Abstract
Natural products are increasingly in demand in dermatology and cosmetology. In the present study, highly valuable supercritical CO2 (sCO2) extracts rich in bioactive compounds with antiradical and antibacterial activity were obtained from the inflorescences of industrial hemp. Volatile compounds were analyzed by gas chromatography in tandem with mass spectrometry (GC-MS), while cannabinoids were determined by high performance liquid chromatography (HPLC-DAD). Extraction yields varied from 0.75 to 8.83%, depending on the pressure and temperature applied. The extract obtained at 320 bar and 40 °C with the highest content (305.8 µg mg−1) of cannabidiolic acid (CBDA) showed the best antiradical properties. All tested extract concentrations from 10.42 µg mL−1 to 66.03 µg mL−1 possessed inhibitory activities against E. coli, P. aeruginosa, B. subtilis, and S. aureus. The sCO2 extract with the highest content of cannabidiol (CBD) and rich in α-pinene, β-pinene, β-myrcene, and limonene was the most effective. The optimal conditions for sCO2 extraction of cannabinoids and volatile terpenes from industrial hemp were determined. The temperature of 60 °C proved to be optimal for all responses studied, while the pressure showed a different effect depending on the compounds targeted. A low pressure of 131.2 bar was optimal for the extraction of monoterpenes, while extracts rich in sesquiterpenes were obtained at 319.7 bar. A high pressure of 284.78 bar was optimal for the extraction of CBD.
1. Introduction
Currently, there is a growing demand for products made from Cannabis sativa L., a medicinal plant that has experienced significant controversy throughout history. The official approval of the medicinal use of cannabis and cannabinoids worldwide has stimulated research in the field of dermatology and cosmetology. Although the topical application of cannabinoids is still in its infancy, there is obviously a growing interest in such preparations. Science has yet to thoroughly research the efficacy of cannabinoids and the safety of their application to the skin, but the production and use of dermatological and cosmetic preparations is increasing daily [1]. A recent study on the prevalence and causes of topical cannabis use in the Canadian population was published by Mahmood et al. [2]. Topical cannabis preparations were used at least once by 24.3% of respondents for the most common dermatologic conditions, such as atopic dermatitis (25%), acne (19%) and anti-ageing skin care (16%) and for non-dermatologic conditions such as joint stiffness (30%) and headaches and migraines (27%). Creams (26%) were the most common form of hemp-based preparations [2].
The main constituents of this plant species are cannabinoids and terpenes, which are mainly contained in the inflorescences. Unlike medicinal cannabis, industrial hemp contains less than 0.3% of the psychoactive cannabinoid tetrahydrocannabinol (THC), while other non-psychoactive cannabinoids are present in much higher proportions [3]. Terpenes also play an important role, as they are volatile components of the plant that have been shown to have synergistic effects with cannabinoids [4]. Nowadays, industrial hemp is a promising renewable resource that can be used not only for the production of textiles, paper, or biofuels, but also in the food, pharmaceutical, and cosmetic industries [5]. Therefore, the production of high quality extracts with a defined composition of desired bioactive compounds is of increasing importance. Today’s research is focused on finding environmentally friendly methods for the extraction, isolation, and purification of cannabinoids, as well as on the development of methods for their qualitative and quantitative analysis.
In the extraction of plant material, the requirements of modern production are almost completely met by supercritical CO2 (sCO2) extraction, as it is one of the “clean technologies” that does not produce environmentally harmful by-products. The sCO2 extraction is based on the fact that CO2 near the critical point or in the supercritical range is a very efficient solvent for certain types of compounds. The fluid is in a supercritical state at a temperature above its critical temperature (Tc) and a pressure above its critical pressure (pc). In this range, the density of the fluid approaches that of liquids, the viscosity approaches that of gases, and diffusion is higher than for conventional solvents. By adjusting the process parameters of pressure and temperature during extraction, the selectivity of the fluids for chemically very sensitive phytochemicals can be influenced [6,7]. Thus, this extraction technique is highly selective and also “green” and is therefore selected in this study for the extraction of cannabinoids and terpenes from industrial hemp. The sCO2 extraction is increasingly used for the isolation of cannabinoids and terpenes from medicinal and industrial hemp. Previous research has focused on the optimization of extraction conditions, solubility of individual cannabinoids in sCO2, their fractionation, and evaluation of the extraction efficiency compared to other extraction techniques [8]. Perrotin-Brunel et al. investigated the solubility of non-psychoactive cannabinoids in sCO2 compared to THC to separate them from the mixture. Lower temperatures were required for good solubility of cannabidiol (CBD) and cannabinol (CBN) than for cannabigerol (CBG) and tetrahydrocannabinol (THC), which dissolve well only at high temperatures, which may be related to differences in chemical structure and melting points [9]. It was found that, in addition to optimizing pressure and temperature, the use of ethanol as a co-solvent significantly contributes to extraction efficiency, shortens extraction time, and reduces the use of solvent and plant material to achieve high yields. Pulsatile addition of co-solvents showed better results than constant concentration of co-solvents [10]. Gallo-Molina et al. optimized the process of extraction, isolation, and purification of THC using supercritical fluid extraction and solid phase extraction [11]. Extracts rich in CBD and related cannabidiolic acid (CBDA) were obtained from biomass residues after industrial hemp processing using an optimized extraction procedure with sCO2 and fractionation [12]. A specially developed method for the supercritical extraction of eleven cannabinoids from medicinal hemp and purification with special filters and additional separation chambers was also described [13].
The sCO2 extracts from industrial hemp, rich in terpenes and cannabinoids without psychoactive effects, have great potential for application in the pharmaceutical and cosmetic industries. The beneficial effects of cannabinoids on the skin and the possibility of their therapeutic action for various skin diseases, such as eczema, acne, itchy skin, systemic sclerosis, and alopecia, have been studied [1,14]. Among cannabinoids, CBD, already included in many topical preparations, stands out for its antioxidant, anti-inflammatory, and analgesic effects, as well as for its positive effect on wrinkle reduction and skin hydration. Although the potential of CBD has been recognized, future clinical trials are expected to provide sufficient evidence for its efficacy and safety [14,15]. Modern methods of CBD extraction, isolation, and purification, including sCO2 extraction, have been researched and developed [16].
The objectives of this research were: (a) to determine the optimal conditions for the isolation of the desired phytochemicals (terpenes and cannabinoids) from the inflorescences of industrial hemp by applying “green” extraction with CO2 under supercritical conditions; (b) to identify the terpenes in the obtained sCO2 extracts by GC-MS; (c) to quantify the content of cannabinoids in the obtained extracts by HPLC; (d) to determine the antioxidant and antimicrobial activities of the sCO2 extracts. As far as we are aware, no one has yet provided detailed insight into the full composition of the volatile terpenes and cannabinoids of the sCO2 extracts from industrial hemp in terms of biological activity, which is the focus of the present research. The complete phytochemical analysis of the extracts from industrial hemp and the analysis of their antioxidant and antibacterial properties as well as the optimization of the extraction may help to ensure their inclusion in new cosmetic products, which is highly desirable.
2. Results and Discussion
2.1. Supercritical CO2 Extraction of Industrial Hemp
Research on sCO2 extraction of bioactive compounds from industrial hemp inflorescences is accessible only through the few published papers, and the demand for such natural extracts is increasing. As detailed in the introduction, most of these studies are related to the solubility of selected cannabinoids or to residues of industrial hemp and medicinal cannabis; only a few hemp compounds have been studied. It is known that sCO2 extraction is a highly selective technique and that varying the pressure at constant temperature changes the solvent density [9], which may affect the solubility of components, such as terpenes and cannabinoids. In order to optimize the extraction, the influence of the two main process parameters of sCO2 extraction (pressure and temperature) on the yield and the content of bioactive extract constituents was investigated. The experimental matrix was prepared according to the Central Composite Design (CCD) (Table 1), and the analysis of variance (ANOVA) evaluated the degree of accuracy of the applied methodology. Extraction yield is calculated as the total mass of extract obtained (g) divided by the mass (g) of plant material placed in the extractor and expressed as a percentage (%).

Table 1.
CCD matrix (levels of independent variables) for further sCO2 optimization.
