Abstract
Hyperprolactinemia is a known cause of amenorrhea and infertility. However, there is an increasing body of evidence suggesting that prolactin is involved in multiple physiological aspects of normal reproduction. Thus, the present paper aims to review the current literature regarding the relationship between serum prolactin level and in vitro fertilization (IVF)/intracytoplasmic sperm injection outcome and the role of dopamine agonists treatment in IVF success. Moreover, the mechanisms by which prolactin may exert its role in fertility and infertility were summarized. Although not all studies agree, the available evidence suggests that higher prolactin levels in follicular fluid are associated with increased oocytes competence, but also with positive effects on corpus luteum formation and survival, endometrial receptivity, blastocyst implantation potential and survival of low-motile sperm. Transient hyperprolactinemia found in IVF cycles was reported in most of the studies not to be related to IVF outcome, although a few reports suggested that it may be associated with higher implantation and pregnancy rates, and better-cumulated pregnancy outcomes. Administration of dopamine agonists for hyperprolactinemia preceding IVF treatment does not seem to negatively impact the IVF results, while treatment of transient hyperprolactinemia during IVF might be beneficial in terms of fertilization rates and conception rates. Due to limited available evidence, future studies are necessary to clarify the optimal level of circulating prolactin in patients performing IVF and the role of dopamine agonist treatment.
1. Introduction
An increasing body of evidence supports the pleiotropic role of prolactin in reproduction, growth, metabolism, electrolyte transport, behaviour, immunity and carcinogenesis [1]. Regarding the physiological effects, aside from the traditional role on lactation, several studies have suggested a more complex role of prolactin in reproduction by its benefic effects on oocytes development [2,3,4,5], corpus luteum formation and survival [6], implantation [7], steroidogenesis [8,9] and immunomodulatory effects [10,11]. Thus, a certain level of circulating prolactin might be necessary for optimal reproductive outcomes. During the menstrual cycle, fluctuations of the prolactin level were noticed, supporting the involvement of prolactin in variate aspects of reproduction. During controlled ovarian stimulation (COS) for assisted reproductive technology a particular dynamic of serum prolactin level, characterized by transient hyperprolactinemia, was also reported [8,12,13] with incompletely known significance and impact on fertility treatment outcome.
Regarding its pathophysiological importance, the most studied implication is hyperprolactinemia-induced infertility. The main mechanism by which hyperprolactinemia leads to infertility is a decrease in gonadotrophin-releasing hormone (GnRH) secretion with subsequent hypogonadotropic hypogonadism and anovulatory infertility. Experimental studies suggest that high levels of prolactin might additionally affect fertility by alteration in endometrial function and implantation through both structural and immunological factors [14]. Whether these effects on endometrium are significant in humans is debated, being suggested that they have only marginal significance [14,15].
Although the exact prevalence of hyperprolactinemia in infertile women is not clearly established, it appears to be at least ten times higher than in the general population [16]. Moreover, females with tubal factor infertility also present more commonly with abnormal prolactin concentration, suggesting that hyperprolactinemia may further decrease fertility potential in this category of patients [17].
Thus, the question arises whether hyperprolactinemia should be treated before and during assisted reproduction techniques (ART) as these procedures may overcome the detrimental effects on ovulation induced by high prolactin levels [14]. On the other hand, treatment for hyperprolactinemia might interfere with the possible beneficial effects of prolactin on other aspects of reproductive function. However, no guidelines address the aspect of optimal prolactin levels during infertility treatment for optimal outcomes. Therefore, we aimed to review the existing literature regarding the relationship between serum prolactin level and in vitro fertilization/intracytoplasmic sperm injection (ICSI) outcome and the role of dopamine agonists treatment in in vitro fertilization (IVF) success. Moreover, the mechanisms by which prolactin may exert its role in fertility and infertility were summarized.
We performed a PubMed database search using the following terms “prolactin” and “in vitro fertilization” or “intracytoplasmic sperm injection”. We retrieved 258 results and we further perfected the search by analyzing the abstracts available for each of the studies and by searching for articles of interest included in the reference list of relevant articles. Finally, 88 studies were included in the current material.
2. The Mechanisms behind Reduced Fertility in Hyperprolactinemic Patients
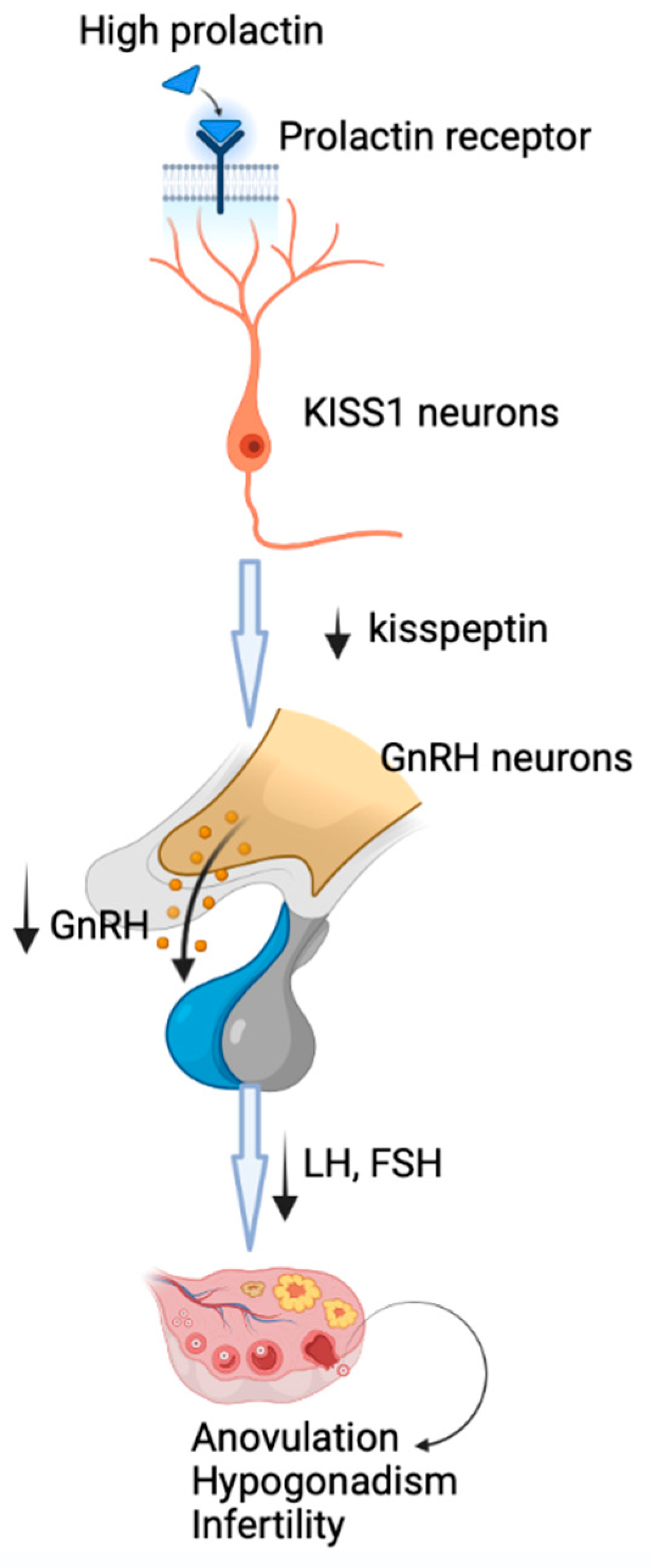
The main mechanism by which prolactin may lead to reproductive dysfunction is the inhibition of GnRH secretion, followed by decreased production of gonadotropins, hypogonadotropic hypogonadism and anovulation. New evidence has shown that the inhibition of hypothalamic secretion of GnRH involves metastasis-suppressor kisspeptin-1 neurons that express prolactin receptors [14] through which prolactin may exert its inhibitory effects at this level, with downstream disruption of GnRH secretion (Figure 1) [1]. The central role of kisspeptin-1 in mediating the prolactin action on GnRH secretion is highlighted by the restoration of fertility by kisspeptin-1 administration [1].
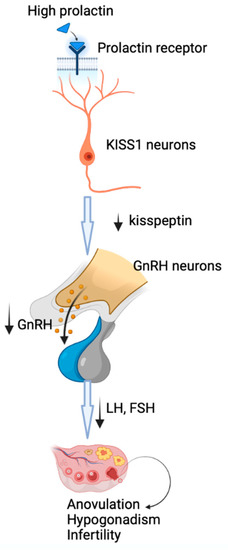
Figure 1.
The mechanism of induction of hypogonadotropic hypogonadism by hyperprolactinemia. Prolactin acts through prolactin receptors on metastasis-suppressor kisspeptin-1 neurons, with downstream disruption of GnRH secretion, followed by decreased production of gonadotropins, hypogonadotropic hypogonadism and anovulation.
