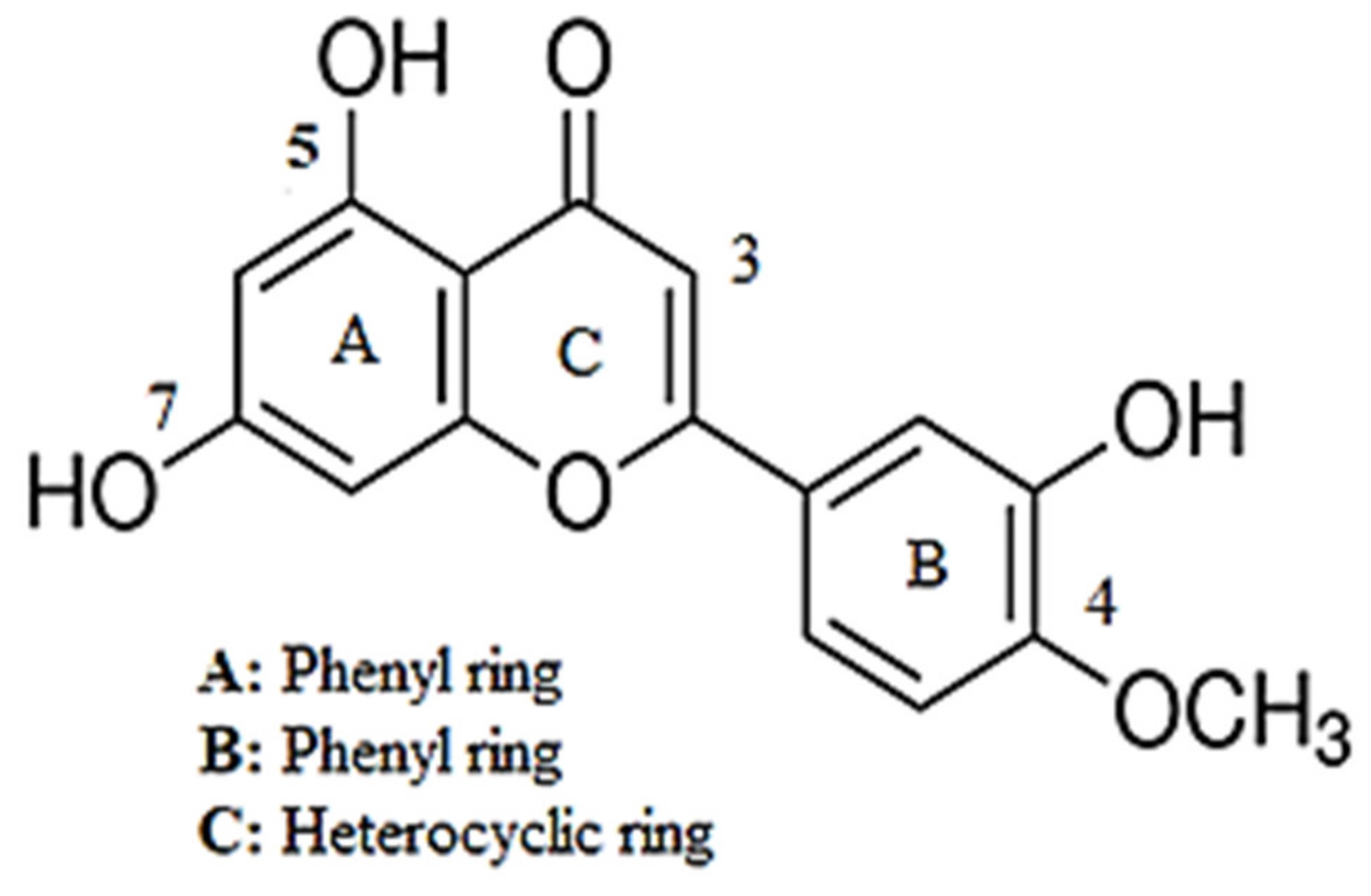
Investigation into the Antihypertensive Effects of Diosmetin and Its Underlying Vascular Mechanisms Using Rat Model
Abstract
1. Introduction
2. Results
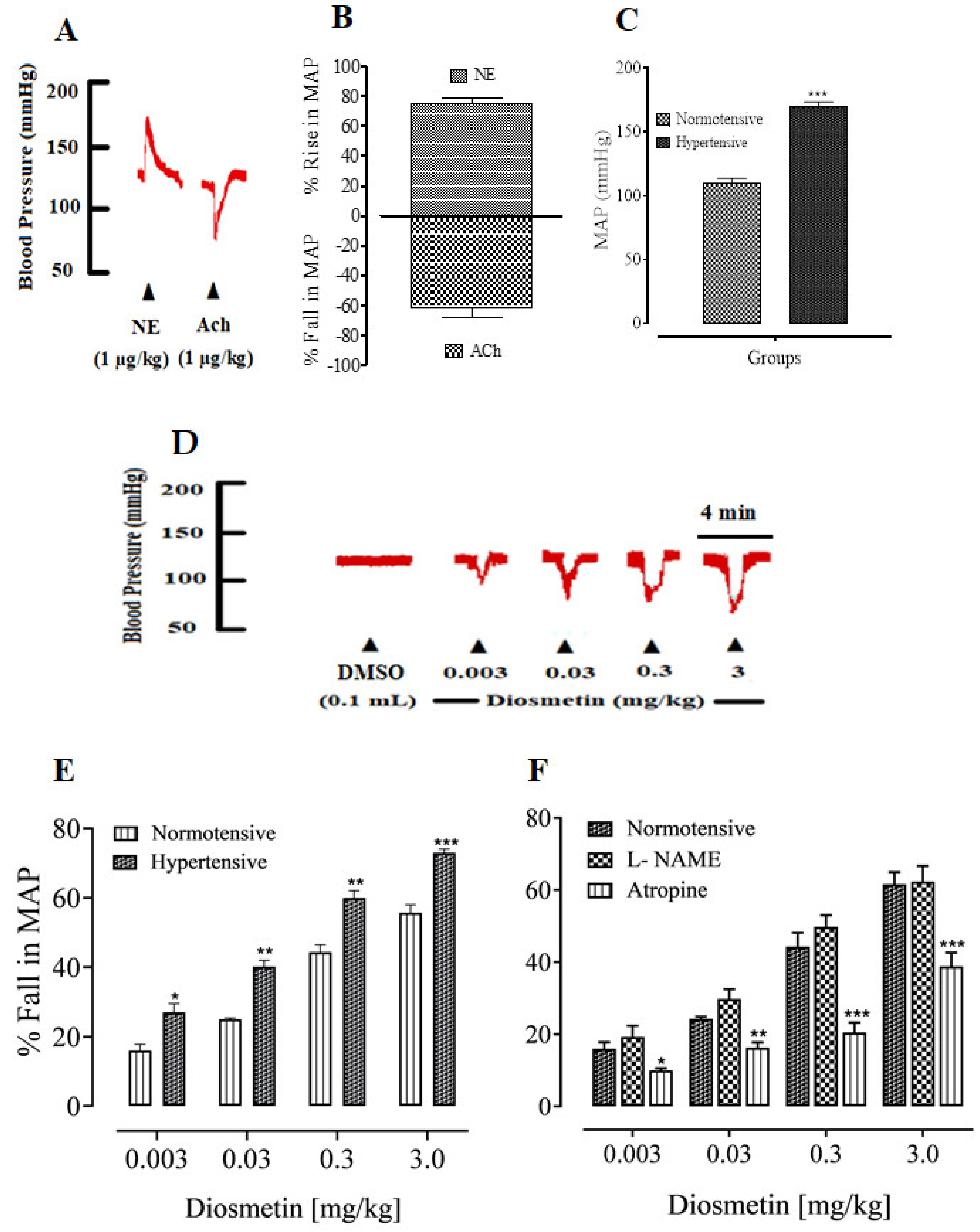
2.1. Antihypertensive Activities of Diosmetin
Effects of Diosmetin on the MAP of SD Rats
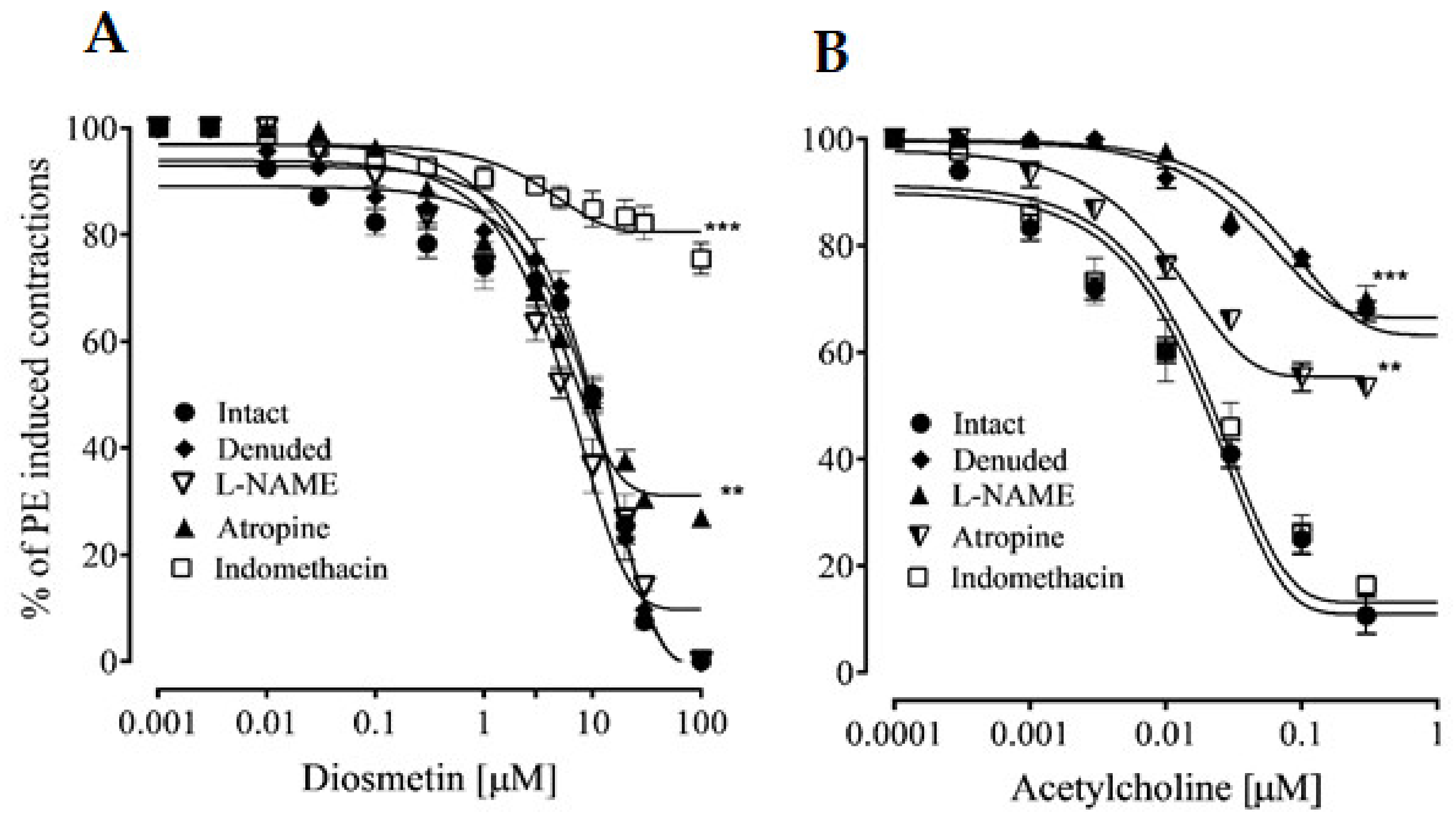
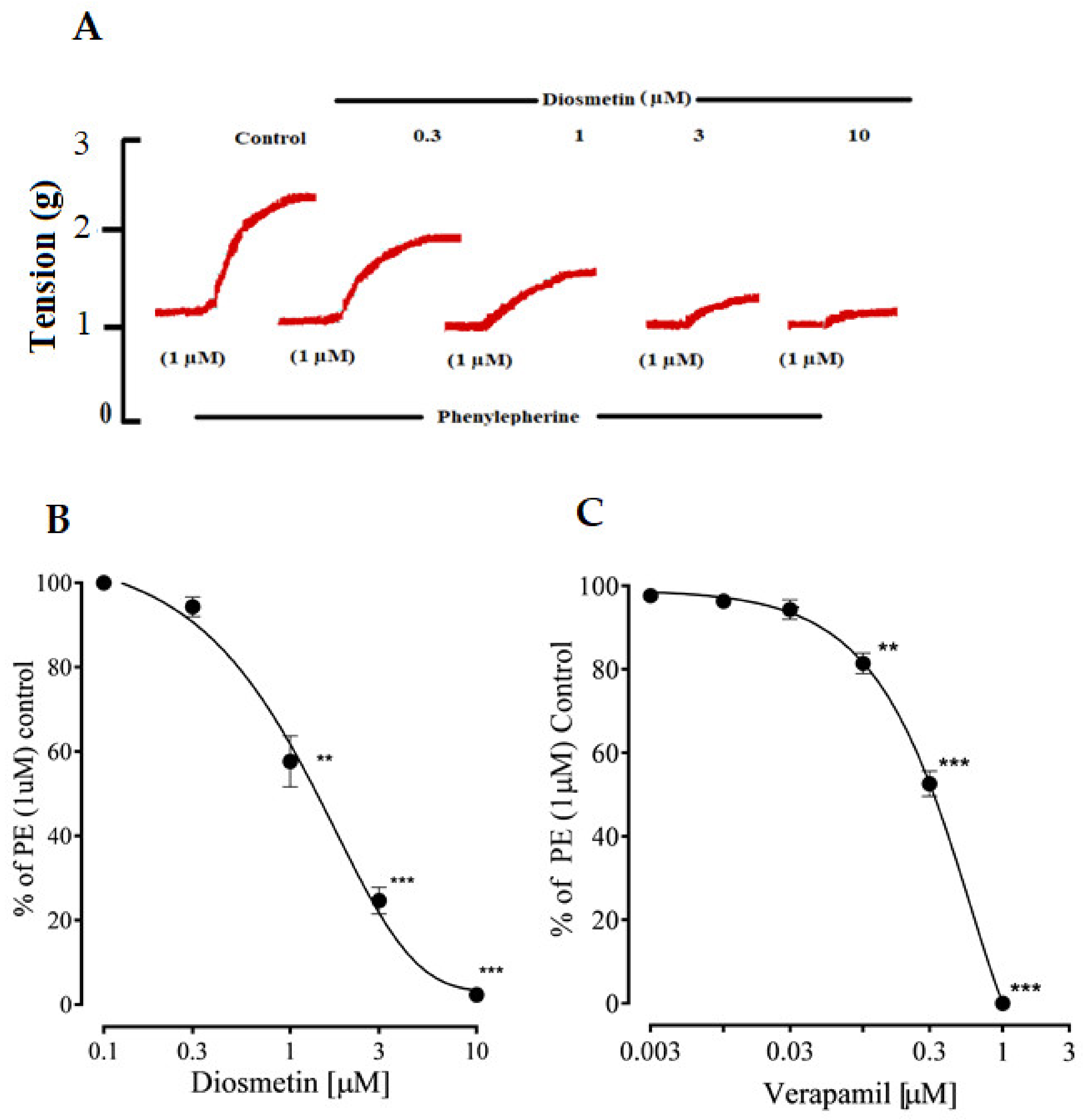
2.2. Vascular Reactivity
2.2.1. Effects of Diosmetin on Aortic Tissues in Rats
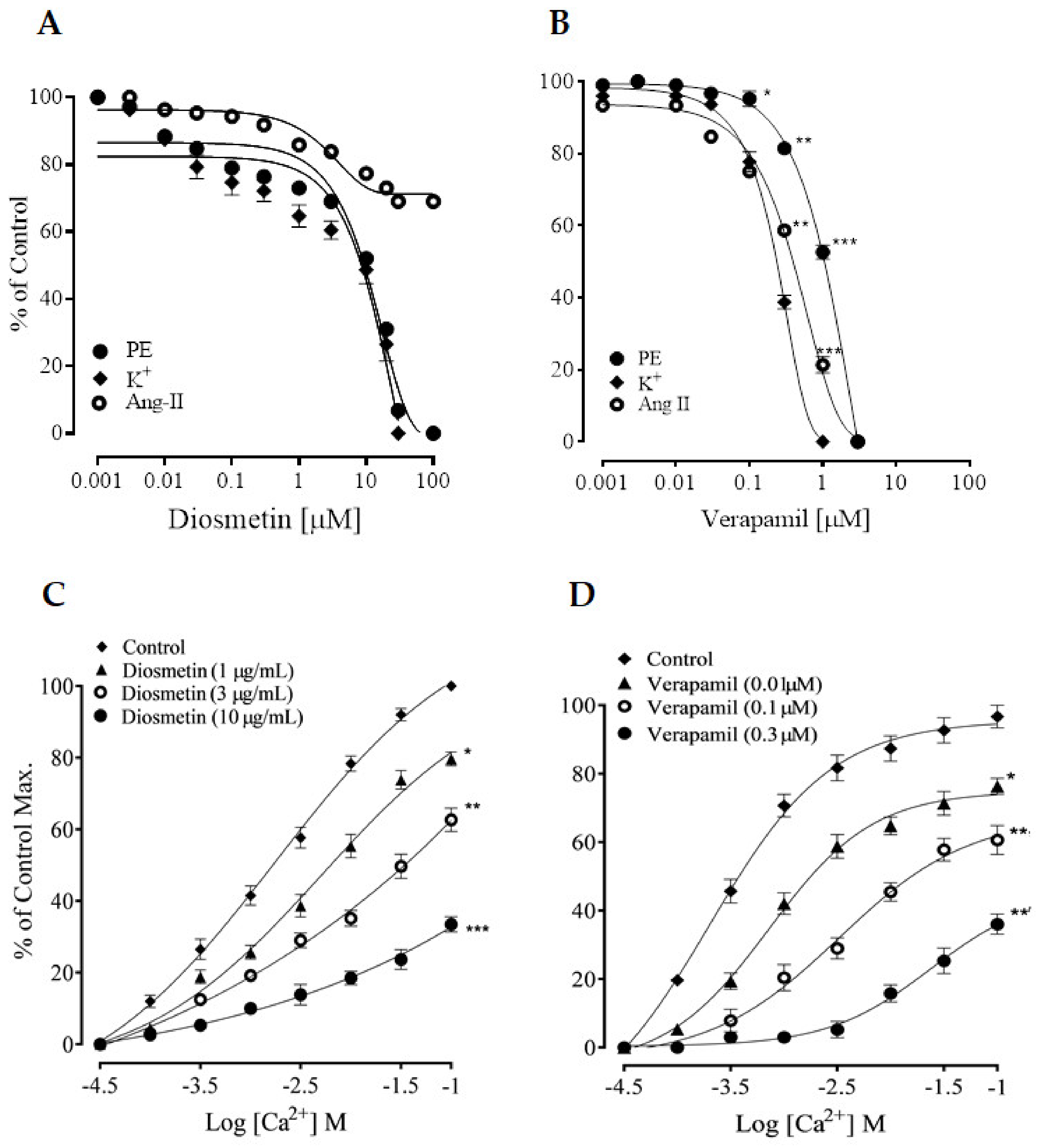
2.2.2. Effects of Diosmetin on Ca2+ Signaling and Ca2+ Channels
2.3. Effects of Diosmetin on Intracellular Calcium Stores
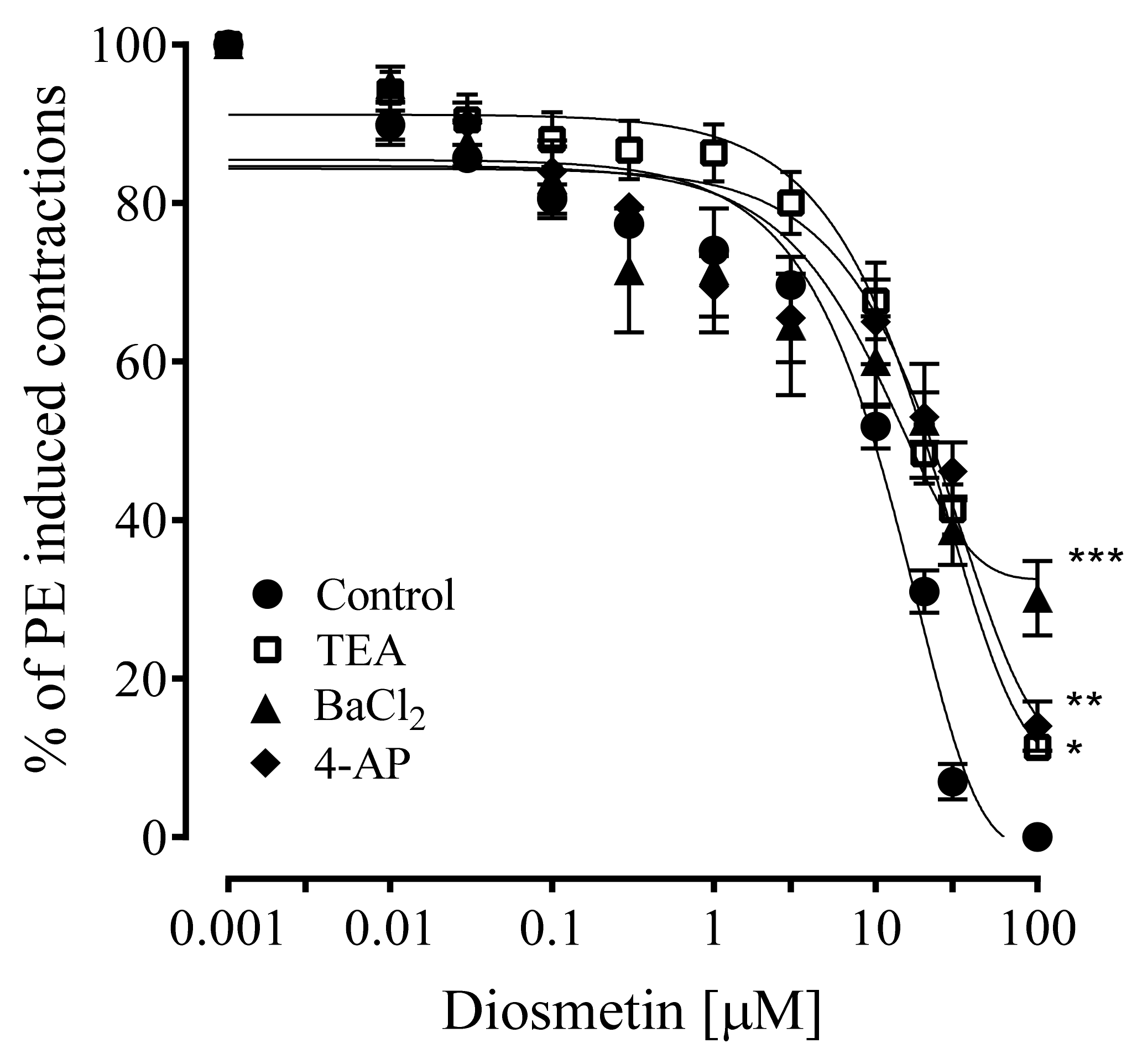
2.4. Effects of Diosmetin on Aortic Tissues of Rats Pretreated with K+ Channel Inhibitors
3. Discussion
4. Methodology
4.1. Drugs and Reagents
4.2. Experimental Rats
4.3. MAP Measurement in Normotensive SD Rats
4.4. MAP Measurement in Hypertensive SD Rats
4.5. Preparation of Isolated Aortic Rings of Rats
4.6. The Effects of Diosmetin on Precontractions Induced by Standard Vasoconstrictors
4.7. Diosmetin Responses in the Presence of Different Vascular Pathway Inhibitors
4.8. Effects of Diosmetin on Ca2+ Channels
4.9. Effects of Diosmetin on Intracellular Ca2+ Stores
4.10. Effects of Diosmetin on K+ Channel Inhibitors
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.; Ouyang, Y.Y.; Liu, J.; Zhao, G. Flavonoid intake and risk of CVD: A systematic review and meta-analysis of prospective cohort studies. Br. J. Nutr. 2014, 111, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.I.; Hayat, M.Q.; Tahir, M.; Mansoor, Q.; Ismail, M.; Keck, K.; Bates, R.B. Pharmacologically active flavonoids from the anticancer; Antioxidant and antimicrobial extracts of Cassia angustifolia Vahl. BMC Complement. Altern Med. 2016, 16, 460. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.H.; Liu, C.; Fan, R.; Zhu, L.F.; Yang, S.X.; Zhu, H.T.; Wang, D.; Yang, C.R.; Zhang, Y.J. Antioxidative Flavan-3-ol Dimers from the Leaves of Camellia fangchengensis. J. Agric. Food Chem. 2018, 66, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Mochizuki, M.; Okada, M.; Hiramitsu, M.; Morimitsu, Y.; Osawa, T. Isolation of Antioxidative Phenolic Glucosides from Lemon Juice and Their Suppressive Effect on the Expression of Blood Adhesion Molecules. Biosci. Biotechnol. Biochem. 