1. Introduction
Somatostatin analogues are an important treatment for well-differentiated, low-grade, gastroenteropancreatic neuroendocrine tumours (GEP-NETs) now. Peptide receptor radionuclide therapy (PRRT) targeting the somatostatin receptor subtype 2 (SST
2) with the SST
2 agonist [
177Lu]Lu-DOTA-TATE, administered in four cycles of 7.4 GBq (200 mCi) per cycle, has become an established treatment modality for patients with advanced, well-differentiated, low-grade, SST
2-positive GEP-NETs [
1]. More recently, SST
2 antagonists, especially [177Lu]Lu-satoreotide tetraxetan (also known as [
177Lu]Lu-IPN01072, [
177Lu]Lu-OPS201, or [
177Lu]Lu-DOTA-JR11), have shown superior non-clinical and early clinical outcomes compared to the agonists and, as a result, research interest has shifted from radiolabelled SST
2 agonists to antagonists, [
2,
3,
4,
5].
In the clinical setting, [
177Lu]Lu-satoreotide tetraxetan has shown 1.1–2.6 times higher tumour uptake compared to [
177Lu]Lu-DOTA-TATE [
5]. In a phase I study in 20 patients with heavily pre-treated NETs, [
177Lu]Lu-satoreotide tetraxetan therapy was associated with a median progression-free survival (mPFS) of 21 months and a disease control rate (DCR) of 85% [
6], which is comparable to the DCR of 79% reported in a meta-analysis of 13 clinical studies in metastatic NETs treated with [
177Lu]Lu-DOTA-TATE [
7]. Compared with the SST
2 agonists [
177Lu]Lu-DOTA-TOC [
4] and [
177Lu]Lu-DOTA-TATE [
3], in vitro and in vivo experiments showed that [
177Lu]Lu-satoreotide tetraxetan had a 10-fold higher activity bound to human carcinoid cells transfected with SST
2 [
4], induced more DNA double-strand breaks (DSBs) [
3], and was associated with a longer median survival [
3,
4] and a longer mean period of tumour stabilisation [
3]. Similar differences between the antagonist [
177Lu]Lu-DOTA-LM3 and the agonist [
177Lu]Lu-DOTA-TOC were also observed [
8].
Despite these promising results, to date, there has been no in vivo study comparing the treatment effects and the toxicity of four repetitive cycles of 177Lu-satoreotide tetraxetan and SST2 agonists such as [177Lu]Lu-DOTA-TATE, which is conventionally applied as four cycles in routine clinical practice. Accordingly, the aim of this present in vivo/ex vivo study was to compare the anti-tumour activity and the potential toxicity of [177Lu]Lu-satoreotide tetraxetan to that of [177Lu]Lu-DOTA-TATE, which were both administered for a total of four cycles in mice bearing AR42J xenografts–a rat pancreatic cancer model expressing endogenous SST2.
3. Discussion
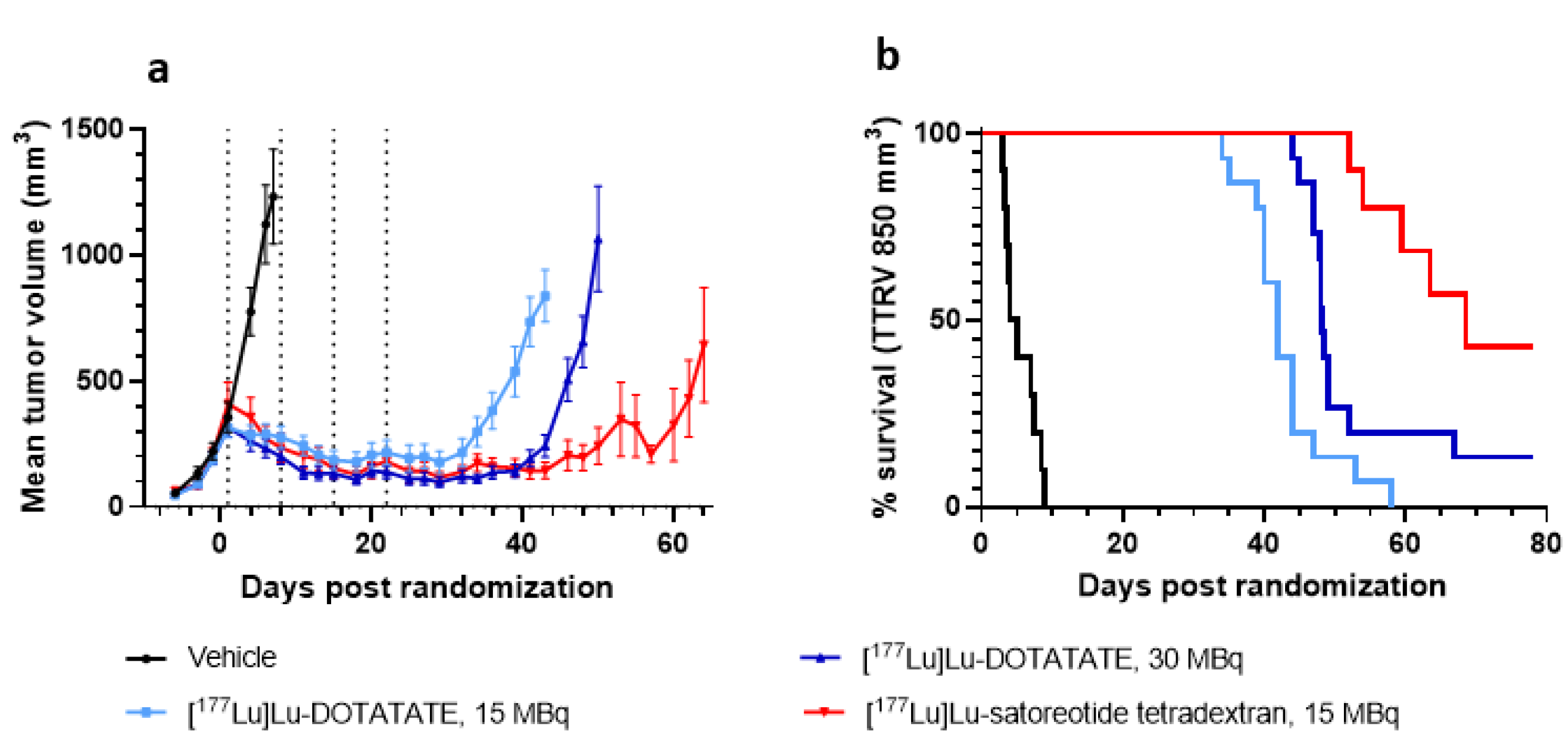
Using a tumour model endogenously expressing high levels of SST
2, the present study demonstrated for the first time that the radiolabelled SST antagonist [
