razzaq, A.; Thiripuranathar, G.; Rana, N.F.; Menaa, F. Green and cost-effective synthesis of metallic nanoparticles by algae: Safe methods for translational medicine. Bioengineering 2020, 7, 129. [Google Scholar] [CrossRef]
- Khavari, F.; Saidijam, M.; Taheri, M.; Nouri, F. Microalgae: Therapeutic potentials and applications. Mol. Biol. Rep. 2021, 48, 4757–4765. [Google Scholar] [CrossRef]
- Dwivedi, S.; Ahmad, I.Z. A review of the emerging role of cyanobacteria-based nanoformulations for skin care: Opportunities and challenges. J. Appl. Biol. Biotechnol. 2022, 10, 210–218. [Google Scholar] [CrossRef]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. The potential use of marine microalgae and cyanobacteria in cosmetics and thalassotherapy. Cosmetics 2017, 4, 46. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuča, K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: An overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, S.A.; Shedid, E.S.; Saied, E.M.; Jassbi, A.R.; Jamebozorgi, F.H.; Rateb, M.E.; Du, M.; Abdel-Daim, M.; Kai, G.-Y.; Al-Hammady, M.; et al. Cyanobacteria—From the oceans to the potential biotechnological and biomedical applications. Mar. Drugs 2021, 19, 241. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, I.; Nardone, G.N.; Zanatta, S.; Bertin, W.; Amadio, E. Spirulina for skin care: A bright blue future. Cosmetics 2021, 8, 7. [Google Scholar] [CrossRef]
- Karimzadeh, K.; Tahergorabi, R.; Zahmatkesh, A. Synthesis of spirulina loaded chitosan nanoparticles from prawn, Macrobrachium nipponense shell for extending the shelf life of pike-perch (Sander lucioperca) fillet during refrigerated storage. J. Sci. Food Agric. 2022, 103, 92–107. [Google Scholar] [CrossRef]
- Capelli, B.; Cysewski, G.R. Potential health benefits of spirulina microalgae. Nutrafoods 2010, 9, 19–26. [Google Scholar] [CrossRef]
- Agrawal, M.; Bansal, S.; Chopra, K. Evaluation of the in vitro and in vivo antioxidant potentials of food grade Phycocyanin. J. Food Sci. Technol. 2021, 58, 4382–4390. [Google Scholar] [CrossRef]
- Menaa, F.; Wijesinghe, U.; Thiripuranathar, G.; Althobaiti, N.A.; Albalawi, A.E.; Khan, B.A.; Menaa, B. Marine Algae-Derived Bioactive Compounds: A New Wave of Nanodrugs? Mar. Drugs 2021, 19, 484. [Google Scholar] [CrossRef]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for high-value products towards human health and nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef]
- Monteverde, D.; Gómez-Consarnau, L.; Suffridge, C.; Sañudo-Wilhelmy, S.A. Life’s utilization of B vitamins on early Earth. Geobiology 2017, 15, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef] [PubMed]
- Deniz, I.; García-Vaquero, M.; Imamoglu, E. Trends in red biotechnology: Microalgae for pharmaceutical applications. In Microalgae-Based Biofuels and Bioproducts; Elsevier: Amsterdam, The Netherlands, 2017; pp. 429–460. [Google Scholar]
