Synthesis, Neuroprotective Effect and Physicochemical Studies of Novel Peptide and Nootropic Analogues of Alzheimer Disease Drug
Abstract
:1. Introduction
2. Results and Discussion
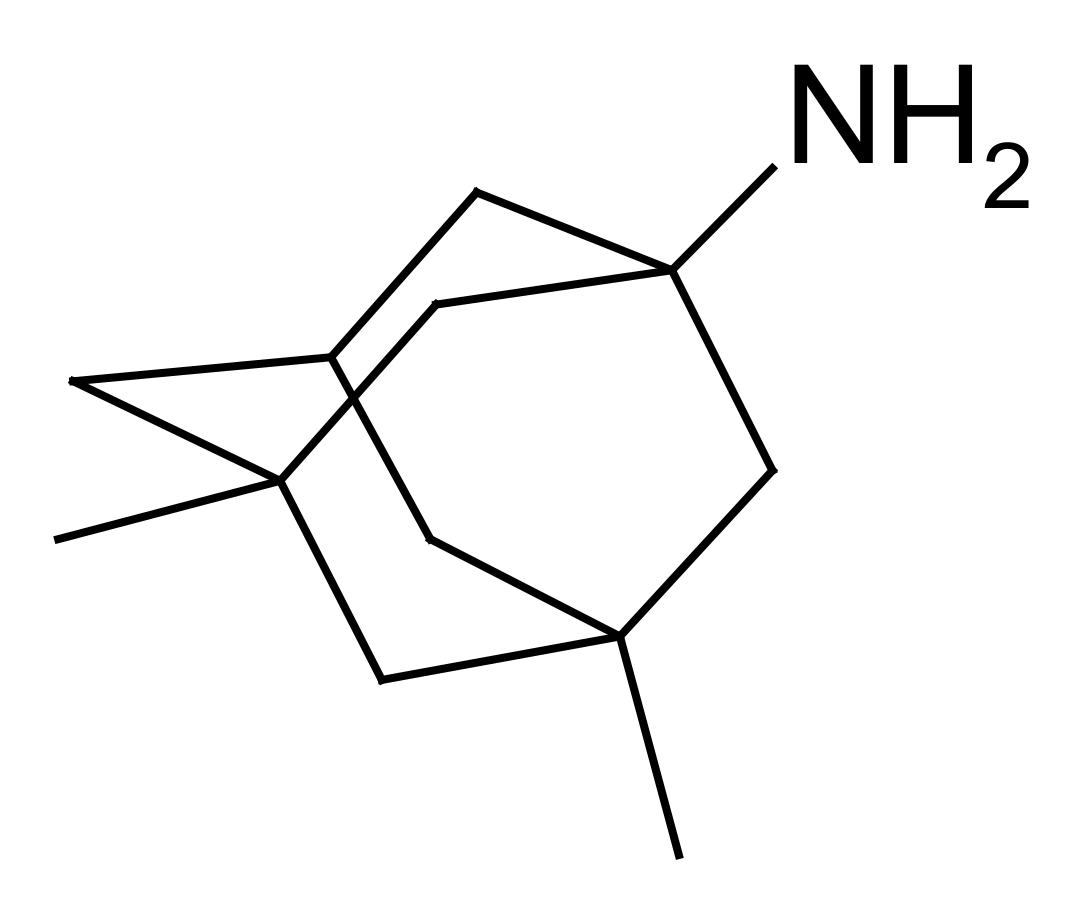
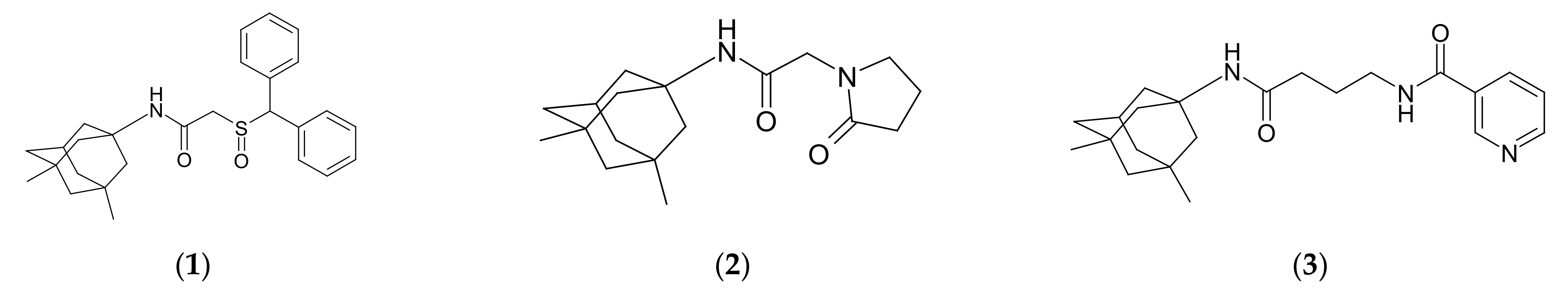
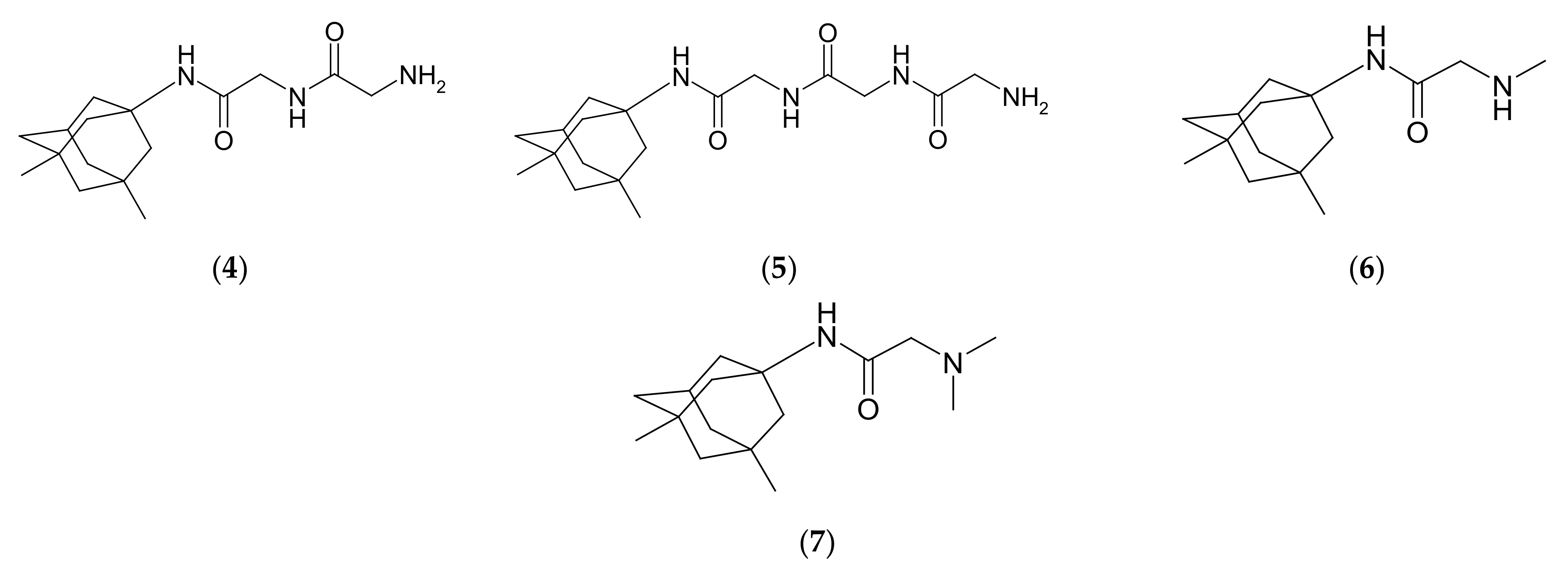
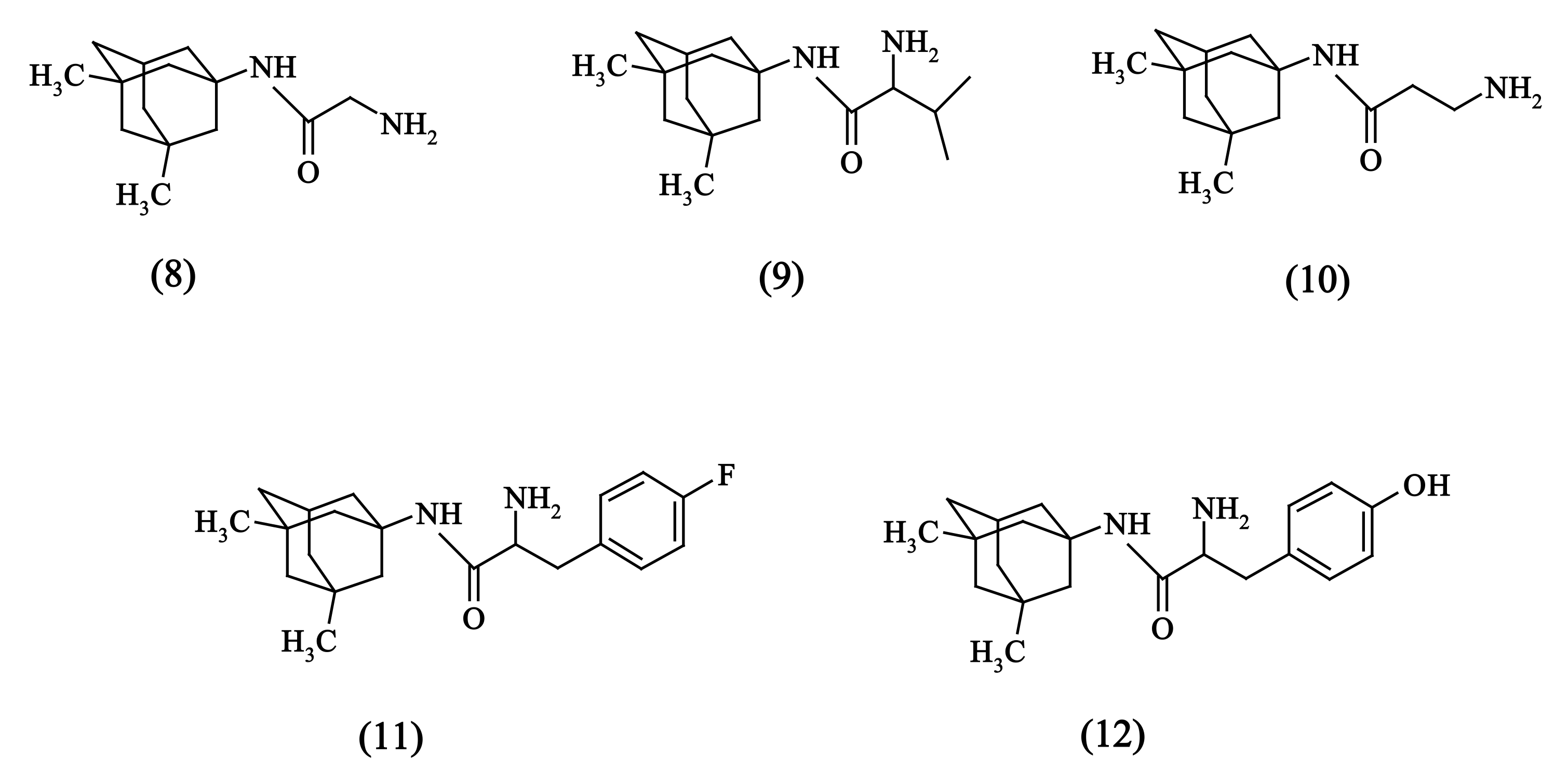
2.1. Chemistry
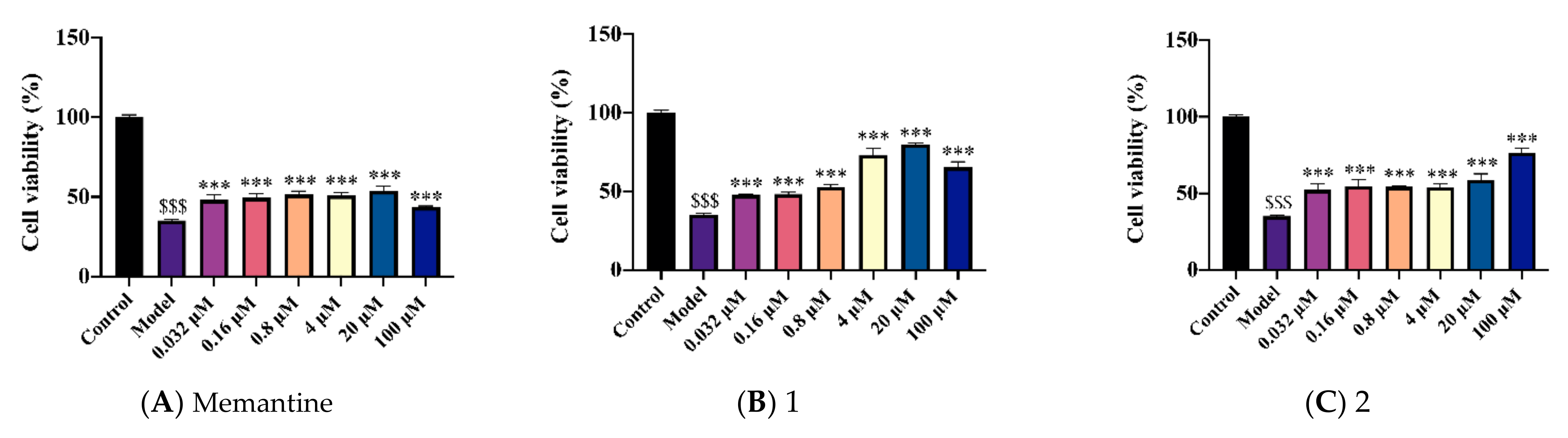
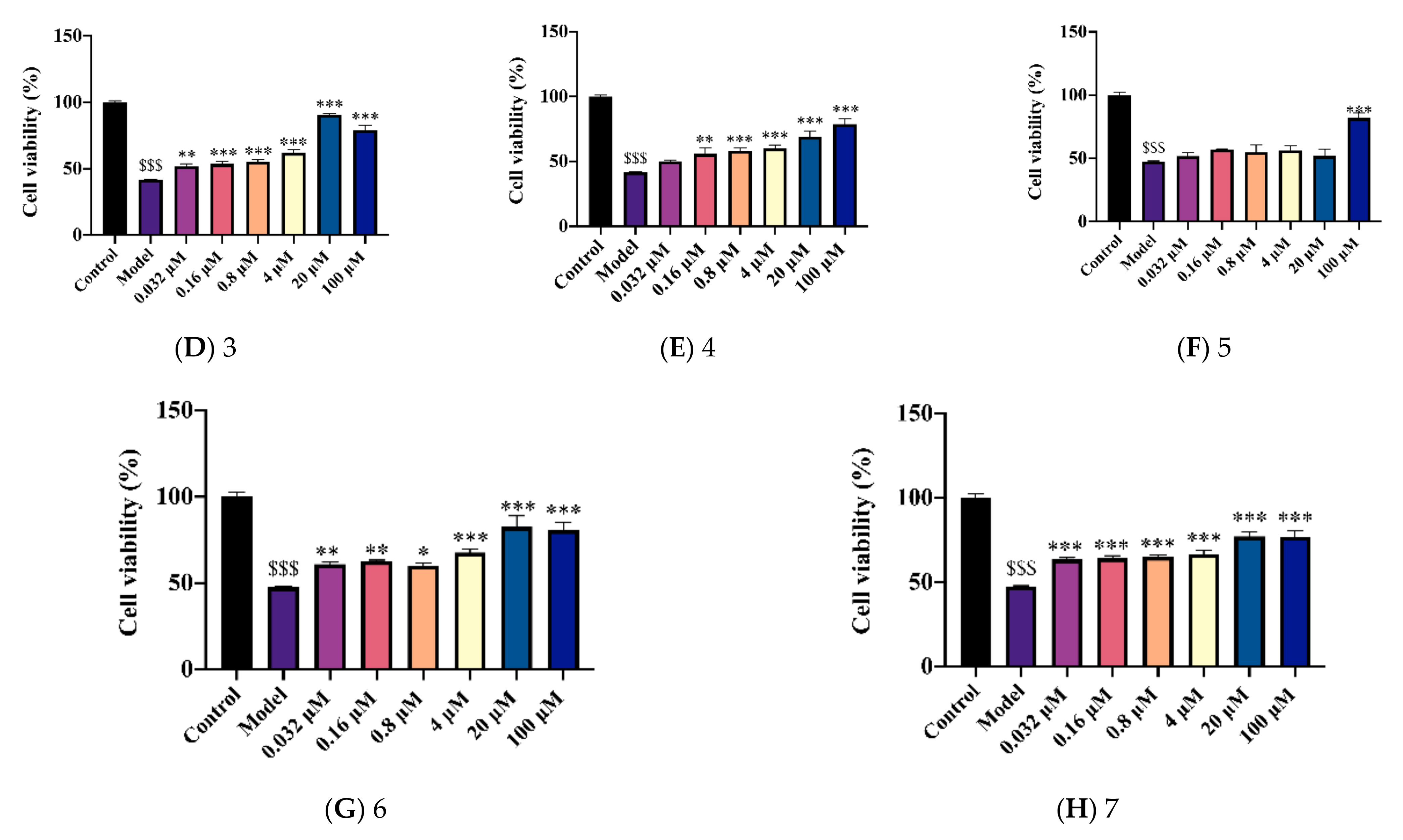
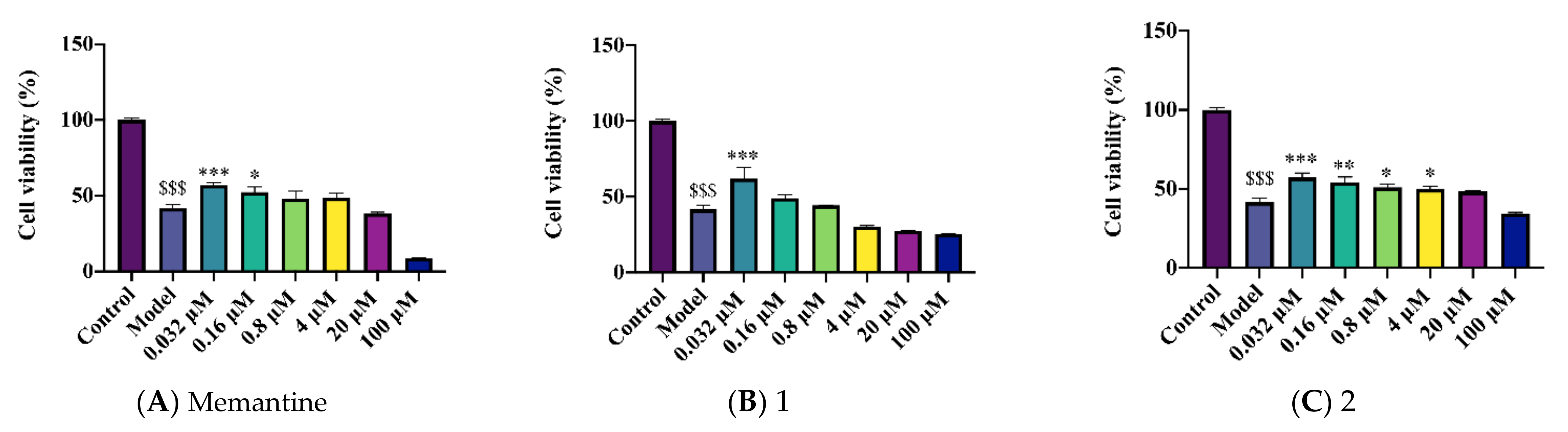
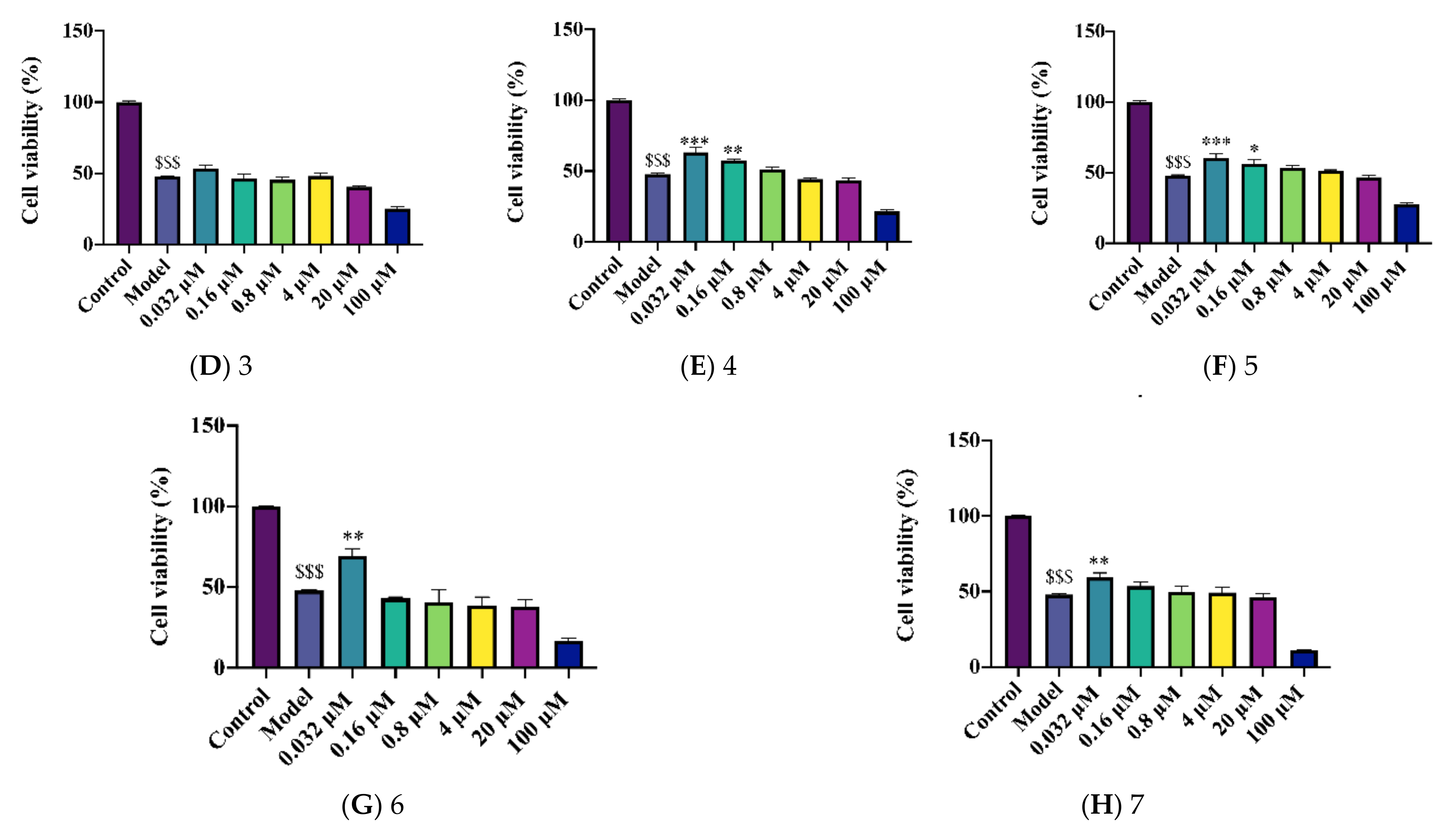
2.2. Novel Structural Derivatives of Memantine Improve Cell Viability against Copper-Induced Neurotoxicity in APPswe Cells
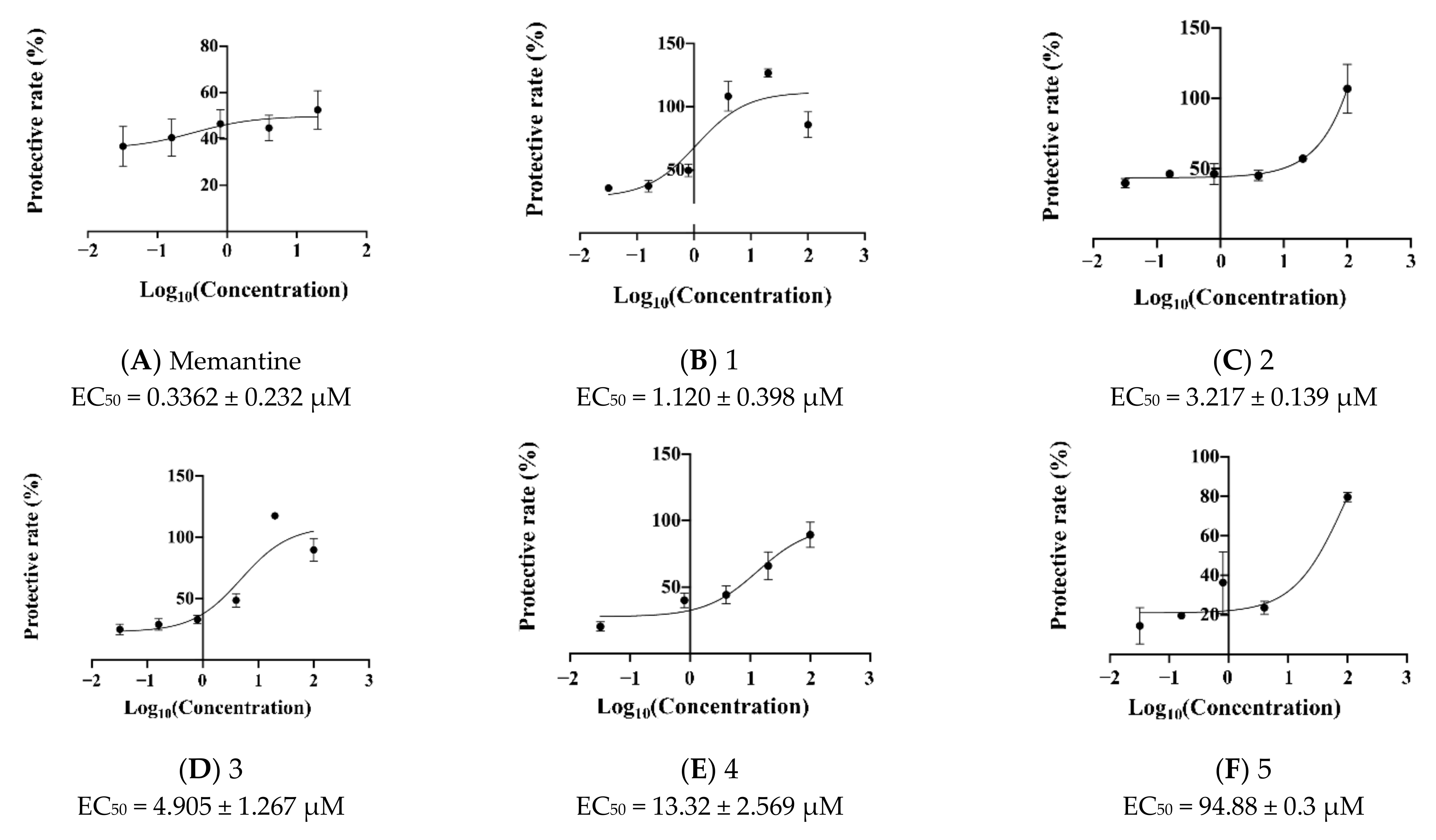
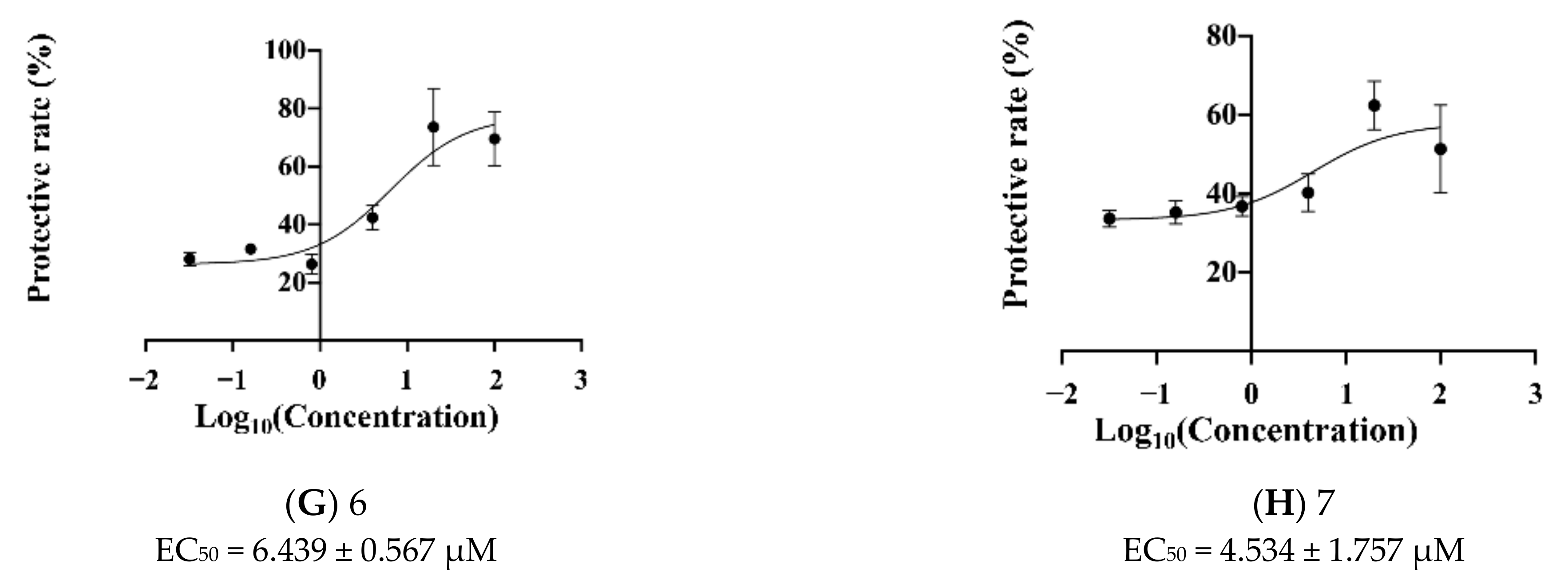
2.3. Novel Structural Derivatives of Memantine Improve Cell Viability against Glutamate-Induced Neurotoxicity in SH-SY5Y Cells
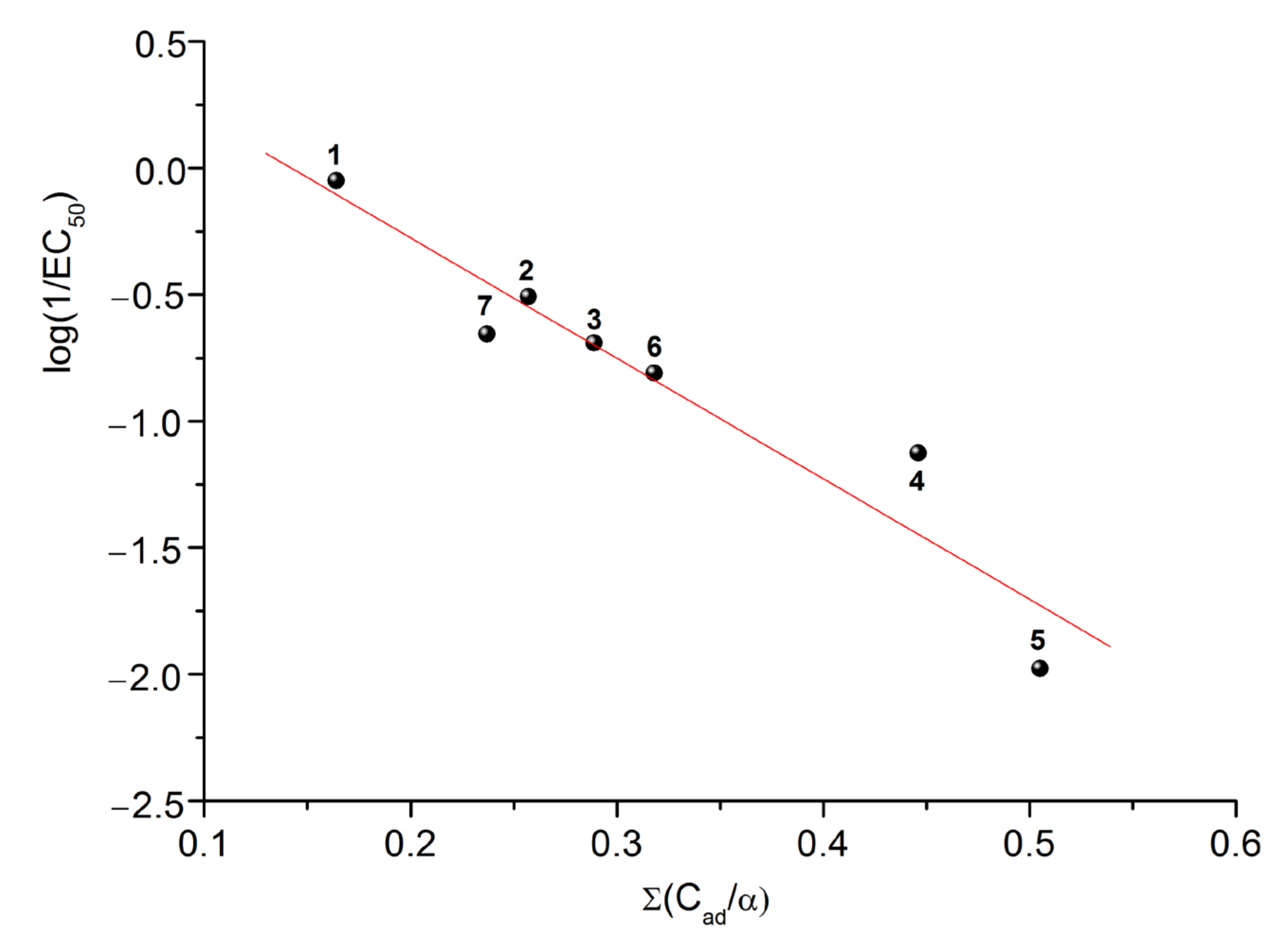
2.4. Prediction of EC50 Using HYBOT Descriptors
2.5. Solubility Experiments
3. Materials and Methods
3.1. Materials
3.1.1. Chemicals
3.1.2. Solvents
3.1.3. Cell Culture and Treatment
3.2. Chemical Synthesis
3.3. Measurement of Neuroprotective Effects against Copper-Induced Toxicity in APPswe Cells
3.4. Measurement of Neuroprotective Effects against Glutamate-Induced Toxicity in SH-SY5Y Cells
3.5. Cell Viability Assay
3.6. Statistical Analysis
3.7. Solubility Determination
3.8. Powder X-ray Diffraction (PXRD) Were Recorded According to SUROV et al. [37]