Table 1 shows that the extraction yield varied from 0.76 (run 6) to 8.83% (run 8). This was expected due to the application of different extraction pressures ranging from 78.6 bar to 361.4 bar. The lower limit of 78.6 bar was only slightly above the critical CO2 solvent pressure of 74 bar, and it is known that volatiles are better extracted at lower pressures. However, higher pressures resulted in much higher yields, so an optimization process is required. In addition, the selected upper temperature limit (64.1 °C) was low enough to avoid changes in thermolabile compounds.
Based on the results of ANOVA, listed in the Supplementary Materials (Table S1), the regression model for yield was highly significant (p < 0.0001), and the obtained coefficient of determination R2 value was 0.9868 with a non-significant lack of fit (p = 0.0557), showing a reasonable representation between the input parameters and the observed extraction yield. In addition, pressure was the most statistically significant parameter for the observed response (p < 0.0001), indicating the expected increase in yield at higher pressure (Figure 1). From Table S1, it can be seen that temperature had a statistically strong influence on yield, but it was not as significant as pressure. In addition, quadratic terms of pressure and temperature variables showed a significant effect on yield, while the interaction between extraction parameters had no influence (p = 0.3578).
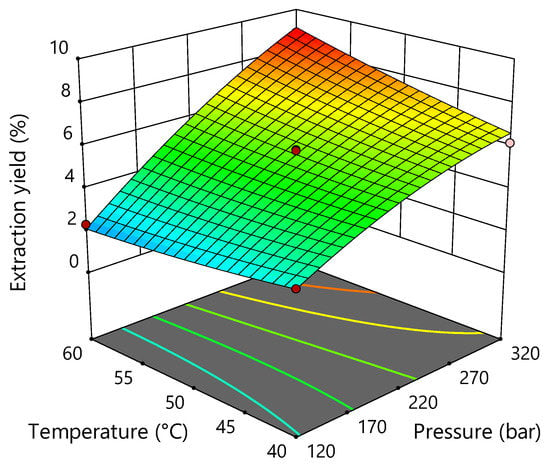
Figure 1.
Three-dimensional plot for obtained extraction yield as a function of extraction pressure and temperature.
Very similar results for extraction yield were also obtained in a previous study [17], where the yield ranged from 0.33 to 7.13% for industrial hemp variety Helena, extracted with sCO2. The extraction yield increased with increasing pressure at constant temperature and ranged from 1.57 to 5.20% at 40 °C, from 0.78 to 5.71 % at 50 °C, and from 0.33 to 7.13% at 60 °C. In several studies investigating the effects of pressure on the yield of supercritical extraction, an increase in yield with increasing pressure was observed in the extraction of oil from industrial hemp seeds [18], as well as cannabinoids from industrial hemp consisting of leaves, flower fragments, and immature seeds [19], and cannabinoids from flowers of different hemp varieties [10]. Kitrytė et al. investigated the effects of pressure (100–500 bar) and temperature (35–70 °C) on the extraction yield from hemp threshing residues. Optimum conditions were found to be 46.5 MPa and 70 °C, resulting in a yield of 8.3%. The response surface diagram for the yield also showed a very similar shape to that in this study. However, it is important to note that the extraction yield depends on the part of the plant to be extracted and also on the plant itself, i.e., plant variety, location, and growing conditions [10].
2.2. Terpenes in sCO2 Extracts of Industrial Hemp
The sCO2 proved to be a good solvent for the extraction of volatile compounds, such as terpenes from C. sativa [20]. Mono- and sesquiterpenes are largely responsible for the characteristic aroma of C. sativa. Their biosynthesis has been studied [21], and terpene synthases from C. sativa have been characterized [22,23]. They were identified ubiquitously in the essential oil of this plant [24]. Recently, comprehensive methods for the isolation of terpene compounds from C. sativa have been presented [20], including hydrodistillation (HD), conventional solvent extraction (SE), and sCO2 extraction. Proven and innovative extraction protocols and chromatographic separation methods, i.e., GC (including GC × GC) and HPLC, have been discussed and their respective advantages and disadvantages highlighted. The use of sCO2 has great advantages compared to HD, such as the use of a low temperature, which prevents the formation of thermal artefacts, and the direct recovery of terpenes without using conventional organic solvents [25].
As expected, the major terpene compounds found in our sCO2 extracts were monoterpenes. From the results presented in Table 2, the main component was β-myrcene (15.55–33.45%), an acyclic monoterpene previously found most abundantly in numerous cannabis cultivars [24]. Thus, the studied sample of C. sativa can be assigned to the β-myrcene chemotype. Other known hemp chemotypes include α-pinene, limonene, terpinolene, linalool, β-caryophyllene, selina-3,7(11)-diene, γ-selinene, 10-epi-γ-eudesmol, β-eudesmol, α-eudesmol, bulnesol, and α-bisabolol [24]. Among the other monoterpenes, α-pinene (5.13–12.88%), β-pinene (2.94–7.78%), and limonene (2.99–6.65%) were the most abundant in the sCO2 extracts. A variety of other monoterpenes was found as a minor constituent. Numerous biological properties are attributed to the main monoterpenes in the study extracts. For example, β-myrcene had antipsychotic, sedative, muscle relaxant, analgesic, antioxidant, anti-inflammatory, and anticancerogenic properties [26,27,28]. In addition to potent antimicrobial and antiseptic effects, α-pinene also showed anti-inflammatory, bronchodilator, and gastroprotective effects [29]. Limonene showed antimicrobial and gastroprotective activities, but anxiolytic, antidepressant, antispasmodic, antiproliferative, and immunostimulatory activities were also highlighted [30,31].

Table 2.
Content and composition of volatile compounds in hemp sCO2 extracts (%).
Among the sesquiterpenes in the studied sCO2 extracts, β-caryophyllene was the most abundant (2.83–10.16%). It has been found to possess various biological activities, such as antimicrobial, antioxidant, anti-inflammatory, gastroprotective, analgesic, anticancerogenic, antiproliferative, antidepressant, anxiolytic, and neuroprotective activities [32,33]. As the only known terpene with this ability, β-caryophyllene interacts with the endogenous cannabinoid system and selectively binds to the CB2 receptor [34]. It also showed high selectivity against herpes simplex virus type 1 in vitro [35]. Table 2 shows that hydrocarbons (γ-selinene, selina-3,7(11)-diene) and alcohols (guaiol, γ-eudesmol, bulnesol), represented in concentrations ranging from 1.26 to 6.59%, also contribute to the sesquiterpene fraction of the studied extracts. Guaiol may act as an anti-inflammatory, antimicrobial, analgesic, and antitumor agent [36,37], while bulnesol has antitussive and expectorant properties [38].
Our research confirmed the previous findings that β-caryophyllene and β-myrcene are often the most abundant terpenes in C. sativa [24]. Due to their high content and various biological properties, they most likely have a significant impact on the medicinal outcomes of cannabis use. The cannabis extracts studied in the present work were also rich in α-pinene, β-pinene, guaiol, γ-eudesmol, and bulnesol, which may also contribute to their biological activities. Therefore, a possible synergistic and/or entourage effect of cannabinoids and terpenes should be considered [39], and further studies are needed to elucidate the possible interactions of cannabinoids and terpenes in humans [24].
The most abundant terpenes were used for further statistical analysis, and the summarized results of ANOVA of the models for each response studied are proposed (Table S2). Based on the obtained results, the regression models for all targeted terpenes were significant (p < 0.05), and the obtained R2 values ranged from 0.7532 to 0.9342 with non-significant fitting deficiencies, indicating an adequate representation between the input parameters and the observed variables. Moreover, an identical statistical effect can be seen for the monoterpenes (α-pinene, β-pinene, β-myrcene, and limonene) and guaiol with respect to the linear term of pressure as well as the quadratic term of temperature, which had a significant effect. For the sesquiterpenes, the quadratic term of pressure and temperature had a significant influence on β-caryophyllene abundance, whereas only the linear term of pressure had a significant influence on γ-eudesmol and bulnesol. For all components, the interaction between pressure and temperature had no effect.
It is interesting to see in Figure 2 and Figure 3 that the response surface plots for all monoterpenes have a similar shape, while all sesquiterpenes have a very similar shape of response plots. Monoterpenes and sesquiterpenes showed completely opposite effects of pressure on their abundance. In the case of monoterpenes (Figure 2), it is obvious that their content decreases with increasing pressure, so lower pressures are recommended for the extraction of monoterpenes.