- Vigliante, I.; Mannino, G.; Maffei, M.E. OxiCyan®, a phytocomplex of bilberry (Vaccinium myrtillus) and spirulina (Spirulina platensis), exerts both direct antioxidant activity and modulation of ARE/Nrf2 pathway in HepG2 cells. J. Funct. Foods 2019, 61, 103508. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Barbieri Moro, G.M.; de Moraes Vaz Batista Filgueira, D.; Corsini, E.; Bertolin, T.E. The potential of spirulina and its bioactive metabolites as ingested agents for skin care. Ind. Biotechnol. 2017, 13, 244–252. [Google Scholar] [CrossRef]
- Dini, I.; Laneri, S. Nutricosmetics: A brief overview. Phytother. Res. 2019, 33, 3054–3063. [Google Scholar] [CrossRef]
- Krausz, A.; Gunn, H.; Friedman, A. The basic science of natural ingredients. J. Drugs Dermatol. JDD 2014, 13, 937–943, quiz 44. [Google Scholar]
- Bajpai, V.K.; Shukla, S.; Kang, S.-M.; Hwang, S.K.; Song, X.; Huh, Y.S.; Han, Y.-K. Developments of cyanobacteria for nano-marine drugs: Relevance of nanoformulations in cancer therapies. Mar. Drugs 2018, 16, 179. [Google Scholar] [CrossRef]
- Ahmed, S.; Kassem, M.A.; Sayed, S. Bilosomes as promising nanovesicular carriers for improved transdermal delivery: Construction, in vitro optimization, ex vivo permeation and in vivo evaluation. Int. J. Nanomed. 2020, 15, 9783. [Google Scholar] [CrossRef]
- Jesumani, V.; Du, H.; Aslam, M.; Pei, P.; Huang, N. Potential use of seaweed bioactive compounds in skincare—A review. Mar. Drugs 2019, 17, 688. [Google Scholar] [CrossRef]
- Conacher, M.; Alexander, J.; Brewer, J.M. Oral immunisation with peptide and protein antigens by formulation in lipid vesicles incorporating bile salts (bilosomes). Vaccine 2001, 19, 2965–2974. [Google Scholar] [CrossRef]
- Abdelalim, L.R.; Abdallah, O.Y.; Elnaggar, Y.S. High efficacy, rapid onset nanobiolosomes of sildenafil as a topical therapy for erectile dysfunction in aged rats. Int. J. Pharm. 2020, 591, 119978. [Google Scholar] [CrossRef] [PubMed]
- El-Nabarawi, M.A.; Shamma, R.N.; Farouk, F.; Nasralla, S.M. Bilosomes as a novel carrier for the cutaneous delivery for dapsone as a potential treatment of acne: Preparation, characterization and in vivo skin deposition assay. J. Liposome Res. 2020, 30, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mosallam, S.; Sheta, N.M.; Elshafeey, A.H.; Abdelbary, A.A. Fabrication of Highly Deformable Bilosomes for Enhancing the Topical Delivery of Terconazole: In Vitro Characterization, Microbiological Evaluation, and In Vivo Skin Deposition Study. AAPS PharmSciTech 2021, 22, 74. [Google Scholar] [CrossRef] [PubMed]
- Khalil, R.M.; Abdelbary, A.; Kocova El-Arini, S.; Basha, M.; El-Hashemy, H.A. Evaluation of bilosomes as nanocarriers for transdermal delivery of tizanidine hydrochloride: In vitro and ex vivo optimization. J. Liposome Res. 2019, 29, 171–182. [Google Scholar] [CrossRef]