3.9. QSAR Modelling and Calculation of HYBOT Descriptors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Czarnecka, K.; Chuchmacz, J.; Wójtowicz, P.; Szymański, P. Memantine in neurological disorders—Schizophrenia and depression. J. Mol. Med. 2021, 99, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.; Albanese, E.; Guerchet, M.; Prina, M. World Alzheimer Report 2014: Dementia and Risk Reduction: An Analysis of Protective and Modifiable Risk Factors; Alzheimer’s Disease International (ADI): London, UK, 2014. [Google Scholar]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Witt, A.; Macdonald, N.; Kirkpatrick, P. Memantine hydrochloride. Nat. Rev. Drug Discov. 2004, 3, 109–110. [Google Scholar] [CrossRef] [PubMed]
- Altevogt, B.M.; Davis, M.; Pankevich, D.E. (Eds.) Glutamate-Related Biomarkers in Drug Development for Disorders of the Nervous System: Workshop Summary; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Bechtholt-Gompf, A.J.; Walther, H.V.; Adams, M.A.; Carlezon, W.A.; Öngür, D.; Cohen, B.M. Blockade of astrocytic glutamate uptake in rats induces signs of anhedonia and impaired spatial memory. Neuropsychopharmacology 2010, 35, 2049–2059. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; Kim, J.P. Butenolide derivatives from the fungus Aspergillus terreus and their radical scavenging activity and protective activity against glutamate-induced excitotoxicity. Appl. Biol. Chem. 2019, 62, 1–5. [Google Scholar] [CrossRef]