2007, 71, 1911. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, R. Flavonoids of Citrus. I. Isolation of Diosmin from Lemons (Citrus Limon). J. Org. Chem. Res. 1956, 21, 1184–1185. [Google Scholar] [CrossRef]
- Caristi, C.; Bellocco, E.; Gargiulli, C.; Toscano, G.; Leuzzi, U. Flavone-Di-C-Glycosides in Citrus Juices from Southern Italy. Food Chem. 2006, 95, 431–437. [Google Scholar] [CrossRef]
- Khayyal, M.T.; El-Ghazaly, M.A.; Abdallah, D.M.; Nassar, N.N.; Okpanyi, S.N.; Kreuter, M.H. Blood pressure lowering effect of an olive leaf extract (Olea europaed) in L-NAME induced hypertension in rats. Arzneimittelforschung 2002, 52, 797–802. [Google Scholar] [CrossRef]
- Meirinhos, J.; Silva, B.M.; Valentao, P.; Seabra, R.M.; Pereira, J.A.; Dias, A.; Andrade, P.B.; Ferreres, F. Analysis and quantification of flavonoidic compounds from Portuguese olive (Olea europaea L.) leaf cultivars. Nat. Prod. Res. 2005, 19, 189–195. [Google Scholar] [CrossRef]
- Kato, Y.; Domoto, T.; Hiramitsu, M.; Katagiri, T.; Sato, K.; Miyake, Y.; Aoi, S.; Ishihara, K.; Ikeda, H.; Umei, N.; et al. Effect on blood pressure of daily lemon ingestion and walking. J. Nutr. Metab. 2014, 2014, 912684. [Google Scholar] [CrossRef]
- Avello, M.; Jofre, P.; Pastene, E.; Fernandez, P. Use of Citrus Limon, L. (lemon) in Treating Blood Pressure Sudden Rises. Int. J. Pharmacog. Phytochem. Res. 2014, 6, 606–611. [Google Scholar]
- Raskovic, A.; Milanovic, I.; Pavlovic, N.; Cebovic, T.; Vukmirovic, S.; Mikov, M. Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential. BMC Complement. Altern. Med. 2014, 14, 225. [Google Scholar] [CrossRef]