- Abbas, H.; El Sayed, N.S.; Ali, M.E.; Elsheikh, M.A. Integrated lecithin–bile salt nanovesicles as a promising approach for effective skin delivery of luteolin to improve UV-induced skin damage in Wistar albino rats. Colloids Surf. B Biointerfaces 2022, 211, 112299. [Google Scholar] [CrossRef]
- Gunes, S.; Tamburaci, S.; Dalay, M.C.; Deliloglu Gurhan, I. In vitro evaluation of Spirulina platensis extract incorporated skin cream with its wound healing and antioxidant activities. Pharm. Biol. 2017, 55, 1824–1832. [Google Scholar] [CrossRef]
- Salem, H.F.; Nafady, M.M.; Ali, A.A.; Khalil, N.M.; Elsisi, A.A. Evaluation of Metformin Hydrochloride Tailoring Bilosomes as an Effective Transdermal Nanocarrier. Int. J. Nanomed. 2022, 17, 1185. [Google Scholar] [CrossRef]
- Abbas, H.; El-Feky, Y.A.; Al-Sawahli, M.M.; El-Deeb, N.M.; El-Nassan, H.B.; Zewail, M. Development and optimization of curcumin analog nano-bilosomes using 21.31 full factorial design for anti-tumor profiles improvement in human hepatocellular carcinoma: In-vitro evaluation, in-vivo safety assay. Drug Deliv. 2022, 29, 714–727. [Google Scholar] [CrossRef]
- Zewail, M.; Gaafar, P.M.E.; Ali, M.M.; Abbas, H. Lipidic cubic-phase leflunomide nanoparticles (cubosomes) as a potential tool for breast cancer management. Drug Deliv. 2022, 29, 1663–1674. [Google Scholar] [CrossRef]
- Salvioni, L.; Morelli, L.; Ochoa, E.; Labra, M.; Fiandra, L.; Palugan, L.; Prosperi, D.; Colombo, M. The emerging role of nanotechnology in skincare. Adv. Colloid Interface Sci. 2021, 293, 102437. [Google Scholar] [CrossRef]
- Elnaggar, Y.S.; Omran, S.; Hazzah, H.A.; Abdallah, O.Y. Anionic versus cationic bilosomes as oral nanocarriers for enhanced delivery of the hydrophilic drug risedronate. Int. J. Pharm. 2019, 564, 410–425. [Google Scholar] [CrossRef] [PubMed]
- Wissing, S.; Müller, R. Solid lipid nanoparticles (SLN)—A novel carrier for UV blockers. Die Pharm. 2001, 56, 783–786. [Google Scholar]
- Yuan, M.; Niu, J.; Xiao, Q.; Ya, H.; Zhang, Y.; Fan, Y.; Li, L.; Li, X. Hyaluronan-modified transfersomes based hydrogel for enhanced transdermal delivery of indomethacin. Drug Deliv. 2022, 29, 1232–1242. [Google Scholar] [CrossRef] [PubMed]
- Elhalmoushy, P.M.; Elsheikh, M.A.; Matar, N.A.; El-Hadidy, W.F.; Kamel, M.A.; Omran, G.A.; Elnaggar, Y.S. Novel berberine-loaded hyalurosomes as a promising nanodermatological treatment for vitiligo: Biochemical, biological and gene expression studies. Int. J. Pharm. 2022, 615, 121523. [Google Scholar] [CrossRef] [PubMed]
- Ammar, H.O.; Mohamed, M.I.; Tadros, M.I.; Fouly, A.A. Transdermal delivery of ondansetron hydrochloride via bilosomal systems: In vitro, ex vivo, and in vivo characterization studies. AAPS PharmSciTech 2018, 19, 2276–2287. [Google Scholar] [CrossRef]