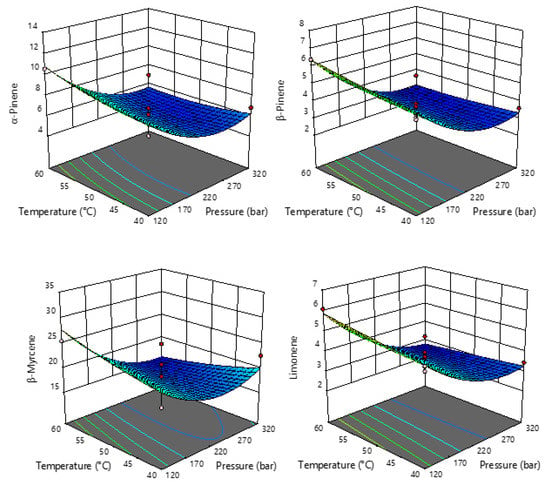
Figure 2.
Response surface plots showing the effects of temperature and pressure on the selected monoterpenes.
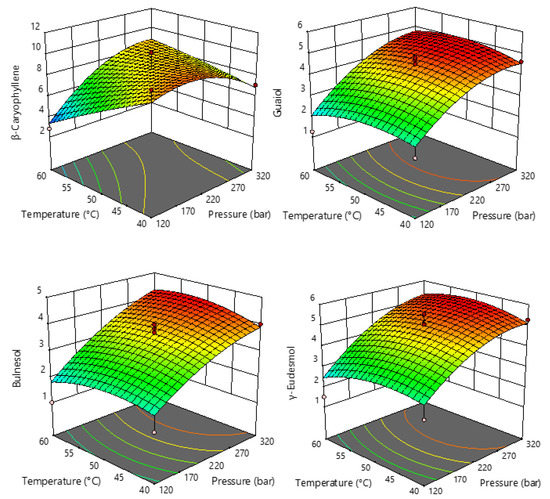
Figure 3.
Response surface plots showing the effects of temperature and pressure on the selected sesquiterpenes.
From Figure 3, it can be seen that pressure has a strong influence and that the contents of guaiol, γ-eudesmol, and bulnesol increase at higher pressure, while the content of β-caryophyllene increases at pressures up to 300 bar and decreases slightly at higher pressures. Therefore, it can be concluded that the application of higher pressures favors the extraction of larger proportions of sesquiterpenes.
Compared to other studies using sCO2 extracts [20,24,25], there is a great similarity between the content and composition of the main terpene compounds. However, our design of sCO2 experiments was appropriate, since a great similarity of the obtained sCO2 extracts and the inflorescence extracts (Table 2) can be observed in terms of abundance of the main compounds and determination of chemotypes. This is a significant improvement over the previous study by Sexton et al. [40], which showed that the products of sCO2 extraction can have a significantly different chemotype fingerprint than the cannabis inflorescence.
2.3. Cannabinoids in sCO2 Extracts of Industrial Hemp
It is well known that C. sativa is the main source of cannabinoids; thus, the most important have been identified in sCO2 extracts, including CBD, CBDA, THC, CBC, CBG, and CBN. However, in the present study, some less represented cannabinoids were also analyzed by the HPLC-DAD, such as cannabigerolic acid (CBGA), tetrahydrocannabivarinic acid (THCVA), cannabichromene acid (CBCA), cannabidivaric acid (CBDVA), and tetrahydrocannabinolic acid A (THCA). Results are presented in Table 3, and the representative chromatograms of the HPLC analysis are given in the Supplementary Materials (Figure S1). In the context of cannabinoid analysis, it is important to understand the biosynthesis of cannabinoids, with CBGA being the major precursor for THCA and CBDA. At high temperatures, both acids tend to break down into their respective decarboxylated analogues, THC and CBD. When THC is oxidized in air and by light, CBN is formed, the result of the chemical decomposition of THC. The more THC that decomposes, the more CBN that is formed. CBN is present in old, dried hemp flowers. When consumed in large quantities, it can lead to paranoia. Therefore, proper storage of the plant is extremely important (hermetically sealed containers); this will extend its shelf life and slow down the natural process of CBN formation. At higher temperatures, the acidic forms are further converted into their neutral degradation products. Therefore, a heat-triggered chemical reaction leading to decarboxylation of these compounds is necessary if the corresponding CBD and THC are the target. All of the above forms possess some biological activity; thus, CBD and CBDA possess analgesic, antibacterial, antidiabetic, antiemetic, antiepileptic, anti-inflammatory, antiproliferative, and antipsychotic properties [41]. CBG is known for its analgesic, antibacterial, and antifungal effects, CBC exhibits anti-inflammatory, antibacterial, and antifungal activities, and CBN possesses sedative properties. The primary psychoactive substance THC has analgesic, anti-inflammatory, and antiemetic effects [4].

Table 3.
Content of cannabinoids in different extraction runs of industrial hemp (according to CCD) expressed as percentage ± SD (%).
The main focus of this research was to obtain natural extracts rich in terpenes and CBD as a targeted cannabinoid. In our recently published review [42], sCO2 plant extracts showed their potential as bioactive ingredients for the development of effective and safe cosmetics. Nowadays, CBD is trending and is preferred in many cosmetic products due to its anti-inflammatory, analgesic, hydrating, moisturizing, and wrinkle–reducing properties [14]. It should be noted that more and more new cosmetic products are appearing on the market that contain only hemp extracts or CBD due to their strong nourishing effects on the skin. Previous research has shown that CBD with good carriers can penetrate the skin and prevent the signs of aging. This is because of the potential ability to protect against free radicals that cause oxidative damage in the skin, which in turn leads to visible damage, wrinkles, or the inevitable aging process of the skin. In addition to its anti-aging effect, the use of CBD products for skin problems such as acne, dermatitis, psoriasis, and other diseases is also being studied, as CBD acts on cellular processes that lead to acne, seborrhea, psoriasis, and dermatitis [1,14,43]. Therefore, these hemp extracts could be effective in treating certain skin problems; however, it is also known that sCO2 extracts can be excellent antioxidants for cosmetic products. Their addition offers numerous benefits for cosmetic products, mainly due to their antioxidant but also anti-inflammatory and antimicrobial effects. Moreover, CO2 in the supercritical state has been shown to be an extractant that ensures safe extracts for further cosmetic use [42,44].
For all these reasons, and especially because of the potential application of sCO2 extracts for topical use, CBD as a cannabinoid is targeted for further optimization processes. In addition, the presence of other cannabinoids in the extracts is also beneficial as they act synergistically. Therefore, CBDA is statistically evaluated as the most important cannabinoid. Table 3 lists eleven cannabinoids detected in the sCO2 extracts and inflorescences of industrial hemp. HPLC analysis showed that the cannabinoids studied were present in the crude plant material, with the exception of THCVA. It was expected that some of them would be present in very low concentrations (THC because industrial hemp contains only small amounts, CBN because it was a fresh sample and it was already mentioned that CBN is present in mature hemp flowers). As expected, CBDA dominated (14.02%), and CBD content in inflorescences was 0.59%.
It can also be seen from the results in Table 3 that the content of total cannabidiols (CBD+CBDA) extracted with sCO2 was very high. The CBDA content ranged from 6.32 to 30.63%, depending on the applied extraction parameters of pressure and temperature, while the CBD content ranged from 0.80 to 6.58%.
The statistical significance of the regression equations for the selected response (CBD and CBDA) was evaluated by ANOVA and is shown in Table S3. The regression models for both studied responses were highly significant according to p-values (0.0009 for CBD and 0.0072 for CBDA), with satisfactory coefficients of determination (R2). The non-significant lack of fit (p > 0.05) for each response indicates that the second order polynomial model is appropriate and can be used for the precision of the experimental values. It is also evident that the linear term of pressure and temperature, as well as the quadratic term of temperature, have a significant effect on the CBD content in the sCO2 extracts. In terms of CBDA content, only pressure had a statistically significant influence (p = 0.0006), which is also evident in Figure 4, where the trend shows that the higher the extraction pressure, the more CBDA that can be extracted.