- Atlante, A.; Calissano, P.; Bobba, A.; Giannattasio, S.; Marra, E.; Passarella, S. Glutamate neurotoxicity, oxidative stress and mitochondria. FEBS Lett. 2001, 497, 1–5. [Google Scholar] [CrossRef]
- Stankova, I.; Stoilkova, A.; Chayrov, R.; Tsvetanova, E.; Georgieva, A.; Alexandrova, A. In Vitro Antioxidant Activity of Memantine Derivatives Containing Amino Acids. Pharm. Chem. J. 2020, 54, 268–272. [Google Scholar] [CrossRef]
- Bai, K.; Jiang, L.; Zhu, S.; Feng, C.; Zhao, Y.; Zhang, L.; Wang, T. Dimethylglycine sodium salt protects against oxidative damage and mitochondrial dysfunction in the small intestines of mice. Int. J. Mol. Med. 2019, 43, 2199–2211. [Google Scholar] [CrossRef]
- Hariganesh, K.; Prathiba, J. Effect of dimethylglycine on gastric ulcers in rats. J. Pharm. Pharmacol. 2000, 52, 1519–1522. [Google Scholar] [CrossRef] [PubMed]
- Bai, K.; Xu, W.; Zhang, J.; Kou, T.; Niu, Y.; Wan, X.; Wang, T. Assessment of free radical scavenging activity of dimethylglycine sodium salt and its role in providing protection against lipopolysaccharide-induced oxidative stress in mice. PLoS ONE 2016, 11, e0155393. [Google Scholar] [CrossRef] [Green Version]
- Friesen, R.W.; Novak, E.M.; Hasman, D.; Innis, S.M. Relationship of dimethylglycine, choline, and betaine with oxoproline in plasma of pregnant women and their newborn infants. J. Nutr. 2007, 137, 2641–2646. [Google Scholar] [CrossRef] [PubMed]