- Zarzuelo, A.; Duarte, J.; Jimenez, J.; Gonzalez, M.; Utrilla, M.P. Vasodilator effect of olive leaf. Planta Med. 1991, 57, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Mendel, M.; Chłopecka, M.; Dziekan, N.; Karlik, W. Antispasmodic effect of selected Citrus flavonoids on rat isolated jejunum specimens. Eur. J. Pharmacol. 2016, 791, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Ning, Z.; Chen, L.; Wei, Q.; Yuan, E.; Yang, J.; Ren, J. Intracellular antioxidant detoxifying effects of diosmetin on 2.; 2-azobis (2-amidinopropane) dihydrochloride (AAPH)-induced oxidative stress through inhibition of reactive oxygen species generation. J. Agric. Food Chem. 2014, 62, 8648–8654. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gong, X.B.; Huang, L.G.; Wang, Z.X.; Wan, R.Z.; Zhang, P.; Zhang, Q.Y.; Chen, Z.; Zhang, B.S. Diosmetin exerts anti-oxidative; Anti-inflammatory and anti-apoptotic effects to protect against endotoxin-induced acute hepatic failure in mice. Oncotarget 2017, 8, 30723. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, O.A.; Ivashev, M.N.; Ozimina, I.I.; Maslikova, G.V. Diosmetin Glycosides from Caucasian Vetch: Isolation and Study of Biological Activity. Pharm. Chem. J. 1998, 32, 595–597. [Google Scholar] [CrossRef]
- Wang, P.; Gao, C.; Wang, W.; Yao, L.P.; Zhang, J.; Zhang, S.D.; Li, J.; Fang, S.H.; Fu, Y.J. Juglone induces apoptosis and autophagy via modulation of mitogen-activated protein kinase pathways in human hepatocellular carcinoma cells. Food Chem. Toxicol. 2018, 116, 40–50. [Google Scholar] [CrossRef]
- Meng, J.C.; Zhu, Q.X.; Tan, R.X. New antimicrobial monoand sesquiterpenes from Soroseris hookeriana subsp.erysimoides. Planta Med. 2000, 44, 541–544. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Uemura, T.; Shimoda, H.; Kishi, A.; Kawahara, Y.; Matsuda, H. Medicinal foodstuffs. XVIII. Phytoestrogens from the aerial part of Petroselinum crispum MIll. (Parsley) and structures of 6″-acetylapiin and a new monoterpene glycoside, petroside. Chem. Pharm. Bull. 2000, 48, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Ge, A.; Liu, Y.; Zeng, X.; Kong, H.; Ma, Y.; Zhang, J.; Bai, F.; Huang, M. Effect of diosmetin on airway remodeling in a murine model of chronic asthma. Acta Biochim. Biophys. Sin. 2015, 47, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Juarez-Reyes, K.; Brindis, F.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Bye, R.; Linares, E.; Mata, R. Hypoglycemic, antihyperglycemic, and antioxidant effects of the edible plant Anoda cristata. J. Ethnopharmacol. 2015, 61, 36–45. [Google Scholar] [CrossRef]