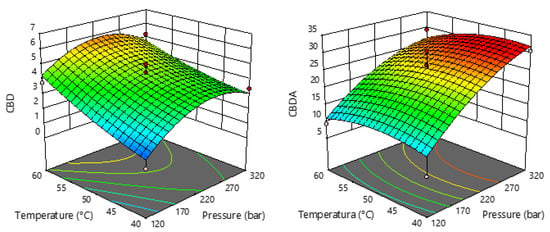
Figure 4.
Response surface plots showing the effects of temperature and pressure on CBD and CBDA.
Figure 4 also shows that higher temperature causes an increase in CBD content, while pressure has dual effects. An increase in pressure up to 250 bar resulted in an increase in CBD content, while pressures above 250 bar caused a slight decrease in content.
Kitrytė et al. [19] described that in sCO2 extracts, CBD (predominantly) and CBDA constituted about 28% of the extract, and 93% of the available CBD and CBDA were extracted. An increase in pressure resulted in a decrease in the content of cannabinoids in the extract. The highest content of cannabinoids was obtained in the extract at the lowest pressure (from 100 bar to 500 bar) and temperature (from 35 to 75 °C)—64.18 mg/g. Vági et al. [45] studied industrial hemp residues and concluded that an extraction pressure of 350 bar and a temperature of 45 °C were suitable parameters for the extraction of cannabinoids with high yields. Further increasing the pressure did not increase the extraction yield and amounts of cannabinoids in the extracts. A study by Rovetto and Aieta [10] showed that sCO2 extraction can be an excellent procedure for the extraction of cannabinoids from C. sativa, and the best results were obtained at 55 °C and 340 bar and without the addition of co-solvents. Omar et al. [46] studied the supercritical extraction of cannabinoids from 13 different samples of medicinal cannabis and analyzed the influence of the process parameters (from 100 bar to 250 bar, from 35 to 55 °C, with ethanol as co-solvent from 0 °C to 40%) on the extraction efficiency and quality. The CBD content in the extracts obtained in this way ranged from 1 mg/g to 13 mg/g, indicating that the plant material used is a good source of CBD. Perrotin-Brunel et al. [47] showed that the solubility of CBD in sCO2 increases with pressure. Interestingly, the highest solubility of CBD is obtained at a medium temperature (53 °C). This particular behavior is theoretically possible and has already been observed for the solubility of CBN in sCO2. The reason for this unusual behavior could be the transition from a solid–supercritical fluid equilibrium to a liquid–supercritical fluid equilibrium. The melting point of pure CBD (67 °C) and pure CBN (77 °C) is close to the experimental temperature of 61 °C. The melting depression effect of CO2 may have caused melting at 61 °C, resulting in lower solubility.
2.4. Antiradical and Antibacterial Activity of Hemp sCO2 Extracts
The antiradical activities of the sCO2 extracts at 250 µg mL−1 were evaluated by the DPPH scavenging assay, as shown in Table 4. Extract run 1 (320 bar; 40 °C), with the highest CBDA content of 30.68%, had the highest antiradical activity (98.06 ± 0.92%). The activity was comparable to extracts 7, 11, and 13 (220 bar; 50 °C). The lowest antiradical activity (87.56 ± 0.69%) was found for extract run 4 (120 bar; 40 °C), with a CBDA content of 6.32%. From these data, as well as data from ANOVA (Table S4), it appears that pressure had the greatest effect on antiradical activity, which is also evident from Figure 5. With the increase of pressure, better antioxidant activity is obtained.

Table 4.
Antioxidant activity of hemp sCO2 extracts expressed as % DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging activity at 250 µg mL−1.
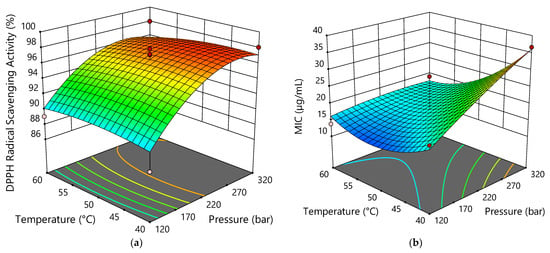
Figure 5.
Response surface plots showing the effects of temperature and pressure on antiradical activity (a) and antibacterial activity (against B. subtilis) (b).
Dawidowicz et al. [48] found that CBG, CBD, Δ9-THC, CBN, CBGA, CBDA, and Δ9-THCA have antioxidant activity due to their ability to scavenge free radicals, protect oxidation processes, and reduce metal ions. Extract run 1 also contained a high level of Δ9-tetrahydrocannabinolic acid (THCA-A) of 1.17%. McPartland et al. [49] suggested that THCA-A may be more stable in herbal cannabis, along with terpenes that serve as protective antioxidants and likely inhibit the oxidative decarboxylation of THCA to THC.
The sCO2 extracts were tested in vitro for antibacterial activity against E. coli, P. aeruginosa, B. subtilis, and S. aureus. The MIC values are shown in Table 5.

Table 5.
Minimum inhibitory concentrations (MIC) of hemp sCO2 extracts against Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis, and Staphylococcus aureus (µg mL−1).
All tested extracts exhibited good antibacterial activities against E. coli, P. aeruginosa, B. subtilis, and S. aureus. MIC values were between 10.42 µg mL−1 and 66.03 µg mL−1. The extracts were more active against Gram-positive bacteria than Gram-negative bacteria. The best antibacterial effect was observed against B. subtilis (these data were used for further analysis and optimization), while P. aeruginosa was the least sensitive to the action of the extracts. The main reason for the differences in bacterial susceptibility could be related to the presence of the outer membrane and lipopolysaccharide; Zhang et al. [50] found that membrane-disrupting drugs or LPS-deficient bacteria increased the susceptibility of Gram-negative bacteria to CBD. As previously reported, cannabinoids are thought to be responsible for the antimicrobial activity of hemp [51,52]. Among these compounds, CBD seems to be the most effective from a pharmaceutical point of view, although CBG and CBC, which are present in female hemp flowers, have remarkable antibacterial activity [53]. Appendino et al. [54] investigated the efficacy of five major cannabinoids (CBD, CBC, CBG, THC, and CBN) against six methicillin-resistant S. aureus (MRSA) strains of current clinical relevance and all showed MIC values in the range of 0.5–2 μg mL−1. The lowest MIC value against S. aureus was exhibited by extract run 6, which corresponds to a CBD content of 0.7 μg mL−1. In addition to cannabinoids, hemp volatile terpenes have been shown to be particularly important, such as α-pinene, β-myrcene and α-terpinolene, the most abundant compounds among the monoterpenes in our extracts, as well as β-caryophyllene and α-humulene as the main sesquiterpenes [51,52,55,56,57]. Extract run 6, with the highest CBD content of 67.15 µg mg−1, α-pinene content of 12.88 µg mg−1, β-pinene content of 7.78 µg mg−1, β-myrcene content of 33.45 µg mg−1, and limonene content of 6.65 µg mg−1 showed the best antibacterial activity, with a MIC value of 10.42 µg mL−1 against B. subtilis, S. aureus, and E. coli and twice the MIC value against P. aeruginosa. In run 6, the lowest pressure of 78.6 bar was used, confirming that lower pressures are desirable to obtain extracts with better antibacterial activity, which is also evident from Figure 5.
The linear terms of pressure and temperature also showed a statistically significant effect on the antibacterial activity of the sCO2 extracts (data in Table S4). According to the results of the present study, CBD content and abundant terpenes appeared to influence the antibacterial activity of the extracts. Interestingly, in the study by Blašković et al. [58], CBD showed antibacterial activity against a broad spectrum of Gram-positive bacteria but was generally inactive against 20 Gram-negative bacterial strains, including E. coli and P. aeruginosa. Issepi et al. [59] found that pure CBD had no remarkable activity against Staphylococcus strains. However, some previous studies support our findings on the synergistic antibacterial activity of volatile terpenes and CBD. Nissen et al. [52] observed good antibacterial activity of CBD and monoterpenes (α-pinene, β-pinene, and β-myrcene), especially against Listeria and Enterococcus strains.