- Clapes, P.; Rosa Infante, M. Amino acid-based surfactants: Enzymatic synthesis, properties and potential applications. Biocatal. Biotransformation 2002, 20, 215–233. [Google Scholar] [CrossRef]
- Curtis, D. A possible role for sarcosine in the management of schizophrenia. Br. J. Psychiatry 2019, 215, 697–698. [Google Scholar] [CrossRef]
- Chang, C.H.; Lin, C.H.; Liu, C.Y.; Chen, S.J.; Lane, H.Y. Efficacy and cognitive effect of sarcosine (N-methylglycine) in patients with schizophrenia: A systematic review and meta-analysis of double-blind randomised controlled trials. J. Psychopharmacol. 2020, 34, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Colucci, L.; Bosco, M.; Ziello, A.R.; Rea, R.; Amenta, F.; Fasanaro, A.M. Effectiveness of nootropic drugs with cholinergic activity in treatment of cognitive deficit: A review. J. Exp. Pharmacol. 2012, 4, 163. [Google Scholar] [CrossRef]
- Wisor, J.P. Modafinil as a catecholaminergic agent: Empirical evidence and unanswered questions. Front. Neurol. 2013, 4, 139. [Google Scholar] [CrossRef]
- Volkow, N.D.; Fowler, J.S.; Logan, J.; Alexoff, D.; Zhu, W.; Telang, F.; Apelskog-Torres, K. Effects of modafinil on dopamine and dopamine transporters in the male human brain: Clinical implications. JAMA 2009, 301, 1148–1154. [Google Scholar] [CrossRef]