- Hathout, R.M.; Mansour, S.; Mortada, N.D.; Guinedi, A.S. Liposomes as an ocular delivery system for acetazolamide: In vitro and in vivo studies. AAPS PharmSciTech 2007, 8, E1–E12. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.; Refai, H.; El Sayed, N.; Rashed, L.A.; Mousa, M.R.; Zewail, M. Superparamagnetic Iron Oxide Loaded Chitosan Coated Bilosomes for Magnetic Nose to Brain Targeting of Resveratrol. Int. J. Pharm. 2021, 610, 121244. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Alruwaili, N.K.; Imam, S.S.; Alotaibi, N.H.; Alharbi, K.S.; Afzal, M.; Ali, R.; Alshehri, S.; Alzarea, S.I.; Elmowafy, M.; et al. Bioactive Apigenin loaded oral nano bilosomes: Formulation optimization to preclinical assessment. Saudi Pharm. J. 2021, 29, 269–279. [Google Scholar] [CrossRef]
- Albash, R.; El-Nabarawi, M.A.; Refai, H.; Abdelbary, A.A. Tailoring of PEGylated bilosomes for promoting the transdermal delivery of olmesartan medoxomil: In-vitro characterization, ex-vivo permeation and in-vivo assessment. Int. J. Nanomed. 2019, 14, 6555. [Google Scholar] [CrossRef] [PubMed]
- Abdelbary, A.A.; Abd-Elsalam, W.H.; Al-Mahallawi, A.M. Fabrication of novel ultradeformable bilosomes for enhanced ocular delivery of terconazole: In vitro characterization, ex vivo permeation and in vivo safety assessment. Int. J. Pharm. 2016, 513, 688–696. [Google Scholar] [CrossRef]
- Clydesdale, G.J.; Dandie, G.W.; Muller, H.K. Ultraviolet light induced injury: Immunological and inflammatory effects. Immunol. Cell Biol. 2001, 79, 547–568. [Google Scholar] [CrossRef] [PubMed]
- Manca, M.L.; Castangia, I.; Caddeo, C.; Pando, D.; Escribano, E.; Valenti, D.; Lampis, S.; Zaru, M.; Fadda, A.M.; Manconi, M. Improvement of quercetin protective effect against oxidative stress skin damages by incorporation in nanovesicles. Colloids Surf. B Biointerfaces 2014, 123, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Castangia, I.; Nácher, A.; Caddeo, C.; Valenti, D.; Fadda, A.M.; Díez-Sales, O.; Ruiz-Saurí, A.; Manconi, M. Fabrication of quercetin and curcumin bionanovesicles for the prevention and rapid regeneration of full-thickness skin defects on mice. Acta Biomater. 2014, 10, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.Y.; Sun, D.S.; Chang, H.H. Silver Nanoparticles Protect Skin from Ultraviolet B-Induced Damage in Mice. Int. J. Mol. Sci. 2020, 21, 7082. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Jung, Y.R.; An, H.J.; Kim, D.H.; Jang, E.J.; Choi, Y.J.; Moon, K.M.; Park, M.H.; Park, C.H.; Chung, K.W.; et al. Anti-wrinkle and anti-inflammatory effects of active garlic components and the inhibition of MMPs via NF-κB signaling. PLoS ONE 2013, 8, e73877. [Google Scholar] [CrossRef]