177Lu]Lu-satoreotide tetraxetan, administered as four fractions of 15 MBq given 1 week apart, suppressed tumour growth and induced tumour regression, prolonged median time taken to reach 850 mm
3, and reduced the rate of tumour relapse to a greater degree than [
177Lu]Lu-DOTA-TATE administered as four fractions of either 15 or 30 MBq 1 week apart, without inducing a higher toxicity. Compared with [
177Lu]Lu-DOTA-TATE at 15 or 30 MBq,
177Lu-satoreotide tetraxetan therapy was also associated with an approximately three times higher tumour uptake as well as a higher rate of DNA DSB damage, with limited effects on body weight, haematological toxicity, or renal toxicity. The enhanced in vivo anti-tumour activity of [
177Lu]Lu-satoreotide tetraxetan compared to [
177Lu]Lu-DOTA-TATE observed in the present study can be partially explained by the ability of [
177Lu]Lu-satoreotide tetraxetan to recognise almost twice as many SST
2 binding sites as [
177Lu]Lu-DOTA-TATE, as shown by our in vitro saturation binding assays using AR42J rat pancreatic tumour membranes (mean Bmax, 3210 fmol/mg protein for [
177Lu]Lu-satoreotide tetraxetan versus 1790 fmol/mg protein for [
177Lu]Lu-DOTA-TATE). These in vitro results are also consistent with previous in vitro receptor binding assays using human embryonic kidney cells transfected with SST
2, in which [
177Lu]Lu-satoreotide tetraxetan was able to recognize about four times more specific binding sites than [
177Lu]Lu-DOTA-TATE assuming that the internalization of both products in AR42J cells had the same profile [
9] i.e., complete internalization of the agonist; whereas, more than 50% of the antagonist remained at the cell membrane, which has not been demonstrated in this model. Although the binding affinity of [
177Lu]Lu-DOTA-TATE was lower than that of the [
177Lu]Lu-satoreotide tetraxetan on AR42J membranes, Mansi et al. have shown a similar affinity for both products in hSST2-HEK-293 membranes [
9]. This difference could be explained by species dissimilarities between human and rat SST
2, as shown with another SST
2 agonist Pasireotide [
10]. In addition, the transfected status of HEK-293 cells with the human SSTR
2 gene may not reflect a normal expression of the receptor at the cell membrane as in a native environment, which is not the case for the AR42J model endogenously expressing SST
2.In the present study, the median tumour growth delay, which was defined as the time taken to reach a tumour volume of at least 850 mm
3, was 68 days in the [
177Lu]Lu-satoreotide tetraxetan-treated group compared with 43 and 48 days with
177Lu-DOTA-TATE at 15 and 30 MBq, respectively. The superiority of
177Lu-satoreotide tetraxetan over [
177Lu]Lu-DOTA-TATE and other SST agonists such as [
177Lu]Lu-DOTA-TOC in inhibiting tumour growth was also noted in other in vivo therapy studies, which applied different animal models and treatment regimens [
3,
4].