- Abbasi, Y.; Shabani, R.; Mousavizadeh, K.; Soleimani, M.; Mehdizadeh, M. Neuroprotective effect of ethanol and Modafinil on focal cerebral ischemia in rats. Metab. Brain Dis. 2019, 34, 805–819. [Google Scholar] [CrossRef]
- Malykh, A.G.; Sadaie, M.R. Piracetam and piracetam-like drugs. Drugs 2010, 70, 287–312. [Google Scholar] [CrossRef] [PubMed]
- Fesenko, U.A. Piracetam improves children’s memory after general anaesthesia. Anestezjol. Intensywna Ter. 2009, 41, 16–21. [Google Scholar]
- Holinski, S.; Claus, B.; Alaaraj, N.; Dohmen, P.M.; Kirilova, K.; Neumann, K.; Konertz, W. Cerebroprotective effect of piracetam in patients undergoing coronary bypass burgery. Med. Sci. Monit. 2008, 14, PI53–PI57. [Google Scholar] [PubMed]
- Nickolson, V.J.; Wolthuis, O.L. Effect of the acquisition-enhancing drug ptracetam on rat cerebral energy metabolism. Comparison with naftidrofuryl and methamphetamine. Biochem. Pharmacol. 1976, 25, 2241–2244. [Google Scholar] [CrossRef]
- Grau, M.; Montero, J.L.; Balasch, J. Effect of Piracetam on electrocorticogram and local cerebral glucose utilization in the rat. Gen. Pharmacol. Vasc. Syst. 1987, 18, 205–211. [Google Scholar] [CrossRef]
- Mirzoian, R.S.; Gan’shina, T.S.; Kim, G.A.; Kurza, E.V.; Maslennikov, D.V.; Il’ya, N.K.; Gorbunov, A.A. The translational potential of experimental pharmacology for cerebrovascular disorders. Ann. Clin. Exp. Neurol. 2019, 13, 34–40. [Google Scholar]
- Mishchenko, O.; Palagina, N. Experimental research of cerebroprotective activity of the new 4-aminobutatanoic acid derivative. EUREKA Health Sci. 2021, 3, 95–100. [Google Scholar] [CrossRef]
