Medicinal Plants from Latin America with Wound Healing Activity: Ethnomedicine, Phytochemistry, Preclinical and Clinical Studies—A Review
Abstract
1. Introduction
2. Results
2.1. Ethnomedicinal Information
2.2. Preclinical Wound Healing Research
| Botanical Family | Plant Name | Solvent/Plant Part/ Vehicle | Method/Cell Line/ Main Outcome/Wound Healing Activity, % | Reference |
|---|---|---|---|---|
| Amaranthaceae | Iresine herbstii Hook. | n-hexane and ethanol, leaves, aerial parts, and flowers | Scratch assay, Swiss 3T3 mouse fibroblasts, 34.33% (ethanolic extract) and 28.26% (hexanic extract) at 10 μg/mL | [15] |
| Anacardiaceae | Schinus molle L. | n-hexane and ethanol, leaves, aerial parts, and flowers | Scratch assay, Swiss 3T3 mouse fibroblasts, 76.22% (ethanolic extract) and 50.76% (hexanic extract) at 10 μg/mL | [15] |
| Annonaceae | Annona crassiflora Mart. | methanol-acetone-water (7:7:6 v/v/v), seeds | Scratch assay, HaCaT cells, 54% at 1.8 μg/mL and 73% at 3.6 μg/mL | [65] |
| Apiaceae | Petroselinum crispum (Mill.) Fuss | Methanol, leaves | Scratch assay, A549 cells, 59.08% at 500 μg/mL | [66] |
| Apocynaceae | Hancornia speciosa Gomes | 96% ethanol, leaves | Scratch assay, primary human gingival fibroblasts, 42.8% at 25 μg/mL | [67] |
| Arecaceae | Attalea speciosa Mart. | Oil, fruit, DMSO (maximal final concentration of 0.5%) | Scratch assay, L929 fibroblasts, concentration dependent manner 3.12–12.5 µg/mL (AUC) | [68] |
| Asteraceae | Achyrocline satureioides (Lam.) DC. | Ethanol, aerial parts | MTT assay, HaCaT cells, stimulated keratinocyte proliferation at 1 μg/mL | [69] |
| Bidens pilosa L. | Ethanol and decoction, aerial parts | MTT assay, HaCaT cells No effect | [69] | |
| Chaptalia nutans (L.) Pol. | Ethanol and decoction, aerial parts | MTT assay, HaCaT cells No effect | [69] | |
| Eupatorium laevigatum Lam. | Ethanol, leaves, aerial parts, and flowers | Scratch assay, Swiss 3T3 mouse fibroblasts, 30.14% at 10 μg/mL | [15] | |
| Galinsoga parviflora Cav. | n-hexane and ethanol, leaves, aerial parts, and flowers | Scratch assay, Swiss 3T3 mouse fibroblasts, 64.3% (hexanic extract) and 59.83% (ethanolic extract) at 10 μg/mL | [15] | |
| Pluchea sagittalis (Lam.) Cabrera | n-hexane and ethanol, leaves, aerial parts, and flowers | Scratch assay, Swiss 3T3 mouse fibroblasts, 40.66% (hexanic extract) and 43.93% (ethanolic extract) at 10 μg/mL | [15] | |
| Wedelia trilobata (L.) Hitchc. (accepted name: Sphagneticola trilobata (L.) Pruski) | 95% ethanol, leaves defatted with hexane: ethyl acetate fraction from column chromatography | Scratch assay, L929 fibroblasts, ethyl acetate fraction: 65.7% migration rate in day 1 and 70.5% closure in day 2 at 3 μg/mL | [70] | |
| Xanthium cavanillesii Schouw (accepted name: Xanthium strumarium L.) | n-hexane and ethanol, leaves, aerial parts | Scratch assay, Swiss 3T3 mouse fibroblasts, 9.94% (hexanic extract) and 41.17% (ethanolic extract) at 10 μg/mL | [15] | |
| Bignoniaceae | Arrabidaea chica (Bonpl.) B. Verl. (accepted name: Fridericia chica (Bonpl.) L.G. Lohmann) | Methanol/0.3% citric acid solution, leaves | MTT assay, confluent primary human fibroblasts, growth stimulation (0.25–250 μg/mL). EC50 = 30 μg/mL | [71] |
| Burseraceae | Bursera morelensis Ramirez | Hydro-distillation, essential oil, stems, DMEM | Scratch assay, human fibroblasts, ↑ cell migration at 0.01 mg/mL | [48] |
| Cactaceae | Pereskia aculeata Mill. | 95% (v/v) ethanol, leaves, DMEM | Scratch assay, L929 mouse fibroblast cells No effect | [72] |
| Cordiaceae | Cordia americana (L.) Gottschling and J.S. Mill. | Ethanol, Leaves (0.084 μg/mL of rosmarinic acid) | Scratch assay, Swiss 3T3 mouse fibroblasts, 9.8% at 1 μg/mL | [73] |
| Crassulaceae | Sedum dendroideum DC. | n-hexane and ethanol, leaves, aerial parts | Scratch assay, Swiss 3T3 mouse fibroblasts, 37.21% (ethanolic extract) and 27.86% (hexanic extract) at 10 μg/mL | [15] |
| Fabaceae | Bauhinia ungulata L. | Stem wood, extracted successively with hexane and ethanol. Liquid-liquid fractioning, ethyl acetate fraction | Scratch assay, A549 human epithelial cells, ↑ cell migration process and ↓ lesion area to approximately 32.6% and 22.0% at 10 and 100 μg/mL, respectively. | [74] |
| Dipteryx alata Vogel | 95% ethanol, nuts, DMEM/F12 medium | Scratch assay, A549 adenocarcinoma cell line, after 72 h 83% and 67% at 0.5 and 1mg/mL, respectively | [75] | |
| Mimosa tenuiflora (Willd.) Poir. | Water, bark, ethanol-precipitated compounds | MTT, WST, and BrdU incorporation assays, primary natural human fibroblasts (pNHDF), stimulated mitochondrial activity and proliferation at 10 μg/mL | [76] | |
| Parapiptadenia rigida (Benth.) Brenan | Ethanol, bark, fractionation of the extract afforded five known catechin derivatives, DMSO | Scratch assay, Swiss 3T3 mouse fibroblasts, ethanolic extract ~40% at 10 μg/mL | [15] | |
| Poincianella pluviosa (DC.) L.P. Queiroz | Ethanol-water (1:1 v/v), bark, and fraction | MTT and BrdU incorporation assays, HaCaT cells and human primary dermal fibroblasts (pNHDF). Stimulation of mitochondrial activity and ↑ keratinocyte proliferation | [77] | |
| Hypericaceae | Hypericum carinatum Griseb. | n-hexane and cold acetone, aerial parts, phloroglucinol-enriched fractions | Scratch assay, HaCaT cells, ↑ 138.7% cell proliferation at 15 μg/mL | [78] |
| Loranthaceae | Struthanthus vulgaris (Vell.) Mart. | Ethanol, leaves, defatted with hexane | Scratch assay, Swiss 3T3 mouse fibroblasts, 56.2% at 100 μg/mL | [79] |
| Lythraceae | Lafoensia pacari A.St.-Hil. | Hydroethanolic solution (1:10, w/v), leaves, DMEM medium | Scratch assay, L929 cells, ↑ proliferation/migration of 23.1% and 35.3% at 0.1 and 0.03 μg/mL, respectively | [80] |
| Malvaceae | Waltheria douradinha A. St.-Hil. (syn. Waltheria communis A. St.-Hil.) | n-hexane and ethanol, leaves, aerial parts, and flowers | Scratch assay, Swiss 3T3 mouse fibroblasts, 79.70% (hexanic extract) and 54.73% (ethanolic extract) at 10 μg/mL | [15] |
| Moraceae | Sorocea houlletiana Gaudich. (accepted name: Sorocea guilleminiana Gaudich.) | Water, leaves, topical, 0.1% DMSO in DMEM | Scratch assay, N3T3 fibroblasts, ~90% proliferation/migration rate at 4 μg/mL | [81] |
| Myrtaceae | Eugenia dysenterica DC. | Essential oil, hydro distillation, leaves | Scratch assay, L929 cells, 100% at 542.2 μg/mL | [82] |
| Plinia peruviana (Poir.) Govaerts | 50% ethanol solution (v/v), fruit peels | Scratch Assay, L929 cells No effect | [83] | |
| Nyctaginaceae | Mirabilis jalapa L. | Ethanol and decoction, aerial parts | MTT assay, HaCaT cells, stimulated keratinocyte proliferation at 25 μg/mL, both extracts | [69] |
| Onagraceae | Fuchsia magellanica Lam. | 50% (v/v) ethanol, leaves | Scratch assay, Swiss 3T3 mouse fibroblasts (120.26%) and HaCaT keratinocytes (114.61%) at 2.5 µg/mL | [84] |
| Petiveriaceae | Petiveria alliacea L. | n-hexane and ethanol, leaves, aerial parts, and flowers | Scratch assay, Swiss 3T3 albino mouse fibroblasts, ethanolic 10.26% at 10 μg/mL | [15] |
| Piperaceae | Piper regnellii (Miq.) C. DC. | Ethanol, leaves, aerial parts, and flowers | Scratch assay, Swiss 3T3 mouse fibroblasts, ethanolic 22.11% at 10 μg/mL | [15] |
| Plantaginaceae | Plantago australis Lam. | Hydroethanolic solution (30% water and 70% ethanol), leaves | Scratch assay, HaCaT cells, 81.06% at 25 µg/mL | [85] |
| Scrophulariaceae | Buddleja cordata Kunth | (CH2Cl2-methanol) (1:1), Leaves, DMSO- DMEM-F12 | Scratch assay, fibroblasts FBH, 33.1% at 50 μg/mL | [86] |
| Solanaceae | Brugmansia suaveolens (Humb. and Bonpl. ex Willd.) Sweet | n-hexane and ethanol, leaves, aerial parts, and flowers | Scratch assay, Swiss 3T3 mouse fibroblasts, hexanic: 26.66% and ethanolic: 9.83% at 10 μg/mL | [15] |
| Solanum diploconos (Mart.) Bohs | 95% ethanol, fresh ripe fruit (peel and pulp with seeds), DMEM | Scratch assay, Murine L929 cells, ↑ fibroblast migration at 1, 10, or 100 μg/mL | [87] |
| Botanical Family | Plant Name | Solvent/Plant Part/ Administration/ Vehicle | Percentage of Wound Healing Activity (Model of Study) | Reference |
|---|---|---|---|---|
| Anacardiaceae | Anacardium occidentale L. | Unripe cashew, fruit pulp, oral, water (juice) 1:1 | Swiss mice pretreated daily for 14 days and post-surgery for 21 days, 86% at 0.2 mL on day 14 (excision wound) | [92] |
| Amphipterygium adstringens (Schltdl.) Standl. | Ethanol-water (70:30), stem bark, topical, propylene glycol:ethanol (90:10) | Wistar rats for 15 days, at 10 mg/wound/day (excision wound) no effect | [89] | |
| Schinus terebinthifolia Raddi | Methanol, leaves, topical, unbuffered physiological saline | Wistar rats for 11 days, 70.88% at 80 mg/mL (excision wound) | [93] | |
| Spondias mombin L. | Fruit, decoction, topical, water | Mice (Strain A) for 2 days, (wound-breaking strength) (incision wound) no effect | [88] | |
| Annonaceae | Annona squamosa L. | 70% ethanol, leaves, topical | Normal and STZ-induced diabetic Wistar rats for 16 days and 20, respectively; 85% of contraction normal rats at 100 mg/kg (excision wound). For 8 days, ↑ 89% in tensile strength in normal rats and 77% in diabetic rats at 100 mg/kg (incision wound) | [21] |
| Apocynaceae | Allamanda cathartica L. | Water, leaves, topical | Sprague Dawley rats for 14 days, 91.12% at 150 mg/kg/day (excision wound). For 10 days, breaking strength 37.44% at 150 mg/kg/day (incision wound) | [94] |
| Himatanthus drasticus (Mart.) Plumel | Latex-water (1:1; v/v), topical, mineral oil | Swiss mice for 14 days, 50 μL on days 3 (37.83%), 7 (69.24%) and 10 (81.56%) (excision wound) | [95] | |
| Asteraceae | Ageratina pichinchensis (Kunth) R.M. King and H. Rob. | Water, aerial parts, topical | Sprague-Dawley rats for 8 days, 60% at 16% (w/v) (incision wound) | [35] |
| Bidens pilosa L. | 90% ethanol, leaves, topical, water | Wistar rats for 9 days, 74.31% at 100 mg/mL (excision wound) | [96] | |
| Mutisia acuminata Ruiz and Pav. | Pulverized stems and leaves, topical, water | Mice (Strain A) for 2 days, (wound-breaking strength) (incision wound) no effect | [88] | |
| Vernonia scorpioides (Lam.) Pers. | Ethanol, flowers and leaves, liquid-liquid fractioning, ethyl acetate fraction | Wistar rats for 7 days, 39.8% at 5 mg/kg (excision wound infected with Staphylococcus aureus) | [97] | |
| Basellaceae | Anredera diffusa (Moq.) Sperling | Leaves infusion, topical, water | Mice (Strain A) for 2 days, 45.1% wound- breaking strength at 200 mg/mL (incision wound) | [88] |
| Bignoniaceae | Arrabidaea chica (Bonpl.) B. Verl. (accepted name: Fridericia chica (Bonpl.) L.G. Lohmann) | Methanol/0.3% citric acid solution, leaves, topical, saline 0.9% | Wistar rats for 10 days, 96% at 100 mg/mL (excision wound) | [71] |
| Pyrostegia venusta (Ker Gawl.) Miers | Methanol, flowers, oral, DMSO | Wistar rats for 19 days, ~38% on 4th day and ~64% on 7th day at 100 mg/kg (excision wound). For 10 days, ↑ breaking strength at 100 mg/kg (incision wound) | [98] | |
| Brassicaceae | Coronopus didymus (L.) Sm. | 95% ethanol and aqueous, entire plant, oral, water | Wistar rats, aqueous and ethanolic ~37% tensile strength at 200 mg/kg (incision wound) | [99] |
| Burseraceae | Bursera morelensis Ramírez | Hydro-distillation, essential oil, stems, topical, cosmetic grade mineral oil | CD-1 mice for 10 days, 67.2% at 10% (excision wound); 36% wound healing efficacy at 10% (incision wound) | [48] |
| Cactaceae | Hylocereus undatus (Haw.) Britton and Rose | Water, leaves, flowers, fruit pulp, topical, water | STZ-induced diabetic Wistar rats for 7 days, 90% flowers extract at 0.5% (w/v); 73% leaves extract at 0.5% (w/v); 59% fruit pulp extract at 0.5% (w/v) on day 7 (excision wound). For 7 days, 81% flowers extract at 0.5% (w/v); 66% leaves extract at 0.5% (w/v); 52% fruit pulp extract at 0.5% (w/v) (incision wound; tensile strength) | [100] |
| Opuntia ficus-indica (L.) Mill. | Water (mucilage extract) and methanol, flowers, topical, methanol extract 10% in glycerol | Wistar rats for 13 days, >98% at 0.5 mg/mm2 mucilage extract and methanol extract (excision wound) | [101] | |
| Combretaceae | Combretum leprosum Mart. | Ethanol/water solution (8:2 v/v), leaves, topical, saline. | Swiss albino mice for 12 days, 86.88% at 100 μg/mL (excision wound) | [102] |
| Cucurbitaceae | Cucurbita pepo L. var. Bejaoui | Cold pressed oil, seeds, topical | Wistar rats for 11 days, 91.6% on day 11 at 0.52 μL/mm2 (excision wound) | [103] |
| Equisetaceae | Chamaesyce hirta (L.) Millsp previously known as Euphorbia hirta L. | 95% Ethanol, whole plant, oral | Alloxan induced diabetic Swiss rats for 16 days, wound contraction 35.92, 44.69 and 61.42% at 100, 200 and 400 mg/kg, respectively (excision wound) | [104] |
| Euphorbiaceae | Acalypha langiana Müll. Arg. | Water, leaves, topical | STZ-induced diabetic rats for 7 days. ED50 = 0.27% (w/v) (excision wound), ED50 = 0.29% (incision wound), and >ED50 = 0.5% (w/v) (tensile strength). | [105] |
| Croton lechleri Müll. Arg. | Latex, alkaloid extraction, topical, PBS | Every 12 h for 2 days, 31% at 10% (wound-breaking strength) | [106] | |
| Jatropha curcas L. | Latex, cortex, topical, | Mice (Strain A) for 2 days, 31.1% wound- breaking strength at 100 mg/mL (incision wound) | [88] | |
| Jatropha neopauciflora Pax | Latex, topical | CD-1 mice for 10 days. 100% at 50, 75, and 100% (w/v) (tensile strength) | [107] | |
| Fabaceae | Bowdichia virgilioides Kunth | Water, stem bark, topical, water | Swiss mice for 9 days, 62.5% on day 6 and 91% on day 9 at 10 mg/kg (w/v) (excision wound); for 9 days, 95.5% at 10 mg/kg (w/v) (excision wound infected with S. aureus) | [108] |
| Caesalpinia ferrea Mart. ex Tul. | Polysaccharide-rich extract, bark, topical, saline 0.9% | Wistar rats for 21 days, 91.67% at 0.1% on day 10 (excision wound) | [109] | |
| Copaifera langsdorffii Desf. | Oleoresin, bark, topical, 4% Tween 80 in normal saline | Wistar rats for 21 days, 84.05% at 4% on day 9 (excision wound) and for 12 days 99% at 4% on day 5 (incision wound; tensile strength) | [110] | |
| Copaifera paupera (Herzog) Dwyer (syn. Copaifera langsdorffii Desf.) | Oleoresin (exudated from the trunk, topical, mineral oil | Alloxan-induced diabetic Swiss Webster mice for 14 days, ~55–60% at 100, 150 or 200 mg/kg from day 7 (excision wound) | [111] | |