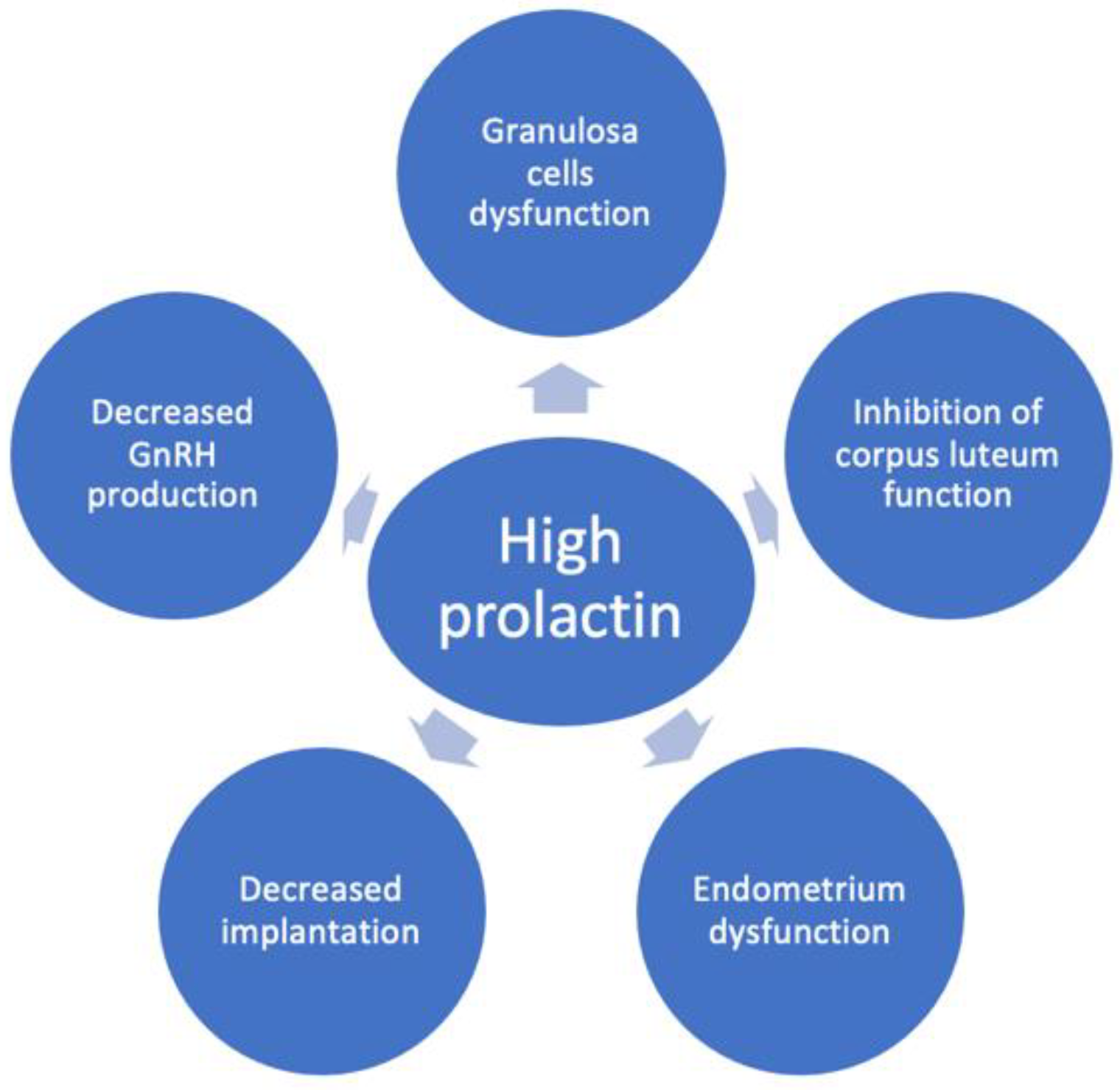
Additionally, accumulating evidence indicates that prolactin also influences directly the granulosa cells’ function (Figure 2) [8], exerting a direct inhibitory effect on gonadotropin action in the ovary [9,18]. In granulosa cells, prolactin inhibits estradiol production [9,19,20,21] and stimulates progesterone production [20,22,23] by activating distinct signalling pathways [24]. The mechanism by which excess prolactin inhibits follicle-stimulating hormone (FSH)-induced estradiol secretion in preovulatory follicles has been reported to be due to the reduction of aromatase activity [20,22,25,26], whereas FSH-induced steroidogenic acute regulatory protein, P450 side-chain cleavage enzyme and 3β-hydroxysteroid dehydrogenase type 2 levels are amplified by prolactin [8]. The bone morphogenic protein system in growing follicles plays a key role in antagonizing prolactin receptor signalling actions in the ovary when exposed to high concentrations of prolactin [27].
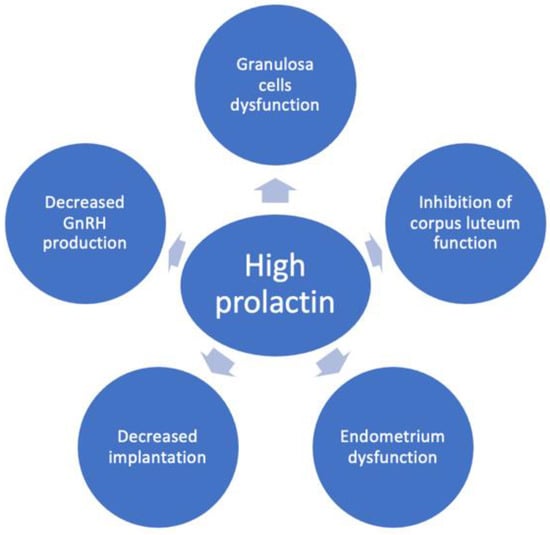
Figure 2.
The mechanisms of reduced female fertility in hyperprolactinemia. Hyperprolactinemia may contribute to infertility by inducing decreased GnRH production, granulosa cell dysfunction, inhibition of corpus luteum function, endometrial dysfunction and decreased implantation.
Some authors found prolactin receptors only in the human luteinized granulosa cells, but not in early follicles, and concluded that prolactin may play a role in the mature follicle during the time of ovulation [28]. Moreover, clinical data support an inhibitory effect of high prolactin levels on the function of the corpus luteum (Figure 2) [29]. Thus, in hyperprolactinemic women with ovulatory cycles, it was shown that hyperprolactinemia leads to luteal phase insufficiency and low progesterone levels [29]. It was demonstrated that the short luteal phase precedes the full clinical picture of hyperprolactinemia, suggesting that luteal phase insufficiency may be the first sign accompanying an increase in prolactin levels or the consequence of less severe hyperprolactinemia [29]. Thus, mild hyperprolactinemia might be associated with an incomplete clinical picture, being more difficult to recognize. Some authors consider that hyperprolactinemic women with regular menstrual cycles, especially when COS is planned, are a problematic patient category to manage due to the lack of clear evidence-based recommendations [30]. Although macroprolactinemia may be the underlying cause of prolactin elevations in some of the cases, and screening for macroprolactinemia is suggested for every value > 25 ng/mL, it was suggested that macroprolactinemia cannot be considered a completely harmless condition as it may be associated with infertility and autoimmune conditions [30,31].
Experimental animal studies offer some insights into the additional mechanism by which high levels of prolactin might contribute to decreased fertility by alteration in endometrial function and implantation (Figure 2) [14]. Thus, metoclopramide-induced hyperprolactinemic mice were found to have fewer pinopodes in the endometrium and a lower pregnancy rate [32]. Moreover, the same mouse model has a different glycosaminoglycan content in the endometrium which might affect the decidualization process [33]. Another suggested mechanism is the immune dysregulation induced by high prolactin which may alter implantation and pregnancy (Figure 2) [10,12,34]. It is debated whether these effects on endometrium are significant in humans since several studies showed that ovulation induction in hyperprolactinemic women resulted in pregnancy in spite of persistently high prolactin levels [14,15].
3. Prolactin Roles in Fertility
Earlier studies demonstrated that follicular fluid prolactin is not synthesized locally but originates in the pituitary gland, reaches the ovary through passive diffusion from systemic circulation and is regulated by estrogen [35]. However, more recent data suggest that the ovary might be an extra-pituitary source of prolactin secretion [6]. Other extra-pituitary sites of the prolactin gene expression have been discovered, among which are the endometrium, T-lymphocytes, brain, skin, breast, follicular fluid, ovarian follicular cells and amniotic fluid. The preponderance of circulating prolactin originates from the pituitary and until now the precise role of extra-pituitary prolactin is unknown [30]. Three major prolactin isoforms have been detected in different biological fluids: monomeric, big prolactin and big, big prolactin (macroprolactin) [36]. Different prolactin isoforms have different functions [1]. The only prolactin encountered in follicular fluid in patients undergoing IVF was the small, potent isoform.
Taking into account the presence of prolactin in the follicular fluid and the expression of the prolactin gene in the ovarian granulosa cells, several studies evaluated prolactin’s influence on oocyte maturation with controversial results. Few studies reported a negative relationship between follicular fluid prolactin levels and oocyte competence. Thus, Lee et al. found higher prolactin concentrations in the follicular fluid of unfertilized oocytes [2] and Reinthaller et al. showed that advanced follicular maturation was associated with decreasing follicular fluid prolactin [3]. Other authors found no correlation between the follicular fluid prolactin level and IVF outcomes, challenging the role of monomeric prolactin in oocyte physiology and fertilization [36].
On the other hand, Lindner et al. demonstrated that oocyte maturation and capacity to be fertilized was predicted by higher prolactin contents in follicular fluid and contested possible adverse effects of prolactin on oocytes [4]. Similarly, Subramanian’s study attested the biological activity of follicular fluid prolactin and found that mature preovulatory follicles contained a significantly greater concentration of prolactin in comparison to immature follicles, but prolactin concentration did not correlate with ovum fertilizability [37]. Laufer et al. linked higher follicular fluid prolactin levels with an increased number of successful pregnancies, supporting the role of prolactin in oocyte maturation [38]. A small study of couples undergoing ICSI for male factor infertility found that prolactin, progesterone, growth hormone, interleukin-1 (IL-1) and tumour necrosis factor-α were higher in follicles yielding oocytes that subsequently fertilized compared with those of oocytes that failed to fertilize [39]. Furthermore, oocytes that underwent a more rapid development had higher levels of follicular LH, prolactin and IL-1 in contrast to those who only reached the 2-cell stage or 3-cell stage 40–44 h after ICSI [39].