- Tabassum, N.; Rasool, S.; Malik, Z.A.; Ahmad, F. Natural cognitive enhancers. J. Pharm. Res. 2012, 5, 153–160. [Google Scholar]
- Rosini, M.; Simoni, E.; Caporaso, R.; Basagni, F.; Catanzaro, M.; Abu, I.F.; Fagiani, F.; Fusco, F.; Masuzzo, S.; Albani, D.; et al. Merging memantine and ferulic acid to probe connections between NMDA receptors, oxidative stress and amyloid-β peptide in Alzheimer’s disease. Eur. J. Med. Chem. 2019, 180, 111–120. [Google Scholar] [CrossRef]
- Turcu, A.; Companys-Alemany, J.; Phillips, M.B.; Patel, D.S.; Griñán-Ferré, C.; Loza, M.I.; Brea, J.M.; Pérez, B.; Soto, D.; Sureda, F.X.; et al. Design, synthesis, and in vitro and in vivo characterization of new memantine analogs for Alzheimer’s disease. Eur. J. Med. Chem. 2022, 236, 114354–114382. [Google Scholar] [CrossRef]
- Glomme, A.; März, J.; Dressman, J.B. Comparison of a miniaturized shake-flask solubility method with automated potentiometric acid/base titrations and calculated solubilities. J. Pharm. Sci. 2005, 94, 1–16. [Google Scholar] [CrossRef]
- Tencheva, A.; Liu, R.; Volkova, T.V.; Chayrov, R.; Mitrev, Y.; Štícha, M.; Li, Y.; Jiang, H.; Li, Z.; Stankova, I.; et al. Synthetic analogues of memantine as neuroprotective and influenza viral inhibitors: In vitro and physicochemical studies. Amino Acids 2020, 52, 1559–1580. [Google Scholar] [CrossRef]