2.5. Optimization of Extraction Conditions
By optimizing the process parameters of pressure and temperature, it is possible to influence the properties of sCO2 and extract specific plant constituents, including those that are chemically sensitive, making this technique very efficient and selective [5,6]. The applied numerical optimization of sCO2 extraction of selected bioactive compounds with certain biological activities from the inflorescences of industrial hemp would allow the process to be carried out in the most efficient way, resulting in maximum output under minimum input conditions.
Our first optimization aimed to obtain extracts with higher extraction yield, rich in CBD, and with good antiradical and antibacterial activities. The maximum value was set for the extraction yield. The best antibacterial activity in the obtained sCO2 extracts was found to be against B. subtilis; the lower value was chosen as the target value in the numerical optimization, and the highest value was chosen for antiradical activity. As for the bioactive components themselves, CBD was included in the optimization to obtain its maximum content in the extracts (for possible topical application). The optimal conditions for all these selected conditions are shown in Table 6.

Table 6.
Optimal sCO2 extraction conditions for extracts of certain characteristics.
The second optimization was performed with the two target components, CBD and CBDA, since both have the desired bioactivity, and CBDA is known to tend to be degraded to the corresponding decarboxylated analogue CBD. It can be seen that very similar values for pressure and temperature were obtained in both cases.
The third optimization was performed to obtain extracts rich in monoterpenes (with a focus on the most abundant: α-pinene, β-pinene, β-myrcene, and limonene), and the fourth focused on extracts rich in sesquiterpenes. Table 6 shows that a temperature of approximately 60 °C was optimal for all terpenes, while the low pressure of 131.2 bar was optimal for the extracts rich in monoterpenes and 319.7 bar for the extracts rich in sesquiterpenes. In the discussion section, we explained that opposite effects of pressure were found for the different terpene groups. For the monoterpenes, it was obvious that their content decreased with increasing pressure, while it increased for the sesquiterpenes.
The final optimal extraction parameters with the expected lower desirability function (0.503), with many parameters included in the calculation, were determined for the case where a maximum of terpenes and cannabinoids are preferred in sCO2 extracts with high extraction yield and high antiradical and antibacterial activity. Omar et al. [46] also performed optimization experiments for marijuana samples, and two opposite trends were observed for terpenes and cannabinoids, leading to the conclusion that different optimal extraction conditions (in terms of extraction parameters) are required depending on the type of target compound. Finally, a pressure of 100 bar and a temperature of 35 °C were chosen as optimal parameters for the extraction of both terpenes and cannabinoids. In our study of industrial hemp, the temperature of 60 °C proved to be optimal for all selected optimization conditions. Thus, each sample is specific (variety, time of harvest, part of the plant from which the extraction is performed, drying method, etc.), and experimental tests must be performed for each sample before conclusions can be drawn.
3. Materials and Methods
3.1. Plant Material
Dried inflorescences of industrial hemp (Cannabis sativa L.) variety Futura 75 obtained from a Croatian company (Hemp Agro d.o.o., Selci) were studied. Preparation of the material for extraction included crushing (grinding) and sieving on a standard series of sieves (Retsch, Grindomix GM200, Germany) [60].
3.2. Chemicals
The standards of cannabinoids purchased as solutions were used for HPLC analysis as follows: cannabidiol (CBD) (Dr. Ehrenstorfer GmbH, Augsburg, Germany), 100 µg/mL in methanol; cannabidiolic acid (CBDA) (Dr. Ehrenstorfer GmbH, Augsburg, Germany), 100.0 µg/mL in acetonitrile; cannabidivarinic acid (CBDVA), 100.0 µg/mL in acetonitrile; cannabidiolic acid (CBDA) (Cerilliant Corporation, Texas, TX, USA), 500 µg/mL in acetonitrile; cannabigerolic acid (CBGA) (Cerilliant Corporation, Texas, TX, USA), 500 µg/mL in acetonitrile; tetrahydrocannabivarinic acid (THCVA) (Cerilliant Corporation, Texas, TX, USA), 500 µg/mL in acetonitrile; tetrahydrocannabinolic acid (THCA-A) (Cerilliant Corporation, Texas, TX, USA), 500 µg/mL in acetonitrile; cannabinol (CBN) (Dr. Ehrenstorfer GmbH, Augsburg, Germany), 100 µg/mL in methanol; cannabichromene (CBC) (Dr. Ehrenstorfer GmbH, Augsburg, Germany), 100 µg/mL in methanol; cannabigerol (CBG) (Dr. Ehrenstorfer GmbH, Augsburg, Germany), 100 µg/mL in methanol; and (-)-delta9-THC solution (THC) (Cerilliant Corporation, Texas, TX, USA), 1.0 mg/mL in methanol. All solvents and chemicals used were of analytical grade.
3.3. Supercritical CO2 Extraction (sCO2)
Experiments were performed in an sCO2 system described in detail elsewhere [61]. A total of 100 g of ground dried inflorescences of industrial hemp was added to the extraction vessel. Extracts were collected in previously weighed glass tubes using a balance with an accuracy of ±0.0001 g. The extraction process took 30 min (determined as optimal time in preliminary studies). Separation conditions were 15 bar and 25 °C. The sCO2 extraction was performed under different pressure and temperature conditions, with a mass flow rate of 1.4 kg/h.
Statistical Experimental Design
The Central Composite Design (CCD) explained by Bas and Boyaci [62] was used to determine the optimal extraction conditions, pressure (X1), and temperature (X2) under which the highest content of desired constituents is obtained. The commercial software Design-Expert® (ver. 9. Stat-Ease Inc., Minneapolis, MN, USA) was used to analyze the obtained results. In addition, the quality of the fitted model was evaluated using the analysis of variance (ANOVA), while the test of statistical differences was based on the total error with a confidence level of 95.0%.
3.4. HPLC Characterization of Cannabinoids
A total of 10 µg ± 2 µg of total sCO2 extract was dissolved in 1 mL of propan-2-ol. Then, 100 μL of this solution was taken and diluted to 2 mL with methanol. For analyses, samples were filtered through a syringe filter (13 mm) with a pore size of 0.45 µm into a vial. For comparison with the sCO2 extracts, analysis of the crude material (hemp inflorescences) was performed by extracting 1 g of plant material in 20 mL of solvent (acetonitrile-methanol, 55:45% v/v) for 1 h at 40 °C on a magnetic stirrer. The mixture was filtered, and 100 µL of the obtained extract was diluted to 2 mL with methanol and then filtered in the same manner as described.
HPLC analysis of cannabinoids was performed on an Agilent 1260 Infinity II (Agilent, Santa Clara, CA, USA) with chromatographic separation on an InfinityLab Poroshell 120-C18 (4.6 mm × 150 mm, 4 µm). The separation of the analyzed compounds was performed by gradient elution at a flow rate of 1 mL/min for 67 min, using 0.1% formic acid in Millipore water (Millipore Simplicity 185, Darmstadt, Germany) as phase A and 0.05% formic acid in methanol as phase B. The gradient was set as follows: 0.00–7.05 min 40% A; 7.05–49.37 min 23% A; 49.37–67.00 min 5% A, followed by a post run time of 10 min during which conditions were reset to baseline. The injection volume was 35 µL, UV detection wavelengths were 210 nm and 230 nm, and analyses were performed at 50 °C. Cannabinoid identification was based on retention time and comparison of the absorbance spectrum in the extracts with the spectrum of standard components, while quantification was based on external calibration. The retention time for CBDVA was 23.236 min, for CBD 31.793 min, for CBG 33.123 min, for CBDA 35.253 min, for CBGA 41.228 min, for CBN 43.252 min, for THC 47.047 min, for THCVA 48.210 min, for CBC 55.261 min, for THCA 60.492 min, and for CBCA 62.358 min.
The standard stock solutions for cannabinoids were prepared in a solvent (methanol), and calibration was performed at eight concentrations, depending on the ingredients. The standard stock solution for CBDVA was prepared in the range of 1.25–125.0 µg/mL, for CBD 5.00–100 µg/mL, for CBG 1.875–75.00 µg/mL, for CBDA 1.00–50.00 µg/mL, for CBGA 1.25–125.00 µg/mL, for CBN 1.00–50.00 µg/mL, for THC 3.125–50.00 µg/mL, for THCVA 1.25–125.00 µg/mL, for CBC 1.875–75.00 µg/mL, for THCA 1.25–125.00 µg/mL, and for 1.25–125.00 µg/mL. The linearity of the calibration curve was confirmed by R2 = 0.99996 for CBDVA, R2 = 0.99868 for CBD, R2 = 0.99913 for CBG, R2 = 0.99921 for CBDA, R2 = 0.99995 for CBGA, R2 = 0.99910 for CBN, R2 = 0.99995 for THC, R2 = 0.99996 for THCVA, R2 = 0.99810 for CBC, R2 = 0.99994 for THCA, and R2 = 0.99996 for CBCA.