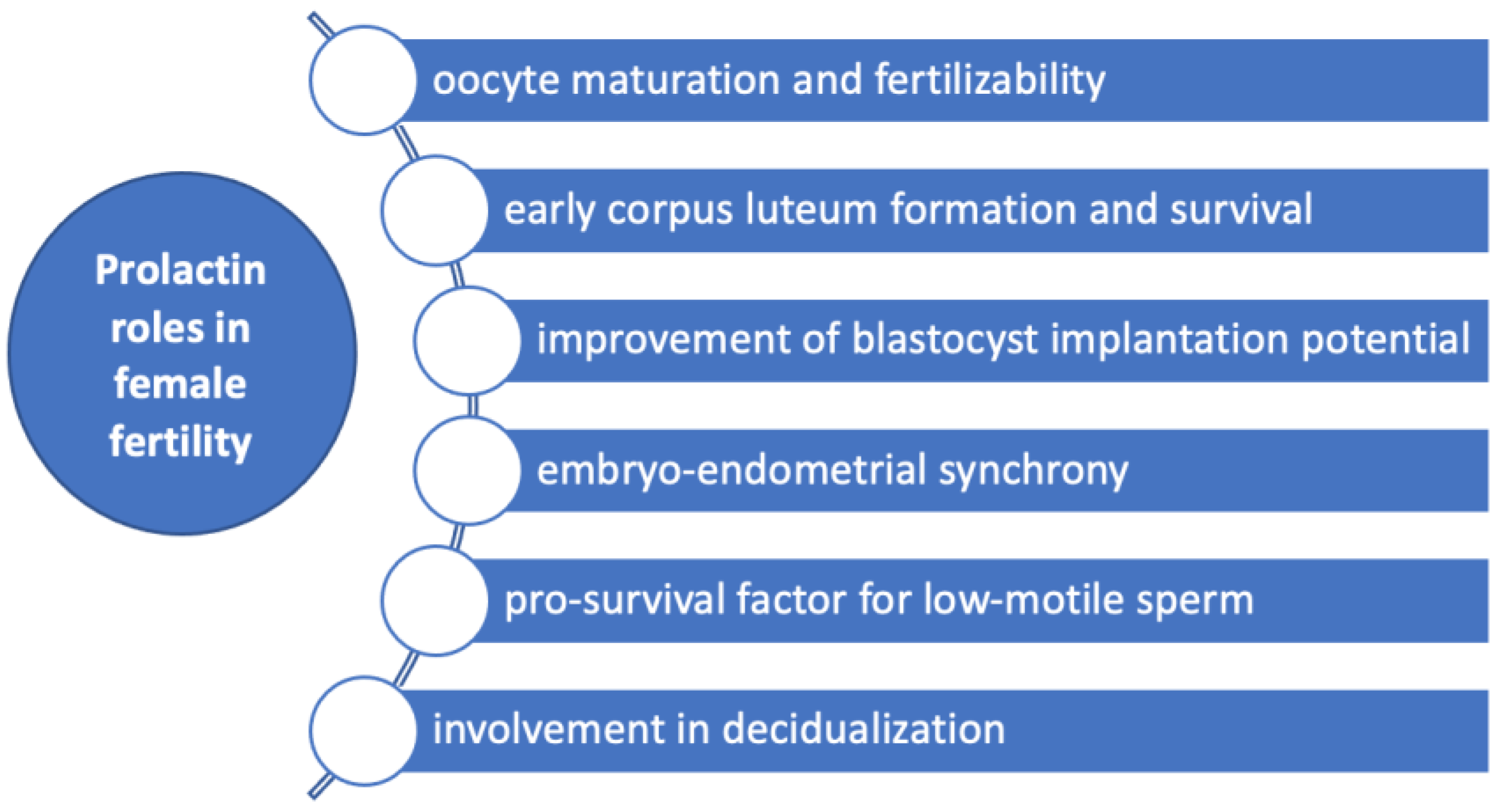
Experimental studies in bovine oocytes showed that both prolactin and growth hormone affect the morphology in metaphase II chromosomes in cumulus-enclosed oocytes in a biphasic dose-dependent manner [40], supporting a role for prolactin in oocyte maturation and developmental competence (Figure 3). Thus, prolactin in concentrations of 50 ng/mL decelerated the abnormal chromosome changes in cumulus-enclosed ageing bovine oocytes [40]. However, this effect was not found in denuded oocytes, suggesting that cumulus cells had a major role in mediating prolactin action probably by the prolactin receptors found in these cells [40,41]. In the opposite manner, concentrations 10–20 times higher than the normal range enhance destructive changes [40]. Animal studies in rats showed that prolactin administration may increase in vitro fertilization rate [42].
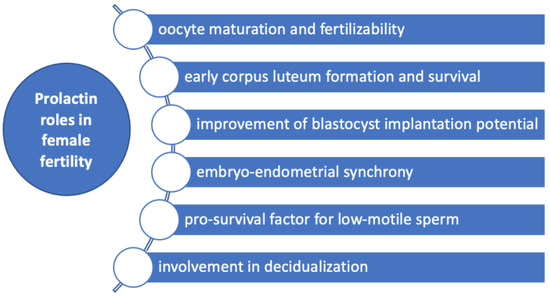
Figure 3.
Prolactin roles in female fertility. Prolactin has several roles in female fertility by influencing oocytes maturation and fertilizability, early corpus luteum formation and survival, improvement of blastocyst implantation potential, decidualization, embryo-endometrial synchrony and acting as a pro-survival factor for low-motile sperm.
Prolactin may be also a significant contributor to early corpus luteum formation and survival through antiapoptotic actions on granulosa cells (Figure 3) [6]. These effects were demonstrated in luteinized granulosa cells due to prior exposure to human chorionic gonadotrophin (HCG), collected from IVF flush [6].
The implantation is the consequence of a complex interplay between the uterus and the blastocyst which are both active parts of this process. Temporal synchronization between blastocyst competency and uterine receptivity is an essential aspect of implantation that occurs in a limited time called ‘’window of implantation’’ and different factors have been demonstrated to interfere with this sensitive, tight-regulated process [6]. During the majority of ART protocols, the administration of hormones is performed in order to achieve an increased number of follicles within the ovary. However, these hormonal influences on the endometrium are drastically different from natural cycles and may alter the “window of implantation”, lowering the chances of achieving pregnancy irrespective of the embryo quality [43]. It was suggested that prolactin might be involved in the implantation by its ability to influence both the uterus and the blastocyst. Thus, it was shown that supplementation of culture medium with prolactin altogether with epithelial growth factor and 4-hydroxyestradiol improves IVF-derived mouse blastocyst implantation potential [6]. Decidualization is the process by which endometrial stromal cells undergo profound changes in preparation for the embryo to implant. Prolactin messenger ribonucleic acid (mRNA) was also found to be expressed in the late-luteal phase endometrium [44,45,46,47,48], being considered a marker of decidualization [49]. Repeated implantation failure (RIF) is a reproductive condition in which women are not able to achieve a pregnancy in at least three consecutive IVF cycles with the transfer of at least one good-quality embryo during each cycle. Some underlying causes have been proposed such as obesity, thrombophilia and autoimmunity, but in a large number of cases, the underlying defect cannot be pinpointed and is termed “unexplained RIF”. An embryo-endometrial asynchrony caused by premature expression of the decidualization marker prolactin during the luteal phase has been associated with RIF [50]. Moreover, decreased expression of prolactin was found in the endometrium of patients with RIF [51].
The interaction between sperm and biological components in the female reproductive tract may be an essential aspect of subfertility [52]. Thus, prolactin was studied among other components of the follicular fluid such as progesterone, myo-inositol and dopamine, regarding its ability to influence sperm parameters [52]. It was found that prolactin supplementation appears to act as a pro-survival factor by reducing reactive oxygen species in the case of low-motile sperm subpopulations [52]. Consequently, the authors concluded that treatment of subfertile semen samples with biological substances present in follicular fluid might be a new treatment strategy in IVF [52]. In regard to other aspects of the fertilization process, prolactin does not appear to have a role in sperm-zona pellucida binding or acrosome reaction [53].
4. The Relationship between Prolactin Levels and ART Outcomes
It was shown that prolactin levels are higher in stimulated than in spontaneous cycles [54]. Similar to natural cycles, there is a transient increase in serum prolactin in the late follicular phase of stimulated cycles [55], but a particular dynamic of prolactin levels in stimulated cycles was also reported, characterized by a transitory increase in prolactin levels. Thus, increases in prolactin levels were found at ovum pick up [13], before and after HCG administration [8,12], in the luteal phase [54], while other authors reported a bimodal serum prolactin profile with peaks 1 and 9 days after the maximal serum LH value [11]. It was shown that the increment on prolactin may reach a maximum level of 93.2 ng/mL after the ovum pickup procedure, but return to normal values before embryo transfer [13]. Moreover, patients undergoing laparoscopic surgery for oocyte retrieval during IVF procedures demonstrate significant, although transient elevations in prolactin levels, which are presumed to be caused by anaesthetic agents and surgical stress [56,57]. Thus, it was demonstrated that intraoperative prolactin levels increase significantly till mean values of 124 ng/mL, with a return to normal levels 48 h after surgery [58]. Other authors found that laparoscopy does not contribute significantly to prolactin rise, the COS being the real cause of prolactin increase [59].
Since prolactin is considered a stress hormone, it was suggested that prolactin increase during IVF treatment might be the consequence of stress. Thus, it was shown that infertile women might have a different personality profile in comparison with fertile women, characterized by more suspicion, guilt and hostility, accompanied by increased stress hormone levels such as prolactin and cortisol [60]. Some authors found that serum prolactin and cortisol levels increase during COS for IVF along with state anxiety score in infertile patients [59], while others found no association between psychological scores and stress hormones among which prolactin in patients performing IVF [61,62].
