1. Introduction
The World Health Organization (WHO) strongly recommends, in its antivenom production guidelines, that antivenoms must be produced at the requested needs of each country, with the venoms of their own medically important venomous animals, based fundamentally on the fact that cross-reactions are not always observed between venoms from different regions, making antivenoms lose their therapeutic efficacy and, therefore, their production in foreign countries is ineffective and not recommended [
1]. According to this, different studies indicate that the available antivenoms in Latin America covering
Tityus envenomation are not effective outside the country of manufacturing [
2]. Currently, Colombia does not have any local production of antivenoms covering scorpion stings, although Colombia possesses an important diversity of medically important scorpions, with six different Class I–Class III species (according to Ward et al. [
3]) classified in the epidemiological and medically important family of Buthidae (genus
Tityus and
Centruroides) [
3]. The harmful scorpion fauna considered epidemiologically and medically important in Colombia correspond to species
Tityus pachyurus,
Tityus asthenes,
Tityus fuhrmanii, and
Centruroides spp. distributed in the central, north-west and north areas [
3,
4,
5]. The only available therapy to treat scorpion stings in Colombia corresponds to an antivenom manufactured in Mexico, which is a third-generation polyclonal horse-derived formulation produced with the hyperimmune plasma of
Centruroides individuals from Mexico [
2].
In addition to the lack of a local antivenom producer, Colombia does not have any official active epidemiological program tracking scorpion stings, and the national incidence is unknown. Epidemiological data collected from two localities in Colombia indicate that the incidence of scorpion stings in Colombia may be estimated to be 4.5 per 100,000 inhabitants/year [
6].
Although Buthidae scorpion venom has not been deeply characterized, previous studies reported their LD
50, their biochemical profile, and protein content, indicating that these scorpions are an important source of peptides affecting mammal ionic channels and peptides with antimicrobial activity [
4,
5,
7,
8,
9,
10]. Their envenoming symptoms cover local and systemic signs, including local pain, tachycardia, vomiting, generalized sweating, drowsiness, abdominal pain, tachypnea, and sialorrhea, among others [
5,
6,
11].
The lack of local antivenom production, despite the recommendation of the WHO, and the presence of harmful scorpion fauna from the Buthidae family, allowed us to propose the objective of this study focused on the characterization of a lab-scale process to produce the first antivenom specific for Colombia, covering scorpion stings produced by Tityus pachyurus, Tityus asthenes, Tityus fuhrmanii, and Centruroides spp.