- Jeon, I.H.; Kim, H.S.; Kang, H.J.; Lee, H.-S.; Jeong, S.I.; Kim, S.J.; Jang, S.I. Anti-inflammatory and antipruritic effects of luteolin from Perilla (P. frutescens L.) leaves. Molecules 2014, 19, 6941–6951. [Google Scholar] [CrossRef]
- Granja, A.; Neves, A.R.; Sousa, C.T.; Pinheiro, M.; Reis, S. EGCG intestinal absorption and oral bioavailability enhancement using folic acid-functionalized nanostructured lipid carriers. Heliyon 2019, 5, e02020. [Google Scholar] [CrossRef]
- Topley, N.; Woodcock, D. Inventors Use of Phospholipids for Wound. Healing. Patent US 2010/0048514 A1, 28 November 2005. [Google Scholar]
- Avadhani, K.S.; Manikkath, J.; Tiwari, M.; Chandrasekhar, M.; Godavarthi, A.; Vidya, S.M.; Hariharapura, R.C.; Kalthur, G.; Udupa, N.; Mutalik, S. Skin delivery of epigallocatechin-3-gallate (EGCG) and hyaluronic acid loaded nano-transfersomes for antioxidant and anti-aging effects in UV radiation induced skin damage. Drug Deliv. 2017, 24, 61–74. [Google Scholar] [CrossRef]
- Pieme, C.; Penlap, V.; Ngogang, J.; Costache, M. In vitro cytotoxicity and antioxidant activities of five medicinal plants of Malvaceae family from Cameroon. Environ. Toxicol. Pharmacol. 2010, 29, 223–228. [Google Scholar] [CrossRef]
- Serra, V.; Von Zglinicki, T.; Lorenz, M.; Saretzki, G. Extracellular superoxide dismutase is a major antioxidant in human fibroblasts and slows telomere shortening. J. Biol. Chem. 2003, 278, 6824–6830. [Google Scholar] [CrossRef]
- Abbas, H.; Kamel, R.; El-Sayed, N. Dermal anti-oxidant, anti-inflammatory and anti-aging effects of Compritol ATO-based Resveratrol colloidal carriers prepared using mixed surfactants. Int. J. Pharm. 2018, 541, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Raza, K.; Singh, B.; Singla, N.; Negi, P.; Singal, P.; Katare, O.P. Nano-lipoidal carriers of isotretinoin with anti-aging potential: Formulation, characterization and biochemical evaluation. J. Drug Target. 2013, 21, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Józsa, L.; Ujhelyi, Z.; Vasvári, G.; Sinka, D.; Nemes, D.; Fenyvesi, F.; Váradi, J.; Vecsernyés, M.; Szabó, J.; Kalló, G.; et al. Formulation of creams containing Spirulina platensis powder with different nonionic surfactants for the treatment of acne vulgaris. Molecules 2020, 25, 4856. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Moneim, A.-M.E.; El-Saadony, M.T.; Shehata, A.M.; Saad, A.M.; Aldhumri, S.A.; Ouda, S.M.; Mesalam, M.N. Antioxidant and antimicrobial activities of Spirulina platensis extracts and biogenic selenium nanoparticles against selected pathogenic bacteria and fungi. Saudi J. Biol. Sci. 2022, 29, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Derm. Endocrinol. 2012, 4, 308–319. [Google Scholar] [CrossRef]