It seems that the variate IVF treatment regimens may have different effects on serum prolactin as reported by several studies. Thus, when comparing prolactin elevation in patients treated for IVF with Clomiphene citrate/HCG, clomiphene citrate/human menopausal gonadotrophin (HMG)/HCG or HMG/HCG, it was found that plasma prolactin concentrations were higher in HMG-treated group [63]. Furthermore, it was noticed that patients treated with HMG-HCG had lower prolactin concentrations when clomiphene citrate was associated in comparison with those treated with GnRH agonist (buserelin) for pituitary suppression [12]. In this study, only in the group treated with the GnRH agonist a significant rise in plasma prolactin was noticed before HCG administration [12]. While a marked prolactin peak after HCG injection was found in both groups, the elevation was more pronounced in the GnRH agonist group [12]. The same study showed that HCG administration is followed by prolactin elevation 48 h after injection in postmenopausal women and that surgical castration is associated with an increase in plasma prolactin which parallels the change in FSH concentration [12]. These data support that lactotroph cell secretion is stimulated by the human gonadotropins, independently from circulating estradiol levels [12]. In another study, the use of leuprolide acetate, a GnRH agonist, in association with human menopausal gonadotropins for oocytes retrieval was associated with higher serum estradiol and prolactin levels in comparison with patients receiving stimulation using only human menopausal gonadotropins [64]. The authors consider that this increase in prolactin level by GnRH and its agonistic analogues is due to the paracrine effects of the alpha and beta subunits of pituitary gonadotropins on lactotroph cells [64]. Women stimulated with GnRH agonist long protocol and HMG were found to have a significant increase in prolactin elevation compared with the patients treated with the short protocol, which was positively correlated with estradiol levels, but independent of midcycle LH surge [65]. Disagreeing, other authors support that LH surge might be involved in the midcycle [31] and luteal phase [11] rise of serum prolactin.
The involvement of increased estradiol levels in the occurrence of transient hyperprolactinemia is debated, with some authors confirming its contribution [66], and other authors founding no relationship between the two hormones [67].
Taking into account the negative impact of high prolactin levels on fertility and the complex involvement of prolactin in reproduction, several studies evaluated the relationship between transient hyperprolactinemia during COS and IVF outcomes. However, most of them, especially early studies, failed to demonstrate a relationship. Thus, Pattinson et al. showed that the percentage rise of prolactin after stimulation did not impact the peak estradiol levels achieved, the number of follicles seen, the number of eggs retrieved, or pregnancies obtained [55]. Similarly, in patients undergoing different ovarian stimulation protocols, Hummel et al. did not observe the injurious effects of transient hyperprolactinemia on the total number of oocytes, the number of mature oocytes, fertilization rates, cleavage rates, and pregnancy rates [68]. Balasch et al. showed that successful and unsuccessful implantation cycles did not differ in estradiol, progesterone and prolactin levels [69]. Moreover, hyperprolactinemia was present in 20% of the patients, but the conception rate and pregnancy outcome were similar in patients with normal prolactin levels and those having hyperprolactinemia [69]. Similar results were obtained by Oda et al. who studied the relationship between serum prolactin level three days before oocytes retrieval and IVF outcome in euprolactinemic patients with tubal infertility [70]. They found similar fertilization and cleavage rates in patients classified as having hyperprolactinemia (prolactin ≥ 30 μg/L) and those with normal prolactin levels. However, significantly lower fertilization rates were found in patients with prolactin < 10 μg/L compared with patients with hyperprolactinemia and lower cleavage rates were achieved in the low prolactin group in contrast with women with normal or elevated serum concentrations [70]. While most pregnancies were obtained in the euprolactinemic group, the sample size was too small to reach statistical significance [70]. Hofmann et al. found a similar number of oocytes and pregnancy rates in patients with transient hyperprolactinemia or without during gonadotropin-stimulated cycles for IVF [66]. Forman et al. also found no association of elevated plasma or follicular fluid prolactin concentration with oocytes or embryonic development and pregnancy occurrence in patients undergoing ovarian stimulation for IVF [67]. In the study of Gonen et al., no difference was noticed in fertilization rates and the number of oocytes retrieved according to prolactin level measured on the day after HCG administration [71].
On the other hand, a few studies showed that transitory hyperprolactinemia might have a detrimental effect on follicular or oocyte development [56,72]. Thus, Reinthaller et al. found lower fertilization rate in patients with transient hyperprolactinemia in comparison with patients without high prolactin levels during gonadotropin stimulation [72], the authors concluding that treatment of transient hyperprolactinemia might be beneficial for IVF outcome. Other authors demonstrated that untreated transient anaesthesia-induced hyperprolactinemia is associated with a lower proportion of fertilized oocytes advancing to cleaving embryos in patients undergoing IVF, although oocyte fertilization and pregnancy rate were unaffected [56]. Tarín et al. developed a predictive model for women’s assisted fecundity before starting the first IVF/ICSI treatment cycle which included prolactin as a prognostic factor which was negatively associated with IVF success [73].
However, based on other authors’ reports, it seems that a higher prolactin level might be positively associated with IVF outcomes. Thus, Zhang et al. showed that patients with better-cumulated pregnancy outcomes had higher prolactin levels and a profoundly increasing trend in prolactin levels during ovarian stimulation before IVF [74]. Moreover, transient elevations of prolactin during the IVF stimulation protocol in the range of more than 200% were found to be associated with an increase in the number of follicles with a mean diameter ≥ 12 millimetres and with more mature oocytes and better IVF success rate [75]. A study which aimed to evaluate the impact of serum prolactin concentration on the day of human chorionic gonadotrophin (HCG) administration of IVF/ICSI-embryo transfer procedures divided patients into groups based on prolactin values: group A values < 30 ng/mL, group B values between 30–60 ng/mL, group C values between 60–90 ng/mL and group D with values ≥ 90 ng/mL [8]. During COS serum prolactin levels increased significantly from baseline values leading to transient hyperprolactinemia [8]. In group A, the number of oocytes was the lowest, suggesting that this could be associated with low estrogen levels [8]. Authors hypothesized that prolactin may be indirectly associated with the number of oocytes obtained and that there might be a feedback loop between the estradiol secreted by the ovarian follicles and prolactin secretion from the pituitary gland [8]. The implantation rate and the pregnancy rate were the highest when prolactin levels were between 30–60 ng/mL [8]. Conversely, significantly lower implantation rates and pregnancy rates were found in group D compared to the other groups [8]. Another study evaluated the hormonal profiles in the mid-luteal phase of super-ovulated IVF cycles and found that patients with successful pregnancy had higher levels of prolactin than those with early pregnancy loss, suggesting a defect in endometrial decidualization associated with lower prolactin [71]. Moreover, the estradiol to prolactin ratio was highest in the group with early pregnancy loss and authors hypothesize that there was a poor response to serum estradiol and that adequate prolactin levels may be mandatory for maintaining a pregnancy after IVF-embryo transfer [76].
Due to better control of male factors such as poor sperm quality, ICSI success largely depends on oocyte quality. Several studies evaluated the relationship between serum levels of prolactin and ICSI outcomes. Kamel et al. found that, in ICSI cycles, transient hyperprolactinemia before ovum pickup was associated with higher-quality embryos [13]. However, no association with the clinical pregnancy rates was observed between prolactin levels in the mid-luteal phase, before and 2 h after the ovum pickup procedure and before embryo transfer [13]. The lack of a relationship between mid-luteal phase prolactin and pregnancy rate was also reported by other authors [77].
The relationship between prolactin level before IVF procedure and IVF outcome is largely unknown, very few studies evaluated this aspect, letting the clinician without a verified tool when facing a hyperprolactinemic patient undergoing IVF. Thus, Gonen et al. found no difference in serum prolactin levels before ovarian stimulation between pregnant and non-pregnant patients [78]. Moreover, Pattinson et al. found no relationship between the initial prolactin levels and the number of follicles, oocytes or pregnancies obtained [55]. Zhang et al. investigated the impact of hyperprolactinemia on the outcomes of IVF/ICSI and observed significantly higher numbers of oocytes and embryos in patients with basal prolactin levels higher than 16.05 ng/mL, but lower than 50 ng/mL [74]. Cumulative clinical pregnancy was positively related to prolactin concentrations between 30 and 50 ng/mL and levels higher than 40 ng/mL were associated with higher cumulative live birth rates [74].
Prolactin Relation with Hyper-Response to COS and Ovarian Hyperstimulation Syndrome
Although clinical studies are lacking, the role of prolactin in mediating the impact of high estrogens on decidualization in high responders was highlighted by an experimental study treating human endometrial stromal cells with a decidualization cocktail with three different estradiol concentrations. The authors noticed that, although endometrial prolactin increased in all three regimens, prolactin was less with the high estradiol dose. Their conclusion was that supraphysiologic estradiol levels associated with high IVF responders may alter endometrial decidualization, implantation and placentation by affecting, among other mediators, the endometrial prolactin [79].
Ovarian hyperstimulation syndrome (OHSS) is the most severe and life-threatening complication of COS that is characterized by the occurrence of endothelial dysfunction, increases in vascular permeability and accumulation of fluid in the third space. In an attempt to identify the risk factors for the development of OHSS, a study evaluating 671 patients found that higher prolactin levels are linked to the development of OHSS in patients undergoing IVF and have been incorporated into a prediction model [80].