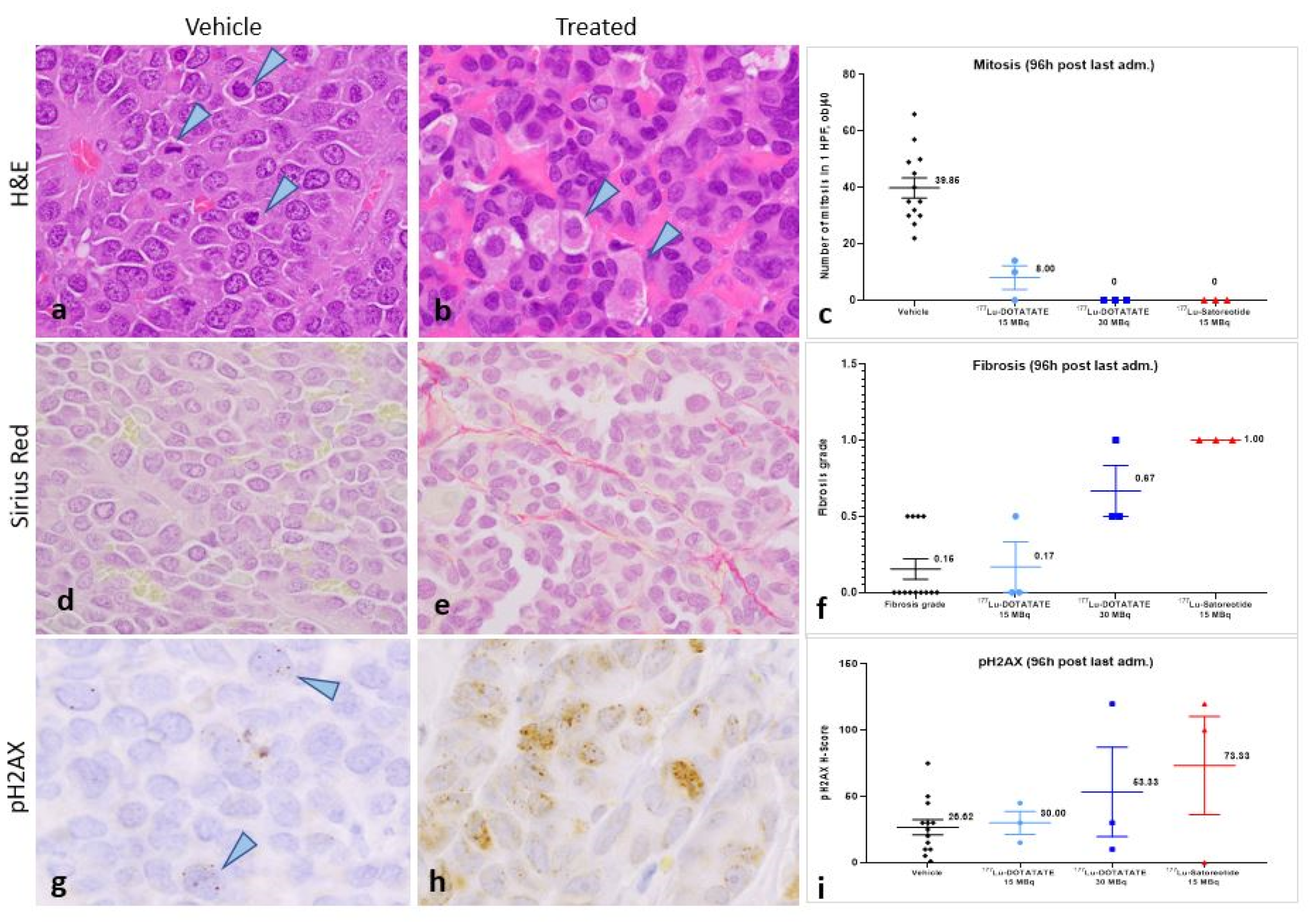
DNA damage detection is one of the most important biological markers in the assessment of pharmacodynamic effects of tumour treatments [
11]. The radionuclide lutetium-
177 is known to emit β-particles that can induce different types of DNA damage, among which DSBs are the most genotoxic [
3]. However, capturing the kinetics of
177Lu-PRRT-induced DNA damage is complex as it is influenced by various factors including the biodistribution of the therapeutic agent within cells and organs, the time course of radiation delivery, and tissue clearance kinetics [
12]. In this study, [
177Lu]Lu-satoreotide tetraxetan produced more DSBs than [
177Lu]Lu-DOTA-TATE, as reflected by a higher mean pH2AX H-score following the administration of four PRRT cycles. Even if radiation most likely induced direct cellular damage (on the DNA and other cell structures) or indirect damages (e.g., production of reactive oxygen species) affecting cancer cell survival, the detection of pH2AX depends on repair mechanisms that prevail over DNA DSB formation. Indeed, as phosphorylation of H2AX is part of the cell cycle checkpoints leading to apoptosis or correcting genetic lesions, pH2AX foci are thus theoretically only detected in dividing cells [
12,
13]. Hence, in organs with no-to-low dividing cell potential, although not detected by pH2AX immunohistochemistry, radioactivity uptake most likely induces DNA damage that might lead to transitory or long-term cell function impairments, but also to an earlier cellular senescence [
14,
15]. Interestingly, two animals treated with [
177Lu]Lu-satoreotide tetraxetan presented with higher levels of DNA damage in relapsing tumours, suggesting a marked alteration of cell cycle regulation mechanism and very high levels of cellular damage.