3.5. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of Volatile Compounds
A total of 10 µg ± 2 µg of total sCO2 extract was dissolved in 1 mL hexane and filtered through a premium syringe filter (13 mm) with polytetrafluoroethylene membrane and 0.45 µm pore size into a 2 mm brown GC vial N 9. The extract of the crude material (hemp inflorescence) was prepared for comparison with the sCO2 extracts. Then, 1 g of the plant material was macerated in 5 mL hexane for 2 h and filtered by the same procedure as described above. The prepared samples were analyzed using an Agilent 7890B gas chromatograph coupled to a mass spectrometer Agilent, series 5977A. The volatile compounds of the extracts were separated on a HP-5MS capillary column (30 m × 0.25 mm, 0.25 μm) (19091 S - 433 UI-INT, Agilent Technologies, Santa Clara, CA, USA). The injector temperature was set at 250 °C, and a 3 μL sample was injected in a 1:50 split mode. Helium with a purity of 99.99% was used as the carrier gas at a constant flow rate of 1 mL/min. The following temperature program was set: 70 °C (2 min), which was increased by 3 °C/min up to 200 °C and kept constant for 18 min. The separated components were analyzed by mass spectrometry (electron energy of 70 eV) with a scan range m/z 30–450. The temperature of the ion source was 230 °C, while the temperature of the quadrupole was 150 °C. The analysis of each sample was performed in three replicates.
3.6. Determination of Antiradical Activity of sCO2 Extracts
The antiradical activity of the extracts was determined using the DPPH radical scavenging assay described previously [63]. Then, 750 μL of diluted extracts (final concentration 250 µg mL−1) were mixed with an equal amount of a 0.2 mM DPPH radical solution so that the final DPPH radical concentration was 0.1 mM. The mixture was stirred well and incubated at room temperature for 30 min. The absorbance decrease was measured at 517 nm. Ascorbic acid (AA) was used as a reference compound in the concentration range of 2–200 μg mL−1. All experiments were performed in triplicate.
3.7. Determination of Antibacterial Activity of sCO2 Extracts
The determination of minimal inhibitory concentrations (MIC) of obtained extracts was performed by the modified broth microdilution method in Mueller Hinton Broth (Fluka, BioChemica, Germany) according to the Clinical Laboratory and Standard Institute (CLSI) M7-A7 document [64]. Bacteria were collected from different clinical samples obtained from the Microbiological Service of the Public Health Institute of Osijek-Baranja County, Croatia. Bacillus subtilis, Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa previously cultured on Mueller Hinton agar were suspended in saline at a density standardized to 0.5 McFarland standard turbidity and added to 100 μL of two-fold serially diluted obtained extracts. Each plate contained a growth control (bacterial inoculum without extracts), background control (broth and ethanol), and the antibacterial standard ciprofloxacin (Hospira, Hurley, Maidenhead, Berkshire, England, UK). After incubation at 37 °C for 24 h, another incubation at 37 °C for three hours was performed using triphenyltetrazolium chloride as a reducing agent indicator for microbial growth. The MIC was defined as the lowest extract concentration at which no color change due to microbial growth occurred, derived from triplicate analyses and normalized against the negative control.
4. Conclusions
Natural pharmaceuticals containing terpenes and especially cannabinoids are in great demand and are a hot topic today. In the present work, “green” extraction with sCO2 was successfully used to extract terpenes and cannabinoids from the inflorescences of industrial hemp. The GC-MS analysis of the sCO2 extracts revealed the chemotype of β-myrcene, with abundant α-pinene and β-pinene and a variety of other monoterpenes as minor constituents, while the most abundant sesquiterpene was β-caryophyllene. The main cannabinoids identified in the sCO2 extracts were CBDA and CBD, followed by minor amounts of CBCA, CBC, THCA-A, THC, CBGA, CBG, and CBDVA, while CBN and THCVA were not detected. The extract with the highest CBDA content, obtained at 320 bar and 40 °C, exhibited the best antiradical properties. All tested extracts showed good antibacterial activities against E. coli, P. aeruginosa, B. subtilis, and S. aureus. The sCO2 extract with the highest CBD content, containing abundant α-pinene, β-pinene, β-myrcene, and limonene, showed the best antibacterial activity. The optimal conditions for sCO2 extraction of cannabinoids and terpenes from industrial hemp were established. The temperature of 60 °C was found to be optimal for all responses studies, while the pressure showed a different effect depending on the target substance. Low pressure was optimal for monoterpenes, while higher pressure was optimal for the extracts rich in sesquiterpenes. In addition, high pressure was also beneficial for CBD.
In the proposed research work, the sCO2 extraction process was optimized to obtain the desired extracts of C. sativa, rich in the target constituents with antiradical and antibacterial properties. This environmentally friendly technological process could be implemented in a production system for the extraction of cannabinoids and terpenes with the aim of their topical application for medicinal and cosmetic purposes in a safe and effective way.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph15091117/s1. Figure S1: Representative chromatogram of the HPLC analysis of the extracts number 4. Table S1: Analysis of variance (ANOVA) for the response surface quadratic models for extraction yield. Table S2: Analysis of variance (ANOVA) for the response surface quadratic models for selected terpenes. Table S3: Analysis of variance (ANOVA) for the response surface quadratic models for CBD and CBDA. Table S4: Analysis of variance (ANOVA) for the response surface quadratic models for antioxidant and antibacterial activity.
Author Contributions
S.J. designed the experiments; K.A. and S.J. performed the extraction experiments; K.A. and I.J. performed GC-MS analysis; M.M. and M.J.K. performed HPLC analysis; V.P. performed biological activity experiments; S.J. performed statistical data analysis; S.J. and I.J. acquired funding; S.V.-K. was responsible for supervision and methodology. All the authors participated in writing, review, and editing of the manuscript and approved the final version of the paper. All authors have read and agreed to the published version of the manuscript.
Funding
The Croatian Government and the European Union (European Regional Development Fund the Competitiveness and Cohesion Operational Programme—KK.01.1.1.02.0010) funded this research through project TEHNOLOŠKO-INOVACIJSKI CENTAR VIROVITICA (KK.01.1.1.02.0010), granted to the Institution for Research and Knowledge Development of Nutrition and Health TEHNOLOŠKO-INOVACIJSKI CENTAR VIROVITICA. The Department of Biology project 3105-12-21 funded the biological investigations in this research. The APC was funded by I.J.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and Supplementary Materials.