3. Discussion
Colombia is one of the most diverse countries for arachnids worldwide, housing around 81 different scorpion species, distributed in 14 different genera [
12]. Despite the presence of these vast number of species, including Buthidae individuals from the
Centruroides and
Tityus genus (causing severe accidents), Colombia does not have a program to produce antivenoms covering scorpion accidents [
2].
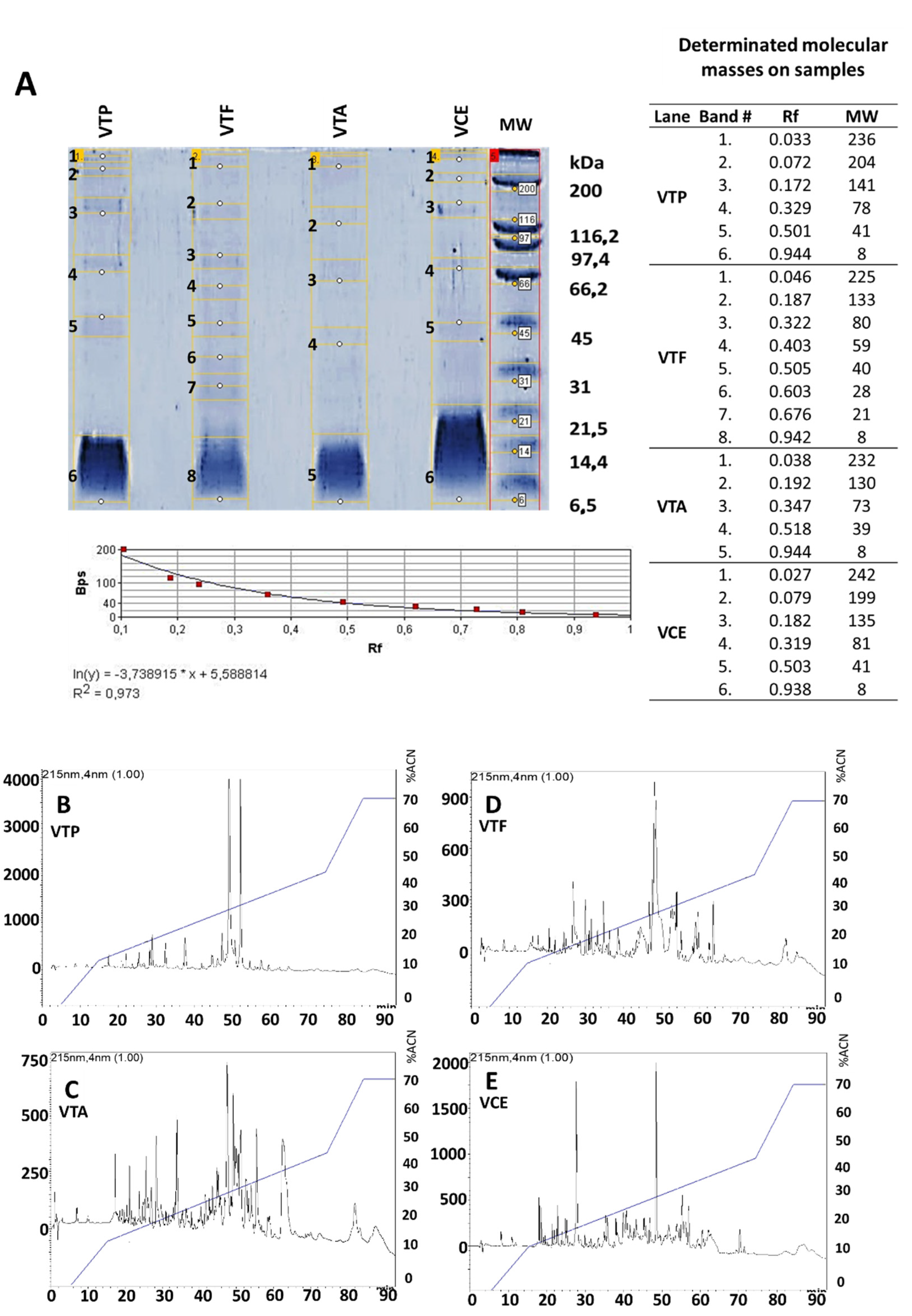
Venoms from Buthidae scorpions from Colombia are a complex mixture of proteins with a molecular mass ranging from 6 kDa up to 210 kDa [
9]. The electrophoretic and chromatographic profiles from all four venoms analyzed in this study showed similar patterns to the previous descriptions of these venoms from Colombia, including the molecular mass ranges and the venom complexity (chromatographic profiles) using similar conditions [
5,
8,
9].
T. pachyurus LD
50 value (53.3 µg/mice or 2.6 mg/kg) showed a lower LD
50 value against the other venoms in concordance with the result published by Otero et al. and Barona et al. [
4,
6]. The LD
50 values from
T. pachyurus,
T. fuhrmanni, and
Centruroides, were similar to previous LD
50 reports (although below) by Mendoza-Tobar et al., Gomez et al., and Estrada-Gomez et al., respectively [
5,
13,
14]. Only
T. asthenes venom showed a difference from the previous report by Gomez et al. [
13]. With the
Centruroides venom batch, we could not calculate the LD
50 value using concentrations up to 25.0 mg/kg, detecting at this concentration only toxic effects characterized by pain, salivation, piloerection, hyperventilation, distress, and excitability. Estrada-Gomez et al. previously described a similar result in the species
C. edwardsii from the Antioquia and Tolima provinces using concentrations of 19.2 mg/kg [
5]. In the same species, Brenes and Gomez established an LD
50 of
C. edwardsii from Costa Rica at 4.4 mg/kg [
15]. In a different
Centruroides species from Colombia, Mendoza-Tobar et al. calculated an LD
50 of 19.5 mg/kg in
C. margaritatus from the Cauca province. The LD
50 discrepancies with previous reports for
T. asthenes and
Centruroides may be due to individual geographical variability and the interspecific differences as previously reported in the venom from
C. edwardsii and
T. pachyurus from Colombia [
5,
16]. In the case of
T. asthenes, individuals used by Gomez et al. were collected exclusively in the Uraba sub-regions (Antioquia). In contrast, we used individuals from the Aburra Valley, North and Uraba sub-regions (Antioquia). In the case of
Centruroides, when comparing our result with Mendoza-Tobar, they used specimens from a different geographical location (Cauca province), while we used individuals from the Antioquia province where the main
Centruroides species distributed are
C. edwardsii [
5,
17] and
C. margaritatus [
17].