| Losaceae | Mentzelia cordifolia Dombey ex Urb. and Gilg | Cortex, decoction, topical, water | Mice (Strain A) for 2 days, (wound-breaking strength) (incision wound) no effect | [88] |
| Lauraceae | Persea americana Mill. | Fruit, paste (extract), topical and oral (water) | Sprague Dawley rats for 21 days, ~90% topical and oral at 300 mg/kg/day on day 13 (excision wound); For 10 days, ↑ wet and dry weight of the granulation tissue (dead space wound) | [112] |
| Martyniaccae | Martynia annua L. | Ethanol, defatted dried powdered, leaves, (methanol soluble fraction from chloroform insoluble fraction), topical | Wistar rats for 20 days, 100% at 100% on day 18 (excision wound); for 9 days, ↑ tensile strength (incision wound) | [113] |
| Meliaceae | Carapa guianensis Aubl. | Ethanol, leaves, topical-petroleum jelly, oral-drinking water | Sprague Dawley rats for 15 days, 100% at 250 mg/kg (excision wounds). for 10 days ~51.7% breaking strength at 250 mg/kg (incision wound); for 10 days ↑ wet and dry weight of the granulation tissue at 250 mg/kg (dead space wound) | [114] |
| Piperaceae | Peperomia galioides Kunth | Pulverized stems and leaves, topical, water | Mice (Strain A) for 2 days, 45.9% wound-breaking strength at a dose of 100 mg/mL (incision wound) | [88] |
| Plantaginaceae | Plantago australis Lam. | Hydroethanolic solution (30% water and 70% ethanol), leaves, oral, saline | Wistar rats for 21 days, ~85–95% wound contraction at 500 and 1000 mg/kg, respectively on day 7 (excision wound) | [85] |
| Polygonaceae | Muehlenbeckia tamnifolia (Kunth) Meisn. | Cortex, decoction, topical, water | Mice (Strain A) for 2 days, (wound-breaking strength) (incision wound) no effect | [88] |
| Solanaceae | Nicotiana tabacum L. | Ethanol, Stems defatted previous, topical | Wistar rats for 14 days, 98.7% at 2% (excision wound) | [115] |
| Urticaceae | Cecropia peltata L. | Leaves, aqueous extract (topical and oral), ethanolic extract (topical), water | Sprague Dawley rats for 10 days, aqueous extract 80.71% (topical) and 72.17% (oral) at 150 mg kg−1/day on day 11; ethanol extract 79.21% (topical) at 150 mg kg−1/day on day 11 (excision wound) | [116] |
| Verbenaceae | Lantana camara L. | Ethanol, leaves, topical | Sprague Dawley rats for 19 days until complete epithelialization, 98% on day 19 at 100 mg/kg daily (excision wound) | [117] |
| Botanical Family | Plant Name | Solvent/Plant Part/ Administration/ Vehicle | Percentage of Wound Healing Activity (Model of Study) | Reference |
|---|---|---|---|---|
| Amaranthaceae | Alternanthera brasiliana (L.) Kuntze | Methanol, leaves, topical, petroleum jelly | Immunocompromised Sprague Dawley rats for 7 days, 77.10% at 5% (w/w) on day 8 (excision wound) | [124] |
| Methanol, leaves, topical, petroleum jelly | STZ-induced diabetic Sprague Dawley rats for 7 days, 89.76% at 5% (w/w) on day 8 (excision wound) | [125] | ||
| Chenopodium ambrosioides L. | Ethanol, aerial parts, topical, ointment base | Wistar rats for 19 days, 72.97% and 98.78%, at 5% on days 14 and 19, respectively (excision wound) | [126] | |
| Annonaceae | Annona crassiflora Mart. | Ethanol 98%, fruit peel, ethyl acetate and n-butanol fractions (1:1)- polyphenol-enriched fraction, topical, petroleum jelly/lanolin (7:3) | C57BL/6 mice for 7 days, 32%, 38%, and 36% at 2%, 4% and 6%, respectively on day 4. 84% at 2% on day 7 (excision wound) | [127] |
| Annona muricata L. | Leaves, aqueous semisolid cream, topical | Sprague Dawley rats for 15 days, 69%, and 77% wound closure at 5% and 10%, respectively (excision wound) | [128] | |
| Asteraceae | Achyrocline alata (Kunth) DC. | Hydroethanolic solution 70%, flowers, topical, ointment base (lanolin and liquid paraffin) | Swiss mice for 17 days, ~60% at 10% (w/w) from day 4 (excision wound) | [129] |
| Flaveria trinervia (Spreng.) C. Mohr | Methanol, leaves, topical, aqueous base (polyethylene glycol and emulsifying wax) | Albino mice for 34 days, 90% at 5% (w/w) on day 18 (excision wound) | [130] | |
| Neurolaena lobata (L.) Cass. | Ethanol, leaves, topical, petroleum jelly | Sprague Dawley rats for 13 days, 87% at 100 mg/kg/day (excision wound) | [131] | |
| Verbesina crocata (Cav.) Less. | Methanol, aerial parts, topical, Vaseline® | CD-1 mice for 14 days, ~33% at 5% w/w (incision wound; tensile strength) | [132] | |
| Vernonia scorpioides (Lam.) Pers. | Ethanol 96%, leaves, topical, hydrogel Carbopol 2%, | Guinea pigs (H–D) for 30 days, at 50% lacked effects on closure time, but stimulated the regeneration of the new tissue (excision wound) | [133] | |
| Cactaceae | Pereskia aculeata Mill. | Methanol and partition with hexane, leaves, topical, gel base: hydroxyethylcellulose (Natrosol 250 HHRs), sodium lauryl sulfate, alcohol, glycerin and methylparaben | C57BL/6 mice for 14 days, 70% hexane fraction at 5% on day 5, 80% both hexane fraction and methanol fraction at 5% on day 7 (excision wound) | [134] |
| Calophyllaceae | Calophyllum brasiliense Cambess. | Ethanol:water (9:1), leaves, topical, non-ionic emulsion: 6% cetostearyl alcohol (w/w), 5% octyldodecanol (w/w), 7% mineral oil (w/w), 12% ethoxylated alcohol stearyl (w/w), 18% glycerin monoestarate (w/w), 10% propylene glycol (w/w), and purified water | Wistar rats for 21 days, 90.67% at 10% (w/w) on day 14 (excision wound) | [135] |
| Caryocaraceae | Caryocar coriaceum Wittm. | Fixed oil from the seeds, topical, Vaseline® and lanolin (1:2) | Swiss albino mice for 14 days, 96.54% at 12% (v/w) on day 7 (excision wound) | [136] |
| Celastraceae | Maytenus ilicifolia Mart. ex Reissek | Ethanol 70%, leaves, topical, Vaseline® and lanolin | BALB/c mice for 7 days, after 3 days, 20.8% at 4%; after 7 days 67.9% at 4% (excision wound) | [137] |
| Convolvulaceae | Ipomoea batatas (L.) Lam. | Tuber flour, topical, Beeler’s base | Wistar rats for 10 days, 43% and 75% re-epithelialization process for 4 and 10 days, respectively, at 2.5% (excision wound) | [138] |
| 1.5 N hydrochloric acid and ethanol 96% (15:85, v/v), peels of the roots, topical, gel formulation: carbopol, liquid glycerin, propylene glycol, triethanolamine and water | Balb/c mice for 14 days, ~70% at 1% on day 6 (incision wound) | [139] | ||
| Equisetaceae | Chamaesyce hirta (L.) Millsp (syn. Euphorbia hirta L.) | Ethanol 95%, whole plant, topical, hydrophobic ointment: povidone iodine/ethanolic extract of Euphorbia hirta whole plants. Petroleum jelly Cetostearyl alcohl PEG 6000 liquid paraffin methyl paraben | Alloxan induced diabetic Swiss rats for 16 days, 32.86 and 36.32% at 5 and 10%, respectively, (excision wound) | [104] |
| Euphorbiaceae | Croton zehntneri Pax and K. Hoffm. | Essential oil, leaves, topical, Pluronic F-127 gels (10% w/w) | Swiss mice for 15 days, 93% at 20% (excision wound) | [140] |
| Jatropha curcas L. | Methanol, leaves, topical, petroleum jelly | White albino rats for 21 days, 100% at 15% w/w (excision wound) | [141] | |
| Jatropha gaumeri Greenm. | Water, latex, topical, glycerin (cream) | Balb/c mice for 20 days, 97.7% at 5% w/w (tensile strength) | [142] | |
| Pedilanthus tithymaloides (L.) Poit. | Defatted with petroleum ether and extracted with methanol, leaves, topical, ointment base: wool fat 5 g, hard paraffin 5 g, cetostearyl alcohol 5 g, soft white paraffin 85 g | Wistar rats for 18 days, 95.88% at 5% (excision wound), for 10 days 41.17% and 37.6% at 2.5% and 5%, respectively (incision wound; tensile strength); ↑ wet and dry weight of the granulation tissue (dead space wound) | [143] | |
| Fabaceae | Copaifera langsdorffii Desf. | Oleoresin and hydroalcoholic, leaves, topical, cream: aqueous phase composed of 75.8% water and 4.0% propylene glycol, and an organic phase contained 17% Lanette cream, 3% hard paraffin, and other components, pH = 6.86. | Wistar rats for 14 days, 95.1% hydroalcoholic at 10% and 94.72% oleoresin at 10% on 14 day (excision wound) | [144] |
| Dipteryx alata Vogel | Bark extracted with ethanol, topical, cream | C57BL/6 mice (excision wound) for 21 days, at 5%, 10%, and 15% no effect | [145] | |
| Mimosa pudica L. | Distilled water, root, defatted by extracting with pet-ether followed by extraction of methanol, topical ointment base B.P. | Wistar albino rats for 19 days, 87.71% on day 12 and 100% on day 16 at 2% (w/w) of methanolic extract (excision wound); for 10 days, ↑ tensile strength at 2% (w/w) (incision wound) | [146] | |
| Prosopis juliflora (Sw.) DC. | Powder, leaves, topical, glycerin | Wistar rats for 21 days, 76% and 85% on day 14 and 21, respectively at 30% (w/w) (excision wound) | [147] | |
| Stryphnodendron adstringens (Mart.) Coville | Me2CO-H2O (7:3), stem bark, redissolved in H2O and extracted with ethyl acetate (fraction), topical, ointment base (Beeler base) | Wistar rats for 10 days, at 1% (excision wound) no effect | [148] | |
| Lauraceae | Persea americana Mill. | Hexane, oil fruit, topical, semisolid formulation petroleum jelly | Wistar rats for 14 days, 100% at 50% on day 13 (excision wound); for 10 days, ~33% at 50% (incision wound; tensile strength) | [149] |
| Methanol, seeds, topical, hydrogel: 1% Carbopol base | Wistar albino rats for 20 days, 100% after 16 days at dose 5 and 10% (excision wound infected with S. aureus) | [150] | ||
| Loganiaceae | Strychnos pseudoquina A. St.-Hil. | Selective sequential extraction n-hexane, ethyl acetate, and ethanol/water (9:1, v/v), stem bark, topical hydroethanolic extract, lanolin | STZ-induced diabetic Wistar rats for 21 days, 90% and 89.9% on day 14 at 5% and 10%, respectively; 98.25% and 98.1% on day 21 at 5% and 10%, respectively; (excision wound) | [151] |
| Samples, ethanol 95%, topical, emulsified in lanolin at 5% and 10% (v/v) | Wistar rats for 21 days, 52.4% and 58.5% on day 7 at 5% and 10%, respectively, (excision wound) | [152] | ||
| Loranthaceae | Struthanthus vulgaris (Vell.) Mart. | Ethanol, leaves, defatted with hexane, topical, lanolin: petrolatum (3:7) | Wistar rats for 21 days, after 7 and 10 days 72% and 79% at 5%, respectively, (excision wound) | [153] |
| Lythraceae | Lafoensia pacari A.St.-Hil. | Hydroethanolic solution (1:10, w/v), leaves, topical, 2% propylene glycol and incorporated into Sepigel® | Wistar rats for 24 days, 57.5% at 30 mg/g on day 6 (excision wound); for 9 days, ~42% at 100 mg/g (incision wound; tensile strength) | [80] |
| Martyniaccae | Martynia annua L. | Ethanol, defatted dried powdered, leaves, topical, ointment base B.P. (ointment) | STZ-induced diabetic Wistar albino rats for 20 days, 100% methanol soluble fraction (from chloroform insoluble fraction) at 5% (w/w) on day 18 (excision wound) | [154] |
| Moraceae | Dorstenia drakena L. | Water, rhizome, topical, petroleum jelly (ointment) | Wistar rats for 30 days. 25% at 20% (w/w) (incision wound) | [155] |
| Sorocea houlletiana Gaudich. (accepted name: Sorocea guilleminiana Gaudich.) | Water, leaves, topical, 1% propylene glycol and incorporated into Polawax™ NF (12%) (from Croda International Plc, Goole, East Yorkshire, UK), mineral oil (5%), propylene glycol (3%), volatile silicone (2%), EDTA (0.1%), methylparaben (0.1%), and propylparaben (0.05%) in distilled water. (cream) | Wistar rats for 23 days, 82.66% on day 7 and 90.08% on day 9 at 2 mg/g (excision wound); for 9 days at doses of 2 mg/g (45.73%) and 50 mg/g (35.27%) (incision wound; tensile strength) | [81] | |
| Orchidaceae | Prosthechea michuacana (Lex.) W.E. Higgins | Hexane, bulbs, 2% (v/v) Tween-80 and simple ointment bases (ointment) | Wistar albino rats for 18 days, 99.2% at 50% (v/v) on day 16 (excision wound); for 7 days, ↑ tensile strength at 50% (incision wound) | [156] |
| Passifloraceae | Passiflora edulis Sims | Ethanol/water (7:3), leaves, liquid/liquid partition (butanol fraction, 6.1% isorientin), topical, chitosan hydrogel | Alloxan-induced diabetic Wistar rats for 14 days, 46.28% on day 2 and 96.26% on day 14 at 0.1% (w/v) (excision wound) | [157] |
| Plantaginaceae | Plantago australis Lam. | 70% ethanol and 30% water, leaves, topical, lanolin:Vaseline® (2:3). | Wistar rats for 14 days, ~80% after 7 days at 4% (w/w) (excision wound) | [158] |
| Rubiaceae | Hamelia patens Jacq. | Ethanol, aerial parts, topical, petroleum jelly (ointment) | Sprague-Dawley rats for 12 days, 16% (double incision; breaking strength) at 10% (w/w) | [159] |
| Sapindaceae | Dodonaea viscosa Jacq. | Ethanol, leaves, topical, petroleum jelly, lanoline, and paraffin (ointment) | Balb/c mice for 7 days. ED50 = 5% (v/v) (tensile strength) | [160] |
| Solanaceae | Capsicum annuum L. | Methanol, fruit, topical, 1% Carbopol (gel) | Wistar rats for 20 days, 100% on day 16 at 5% and 10% (excision wound infected with S. aureus) | [161] |
| Physalis angulata L. | 70% methanol, leaves, topical, aqueous cream (British Pharmacopoeia) | Wistar rats for 15 days, ~95% at 2.5, 5 and 10% from day 10 (excision wound) | [162] | |
| Solanum diploconos (Mart.) Bohs | 95% ethanol, fresh ripe fruit (peel and pulp with seeds), topical, semisolid formulation. | Swiss mice for 7 days, reduction (~35%) at 1% on day 7 (excision wound) | [87] | |
| Vernenaceae | Lippia gracilis Schauer | Essential oil, leaves, topical, 70% petroleum jelly and 30% anhydrous lanolin | Wistar rats for 21 days, ~72.5% at 10% from day 7 (excision wound) | [163] |
| Lippia sidoides Cham. (accepted name: Lippia origanoides Kunth) | Essential oil, topical, Vaseline® and Lanolin (1:2) | Wistar rats for 21 days, at 6% and 12% (v/w) (excision wound) no effect | [123] | |
| Ximeniaceae | Ximenia americana L. | 70% hydroalcoholic, bark and wood, topical, Lanette base | Wistar rats for 14 days, 71% and 86.9% on day 7 and 14, respectively, at 10% (excision wound) | [164] |
| No. | Compound | Plant Source | Formulation or Vehicle | Percentage of Wound/ Healing Activity (Model of Study) | Reference |
|---|---|---|---|---|---|
| 1 | 3α-hydroxymasticadienoic acid | Amphipterygium adstringens (Schltdl.) Standl. | Topical, propylene glycol:ethanol (90:10) | Wistar rats for 15 days, ~80% on day 7 at 300 μg/wound/day (excision wound) | [89] |
| 2–6 | Anacardic acids | Amphipterygium adstringens (Schltdl.) Standl. | Topical, propylene glycol:ethanol (90:10) | Wistar rats for 15 days, ~80% on day 7 at 300 μg/wound/day (excision wound) | [89] |