Acknowledgments
We would like to thank the Croatian Government and the European Union (European Regional Development Fund the Competitiveness and Cohesion Operational Programme—KK.01.1.1.02.0010) for funding this research through project TEHNOLOŠKO-INOVACIJSKI CENTAR VIROVITICA (KK.01.1.1.02.0010), granted to the Institution for Research and Knowledge Development of Nutrition and Health TEHNOLOŠKO-INOVACIJSKI CENTAR VIROVITICA. The Department of Biology project 3105-12-21 funded biological investigations in this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gupta, A.K.; Talukder, M. Cannabinoids for skin diseases and hair regrowth. J. Cosmet. Dermatol. 2021, 20, 2703–2711. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, F.; Lim, M.M.; Kirchhof, M.G. A Survey of topical cannabis use in Canada. J. Cutan. Med. Surg. 2022, 26, 156–161. [Google Scholar] [CrossRef]
- Montero, L.; Meckelmann, S.W.; Kim, H.; Ayala-Cabrera, J.F.; Schmitz, O.J. Differentiation of industrial hemp strains by their cannabinoid and phenolic compounds using LC LC-HRMS. Anal. Bioanal. Chem. 2022, 414, 5445–5459. [Google Scholar] [CrossRef] [PubMed]
- Citti, C.; Braghiroli, D.; Vandelli, M.A.; Cannazza, G. Pharmaceutical and biomedical analysis of cannabinoids: A critical review. J. Pharm. Biomed. Anal. 2018, 147, 565–579. [Google Scholar] [CrossRef]
- Ahmed, A.T.M.F.; Islam, M.Z.; Mahmud, M.S.; Sarker, M.E.; Islam, M.R. Hemp as a potential raw material toward a sustainable world: A review. Heliyon 2022, 8, e08753. [Google Scholar] [CrossRef]
- Jokić, S. Matematičko Modeliranje Ekstrakcije Ulja Iz Zrna Soje Superkritičnim CO2. Ph.D. Thesis, Faculty of Food Technology Osijek, Osijek, Croatia, 2011. [Google Scholar]
- Valizadehderakhshan, M.; Shahbazi, A.; Kazem-Rostami, M.; Todd, M.S.; Bhowmik, A.; Wang, L. Extraction of cannabinoids from Cannabis sativa L. (Hemp)—Review. Agriculture 2021, 11, 384. [Google Scholar] [CrossRef]
- Al Ubeed, H.M.S.; Bhuyan, D.J.; Alsherbiny, M.A.; Basu, A.; Vuong, Q.V. A comprehensive review on the techniques for extraction of bioactive compounds from medicinal cannabis. Molecules 2022, 27, 604. [Google Scholar] [CrossRef]
- Perrotin-Brunel, H.; Kroon, M.C.; van Roosmalen, M.J.E.; van Spronsen, J.; Peters, C.J.; Witkamp, G.J. Solubility of non-psychoactive cannabinoids in supercritical carbon dioxide and comparison with psychoactive cannabinoids. J. Supercrit. Fluids 2010, 55, 603–608. [Google Scholar] [CrossRef]
- Rovetto, L.J.; Aieta, N.V. Supercritical carbon dioxide extraction of cannabinoids from Cannabis sativa L. J. Supercrit. Fluids 2017, 129, 16–27. [Google Scholar] [CrossRef]
- Gallo-Molina, A.C.; Castro-Vargas, H.I.; Garzón-Méndez, W.F.; Ramírez, J.A.M.; Monroy, Z.J.R.; King, J.W.; Parada-Alfonso, F. Extraction, isolation and purification of tetrahydrocannabinol from the Cannabis sativa L. plant using supercritical fluid extraction and solid phase extraction. J. Supercrit. Fluids 2019, 146, 208216. [Google Scholar] [CrossRef]
- Vági, E.; Balázs, M.; Komoczi, A.; Mihalovits, M.; Székely, E. Fractionation of phytocannabinoids from industrial hemp residues with high-pressure technologies. J. Supercrit. Fluids 2020, 164, 104898. [Google Scholar] [CrossRef]
- Qamar, S.; Manrique, Y.J.; Parekh, H.S.; Falconer, J.R. Development and optimization of supercritical fluid extraction setup leading to quantification of 11 cannabinoids derived from medicinal cannabis. Biology 2021, 10, 481. [Google Scholar] [CrossRef] [PubMed]
- Jhawar, N.; Schoenberg, E.; Wang, J.V.; Saedi, N. The growing trend of cannabidiol in skincare products. Clin. Dermatol. 2019, 37, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and anti-inflammatory properties of cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef]
- Marzorati, S.; Friscione, D.; Picchi, E.; Verotta, L. Cannabidiol from inflorescences of Cannabis sativa L.: Green extraction and purification processes. Ind. Crops Prod. 2020, 155, 112816. [Google Scholar] [CrossRef]
- Drinić, Z. Extraction of Industrial Hemp (Cannabis sativa L.). Ph.D. Thesis, Faculty of Technology, University of Novy Sad, Novi Sad, Serbia, 2020. [Google Scholar]
- Aladić, K.; Jarni, K.; Barbir, T.; Vidović, S.; Vladić, J.; Bilić, M.; Jokić, S. Cold pressing and supercritical CO2 extraction of hemp (Cannabis sativa) seed oil. Ind. Crops Prod. 2015, 76, 472–478. [Google Scholar] [CrossRef]
- Kitrytė, V.; Bagdonaitė, D.; Venskutonis, P.R. Biorefining of industrial hemp (Cannabis sativa L.) threshing residues into cannabinoid and antioxidant fractions by supercritical carbon dioxide, pressurized liquid and enzyme-assisted extractions. Food Chem. 2018, 267, 420–429. [Google Scholar] [CrossRef]
- Isidore, E.; Karim, H.; Ioannou, I. Extraction of phenolic compounds and terpenes from Cannabis sativa L. by-products: From conventional to intensified processes. Antioxidants 2021, 10, 942. [Google Scholar] [CrossRef]
- Kovalchuk, I.; Pellino, M.; Rigault, P.O.; van Velzen, R.; Ebersbach, J.; Ashnest, J.R.; Mau, M.; Schranz, M.E.; Alcorn, J.; Laprairie, R.B.; et al. The genomics of cannabis and its close relatives. Ann. Rev. Plant. Biol. 2020, 71, 713–739. [Google Scholar] [CrossRef]
- Allen, K.D.; McKernan, K.; Pauli, C.; Roe, J.; Torres, A.; Gaudino, R. Genomic characterization of the complete terpene synthase gene family from Cannabis sativa. PLoS ONE 2019, 14, e0222363. [Google Scholar]
- Booth, J.K.; Page, J.E.; Bohlmann, J. Terpene synthases from Cannabis sativa. PLoS ONE 2017, 12, e0173911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanuš, L.O.; Hod, Y. Terpenes/Terpenoids in Cannabis: Are they important? Med. Cannabis Cannabinoids 2020, 3, 25–60. [Google Scholar] [CrossRef] [PubMed]
- Micalizzi, G.; Vento, F.; Alibrando, F.; Donnarumma, D.; Dugo, P.; Mondello, L. Cannabis Sativa L.: A comprehensive review on the analytical methodologies for cannabinoids and terpenes characterization. J. Chromatogr. A 2021, 1637, 461864. [Google Scholar] [CrossRef]
- Gulluni, N.; Re, T.; Loiacono, I.; Lanzo, G.; Gori, L.; Macchi, C.; Epifani, F.; Bragazzi, N.; Firenzuoli, F. Cannabis essential oil: A preliminary study for the evaluation of the brain effects. Evidence-Based Complement. Alternat. Med. 2018, 2018, 1709182. [Google Scholar] [CrossRef]
- Jansen, C.; Shimoda, L.M.N.; Kawakami, J.K.; Ang, L.; Bacani, A.J.; Baker, J.D.; Badowski, C.; Speck, M.; Stokes, A.J.; Small-Howard, A.L.; et al. Myrcene and terpene regulation of TRPV1. Channels 2019, 13, 344–366. [Google Scholar] [CrossRef] [PubMed]
- Kozioł, A.; Stryjewska, A.; Librowski, T.; Sałat, K.; Gaweł, M.; Moniczewski, A.; Lochyński, S. An overview of the pharmacological properties and potential applications of natural monoterpenes. Mini Rev. Med. Chem. 2014, 14, 1156–1168. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Orhan, I.E.; Jugran, A.K.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic potential of α- and β-pinene: A miracle gift of nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef]
- Erasto, P.; Viljoen, A.M. Limonene: A review: Biosynthetic, ecological and pharmacological relevance. Nat. Prod. Commun. 2008, 3, 1193–1202. [Google Scholar] [CrossRef]
- Sun, J. D-limonene: Safety and clinical applications. Altern. Med. Rev. 2007, 12, 259–264. [Google Scholar]