5. The Role of Dopamine Agonist Administration in IVF Treatment
It was shown that women with hyperprolactinemia, even with treatment, had lower parity, and older age at first pregnancy, although there appears to be no increased risk of pregnancy complications or adverse outcomes. [81] Thus, the possible impact of treated hyperprolactinemia on IVF outcome was evaluated in several studies, with divergent results. Therefore, Duan et al. [14] performed a retrospective study and found that the cumulative live birth rates, number of pregnancies and perinatal outcomes were similar between patients with treated hyperprolactinemia and those with normal prolactin levels undergoing IVF (Table 1) [14]. In patients with persistently elevated prolactin after treatment (median levels 88.35 ng/mL) the number of metaphase II oocytes was lower, but no other differences were observed between the groups [14]. Median prolactin levels before dopamine agonist treatment in the case group were 80 ng/mL and it reduced to around 15 ng/mL with treatment, similar to levels in the control group [14]. Doldi et al. divided patients with mild hyperprolactinemia before COS (mean values of serum prolactin in the study group of 24.7 ng/mL), undergoing ICSI, into two groups: one group received dopamine agonists, either cabergoline or bromocriptine, and the other group did not receive treatment. They found that in the group with untreated hyperprolactinemia a decreased FSH requirement, a higher number of good-quality oocytes (metaphase 2, mature oocytes), increased fertilization rate and higher numbers of embryos transferred were noticed (Table 1) [82].
Since the transient hyperprolactinemia occurring during COS was considered by some to negatively influence fertilization and/or implantation [72,83], the effect of treatment with dopamine agonists on IVF outcome was evaluated in a few studies (Table 1). Thus, administration of bromocriptine in patients with transient hyperprolactinemia in previous stimulated cycles was associated with higher fertilization rate in comparison with patients with untreated transient prolactin increase, but similar fertilization rates in patients without prolactin increase during ovarian stimulation [72]. Similarly, in unstimulated cycles of women with longstanding idiopathic infertility and with mild transitory elevations of prolactin in the range of 27–70 ng/mL lasting for 1–3 days and coinciding with preovulatory estradiol increases, bromocriptine administration was reported to be associated with higher conception rates [84]. However, other authors did not recommend dopamine agonist treatment in patients with transient hyperprolactinemia [78].
In patients with transient hyperprolactinemia during laparoscopy for oocyte collection, treatment with bromocriptine did result in a positive influence on embryonic development after IVF, although fertilization and pregnancy rates were similar (Table 1) [56]. On the other hand, another study examining the impact of anaesthesia-induced hyperprolactinemia and bromocriptine administration on IVF outcomes did not relate the change in prolactin concentrations with the occurrence of pregnancy [57].
Bromocriptine administration was studied in patients without hyperprolactinemia undergoing ovarian hyperstimulation for IVF, for prolactin suppression, but no impact was observed in terms of the number of oocytes, embryo quality and pregnancies in a double-blind placebo-controlled study [85]. However, it was noted that low prolactin concentration (median value of 5 μg/L) in association with higher estradiol levels during the follicular phase was correlated with an increase in placental protein 14, a glycoprotein which rises in the luteal phase, inhibits cell immune function and plays an essential role in the pregnancy process [85].
Bromocriptine administration was also studied in other situations assumed to be associated with a transitory increase in prolactin levels. Thus, normoprolactinemic clomiphene citrate-resistant women were suggested to have exaggerated prolactin response to TSH-releasing hormone and latent or intermittent hyperprolactinemia [86]. Thus, bromocriptine treatment was studied as adjunctive therapy in clomiphene-resistant patients with polycystic ovary syndrome and normal prolactin levels, but no significant effect was observed, except for the lowering of prolactin levels (Table 1) [86].
The role of prolactin in restoring the responsiveness of granulosa cells to stimulation was explored in an older study. Thus, Jinno et al. [87] proposed a new method of ovarian stimulation for IVF in patients with previous IVF failure, the bromocriptine rebound (BR) method [87]. They administrated bromocriptine from day 4 of the preceding cycle until 7 days before stimulation in patients treated with long agonist protocol and HMG. They reported that patients treated with the BR method had higher numbers of follicles, fertilized oocytes, embryos with superior morphology, clinical pregnancy and live birth rates in comparison with patients who did not receive bromocriptine (Table 1) [87]. Prolactin levels were also significantly higher in patients treated with bromocriptine and were correlated with the number of superior embryos [87]. They also noticed that the mRNA prolactin receptors in granulosa cells were higher in non-pregnant patients before bromocriptine administration and that the mRNA prolactin receptors decreased after BR method treatment. Thus, the authors concluded that IVF failure is the consequence of a granulosa cell resistance to prolactin action and that the bromocriptine-induced hypoprolactinemia contributes to the restoration of prolactin responsiveness and, subsequently, improved oocytes maturation [87]. A more recent study confirmed the results with the BR method [88]. Bromocriptine was administered daily from day 5 of the preceding cycle until 7 days before ovarian stimulation in women with normal prolactin levels and previous repeated unsuccessful ART in a new long agonist protocol cycle and the results were compared with those of women treated with long agonist protocol only [88]. The number of retrieved oocytes was the same in both groups; however, superior results were obtained with the BR method in terms of the number of fertilized oocytes, cleaved embryos, transplanted embryos, the ratio of good embryos, embryo score, and clinical pregnancy rates. The authors concluded that this method could be of value in attaining superior-quality embryos and transplantation rates in women with previous unsuccessful IVF/ICSI (Table 1) [88].

Table 1.
Studies evaluating the efficacy of dopamine agonist administration on in vitro fertilization outcome.
Table 1.
Studies evaluating the efficacy of dopamine agonist administration on in vitro fertilization outcome.
| Study | No of Patients | Type of the Study | Study Population | Type of HPRL | IVF Outcome | Prolactin Levels before Dopamine Agonist Treatment |
|---|---|---|---|---|---|---|
| Duan et al. (2019) [14] | 535 pts | Retrospective | 123 pts with treated HPRL and 369 matched controls | Preexistent to COS | CLBR similar in the two groups (69.1% versus s 66.4%, p = 0.58) | Median prolactin 80 ng/mL (study group) vs. 15.17 ng/mL (controls) |
| Doldi et al. (2000) [82] | 135 pts | Prospective | 59 pts with HPRL treated with BRC and 76 pts with HPRL without treatment | Preexistent to COS, mild HPRL in a cycle before COS | In the group with untreated HPRL a higher number of mature oocytes (87.9% versus 80.4%; p < 0.05), increased fertilization rate (70.8 +/− 28.0 versus 60.8 +/− 28.5; p < 0.03) and higher numbers of embryos transferred (3.6 +/− 1.6 versus 3.2 +/− 1.5; p < 0.05) | Mean 24.74 ± 3.17 ng/mL |
| Reinthaller et al. (1988) [72] | 50 cycles, 40 pts | Prospective | 18 pts with PRL ≤ 25 ng/mL (group 1), 15 pts with PRL > 25 ng/mL (group 2), 17 pts treated with BRC 3.75 mg/day (group 3) | Transient during COS | Higher fertilization rate (81.5%) in group 3 in comparison with group 2 (40.9%), but similar fertilization rates to group 1 (65.9%) | |
| Sopelak et al. (1989) [56] | 32 pts | Prospective | 19 controls and 13 pts treated with BRC 2.5 mg at 1 h (7 pts) and 12 h (6 pts) before anaesthesia | Transient, during anaesthesia for oocyte retrieval laparoscopy | 95% cleaving embryo in treated pts vs. 63% in controls, p < 0.001, similar fertilization and pregnancy rates | 22.4 ± 5.2 ng/mL in controls, 7 ± 1.7 ng/mL (1 h), 3.1 ± 0.3 ng/mL (12 h) |
| Taylor et al. (1986) [57] | 20 pts | Prospective | 10 pts treated with BRC, 10 pts without treatment | Transient, during anaesthesia for oocyte retrieval laparoscopy | No relationship between change in PRL and occurence of pregnancy | |
| Seppälä et al. (1989) [85] | 35 pts | Double-blind placebo-controlled study | 17 pts treated with BRC 1.25 mg/day from day 2 till HCG administration 18 pts treated with placebo | Normal PRL levels | Number of oocytes, embryo quality and pregnancies were similar between groups | |
| Parsanezhad et al. (2004) [86] | 100 pts | Prospective, double-blind, placebo-controlled study | CC-resistant normoprolactinemic PCOS women 47 pts treated with CC 200 mg × 5 days plus BRC 7.5 mg/day and 53 pts with placebo | Normal PRL levels | Similar ovulation rate in treated vs. placebo | |
| Jinno et al. (1997) [87] | 162 cycles | Prospective randomized study | Ovulatory women with previous IVF failure (82 cycles treated with BRC rebound method and 80 cycles with long protocol) | Normal PRL levels | Clinical pregnancy and live birth rates were higher (38% and 33%) in BRC rebound group versus long protocol (21% and 19%) | |
| Moride et al. (2018) [88] | 121 pts | Retrospective | Pts with repeated unsuccessful ART (22 women treated with BRC rebound method and 99 pts with long protocol) | Normal PRL levels | superior results were obtained with the BR method in terms of higher number of fertilized oocytes, cleaved embryos, transplanted embryos and clinical pregnancy rates (27.2% vs. 10.1%) in BRC rebound group | 5.9 ± 3.3 ng/mL (BRC rebound) vs. 7.5 ± 8.3 ng/mL (long agonist) |
HPRL: hyperprolactinemia; Pts: patients; IVF: in vitro fertilization; COS: controlled ovarian stimulation; CLBR: cumulative live birth rate; BRC: bromocriptine; PRL: prolactin; HCG: human chorionic gonadotropin; PCOS: polycystic ovary syndrome; CC: clomiphene citrate; ART: assisted reproductive technologies.