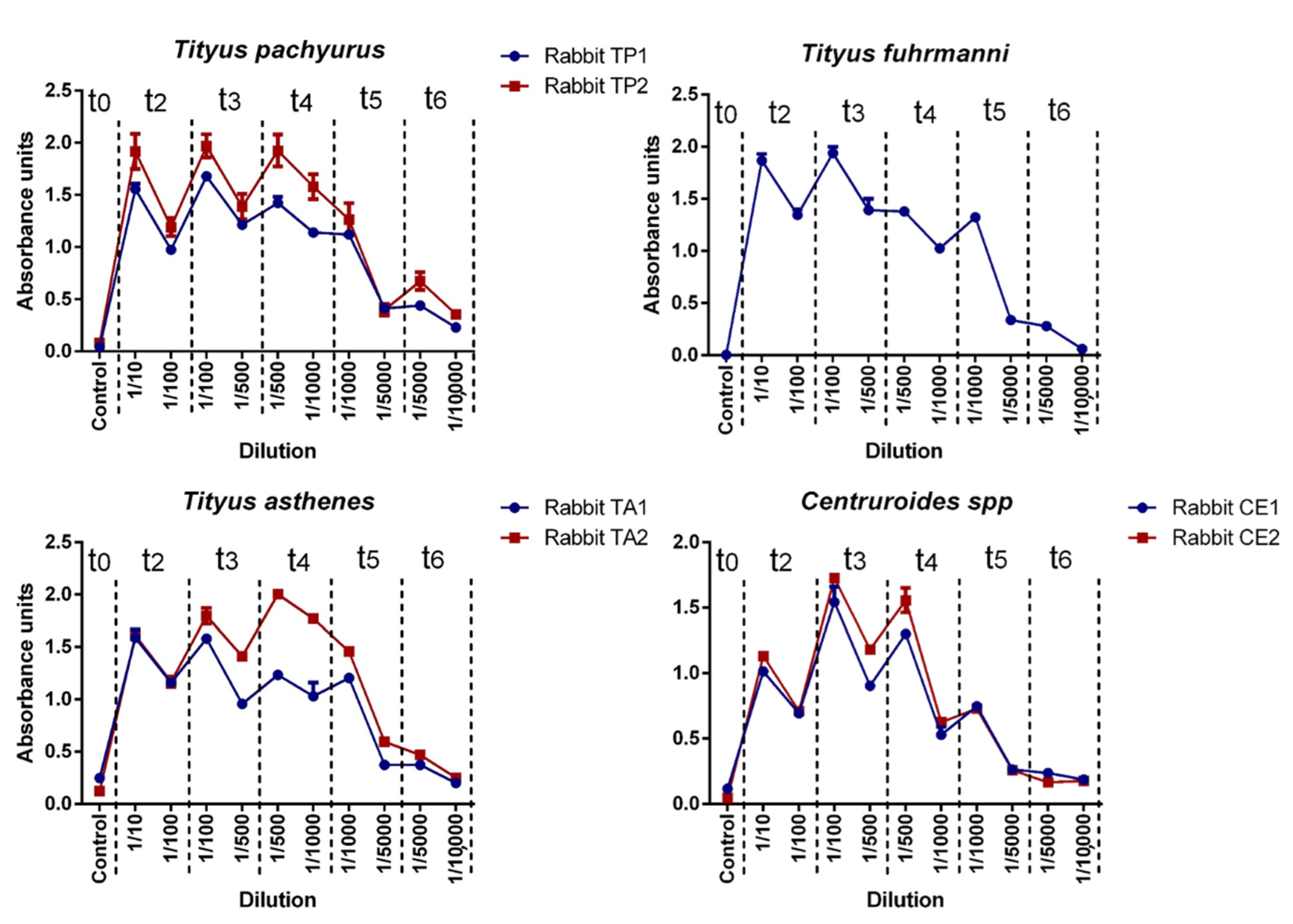
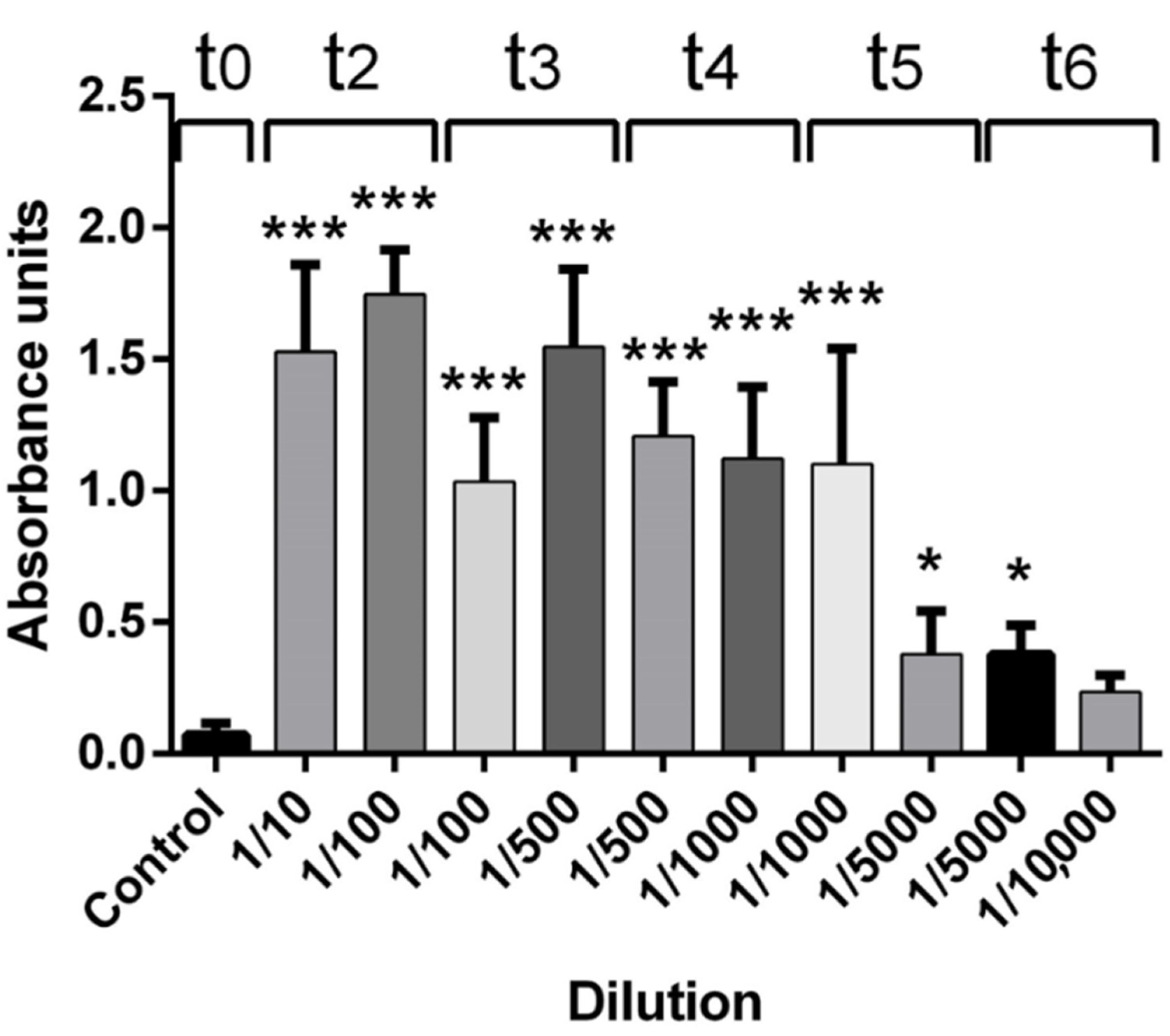
There are no previous scientific reports of antibodies against scorpion venom production in Colombia. The presence of antibodies against each venom batch (antigens) was tested by ELISA assay. All rabbits were able to produce IgG according to the respective stimuli used (venom), with titers ranging from 1 × 10
1 U-ELISA/mL and 1 × 10
4 U-ELISA/mL. Anti-scorpion IgG antibody production in rabbits performed a similar time-dependent IgG production (in different magnitudes) and constant IgG production in each individual (in the same rank), as previously reported by Guidolin et al. in the production of equine IgG against snake venoms [
18,
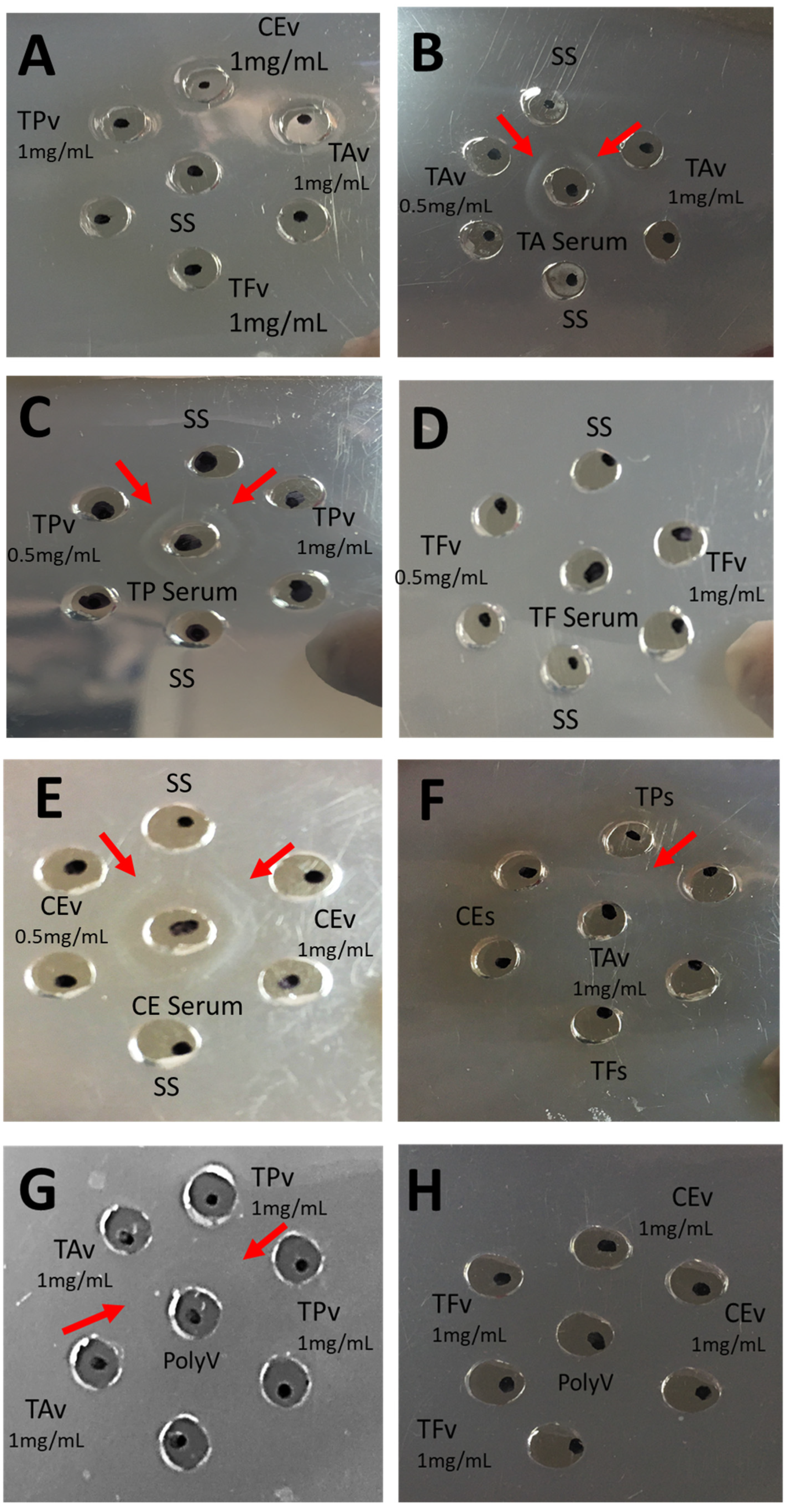
19]. Although we observed statistically significant differences in IgG production in each rabbit group, the general production behavior was similar. Ouchterlony indicates that the rabbit IgGs produced showed specificity (recognition) against the respective immunization venoms. In the case of
T. asthenes, hyperimmune serum showed cross-recognition against
T. pachyurus venom, as reported by Borges et al. [
2]. Scorpion venoms from the same genera share similitude in their venom content, especially in the
Tityus genus; as previously reported by Estrada
et al., where
T. asthenes venom showed similarity with
T. serrulatus,
T. bahuiensis,
T. discrepans and
T. pachyurus [
9]. The IgG production could be improved by using higher venom concentrations to reach titers over 1 × 10
4 U-ELISA/mL before final bleeding. To do so, it is very important to consider that we did not detect any toxic effect at the venom doses used on each rabbit (we used doses 9.2 times for
T. pachyurus, 12 times for
T. asthenes, and 11.4 times for
T. fuhrmanni below their respective LD
50).