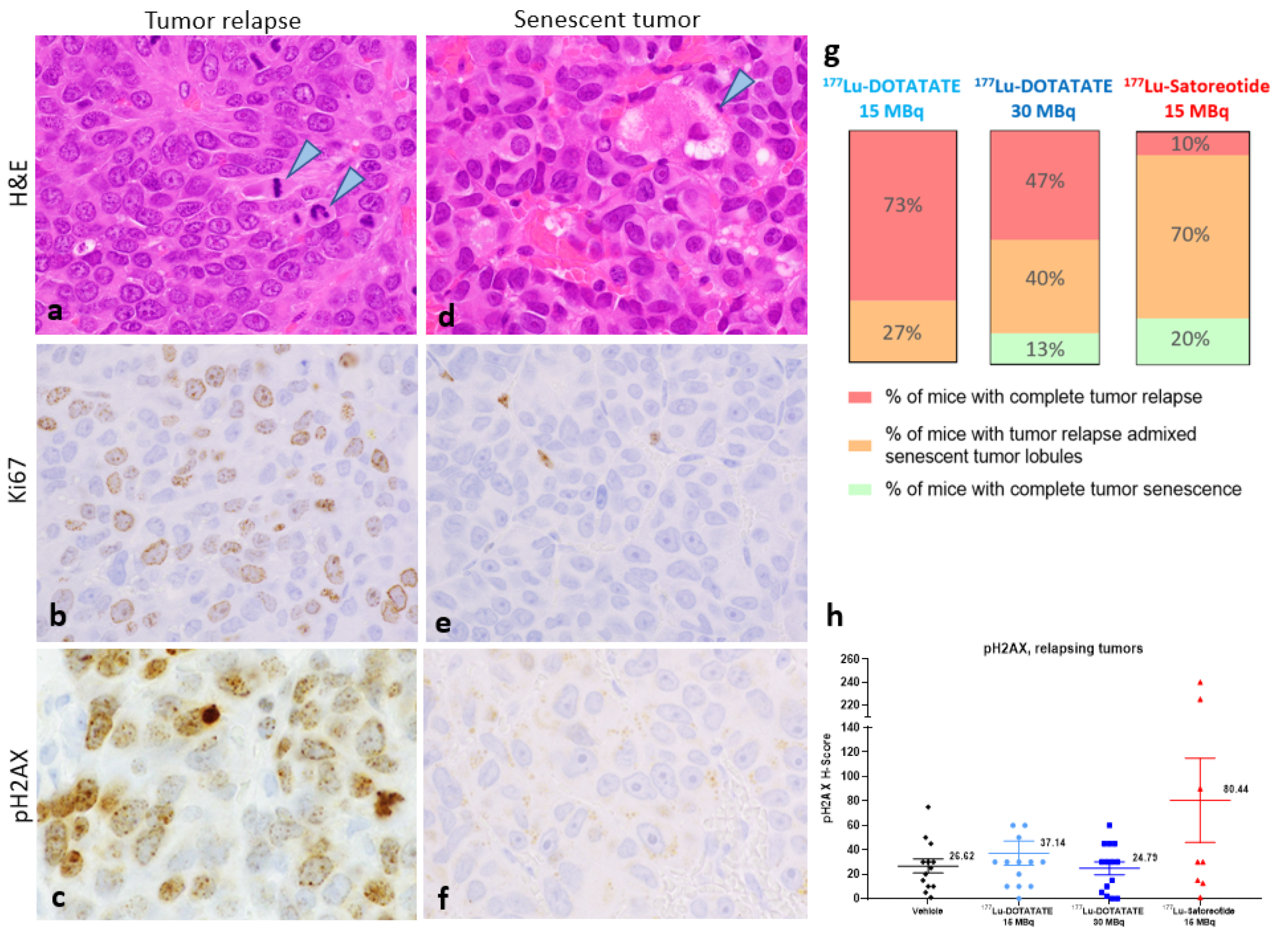
Cellular senescence is a common outcome of various anti-cancer interventions [
14]. In this study, compared with [
177Lu]Lu-DOTA-TATE treatments, [
177Lu]Lu-satoreotide tetraxetan therapy was associated with a greater rate of complete tumour senescence as well as partial tumour senescence. In addition, the absence of mitosis and lack of Ki67 immunostaining in senescent tumours is strong evidence supporting the senescent status of some tumours at the end of the study, several days after the last treatment administration. In our opinion, senescent cancer cells have lost their dividing capacities due to radiation therapy and they will most likely slowly degenerate. As observed in
Figure 5d, cancer cell vacuolation and denegation was noted in all senescent tumors/tumor lobules in our study. Recently, it has been shown in a mouse model of pancreatic ductal adenocarcinoma that therapy-induced tumour cell senescence promotes vascular remodelling through induction of a pro-angiogenic senescence-associated secretory phenotype, leading to enhanced drug delivery and T-cell infiltration that can sensitize tumours to different therapies such as chemotherapy and immunotherapy [
16]. Hence, the tumour senescence outcomes obtained in our study with
177Lu-satoreotide tetraxetan therapy are encouraging and may translate clinically into improved therapeutic responses [
17].