| 7 | Bornesitol | Hancornia speciosa Gomes | DMSO, 0.5% | Scratch assay, primary human gingival fibroblasts, 80.8% at 50 μM (9.71 μg/mL) | [67] |
| 8 | Quinic acid | Hancornia speciosa Gomes | DMSO, 0.5% | Scratch assay, primary human gingival fibroblasts, 69.1% at 50 μM (9.61 μg/mL) | [67] |
| 9 | Rutin | Hancornia speciosa Gomes | DMSO, 0.5% | Scratch assay, primary human gingival fibroblasts, 39.6% at 50 μM (30.53 μg/mL) | [67] |
| 10 | Oleanolic acid | Anredera diffusa (Moq.) Sperling | Topical, DMSO | Mice (strain A) for 2 days, 42.9% at 12.5 mg/mL (incision wound; tensile strength) | [44] |
| 11 | 3β, 6β, 16β-trihydroxylup-20(29)-ene | Combretum leprosum Mart. | Topical, saline | Swiss albino mice for 12 days, 99.65% at 100 μg/mL (excision wound) | [102] |
| 12 | Kaempferol | Ipomoea carnea Jacq. | Topical | Wistar rats for 14 days, 91.1% at 200 mg/kg body weight/day (excision wound) 22.6% at 200 mg/kg body weight/day (incision wound; breaking strength) | [167] |
| 13 | Kaempferol 3-O-α-D-glucoside | Ipomoea carnea Jacq. | Topical | Wistar rats for 14 days, 90.2% at 200 mg/kg body weight/day (excision wound); 38.3% at 200 mg/kg body weight/day (incision wound; breaking strength) | [167] |
| 14 | Rosmarinic acid | Cordia americana (L.) Gottschling and J.S. Mill. | DMSO, 0.5% | Scratch assay, Swiss 3T3 albino mouse fibroblasts cells, 11.8% at 10 μg/mL | [73] |
| 15 | Taspine hydrochloride | Croton lechleri Müll. Arg. | Topical, PBS | Mice (strain A) every 12 h for 2 days, wound-breaking strength, 58.2% at a dose of 0.1 mg/mL (incision wound) ED50 = 0.375 mg/kg | [106] |
| PBS | Scratch assay, human foreskin fibroblast, 27 ± 1.83 no. cells/cm at 2 ng/mL | [106] | |||
| 16 | Trans-anethole | Croton zehntneri Pax and K. Hoffm. | Topical, Pluronic F-127 gels (10% w/w) | Swiss mice for 15 days, 90% at a dose of 20% (excision wound) | [140] |
| 17 | 1,2 tetradecanediol, 1-(hydrogen sulfate), sodium sal | Pedilanthus tithymaloides (L.) Poit. | Topical, ointment base: wool fat 5 g, hard paraffin 5 g, cetostearyl alcohol 5 g, soft white paraffin 85 g | Wistar rats for 18 days, 100% at a dose of 0.25% w/w (excision wound); for 10 days 50.8% at 0.25% w/w (incision wound; breaking strength) | [143] |
| 18 | 2-(3,4-dihydroxy-phenyl)-5,7-dihydroxy-chromen-4-one | Pedilanthus tithymaloides (L.) Poit. | Topical, ointment base: wool fat 5 g, hard paraffin 5 g, cetostearyl alcohol 5 g, soft white paraffin 85 g | Wistar rats for 18 days, 100% at a dose of 0.25% w/w (excision wound); for 10 days 51.8% at 0.25% w/w (incision wound; breaking strength) | [143] |
| Martynia annua L. | Topical, ointment base B.P. (ointment) | STZ-induced diabetic Wistar albino rats for 20 days, 87.67% at 0.5% w/w on day 18 (excision wound) | [154] | ||
| 19 | Epicatechin-3-O-gallate | Parapiptadenia rigida (Benth.) Brenan | DMSO | Scratch assay, Swiss 3T3 albino mouse fibroblasts, ~58% increased cell numbers at 1 μM | [168] |
| 20 | 4′-O-methylepicatechin-3-O-gallate | Parapiptadenia rigida (Benth.) Brenan | DMSO | Scratch assay, Swiss 3T3 albino mouse fibroblasts, ~60% increased cell numbers at 1 μM | [168] |
| 21 | (+)-epi-α-bisabolol Some authors referred (+)-anymol | Peperomia galioides Kunth | Topical | Mice (strain A) every 12 h for 2 days, wound-breaking strength, 62% using 4 mg per mouse (incision wound), ED50 = 155 μg/g | [166] |
| 22 | Verbascoside | Plantago australis Lam. | DMSO | Scratch assay, HaCaT cells, 58.7% and 57.77% at 5 and 10 µg/mL, respectively | [85] |
| 23 | N-Methyl-(2S,4R)-trans- 4-Hydroxy-L-Proline | Sideroxylon obtusifolium (Humb. ex Roem. and Schult.) T.D. Penn. | Topical, 1% carboxyvinyl polymer, 5% glycerin, and 1% polypropy- lene glycol. Methylparaben (0.15%) and propilparaben (0.05%) (gel) | Swiss mice for 12 days, reductions around 58% at 3% and 10% (excision wound) | [169] |
| 24 | 3-O-α-L-rhamnopyranosyl-(1→4)-β-D-glucopyranosyl- (1→3)-[β-D-glucopyranosyl-(1→2)]-β-D-fucopyranosyl- 23,28-dihydroxyoleane-11,13(18)-diene | Buddleja scordioides Kunth | Topical, water | STZ-induced diabetic Wistar rats for 7 days, 70% compound 1 at 0.5% (excision wound), 75% compound 1 at 0.5% (incision wound) | [170] |
| 25 | 3-O-α-L-rhamnopyranosyl-(1→4)-β-D-glucopyranosyl-(1→3)-[β-D-glucopyranosyl-(1→2)]-β-D-fucopyranosyl-16,23,28-trihydroxyoleane-11,13(18)-diene | Buddleja scordioides Kunth | Topical, water | STZ-induced diabetic Wistar rats for 7 days, 81% compound 2 at 0.5% (excision wound), 85% compound 2 at 0.5%, (incision wound) | [170] |
2.3. Clinical Wound Healing Research
| Plant Name | Solvent/Plant Part/ Administration/ Vehicle | Study Design | Dose, Duration, and Patient Type | Main Outcome | Adverse Effect | Reference |
|---|---|---|---|---|---|---|
| Ageratina pichinchensis (Kunth) R.M. King and H. Rob. | Hexane-ethyl acetate extract, aerial parts, topical, carboxymethyl cellulose (AKUCELL 2201) | Double-blind, prospective, and randomized study |
| Ulcer size reduction 79.1% in the second treatment month. Heal completely 8 months | Tolerability | [171] |
| Hexane-ethyl acetate extract, aerial parts, topical, cold cream | Randomized, double-blind, controlled pilot study |
| At week 8 90.78% wound size reduction | No adverse effects | [176] | |
| Croton lechleri Müll. Arg. | 80% ethanol, bark latex, topical, 10% cetyl alcohol, 7% isopropylmeristat, and 21% Vaseline (cream base); span 20 and tween 80 (emulsifying agent) parabens (preservative); and propylene glycol (humectant) | Double-blind, placebo-controlled, randomized |
| At day 14 95.73% of wound healing | Not mentioned | [177] |
| Rhizophora mangle L. | Aqueous extract, bark | Single blind, randomized and comparative with an antiseptic |
| At day 30, 32% of wound healing | No adverse effects | [178] |
| Mimosa tenuiflora (Willd.) Poir. | Ethanol, cortex, topical, each 100 g of the hydrogel had Carbopol 940 (0.75 g), triethanolamine (0.85 g), M. tenuiflora extract (5 g), ethanol (10 g), propylene glycol (16.5 g), methylparaben (0.05 g) and vehicle (100 g). | Randomized, placebo-controlled, double blind |
| No effect | Pain and burning in four patients | [179] |
| Ethanol, Bark, topical, polyethylene glycol (PEG-200) and incorporated into Carbopol® 940 | Randomized, double-blind, placebo-controlled clinical trial |
| 93% ulcer-size reduction at the 8th treatment week | No adverse effects | [173] | |
| Achillea millefolium L. | 90% ethanol, topical, Vaseline | Double-blind clinical trial study |
| Reduce perineal pain level, redness, edema, and ecchymosis | Not mentioned | [180] |
2.4. Mechanism of Action
2.5. Future Considerations
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schreml, S.; Szeimies, R.-M.; Prantl, L.; Landthaler, M.; Babilas, P. Wound Healing in the 21st Century. J. Am. Acad. Dermatol. 2010, 63, 866–881. [Google Scholar] [CrossRef] [PubMed]
- Enoch, S.; Leaper, D.J. Basic Science of Wound Healing. Surgery 2008, 26, 31–37. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, R.A.; Stephens, C. Indigenous Health in Latin America and the Caribbean. Lancet 2006, 367, 1859–1869. [Google Scholar] [CrossRef]
- Del Carmen Juárez-Vázquez, M.; Carranza-Álvarez, C.; Alonso-Castro, A.J.; González-Alcaraz, V.F.; Bravo-Acevedo, E.; Chamarro-Tinajero, F.J.; Solano, E. Ethnobotany of Medicinal Plants Used in Xalpatlahuac, Guerrero, México. J. Ethnopharmacol. 2013, 148, 521–527. [Google Scholar] [CrossRef]
- Süntar, I. Importance of Ethnopharmacological Studies in Drug Discovery: Role of Medicinal Plants. Phytochem. Rev. 2020, 19, 1199–1209. [Google Scholar] [CrossRef]
- Parmar, V.S.; Jain, S.C.; Bisht, K.S.; Jain, R.; Taneja, P.; Jha, A.; Tyagi, O.D.; Prasad, A.K.; Wengel, J.; Olsen, C.E. Phytochemistry of the Genus Piper. Phytochemistry 1997, 46, 597–673. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Maldonado-Miranda, J.J.; Zarate-Martinez, A.; del Rosario Jacobo-Salcedo, M.; Fernández-Galicia, C.; Figueroa-Zuñiga, L.A.; Rios-Reyes, N.A.; de León-Rubio, M.A.; Medellín-Castillo, N.A.; Reyes-Munguia, A. Medicinal Plants Used in the Huasteca Potosina, Mexico. J. Ethnopharmacol. 2012, 143, 292–298. [Google Scholar] [CrossRef]
- Scarpa, G.F. Medicinal Plants Used by the Criollos of Northwestern Argentine Chaco. J. Ethnopharmacol. 2004, 91, 115–135. [Google Scholar] [CrossRef]
- Tribess, B.; Pintarelli, G.M.; Bini, L.A.; Camargo, A.; Funez, L.A.; de Gasper, A.L.; Zeni, A.L.B. Ethnobotanical Study of Plants Used for Therapeutic Purposes in the Atlantic Forest Region, Southern Brazil. J. Ethnopharmacol. 2015, 164, 136–146. [Google Scholar] [CrossRef]
- Magalhães, P.K.A.; Araujo, E.N.; Santos, A.M.; Vanderlei, M.B.; Souza, C.C.L.; Correia, M.S.; Fonseca, S.A.; Pavão, J.; Souza, M.A.; Costa, J.G. Ethnobotanical and Ethnopharmacological Study of Medicinal Plants Used by a Traditional Community in Brazil’s Northeastern. Braz. J. Biol. 2021, 82. [Google Scholar] [CrossRef]
- Monigatti, M.; Bussmann, R.W.; Weckerle, C.S. Medicinal Plant Use in Two Andean Communities Located at Different Altitudes in the Bolívar Province, Peru. J. Ethnopharmacol. 2013, 145, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, I.B.C.; Bonfim, F.P.G.; Pasa, M.C.; Montero, D.A. Ethnobotanical Survey of Medicinal Flora in the Rural Community Rio Dos Couros, State of Mato Grosso, Brazil. Bol. Latinoam. Caribe Plantas Med. Aromat. 2017, 16, 53–67. [Google Scholar]
- Scarpa, G.F.; Rosso, C.N. Etnobotánica Histórica de Grupos Criollos de Argentina IV: Identificación Taxonómica de Las Plantas y Análisis de Datos Medicinales Del Chaco Húmedo Provenientes de La Encuesta Nacional de Folklore de 1921. Bonplandia 2019, 28, 5–42. [Google Scholar] [CrossRef]
- Schmidt, C.; Fronza, M.; Goettert, M.; Geller, F.; Luik, S.; Flores, E.M.M.; Bittencourt, C.F.; Zanetti, G.D.; Heinzmann, B.M.; Laufer, S. Biological Studies on Brazilian Plants Used in Wound Healing. J. Ethnopharmacol. 2009, 122, 523–532. [Google Scholar] [CrossRef] [PubMed]
- De O Mesquita, U.; Tavares-Martins, A.C.C. Ethnobotany of Medicinal Plants in the Community of Caruaru, Mosqueiro Island, Belem-PA, Brazil. Bol. Latinoam. Caribe Plantas Med. Aromat. 2018, 17, 130–159. [Google Scholar]
- Canales, M.; Hernández, T.; Caballero, J.; De Vivar, A.R.; Avila, G.; Duran, A.; Lira, R. Informant Consensus Factor and Antibacterial Activity of the Medicinal Plants Used by the People of San Rafael Coxcatlán, Puebla, México. J. Ethnopharmacol. 2005, 97, 429–439. [Google Scholar] [CrossRef] [PubMed]
- De Albuquerque, U.P.; Monteiro, J.M.; Ramos, M.A.; de Amorim, E.L.C. Medicinal and Magic Plants from a Public Market in Northeastern Brazil. J. Ethnopharmacol. 2007, 110, 76–91. [Google Scholar] [CrossRef]
- Di Stasi, L.C.; Oliveira, G.P.; Carvalhaes, M.A.; Queiroz-Junior, M.; Tien, O.S.; Kakinami, S.H.; Reis, M.S. Medicinal Plants Popularly Used in the Brazilian Tropical Atlantic Forest. Fitoterapia 2002, 73, 69–91. [Google Scholar] [CrossRef]
- Carhuanca, K.M.; Salgado, E.L.R. Plantas Medicinales de Uso Popular en la Amazonía Peruana; Spanish Agency for International Development Cooperation: Madrid, Spain, 2000. [Google Scholar]
- Ponrasu, T.; Suguna, L. Efficacy of Annona squamosa on Wound Healing in Streptozotocin-induced Diabetic Rats. Int. Wound J. 2012, 9, 613–623. [Google Scholar] [CrossRef]
- Medrano-Guerrero, A.; Carranza, E.; Juárez-Vázquez, M.C.; Solano, E.; Ruiz-Padilla, A.J.; Ruiz-Noa, Y.; Deveze-Alvarez, M.A.; Brennan-Bourdon, L.M.; Alonso-Castro, A.J. Medicinal Plants Used in Rural Communities from the Municipality of Dolores Hidalgo, Guanajuato, Mexico. Bol. Latinoam. Caribe Plantas Med. Aromat. Available online: http://www.scielo.org.co/scielo.php?script=sci_abstract&pid=S0124-00642021000400204 (accessed on 18 July 2022).
- Tene, V.; Malagon, O.; Finzi, P.V.; Vidari, G.; Armijos, C.; Zaragoza, T. An Ethnobotanical Survey of Medicinal Plants Used in Loja and Zamora-Chinchipe, Ecuador. J. Ethnopharmacol. 2007, 111, 63–81. [Google Scholar] [CrossRef]
- De-la-Cruz, H.; Vilcapoma, G.; Zevallos, P.A. Ethnobotanical Study of Medicinal Plants Used by the Andean People of Canta, Lima, Peru. J. Ethnopharmacol. 2007, 111, 284–294. [Google Scholar] [CrossRef]
- Edelmira, L.M.; Beatriz, F.P.; Robert, B. Selección de Plantas Medicinales de México; Editorial Limusa: Ciudad de Mexico, Mexico, 1988. [Google Scholar]
- Estrada-Castillón, E.; Villarreal-Quintanilla, J.Á.; Rodríguez-Salinas, M.M.; Encinas-Domínguez, J.A.; González-Rodríguez, H.; Figueroa, G.R.; Arévalo, J.R. Ethnobotanical Survey of Useful Species in Bustamante, Nuevo León, Mexico. Hum. Ecol. 2018, 46, 117–132. [Google Scholar] [CrossRef]
- Hirschmann, G.S.; de Arias, A.R. A Survey of Medicinal Plants of Minas Gerais, Brazil. J. Ethnopharmacol. 1990, 29, 159–172. [Google Scholar] [CrossRef]
- Pedrollo, C.T.; Kinupp, V.F.; Shepard, G., Jr.; Heinrich, M. Medicinal Plants at Rio Jauaperi, Brazilian Amazon: Ethnobotanical Survey and Environmental Conservation. J. Ethnopharmacol. 2016, 186, 111–124. [Google Scholar] [CrossRef]
- Bourdy, G.; DeWalt, S.J.; De Michel, L.R.C.; Roca, A.; Deharo, E.; Muñoz, V.; Balderrama, L.; Quenevo, C.; Gimenez, A. Medicinal Plants Uses of the Tacana, an Amazonian Bolivian Ethnic Group. J. Ethnopharmacol. 2000, 70, 87–109. [Google Scholar] [CrossRef]
- Odonne, G.; Valadeau, C.; Alban-Castillo, J.; Stien, D.; Sauvain, M.; Bourdy, G. Medical Ethnobotany of the Chayahuita of the Paranapura Basin (Peruvian Amazon). J. Ethnopharmacol. 2013, 146, 127–153. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, P. Medicinal Plants of the Achuar (Jivaro) of Amazonian Ecuador: Ethnobotanical Survey and Comparison with Other Amazonian Pharmacopoeias. J. Ethnopharmacol. 2015, 164, 78–88. [Google Scholar] [CrossRef]
- Polesna, L.; Polesny, Z.; Clavo, M.Z.; Hansson, A.; Kokoska, L. Ethnopharmacological Inventory of Plants Used in Coronel Portillo Province of Ucayali Department, Peru. Pharm. Biol. 2011, 49, 125–136. [Google Scholar] [CrossRef]
- Scarpa, G.F. Etnobotánica Médica de Los Indígenas Chorote y Su Comparación Con La de Los Criollos Del Chaco Semiárido (Argentina). Darwiniana Nueva Ser. 2009, 47, 92–101. [Google Scholar]
- Martínez, M. Las Plantas Medicinales de Mexico. Ediciones Botas. Mex. DF 1989, 656. Available online: https://books.google.com.cu/books/about/Las_plantas_medicinales_de_M%C3%A9xico.html?id=811gAAAAMAAJ (accessed on 18 July 2022).