6. Conclusions and Future Directions
Although an increasing body of evidence supports the involvement of prolactin in the physiology and pathology of reproduction by several mechanisms, the data available in the literature regarding the optimal level of circulating prolactin for the best IVF treatment outcome are scarce. Hyperprolactinemia is a well-known cause of infertility throiugh an inhibitory effect on gonadotropin production. However, other mechanisms may also be involved such as endometrial dysfunction and immune dysregulation. On the other hand, prolactin may be involved in the physiology of reproduction. Thus, although not all studies agree, there are data supporting that higher levels of prolactin in follicular fluid are associated with increased oocyte competence. It is also possible that prolactin contributes to corpus luteum formation and survival, endometrial receptivity, blastocyst implantation potential and survival of low-motile sperm. During IVF, variations in prolactin levels were reported, characterized as transient hyperprolactinemia, possible causes being gonadotropins and GnRH agonists administration, increased estradiol levels and procedure-related stress. The association of transient hyperprolactinemia with IVF results was variably reported by different studies, most of the studies failing to demonstrate a relationship. However, a limited number of studies showed that higher prolactin levels during IVF are associated with higher implantation and pregnancy rates, and better-cumulated pregnancy outcomes. It is possible that the moment of the measurement of prolactin level is a significant factor in explaining the differences in the results. Regarding the benefits of treatment of hyperprolactinemia preceding IVF, the data are scarce and divergent. It seems that treatment of hyperprolactinemia does not negatively impact the pregnancy rate, although the effect of persistently high prolactin levels on oocyte quality is controversial. Treatment of transient hyperprolactinemia during IVF or spontaneous cycles was suggested to be beneficial in terms of fertilization rates and conception rates, although a limited amount of evidence is available. Administration of bromocriptine followed by withdrawal of treatment might be successful for obtaining a pregnancy in previous IVF failure patients by restoring granulosa cells’ responsiveness to prolactin.
Future studies are necessary to clarify apparently divergent laboratory and clinical data and to establish the optimal serum prolactin level for the best IVF outcome in patients with hyperprolactinemia preceding the IVF treatment and those with transient hyperprolactinemia during IVF.
Author Contributions
Conceptualization, A.I.A. and M.E.I.; methodology, A.I.A. and M.E.I.; validation, A.I.A., M.E.I. and D.N.A.; investigation, A.I.A., M.E.I. and D.N.A.; resources, A.I.A., M.E.I. and D.N.A.; data curation, A.I.A., M.E.I. and D.N.A.; writing—original draft preparation, M.E.I.; writing—review and editing, A.I.A., M.E.I. and D.N.A.; visualization, A.I.A., M.E.I. and D.N.A.; supervision, A.I.A. and D.N.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
ART: assisted reproductive techniques; BR, bromocriptine rebound; COS, controlled ovarian stimulation; GnRH, gonadotrophin-releasing hormone; FSH, follicle-stimulating hormone; HCG, human chorionic gonadotrophin; HMG, human menopausal gonadotrophin; ICSI, intracytoplasmic sperm injection; IL-1, interleukin 1; IVF, in vitro fertilization; LH, luteinizing hormone; mRNA, messenger ribonucleic acid; OHSS, ovarian hyperstimulation syndrome; RIF, repeated implantation failure.
References
- Bernard, V.; Young, J.; Chanson, P.; Binart, N. New insights in prolactin: Pathological implications. Nat. Rev. Endocrinol. 2015, 11, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Ben-Rafael, Z.; Meloni, F.; Mastroianni, L.; Flickinger, G.L. Relationship of human oocyte maturity, fertilization, and cleavage to follicular fluid prolactin and steroids. J. In Vitro Fertil. Embryo Transf. 1987, 4, 168–172. [Google Scholar] [CrossRef]
- Reinthaller, A.; Deutinger, J.; Riss, P.; Müller-Tyl, E.; Fischl, F.; Bieglmayer, C.; Janisch, H. Relationship between the steroid and prolactin concentration in follicular fluid and the maturation and fertilization of human oocytes. J. In Vitro Fertil. Embryo Transf. 1987, 4, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Lindner, C.; Lichtenberg, V.; Westhof, G.; Braendle, W.; Bettendorf, G. Endocrine Parameters of Human Follicular Fluid and Fertilization Capacity of Oocytes. Horm. Metab. Res. 1988, 20, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Wyse, B.A.; Fuchs, W.N.; Defer, M.; Montbriand, J.; Szaraz, P.; Librach, C. The follicular fluid adipocytokine milieu could serve as a prediction tool for fertility treatment outcomes. Reprod. Biomed. Online 2021, 43, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Perks, C.M.; Newcomb, P.V.; Grohmann, M.; Wright, R.J.; Mason, H.D.; Holly, J.M. Prolactin acts as a potent survival factor against C2-ceramide-induced apoptosis in human granulosa cells. Hum. Reprod. 2003, 18, 2672–2677. [Google Scholar] [CrossRef]
- Takeuchi, M.; Seki, M.; Furukawa, E.; Takahashi, A.; Saito, K.; Kobayashi, M.; Ezoe, K.; Fukui, E.; Yoshizawa, M.; Matsumoto, H. Improvement of implantation potential in mouse blastocysts derived from IVF by combined treatment with prolactin, epidermal growth factor and 4-hydroxyestradiol. Mol. Hum. Reprod. 2017, 23, 557–570. [Google Scholar] [CrossRef]
- Zhong, Y.-P.; Shen, X.-T.; Ying, Y.; Wu, H.-T.; Li, J.; Qi, Q.; Zhou, C.-Q.; Zhuang, G.-L. Impact of Transitory Hyperprolactinemia on Clinical Outcome of In Vitro Fertilization and Embryo Transfer. J. Med Biochem. 2011, 31, 27–33. [Google Scholar] [CrossRef]
- McNeilly, A.S.; Glasier, A.; Jonassen, J.; Howie, P.W. Evidence for direct inhibition of ovarian function by prolactin. Reproduction 1982, 65, 559–569. [Google Scholar] [CrossRef]
- Borba, V.V.; Zandman-Goddard, G.; Shoenfeld, Y. Prolactin and autoimmunity. Front. Immunol 2018, 9, 73. [Google Scholar] [CrossRef]
- Healy, D.L.; Burger, H.G. Serum Follicle-Stimulating Hormone, Luteinizing Hormone, and Prolactin during the Induction of Ovulation with Exogenous Gonadotropin. J. Clin. Endocrinol. Metab. 1983, 56, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Crosignani, P.G.; Maini, M.C.; Negri, E.; Ragni, G. Human prolactin release induced by follicle stimulating hormone, luteinizing hormone and human chorionic gonadotrophin. Hum. Reprod. 1991, 6, 1070–1073. [Google Scholar] [CrossRef] [PubMed]
- Kamel, A.; Halim, A.A.; Shehata, M.; Alfarra, S.; El-Faissal, Y.; Ramadan, W.; Hussein, A.M. Changes in serum prolactin level during intracytoplasmic sperm injection, and effect on clinical pregnancy rate: A prospective observational study. BMC Pregnancy Childbirth 2018, 18, 141. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Liu, X.; Hou, W.; Deng, M.; Gao, J.; Zhou, C.; Xu, Y. No impact of treated hyperprolactinemia on cumulative live birth rate and perinatal outcomes in in vitro fertilization-embryo transfer. J. Obstet. Gynaecol. Res. 2019, 45, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, P.; Lecomte, C.; Lansac, J.; Gallier, J.; Sonier, C.B.; Simonetta, C. Pregnancy after intravenous pulsatile gonadotropin-releasing hormone in a hyperprolactinemic woman resistant to treatment with dopamine agonist. Eur. J. Obstet. Gynecol. 1997, 74, 219–221. [Google Scholar] [CrossRef]
- Souter, I.; Baltagi, L.M.; Toth, T.L.; Petrozza, J.C. “âPrevalence of hyperprolactinemia and abnormal magnetic resonance imaging findings in a population with infertility. Fertil Steril 2010, 94, 1159–1162. [Google Scholar] [CrossRef]
- Laufer, M.R.; Floor, A.E.; Parsons, K.E.; Kuntz, K.M.; Barbieri, R.L.; Friedman, A.J. Evaluation of hormonal testing in the screening for in vitro fertilization (IVF) of women with tubal factor infertility. J. Assist. Reprod. Genet. 1995, 12, 93–96. [Google Scholar] [CrossRef]
- Hsueh, A.J.W.; Adashi, E.Y.; Jones, P.B.C.; Welsh, J.T.H. Hormonal Regulation of the Differentiation of Cultured Ovarian Granulosa Cells*. Endocr. Rev. 1984, 5, 76–127. [Google Scholar] [CrossRef]
- Tsai-Morris, C.-H.; Ghosh, M.; Hirshfield, A.N.; Wise, P.M.; Brodie, A.M.H. Inhibition of Ovarian Aromatase by Prolactin In Vivo. Biol. Reprod. 1983, 29, 342–346. [Google Scholar] [CrossRef]
- Fortune, J.E.; Wissler, R.N.; Vincent, S.E. Prolactin Modulates Steroidogenesis by Rat Granulosa Cells: II. Effects on Estradiol. Biol. Reprod. 1986, 35, 92–99. [Google Scholar] [CrossRef]