- Ahmad, T.; Shah, A.J.; Khan, T.; Roberts, R. Mechanism underlying the vasodilation induced by diosmetin in porcine coronary artery. Eur. J. 2020, 884, 173400. [Google Scholar] [CrossRef]
- Vasanthi, H.R.; ShriShriMal, N.; Das, D.K. Phytochemicals from Plants to Combat Cardiovascular Disease. Curr. Med. Chem. 2012, 19, 2242–2251. [Google Scholar] [CrossRef] [PubMed]
- Arunlakhshana, O.; Schild, H.O. Some quantitative uses of drug antagonists. Brit. J. Pharmacol. 1959, 14, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Gross, G.J. Role of nitric oxide; Muscarinic receptors; And the ATP-sensitive K+ channel in mediating the effects of acetylcholine to mimic preconditioning in dogs. Circ. Res. 1993, 73, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, S.; Leopold, E.; Schmidt, K.; Brunner, F.; Mayer, B. Inhibition of nitric oxide synthesis by NG-nitro-L-arginine methyl ester (L-NAME): Requirement for bioactivation to the free acid, NG-nitro-L-arginine. Br. J. Pharmacol. 1996, 118, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Kruger-Genge, A.; Blocki, A.; Franke, R.P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.C.; Tsou, H.H.; Lin, R.J.; Wu, D.C.; Wu, B.N.; Lin, Y.T.; Chen, J. Endothelium-dependent and-independent vasorelaxation by a theophylline derivative MCPT: Roles of cyclic nucleotides, potassium channel opening and phosphodiesterase inhibition. Life Sci. 2005, 76, 931–944. [Google Scholar] [CrossRef] [PubMed]
- Onsa-ard, A.; Shimbhu, D.; Tocharus, J.; Sutheerawattananonda, M.; Pantan, R.; Tocharus, C. Hypotensive and vasorelaxant effects of sericin-derived oligopeptides in rats. ISRN Pharmacol. 2013, 1, 717529. [Google Scholar] [CrossRef]