- Romero-Cerecero, O.; Zamilpa-Álvarez, A.; Ramos-Mora, A.; Alonso-Cortés, D.; Jiménez-Ferrer, J.E.; Huerta-Reyes, M.E.; Tortoriello, J. Effect on the Wound Healing Process and in vitro Cell Proliferation by the Medicinal Mexican Plant Ageratina pichinchensis. Planta Med. 2011, 77, 979–983. [Google Scholar] [CrossRef]
- Andrade-Cetto, A. Ethnobotanical Study of the Medicinal Plants from Tlanchinol, Hidalgo, México. J. Ethnopharmacol. 2009, 122, 163–171. [Google Scholar] [CrossRef]
- Ocampo, R.; Balick, M.J. Plants of Semillas Sagradas: An Ethnomedicinal Garden in Costa Rica; Finca Luna Nueva Lodge: Peñas Blancas La Fortuna, Costa Rica, 2009; ISBN 0615274153. [Google Scholar]
- Hammond, G.B.; Fernández, I.D.; Villegas, L.F.; Vaisberg, A.J. A Survey of Traditional Medicinal Plants from the Callejón de Huaylas, Department of Ancash, Perú. J. Ethnopharmacol. 1998, 61, 17–30. [Google Scholar] [CrossRef]
- García-Regalado, G. Plantas Medicinales de Aguascalientes, 1st ed.; García-Regalado, G., Ed.; Universidad Autónoma de Aguascalientes: Aguascalientes, México, 2014; ISBN 978-607-8359-83-7. [Google Scholar]
- Lara Reimers, E.A.; Lara Reimers, D.J.; Chaloupkova, P.; del Valle, J.M.Z.; Milella, L.; Russo, D. An Ethnobotanical Survey of Medicinal Plants Used in Papantla, Veracruz, Mexico. Plants 2019, 8, 246. [Google Scholar] [CrossRef]
- Sánchez, M.P.O. Plantas Medicinales Del Estado de Chihuahua; UACJ: Chiyoda City, Japan, 1999; Volume 1, ISBN 9687485825. [Google Scholar]
- Nava, R.F.; Zamora, D.R.; González, E.C. Notas Sobre Plantas Medicinales Del Estado de Querétaro, México. Polibotánica 2001, 12, 1–39. [Google Scholar]
- De Feo, V.; Soria, R.M.U. Medicinal Plants and Phytotherapy in Traditional Medicine of Paruro Province, Cusco Department, Peru. Pharmacol. Online 2012, 1, 154–219. [Google Scholar]
- Moura-Letts, G.; Villegas, L.F.; Marçalo, A.; Vaisberg, A.J.; Hammond, G.B. In vivo Wound-Healing Activity of Oleanolic Acid Derived from the Acid Hydrolysis of Anredera diffusa. J. Nat. Prod. 2006, 69, 978–979. [Google Scholar] [CrossRef] [PubMed]
- Godínez-Caraballo, D.; Volpato, G. Plantas Medicinales Que Se Venden En El Mercado El Río, Camagüey, Cuba. Rev. Mex. Biodivers. 2008, 79, 217–241. [Google Scholar] [CrossRef]
- Hilgert, N.I. Plants Used in Home Medicine in the Zenta River Basin, Northwest Argentina. J. Ethnopharmacol. 2001, 76, 11–34. [Google Scholar] [CrossRef]
- Hajdu, Z.; Hohmann, J. An Ethnopharmacological Survey of the Traditional Medicine Utilized in the Community of Porvenir, Bajo Paraguá Indian Reservation, Bolivia. J. Ethnopharmacol. 2012, 139, 838–857. [Google Scholar] [CrossRef] [PubMed]
- Salas-Oropeza, J.; Jimenez-Estrada, M.; Perez-Torres, A.; Castell-Rodriguez, A.E.; Becerril-Millan, R.; Rodriguez-Monroy, M.A.; Canales-Martinez, M.M. Wound Healing Activity of the Essential Oil of Bursera morelensis, in Mice. Molecules 2020, 25, 1795. [Google Scholar] [CrossRef] [PubMed]
- Bueno, N.R.; Castilho, R.O.; da Costa, R.B.; Pott, A.; Pott, V.J.; Scheidt, G.N.; da Silva Batista, M. Medicinal Plants Used by the Kaiowá and Guarani Indigenous Populations in the Caarapó Reserve, Mato Grosso Do Sul, Brazil. Acta Bot. Bras. 2005, 19, 39–44. [Google Scholar] [CrossRef]
- Bitu, V.D.C.N.; Bitu, V.D.C.N.; Matias, E.F.F.; de Lima, W.P.; da Costa Portelo, A.; Coutinho, H.D.M.; de Menezes, I.R.A. Ethnopharmacological Study of Plants Sold for Therapeutic Purposes in Public Markets in Northeast Brazil. J. Ethnopharmacol. 2015, 172, 265–272. [Google Scholar] [CrossRef]
- Milliken, W.; Albert, B. The Use of Medicinal Plants by the Yanomami Indians of Brazil. Econ. Bot. 1996, 50, 10–25. [Google Scholar] [CrossRef]
- De Fátima Agra, M.; Silva, K.N.; Basílio, I.J.L.D.; de Freitas, P.F.; Barbosa-Filho, J.M. Survey of Medicinal Plants Used in the Region Northeast of Brazil. Rev. Bras. Farmacogn. 2008, 18, 472–508. [Google Scholar] [CrossRef]
- Do Nascimento Magalhães, K.; Guarniz, W.A.S.; Sá, K.M.; Freire, A.B.; Monteiro, M.P.; Nojosa, R.T.; Bieski, I.G.C.; Custódio, J.B.; Balogun, S.O.; Bandeira, M.A.M. Medicinal Plants of the Caatinga, Northeastern Brazil: Ethnopharmacopeia (1980–1990) of the Late Professor Francisco José de Abreu Matos. J. Ethnopharmacol. 2019, 237, 314–353. [Google Scholar] [CrossRef]
- Desmarchelier, C.; Mongelli, E.; Coussio, J.; Giulietti, A.; Ciccia, G. Etnobotánica y Bioactividad de Plantas Medicinales Utilizadas Por Un Grupo Indígena Takana de La Amazonía Peruana. Acta Farm. Bonaer. 1995, 14, 195–208. [Google Scholar]
- Estudillo, R.L.; García, A.H. Catálogo de Plantas Medicinales Sonorenses; Universidad de Sonora: Sonora, Mexico, 1988. [Google Scholar]
- Madaleno, I.M.; El Montero, M.C. Cultivo Urbano de Plantas Medicinales, Su Comercialización y Usos Eitoterapeuticos En La Ciudad de Río Cuarto, Provincia de Córdoba, Argentina. Cuad. Geogr. 2012, 50, 63–85. [Google Scholar] [CrossRef]
- Hernández, R.; Jordá, M.G. Plantas Medicinales; Editorial Pax Mexico: Mexico City, Mexico, 1981; ISBN 968461005X. [Google Scholar]
- Milliken, W.; Albert, B. The Use of Medicinal Plants by the Yanomami Indians of Brazil, Part II. Econ. Bot. 1997, 51, 264–278. [Google Scholar] [CrossRef]
- Molina-Mendoza, J.L.; Galván-Villanueva, R.; Patiño-Siciliano, A.; Fernández-Nava, R. Plantas Medicinales y Listado Florístico Preliminar Del Municipio de Huasca de Ocampo, Hidalgo, México. Polibotánica 2012, 34, 259–291. [Google Scholar]
- Saraiva, M.E.; de Alencar Ulisses, A.V.R.; Ribeiro, D.A.; de Oliveira, L.G.S.; de Macedo, D.G.; de Sousa, F.D.F.S.; de Menezes, I.R.A.; Sampaio, E.V.D.S.B.; de Almeida Souza, M.M. Plant Species as a Therapeutic Resource in Areas of the Savanna in the State of Pernambuco, Northeast Brazil. J. Ethnopharmacol. 2015, 171, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Argueta, A.; Cano Asseleih, L.M.; Rodarte García, M.E. Atlas de Las Plantas de La Medicina Tradicional Mexicana; Academia: Prague, Czechoslovakia, 1994; ISBN 9682973236. [Google Scholar]
- De la Cruz, M.G.; Malpartida, S.B.; Santiago, H.B.; Jullian, V.; Bourdy, G. Hot and Cold: Medicinal Plant Uses in Quechua Speaking Communities in the High Andes (Callejón de Huaylas, Ancash, Perú). J. Ethnopharmacol. 2014, 155, 1093–1117. [Google Scholar] [CrossRef] [PubMed]
- Consolini, A.E.; Ragone, M.I.; Tambussi, A.; Paura, A. Estudio Observacional Del Consumo de Plantas Medicinales En La Provincia de Buenos Aires, Argentina, En El Período Diciembre de 2004-Noviembre de 2005. Lat. Am. J. Pharm. 2007, 26, 924. [Google Scholar]
- Pastar, I.; Liang, L.; Sawaya, A.P.; Wikramanayake, T.C.; Glinos, G.D.; Drakulich, S.; Chen, V.; Stojadinovic, O.; Davis, S.C.; Tomic-Canic, M. Preclinical Models for Wound-Healing Studies. In Skin Tissue Models; Elsevier: Amsterdam, The Netherlands, 2018; pp. 223–253. [Google Scholar] [CrossRef]
- Prado, L.G.; Arruda, H.S.; Araujo, N.M.P.; de Oliveira Braga, L.E.; Banzato, T.P.; Pereira, G.A.; Figueiredo, M.C.; Ruiz, A.L.T.G.; Eberlin, M.N.; de Carvalho, J.E. Antioxidant, Antiproliferative and Healing Properties of Araticum (Annona crassiflora Mart.) Peel and Seed. Food Res. Int. 2020, 133, 109168. [Google Scholar] [CrossRef] [PubMed]
- Thangavelu, S.; Balasubramanian, B.; Palanisamy, S.; Shanmugam, V.; Natchiappan, S.; Kalibulla, S.I.; Rathinasamy, B.; Arumugam, V.A. Characterization and Phytoconstituents of Petroselinum crispum (Mill) and Coriandrum sativum (Linn) and Their Impacts on Inflammation—An in vitro Analysis against Human Adenocarcinoma Cells with Molecular Docking. S. Afr. J. Bot. 2022, 146, 776–788. [Google Scholar] [CrossRef]
- Geller, F.C.; Teixeira, M.R.; Pereira, A.B.D.; Dourado, L.P.A.; Souza, D.G.; Braga, F.C.; Simões, C.M.O. Evaluation of the Wound Healing Properties of Hancornia speciosa Leaves. Phyther. Res. 2015, 29, 1887–1893. [Google Scholar] [CrossRef]
- Santos, J.A.A.; da Silva, J.W.; dos Santos, S.M.; de Rodrigues, M.F.; Silva, C.J.A.; da Silva, M.V.; Correia, M.T.S.; Albuquerque, J.F.C.; Melo, C.M.L.; Silva, T.G. In vitro and in vivo Wound Healing and Anti-Inflammatory Activities of Babassu Oil (Attalea speciosa Mart. Ex Spreng., Arecaceae). Evid.-Based Complement. Altern. Med. 2020, 2020, 8858291. [Google Scholar] [CrossRef]
- Alerico, G.C.; Beckenkamp, A.; Vignoli-Silva, M.; Buffon, A.; von Poser, G.L. Proliferative Effect of Plants Used for Wound Healing in Rio Grande Do Sul State, Brazil. J. Ethnopharmacol. 2015, 176, 305–310. [Google Scholar] [CrossRef]
- Balekar, N.; Katkam, N.G.; Nakpheng, T.; Jehtae, K.; Srichana, T. Evaluation of the Wound Healing Potential of Wedelia trilobata (L.) Leaves. J. Ethnopharmacol. 2012, 141, 817–824. [Google Scholar] [CrossRef]
- Jorge, M.P.; Madjarof, C.; Ruiz, A.L.T.G.; Fernandes, A.T.; Rodrigues, R.A.F.; de Oliveira Sousa, I.M.; Foglio, M.A.; de Carvalho, J.E. Evaluation of Wound Healing Properties of Arrabidaea chica Verlot Extract. J. Ethnopharmacol. 2008, 118, 361–366. [Google Scholar] [CrossRef]
- Carvalho, E.G.; Soares, C.P.; Blau, L.; Menegon, R.F.; Joaquim, W.M. Wound Healing Properties and Mucilage Content of Pereskia aculeata from Different Substrates. Rev. Bras. Farmacogn. 2014, 24, 677–682. [Google Scholar] [CrossRef]
- Geller, F.; Schmidt, C.; Göttert, M.; Fronza, M.; Schattel, V.; Heinzmann, B.; Werz, O.; Flores, E.M.M.; Merfort, I.; Laufer, S. Identification of Rosmarinic Acid as the Major Active Constituent in Cordia americana. J. Ethnopharmacol. 2010, 128, 561–566. [Google Scholar] [CrossRef]
- De Oliveira Rodrigues, R.; Yaochite, J.N.U.; Sasahara, G.L.; Albuquerque, A.A.; da Cruz Fonseca, S.G.; de Vasconcelos Araújo, T.D.; Santiago, G.M.P.; de Sousa, L.M.; de Carvalho, J.L.; Alves, A.P.N.N. Antioxidant, Anti-Inflammatory and Healing Potential of Ethyl Acetate Fraction of Bauhinia ungulata L.(Fabaceae) on in vitro and in vivo Wound Model. Mol. Biol. Rep. 2020, 47, 2845–2859. [Google Scholar] [CrossRef]
- Coco, J.C.; Ataide, J.A.; Sake, J.A.; Tambourgi, E.B.; Ehrhardt, C.; Mazzola, P.G. In vitro Antioxidant and Wound Healing Properties of Baru Nut Extract (Dipteryx alata Vog.) in Pulmonary Epithelial Cells for Therapeutic Application in Chronic Pulmonary Obstructive Disease (COPD). Nat. Prod. Res. 2021, 1, 1–7. [Google Scholar] [CrossRef]
- Zippel, J.; Deters, A.; Hensel, A. Arabinogalactans from Mimosa tenuiflora (Willd.) Poiret Bark as Active Principles for Wound-Healing Properties: Specific Enhancement of Dermal Fibroblast Activity and Minor Influence on HaCaT Keratinocytes. J. Ethnopharmacol. 2009, 124, 391–396. [Google Scholar] [CrossRef]
- Bueno, F.G.; Panizzon, G.P.; de Leite Mello, E.V.S.; Lechtenberg, M.; Petereit, F.; de Mello, J.C.P.; Hensel, A. Hydrolyzable Tannins from Hydroalcoholic Extract from Poincianella pluviosa Stem Bark and Its Wound-Healing Properties: Phytochemical Investigations and Influence on in vitro Cell Physiology of Human Keratinocytes and Dermal Fibroblasts. Fitoterapia 2014, 99, 252–260. [Google Scholar] [CrossRef]
- Bridi, H.; Beckenkamp, A.; Ccana-Ccapatinta, G.V.; de Loreto Bordignon, S.A.; Buffon, A.; von Poser, G.L. Characterization of Phloroglucinol-enriched Fractions of Brazilian Hypericum Species and Evaluation of Their Effect on Human Keratinocytes Proliferation. Phyther. Res. 2017, 31, 62–68. [Google Scholar] [CrossRef]
- Vittorazzi, C.; Endringer, D.C.; de Andrade, T.U.; Scherer, R.; Fronza, M. Antioxidant, Antimicrobial and Wound Healing Properties of Struthanthus vulgaris. Pharm. Biol. 2016, 54, 331–337. [Google Scholar] [CrossRef]
- Pereira, L.O.M.; Vilegas, W.; Tangerina, M.M.P.; Arunachalam, K.; Balogun, S.O.; Orlandi-Mattos, P.E.; Colodel, E.M.; de Oliveira Martins, D.T. Lafoensia pacari A. St.-Hil.: Wound Healing Activity and Mechanism of Action of Standardized Hydroethanolic Leaves Extract. J. Ethnopharmacol. 2018, 219, 337–350. [Google Scholar] [CrossRef]
- De Freitas Figueiredo, F.; Cechinel Filho, V.; Damazo, A.S.; Arunachalam, K.; Colodel, E.M.; Ribeiro, M.; Venturini, C.L.; Oliveira, D.M.; Machado, M.T.M.; Pavan, E. Sorocea guilleminiana Gaudich.: Wound Healing Activity, Action Mechanisms, and Chemical Characterization of the Leaf Infusion. J. Ethnopharmacol. 2020, 248, 112307. [Google Scholar] [CrossRef] [PubMed]
- Mazutti da Silva, S.M.; Rezende Costa, C.R.; Martins Gelfuso, G.; Silva Guerra, E.N.; de Medeiros Nóbrega, Y.K.; Gomes, S.M.; Pic-Taylor, A.; Fonseca-Bazzo, Y.M.; Silveira, D.; de Oliveira Magalhães, P. Wound Healing Effect of Essential Oil Extracted from Eugenia dysenterica DC (Myrtaceae) Leaves. Molecules 2018, 24, 2. [Google Scholar] [CrossRef]
- Da Pitz, H.S.; Pereira, A.; Blasius, M.B.; Voytena, A.P.L.; Affonso, R.C.L.; Fanan, S.; Trevisan, A.C.D.; Ribeiro-do-Valle, R.M.; Maraschin, M. In vitro Evaluation of the Antioxidant Activity and Wound Healing Properties of Jaboticaba (Plinia peruviana) Fruit Peel Hydroalcoholic Extract. Oxid. Med. Cell. Longev. 2016, 2016, 3403586. [Google Scholar] [CrossRef]
- Csepregi, R.; Temesfői, V.; Das, S.; Alberti, Á.; Tóth, C.A.; Herczeg, R.; Papp, N.; Kőszegi, T. Cytotoxic, Antimicrobial, Antioxidant Properties and Effects on Cell Migration of Phenolic Compounds of Selected Transylvanian Medicinal Plants. Antioxidants 2020, 9, 166. [Google Scholar] [CrossRef]