- Jonassen, J.A.; Baker, S.P.; McNeilly, A.S. Long-term hyperprolactinaemia reduces basal but not androgen-stimulated oestradiol production in small antral follicles of the rat ovary. J. Endocrinol. 1991, 129, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Veldhuis, J.D.; Klase, P.; Hammond, J.M. Divergent Effects of Prolactin upon Steroidogenesis by Porcine Granulosa Cells in Vitro: Influence of Cytodifferentiation. Endocrinology 1980, 107, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.B.C.; Valk, C.A.; Hsueh, A.J.W. Regulation of Progestin Biosynthetic Enzymes in Cultured Rat Granulosa Cells: Effects of Prolactin, β 2-Adrenergic Agonist, Human Chorionic Gonadotropin and Gonadotropin Releasing Hormone 1. Biol. Reprod. 1983, 29, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Russell, D.L.; Richards, J.S. Differentiation-dependent prolactin responsiveness and stat (signal transducers and activators of transcription) signaling in rat ovarian cells. Mol. Endocrinol. 1999, 13, 2049–2064. [Google Scholar] [CrossRef] [PubMed]
- Dorrington, J.H.; Gore-Langton, R.E. Antigonadal Action of Prolactin: Further Studies on the Mechanism of Inhibition of Follicle-Stimulating Hormone-Induced Aromatase Activity in Rat Granulosa Cell Cultures. Endocrinology 1982, 110, 1701–1707. [Google Scholar] [CrossRef]
- Uilenbroek, J.; van der Schoot, P.; Besten, D.D.; Lankhorst, R.R. A Possible Direct Effect of Prolactin on Follicular Activity. Biol. Reprod. 1982, 27, 1119–1125. [Google Scholar] [CrossRef]
- Nakamura, E.; Otsuka, F.; Inagaki, K.; Miyoshi, T.; Yamanaka, R.; Tsukamoto, N.; Suzuki, J.; Ogura, T.; Makino, H. A Novel Antagonistic Effect of the Bone Morphogenetic Protein System on Prolactin Actions in Regulating Steroidogenesis by Granulosa Cells. Endocrinology 2010, 151, 5506–5518. [Google Scholar] [CrossRef]
- Vlahos, N.P.; Bugg, E.M.; Shamblott, M.J.; Phelps, J.Y.; Gearhart, J.D.; Zacur, H.A. Prolactin receptor gene expression and immunolocalization of the prolactin receptor in human luteinized granulosa cells. Mol. Hum. Reprod. 2001, 7, 1033–1038. [Google Scholar] [CrossRef]
- Seppälä, M.; Ranta, T.; Hirvonen, E. Hyperprolactinaemia and luteal insufficiency. Lancet 1976, 307, 229–230. [Google Scholar] [CrossRef]
- Nawroth, F. Hyperprolactinaemia and the regular menstrual cycle in asymptomatic women: Should it be treated during therapy for infertility? Reprod. Biomed Online 2005, 11, 581–588. [Google Scholar] [CrossRef]
- Shimatsu, A.; Hattori, N. Macroprolactinemia: Diagnostic, Clinical, and Pathogenic Significance. Clin. Dev. Immunol. 2012, 2012, 167132. [Google Scholar] [CrossRef] [PubMed]
- Panzan, M.Q.; Junior, J.M.S.; Da Motta, E.L.A. Metoclopramide-induced hyperprolactinemia caused marked decline in pinopodes and pregnancy rates in mice. Hum. Reprod. 2006, 21, 2514–2520. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.C.; Maioral, G.C.; Verna, C. Hyperprolactinemia changes the sulfated gycosaminoglycan amount on the murine uterus during estrous cycle. Fertil Steril 2013, 100, 1419–1427. [Google Scholar] [CrossRef]
- Shelly, S.; Boaz, M.; Orbach, H. Prolactin and autoimmunity. Autoimmun. Rev. 2012, 1, A465–A470. [Google Scholar] [CrossRef] [PubMed]
- Ohwaki, M.; Suganuma, N.; Seo, H.; Nawa, A.; Kikkawa, F.; Narita, O.; Matsui, N.; Tomoda, Y. Source of Prolactin in Human Follicular Fluid. Endocrinol. JPN 1992, 39, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Romão, G.S.; Ferriani, R.A.; Moura, M.D.; Martins, A.R. Screening for prolactin isoforms in the follicular fluid of patients undergoing in vitro fertilization. Gynecol. Obstet. Investig. 2002, 54, 46–49. [Google Scholar] [CrossRef]
- Subramanian, M.G.; Sacco, A.G.; Moghissi, K.S.; Magyar, D.M.; Hayes, M.F.; Lawson, D.M.; Gala, R.R. Human follicular fluid: Prolactin is biologically active and ovum fertilization correlates with estradiol concentration. J. In Vitro Fertil. Embryo Transf. 1988, 5, 129–133. [Google Scholar] [CrossRef]
- Laufer, N.; Botero-Ruiz, W.; DeCherney, A.H.; Haseltine, F.; Polan, M.L.; Behrman, H.R. Gonadotropin and Prolactin Levels in Follicular Fluid of Human Ova Successfully Fertilized in Vitro. J. Clin. Endocrinol. Metab. 1984, 58, 430–434. [Google Scholar] [CrossRef]
- Mendoza, C.; Cremades, N.; Ruiz-Requena, E.; Martinez, F.; Ortega, E.; Bernabeu, S.; Tesarik, J. Relationship between fertilization results after intracytoplasmic sperm injection, and intrafollicular steroid, pituitary hormone and cytokine concentrations. Hum. Reprod. 1999, 14, 628–635. [Google Scholar] [CrossRef]
- Lebedeva, I.Y.; Singina, G.N.; Lopukhov, A.V.; Shedova, E.N.; Zinovieva, N.A. Prolactin and growth hormone affect metaphase-II chromosomes in aging oocytes via cumulus cells using similar signaling pathways. Front. Genet. 2015, 6, 274. [Google Scholar] [CrossRef]
- Lebedeva, I.Y.; Singina, G.N.; Volkova, N.A.; Vejlsted, M.; Zinovieva, N.A.; Schmidt, M. Prolactin affects bovine oocytes through direct and cumulus-mediated pathways. Theriogenology 2014, 82, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Starowicz, A.; Galas, J.; Duda, M.; Tabarowski, Z.; Szołtys, M. Effects of testosterone and prolactin on steroidogenesis in post-ovulatory cumuli oophori and on in vitro oocyte fertilisation in the rat. Reprod. Fertil. Dev. 2017, 29, 406. [Google Scholar] [CrossRef]
- Evans, J.; Hannan, N.J.; Hincks, C.; Rombauts, L.J.F.; Salamonsen, L.A. Defective Soil for a Fertile Seed? Altered Endometrial Development Is Detrimental to Pregnancy Success. PLoS ONE 2012, 7, e53098. [Google Scholar] [CrossRef] [PubMed]
- Kauma, S.; Shapiro, S.S. Immunoperoxidase localization of prolactin in endometrium during normal menstrual, luteal phase defect, and corrected luteal phase defect cycles. Fertil. Steril. 1986, 46, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Garzia, E.; Borgato, S.; Cozzi, V. Lack of expression of endometrial prolactin in early implantation failure: A pilot study. Hum. Reprod. 2004, 19, 1911–1916. [Google Scholar] [CrossRef]
- Altmäe, S.; Koel, M.; Võsa, U.; Adler, P.; Suhorutšenko, M.; Laisk-Podar, T.; Kukushkina, V.; Saare, M.; Velthut-Meikas, A.; Krjutškov, K.; et al. Meta-signature of human endometrial receptivity: A meta-analysis and validation study of transcriptomic biomarkers. Sci. Rep. 2017, 7, 10077. [Google Scholar] [CrossRef]
- Critchley, H.O.; Robertson, K.A.; Forster, T.; Henderson, T.A.; Williams, A.R.; Ghazal, P. Gene expression profiling of mid to late secretory phase endometrial biopsies from women with menstrual complaint. Am. J. Obstet. Gynecol. 2006, 195, 406–416. [Google Scholar] [CrossRef]
- Wu, W.-X.; Brooks, J.; Glasier, A.F.; McNeilly, A.S. The relationship between decidualization and prolactin mRNA and production at different stages of human pregnancy. J. Mol. Endocrinol. 1995, 14, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Gellersen, B.; Brosens, J. Cyclic Decidualization of the Human Endometrium in Reproductive Health and Failure. Endocr. Rev. 2014, 35, 851–905. [Google Scholar] [CrossRef]
- Berkhout, R.P.; Lambalk, C.B.; Repping, S.; Hamer, G.; Mastenbroek, S. Premature expression of the decidualization marker prolactin is associated with repeated implantation failure. Gynecol. Endocrinol. 2020, 36, 360–364. [Google Scholar] [CrossRef]
- Fu, Y.-X.; Yang, H.-M.; OuYang, X.-E.; Hu, R.; Hu, T.; Wang, F.-M. Assessment of Anti-Mullerian Hormone and Anti-Mullerian Hormone Type II Receptor Variants in Women with Repeated Implantation Failures. Reprod. Sci. 2021, 28, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Keyser, S.; van der Horst, G.; Maree, L. Progesterone, Myo-Inositol, Dopamine and Prolactin Present in Follicular Fluid Have Differential Effects on Sperm Motility Subpopulations. Life 2021, 11, 1250. [Google Scholar] [CrossRef] [PubMed]
- Huyser, C.; Fourie, F.R.; Moolman, H. The influence of sera, follicular fluids and seminal plasma on human sperm-zona pellucida binding. Hum. Reprod. 1997, 12, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Molina, R.; Castilla, J.A.; Vergara, F.; Pérez, M.; Garrido, F.; Herruzo, A.J. Luteal cytoplasmic estradiol and progesterone receptors in human endometrium: In vitro fertilization and normal cycles. Fertil. Steril. 1989, 51, 976–979. [Google Scholar] [CrossRef]
- Pattinson, H.A.; Taylor, P.J.; Fleetham, J.A.; Servis, S.A. Transient hyperprolactinemia has no effect on endocrine response and outcome in in vitro fertilization (IVF). J. In Vitro Fertil. Embryo Transf. 1990, 7, 89–93. [Google Scholar] [CrossRef]