The acute toxicity of radiopharmaceuticals can be attributed to the targeting of rapidly dividing cells (e.g., bone marrow and other lymphoid organs) [
18]. In our study, the weekly intravenous administration of [
177Lu]Lu-satoreotide tetraxetan at 15 MBq, [
177Lu]Lu-DOTA-TATE at 15 MBq, or 30 MBq for 4 weeks induced overall similar haematological and histological changes in the bone marrow, spleen, and kidneys, and these observations were considered of minimal toxicological significance. Whole body irradiation (WBI) was needed to optimize cancer cell survival and homogeneous tumour development after subcutaneous implantation to prevent immune-dependent rejection. This procedure could have impacted the effects of both agents, leading to increased toxicity and changes in tumour responses to radiotherapies. Considering that all mice were exposed to the same body irradiation dose 8 days before the first treatments, the comparison of toxicity and efficacy of [177Lu]Lu-DOTA-TATE and [177Lu]Lu-satoreotide tetraxetan remains relevant in our opinion. Indeed, even if WBI toxicity cannot be excluded completely, the impact of this procedure on the evaluation of the toxicity of radiotherapeutics is likely minimal, although it cannot be discarded completely. Regarding the impact of WBI on tumour response to the treatments, anti-tumour effects of both radiotherapeutics were well-correlated to the radioactivity uptake in tumours, indicating that differences between the treatments are unlikely influenced by WBI.