- Prins, B.A.; Hu, R.M.; Nazario, B.; Pedram, A.; Frank, H.J.; Weber, M.A.; Levin, E.R. Prostaglandin E2 and prostacyclin inhibit the production and secretion of endothelin from cultured endothelial cells. J. Biol. Chem. 1994, 269, 11938–11944. [Google Scholar] [CrossRef]
- Roghani-Dehkordi, F.; Roghani, M. The vasorelaxant effect of simvastatin in isolated aorta from diabetic rats. ARYA Atheroscler. 2016, 12, 104–108. [Google Scholar] [PubMed]
- Silver, K.; Littlejohn, A.; Thomas, L.; Marsh, E.; Lillich, J.D. Inhibition of Kv channel expression by NSAIDs depolarizes membrane potential and inhibits cell migration by disrupting calpain signaling. Biochem. Pharmacol. 2015, 98, 614–628. [Google Scholar] [CrossRef]
- Toroudi, H.P.; Rahgozar, M.; Bakhtiarian, A.; Djahanguiri, B. Potassium channel modulators and indomethacin-induced gastric ulceration in rats. Scand. J. Gastroenterol. 1999, 34, 962–966. [Google Scholar] [PubMed]
- Menozzi, A.; Pozzoli, C.; Poli, E.; Passeri, B.; Gianelli, P.; Bertini, S. Diazoxide attenuates indomethacin-induced small intestinal damage in the rat. Eur. J. Pharmacol. 2011, 650, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Tian, J.; Zhu, Y.; Wang, C.; Xiao, R.; Herz, J.M.; Wood, J.D.; Zhu, M.X. Activation of TRPA1 channels by fenamate nonsteroidal anti-inflammatory drugs. Pflug. Arch. Eur. J. Phy. 2010, 459, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Earley, S.; Brayden, J.E. Transient Receptor Potential Channels in the Vasculature. Physiol. Rev. 2015, 95, 645–690. [Google Scholar] [CrossRef]
- Bohr, D.F. Vascular smooth muscle: Dual effects of calcium. Science 1963, 139, 597–599. [Google Scholar] [CrossRef] [PubMed]
- Yen, M.H.; Wu, C.C.; Chiou, W.F. Partially endothelium-dependent vasodilator effect of adenosine in rat aorta. Hypertension 1988, 11, 514–518. [Google Scholar] [CrossRef]
- Karaki, H.; Ozaki, H.; Hori, M.; Mitsui-Saito, M.; Amano, K.; Harada, K.; Miyamoto, S.; Nakazawa, H.; Won, K.J.; Sato, K. Calcium movements, distribution, and functions in smooth muscle. Pharmacol. Rev. 1997, 49, 157–230. [Google Scholar]
- Chan, S.S.K.; Choi, A.O.K.; Jones, R.L.; Lin, G. Mechanisms underlying the vasorelaxing effects of butylidenephthalide, an active constituent of Ligusticum chuanxiong, in rat isolated aorta. Eur. J. Pharmacol. 2006, 537, 111–117. [Google Scholar] [CrossRef]