- Knorr, R.; Trzeciak, A.; Bannwarth, W.; Gillessen, D. New coupling reagents in peptide chemistry. Tetrahedron Lett. 1989, 30, 1927–1930. [Google Scholar] [CrossRef]
- Higuchi, T.; Connors, K. Phase-solubility techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–123. [Google Scholar]
- Zeng, L.; Jiang, H.; Ashraf, G.M.; Liu, J.; Wang, L.; Zhao, K.; Liu, M.; Li, Z.; Liu, R. Implications of miR-148a-3p/p35/PTEN signaling in tau hyperphosphorylation and autoregulatory feedforward of Akt/CREB in Alzheimerés disease. Mol. Ther. Nucleic Acids 2021, 27, 256–275. [Google Scholar] [CrossRef]
- Raevsky, O.A.; Grigor’ev, V.J.; Trepalin, S.V. HYBOT Program Package. Registration by Russian State Patent Agency. No. 990090 26 February 1999. [Google Scholar]
- Surov, A.O.; Volkova, T.V. Solubility/distribution thermodynamics and permeability of two anthelmintics in biologically relevant solvents. J. Mol. Liq. 2022, 354, 118835–118862. [Google Scholar] [CrossRef]

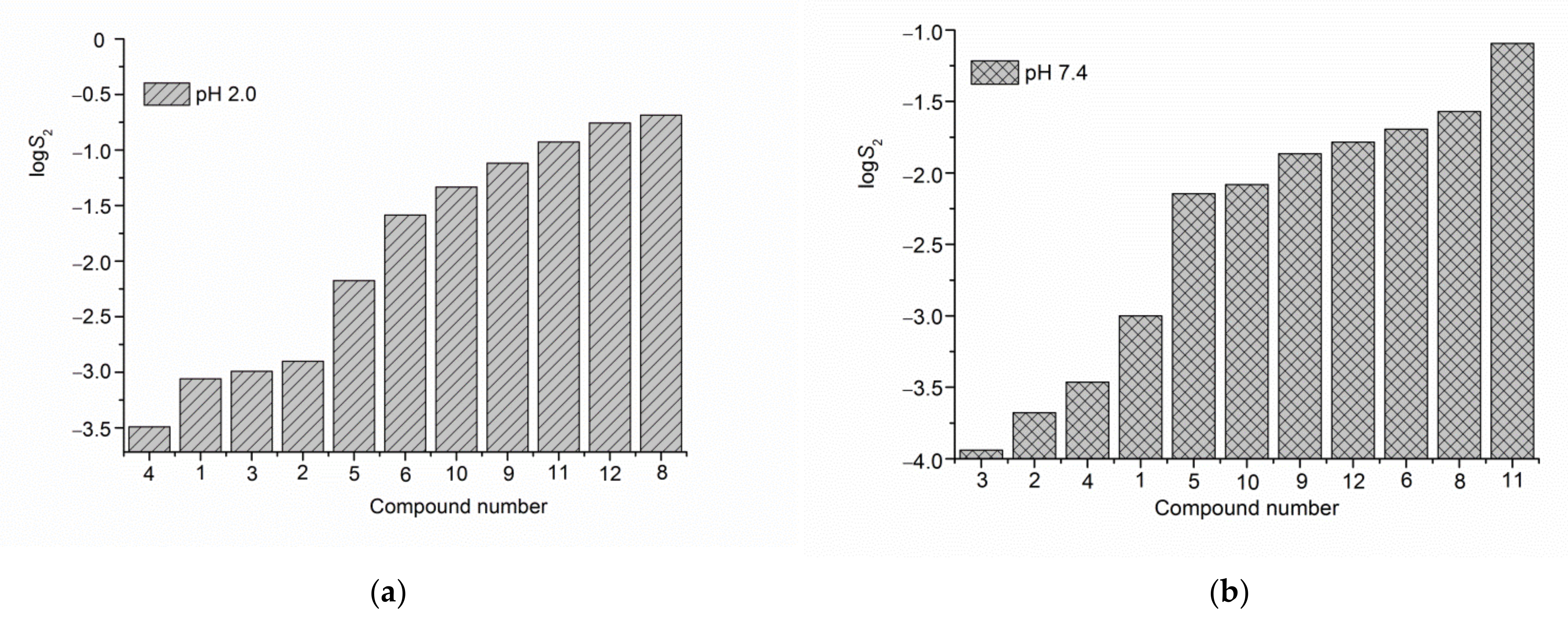
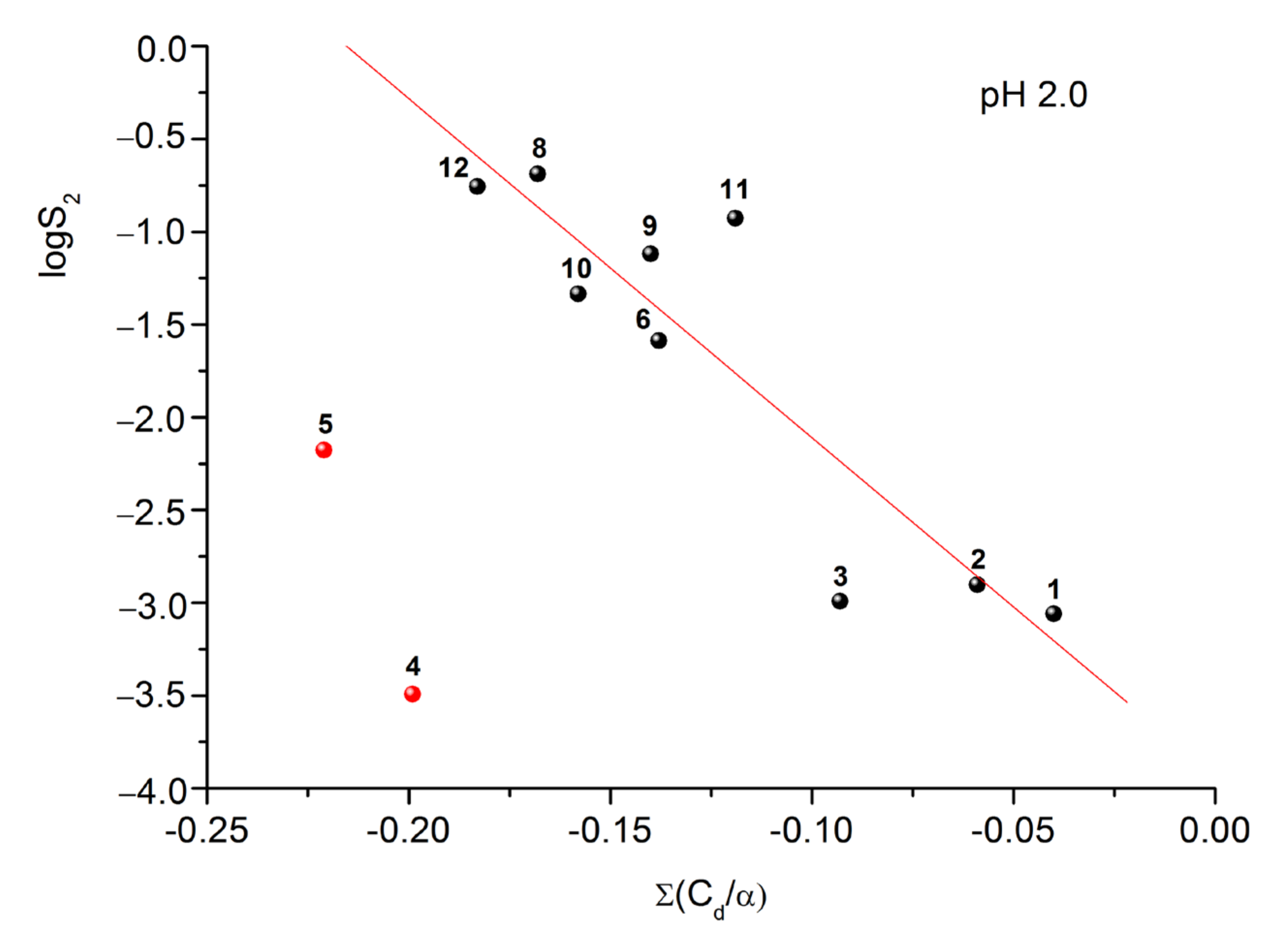
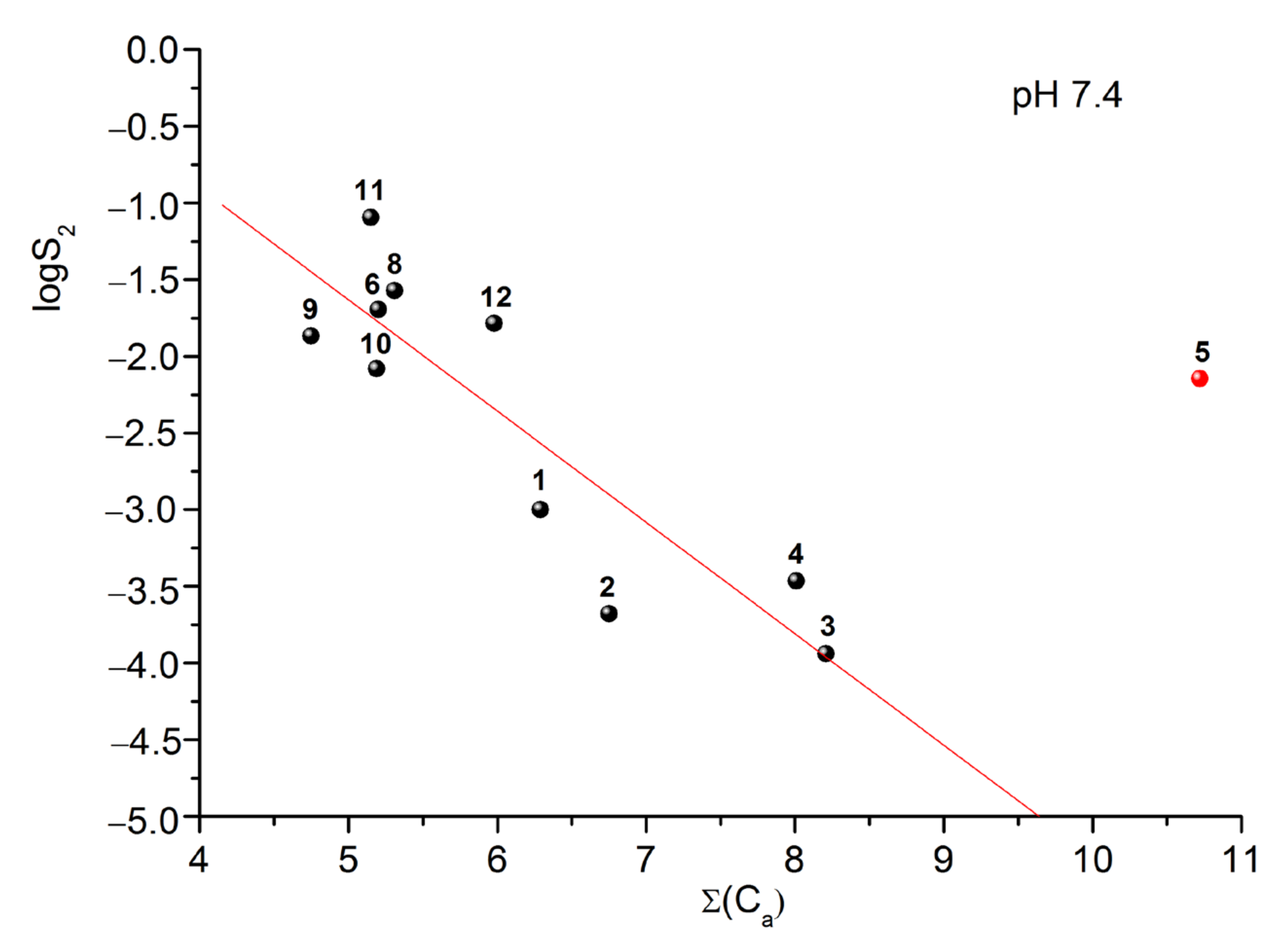
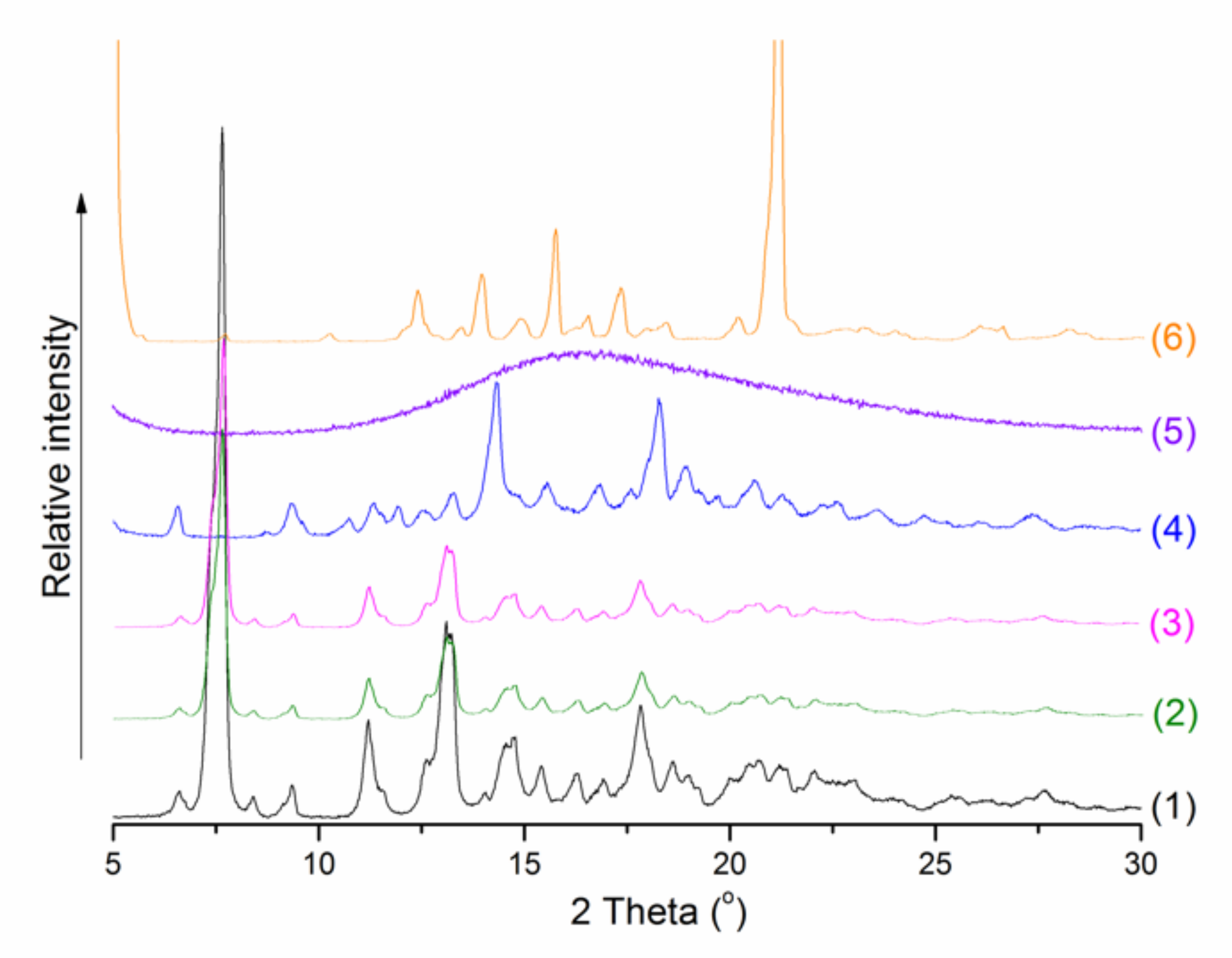
| № Compound | pH 2.0 | pH 7.4 |
|---|---|---|
| S2·103 (M) | S2·104 (M) | |
| (1) | 0.873 ± 0.022 | 9.93 ± 0.25 |
| (2) | 1.25 ± 0.03 | 2.10 ± 0.05 |
| (3) | 1.02 ± 0.03 | 1.15 ± 0.03 |
| (4) | 0.321 ± 0.008 | 3.44 ± 0.09 |
| (5) | 6.67 ± 0.17 | 71.5 ± 1.8 |
| (6) | 25.88 ± 0.65 | 201.8 ± 5.1 |
| (8) a | 205.1 ± 5.1 | 269.2 ± 6.7 |