- Tsukahara, K.; Nakagawa, H.; Moriwaki, S.; Takema, Y.; Fujimura, T.; Imokawa, G. Inhibition of ultraviolet-B-induced wrinkle formation by an elastase-inhibiting herbal extract: Implication for the mechanism underlying elastase-associated wrinkles. Int. J. Dermatol. 2006, 45, 460–468. [Google Scholar] [CrossRef]
- Imokawa, G. Epithelial-mesenchymal interaction mechanisms leading to the over-expression of neprilysin are involved in the UVB-induced formation of wrinkles in the skin. Exp. Dermatol. 2016, 25, 2–13. [Google Scholar] [CrossRef]
- Imokawa, G.; Nakajima, H.; Ishida, K. Biological mechanisms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging II: Over-expression of neprilysin plays an essential role. Int. J. Mol. Sci. 2015, 16, 7776–7795. [Google Scholar] [CrossRef]
- Abbas, H.; Kamel, R. Potential role of resveratrol-loaded elastic sorbitan monostearate nanovesicles for the prevention of UV-induced skin damage. J. Liposome Res. 2020, 30, 45–53. [Google Scholar] [CrossRef]
- Elnaggar, Y.S.R. Multifaceted applications of bile salts in pharmacy: An emphasis on nanomedicine. Int. J. Nanomed. 2015, 10, 3955–3971. [Google Scholar] [CrossRef]
- El Menshawe, S.F.; Aboud, H.M.; Elkomy, M.H.; Kharshoum, R.M.; Abdeltwab, A.M. A novel nanogel loaded with chitosan decorated bilosomes for transdermal delivery of terbutaline sulfate: Artificial neural network optimization, in vitro characterization and in vivo evaluation. Drug Deliv. Transl. Res. 2020, 10, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.; Campos, P.M.M. Development and photoprotective effect of a sunscreen containing the antioxidants Spirulina and dimethylmethoxy chromanol on sun-induced skin damage. Eur. J. Pharm. Sci. 2017, 104, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Fais, G.; Manca, A.; Bolognesi, F.; Borselli, M.; Concas, A.; Busutti, M.; Broggi, G.; Sanna, P.; Castillo-Aleman, Y.M.; Rivero-Jiménez, R.A.; et al. Wide Range Applications of Spirulina: From Earth to Space Missions. Mar. Drugs 2022, 20, 299. [Google Scholar] [CrossRef] [PubMed]
- Delsin, S.; Mercurio, D.; Fossa, M.; Maia Campos, P. Clinical efficacy of dermocosmetic formulations containing Spirulina extract on young and mature skin: Effects on the skin hydrolipidic barrier and structural properties. Clin. Pharm. Biopharm. 2015, 4, 2. [Google Scholar]
- Teng, Y.; Fan, Y.; Ma, J.; Lu, W.; Liu, N.; Chen, Y.; Pan, W.; Tao, X. The PI3K/Akt pathway: Emerging roles in skin homeostasis and a group of non-malignant skin disorders. Cells 2021, 10, 1219. [Google Scholar] [CrossRef]
- Noh, E.-M.; Park, J.; Song, H.-R.; Kim, J.-M.; Lee, M.; Song, H.-K.; Hong, O.Y.; Whang, P.H.; Han, M.K.; Kwon, K.B.; et al. Skin aging-dependent activation of the PI3K signaling pathway via downregulation of PTEN increases intracellular ROS in human dermal fibroblasts. Oxid. Med. Cell. Longev. 2016, 2016, 6354261. [Google Scholar] [CrossRef]