- Sharma, C.; Al Kaabi, J.M.; Nurulain, S.M.; Goyal, S.N.; Kamal, M.A.; Ojha, S. Polypharmacological properties and therapeutic potential of β-caryophyllene: A dietary phytocannabinoid of pharmaceutical promise. Curr. Pharm. Des. 2016, 22, 3237–3264. [Google Scholar] [CrossRef]
- Francomano, F.; Caruso, A.; Barbarossa, A.; Fazio, A.; La Torre, C.; Ceramella, J.; Mallamaci, R.; Saturnino, C.; Iacopetta, D.; Sinicropi, M.S. β-Caryophyllene: A sesquiterpene with countless biological properties. Appl. Sci. 2019, 9, 5420. [Google Scholar] [CrossRef]
- Gertsch, J.; Leonti, M.; Raduner, S.; Racz, I.; Jian-Zhong, C.; Xiang-Qun, X.; Altmann, K.H.; Karsak, M.; Zimmer, A. Beta-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astani, A.; Reichling, J.; Schnitzler, P. Screening for antiviral activities of isolated compounds from essential oils. Evidence-Based Complement. Altern. Med. 2011, 2011, 253643. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wu, J.; Luo, Y.; Huang, N.; Zhen, N.; Zhou, Y.; Sun, F.; Li, Z.; Pan, Q.; Li, Y. (–)-Guaiol regulates RAD51 stability via autophagy to induce cell apoptosis in nonsmall cell lung cancer. Oncotarget 2016, 7, 62585–62597. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhu, J.; Wu, J.; Huang, N.; Cui, Z.; Luo, Y.; Sun, F.; Pan, Q.; Li, Y.; Yanga, Q. (–)-Guaiol regulates autophagic cell death depending on mTOR signaling in NSCLC. Cancer Biol. Ther. 2018, 19, 706–714. [Google Scholar] [CrossRef]
- Lu, Y.-C. Studies on the chemical constituents of the essential oil of Rhododendron tsinghaiense Ching. Huaxue Xuebao 1980, 38, 241–249. [Google Scholar]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef]
- Sexton, M.; Shelton, K.; Haley, P.; West, M. Evaluation of cannabinoid and terpenoid content: Cannabis flower compared to supercritical CO2 concentrate. Planta Med. 2018, 84, 234–241. [Google Scholar] [CrossRef]
- Chandra, S.; Lata, H.; ElSohly, M.A. Cannabis Sativa L.-Botany and Biotechnology; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Zorić, M.; Banožić, M.; Aladić, K.; Vladimir-Knežević, S.; Jokić, S. Supercritical CO2 extracts in cosmetic industry: Current status and future perspectives. Sustain. Chem. Pharm. 2022, 27, 100688. [Google Scholar] [CrossRef]
- Eagleston Lauren, R.M.; Kalani, N.K.; Patel, R.R.; Flaten, H.K.; Dunnick, C.A.; Dellavalle, R.P. Cannabinoids in dermatology: A scoping review. Dermatol. Online J. 2018, 24, 1. [Google Scholar] [CrossRef]
- Cabaleiro, N.; De la Calle, I.; Bendicho, C.; Lavilla, I. Current trends in liquid–liquid and solid–liquid extraction for cosmetic analysis: A review. Anal. Methods 2013, 5, 323–340. [Google Scholar] [CrossRef]
- Vági, E.; Balázs, M.; Komoczi, A.; Kiss, I.; Mihalovits, M.; Székely, E. Cannabinoids enriched extracts from industrial hemp residues. Period. Polytech. Chem. Eng. 2019, 63, 357–363. [Google Scholar] [CrossRef]
- Omar, J.; Olivares, M.; Alzaga, M.; Etxebarria, N. Optimisation and characterisation of marihuana extracts obtained by supercritical fluid extraction and focused ultrasound extraction and retention time locking GC–MS. J. Sep. Sci. 2013, 36, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Perrotin-Brunel, H.; van Roosmalen, M.J.E.; Kroon, M.C.; van Spronsen, J.; Witkamp, G.J.; Peters, C.J. Solubility of cannabinol in supercritical carbon dioxide. J. Chem. Eng. Data 2010, 55, 3704–3707. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Olszowy-Tomczyk, M.; Typek, R. CBG, CBD, Δ9-THC, CBN, CBGA, CBDA and Δ9-THCA as antioxidant agents and their intervention abilities in antioxidant action. Fitoterapia 2021, 152, 104915. [Google Scholar] [CrossRef]
- McPartland, J.M.; MacDonald, C.; Young, M.; Grant, P.S.; Furkert, D.P.; Glass, M. Affinity and efficacy studies of tetrahydrocannabinolic acid a at cannabinoid receptor types one and two. Cannabis Cannabinoid Res. 2017, 2, 87–95. [Google Scholar] [CrossRef]
- Zhang, G.; Meredith, T.C.; Kahne, D. On the essentiality of lipopolysaccharide to Gram-negative bacteria. Curr. Opin. Microbiol. 2013, 16, 779–785. [Google Scholar] [CrossRef]
- Ibrahim, E.A.; Wang, M.; Radwan, M.M.; Wanas, A.S.; Majumdar, C.G.; Avula, B.; Wang, Y.H.; Khan, I.A.; Chandra, S.; Lata, H.; et al. Analysis of terpenes in Cannabis sativa L. using GC/MS: Method development, validation and application. Planta Med. 2019, 85, 431–438. [Google Scholar] [CrossRef]
- Nissen, L.; Zatta, A.; Stefanini, I.; Grandi, S.; Sgorbati, S.; Biavati, B.; Monti, A. Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.). Fitoterapia 2010, 81, 413–419. [Google Scholar] [CrossRef]
- Leghissa, A.; Hildenbr, Z.L.; Schug, K.A. A review of methods for the chemical characterization of cannabis natural products. J. Sep. Sci. 2018, 41, 398–415. [Google Scholar] [CrossRef]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial cannabinoids from Cannabis sativa: A structure-activity study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Fiorini, D.; Molle, A.; Nabissi, M.; Santini, G.; Benelli, G.; Maggi, G.M. Valorizing industrial hemp (Cannabis sativa L.) by-products: Cannabidiol enrichment in the inflorescence essential oil optimizing sample pre-treatment prior to distillation. Ind. Crops Prod. 2019, 128, 581–589. [Google Scholar] [CrossRef]
- Hazekamp, A.; Fischedick, J.T.; Llano Dìez, M.; Lubbe, A.; Ruhaak, R.L. Chemistry of Cannabis. In Comprehensive Natural Products II Chemistry and Biology, 1st ed.; Mander, L., Liu, H.-W., Eds.; Elsevier: Kidlington, UK, 2010; Volume 3, pp. 1034–1077. [Google Scholar]
- Wang, C.-T.; Wiedinmyer, C.; Ashworth, K.; Harley, P.C.; Ortega, J.; Vizuete, W. Leaf enclosure measurements for determining volatile organic compound nemission capacity from Cannabis spp. Atmos. Environ. 2019, 199, 80–87. [Google Scholar] [CrossRef]
- Blaskovich, M.A.T.; Kavanagh, A.M.; Elliott, A.G.; Zhang, B.; Ramu, S.; Amado, M.; Lowe, G.J.; Hinton, A.O.; Thu Pham, D.M.; Zuegg, J.; et al. The antimicrobial potential of cannabidiol. Commun. Biol. 2021, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Iseppi, R.; Brighenti, V.; Licata, M.; Lambertini, A.; Sabia, C.; Messi, P.; Pellati, F.; Benvenuti, S. Chemical characterization and evaluation of the antibacterial activity of essential oils from fibre-type Cannabis sativa L. (Hemp). Molecules 2019, 24, 2302. [Google Scholar] [CrossRef]
- Jokić, S.; Molnar, M.; Jakovljević, M.; Aladić, K.; Jerković, I. Optimization of supercritical CO2 extraction of Salvia officinalis L. leaves targeted on oxygenated monoterpenes, α-humulene, viridiflorol and manool. J. Supercrit. Fluid 2018, 133, 253–262. [Google Scholar] [CrossRef]
- Jokić, S.; Horvat, G.; Aladić, K. Design of SFE system using a holistic approach–problems and challenges. In Supercritical Fluid Extraction: Technology, Applications and Limitations; Lindy, J., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2015; p. 95. [Google Scholar]
- Bas, D.; Boyaci, I.H. Modeling and optimization I: Usability of response surface methodology. J. Food Eng. 2007, 78, 836–845. [Google Scholar] [CrossRef]
- Chang, S.T.; Wu, J.H.; Wang, S.Y.; Kang, P.L.; Yang, N.S.; Shyur, L.F. Antioxidant activity of extracts from Acacia confusa Bark and Heartwood. J. Agric. Food Chem. 2001, 49, 3420–3424. [Google Scholar] [CrossRef]
- Weinstein, M.P.; Patel, J.B. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: M07-A11, 11th ed.; Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2018. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).