- Zhang, Y.; Hermanson, M.E.; Eddinger, T.J. Tonic and Phasic Smooth Muscle Contraction Is Not Regulated by the PKCα—CPI-17 Pathway in Swine Stomach Antrum and Fundus. PLoS ONE 2013, 8, e74608. [Google Scholar] [CrossRef]
- Bolton, T.B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol. Rev. 1979, 59, 606–718. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.L.; Wang, Y.Y.; Cheng, J.; Zhao, Y.Y. Calcium channel blocking activity of calycosin, a major active component of Astragali Radix, on rat aorta. Acta Pharmacol. Sin. 2006, 27, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Bouchie, J.L.; Perez, A.S.; Clermont, A.C.; Izumo, S.; Hampe, J.; Feener, E.P. Role of the angiotensin AT1 receptor in rat aortic and cardiac PAI-1 gene expression. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2297–2302. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Adeagbo, A.; Triggle, C. Varying extracellular [K+]: A functional approach to separating EDHF- and EDNO-related mechanisms in perfused rat mesenteric arterial bed. J. Cardiovasc. Pharmacol. 1993, 21, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Ross, G.R.; Yallampalli, C. Endothelium-independent relaxation by adrenomedullin in pregnant rat mesenteric artery: Role of cAMP-dependent protein kinase A and calcium-activated potassium channels. J. Pharmacol. Exp. Ther. 2006, 317, 1269–1275. [Google Scholar] [CrossRef]
- Iozzi, D.; Schubert, R.; Kalenchuk, V.U.; Neri, A.; Sgaragli, G.; Fusi, F.; Saponara, S. Quercetin relaxes rat tail main artery partly via a PKG-mediated stimulation of KCa 1.1 channels. Acta Physiol. 2013, 208, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.M.; Fang, L.H.; Li, Y.J.; Du, G.H. Endothelium-dependent and -independent relaxation induced by pinocembrin in rat aortic rings. Vascul. Pharmacol. 2007, 46, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Novakovic, A.; Bukarica, L.G.; Kanjuh, V.; Heinle, H. Potassium channels-mediated vasorelaxation of rat aorta induced by resveratrol. Basic Clin. Pharmacol. Toxicol. 2006, 99, 360–364. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (NRC). Guide for the Care and Use of Laboratory Animals; National Academy Press: Washington, DC, USA, 1996. [Google Scholar]