- De Moura Sperotto, N.D.; Steffens, L.; Veríssimo, R.M.; Henn, J.G.; Péres, V.F.; Vianna, P.; Chies, J.A.B.; Roehe, A.; Saffi, J.; Moura, D.J. Wound Healing and Anti-Inflammatory Activities Induced by a Plantago australis Hydroethanolic Extract Standardized in Verbascoside. J. Ethnopharmacol. 2018, 225, 178–188. [Google Scholar] [CrossRef]
- Hernández-Pasteur, G.; Silva-Bermúdez, P.S.; Reyes-Chilpa, R.; Vibrans, H.; Soto-Hernández, M. E Evaluación in vitro de la Actividad Cicatrizante y Antibacteriana de Extractos de Buddleja cordata Kunth y Vismia baccifera (L.) Triana & Planch. Rev. Fitotec. Mex. 2019, 42, 93–99. [Google Scholar]
- Benvenutti, L.; Nunes, R.; Venturi, I.; Ramos, S.A.; Broering, M.F.; Goldoni, F.C.; Pavan, S.E.; Pastor, M.V.D.; Malheiros, A.; Quintão, N.L.M. Anti-Inflammatory and Healing Activity of the Hydroalcoholic Fruit Extract of Solanum diploconos (Mart.) Bohs. J. Immunol. Res. 2021, 2021, 9957451. [Google Scholar] [CrossRef]
- Villegas, L.F.; Fernández, I.D.; Maldonado, H.; Torres, R.; Zavaleta, A.; Vaisberg, A.J.; Hammond, G.B. Evaluation of the Wound-Healing Activity of Selected Traditional Medicinal Plants from Peru. J. Ethnopharmacol. 1997, 55, 193–200. [Google Scholar] [CrossRef]
- Pérez-Contreras, C.V.; Alvarado-Flores, J.; Orona-Ortiz, A.; Balderas-López, J.L.; Salgado, R.M.; Zacaula-Juárez, N.; Krötzsch, E.; Navarrete, A. Wound Healing Activity of the Hydroalcoholic Extract and the Main Metabolites of Amphipterygium adstringens (Cuachalalate) in a Rat Excision Model. J. Ethnopharmacol. 2022, 293, 115313. [Google Scholar] [CrossRef]
- Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Extraction of Polyphenols from Aromatic and Medicinal Plants: An Overview of the Methods and the Effect of Extraction Parameters. In Polyphenols in Plants; Academic Press: Cambridge, MA, USA, 2019; pp. 243–259. [Google Scholar] [CrossRef]
- Guimarães, I.; Baptista-Silva, S.; Pintado, M.; Oliveira, A.L. Polyphenols: A Promising Avenue in Therapeutic Solutions for Wound Care. Appl. Sci. 2021, 11, 1230. [Google Scholar] [CrossRef]
- Da Silveira Vasconcelos, M.; Gomes-Rochette, N.F.; de Oliveira, M.L.M.; Nunes-Pinheiro, D.C.S.; Tomé, A.R.; de Sousa, F.Y.M.; Pinheiro, F.G.M.; Moura, C.F.H.; Miranda, M.R.A.; Mota, E.F. Anti-Inflammatory and Wound Healing Potential of Cashew Apple Juice (Anacardium occidentale L.) in Mice. Exp. Biol. Med. 2015, 240, 1648–1655. [Google Scholar] [CrossRef]
- Fedel-Miyasato, L.E.S.; Kassuya, C.A.L.; Auharek, S.A.; Formagio, A.S.N.; Cardoso, C.A.L.; Mauro, M.O.; Cunha-Laura, A.L.; Monreal, A.C.D.; Vieira, M.C.; Oliveira, R.J. Evaluation of Anti-Inflammatory, Immunomodulatory, Chemopreventive and Wound Healing Potentials from Schinus terebinthifolius Methanolic Extract. Rev. Bras. Farmacogn. 2014, 24, 565–575. [Google Scholar] [CrossRef]
- Nayak, S.; Nalabothu, P.; Sandiford, S.; Bhogadi, V.; Adogwa, A. Evaluation of Wound Healing Activity of Allamanda cathartica. L. and Laurus nobilis. L. Extracts on Rats. BMC Complement. Altern. Med. 2006, 6, 12. [Google Scholar] [CrossRef]
- Santos, G.J.L.; Ferreira, T.C.; Rodrigues, A.L.M.; Freitas, J.C.C.; Morais, S.M.; Girão, V.C.C.; Nunes-Pinheiro, D.C.S. Involvement of Mast Cells, CD68+ and VEGF+ Expressions in Response to Himatanthus drasticus Commercial Latex in Mice Wound Healing Model. Arq. Bras. Med. Vet. Zootec. 2017, 69, 513–522. [Google Scholar] [CrossRef][Green Version]
- Kyakulaga, A.H.; Olila, D.; Jane, F.N.; Omujal, F.; Ogwang, P.E. Wound Healing Potential of the Ethanolic Extracts of Bidens pilosa and Ocimum suave. African J. Pharm. Pharmacol. 2011, 5, 132–136. [Google Scholar]
- Kreuger, M.R.O.; Farias, B.G.; Moreira, J.; Blind, L.Z.; Amoah, S.K.S.; Leite, A.S.; Biavatti, M.W.; Van Hoof, T.; D’Herde, K.; Cruz, A.B. Effects of the Topical Application of an Ethyl Acetate Fraction from Vernonia scorpioides on Excisional Wounds Infected with Staphylococcus aureus in Rats. Rev. Bras. Farmacogn. 2012, 22, 123–130. [Google Scholar] [CrossRef]
- Roy, P.; Amdekar, S.; Kumar, A.; Singh, R.; Sharma, P.; Singh, V. In vivo Antioxidative Property, Antimicrobial and Wound Healing Activity of Flower Extracts of Pyrostegia venusta (Ker Gawl) Miers. J. Ethnopharmacol. 2012, 140, 186–192. [Google Scholar] [CrossRef]
- Prabhakar, K.R.; Srinivasan, K.K.; Rao, P.G.M. Chemical Investigation, Anti-Inflammatory and Wound Healing Properties of Coronopus didymus. Pharm. Biol. 2002, 40, 490–493. [Google Scholar] [CrossRef]
- Perez, R.M.G.; Vargas, R.S.; Ortiz, Y.D.H. Wound Healing Properties of Hylocereus undatus on Diabetic Rats. Phyther. Res. 2005, 19, 665–668. [Google Scholar] [CrossRef]
- Ammar, I.; Bardaa, S.; Mzid, M.; Sahnoun, Z.; Rebaii, T.; Attia, H.; Ennouri, M. Antioxidant, Antibacterial and in vivo Dermal Wound Healing Effects of Opuntia Flower Extracts. Int. J. Biol. Macromol. 2015, 81, 483–490. [Google Scholar] [CrossRef]
- Do Nascimento-Neto, L.G.; Evaristo, F.F.V.; de Alencar Alves, M.F.; Albuquerque, M.R.J.R.; dos Santos, H.S.; Bandeira, P.N.; Arruda, F.V.S.; Teixeira, E.H. Effect of the Triterpene 3β, 6β, 16β-Trihydroxylup-20 (29)-Ene Isolated from the Leaves of Combretum leprosum Mart. on Cutaneous Wounds in Mice. J. Ethnopharmacol. 2015, 171, 116–120. [Google Scholar] [CrossRef]
- Bardaa, S.; Ben Halima, N.; Aloui, F.; Ben Mansour, R.; Jabeur, H.; Bouaziz, M.; Sahnoun, Z. Oil from Pumpkin (Cucurbita pepo L.) Seeds: Evaluation of Its Functional Properties on Wound Healing in Rats. Lipids Health Dis. 2016, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Tuhin, R.H.; Begum, M.; Rahman, M.; Karim, R.; Begum, T.; Ahmed, S.U.; Mostofa, R.; Hossain, A.; Abdel-Daim, M.; Begum, R. Wound Healing Effect of Euphorbia hirta Linn.(Euphorbiaceae) in Alloxan Induced Diabetic Rats. BMC Complement. Altern. Med. 2017, 17, 423. [Google Scholar] [CrossRef]
- Gutierrez, R.M.P. Evaluation of the Wound Healing Properties of Acalypha langiana in Diabetic Rats. Fitoterapia 2006, 77, 286–289. [Google Scholar] [CrossRef]
- Vaisberg, A.J.; Milla, M.; del Carmen Planas, M.; Cordova, J.L.; de Agusti, E.R.; Ferreyra, R.; del Carmen Mustiga, M.; Carlin, L.; Hammond, G.B. Taspine Is the Cicatrizant Principle in Sangre de Grado Extracted from Croton lechleri. Planta Med. 1989, 55, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Hernandez, A.B.; Alarcon-Aguilar, F.J.; Almanza-Perez, J.C.; Nieto-Yañez, O.; Olivares-Sanchez, J.M.; Duran-Diaz, A.; Rodriguez-Monroy, M.A.; Canales-Martinez, M.M. Antimicrobial and Anti-Inflammatory Activities, Wound-Healing Effectiveness and Chemical Characterization of the Latex of Jatropha neopauciflora Pax. J. Ethnopharmacol. 2017, 204, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Agra, I.K.R.; Pires, L.L.S.; Carvalho, P.S.M.; Silva-Filho, E.A.; Smaniotto, S.; Barreto, E. Evaluation of Wound Healing and Antimicrobial Properties of Aqueous Extract from Bowdichia virgilioides Stem Barks in Mice. An. Acad. Bras. Cienc. 2013, 85, 945–954. [Google Scholar] [CrossRef]
- De Pereira, L.P.; Mota, M.R.L.; Brizeno, L.A.C.; Nogueira, F.C.; Ferreira, E.G.M.; Pereira, M.G.; Assreuy, A.M.S. Modulator Effect of a Polysaccharide-Rich Extract from Caesalpinia ferrea Stem Barks in Rat Cutaneous Wound Healing: Role of TNF-α, IL-1β, NO, TGF-β. J. Ethnopharmacol. 2016, 187, 213–223. [Google Scholar] [CrossRef]
- Paiva, L.A.F.; de Alencar Cunha, K.M.; Santos, F.A.; Gramosa, N.V.; Silveira, E.R.; Rao, V.S.N. Investigation on the Wound Healing Activity of Oleo-resin from Copaifera langsdorffi in Rats. Phytother. Res. 2002, 16, 737–739. [Google Scholar] [CrossRef]
- Amorim, J.L.; de Barros Figueiredo, J.; Amaral, A.C.F.; de Oliveira Barros, E.G.; Palmero, C.; MPalantinos, M.A.; de Souza Ramos, A.; Ferreira, J.L.P.; de Andrade Silva, J.R.; Benjamim, C.F.; et al. Wound Healing Properties of Copaifera paupera in Diabetic Mice. PLoS ONE 2017, 12, e0187380. [Google Scholar] [CrossRef]
- Nayak, B.S.; Raju, S.S.; Chalapathi Rao, A. V Wound Healing Activity of Persea americana (Avocado) Fruit: A Preclinical Study on Rats. J. Wound Care 2008, 17, 123–125. [Google Scholar] [CrossRef]
- Lodhi, S.; Singhai, A.K. Preliminary Pharmacological Evaluation of Martynia annua Linn Leaves for Wound Healing. Asian Pac. J. Trop. Biomed. 2011, 1, 421–427. [Google Scholar] [CrossRef]
- Nayak, B.S.; Kanhai, J.; Milne, D.M.; Pereira, L.P.; Swanston, W.H. Experimental Evaluation of Ethanolic Extract of Carapa guianensis L. Leaf for Its Wound Healing Activity Using Three Wound Models. Evid.-Based Complement. Altern. Med. 2011, 2011, 419612. [Google Scholar] [CrossRef]
- Sharma, Y.; Kaur, A.; Bhardwaj, R.; Srivastava, N.; Lal, M.; Madan, S.; Bala, K. Preclinical Assessment of Stem of Nicotiana tabacum on Excision Wound Model. Bioorg. Chem. 2021, 109, 104731. [Google Scholar] [CrossRef]
- Nayak, B.S. Cecropia peltata L. (Cecropiaceae) Has Wound-Healing Potential: A Preclinical Study in a Sprague Dawley Rat Model. Int. J. Low. Extrem. Wounds 2006, 5, 20–26. [Google Scholar] [CrossRef]
- Nayak, B.S.; Raju, S.S.; Eversley, M.; Ramsubhag, A. Evaluation of Wound Healing Activity of Lantana camara L.—A Preclinical Study. Phytother. Res. 2009, 23, 241–245. [Google Scholar] [CrossRef]
- Budovsky, A.; Yarmolinsky, L.; Ben-Shabat, S. Effect of Medicinal Plants on Wound Healing. Wound Repair Regen. 2015, 23, 171–183. [Google Scholar] [CrossRef]
- Masson-Meyers, D.S.; Andrade, T.A.M.; Caetano, G.F.; Guimaraes, F.R.; Leite, M.N.; Leite, S.N.; Frade, M.A.C. Experimental Models and Methods for Cutaneous Wound Healing Assessment. Int. J. Exp. Pathol. 2020, 101, 21–37. [Google Scholar] [CrossRef]
- Cross, S.E.; Naylor, L.; Coleman, R.A.; Teo, T.C. An Experimental Model to Investigate the Dynamics of Wound Contraction. Br. J. Plast. Surg. 1995, 48, 189–197. [Google Scholar] [CrossRef]
- Abarca, L.F.S.; Klinkhamer, P.G.L.; Choi, Y.H. Plant Latex, from Ecological Interests to Bioactive Chemical Resources. Planta Med. 2019, 85, 856–868. [Google Scholar] [CrossRef]
- Lovell, C.; Paulsen, E.; Lepoittevin, J.-P. Adverse Skin Reactions to Plants and Plant Products. In Contact Dermatitis; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–49. [Google Scholar] [CrossRef]
- De Oliveira, M.L.M.; Bezerra, B.M.O.; Leite, L.O.; Girão, V.C.C.; Nunes-Pinheiro, D.C.S. Topical Continuous Use of Lippia sidoides Cham. Essential Oil Induces Cutaneous Inflammatory Response, but Does Not Delay Wound Healing Process. J. Ethnopharmacol. 2014, 153, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Barua, C.C.; Ara Begum, S.; Talukdar, A.; Datta Roy, J.; Buragohain, B.; Chandra Pathak, D.; Kumar Sarma, D.; Saikia Bora, R.; Gupta, A. Influence of Alternanthera brasiliana (L.) Kuntze on Altered Antioxidant Enzyme Profile during Cutaneous Wound Healing in Immunocompromised Rats. Int. Sch. Res. Not. 2012, 2012, 948792. [Google Scholar] [CrossRef]
- Barua, C.C.; Begum, S.A.; Pathak, D.C.; Bora, R.S. Wound Healing Activity of Alternanthera brasiliana Kuntze and Its Anti Oxidant Profiles in Experimentally Induced Diabetic Rats. J. Appl. Pharm. Sci. 2013, 3, 161–166. [Google Scholar] [CrossRef]
- TrivellatoGrassi, L.; Malheiros, A.; Meyre-Silva, C.; da Silva Buss, Z.; Monguilhott, E.D.; Fröde, T.S.; da Silva, K.A.B.S.; de Souza, M.M. From Popular Use to Pharmacological Validation: A Study of the Anti-Inflammatory, Anti-Nociceptive and Healing Effects of Chenopodium ambrosioides Extract. J. Ethnopharmacol. 2013, 145, 127–138. [Google Scholar] [CrossRef] [PubMed]
- De Moura, F.B.R.; Justino, A.B.; Ferreira, B.A.; Espindola, F.S.; de Assis Araújo, F.; Tomiosso, T.C. Pro-Fibrogenic and Anti-Inflammatory Potential of a Polyphenol-Enriched Fraction from Annona crassiflora in Skin Repair. Planta Med. 2019, 85, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Moghadamtousi, S.Z.; Rouhollahi, E.; Hajrezaie, M.; Karimian, H.; Abdulla, M.A.; Kadir, H.A. Annona muricata Leaves Accelerate Wound Healing in Rats via Involvement of Hsp70 and Antioxidant Defence. Int. J. Surg. 2015, 18, 110–117. [Google Scholar] [CrossRef]
- Pereira, L.X.; Silva, H.K.C.; Longatti, T.R.; Silva, P.P.; Oliveira, C.D.L.; de Proietti, A.B.F.C.; Thomé, R.G.; do Carmo Vieira, M.; Carollo, C.A.; Demarque, D.P. Achyrocline alata Potentiates Repair of Skin Full Thickness Excision in Mice. J. Tissue Viability 2017, 26, 289–299. [Google Scholar] [CrossRef]
- Umadevi, S.; Mohanta, G.P.; Kalaichelvan, V.K.; Manavalan, R. Studies on Wound Healing Effect of Flaveria trinervia Leaf in Mice. Indian J. Pharm. Sci. 2006, 68, 106. [Google Scholar] [CrossRef]
- Nayak, B.S.; Ramlogan, S.; Rao, A.V.C.; Maharaj, S. Neurolaena lobata L. Promotes Wound Healing in Sprague Dawley Rats. Int. J. Appl. Basic Med. Res. 2014, 4, 106. [Google Scholar] [CrossRef]
- García-Bores, A.M.; Álvarez-Santos, N.; López-Villafranco, M.E.; Jácquez-Ríos, M.P.; Aguilar-Rodríguez, S.; Grego-Valencia, D.; Espinosa-González, A.M.; Estrella-Parra, E.A.; Hernández-Delgado, C.T.; Serrano-Parrales, R. Verbesina crocata: A Pharmacognostic Study for the Treatment of Wound Healing. Saudi J. Biol. Sci. 2020, 27, 3113–3124. [Google Scholar] [CrossRef]
- Leite, S.N.; Palhano, G.; Almeida, S.; Biavatti, M.W. Wound Healing Activity and Systemic Effects of Vernonia scorpioides Extract in Guinea Pig. Fitoterapia 2002, 73, 496–500. [Google Scholar] [CrossRef]