| (9) a | 75.9 ± 1.9 | 136.1 ± 3.4 |
| (10) a | 46.2 ± 1.1 | 83.0 ± 2.1 |
| (11) a | 118.0 ± 3.0 | 804 ± 20 |
| (12) a | 175.0 ± 4.4 | 164.1 ± 4.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chayrov, R.; Volkova, T.; Perlovich, G.; Zeng, L.; Li, Z.; Štícha, M.; Liu, R.; Stankova, I. Synthesis, Neuroprotective Effect and Physicochemical Studies of Novel Peptide and Nootropic Analogues of Alzheimer Disease Drug. Pharmaceuticals 2022, 15, 1108. https://doi.org/10.3390/ph15091108
Chayrov R, Volkova T, Perlovich G, Zeng L, Li Z, Štícha M, Liu R, Stankova I. Synthesis, Neuroprotective Effect and Physicochemical Studies of Novel Peptide and Nootropic Analogues of Alzheimer Disease Drug. Pharmaceuticals. 2022; 15(9):1108. https://doi.org/10.3390/ph15091108
Chicago/Turabian StyleChayrov, Radoslav, Tatyana Volkova, German Perlovich, Li Zeng, Zhuorong Li, Martin Štícha, Rui Liu, and Ivanka Stankova. 2022. "Synthesis, Neuroprotective Effect and Physicochemical Studies of Novel Peptide and Nootropic Analogues of Alzheimer Disease Drug" Pharmaceuticals 15, no. 9: 1108. https://doi.org/10.3390/ph15091108
APA StyleChayrov, R., Volkova, T., Perlovich, G., Zeng, L., Li, Z., Štícha, M., Liu, R., & Stankova, I. (2022). Synthesis, Neuroprotective Effect and Physicochemical Studies of Novel Peptide and Nootropic Analogues of Alzheimer Disease Drug. Pharmaceuticals, 15(9), 1108. https://doi.org/10.3390/ph15091108