- Shah, A.J.; Gilani, A.H. Blood pressure lowering and vascular modulator effects of Acorus calamus extract are mediated through multiple pathways. Cardiovasc. Pharmacol. 2009, 54, 38–46. [Google Scholar] [CrossRef]
- Parasuraman, S.; Raveendran, R. Measurement of invasive blood pressure in rats. J. Pharmacol. Pharmacother. 2012, 3, 172–177. [Google Scholar] [PubMed]
- Van Vliet, B.N.; Montani, J.P. The time course of salt-induced hypertension, and why it matters. Int. J. Obes. 2008, 32, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, R.; Qamar, H.M.D.; Khan, S.; Salma, U.; Khan, T.; Shah, A.J. Mechanisms underlying the antihypertensive properties of Urtica dioica. J. Transl. Med. 2016, 14, 254. [Google Scholar] [CrossRef] [PubMed]
- Furchgott, R.F.; Zawadzki, J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980, 299, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Faraci, F.M.; Heistad, D.D. Regulation of the Cerebral Circulation: Role of Endothelium and Potassium Channels. Physiol. Rev. 1998, 78, 53–97. [Google Scholar] [CrossRef] [PubMed]
- Yam, M.F.; Tan, C.S.; Ahmad, M.; Ruan, S. Vasorelaxant action of the chloroform fraction of Orthosiphon stamineus via NO/cGMP pathway, potassium and calcium channels. Am. J. Chin. Med. 2016, 44, 1413–1439. [Google Scholar] [CrossRef] [PubMed]
- Senejoux, F.; Girard, C.; Kerram, P.; Aisa, H.A.; Berthelot, A.; Bévalot, F.; Demougeot, C. Mechanisms of vasorelaxation induced by Ziziphora clinopodioides Lam. (Lamiaceae) extract in rat thoracic aorta. J. Ethnopharmacol. 2010, 32, 268–273. [Google Scholar] [CrossRef]