- De Castro Campos, N.P.; Cassini-Vieira, P.; de Souza-Fagundes, E.M.; Barcelos, L.S.; Castañon, M.C.M.N.; Scio, E. Pereskia aculeata Miller Leaves Accelerate Excisional Wound Healing in Mice. J. Ethnopharmacol. 2016, 194, 131–136. [Google Scholar] [CrossRef]
- Lordani, T.V.A.; Brenzan, M.A.; Cortez, L.E.R.; Lordani, C.R.F.; Honda, P.A.; Lonardoni, M.V.C.; Cortez, D.A.G. Effect of a Topical Formulation Containing Calophyllum brasiliense Camb. Extract on Cutaneous Wound Healing in Rats. Nat. Prod. Res. 2015, 29, 953–957. [Google Scholar] [CrossRef]
- De Oliveira, M.L.M.; Nunes-Pinheiro, D.C.S.; Tomé, A.R.; Mota, E.F.; Lima-Verde, I.A.; de Melo Pinheiro, F.G.; Campello, C.C.; De Morais, S.M. In vivo Topical Anti-Inflammatory and Wound Healing Activities of the Fixed Oil of Caryocar coriaceum Wittm. Seeds. J. Ethnopharmacol. 2010, 129, 214–219. [Google Scholar] [CrossRef]
- De Moura, F.B.R.; Ferreira, B.A.; Deconte, S.R.; Landim, B.C.; Justino, A.B.; de Aro, A.A.; Espindola, F.S.; Rodrigues, R.A.F.; Ribeiro, D.L.; de Assis Araújo, F. Wound Healing Activity of the Hydroethanolic Extract of the Leaves of Maytenus ilicifolia Mart. Ex Reis. J. Tradit. Complement. Med. 2021, 11, 446–456. [Google Scholar] [CrossRef]
- Hermes, D.; Dudek, D.N.; Maria, M.D.; Horta, L.P.; Lima, E.N.; de Fatima, A.; Sanches, A.C.C.; Modolo, L.V. In vivo Wound Healing and Antiulcer Properties of White Sweet Potato (Ipomoea batatas). J. Adv. Res. 2013, 4, 411–415. [Google Scholar] [CrossRef][Green Version]
- Silva-Correa, C.R.; Ortiz-Noriega, C.M.; Villarreal-La Torre, V.E.; Calderón-Peña, A.A. Effect of a Gel Based on Ipomoea batatas (Purple Sweet Potato) on Dermal Wound Healing in Mice. Pharmacogn. J. 2021, 13, 1720–1726. [Google Scholar] [CrossRef]
- Cavalcanti, J.M.; Leal-Cardoso, J.H.; Diniz, L.R.L.; Portella, V.G.; Costa, C.O.; Linard, C.F.B.M.; Alves, K.; de Paula Rocha, M.V.A.; Lima, C.C.; Cecatto, V.M. The Essential Oil of Croton zehntneri and Trans-Anethole Improves Cutaneous Wound Healing. J. Ethnopharmacol. 2012, 144, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Esimone, C.O.; Nworu, C.S.; Jackson, C.L. Cutaneous Wound Healing Activity of a Herbal Ointment Containing the Leaf Extract of Jatropha curcas L.(Euphorbiaceae). Int. J. Appl. Res. Nat. Prod. 2008, 1, 1–4. [Google Scholar]
- León, F.; Hernandez-Zapata, V.; Bacab, M.C.; Maldonado, G.; Lezama, J.A.; Monteon, V. The Wound Healing Action of a Cream Latex Formulation of Jatropha gaumeri Greenm. in a Pre-Clinical Model. Vet. World 2020, 13, 2508. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Samanta, A.; Mandal, N.B.; Bannerjee, S.; Chattopadhyay, D. Evaluation of the Wound Healing Activity of Methanol Extract of Pedilanthus tithymaloides (L.) Poit Leaf and Its Isolated Active Constituents in Topical Formulation. J. Ethnopharmacol. 2012, 142, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Gushiken, L.F.S.; Hussni, C.A.; Bastos, J.K.; Rozza, A.L.; Beserra, F.P.; Vieira, A.J.; Padovani, C.R.; Lemos, M.; Polizello Junior, M.; da Silva, J.J.M. Skin Wound Healing Potential and Mechanisms of the Hydroalcoholic Extract of Leaves and Oleoresin of Copaifera langsdorffii Desf. Kuntze in Rats. Evid.-Based Complement. Altern. Med. 2017, 2017, 6589270. [Google Scholar] [CrossRef]
- Gouveia, M.C.P.; Minto, B.W.; Sargi, L.F.; Souza, R.L.; Pazzini, J.M.; Colodel, E.M.; Silva, V.C.P.; Cassino, P.C.; Dias, L. Evaluation of the Alcoholic Extract of Dipteryx alata Vogel Almonds and Bark in Skin Wound Healing in C57BL6 Mice. Arq. Bras. Med. Vet. Zootec. 2021, 73, 1315–1322. [Google Scholar] [CrossRef]
- Kokane, D.D.; More, R.Y.; Kale, M.B.; Nehete, M.N.; Mehendale, P.C.; Gadgoli, C.H. Evaluation of Wound Healing Activity of Root of Mimosa pudica. J. Ethnopharmacol. 2009, 124, 311–315. [Google Scholar] [CrossRef]
- Costa, A.C.F.; Cavalcante, G.M. Evaluating the Use of an Ointment Based on Prosopis juliflora Leaves in the Topical Therapy of Wounds. Adv. Res. 2015, 5, 17440. [Google Scholar] [CrossRef]
- Hernandes, L.; da Silva Pereira, L.M.; Palazzo, F.; de Mello, J.C.P. Wound-Healing Evaluation of Ointment from Stryphnodendron adstringens (Barbatimão) in Rat Skin. Braz. J. Pharm. Sci. 2010, 46, 431–436. [Google Scholar] [CrossRef]
- De Oliveira, A.P.; de Franco, E.S.; Barreto, R.R.; Cordeiro, D.P.; de Melo, R.G.; de Aquino, C.M.F.; de Medeiros, P.L.; da Silva, T.G.; da Silva Góes, A.J.; de Sousa Maia, M.B. Effect of Semisolid Formulation of Persea americana Mill (Avocado) Oil on Wound Healing in Rats. Evid.-Based Complement. Altern. Med. 2013, 2013, 472382. [Google Scholar] [CrossRef]
- Ekom, S.E.; Kuete, V. Methanol Extract from the Seeds of Persea americana Displays Antibacterial and Wound Healing Activities in Rat Model. J. Ethnopharmacol. 2022, 282, 114573. [Google Scholar] [CrossRef]
- Sarandy, M.M.; Novaes, R.D.; Xavier, A.A.; Vital, C.E.; Leite, J.P.V.; Melo, F.C.S.A.; Gonçalves, R.V. Hydroethanolic Extract of Strychnos pseudoquina Accelerates Skin Wound Healing by Modulating the Oxidative Status and Microstructural Reorganization of Scar Tissue in Experimental Type I Diabetes. Biomed Res. Int. 2017, 2017, 9538351. [Google Scholar] [CrossRef]
- Sarandy, M.M.; Miranda, L.L.; Altoé, L.S.; Novaes, R.D.; Zanuncio, V.V.; Leite, J.P.V.; Goncalves, R.V. Strychnos pseudoquina Modulates the Morphological Reorganization of the Scar Tissue of Second Intention Cutaneous Wounds in Rats. PLoS ONE 2018, 13, e0195786. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Gramma, L.S.; Marques, F.M.; Vittorazzi, C.; de Andrade, T.A.M.; Frade, M.A.C.; de Andrade, T.U.; Endringer, D.C.; Scherer, R.; Fronza, M. Struthanthus vulgaris Ointment Prevents an over Expression of Inflammatory Response and Accelerates the Cutaneous Wound Healing. J. Ethnopharmacol. 2016, 190, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, S.; Singhai, A.K. Wound Healing Effect of Flavonoid Rich Fraction and Luteolin Isolated from Martynia annua Linn. on Streptozotocin Induced Diabetic Rats. Asian Pac. J. Trop. Med. 2013, 6, 253–259. [Google Scholar] [CrossRef]
- Martínez, M.G.M.; Montalvo-Javé, E.E.; Toledo, E.W.; Sánchez, M.T.; Rodríguez, A.C.; Campos, A.G.; Hernández, G.L.; Fernández, R.O.; Franco, A.E.B. Efecto Cicatrizante de La Pomada Preparada Con Dorstenia drakena L.(Moraceae) En Heridas Cutáneas. Cir. Gen. 2008, 30, 204–210. [Google Scholar]
- Gutierrez, R.M.P.; Solis, R.V. Anti-Inflammatory and Wound Healing Potential of Prosthechea michuacana in Rats. Pharmacogn. Mag. 2009, 5, 219. [Google Scholar]
- Soares, R.D.F.; Campos, M.G.N.; Ribeiro, G.P.; Salles, B.C.C.; Cardoso, N.S.; Ribeiro, J.R.; Souza, R.M.; Leme, K.C.; Soares, C.B.; de Oliveira, C.M. Development of a Chitosan Hydrogel Containing Flavonoids Extracted from Passiflora edulis Leaves and the Evaluation of Its Antioxidant and Wound Healing Properties for the Treatment of Skin Lesions in Diabetic Mice. J. Biomed. Mater. Res. Part A 2020, 108, 654–662. [Google Scholar] [CrossRef]
- Reinhardt, L.S.; Henn, J.G.; Moras, A.M.; de Moura Sperotto, N.D.; Ferro, M.B.; Cao, Z.; Roehe, A.V.; Petry, A.U.S.; Nugent, M.; Moura, D.J. Plantago australis Hydroethanolic Extract-Loaded Formulations: Promising Dressings for Wound Healing. Rev. Bras. Farmacogn. 2021, 31, 91–101. [Google Scholar] [CrossRef]
- Gomez-Beloz, A.; Rucinski, J.C.; Balick, M.J.; Tipton, C. Double Incision Wound Healing Bioassay Using Hamelia patens from El Salvador. J. Ethnopharmacol. 2003, 88, 169–173. [Google Scholar] [CrossRef]
- Mora, C.A.; Chamorro, K.L.; Yupanqui, D.M.L.; Unzueta, R.J.S.; Mestanza, R.C.; Perez, P.R. Efecto Cicatrizante Del Ungüento de Dodonaea viscosa Jacq “Chamisa” En Ratones Balb/C 53. Ágora Rev. Cient. 2018, 4, e2. [Google Scholar] [CrossRef]
- Ekom, S.E.; Tamokou, J.-D.-D.; Kuete, V. Antibacterial and Therapeutic Potentials of the Capsicum annuum Extract against Infected Wound in a Rat Model with Its Mechanisms of Antibacterial Action. Biomed Res. Int. 2021, 2021, 4303902. [Google Scholar] [CrossRef]
- Abdul-Nasir-Deen, A.-Y.; Boakye, Y.D.; Osafo, N.; Agyare, C.; Boamah, D.; Boamah, V.E.; Agyei, E.K. Anti-Inflammatory and Wound Healing Properties of Methanol Leaf Extract of Physalis angulata L. S. Afr. J. Bot. 2020, 133, 124–131. [Google Scholar] [CrossRef]
- De Carvalho Bulhões, A.A.V.; de Moura Estevão, L.R.; Florencio-Silva, R.; Simoes, R.S.; Leite, A.G.B.; da Silva Cunha, D.M.S.; Ramos, C.S.; de Andrade Soares, E.B.; D’Emery, M.B.; da Câmara, C.A.G.; et al. Effects of the Healing Activity of Rosemary-of-Chapada (Lippia gracilis Schauer) on Cutaneous Lesions in Rats. Acta Cir. Bras. 2022, 37. [Google Scholar] [CrossRef]
- De Castro Souza, J.; de Moura Estevão, L.R.; Baratella-Evêncio, L.; Vieira, M.G.F.; Simões, R.S.; Florencio-Silva, R.; Evêncio-Luz, L.; Evêncio-Neto, J. Mast Cell Concentration and Skin Wound Contraction in Rats Treated with Ximenia americana L. Acta Cir. Bras. 2017, 32, 148–156. [Google Scholar] [CrossRef]
- Faccio, G. Plant Complexity and Cosmetic Innovation. iScience 2020, 23, 101358. [Google Scholar] [CrossRef]
- Villegas, L.F.; Marçalo, A.; Martin, J.; Fernández, I.D.; Maldonado, H.; Vaisberg, A.J.; Hammond, G.B. (+)-Ep i-α-Bisbolol Is the Wound-Healing Principle of Peperomia galioides: Investigation of the in vivo Wound-Healing Activity of Related Terpenoids. J. Nat. Prod. 2001, 64, 1357–1359. [Google Scholar] [CrossRef]
- Ambiga, S.; Narayanan, R.; Gowri, D.; Sukumar, D.; Madhavan, S. Evaluation of Wound Healing Activity of Flavonoids from Ipomoea carnea Jacq. Anc. Sci. Life 2007, 26, 45. Available online: http://www.ncbi.nlm.nih.gov/pmc/articles/pmc3330873/ (accessed on 28 April 2022).
- Schmidt, C.A.; Murillo, R.; Bruhn, T.; Bringmann, G.; Goettert, M.; Heinzmann, B.; Brecht, V.; Laufer, S.A.; Merfort, I. Catechin Derivatives from Parapiptadenia rigida with in vitro Wound-Healing Properties. J. Nat. Prod. 2010, 73, 2035–2041. [Google Scholar] [CrossRef]
- De Aquino, P.E.A.; de Souza, T.d.F.G.; Santos, F.A.; Viana, A.F.S.C.; Louchard, B.O.; Leal, L.K.A.M.; Rocha, T.M.; Evangelista, J.S.A.M.; de Aquino, N.C.; de Alencar, N.M.N. The Wound Healing Property of N-Methyl-(2 S, 4 R)-Trans-4-Hydroxy-L-Proline from Sideroxylon obtusifolium Is Related to Its Anti-Inflammatory and Antioxidant Actions. J. Evid.-Based Integr. Med. 2019, 24, 2515690X19865166. [Google Scholar] [CrossRef]
- Perez-Gutierrez, R.M.; Vargas-Solis, R. Wound Healing Properties of Triterpenes from Buddleia scordioides in Diabetic Rats. Pharm. Biol. 2008, 46, 647–653. [Google Scholar] [CrossRef][Green Version]
- Romero-Cerecero, O.; Zamilpa-Álvarez, A.; Jiménez-Ferrer, E.; Tortoriello, J. Exploratory Study on the Effectiveness of a Standardized Extract from Ageratina pichinchensis in Patients with Chronic Venous Leg Ulcers. Planta Med. 2012, 78, 304–310. [Google Scholar] [CrossRef]
- Hoversten, K.P.; Kiemele, L.J.; Stolp, A.M.; Takahashi, P.Y.; Verdoorn, B.P. Prevention, Diagnosis, and Management of Chronic Wounds in Older Adults. Mayo Clin. Proc. 2020, 95, 2021–2034. [Google Scholar] [CrossRef]
- Rivera-Arce, E.; Chavez-Soto, M.A.; Herrera-Arellano, A.; Arzate, S.; Agüero, J.; Feria-Romero, I.A.; Cruz-Guzmán, A.; Lozoya, X. Therapeutic Effectiveness of a Mimosa tenuiflora Cortex Extract in Venous Leg Ulceration Treatment. J. Ethnopharmacol. 2007, 109, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Phillips, P.; Lumley, E.; Duncan, R.; Aber, A.; Woods, H.B.; Jones, G.L.; Michaels, J. A Systematic Review of Qualitative Research into People’s Experiences of Living with Venous Leg Ulcers. J. Adv. Nurs. 2018, 74, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.L.; Santos, C.A.; Souza, C.A.S.; Nunes, M.A.P.; Antoniolli, Â.R.; da Silva, W.B.; da Silva, F.A. The Use of Medicinal Plants in Venous Ulcers: A Systematic Review with Meta-analysis. Int. Wound J. 2017, 14, 1019–1024. [Google Scholar] [CrossRef]
- Romero-Cerecero, O.; Zamilpa, A.; Tortoriello, J. Effectiveness and Tolerability of a Standardized Extract from Ageratina pichinchensis in Patients with Diabetic Foot Ulcer: A Randomized, Controlled Pilot Study. Planta Med. 2015, 81, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Namjoyan, F.; Kiashi, F.; Moosavi, Z.B.; Saffari, F.; Makhmalzadeh, B.S. Efficacy of Dragon’s Blood Cream on Wound Healing: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Tradit. Complement. Med. 2016, 6, 37–40. [Google Scholar] [CrossRef]
- Fernandez, O.; Capdevila, J.Z.; Dalla, G.; Melchor, G. Efficacy of Rhizophora mangle Aqueous Bark Extract in the Healing of Open Surgical Wounds. Fitoterapia 2002, 73, 564–568. [Google Scholar] [CrossRef]
- Lammoglia-Ordiales, L.; Vega-Memije, M.E.; Herrera-Arellano, A.; Rivera-Arce, E.; Agüero, J.; Vargas-Martinez, F.; Contreras-Ruiz, J. A Randomised Comparative Trial on the Use of a Hydrogel with Tepescohuite Extract (Mimosa tenuiflora Cortex Extract-2G) in the Treatment of Venous Leg Ulcers. Int. Wound J. 2012, 9, 412–418. [Google Scholar] [CrossRef]
- Hajhashemi, M.; Ghanbari, Z.; Movahedi, M.; Rafieian, M.; Keivani, A.; Haghollahi, F. The Effect of Achillea millefolium and Hypericum perforatum Ointments on Episiotomy Wound Healing in Primiparous Women. J. Matern. Neonatal Med. 2018, 31, 63–69. [Google Scholar] [CrossRef]
- Ritsu, M.; Kawakami, K.; Kanno, E.; Tanno, H.; Ishii, K.; Imai, Y.; Maruyama, R.; Tachi, M. Critical Role of Tumor Necrosis Factor-α in the Early Process of Wound Healing in Skin. J. Dermatol. Dermatol. Surg. 2017, 21, 14–19. [Google Scholar] [CrossRef]