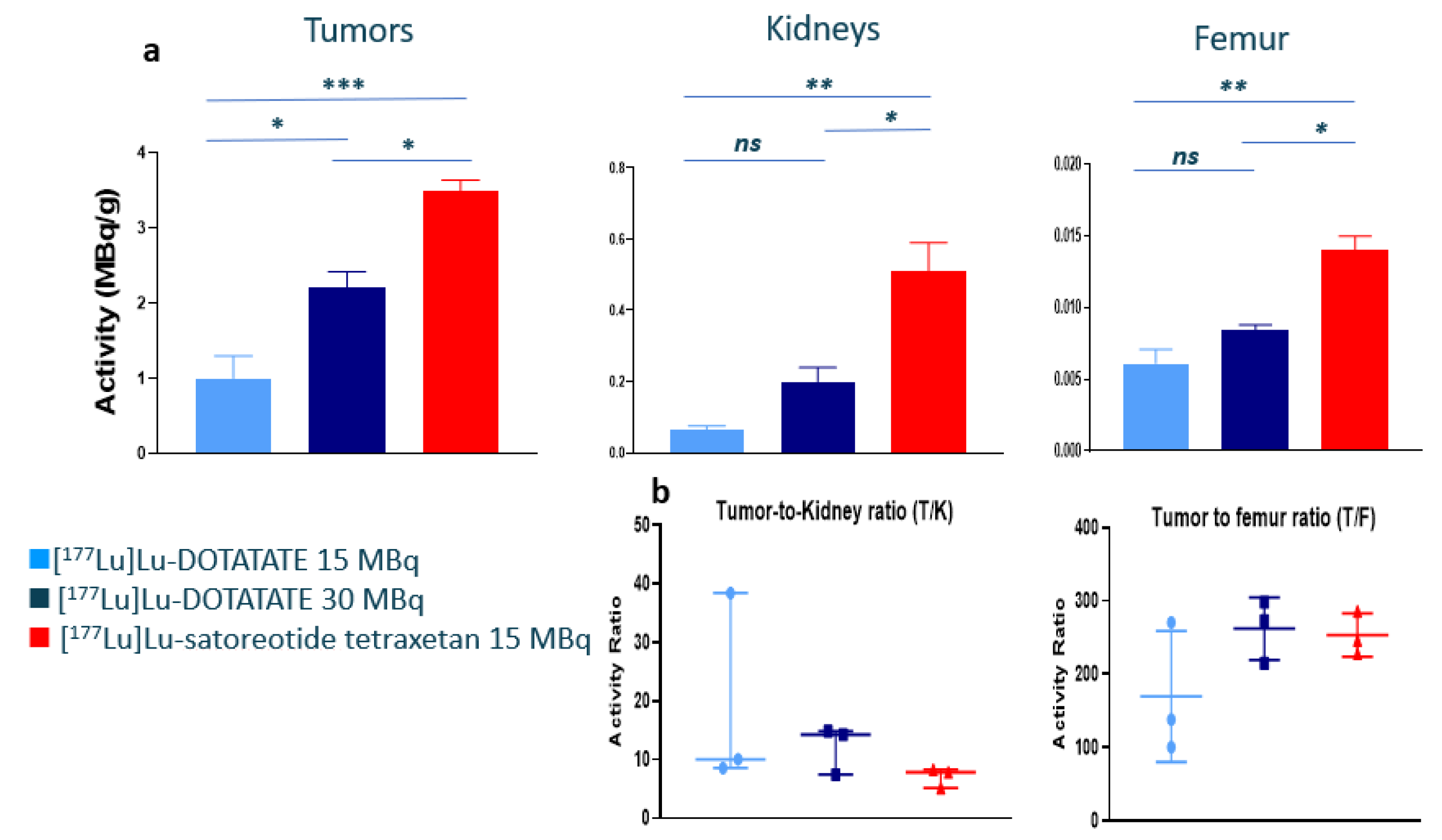
Interestingly, organ uptakes measured at 96 h after the last treatment administration were well-correlated with SST
2 expression levels, except in the kidneys (
Table S2). Indeed, despite notable radioactivity uptake in the kidneys, there was no evidence of SST
2 expression in this tissue. Renal uptake is most likely due to a non-specific reabsorption of peptides in the proximal part of the nephron by megalin and cubilin receptors [
19]. In humans, as an amino acid solution containing arginine and lysine is used to saturate these renal receptors, the renal uptake and potential renal toxicity of radiopharmaceuticals is limited, as noted in several clinical studies of [
177Lu]Lu-satoreotide tetraxetan [
5,
6,
20].The low toxicity induced by both agonist and antagonist products could be due to the high SST2 expression in AR42J cells and a potential sink effect in the tumour limiting the radioactivity uptake in other organs. Indeed, the %ID/g of tissue (
Table 1) or MBq/g of tissue (
Table S2) were higher in tumours compared to other organs for both products likely reducing other organs’ radioactivity exposure. However, our results at 96h could be compared with the results formerly published in the H69 cancer model with a lower SST2 expression with [177Lu]Lu-satoreotide tetraxetan [
3]. In the AR42J model, the %ID/g in tumours was twice as high, reflecting a high SST2 expression in AR42J cells compared to H69. However, the %ID/g in other tissues was not consistent with a sink effect as the radioactivity uptake was higher or equivalent in the adrenal glands and kidneys, respectively, in our AR42J model and lower in the spleen when compared to the H69 model [
3]. Therefore, even if there is more radioactivity uptake in tumours, the level of SST2 expression does not seem to be clearly associated with a change in radioactivity uptake in other organs. Finally, depending on the tumour size, degree of tumour fibrosis and blood perfusion, level of SST2 expression, and the amount of drug used to treat a patient, a sink effect in the tumour remains possible and expected but difficult to estimate in practice.