- Sonkusare, S.; Palade, P.T.; Marsh, J.D.; Telemaque, S.; Pesic, A.; Rusch, N.J. Vascular calcium channels and high blood pressure: Pathophysiology and therapeutic implications. Vasc. Pharmacol. 2006, 44, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.G.; Zhang, M.S.; Liu, Y.; Xue, W.X.; Liu, D.B.; Zhang, J.; Liang, Y.Q. Vasorelaxant effect of taurine is diminished by tetraethylammonium in rat isolated arteries. Eur. J. Pharmacol. 2008, 580, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Cole, W.C.; Clément-Chomienne, O.; Aiello, E.A. Regulation of 4-aminopyridine-sensitive.; delayed rectifier K+ channels in vascular smooth muscle by phosphorylation. Biochem. Cell Biol. 1996, 74, 439–447. [Google Scholar] [CrossRef]
- Tep-areenan, P.; Kendall, D.A.; Randall, M.D. Mechanisms of vasorelaxation to testosterone in the rat aorta. Eur. J. Pharmacol. 2003, 465, 125–132. [Google Scholar] [CrossRef]
| Dose (mg/kg) | Diosmetin | |
|---|---|---|
| BP (%) | HR (%) | |
| Control | 99.8 ± 0.07 | 99.5 ± 0.10 |
| 0.003 | 16 ± 2.47 * | 13 ± 1.90 * |
| 0.03 | 24 ± 1.77 * | 28 ± 2.50 * |
| 0.3 | 54 ± 2.03 *** | 38 ± 3.05 ** |
| 3 | 61 ± 1.03 *** | 50 ± 2.04 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, T.; Javed, A.; Khan, T.; Althobaiti, Y.S.; Ullah, A.; Almutairi, F.M.; Shah, A.J. Investigation into the Antihypertensive Effects of Diosmetin and Its Underlying Vascular Mechanisms Using Rat Model. Pharmaceuticals 2022, 15, 951. https://doi.org/10.3390/ph15080951
Ahmad T, Javed A, Khan T, Althobaiti YS, Ullah A, Almutairi FM, Shah AJ. Investigation into the Antihypertensive Effects of Diosmetin and Its Underlying Vascular Mechanisms Using Rat Model. Pharmaceuticals. 2022; 15(8):951. https://doi.org/10.3390/ph15080951
Chicago/Turabian StyleAhmad, Taseer, Adil Javed, Taous Khan, Yusuf S. Althobaiti, Aman Ullah, Farooq M. Almutairi, and Abdul Jabbar Shah. 2022. "Investigation into the Antihypertensive Effects of Diosmetin and Its Underlying Vascular Mechanisms Using Rat Model" Pharmaceuticals 15, no. 8: 951. https://doi.org/10.3390/ph15080951
APA StyleAhmad, T., Javed, A., Khan, T., Althobaiti, Y. S., Ullah, A., Almutairi, F. M., & Shah, A. J. (2022). Investigation into the Antihypertensive Effects of Diosmetin and Its Underlying Vascular Mechanisms Using Rat Model. Pharmaceuticals, 15(8), 951. https://doi.org/10.3390/ph15080951