- Lin, Z.; Kondo, T.; Ishida, Y.; Takayasu, T.; Mukaida, N. Essential Involvement of IL-6 in the Skin Wound-healing Process as Evidenced by Delayed Wound Healing in IL-6-deficient Mice. J. Leukoc. Biol. 2003, 73, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.; Jayaraman, V.; Huelsmann, E.J.; Bonish, B.; Burgad, D.; Sivaramakrishnan, G.; Qin, S.; DiPietro, L.A.; Zloza, A.; Zhang, C. Pro-Inflammatory Chemokine CCL2 (MCP-1) Promotes Healing in Diabetic Wounds by Restoring the Macrophage Response. PLoS ONE 2014, 9, e91574. [Google Scholar] [CrossRef]
- Malone-Povolny, M.J.; Maloney, S.E.; Schoenfisch, M.H. Nitric Oxide Therapy for Diabetic Wound Healing. Adv. Healthc. Mater. 2019, 8, 1801210. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.S.; Spitzer, V.; Schapoval, E.E.S.; Schenkel, E.P. Antiinflammatory Activity of Extracts and Fractions from the Leaves of Gochnatia polymorpha. Phytother. Res. 2000, 14, 638–640. [Google Scholar] [CrossRef]
- Salazar-Gómez, A.; Vargas-Díaz, M.E.; Meléndez-Camargo, M.E.; Pablo-Pérez, S.S. Antinociceptive Activity of the Ethanolic Extract of Trixis angustifolia DC. Nat. Prod. Res. 2021, 1–4. [Google Scholar] [CrossRef]
- Sharma, G.-D.; He, J.; Bazan, H.E.P. P38 and ERK1/2 Coordinate Cellular Migration and Proliferation in Epithelial Wound Healing: Evidence of Cross-Talk Activation between MAP Kinase Cascades. J. Biol. Chem. 2003, 278, 21989–21997. [Google Scholar] [CrossRef] [PubMed]
- Palloni, A.; McEniry, M. Aging and Health Status of Elderly in Latin America and the Caribbean: Preliminary Findings. J. Cross. Cult. Gerontol. 2007, 22, 263–285. [Google Scholar] [CrossRef] [PubMed]

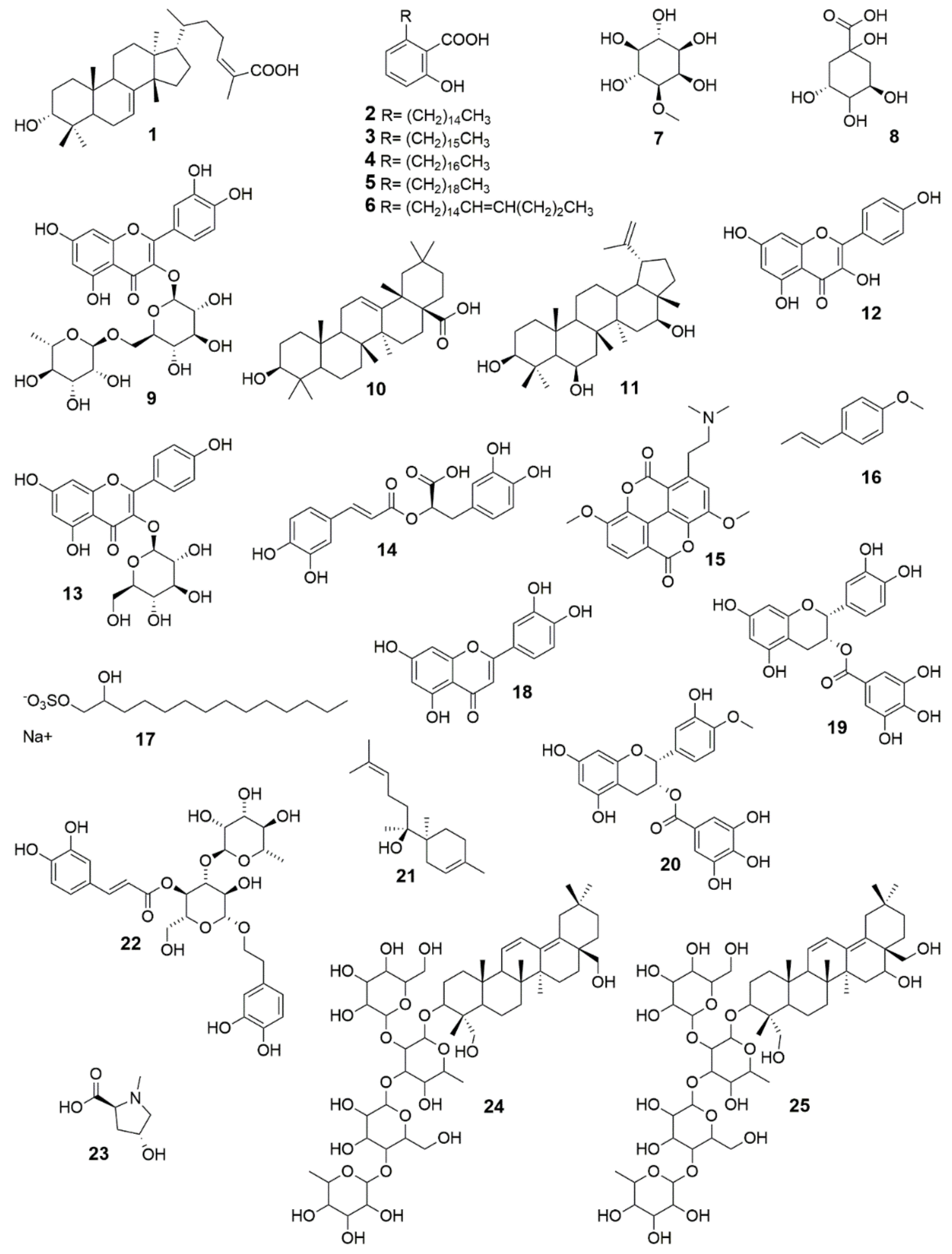
| Botanical Family | Plant Name | Plant Part, Way of Administration | Reference |
|---|---|---|---|
| Acanthaceae | Justicia spicigera Schltdl. | Infusion of leaves, oral | [8] |
| Ruellia hygrophila Mart. | Decoction of leaves, topical | [9] | |
| Alismataceae | Sagittaria montevidensis Cham. and Schltdl. | Infusion of leaves, oral | [10] |
| Amaranthaceae | Achyranthes brasiliana (L.) Standl. (accepted name: Alternanthera brasiliana (L.) Kuntze) | Infusion of aerial parts, oral | [11] |
| Alternanthera pungens Kunth | Decoction of aerial parts, topical | [9] | |
| Chenopodium ambrosioides L. | Pulverized leaves, topical | [12] | |
| Dysphania ambrosioides (L.) Mosyakin and Clemants | Maceration of leaves, topical | [13] | |
| Gomphrena celosioides Mart. | Cataplasm of leaves, topical | [14] | |
| Iresine herbstii Hook. | Infusion of leaves, topical | [15] | |
| Anacardiaceae | Anacardium occidentale L. | Maceration of fruits, topical | [16] |
| Amphipterygium adstringens (Schltdl.) Standl. | Pulverized bark, topical | [17] | |
| Myracrodruon urundeuva Allemão | Infusion of stem, topical | [18] | |
| Schinopsis lorentzii (Griseb.) Engl. | Bark infusion, topical | [9] | |
| Schinus molle L. | Infusion of leaves, topical | [15] | |
| Schinus terebinthifolia Raddi | Maceration of leaves, topical | [19] | |
| Spondias mombin L. | Resin, topical | [20] | |
| Annonaceae | Annona squamosa L. | Pulverized leaves, topical | [21] |
| Apiaceae | Cyclospermum leptophyllum (Pers.) Sprague ex Britton and P. Wilson | Decoction of aerial parts, topical | [14] |
| Eryngium carlinae F. Delaroche | Infusion of aerial parts, oral | [22] | |
| Petroselinum crispum (Mill.) Fuss | Decoction of whole plant, topical | [23] | |
| Apocynaceae | Asclepias curassavica L. | Latex, topical | [24] |
| Asclepias linaria Cav. | Latex, topical | [25] | |
| Cascabela thevetioides (Kunth) Lippold | Sap, topical | [26] | |
| Hancornia speciosa Gomes | Decoction of roots, topical | [27] | |
| Himatanthus drasticus (Mart.) Plumel | Latex, topical | [28] | |
| Plumeria sucuuba Spruce ex Müll. Arg. | Latex, topical | [29] | |
| Prestonia glabrata Kunth | Infusion of whole plant, topical | [23] | |
| Tabernaemontana catharinensis A. DC. | Bark infusion, oral | [14] | |
| Tabernaemontana sananho Ruiz and Pav. | Bark latex, topical | [30] | |
| Vallesia glabra (Cav.) Link | Decoction of branches, topical | [9] | |
| Aquifoliaceae | Ilex guayusa Loes. | Cataplasm of leaves, topical | [31] |
| Araceae | Dracontium loretense K. Krause | Cataplasm of tuber, topical | [32] |
| Monstera lechleriana Schott | Latex, topical | [30] | |
| Synandrospadix vermitoxicus (Griseb.) Engl. | Tubercle, topical | [33] | |
| Araliaceae | Oreopanax andreanus Marchal | Decoction of leaves, topical | [23] |
| Arecaceae | Attalea speciosa Mart. | Seed oil, topical | [29] |
| Socratea exorrhiza (Mart.) H. Wendl. | Pulverized root, topical | [29] | |
| Aristolochiaceae | Prosopanche americana (R. Br.) Baill. | Ashes of whole plant, topical | [33] |
| Asparagaceae | Agave atrovirens Karw. ex Salm-Dyck | Leaves, topical | [34] |
| Asteraceae | Acanthospermum australe (Loefl.) Kuntze | Decoction of leaves, topical | [19] |
| Achillea millefolium L. | Infusion of whole plant, topical | [22] | |
| Ageratina pichinchensis (Kunth) R.M. King and H. Rob. | Maceration of leaves and root, topical | [35] | |
| Baccharis genistelloides (Lam.) Pers. | Decoction of aerial parts, topical | [12] | |
| Baccharis odorata Kunth | Decoction of leaves, topical | [24] | |
| Baccharis salicifolia (Ruiz and Pav.) Pers. | Decoction of leaves, topical | [24] | |
| Bidens pilosa L. | Infusion of aerial parts, topical | [36] | |
| Chaptalia nutans (L.) Pol. | Infusion of aerial parts, topical | [37] | |
| Eupatorium laevigatum Lam. | Infusion of leaves, topical | [15] | |
| Eupatorium subhastatum Hook. and Arn. | Decoction of aerial parts, topical | [14] | |
| Flaveria trinervia (Spreng.) C. Mohr | Infusion of aerial parts, topical | [17] | |
| Galinsoga parviflora Cav. | Infusion of flowers, oral | [22] | |
| Gamochaeta coarctata (Willd.) Kerguélen | Decoction of whole plant, topical | [38] | |
| Grindelia oxylepis Greene | Infusion of whole plant, topical | [39] | |
| Haplopappus venetus (Kunth) S.F. Blake | Decoction of branches, topical | [39] | |
| Heterotheca inuloides Cass. | Infusion of whole plant, topical | [39] | |
| Jungia paniculata (DC.) A. Gray | Infusion of leaves, topical | [38] | |
| Molina latifolia Ruiz and Pav | Decoction of leaves and bark, topical | [24] | |
| Mutisia acuminata Ruiz and Pav. | Pulverized leaves, topical | [38] | |
| Parthenium hysterophorus L. | Decoction of leaves, topical | [40] | |
| Pluchea microcephala R.K. Godfrey | Decoction of aerial parts, topical | [9] | |
| Pluchea sagittalis (Lam.) Cabrera | Infusion of leaves, topical | [15] | |
| Porophyllum ruderale (Jacq.) Cass. | Pulverized leaves, topical | [30] | |
| Psacalium decompositum (A. Gray) H. Rob. and Brettell | Root infusion, oral | [41] | |
| Pseudognaphalium canescens (DC.) Anderb. | Maceration of leaves and roots, topical | [26] | |
| Pseudelephantopus spicatus (B. Juss. ex Aubl.) C.F. Baker | Cataplasm of aerial parts, topical | [30] | |
| Pterocaulon virgatum (L.) DC. | Decoction of aerial parts, topical | [14] | |
| Senecio angulifolius DC. | Pulverized leaves, topical | [42] | |
| Senecio rhizomatus Rusby | Infusion of leaves, oral | [38] | |
| Schkuhria pinnata (Lam.) Kuntze ex Thell. | Infusion of leaves, oral | [8] | |
| Smallanthus sonchifolius (Poepp.) H. Rob. | Infusion of roots, topical | [23] | |
| Solidago chilensis Meyen | Maceration of leaves, topical | [11] | |
| Sphagneticola trilobata (L.) Pruski | Infusion of aerial parts, topical | [10] | |
| Spilanthes alba L’Hér. | Infusion of aerial parts, topical | [23] | |
| Tagetes erecta L. | Maceration of leaves, topical | [36] | |
| Tagetes mandonii Sch. Bip. ex Klatt | Infusion of leaves, topical | [43] | |
| Tagetes terniflora Kunth | Decoction of leaves and roots, topical | [23] | |
| Trixis angustifolia DC. | Infusion of whole plant, oral | [39] | |
| Verbesina crocata (Cav.) Less. | Infusion of aerial parts, topical | [17] | |
| Verbesina scabra Benth. | Maceration of leaves with oil, topical | [9] | |
| Werneria nubigena Kunth | Infusion of whole plant, topical | [43] | |
| Xanthium strumarium L. | Infusion of whole plant, topical | [15] | |
| Basellaceae | Anredera diffusa (Moq.) Sperling | Infusion of leaves, topical | [44] |
| Begoniaceae | Begonia glabra Aubl | Cataplasm of leaves, topical | [30] |
| Bignoniaceae | Crescentia cujete L. | Fruit pulp, oral | [45] |
| Fridericia chica (Bonpl.) L.G. Lohmann | Cataplasm of leaves, topical | [30] | |
| Mussatia hyacinthina (Standl.) Sandwith | Decoction of bark, oral | [29] | |
| Tabebuia serratifolia (Vahl) G. Nicholson | Cataplasm of bark, topical | [20] | |
| Tanaecium nocturnum (Barb. Rodr.) Bureau and K. Schum. | Decoction of bark, oral | [29] | |
| Tecoma stans (L.) Juss. ex Kunth | Cataplasm of leaves and bark, topical | [46] | |
| Bixaceae | Bixa orellana L. | Infusion of stem bark, topical | [47] |
| Brassicaceae | Coronopus didymus (L.) Sm. | Infusion of whole plant, topical | [46] |
| Burseraceae | Bursera arida (Rose) Standl. | Latex, topical | [17] |
| Bursera morelensis Ramírez | Infusion of bark, oral | [48] | |
| Bursera simaruba (L.) Sarg. | Resin, topical | [37] | |
| Cactaceae | Cephalocereus senilis (Haw.) Pfeiff. | Pulp, topical | [26] |
| Echinocereus poselgeri Lem. | Maceration of roots, topical | [26] | |
| Nopalea cochenillifera (L.) Salm-Dyck | Maceration of roots, topical | [8] | |
| Pereskia sacharosa Griseb. | Infusion of leaves, topical | [47] | |
| Calophyllaceae | Calophyllum brasiliense Cambess. | Resin/topical | [45] |
| Capparaceae | Capparis osmantha Diels | Cataplasm of bark, topical | [30] |
| Caryophyllaceae | Paronychia chilensis DC. | Infusion of branches, oral | [46] |
| Celastraceae | Maytenus ilicifolia Mart. ex Reissek | Infusion of whole plant, topical | [49] |
| Maytenus macrocarpa (Ruiz and Pav.) Briq. | Decoction of bark, topical | [32] | |
| Cleomaceae | Cleome spinosa Jacq. | Decoction of roots, topical | [50] |
| Clusiaceae | Clusia nemorosa G. Mey. | Resin, topical | [51] |
| Garcinia macrophylla Mart. | Resin, topical | [52] | |
| Combretaceae | Combretum leprosum Mart. | Infusion of aerial parts, topical | [53] |
| Commelinaceae | Commelina coelestis Willd. | Cataplasm of leaves, topical | [5] |
| Dichorisandra ulei J.F. Macbr. | Cataplasm of aerial parts, topical | [30] | |
| Tradescantia spathacea Sw. | Cataplasm of leaves, topical | [40] | |
| Convolvulaceae | Convolvulus crenatifolius var. peruviana Hallier f. | Decoction of whole plant, oral | [43] |
| Cuscuta grandiflora Kunth | Decoction of whole plant, topical | [43] | |
| Dichondra argentea Humb. and Bonpl. ex Willd | Decoction of whole plant, topical | [39] | |
| Ipomoea asarifolia (Desr.) Roem. and Schult. | Decoction of aerial parts, topical | [52] | |
| Ipomoea batatas (L.) Lam. | Infusion of leaves, topical | [19] | |
| Ipomoea carnea Jacq. | Pulverized leaves, topical | [9] | |
| Ipomoea pes-caprae (L.) R. Br. | Decoction of whole plant, topical | [52] | |
| Operculina hamiltonii (G. Don) D.F. Austin and Staples | Infusion of roots, topical | [50] | |
| Cordiaceae | Cordia americana (L.) Gottschling and J.S. Mill. | Decoction of leaves, topical | [14] |
| Cordia ecalyculata Vell. | Infusion of leaves, oral | [27] | |
| Crassulaceae | Sedum dendroideum DC. | Infusion of leaves, topical | [15] |
| Cucurbitaceae | Cucurbita maxima Duchesne | Sap fruit, topical | [14] |
| Cucurbita pepo L. | Latex, topical | [40] | |
| Cyatheaceae | Cyathea cuspidate Kunze | Decoction of leaves, topical | [54] |
| Cyperaceae | Cyperus articulates L. | Decoction of tubercule, topical | [54] |
| Scirpus californicus (C.A. Mey.) Steud. | Decoction of the aerial rhizome, oral | [43] | |
| Equisetaceae | Equisetum bogotense Kunth | Infusion of leaves, topical | [12] |
| Euphorbiaceae | Acalypha monostachya Cav. | Infusion of leaves, topical | [26] |
| Chamaesyce hirta (L.) Millsp | Latex, topical | [24] | |
| Chamaesyce hypericifolia (L.) Millsp. | Latex, topical | [24] | |
| Croton antisyphiliticus Mart. | Pulverized roots, topical | [27] | |
| Croton draconoides Müll. Arg. | Resin, topical | [47] | |
| Croton lechleri Müll. Arg. | Resin, topical | [32] | |
| Croton mutisianus Kunth | Latex, topical | [23] | |
| Croton pottsii (Klotzsch) Müll. Arg. | Decoction of whole plant, topical | [39] | |
| Croton suaveolens Torr. | Cataplasm of leaves, topical | [26] | |
| Hura crepitans L. | Infusion of bark, topical | [32] | |
| Jatropha curcas L. | Latex, topical | [45] | |
| Jatropha dioica Sessé ex Cerv. | Infusion of aerial parts, topical | [39] | |
| Jatropha gossypiifolia L. | Latex, topical | [53] | |
| Jatropha neopauciflora Pax | Latex, topical | [17] | |
| Machaerium complanatum Ducke | Cataplasm of bark, topical | [30] | |
| Manihot tripartita (Spreng.) Müll. Arg | Infusion of whole plant, oral | [27] | |
| Pedilanthus tithymaloides (L.) Poit. | Infusion of aerial parts, oral | [36] | |
| Sapium haematospermum Müll. Arg. | Decoction of leaves, topical | [9] | |
| Sapium laurifolium (A. Rich.) Griseb. | Latex, topical | [29] | |
| Sapium marmieri Huber | Latex, topical | [30] | |
| Fabaceae | Acacia albicorticata Burkart | Pulverized bark, topical | [33] |
| Acacia aroma Gillies ex Hook. and Arn. | Decoction of leaves, topical | [33] | |