Overall, our encouraging efficacy and safety findings support the application of repeated treatment cycles of [
177Lu]Lu-satoreotide tetraxetan. Fractionated dose PRRT protocols have been found to inhibit tumour growth and lead to reductions in tumour burden with decreased toxicity, by delivering a sufficient cumulative radioactivity to the tumour while allowing non-target tissues to recover [
21]. Supporting the current clinical practice of performing several spaced cycles of PRRT, administration of [
177Lu]Lu-satoreotide tetraxetan, at a median cumulative radioactivity of 13.0 GBq over three cycles separated by an 8–12-week interval, had a manageable safety profile with a 12-month DCR of 90% in an ongoing phase I/II study (
ClinicalTrials.gov: NCT02592707) in 40 patients with progressive SST
2-positive NETs [
20]. The results of the current study suggest that PRRT with [
177Lu]Lu-satoreotide tetraxetan may also be performed in four cycles at a higher median cumulative radioactivity, divided over four instead of three cycles, with a lower radioactivity per cycle compared to the radioactivity level administered in the ongoing phase I/II study.
4. Materials and Methods
4.1. Radioligands
The peptide conjugate satoreotide tetraxetan was supplied by Octreopharm/Ipsen (Berlin, Germany), and DOTA-TATE by Bachem (Bubendorf, Switzerland).
177LuCl3 (non-carrier added) was obtained from ITM (Munich, Germany). The preparation of [
177Lu]Lu-DOTA-TATE and [
177Lu]Lu-satoreotide tetraxetan has been described elsewhere [
22]. For both compounds, the radiochemical purities were above 90%, the radiochemical yields above 97%, and their molar activities were identical (around 50 MBq/nmol).
4.2. Cell Culture and Membrane Preparation
The SST2-expressing AR42J, acinar pancreatic tumour cell line derived from a transplantable tumour of a rat exocrine pancreas, was provided by ATCC (LGC Standards, Molsheim, France). Cells were cultured in RPMI 1640 medium (Dutscher, Bernolsheim, France), supplemented with 10% foetal calf serum (PAN-Biotech, Aidenbach, Germany), in a humidified atmosphere at 37 °C with 5% CO2. AR42J membranes were obtained by sonication in 50 mM Tris–HCl, pH 7.4, and centrifugation at 39,000× g for 10 min at 4 °C. The pellet was resuspended in the same buffer and centrifuged at 50,000 ×g for 10 min at 4 °C, and membranes in the resulting pellet were stored at −80 °C until further use.
4.3. Saturation Binding Assays
Saturation binding assays were performed in vitro using AR42J cell membrane suspension (10 µg/well) incubated with increased concentrations (ranging between 0.075 and 10 nM) of the radioligand. Non-specific binding of [177Lu]Lu-satoreotide tetraxetan was determined using 1000-fold excess of the unlabelled SST2 antagonist OPS202, also known as NODAGA-JR11. Non-specific binding of [177Lu]Lu-DOTA-TATE was determined using the same excess of the natural somatostatin-14 or DOTA-TATE. Membrane-bound radio-peptide was counted in a γ-counter (COBRA 5003, Packard Instruments). The number of binding sites per cell was calculated and fitted to a one-site binding model in GraphPad Prism 8 to determine the dissociation constant (KD) and the number of binding sites (Bmax).
4.4. Animals and Ethics
Healthy female Swiss Nude-Foxn1nu mice, of 7 weeks old, were obtained from Charles River (Ecully, France). The animals were housed and cared for according to French and European regulations on animal experimentation. All procedures with animals were submitted to the Institutional Animal Care and Use Committee of Oncodesign (CNREEA Agreement N°91). Animal physical well-being was monitored on a daily basis, and animal weight was measured at least twice a week. Tumours were induced by subcutaneously injecting 1 × 107 AR42J cells in 200 μL of RPMI 1640 into the right flank of the mice. In order to improve cell engraftment, AR42J tumour cell implantation was performed 72 h after whole-body irradiation with a γ-source (2 Gy 60Co; BioMep, Bretenières, France).