- Avila Acevedo, J.G.; Espinosa González, A.M.; Benitez Flores, J.d.C.; Delgado, T.H.; Maya, S.F.; Contreras, J.C.; López, J.L.M.; García Bores, A.M. Photoprotection of Buddleja cordata extract against UVB-induced skin damage in SKH-1 hairless mice. BMC Complement. Altern. Med. 2014, 14, 1–9. [Google Scholar] [CrossRef][Green Version]
- Kim, H.K. Garlic supplementation ameliorates UV-induced photoaging in hairless mice by regulating antioxidative activity and MMPs expression. Molecules 2016, 21, 70. [Google Scholar] [CrossRef]
- Abdel-Daim, M.; Funasaka, Y.; Kamo, T.; Ooe, M.; Matsunaka, H.; Yanagita, E.; Itoh, T.; Nishigori, C. Effect of chemical peeling on photocarcinogenesis. J. Dermatol. 2010, 37, 864–872. [Google Scholar] [CrossRef]
- Jang, Y.A.; Kim, B.A. Protective effect of spirulina-derived C-phycocyanin against ultraviolet B-induced damage in HaCaT cells. Medicina 2021, 57, 273. [Google Scholar] [CrossRef]
- Elnaggar, Y.S.; Elsheikh, M.A.; Abdallah, O.Y. Phytochylomicron as a dual nanocarrier for liver cancer targeting of luteolin: In vitro appraisal and pharmacodynamics. Nanomedicine 2018, 13, 209–232. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, Y.S.; Etman, S.M.; Abdelmonsif, D.A.; Abdallah, O.Y. Intranasal piperine-loaded chitosan nanoparticles as brain-targeted therapy in Alzheimer’s disease: Optimization, biological efficacy, and potential toxicity. J. Pharm. Sci. 2015, 104, 3544–3556. [Google Scholar] [CrossRef] [PubMed]
- Byeon, S.Y.; Cho, M.K.; Shim, K.H.; Kim, H.J.; Song, H.G.; Shin, H.S. Development of a spirulina extract/alginate-imbedded pcl nanofibrous cosmetic patch. J. Microbiol. Biotechnol. 2017, 27, 1657–1663. [Google Scholar] [CrossRef] [PubMed]
- Zewail, M.; EL-Deeb, N.M.; Mousa, M.R.; Abbas, H. Hyaluronic acid coated Teriflunomide (A771726) Loaded Lipid Carriers for the Oral Management of Rheumatoid Arthritis. Int. J. Pharm. 2022, 623, 121939. [Google Scholar] [CrossRef] [PubMed]
- Nair, K.G.; Ravikumar, Y.; Sukumaran, S.K.; Velmurugan, R. Fabrication, Optimization and Characterization of Paclitaxel and Spirulina Loaded Nanoparticles for Enhanced Oral Bioavailability. Curr. Nanosci. 2020, 16, 723–733. [Google Scholar] [CrossRef]
- Elsayed, I.; Sayed, S. Tailored nanostructured platforms for boosting transcorneal permeation: Box–Behnken statistical optimization, comprehensive in vitro, ex vivo and in vivo characterization. Int. J. Nanomed. 2017, 12, 7947. [Google Scholar] [CrossRef] [PubMed]
- Gilchrest, B.A.; Yaar, M. Ageing and photoageing of the skin: Observations at the cellular and molecular level. Br. J. Dermatol. 1992, 127, 25–30. [Google Scholar] [CrossRef]
- Mandawgade, S.D.; Patravale, V.B. Development of SLNs from natural lipids: Application to topical delivery of tretinoin. Int. J. Pharm. 2008, 363, 132–138. [Google Scholar] [CrossRef]