- Sopelak, V.M.; Whitworth, N.S.; Norman, P.F.; Cowan, B.D. Bromocriptine inhibition of anesthesia-induced hyperprolactinemia: Effect on serum and follicular fluid hormones, oocyte fertilization, and embryo cleavage rates during in vitro fertilization. Fertil. Steril. 1989, 52, 627–632. [Google Scholar] [CrossRef]
- Taylor, P.J.; Trounson, A.; Besanko, M.; Burger, H.G.; Stockdale, J. Plasma progesterone and prolactin changes in superovulated women before, during, and immediately after laparoscopy for in vitro fertilization and their relation to pregnancy. Fertil. Steril. 1986, 45, 680–686. [Google Scholar] [CrossRef]
- Boyers, S.P.; Lavy, G.; Russell, J.B.; Polan, M.L.; DeCherney, A.H. Serum prolactin response to embryo transfer during human in vitro fertilization and embryo transfer. J. In Vitro Fertil. Embryo Transf. 1987, 4, 269–272. [Google Scholar] [CrossRef]
- Harlow, C.; Fahy, U.; Talbot, W.; Wardle, P.; Hull, M. Stress and stress-related hormones during in-vitro fertilization treatment. Hum. Reprod. 1996, 11, 274–279. [Google Scholar] [CrossRef]
- Csemiczky, G.; Landgren, B.M.; Collins, A. The influence of stress and state anxiety on the outcome of IVF-treatment: Psychological and endocrinological assessment of Swedish women entering IVF-treatment. Acta Obstet. Gynecol. Scand. 2000, 79, 113–118. [Google Scholar] [CrossRef]
- Milad, M.; Klock, S.C.; Moses, S.; Chatterton, R. Stress and anxiety do not result in pregnancy wastage. Hum. Reprod. 1998, 13, 2296–2300. [Google Scholar] [CrossRef]
- Trikoilis, Ν.; Mavromatidis, G.; Tzafetas, M.; Deligeoroglou, Ε.; Tzafetta, M.; Loufopoulos, A.; Dafoulis, V.; Tziomalos, K.; Goulis, D.G. The association of in vitro fertilization/intracytoplasmic sperm injection results with anxiety levels and stress biomarkers: An observational, case-control study. J. Gynecol. Obstet. Hum. Reprod. 2022, 51, 102254. [Google Scholar] [CrossRef]
- Shin, M.T.; Chu, T.Y.; Hsu, C.H.; Yu, M.H.; Chang, J.S.; Sun, D.; Lao, Z.H.; Wu, K.T.; Lee, C.K.; Yang, C.L. FSH, LH, PRL and E2 levels in follicular fluid and serum of patients undergoing follicle stimulation with different protocols for IVF. Asia Ocean. J. Obs. Gynaecol. 1988, 14, 227–232. [Google Scholar] [CrossRef]
- Meldrum, D.R.; Cedars, M.I.; Hamilton, F.; Huynh, D.; Wisot, A.; Marr, B. Leuprolide acetate elevates prolactin during ovarian stimulation with gonadotropins. J. Assist. Reprod. Genet. 1992, 9, 251–253. [Google Scholar] [CrossRef]
- Kamel, M.A.; Zabel, G.; Bernart, W.; Neulen, J.; Breckwoldt, M. Comparison between prolactin, gonadotrophins and steroid hormones in serum and follicular fluid after stimulation with gonadotrophin-releasing hormone agonists and human menopausal gonadotrophin for an in-vitro fertilization programme. Hum. Reprod. 1994, 9, 1803–1806. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, G.E.; Denis, A.L.; Scott, R.T.; Muasher, S.J. The incidence of transient hyperprolactinemia in gonadotropin-stimulated cycles for in vitro fertilization and its effect on pregnancy outcome. Fertil Steril 1989, 4, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Forman, R.; Fishel, S.; Edwards, S.; Walters, E. The Influence of Transient Hyperprolactinemia on in Vitro Fertilization in Humans. J. Clin. Endocrinol. Metab. 1985, 60, 517–522. [Google Scholar] [CrossRef]
- Hummel, W.P.; Clark, M.R.; Talbert, L.M. Transient hyperprolactinemia during cycle stimulation and its influence on oocyte retrieval and fertilization rates. Fertil. Steril. 1990, 53, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Balasch, J.; Creus, M.; Fábregues, F.; Carmona, F.; Casamitjana, R.; Peñarrubia, J.; Rivera, F.; Vanrell, J.A. Hormonal profiles in successful and unsuccessful implantation in IVF–ET after combined GnRH agonist/gonadotropin treatment for superovulation and hCG luteal support. Gynecol. Endocrinol. 1995, 9, 51–58. [Google Scholar] [CrossRef]
- Oda, T.; Yoshimura, Y.; Takehara, Y.; Kohriyama, S.; Sano, Y.; Tanabe, K.; Kobayashi, T.; Nakamura, Y.; Ohno, T.; Nozawa, S. Effects of prolactin on fertilization and cleavage of human oocytes. Horm. Res. 1991, 35, 33–38. [Google Scholar] [CrossRef]
- Gonen, Y.; Casper, R.F. The influence of transient hyperprolactinemia on hormonal parameters, oocyte recovery, and fertilization rates in in vitro fertilization. J. In Vitro Fertil. Embryo Transf. 1989, 6, 155–159. [Google Scholar] [CrossRef]
- Reinthaller, A.; Bieglmayer, C.; Deutinger, J.; Csaicsich, P. Transient hyperprolactinemia during cycle stimulation: Influence on the endocrine response and fertilization rate of human oocytes and effects of bromocriptine treatment. Fertil Steril 1988, 3, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Tarín, J.J.; Pascual, E.; García-Pérez, M.A.; Gómez, R.; Hidalgo-Mora, J.J.; Cano, A. A predictive model for women’s assisted fecundity before starting the first IVF/ICSI treatment cycle. J. Assist. Reprod. Genet. 2020, 37, 171–180. [Google Scholar] [CrossRef]
- Zhang, D.; Yuan, X.; Zhen, J.; Sun, Z.; Deng, C.; Yu, Q. Mildly Higher Serum Prolactin Levels Are Directly Proportional to Cumulative Pregnancy Outcomes in in-vitro Fertilization/Intracytoplasmic Sperm Injection Cycles. Front. Endocrinol. 2020, 11, 584. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.C.; Ferriani, R.A.; Sala, M.M.; Moura, M.D.; Carrara, H.H.; de Sá, M.F. Effect of transitory hyperprolactinemia on in vitro fertilization of human oocytes (abstract). J. Reprod. Med. 2001, 45, 444–450. [Google Scholar]
- Ozaki, T.; Takahashi, K.; Kurioka, H.; Miyazaki, K. Clinical Assisted Reproduction: Influence of Midluteal Serum Prolactin on Outcome of Pregnancy After IVF-ET: A Preliminary Study. J. Assist. Reprod. Genet. 2001, 18, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Pérez, P.A.S.; Ceschin, Á.P.; Moraes, D.M.P.; Oliveira, L.K.S.N.; Ceschin, N.I.; Ichikawa, N. Early serum progesterone and prolactin analysis at day 9 of oocyte retrieval as a predictor of success in fresh ICSI cycles. JBRA Assist. Reprod. 2018, 2, 95–98. [Google Scholar] [CrossRef]
- Gonen, Y.; Casper, R.F. Does transient hyperprolactinemia during ovarian hyperstimulation interfere with conception or pregnancy outcome? Fertil. Steril. 1989, 6, 1007–1010. [Google Scholar] [CrossRef]
- Cottrell, H.N.; Deepak, V.; Spencer, J.B.; Sidell, N.; Rajakumar, A. Effects of Supraphysiologic Levels of Estradiol on Endometrial Decidualization, sFlt1, and HOXA10 Expression. Reprod. Sci. 2019, 12, 1626–1632. [Google Scholar] [CrossRef]
- Pakhomov, S.P.; Orlova, V.S.; Verzilina, I.N.; Sukhih, N.V.; Nagorniy, A.V.; Matrosova, A.V. Risk Factors and Methods for Predicting Ovarian Hyperstimulation Syndrome (OHSS) in the in vitro Fertilization. Arch. Razi Inst. 2020, 5, 1461–1468. [Google Scholar]
- Berinder, K.; Hulting, A.-L.; Granath, F.; Hirschberg, A.L.; Akre, O. Parity, pregnancy and neonatal outcomes in women treated for hyperprolactinaemia compared with a control group. Clin. Endocrinol. 2007, 67, 393–397. [Google Scholar] [CrossRef]
- Doldi, N.; Papaleo, E.; De Santis, L.; Ferrari, A. Treatment versus no treatment of transient hyperprolactinemia in patients undergoing intracytoplasmic sperm injection programs. Gynecol. Endocrinol. 2000, 6, 437–441. [Google Scholar] [CrossRef]
- Del Pozo, E. Management of Borderline Hyperprolactinemia. Horm. Res. 1985, 22, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Ben-David, M.; Schenker, J. Transient hyperprolactinemia: A correctable cause of idiopathic female infertility. J. Clin. Endocrinol. Metab. 1983, 57, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Seppälä, M.; Martikainen, H.; Rönnberg, L.; Riittinen, L.; Kauppila, A. Suppression of prolactin secretion during ovarian hyperstimulation is followed by elevated serum levels of endometrial protein PP14 in the late luteal phase. Hum. Reprod. 1989, 4, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Parsanezhad, M.E.; Alborzi, S.; Jahromi, B.N. A prospective, double-blind, randomized, placebo-controlled clinical trial of bromocriptin in clomiphene-resistant patients with polycystic ovary syndrome and normal prolactin level. Arch. Gynecol. Obstet. 2004, 269, 125–129. [Google Scholar] [CrossRef]
- Jinno, M.; Katsumata, Y.; Hoshiai, T.; Nakamura, Y.; Matsumoto, K.; Yoshimura, Y. A therapeutic role of prolactin supplementation in ovarian stimulation for in vitro fertilization: The bromocriptine-rebound method. J. Clin. Endocrinol. Metab. 1997, 11, 3603–3611. [Google Scholar] [CrossRef]
- Moride, N.; Kuwahara, A.; Yamashita, M.; Tanaka, Y.; Matsuzaki, T.; Yasui, T.; Irahara, M. Does the bromocriptine-rebound method improve embryo quality? J. Med. Investig. 2018, 1–2, 63–66. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).