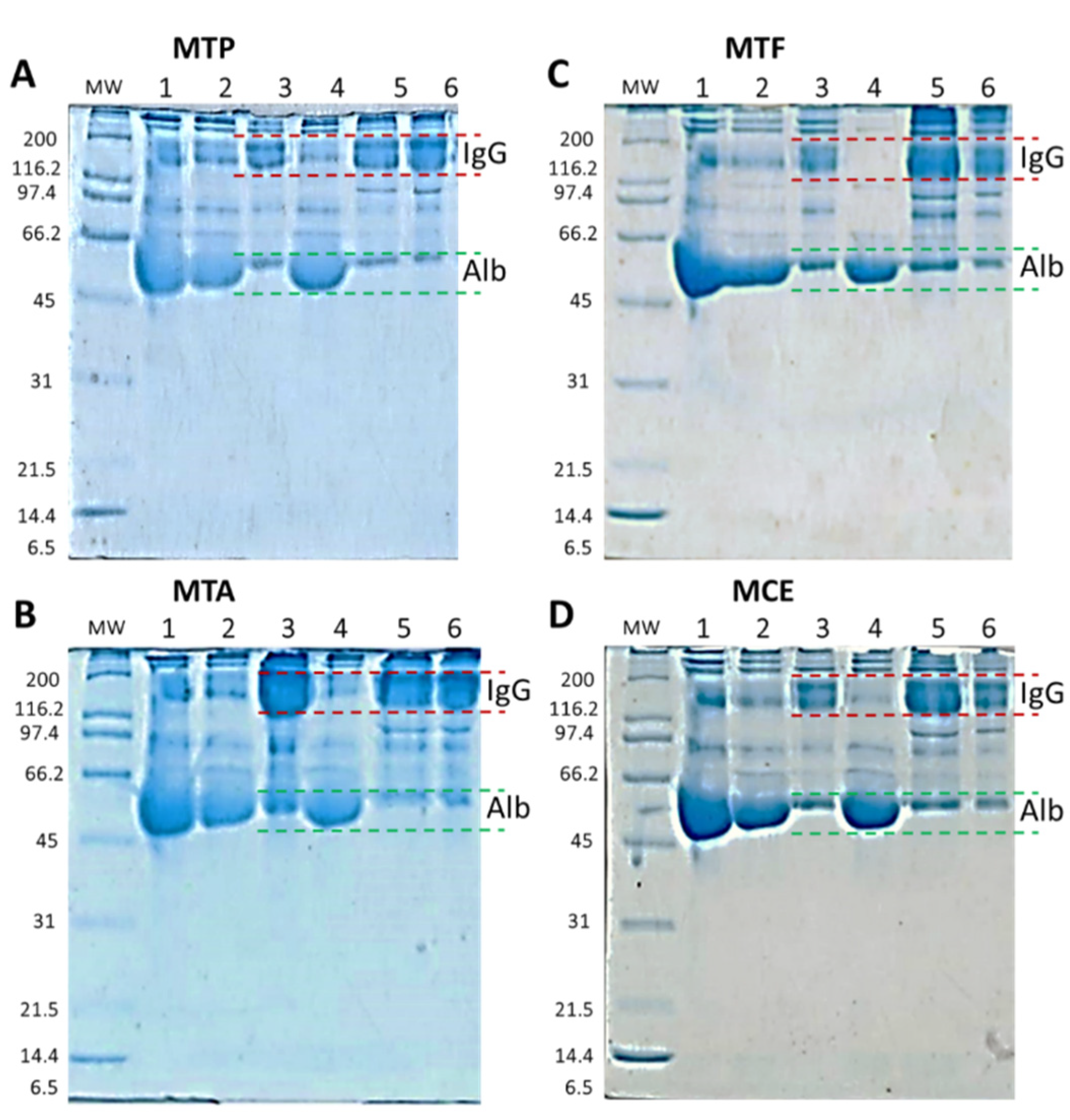
The specificity of the antibodies produced and the approach of the IgG purification process in monovalent batches allowed the further purification of specific IgG with slight albumin contamination. In all cases, we recovered an average of 6.9% ± 3.9% of protein, and after subtracting the concentration of albumin detected, the total amount of protein corresponds mainly to total IgG (6.4% ± 3.9%). Electrophoretic profiles corroborate this result since, in the final product, we detected bands with molecular masses similar to IgG and albumin. The
T. asthenes batch (M.T.A.) showed lower values of recuperated proteins at the end of the process, with strong losses after the precipitation and thermocoagulation processes. However, the volume of processed plasma and IgG titers was similar to the other groups. The most significant loss is observed in the thermocoagulation process just before the filtration, indicating a possible loss of IgG by precipitation.