- Schaffler, K.; Nicolas, L.B.; Borta, A.; Brand, T.; Reitmeir, P.; Roebling, R.; Scholpp, J. Investigation of the predictive validity of laser-EPs in normal, UVB-inflamed and capsaicin-irritated skin with four analgesic compounds in healthy volunteers. Br. J. Clin. Pharmacol. 2017, 83, 1424–1435. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, S.-q.; Peng, H.; Yu, L.; He, B.; Zhao, Q. In vivo anti-apoptosis activity of novel berberine-loaded chitosan nanoparticles effectively ameliorates osteoarthritis. Int. Immunopharmacol. 2015, 28, 34–43. [Google Scholar] [CrossRef]
- Bancroft, J.D. Histochemical Techniques; Butterworth-Heinemann: Oxford, UK, 2013. [Google Scholar]
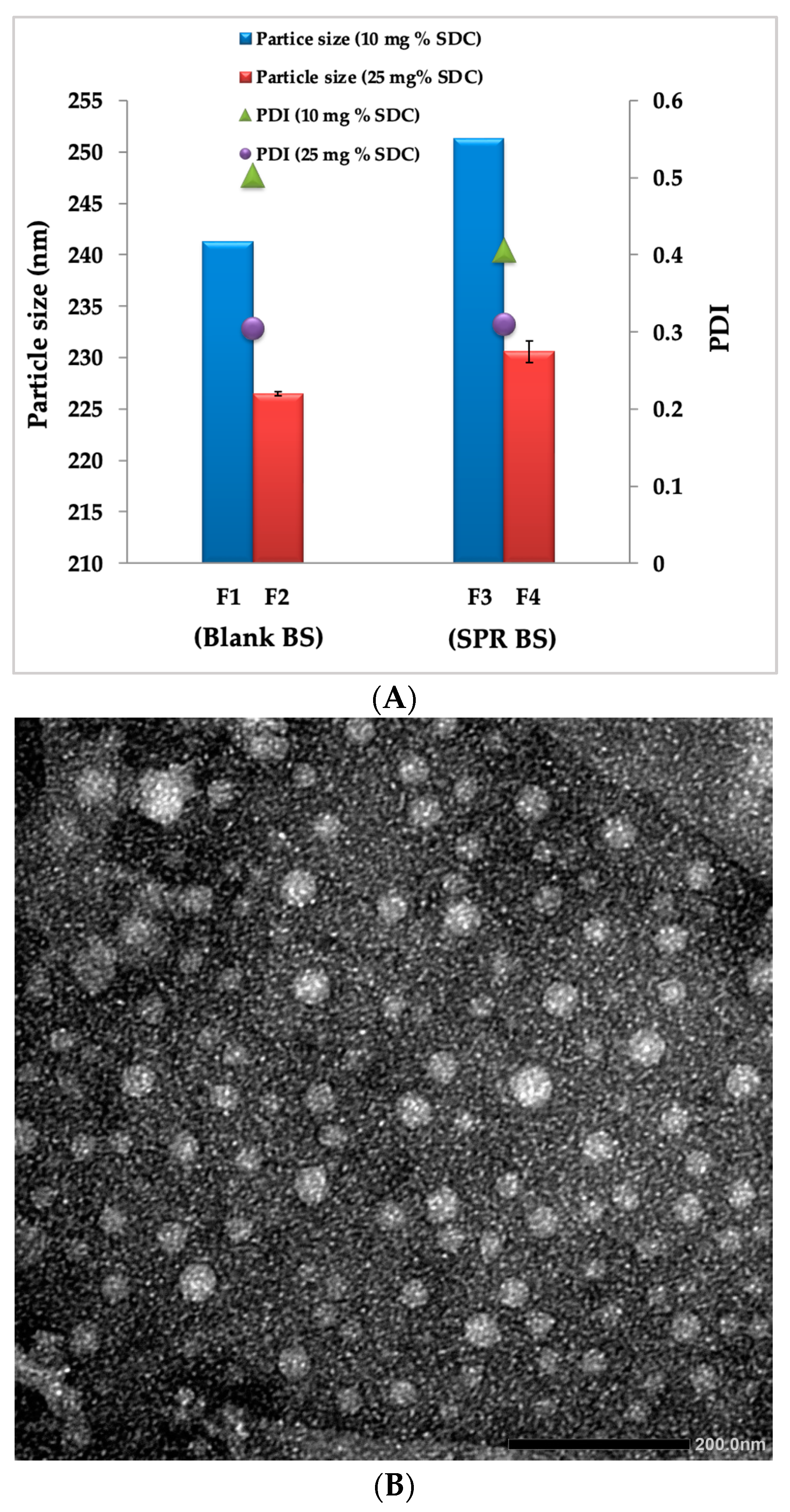









| Formulation Code | Particle Size (nm) | PDI | Zeta Potential (mV) | Entrapment Efficiency% |
|---|---|---|---|---|
| F1 | 241.3 ± 1.87 | 0.503 ± 0.04 | −28.4 ± 1.60 | NA |
| F2 | 226.5 ± 1.65 | 0.406 ± 0.05 | −33.5 ± 2.03 | NA |
| F3 | 251.3 ± 1.21 | 0.310 ± 0.06 | −30.6 ± 1.08 | 85.2 ± 1.50 |
| F4 | 230.6 ± 1.30 | 0.305 ± 0.02 | −34.5 ± 1.30 | 89.5 ± 1.08 |
| Formulation | R2 | |||
|---|---|---|---|---|
| Zero Order | First Order | Higuchi | Krosmeyer Peppas | |
| SPR suspension | 0.657 | 0.986 | 0.484 | 0.864 |
| F3 | 0.780 | 0.977 | 0.861 | 0.893 |
| F4 | 0.776 | 0.973 | 0.839 | 0.886 |
| Ingredients | F1 | F2 | F3 | F4 |
|---|---|---|---|---|
| Phosphatidyl choline (PC) mg %w/v | 100 | 100 | 100 | 100 |
| Cholesterol (Chol) mg %w/v | 25 | 25 | 25 | 25 |
| Sodium deoxycholate (SDC) mg %w/v | 10 | 25 | 10 | 25 |
| Spirulina (SPR) mg %w/v | -------- | -------- | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zewail, M.; Gaafar, P.M.E.; Youssef, N.A.H.A.; Ali, M.E.; Ragab, M.F.; Kamal, M.F.; Noureldin, M.H.; Abbas, H. Novel Siprulina platensis Bilosomes for Combating UVB Induced Skin Damage. Pharmaceuticals 2023, 16, 36. https://doi.org/10.3390/ph16010036
Zewail M, Gaafar PME, Youssef NAHA, Ali ME, Ragab MF, Kamal MF, Noureldin MH, Abbas H. Novel Siprulina platensis Bilosomes for Combating UVB Induced Skin Damage. Pharmaceuticals. 2023; 16(1):36. https://doi.org/10.3390/ph16010036
Chicago/Turabian StyleZewail, Mariam, Passent M. E. Gaafar, Nancy Abdel Hamid Abou Youssef, Merhan E. Ali, Mai F. Ragab, Miranda F. Kamal, Mohamed H. Noureldin, and Haidy Abbas. 2023. "Novel Siprulina platensis Bilosomes for Combating UVB Induced Skin Damage" Pharmaceuticals 16, no. 1: 36. https://doi.org/10.3390/ph16010036
APA StyleZewail, M., Gaafar, P. M. E., Youssef, N. A. H. A., Ali, M. E., Ragab, M. F., Kamal, M. F., Noureldin, M. H., & Abbas, H. (2023). Novel Siprulina platensis Bilosomes for Combating UVB Induced Skin Damage. Pharmaceuticals, 16(1), 36. https://doi.org/10.3390/ph16010036