| Acacia caven Molina | Infusion of bark, topical | [46] | |
| Amburana cearensis (Allemão) A.C. Sm. | Decoction of bark, topical | [50] | |
| Bauhinia divaricata L. | Infusion of leaves, oral | [55] | |
| Caesalpinia paraguariensis (D. Parodi) Burkart | Pulverized bark, topical | [14] | |
| Caesalpinia spinosa (Molina) Kuntze | Decoction of fruit, topical | [24] | |
| Cassia tomentosa L. f. | Pulverized leaves, topical | [38] | |
| Copaifera langsdorffii Desf. | Infusion of shell, oral | [16] | |
| Copaifera reticulata Ducke | Sap, topical | [29] | |
| Cercidium praecox (Ruiz and Pav. ex Hook.) Harms | Ashes of stem and bark, topical | [14] | |
| Deguelia rariflora (Mart. ex Benth.) G.P. Lewis and Acev.-Rodr. | Infusion of roots, oral | [28] | |
| Desmodium mexicanum Sweet | Infusion of whole plant, oral | [12] | |
| Erythrina crista-galli L. | Pulverized bark and leaves, topical | [14] | |
| Erythrina dominguezii Hassl. | Decoction of bark, oral | [29] | |
| Erythrina edulis Triana ex Micheli | Infusion of bark, oral | [23] | |
| Geoffroea decorticans (Gillies ex Hook. and Arn.) Burkart | Pulverized leaves, topical | [14] | |
| Gliricidia sepium (Jacq.) Kunth ex Walp. | Cataplasm of leaves, topical | [37] | |
| Indigofera suffruticosa Mill. | Infusion of leaves, oral | [8] | |
| Macrolobium acaciifolium (Benth.) Benth | Decoction of stem bark, topical | [47] | |
| Mimosa albida Humb. and Bonpl. ex Willd. | Infusion of leaves, oral | [5] | |
| Mimosa tenuiflora (Willd.) Poir. | Maceration of whole plant, topical | [22] | |
| Myrocarpus frondosus Allemão | Maceration of aerial parts, topical | [19] | |
| Myroxylon balsamum (L.) Harms | Infusion of bark, topical | [23] | |
| Myroxylon peruiferum L. f. | Infusion of bark, topical | [46] | |
| Parkia multijuga Benth. | Powdered bark, topical | [31] | |
| Peltophorum dubium (Spreng.) Taub. | Pulverized bark, topical | [49] | |
| Poeppigia procera C. Presl | Decoction of bark, topical | [45] | |
| Prosopis juliflora (Sw.) DC. | Decoction of leaves and seed, topical | [23] | |
| Prosopis ruscifolia Griseb. | Decoction of leaves, topical | [9] | |
| Prosopis vinalillo Stuck. | Decoction of leaves, topical | [9] | |
| Psoralea pubescens Poir | Decoction of aerial parts, topical | [43] | |
| Senna morongii (Britton) H.S. Irwin and Barneby | Decoction of aerial parts, topical | [9] | |
| Senna pendula (Humb. and Bonpl. ex Willd.) H.S. Irwin and Barneby | Ashes of leaves, topical | [33] | |
| Stryphnodendron adstringens (Mart.) Coville | Pulverized bark, topical | [27] | |
| Stryphnodendron coriaceum Benth. | Decoction of bark, topical | [53] | |
| Stryphnodendron rotundifolium Mart. | Infusion of bark, topical | [50] | |
| Trifolium amabile Kunth | Decoction of roots, topical | [43] | |
| Vachellia caven (Molina) Seigler and Ebinger | Pulverized bark, topical | [56] | |
| Geraniaceae | Geranium mexicanum Kunth | Pulverized aerial parts, topical | [57] |
| Pelargonium odoratissimum (L.) L’Hér. | Infusion of leaves, topical | [23] | |
| Heliotropiaceae | Heliotropium angiospermum Murray | Decoction of whole plant, topical | [42] |
| Heliotropium curassavicum var. argentinum I.M. Johnst. | Decoction of aerial parts, topical | [14] | |
| Heliotropium procumbens Mill. | Decoction of aerial parts, topical | [9] | |
| Hypericaceae | Vismia guianensis (Aubl.) Choisy | Latex, topical | [58] |
| Juglandaceae | Juglans neotropica Diels | Decoction of leaves, topical | [43] |
| Lamiaceae | Leonurus sibiricus L. | Infusion of leaves, oral | [11] |
| Salvia mexicana L. | Infusion of leaves, topical | [36] | |
| Salvia stachydifolia Benth | Pulverized leaves, topical | [56] | |
| Lecythidaceae | Bertholletia excelsa Bonpl. | Infusion of stem bark, oral | [28] |
| Loranthaceae | Struthanthus crassipes (Oliv.) Eichler | Infusion of leaves, topical | [40] |
| Struthanthus densiflorus (Benth.) Standl. | Infusion of flowers, oral | [8] | |
| Losaceae | Mentzelia cordifolia Dombey ex Urb. and Gilg | Decoction of aerial parts, topical | [38] |
| Lythraceae | Cuphea aequipetala Cav. | Pulverized aerial parts, topical | [59] |
| Malpighiaceae | Banisteriopsis caapi (Spruce ex Griseb.) C.V. Morton | Decoction of stem, topical | [23] |
| Byrsonima sericea DC | Maceration of bark, topical | [60] | |
| Malvaceae | Ceiba aesculifolia subsp. parvifolia (Rose) P.E. Gibbs and Semir | Pulverized bark, topical | [17] |
| Gossypium barbadense L. | Seeds, oral | [50] | |
| Heliocarpus appendiculatus Turcz. | Latex, topical | [40] | |
| Malachra alceifolia Jacq. | Decoction of whole plant, topical | [37] | |
| Modiola caroliniana (L.) G. Don | Decoction of leaves, topical | [14] | |
| Sida rhombifolia L. | Infusion of roots, oral | [23] | |
| Sphaeralcea angustifolia (Cav.) G. Don | Decoction of leaves, oral | [36] | |
| Waltheria communis A. St.-Hil. | Infusion of whole plant, topical | [15] | |
| Marantaceae | Maranta arundinacea L. | Pulverized rhizome, topical | [58] |
| Meliaceae | Carapa guianensis Aubl. | Seed oil, topical | [28] |
| Guarea kunthiana A. Juss. | Infusion of leaves, topical | [23] | |
| Menispermaceae | Cissampelos pareira L. | Pulverized leaves, topical | [14] |
| Myrsinaceae | Ardisia compressa Kunth | Infusion of leaves, topical | [40] |
| Myrtaceae | Blepharocalyx tweediei (Hook. and Arn.) O. Berg | Infusion of leaves, topical | [8] |
| Campomanesia rufa (O. Berg) Nied. | Infusion of roots, oral | [27] | |
| Psidium guajava L. | Infusion leaves, topical | [57] | |
| Psidium guineense Sw. | Decoction of leaves, topical | [19] | |
| Moraceae | Dorstenia houstonii L | Latex, topical | [37] |
| Ficus americana Aubl. | Resin, topical | [47] | |
| Ficus insipida Willd. | Pulverized bark and leaves, topical | [49] | |
| Sorocea houlletiana Gaudich. (accepted name: Sorocea guilleminiana Gaudich.) | Cataplasm of leaves, topical | [30] | |
| Nyctaginaceae | Mirabilis jalapa L. | Infusion of leaves, topical | [10] |
| Onagraceae | Fuchsia hypoleuca I.M. Johnst. | Infusion of aerial parts, topical | [23] |
| Fuchsia magellanica Lam. | Infusion of leaves, topical | [23] | |
| Gaura hexandra Ortega | Decoction of whole plant, topical | [39] | |
| Oenothera rosea L’Hér. ex Aiton | Decoction of whole plant, topical | [8] | |
| Papaveraceae | Argemone mexicana L. | Infusion of flowers and seeds, oral | [22] |
| Argemone ochroleuca Sweet | Decoction of roots, topical | [61] | |
| Argemone subfusiformis G.B. Ownbey | Decoction of aerial parts, topical | [14] | |
| Bocconia frutescens L. | Infusion of leaves, oral | [36] | |
| Petiveriaceae | Petiveria alliacea L. | Infusion of aerial parts, topical | [15] |
| Phytolaccaceae | Phytolacca icosandra L. | Infusion of leaves, topical | [51] |
| Picramniaceae | Picramnia spruceana Engl. | Infusion of leaves, topical | [58] |
| Piperaceae | Peperomia congona Sodiro | Infusion of whole plant, oral | [62] |
| Peperomia galioides Kunth | Pulverized whole plant, topical | [38] | |
| Piper aduncum L. | Infusion of leaves, oral | [8] | |
| Piper affictum Trel. | Cataplasm of leaves, topical | [30] | |
| Piper barbatum Kunth | Infusion of whole plant, topical | [23] | |
| Piper confusionis Trel. | Cataplasm of leaves, topical | [30] | |
| Piper dichotomum Ruiz and Pav | Cataplasm of leaves, topical | [30] | |
| Piper ecuadorense Sodiro | Infusion of leaves, oral | [23] | |
| Piper loretoanum Trel. | Cataplasm of leaves, topical | [30] | |
| Piper peltatum L. | Decoction of leaves, topical | [54] | |
| Piper regnellii (Miq.) C. DC. | Infusion of leaves, topical | [15] | |
| Piper sanctum (Miq.) Schltdl. ex C. DC. | Infusion of leaves, oral | [36] | |
| Plagiochilaceae | Plagiochila hondurensis Herzog ex Carl | Cataplasm of whole plant, topical | [31] |
| Plantaginaceae | Mecardonia procumbens (Mill.) Small | Pulverized aerial parts, topical | [59] |
| Plantago tomentosa Lam. | Decoction of leaves, topical | [10] | |
| Scoparia dulcis L. | Infusion of whole plant, topical | [23] | |
| Poaceae | Gynerium sagittatum (Aubl.) P. Beauv. | Pulverized leaves, topical | [29] |
| Polygonaceae | Muehlenbeckia tamnifolia (Kunth) Meisn. | Pulverized leaves, topical | [38] |
| Muehlenbeckia volcanica (Benth.) Endl. | Infusion of whole plant, oral | [62] | |
| Polygonum punctatum Elliott | Ashes of leaves, topical | [14] | |
| Polypodiaceae | Campyloneurum fuscosquamatum Lellinger | Pulverized leaves, topical | [29] |
| Microgramma squamulosa (Kaulf.) de la Sota | Infusion of whole plant, oral | [46] | |
| Primulaceae | Cybianthus anthuriophyllus Pipoly | Cataplasm of leaves and roots | [30] |
| Pteridaceae | Adiantum poiretii Wikstr. | Cataplasm of whole plant, topical | [39] |
| Rhamnaceae | Ceanothus azureus Desf. ex DC | Infusion of whole plant, oral | [5] |
| Rhizophoraceae | Rhizophora mangle L. | Decoction of bark, topical | [45] |
| Rosaceae | Rubus glaucus Benth. | Infusion of leaves, topical | [23] |
| Rubiaceae | Carapichea ipecacuanha (Brot.) L. Andersson | Infusion of roots, topical | [50] |
| Calycophyllum spruceanum (Benth.) Hook. f. ex K. Schum. | Decoction of bark, topical | [32] | |
| Genipa americana L. | Decoction of fruit, topical | [20] | |
| Hamelia patens Jacq. | Infusion of leaves, oral | [8] | |
| Hintonia latiflora (DC.) Bullock | Pulverized bark, topical | [39] | |
| Uncaria guianensis (Aubl.) J.F. Gmel. | Decoction of bark, oral | [32] | |
| Rutaceae | Helietta apiculate Benth. | Infusion of bark, oral | [49] |
| Salicaceae | Salix humboldtiana Willd. | Decoction of bark, topical | [9] |
| Sapindaceae | Acer negundo L. | Sap, topical | [26] |
| Cardiospermum halicacabum L. | Cataplasm of leaves, topical | [5] | |
| Sapotaceae | Sideroxylon obtusifolium (Humb. ex Roem. and Schult.) T.D. Penn. | Infusion of aerial parts, oral | [18] |
| Saururaceae | Anemopsis californica (Nutt.) Hook. and Arn. | Decoction of whole plant, topical | [39] |
| Scrophulariaceae | Buddleja cordata Kunth | Decoction of leaves, topical | [36] |
| Buddleja sessiliflora Kunth | Infusion of leaves, topical | [55] | |
| Smilacaceae | Smilax bona-nox L. | Infusion of roots, oral | [26] |
| Solanaceae | Brugmansia candida Pers. | Maceration of leaves, topical | [36] |
| Brugmansia suaveolens (Humb. and Bonpl. ex Willd.) Sweet | Maceration of leaves, topical | [15] | |
| Capsicum annuum var. glabriusculum (Dunal) Heiser and Pickersgill | Cataplasm of fruit, topical | [26] | |
| Cestrum auriculatum L’Hér. | Pulverized leaves, topical | [38] | |
| Datura ferox L. | Maceration of leaves with oil, topical | [9] | |
| Lycopersicon esculentum Mill. | Cataplasm of fruit peel, topical | [26] | |
| Nicotiana glauca Graham | Infusion of leaves, topical | [56] | |
| Nicotiana tabacum L. | Infusion of leaves, topical | [11] | |
| Physalis chenopodifolia Lam. | Decoction of flowers, topical | [23] | |
| Physalis angulata L. | Cataplasm of leaves, topical | [32] | |
| Solanum erianthum D. Don | Pulverized whole plant, topical | [19] | |
| Solanum glaucophyllum Desf. | Ashes of stem, topical | [33] | |
| Solanum mammosum L. | Cataplasm of fruit, topical | [32] | |
| Solanum nigrescens M. Martens and Galeotti | Maceration of fruit, topical | [36] | |
| Solanum sessiliflorum Dunal | Infusion of fruit, oral | [32] | |
| Tectariaceae | Tectaria heracleifolia (Willd.) Underw. | Infusion of whole plant, oral | [8] |
| Urticaceae | Cecropia peltata L. | Pulverized leaves, topical | [58] |
| Vernenaceae | Lippia alba (Mill.) N.E. Br. ex Britton and Wilson, P. | Infusion of leaves, topical | [19] |
| Lippia gracilis Schauer | Infusion of leaves, topical | [50] | |
| Stachytarpheta cayennensis (Rich.) Vahl | Maceration of leaves, topical | [11] | |
| Verbena hispida Ruiz and Pav. | Infusion of branches, oral | [46] | |
| Verbena litoralis Kunth | Infusion of whole plant, topical | [23] | |
| Viburnaceae | Sambucus mexicana C. Presl ex DC. | Infusion of leaves, oral | [5] |
| Sambucus peruviana Kunth | Pulverized leaves, topical | [38] | |
| Ximeniaceae | Ximenia americana L. | Infusion of bark, topical | [50] |
| Zamiaceae | Zamia ulei Dammer | Pulverized stem, topical | [20] |
| Zygophyllaceae | Bulnesia sarmientoi Lorentz ex Griseb. | Pulverized bark, topical | [63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar-Gómez, A.; Alonso-Castro, A.J. Medicinal Plants from Latin America with Wound Healing Activity: Ethnomedicine, Phytochemistry, Preclinical and Clinical Studies—A Review. Pharmaceuticals 2022, 15, 1095. https://doi.org/10.3390/ph15091095
Salazar-Gómez A, Alonso-Castro AJ. Medicinal Plants from Latin America with Wound Healing Activity: Ethnomedicine, Phytochemistry, Preclinical and Clinical Studies—A Review. Pharmaceuticals. 2022; 15(9):1095. https://doi.org/10.3390/ph15091095
Chicago/Turabian StyleSalazar-Gómez, Anuar, and Angel Josabad Alonso-Castro. 2022. "Medicinal Plants from Latin America with Wound Healing Activity: Ethnomedicine, Phytochemistry, Preclinical and Clinical Studies—A Review" Pharmaceuticals 15, no. 9: 1095. https://doi.org/10.3390/ph15091095
APA StyleSalazar-Gómez, A., & Alonso-Castro, A. J. (2022). Medicinal Plants from Latin America with Wound Healing Activity: Ethnomedicine, Phytochemistry, Preclinical and Clinical Studies—A Review. Pharmaceuticals, 15(9), 1095. https://doi.org/10.3390/ph15091095