Figure 7B, line 6, shows this is likely lost with the high concentration of proteins with a molecular mass similar to IgG in the thermocoagulation precipitate. At the end of the M.T.A. batch, we were able to obtain lower values of total protein concentration—1.7% of the total protein—with albumin corresponding to 21% on the protein measure. The precipitation with (NH
4)
2SO
4 allowed a deep purification of IgG, precipitating 98% ± 3.7% of the total albumin present in the plasma. This is an exceptionally important characteristic in antivenoms since albumin is the main contaminant in final products and its presence compromises the safety of the product since it is responsible for early adverse reactions after antivenom administration [
1]. Despite this important decrease in total albumin, we were not able to reach the WHO standards, which indicates that albumin concentration should not exceed 1% of total protein content [
1]. To obtain the requested concentrations suggested by the WHO, further improvements could be performed in the T.F.F. step to diminish the concentration of albumin in the final product, adding a specific polyethersulfone albumin cassette just after the hydrosart filtration.
Although the purified IgG anti-
T. pachyurus was able to neutralize the toxic effects, diminish symptoms, and prolong mice lifetime, the venom/IgG ratio (0.3 LD
50/1 mg) used in this research was low compared with commercial antivenoms, which can neutralize 0.3 LD
50/1 mg of
Centruroides venoms from North America or
T. pachyurus venom from Colombia [
4,
20]. Further studies are required to test higher concentrations of IgG. Obtaining higher amounts of IgG to be processed could be achieved by obtaining plasma with higher titers of IgG. To raise the IgG titers on rabbits, they can be immunized with higher concentrations of venoms since we used doses 9.2 times, 12 times and11.4 times below the respective LD
50 for
T. pachyurus,
T. asthenes, and
T. fuhrmanni, respectively. The fractionation method is efficient in separating and purifying the specific IgG. Processing hyperimmune plasmas with higher titers of IgG should allow the purification of higher concentrations of IgG in the final product.
4. Materials and Methods
4.1. Venoms and Antigen Preparation
Scorpions with epidemiologic and clinical importance in Colombia, according to [
3,
6,
11], were selected for this research and included
Tityus pachyurus,
Tityus asthenes,
Tityus fuhrmanni, and
Centruroides spp., collected in the provinces of Antioquia and Tolima, and kept in captivity in the serpentarium of the University of Antioquia (COLBIOFAR-149) with water ad libitum and fed with insects (
Periplaneta americana and
Tenebrio molitor). Tityus pachyurus venom from Tolima, including 31 individuals was pooled in the
T. pachyurus batch;
Tityus asthenes venom from the Aburra Valley, North, and Urabá sub-regions (Antioquia) included 27 individuals;
Tityus fuhrmanni venom from the Aburrá Valley and South-west sub-regions (Antioquia) included 140 individuals and
Centruroides spp. venom from the Aburrá Valley, Urabá, South-west and West sub-regions (Antioquia) included 67 individuals, females, and males in all cases. Each antigen mixture was identified with a batch number composed of the letter V of “venom” and the first genus letter followed by a second letter corresponding to the scorpion species. Each antigen batch preparation was characterized using protein quantification, electrophoresis, rp-HPLC, and LD
50, as described below.
4.2. Electrophoretic Profile
Antigens batches (venoms) were analyzed using 10% TRIS-TRICINE gels and stained with Coomassie blue R-250, and molecular weights were estimated using standard broad range markers (unstained SDS-PAGE standards broad range # 1,610,317 Bio-Rad, Hercules, CA, USA). Quantification of volumes and calculation of molecular weights were performed using the software GelAnalyzer 19.1, available at:
http://www.gelanalyzer.com/ (accessed on 2 February 2022) [
21]. Molecular weights were calculated using the known values of the standard broad rank markers (Bio-Rad): 200 kDa, 116 kDa, 97 kDa, 66 kDa, 45 kDa, 31 kDa, 21 kDa, 14 kDa, 6 kDa. To estimate the molecular weight, we used a simple exponential fit approximation of the Rf (retention factor, measured as the band distance migrated/gel length) of each analyzed band. Samples were loaded at a concentration of 1.5 mg/mL and a final volume of 20 µL. Each antibody purification/preparation stage and antivenoms batches were analyzed using 12% sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE) according to Laemmli [
22] and stained with Coomassie blue R-250. Molecular weights were estimated using standard broad range standards (Bio-Rad). Samples were loaded at a concentration of 1.5 mg/mL.