4.5. In Vivo PRRT Studies
PRRT with either 177Lu-satoreotide tetraxetan or [177Lu]Lu-DOTA-TATE started once the tumours reached a mean volume of 150–250 mm3. A total of 62 mice were randomised according to their individual tumour volume into 4 groups: vehicle control group (group 1; n = 13), 15 MBq [177Lu]Lu-DOTA-TATE-treated group (group 2; n = 18), 30 MBq 177Lu-DOTA-TATE-treated group (group 3; n = 18), and 15 MBq 177Lu-satoreotide tetraxetan-treated group (group 4; n = 13). 15 MBq and 30 MBq of [177Lu]Lu-DOTA-TATE corresponded to 0.48 µg and 0.96 µg or 0.29 and 0.58 nano moles of peptide per mice, respectively (i.e., 24 µg/kg and 48 µg/kg or 14.5 nmol/kg and 29 nmol/kg, respectively), 15 MBq 177Lu-satoreotide tetraxetan corresponded to 0.59 µg or 0.29 nano moles of peptide per mice (i.e., 29.5 µg/kg or 14.5 nmol/kg). All test substances, including the mixture of radiolabelling buffer and 0.9% NaCl solution administered in the vehicle group, were intravenously injected into the caudal vein once a week for 4 consecutive weeks. The treatment schedule was determined by the growth kinetics of the AR42J tumour model. The day of randomisation was considered as day 0. In order to evaluate the anti-tumour activity of [177Lu]Lu-satoreotide tetraxetan and [177Lu]Lu-DOTA-TATE, the length and width of the tumours were measured thrice a week with calipers to determine the tumour volumes. Tumour growth delay was estimated by calculating the median time taken to reach a tumour volume of 850 mm3. Animals were sacrificed once their tumour volume exceeded 1500 mm3. The study was stopped on day 83 because only 1 and 3 animals remained with non-progressing tumours in groups 3 and 4, respectively.
4.6. In Vivo Biodistribution Studies
Twelve mice (3 in each group) were sacrificed at 96 h after the fourth administration of the test substances to evaluate at the same time toxicity and haematological parameters, and radioactivity uptake in tumours and organs after 4 cycles. The remaining 50 mice (10 in both groups 1 and 4, and 15 in both groups 2 and 3) were either sacrificed for ethical reasons on the last day of the study, on day 83. At 96 h, following the last treatment administration, individual tumours and organs of interest were collected, blotted dry, weighted, fixed in formalin, and the amount of radioactivity present in each organ was quantified using a γ-counter. The radioactivity uptake in tumour and organs was determined and expressed as MBq/g of tissue as well as percentage injected dose/g of tissue (%ID/g).
4.7. Haematology, Histopathology and Immunohistochemistry
At each of the sacrifice timepoints, blood samples were collected in EDTA tubes and complete heamatological analyses were performed using a VetScan HM5 (Abaxis) to investigate white blood cell, red blood cell and platelet parameters.
Once fixed, tumours, spleen, bone marrow (femur), jejunum, colon, tail (injection site), and kidneys were processed to paraffin blocks and tissues slides produced for immunohistochemical analyses or stained with haematoxylin and eosin (H&E) or sirius red (collagen staining) for histopathological examination.
Regular immunohistochemistry was performed using heat-induced antigen retrieval, as previously described [
23,
24]. Briefly, sections were incubated with primary antibodies–pH2AX (ab81299 from, Abcam, Paris, France) and SST
2 (ab134152, Abcam, Paris, France) or with an unspecific isotypic control antibody to assess the specificity of the labelling. Afterwards, sections were incubated with a biotinylated secondary antibody and then an amplification system (streptavidin-biotin-peroxidase, ABC vector) was used before revelation with 0.02% diaminobenzidine. For the streptavidin-biotin-alkaline-phosphatase procedure, the same steps were followed, with a fast red solution used as chromogen. Counterstaining was done using aqueous haematoxylin.
All the slides were evaluated by a board-certified veterinary pathologist (S.L.) using a light microscope (Olympus BX41). The pH2AX and SST2 signal intensities were scored using a semi-quantitative approach (H-score), by multiplying the signal intensity score (0, absent; 1, weak; 2, moderate; 3, strong) with the percentage of labelled cells. The H-score, which ranges from 0 to 300, gives the relative weight of staining in a given tumour sample. Regarding the histopathological examination, the number of mitoses was counted in the areas of tumours with the highest mitotic figures in 1 high-power field at the objective 40. The severity of fibrosis was evaluated on a classical toxicologic pathology scale ranging from 0 (absent) to 5 (severe).
4.8. Statistics
Statistical analysis was performed using GraphPad Prism v8 software (La Jolla, CA, USA), as well as JMP version 14.2 (SAS Institute Inc., Cary, NC, USA), R version 3.5.1 (
https://www.r-project.org/) (accessed on date 1 October 2020)., and SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) for the tumour growth and time to reach 850 mm
3 analyses. The time for a tumour to reach a volume of at least 850 mm
3 and was estimated using the Kaplan-Meier method. For comparisons, one- or two-way ANOVA with Tukey’s or Dunnett’s multiple comparison was used when appropriate. Fisher’s exact test was also used to compare total and partial senescence in tumours between the 4 groups at the end of the study. Data are presented as mean, standard deviation, standard error of the mean (SEM), median, and range. Asterisks were used to specify significance level, where * denotes
p ≤ 0.05, ** ≤0.01, and *** ≤0.001.