4.3. Chromatographic Profile
Antigens batches (venoms) profiles were obtained using reverse-phase high-pressure liquid chromatography (RP-HPLC) using a Shimadzu Prominence chromatograph LC-20AT (pump unit). One mg of crude venom was dissolved in 200 μL of solution A (0.1% TFA in water) and centrifuged at 3500×
g for 5 min at room temperature. The supernatant was fractionated using a C18 RP-HPLC analytical column (250 × 4.6 mm), equilibrated, and eluted at a flow rate of 1.0 mL/min first isocratically (5% B for 5 min), followed by a linear gradient of 5–15% B for 10 min, 15–45% B for 60 min, and 45–70% B for 12 min according to with [
9,
23]. The chromatographic separation was monitored at 215 nm.
4.4. Median Lethal Dose
All animal experiments were carried out in Swiss-Webster mice (18–20 g body weight). The lethality induced by each scorpion venom was evaluated by injecting doses ranging from 25 µg up to 121 µg, all diluted in 250 µL of PBS and administrated by the intraperitoneal (i.p.) route, in groups of four mice. The control group received PBS alone. Deaths were recorded during 48 h, and the Spearman–Karmer method was used to estimate the median lethal dose. All procedures were in accordance with the guidelines of the Ethics Committee of Universidad de Antioquia.
4.5. Animals
Adult rabbits weighing between 3.0–4.0 kg were used to produce the antivenoms. They were divided into 4 groups: T. pachyurus group n = 2, T. asthenes group n = 2, T. fuhrmanii group n = 1, and Centruroides spp. group n = 2. Animals were housed at serpentarium bioterium of the Universidad de Antioquia with water and food ad libitum. Before the immunization process, all animals were housed for 40 days, submitted to a deparasitation, and included in a clinical and para-clinical surveillance program. All procedures followed the guidelines of the Universidad de Antioquia Ethics Committee.
4.6. Immunization and Bleeding
Polyclonal antibodies were obtained by the continuous s.c. injection, during 93 days, with injections every 21 days of the antigen mixture across the back area (around the lymphatic back area). Each rabbit group was immunized using 857 µg of T. pachyurus (Batch number: TP200219), T. asthenes (Batch number: TA200219), T. fuhrmanii (Batch number: TF200219), and Centruroides spp. (Batch number: CE200219). All antigens were diluted in Marco Montanide I.S.A. 50 (Brazil) and mixed with Tween 80 (complete MMT80) for injection. Rabbits were bled to collect exploratory samples in the central ear vein using dry tubes (red cap) to collect serum samples for antibodies quantification every eight days after each antigen injection for two months (t0, t1, t2, t3, and t4–t0 was used as a baseline or negative control). With the appropriate antibody concentration, rabbits were anesthetized using ketamine and xylazine (1:1) and bled by intracardiac punction using a 20 Gauge × 1 1/2 inch needle connected to a 20 mL syringe containing an anticoagulant solution. Plasma and cells were separated by centrifugation using 500× g (2000 revolutions per minute) for 15 min at room temperature. The collected plasma was measured and stored at 2–8 °C until use.
4.7. Antibodies Quantification
Rabbit IgG titers were determined by enzyme-linked immunosorbent assay (ELISA), according to [
24], with some modifications. Plates (Corning Costar microplates ref 3591) were coated overnight, at 4 °C, with 100 µL/well of the respective antivenom batch antigen (4000 ng/mL in Carbonate/Bicarbonate Buffer, pH 9.6). After a washing step, the remaining binding sites were blocked with coated buffer containing 2% B.S.A. (Bovine Serum albumin, Low Heavy Metals US112659-100GM Albumin, Calbiochem) for two h at 37 °C, followed by a washing step. Rabbit hyperimmune serum was diluted in sample buffer (PBS pH 7.4, containing 1% B.S.A.) in concentrations from 1:10 to 1:10,000 (serial dissolutions). Then 100 µL of commercial antivenom dilution or similar preparation of immunoglobulins from a non-immunized rabbit (as controls) were added to the respective well according to the antigen, incubated for two h at 37 °C, and washed. IgG anti-rabbit and IgG- rabbit radish peroxidase conjugate diluted 1:8000 in sample buffer, was added (100 µL/well), incubated for 2 h at 37 °C, and washed. Finally, 100 µL O.P.D. -ortho-phenylenediamine (Amresco) diluted at 0.1% in citrate buffer, pH 6.0, containing 0.1% hydrogen peroxide, was added. Plates were protected from the light, incubated for 20 min at 37 °C, and absorbances were recorded using a Thermo Scientific Multiskan FC microplate reader at 450 nm.
4.8. Double Immunodiffusion
The immunological properties of each antigen were evaluated using the double immunodiffusion technique described by Ouchterlony [
25]. Serum samples were collected, as explained above. Using acetate sheets covered with agarose 1%, antigens solution (20 µL) was placed in peripheral wells (antigen concentrations used were 0.5 mg/mL and 1 mg/mL), whereas the central well was filled with 20 µL of hyperimmune serum. The antibodies in the antiserum react with both the antigens resulting in a smooth line of precipitate.
4.9. Antibody Purification and IgG Process Characterization
IgG purification and fractionation were performed according to the process described by Guidlolin et al., with some modifications [
18]. Each rabbit group plasma was purified and fractionated separately in monovalent batches according to the used antigen (venom). For this, a saturated solution of (NH
4)
2SO
4 (Ammonium sulfate EMPROVE
® EXPERT—Merck, Darmstadt, Germany) was added to each pooled plasma obtained from the rabbits immunized with the respective scorpion venoms. After 2 h of stirring, the mixture mainly containing immunoglobulins was separated by filtration using 10 µm pore size nitrocellulose membranes (Ahlstrom Munktell Quantitative Filter Paper, Sheets, Grade 1602N). Membrane cake was re-suspended, and the pH was adjusted to a neutral value with a low protein donor acid. Non-IgG proteins were precipitated by adding a second round of a saturated solution of (NH
4)
2SO
4 (Ammonium sulfate EMPROVE
® EXPERT—Merck) and incubated at 55 °C for 1 h under stirring. The mixture was filtered again using 10 µm pore size nitrocellulose membranes (Ahlstrom Munktell Quantitative Filter Paper, Sheets, Grade 1602N), and the membrane cake was discarded. The permeate was subsequently filtrated using a tangential flow filtration (T.F.F.) process using a ready-to-use and reusable laboratory crossflow tangential cassette with a polyethersulfone membrane (Vivaflow 50R—Sartorius) to remove salts and concentrate the resultant IgG antibodies in the same equipment. Each monovalent batch was placed at 4 °C until use with a batch number assigned (see below). In the end, the resultant IgG solution was sterilized by passing through a 0.22 µm filter membrane and freeze-dried using a Labconco Lyph-lock 6 L, Model 77,535 (Labconco Corporación, Kansas City, MO, USA). The freeze-dry conditions included a temperature delta from −41 °C to 15 °C and a pressure of 50 × 10
−3 mBar. Final products and each production process stage of each monovalent batch were analyzed to confirm the presence of IgG by comparing their relative molecular weight with standard molecular weight markers using gel electrophoresis and the software GelAnalyzer 19.1. Total protein concentration and albumin concentration were also quantified at each production process stage, including final products in each monovalent batch. Each monovalent batch was identified with a batch number composed of the letter M (indicating a monovalent batch) and the first genus letter followed by a second letter corresponding to the scorpion species.
4.10. Protein Quantification
Protein concentration of each production process stage, including final products in monovalent batches, was determined, following the Biuret method using Bio-Rad Protein Assay reagent and B.S.A. (Biosystems ref 11698) as standard [
26,
27,
28]. Briefly, 750 µL of Biuret reactive (MOL LABS, Bogotá, Colombia) were added to 250 µL of 1:20 dilution of each sample. The mixtures were incubated for 30 min at 37 °C, and the absorbance was measured at 540 nm. Additionally, a calibration curve was created with different albumin concentrations ranging from 0.1 mg/mL to 50 mg/mL, working under the conditions previously described (y = 0.0592X + 0.0204—R
2 = 0.9944). Once the sample was read, the value was interpolated in the calibration curve to measure the albumin quantity.
4.11. Albumin Quantification
Albumin concentration of each production process stage, including final products in monovalent batches, was determined following the method described by Doumas et al. [
27] using a specific commercial kit for albumin determination (Biosystems Albumin Reagent with bromocresol green ref 11547). Briefly, 10 µL samples were mixed with 1000 µL of albumin reactive. The mixtures were mixed using a vortex, and the absorbance was measured at 630 nm. Additionally, a calibration curve was created with different albumin (BSA) concentrations ranging from 5 mg/mL to 50 mg/mL, working under the conditions previously described (y = 0.0068X + 0.001—R
2 = 0.9978). Once the sample was read, the value was interpolated in the calibration curve to measure the albumin quantity.
4.12. Efficacy Test
A preliminary efficacy test was performed to test the ability of the specific IgG to neutralize the venom toxic effects. From all monovalent batches, the batch antivenom from Tityus pachyurus was tested since this venom presented the lowest LD50 among all other venoms. Different amounts of Tityus pachyurus of monovalent batches (1 mg, 5 mg, and 10 mg) were tested against 3 LD50. Mixtures of antivenom and venom were incubated at 37 °C for 30 min. After incubation, the mixture was injected intraperitoneally into Swiss mice weighing 18–20 g. One control group was injected only with the respective 3 LD50.
4.13. Ethical Statement
All animals used in this study were maintained and treated under strict ethical conditions following the WHO Guidelines for the Production, Control, and Regulation of Snake Antivenom Immunoglobulins [
1]. All procedures involving animals were carried out with the support of the veterinaries Laura lopez Diez, Angélica Zuluaga Cabrera PhD and Javier Murillo PhDc. Protocols were approved by the Ethics Committee of Animal Usage in Research (CEEA, University of Antioquia—Rectoral Resolution 18,084) Minute No.: 123-2019.
4.14. Statistical Analysis
Results were expressed as mean ± standard error media (S.E.M.), and statistical comparisons were made using a one-way ANOVA with a Dunnet’s post-test when comparing the median of each group or a Tukey’s post-test when comparing the median of each group with the media control group. In all cases, a difference statistically significant was assumed when p < 0.05. All data analysis was carried out using GraphPad PRISM 5 (GraphPad Software, Inc.; La Jolla, CA, USA).